Abstract
The aim of this study was to investigate the effect of sodium dodecyl sulfate (SDS), citric acid, and hydrogen peroxide (H2O2), alone or in combination, on reducing the population of four foodborne pathogens, including Escherichia coli, Listeria monocytogenes, Salmonella typhimurium, and Staphylococcus aureus on eggshells. In each series of tests, eight fresh eggs were inoculated with each bacterial strain by being immersed in a bacterial suspension and exposed to SDS (1.5%), H2O2 (0.5%), citric acid (1%), or sequential treatments with SDS + citric acid and SDS + H2O2. Viable cell counts were made and the bacterial concentrations results compared to pre-treatment levels. Results showed that all washing solutions except citric acid significantly (P<0.05) reduced the concentration of all tested bacteria (~2-4 log reductions). The sensitivity of S. typhimurium and E. coli to SDS and H2O2 was similar (~2.5 log reduction). Listeria monocytogenes (4.1 Log reduction) and S. aureus (4.3 Log reduction) were more sensitive to SDS and H2O2, respectively. The combination of SDS and citric acid or H2O2 in comparison to SDS alone, generally did not produce significant additive reductions in the viability of the bacteria on eggshells. These data suggest that SDS potentially could be used alone or in combination with citric acid or H2O2 as an effective and inexpensive method to reduce bacteria, such as L. monocytogenes, on eggshells. Additionally, application of SDS may be useful for bacterial decontamination of other materials and surfaces in food industries.
Key Words: Decontamination, Eggshells, Foodborne pathogens, Sodium dodecyl sulfate
Introduction
Eggs are highly nutrient dense foods. They contain many vitamins and trace nutrients that are essential for health. Intact eggs, however, can be contaminated by various pathogens during laying (vertical transmission) (Gantois et al., 2009 ▶) or processing, transportation, and storage (horizontal transmission) (Davies and Breslin, 2003 ▶; Messens et al., 2007 ▶). The most common sources of microbial contamination of eggshells are soil, faeces, nesting particles, and hands of workers. Contamination can adversely affect the shelf life and safety of eggs. Washing of eggs with an appropriate detergent can reduce the microbial load at the eggshells surface (Gole et al., 2014 ▶).
Previously, various chemical and physical sanitation procedures have been investigated against foodborne pathogens such as Salmonella, Listeria and Escherichia coli on eggshells. Example decontamination procedures include electrolyzed water (Cao et al., 2009 ▶), UV irradiation (De Reu et al., 2006 ▶), hydrogen peroxide (H2O2) (Padron, 1995 ▶), and combinations of ozone and UV irradiation (Rodriguez-Romo and Yousef, 2005 ▶).
Sodium dodecyl sulfate (SDS) is an anionic surfactant used to disrupt membranes and denature proteins (Woo et al., 2000 ▶). Sodium dodecyl sulfate also is a common ingredient in cosmetics, washing detergents, and personal-care products, and is used in the laboratory environment as a denaturing agent in gel electrophoresis and other protein solubilization techniques. It is considered by the United Nations Environment Program to be “of no concern with respect to human health” (Morales-delaNuez et al., 2011 ▶). Use of SDS as a disinfectant in foodstuffs, equipment, and surfaces associated with food industry has received more attention in recent years. To date, the efficacy of SDS alone, or in combination with other materials, in reducing bacterial contamination has been studied in beef (Stelzleni et al., 2013 ▶), chicken breast meat (Lu and Wu, 2012 ▶), and blueberries (Li and Wu, 2013 ▶).
Under standard doses, the use of citric acid as a flavoring in foodstuffs is common and harmless. The combination of citric acid with other disinfectants has a synergistic effect on reduction of microorganisms (Park et al., 2009). Hydrogen peroxide is another inexpensive chemical substance with strong bactericidal properties, and has been used to reduce microbial populations in different foods (Lin et al., 2002 ▶; Ukuku et al., 2005 ▶).
Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, and Listeria monocytogenes are among the most common foodborne pathogens throughout the globe. These bacteria are associated with faeces and soil and, as such, often contaminate eggshells. The aim of this study was to evaluate the capacity of SDS, alone or in combination with citric acid or H2O2, in reducing bacterial concentrations on eggshells experimentally inoculated with four foodborne pathogens: E. coli, S. aureus, S. typhimurium, and L. monocytogenes at ambient temperature (25°C).
Materials and Methods
Preparation of bacterium suspension for inoculation
Single, isolated colonies were transferred from agar plates to inoculation tubes containing 5 ml trypticase soy broth (TSB) and incubated for 20 h at 37°C with shaking (10 RPM). An aliquot (1.5 ml) was transferred to 60 ml TSB and incubated for an additional 20 h under the same conditions. The bacterial suspension was centrifuged (4000 RPM for 7 min), the supernatant was discarded, and the pellet was resuspended in 10 ml phosphate-buffered saline (PBS). For enumeration of bacterial populations, cultures were serially diluted and aliquots (0.1 ml) were transferred to nutrient agar. This concentrated suspension was used for eggs inoculation as described below.
Preparation and inoculation of eggs
In each series of tests, eight medium-sized fresh eggs (total of 96 eggs) were obtained from local markets. Eggs were prepared using a previously described protocol (Rodriguez-Romoand and Youssef, 2005 ▶). Briefly, all eggs were washed under tap water and then were placed in 70% ethanol for 30 min. Sanitized eggs were rinsed thoroughly with sterile distilled water, transferred to a reticular plastic tray, and aseptically dried under laminar flow for approximately 30 min before inoculation. The concentrated bacterial suspension was added to 500 ml of sterile PBS to reach concentrations outlined in Table 1. Eggs were placed in the different bacterial suspensions for 30 min. Subsequently, the eggs were put under a laminar flow for 1 h to dry at ambient temperature (Upadhyaya et al., 2013 ▶).
Table 1.
List of the actual bacterial concentrations
| Strain | Actual bacterial concentration (Log CFU/ml) |
| Escherichia coli | 9 ± 0.2 |
| Listeria monocytogenes | 8.1 ± 0.5 |
| Salmonella typhimurium | 8.5 ± 0.1 |
| Staphylococcus aureus | 8.7 ± 0.1 |
Eggs treatments
Each egg was placed in individual sterile stomacher plastic bags containing 200 ml disinfectant treatment (SDS [1.5%], H2O2 [0.5%], citric acid [1.0%]) or PBS (as control 1) and shaken gently for 5 min at ambient temperature (25°C). To examine the combined effects of the solutions (SDS [1.5%] + citric acid [1.0%] or SDS [1.5%] + H2O2 [0.5%]), inoculated eggs were immersed in the first solution as described above and then aseptically transferred into another sterile stomacher bag containing the second solution and further incubated for 5 min with shaking (Park et al., 2005 ▶). Concurrently, individual eggs were also immersed two times in PBS as control 2, and another inoculated egg receiving no disinfectant treatment (PBS or solution) served as an additional negative control.
Enumeration of bacteria
After treatment, each egg was transferred to a sterile plastic bag containing 100 ml of PBS and was gently rubbed by hand for 1 min (Park et al., 2005 ▶). Eggs were removed, the rinsate was serially diluted and bacterial survival was assessed by viable counts on trypticase soy agar (TSA) plates. The results were presented as CFU/ml rinsate.
Statistical analysis
All experiments were performed in triplicate. Final bacterial concentrations in treated eggs were compared to bacterial concentrations in eggs immersed once in PBS (control 1), eggs immersed twice (control 2), and inoculated eggs receiving no treatments. Results were analyzed using a one-way analysis of variance (ANOVA) and the least significant difference test (LSD) using SPSS (version 16; SPSS Inc., Chicago, USA). Results were considered significantly different at P<0.05.
Results
Effect of various treatments on E. coli
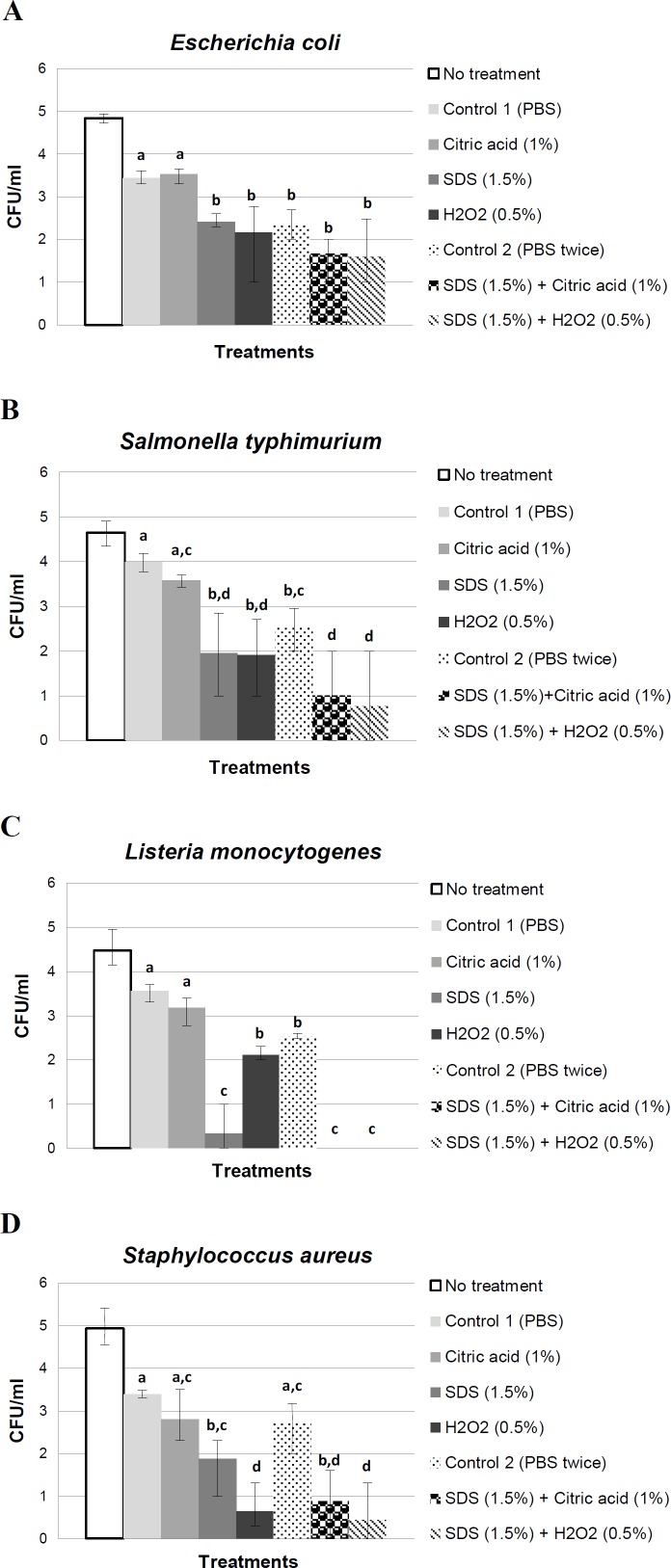
Changes in the viable count of the bacterium after each treatment are shown in Fig. 1A. In eggs inoculated with E. coli, treatment with SDS, H2O2, or citric acid reduced bacterial concentrations by 1, 1.3 and -0.1 Log CFU/ml, respectively. Treatment with SDS and H2O2 resulted in significantly greater (P<0.05) reductions in bacterial concentrations compared to treatment with PBS alone. Treatment with SDS and citric acid or SDS and H2O2 significantly reduced bacterial concentrations, but the differences were not significant when compared to eggs washed twice with PBS alone or eggs treated with SDS or H2O2 alone.
Fig. 1.
Effect of different treatments on reducing. (A) E. coli, (B) S. typhimurium, (C) L. monocytogenes, and (D) S. aureus on experimentally inoculated on eggshells. Bars with different subscripts are considered statistically different at P<0.05
Effect of various treatments on Salmonella typhimurium
Changes in the viable count of the bacterium after each treatment are shown in Fig. 1B. Salmonella typhimurium concentrations in egg treated with SDS, H2O2, and citric acid were 2, 2.1 and 0.4 Log CFU/ml lower than S. typhimurium concentrations in eggs washed with PBS alone indicating a significant (P<0.05). Additionally, the combination of SDS and citric acid or SDS and H2O2 treatments was more effective in reducing bacterial concentrations (P<0.05) compared to eggs washed either once or twice with PBS.
Effect of various treatments on Listeria monocytogenes
As is shown in Fig. 1C, SDS had a potentially greater impact in reducing Listeria on contaminated eggs. Treatment with SDS or H2O2 significantly (P<0.05) reduced Listeria concentrations by 3.2 and 1.4 Log CFU/ml, respectively, compared to control eggs. Additionally, treatment of Listeria contaminated eggs with SDS and citric acid or SDS and H2O2 reduced bacterial concentrations to undetectable levels. These results indicate that SDS may have a greater antibacterial effect on Listeria compared to other bacterial species tested here.
Effect of various treatments on Staphylococcus aureus
Staphylococcus aureus appeared more sensitive to H2O2 than other solutions tested. As detailed in Fig. 1D, S. aureus concentrations in H2O2 treated eggs were significantly reduced (~3 Log CFU/ml; P<0.05) compared to S. aureus concentrations in eggs washed either once or twice with PBS alone. Treatment with SDS was also effective compared to treatment with PBS alone; however, S. aureus concentrations were not reduced in eggs treated with citric acid. There was no additive effects with the various treatments that were used in combination.
Discussion
Contamination of eggshells can reduce shelf-life and safety of eggs and their byproducts. Therefore, application of appropriate antimicrobial agents for decontamination of egg surfaces can play an important role in achieving public health goals. In this study, three different disinfectants, alone and in combination, were used to evaluate the effectiveness of sanitizers in reducing bacterial concentrations on eggshells experimentally inoculated with four different foodborne pathogens.
According to the results of this study, sequential immersion of eggs in SDS and either citric acid or H2O2 resulted in the largest reductions in E. coli on eggshells compared to other tested treatments. Similar results were observed on eggshells inoculated with S. typhimurium. Both bacteria were not sensitive to citric acid alone, but notable reductions in target bacteria were observed when citric acid was used in combination with SDS. These similar responses to treatments by E. coli and S. typhimurium could be due to similarity of the bacteria as both pathogens are gram-negative with similar phenotypic (e.g., cell membranes) and genetic makeup.
Other groups have shown similar effects of SDS, H2O2, and citric acid, or similar compounds in reducing Salmonella spp. or E. coli in other food types. For example, treatment of alfalfa seeds with levulinic acid and SDS for 5 min resulted in 3 Log reductions of E. coli O157: H7 and S. typhimurium (Zaho et al., 2010 ▶). Other groups, however, have reported reduced efficacy of SDS as a bactericide. Lu and Wu (2012) ▶ treated chicken breasts with thymol-based washing solutions with and without SDS. Both solutions achieved approximately 2.2 log reductions of Salmonella on chicken breasts. The authors mentioned that the combination of thymol and acetic acid had great potential to be a natural alternative to chlorine-based washing solution for reducing Salmonella contamination in chicken breast meat, and the addition of SDS did not result in an additive bactericidal effect. In contrast, Li and Wu (2013) ▶ evaluated Salmonella inactivation on blueberries washed with SDS in combination with chlorine, lactic acid, acetic acid, citric acid, and/or H2O2. Their results showed that the use of acetic acid or H2O2 in combination with SDS may have practical potential as an alternative to the use of chlorine-based washing solution for blueberries (Li and WU, 2013 ▶). In another study, Stelvani et al. (2013) ▶ examined the effect of vinegar and SDS with levulinic acid on S. typhimurium and shelf-life and sensory characteristics of ground beef. SDS plus levulonic acid resulted in the largest reductions of Salmonella. However, beef samples treated with liquid buffered vinegar and powdered buffered vinegar had the least psychrotrophic growth (Stelzleni et al., 2013 ▶). Sodium dodecyl sulfate has also been used to enhance the lethality of organic acids against S. enterica inoculated on chicken skin. Results showed that combining organic acids, especially lactic or acetic acid, with SDS might be suitable for application by chicken processors to effectively decontaminate chicken carcasses or cuts (Zaki et al., 2015 ▶).
Compared to other pathogens, SDS was most effective in reducing L. monocytogenes on eggshells, reducing bacterial concentrations to undetectable levels. Listeria monocytogenes was resistant to treatment with citric acid alone and it could be due to the inherent resistance of the bacterium to acidic conditions (Koutsoumanis et al., 2003 ▶). Various groups have examined the sensitivity of L. monocytogenes to SDS (Maktabi, 2003 ▶; Byelashov et al., 2008 ▶; Kennedy et al., 2011 ▶). A combination of SDS with citric acid or H2O2, however, resulted in significant reductions in bacterial concentrations. These results suggest that both sanitizers may contribute to the enhanced effectiveness of the sequential treatments, probably by mutual reinforcement. The U.S. Department of Agriculture Food Safety and Inspection Service (FSIS) enforces a zero-tolerance rule for L. monocytogenes in ready-to-eat meats (Byelashov et al., 2008 ▶). Thus, effective and alternative means to reduce L. monocytogenes, such as those described here, would be advantageous.
In our studies, S. aureus showed more sensitivity to H2O2 compared with SDS. Sequential treatments by SDS and H2O2 or SDS and citric acid did not provide additional significant reduction in the viability of S. aureus on eggshells. Sensitivity of the S. aureus to H2O2 has been reported before. Sander and Wilson (1999 ▶) observed that H2O2 (3%) caused significant reductions in the number of S. aureus on eggs placed in incubators. The authors did, however, report that the eggs lost a great amount of their moisture during the incubation period, but hatchability was not affected. Additionally, the use of H2O2 as a hatchery sanitizer did not affect broiler livability, body weight, or feed conversion. In 2004, it was reported that H2O2 vapor decontamination effectively reduced methicillin-resistant S. aureus (MRSA) from rooms, furniture, and equipment (French et al., 2004 ▶).
The results of our study showed that SDS, H2O2, and citric acid, either alone or in combination, each show promise as potential disinfectant egg washes. The efficacy of each treatment was dependent on the targeted pathogen (e.g., L. monocytogenes was highly sensitive to SDS in vitro and on the eggshell). Our results extend beyond eggshells as SDS may be useful for decontamination of other materials and surfaces in the food industry.
Acknowledgements
We would like to express our appreciation of the Research Council of Shahid Chamran University of Ahvaz, Iran, for their financial support.
References
- Byelashov, OA , Kendall, PA , Belk, KE , Scanga, JA , Sofos, JN Control of Listeria monocytogenes on vacuum-packaged frankfurters sprayed with lactic acid alone or in combination with sodium lauryl sulfate. J. Food Prot. 2008;71:728–734. doi: 10.4315/0362-028x-71.4.728. [DOI] [PubMed] [Google Scholar]
- Cao, W , Zhu, ZW , Shi, ZX , Wang, CY , Li, BM Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int. J. Food Microbiol. 2009;130:88–93. doi: 10.1016/j.ijfoodmicro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Davies, RH , Breslin, M Investigation of Salmonella contamination and disinfection in farm egg-packing plants. J. Appl. Microbiol. 2003;94:191–196. doi: 10.1046/j.1365-2672.2003.01817.x. [DOI] [PubMed] [Google Scholar]
- De Reu, K , Grijspeerdt, K , Herman, L , Heyndrickx, M , Uyttendaele, M , Debevere, J , Bolder, NM The effect of a commercial UV disinfection system on the bacterial load of shell eggs. Let. Applied Microbiol. 2006;42:144–148. doi: 10.1111/j.1472-765X.2005.01825.x. [DOI] [PubMed] [Google Scholar]
- French, GL , Otter, JA , Shannon, KP , Adams, NMT , Watling, D , Parks, MJ Tackling con-tamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hospital Infe. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gantois, I , Ducatelle, R , Pasmans, F , Haesebrouck, F , Gast, R , Humphrey, TJ , Van Immerseel, F Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009;33:718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- Gole, VC , Chousalkar, KK , Roberts, JR , Sexton, M , May, D , Tan, J , Kiermeier, A Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella typhimurium strains. PLoS One. 2014;9:e90987. doi: 10.1371/journal.pone.0090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D , Cronin, UP , Wilkinson, MG Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to simulated food processing treatments, determined using fluorescence-activated cell sorting and plate counting. Appl. Environ. Microbiol. 2011;77:4657–4668. doi: 10.1128/AEM.00323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoumanis, KP , Kendall, PA , Sofos, JN Effect of food processing-related stresses on acid tolerance of Listeria monocytogenes. Appl. Envion. Microbiol. 2003;69:7514–7516. doi: 10.1128/AEM.69.12.7514-7516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y , Wu, C Enhanced inactivation of Salmonella typhimurium from blueberries by combinations of sodium dodecyl sulfate with organic acids or hydrogen peroxide. Food Res. Int. 2013;54:1553–1559. [Google Scholar]
- Lin, CM , Moon, SS , Doyle, MP , McWatters, KH Inactivation of Escherichia coli O157:H7, Salmonella enterica serotype enteritidis, and Listeria monocytogenes on lettuce by hydrogen peroxide and lactic acid and by hydrogen peroxide with mild heat. J. Food Prot. 2002;65:1215–1220. doi: 10.4315/0362-028x-65.8.1215. [DOI] [PubMed] [Google Scholar]
- Lu, Y , Wu, C Reductions of Salmonella enterica on chicken breast by thymol, acetic acid, sodium dodecyl sulfate or hydrogen peroxide combinations as compared to chlorine wash. Int. J. Food Microbiol. 2012;152:31–34. doi: 10.1016/j.ijfoodmicro.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Maktabi, S. Combination methods for microbial decontamination. Doctoral Dissertation. University of Glasgow; 2003. pp. 237–238. [Google Scholar]
- Messens, W , Grijspeerdt, K , De Reu, K , De Ketelaere, B , Mertens, K Eggshell penetration of various types of hens’ eggs by Salmonella enterica serovar enteritidis. J. Food Prot. 2007;70:623–628. doi: 10.4315/0362-028x-70.3.623. [DOI] [PubMed] [Google Scholar]
- Morales-DelaNuez, A , Moreno-Indias, I , Sánchez-Macías, D , Capote, J , Juste, MC , Castro, N , Argüello, A Sodium dodecyl sulfate reduces bacterial contamination in goat colostrum without negative effects on immune passive transfer in goat kids. J. Dairy Sci. 2011;94:410–415. doi: 10.3168/jds.2010-3624. [DOI] [PubMed] [Google Scholar]
- Padron, M Egg dipping in hydrogen peroxide solution to eliminate Salmonella typhimurium from eggshell membranes. Avian Dis. 1995;10:627–630. [PubMed] [Google Scholar]
- Park, YB , Guo, JY , Rahman, SME , Ahn, J , Oh, DH Synergistic effect of electrolyzed water and citric acid against Bacillus cereus cells and spores on cereal grains. J. Food Sci. 2009;74:185–189. doi: 10.1111/j.1750-3841.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- Park, CM , Hung, YC , Lin, CS , Brackett, RE Efficacy of electrolyzed water in inactivating Salmonella enteritidis and Listeria monocytogenes on shell eggs. J. Food Prot. 2005;68:986–990. doi: 10.4315/0362-028x-68.5.986. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romo, LA , Yousef, AE Inactivation of Salmonella enterica serovar enteritidis on shell eggs by ozone and UV radiation. J. Food Prot. 2005;68:711–717. doi: 10.4315/0362-028x-68.4.711. [DOI] [PubMed] [Google Scholar]
- Sander, JE , Wilson, JL Effect of hydrogen peroxide disinfection during incubation of chicken eggs on microbial levels and productivity. Avian Dis. 1999;28:227–233. [PubMed] [Google Scholar]
- Stelzleni, AM , Ponrajan, A , Harrison, MA Effects of buffered vinegar and sodium dodecyl sulfate plus levulinic acid on Salmonella typhimurium survival, shelf-life, and sensory characteristics of ground beef patties. Meat Sci. 2013;95:1–7. doi: 10.1016/j.meatsci.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Ukuku, DO , Bari, ML , Kawamoto, S , Isshiki, K Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int. J. Food Microbiol. 2005;104:225–233. doi: 10.1016/j.ijfoodmicro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Upadhyaya, I , Upadhyay, A , Kollanoor-Johny, A , Baskaran, SA , Mooyottu, S , Darre, MJ , Venkitanarayanan, K Rapid inactivation of Salmonella enteritidis on shell eggs by plant-derived antimicrobials. Poult. Sci. 2013;92:3228–3235. doi: 10.3382/ps.2013-03126. [DOI] [PubMed] [Google Scholar]
- Woo, IS , Rhee, IK , Park, HD Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl. Environ. Microbiol. 2000;66:2243–2247. doi: 10.1128/aem.66.5.2243-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, HM , Mohamed, HM , El-Sherif, AM Improving the antimicrobial efficacy of organic acids against Salmonella enterica attached to chicken skin using SDS with acceptable sensory quality. LWT-Food Sci. Technol. 2015;64:558–564. [Google Scholar]
- Zhao, T , Zhao, P , Doyle, MP Inactivation of Escherichia coli O157: H7 and Salmonella typhimurium DT 104 on alfalfa seeds by levulinic acid and sodium dodecyl sulfate. J. Food Prot. 2010;73:2010–2017. doi: 10.4315/0362-028x-73.11.2010. [DOI] [PubMed] [Google Scholar]