Abstract
Natural killer (NK) cells play a crucial role in regulating immune functions. Few studies have characterized canine NK cells. We previously demonstrated that canine peripheral blood lymphocytes (PBLs) with a low surface CD5 density (CD5lo) are considered a critical NK population. Natural killer cells in most mammals do not express T-cell markers, but canine CD5lo cells express surface molecules, such as CD3 T-cell receptors. These features make canines unique models for the study of comparative immunology in NK cells. In this study, we discovered that CD5lo and CD21 double-negative (CD5lo-ne/CD2−) cells were originally low in NK cytotoxicity and their NK cytotoxicity was highly activated when co-cultured with CD5lo NK cells. The cytotoxicity was not activated when co-cultured with other cell types, such as high surface CD5 density (CD5hi) cells. The CD5lo-negative (CD5lo-ne) population comprises CD5− and CD5hi cells. CD5-cells were low in NK cytotoxicity initially or after culturing with interleukin-2 (IL-2) without CD5lo cells; however, the addition of CD5lo cells in a similar medium markedly enhanced the NK activity. By contrast, CD5hi cells were always NK inactive, irrespective of them being cultured with CD5lo cells or not. We further verified that only the CD5−CD21− cells, which were separated from CD5−CD21+ cells in the entire CD5− population, showed activated NK activity through CD5lo cell induction. This study is the first to reveal that canine NK cells enhanced NK-inert cells to become NK-cytotoxic cells. Additionally, it is concluded that in beagles, except for CD5lo cells, CD5−CD21− cells show NK activity.
Key Words: CD5−CD21− cells, Cytotoxicity, Low surface CD5 density, Natural killer cells
Introduction
Natural killer (NK) cells are major effect or cells that are critical to the innate immune system and are typically considered to play a fundamental role, particularly in antiviral and antitumor host responses (Orange, 2006 ▶). In humans and mice, NK cells are a subset of large granular lymphocytes (LGLs) that are absent in both T-cell (CD3, CD4, and CD8) and B-cell (CD21) markers (Ribatti, 2017 ▶). These cells were recently reported to play pivotal roles in connecting innate and adaptive immune responses (Biron, 2010 ▶). Activated NK cells represent the primary arm of the innate immune response by killing infected or transformed cells or producing inflammatory cytokines, such as interferon (IFN)-γ (Christaki et al., 2015 ▶); therefore, they influence adaptive immune responses by modulating dendritic cells to produce cytokines and chemokines (Stojanovic et al., 2014 ▶). The NK cell activity is tightly controlled by a series of activating and inhibitory signals. Different NK phenotypes in different anatomical locations from various species determine the activation status and functions of NK cells (Biron, 2010 ▶).
Regarding phenotypic expression, NK cells typically do not express the CD3 antigen or any of the known T-cell receptor (TCR) chains (α, β, γ, or δ) and do not have detectable surface markers for B-cells (Brudno et al., 2016 ▶). Thus, typical NK cells are a lymphocytic population that is characterized as non-T and non-B (NTNB) cells (Macêdo et al., 2013 ▶).
Canine NK cell markers are incompletely characterized. Canine peripheral blood lymphocytes (PBLs) can be categorized as three distinct populations, including low surface CD5 density (CD5lo), high surface CD5 density (CD5hi), and CD5 negative (CD5−) cells. As for CD5− cells, we observed two groups: one did not express T- and B-cell markers, the so-called NTNB cells (CD3−, CD4−, CD8−, and CD21−), and the other expressed B-cell markers (CD3−, CD4−, CD8−, and CD21+). One study reported that the characteristics of canine CD5lo cells in PBLs are closely associated with those of NK cells (Huang et al., 2008 ▶). Nevertheless, CD5lo cells express T-cell surface markers (CD3, CD8, and α/βTCR). Although CD5lo cells are not NTNB cells, they can develop high NK cytotoxicity and express high levels of NK cell-related receptors (NKp30, CD16, and CD94), particularly when stimulated with interleukin-2 (IL-2). Significant interferon (IFN)-γ production is observed in IL-12-activated CD5lo cells, revealing typical evidence of NK cell properties. Therefore, it is interesting and necessary to determine whether other types of NK cells in canines are similar to those in other mammals.
According to our review of relevant literature, this study is the first to indicate that the NK activity of CD5lo-ne cells with NTNB surface expression is activated by another type of NK cell (CD5lo). By defining the phenotypes and functions of NK subpopulations in beagles, the properties of mammalian NK cells can be more comprehensively understood.
Materials and Methods
Preparation of canine peripheral blood lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of 15 adult healthy beagles that had been dewormed and vaccinated on a regular basis merely for the purpose of blood draws of this study. All experiments were performed according to the guidelines of the National Taiwan University Animal Experimental Ethics Committee. The dogs were adopted or well cared for in our animal facility laboratory after the study was completed. Through standard gradient centrifugation with Ficoll-Hypaque (density: 1.077; GE Healthcare Bio-Sciences, Sweden), PBMCs were isolated and resuspended in RPMI 1640 medium (Invitrogen, USA) supplemented with 100 U/ml of penicillin, 100 mg/ml of streptomycin (Gibco, USA), and 10% fetal bovine serum (FBS; Perbio, USA). The cells were subsequently incubated in a 75-cm2 flask (5-10 × 106 cells/ml) for 2 h to remove adherent cells. The nonadherent cells with a lymphocyte morphology were used as PBLs.
Lymphokine-activated killer cell preparation
To generate lymphokine-activated killer (LAK) cells, canine PBLs (106 cells/ml) were cultured for 6 days in RPMI 1640 medium supplemented with 100 U/ml of penicillin, 100 mg/ml of streptomycin, 50 mM2-mercaptoethanol (Sigma, USA), 2500 U/ml of recombinant human IL-2 (rhIL-2; Aldesleukin, The Netherlands), and 10% FBS. Fresh rhIL-2 was added to cultured cells every 3 days (Hsiao et al., 2004 ▶). Canine thyroid adenocarcinoma (CTAC) cells, the target of canine NK cells, were purchased from the European Collection of Cell Cultures (Salisbury, UK). Canine thyroid adenocarcinoma cells lines were maintained in a RPMI-1640 medium with 2 mM glutamine supplemented with 10% FBS.
Isolation of CD5hi, CD5 − , and CD5lo-ne cells
Canine NK cells carry fewer surface CD5 molecules (Huang et al., 2008 ▶); therefore, to isolate the cells, canine PBLs or LAK cells were stained with a fluorescein isothiocyanate (FITC)-conjugated rat anticanine CD5 antibody (AbDSerotec, UK) and were subsequently washed and suspended in phosphate-buffered saline (PBS) containing 2% FBS. Fluorescence intensities of viable cells were measured, and the cells were separated using a FACSAriaTM flow cytometer (Becton Dickinson, USA) through a service provided by the Cell Sorting Core Facility (National Taiwan University, College of Medicine). Low surface CD5 density cells were sorted from PBLs or LAK cells, and CD5lo-ne cells were collected. The average purity of every cell population was more than 95%. These cells were used as effect or cells in the NK cytotoxicity assay.
Natural killer cytotoxicity assay
The NK cytotoxic assay was performed using a CytoTox96® nonradioactive cytotoxicity assay (Promega, Madison, USA), according to previous studies (Odeberg et al., 2003 ▶; Vankayalapati et al., 2004 ▶; Huang et al., 2008 ▶). The target 2 × 103 CTAC cells were grown in 50 μL of CTAC culture medium in a 96-well flat-bottom plate. The effector cells were added using effector: target cell (E:T) ratios of 50:1, 25:1, 12.5:1, and 6.25:1, as indicated. After 16 h of incubation at 37°C, the culture medium was harvested in order to analyze lactate dehydrogenase (LDH) production. Lactate dehydro-genase, a stable cytosolic enzyme, was released upon cell lysis. Culture supernatants were measured by reading the absorbance (490 nm) of the red formazon formed in the reaction mixture. The intensity of the formed color is proportional to the number of lysed cells. The percent cytotoxicity was calculated as follows:
Phenotype analysis of CD5 − cells in peripheral blood lymphocytes and lymphokine-activated killer cells
Numerous commercial mouse and rat monoclonal antibodies (mAb) (Table 1) were used to analyze CD5 cell surface antigens in PBLs and LAK cells. For indirect immunofluorescence staining, 106 cells were incubated with an isotype control or specific mAb raised against CD3, CD4, CD8α, CD8β, CD21, α/βTCR, γ/δTCR, MHC classes I and II on ice for 30 min. The cells were subsequently washed and further stained with phycoerythrin-conjugated goat antimouse IgG (Becton Dickinson, USA) for 30 min, after which the cells were washed again three times. For direct immuno-fluorescence, the cells were incubated with an FITC-conjugated isotype control or FITC-conjugated rat anticanine CD5 mAb (Serotec) for 30 min. Finally, all the cells were washed and suspended in a FACS buffer [2% FBS and 0.02% sodium azide in PBS (pH = 7.4)]. The surface immunofluorescence intensity of 104 viable cells was measured using a FACSCaliburTM flow cytometer (Becton Dickinson). Data were analyzed using CellQuest software (Becton Dickinson).
Table 1.
List of antibodies used for surface phenotype assays
| Specificity | Clone | Source |
|---|---|---|
| Canine CD3 | CA17.2A12 | Dr. P. F. Moorea |
| Canine CD4 | CD13.1E4 | Serotecb |
| Canine CD5-FITC | YKIX322.3 | Serotec |
| Canine CD8α-RPE | YCATE55.9 | Serotec |
| Canine CD8β | CA15.4G2 | Serotec |
| Canine CD21 | CA2.1D6 | Serotec |
| Canine MHC I | 2G5 | Serotec |
| Canine MHC II | CA2.1C12 | Serotec |
| Canineα/βTCR | CA15.8G7 | Dr. P. F. Moore |
| Canine γ/δTCR | CA20.8H1 | Dr. P. F. Moore |
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
For RT-PCR analysis, total RNA was extracted from CD5lo, CD5hi, and CD5− cells using TRIzol (Invitrogen) and treated with DNase I (Fermentas, Canada) to remove residual genomic DNA. Complementary DNA was synthesized with SuperScript II RT (Invitrogen), and the RT reaction was performed using a Mastercycler Personal thermal cycler (Eppendorf, Germany). The primer sequences used for all the experiments were designed to specifically bind to canine cDNA by using Primer Express software (Applied Biosystems, USA), according to published canine mRNA sequences (GenBank) and the Ensemblgenome browser (www. ensembl.org). The housekeeping gene, β-actin, was used as an internal control. The GenBank accession numbers of the targets as well as the primer sequences are shown in Table 2. Real-time RT-PCR was performed using a Bio-Rad real-time PCR machine with SYBR Green PCR master mix, according to the manufacturer’s instructions (Bio-Rad, USA) (Chiang et al., 2013 ▶). To analyze real-time RT-PCR data, the relative mRNA amount in each sample was calculated according to its threshold cycle (Ct) and compared with the Ct of β-actin. The results were presented as 2−(Ct of the target gene − Ct of β-actin), (2–DCT) in arbitrary units.
Table 2.
Sequences of PCR primers
| Target gene | Primer name | Sequences (5´→3´) | GenBank accession No. |
|---|---|---|---|
| β-actin | Forward primer | GACCCTGAAGTACCCCATTGAG | Z70044 |
| Reverse primer | TTGTAGAATGTGTGGTGCCAGAT | ||
| NKp30 | Forward primer | ACTGCTGCCTTCTTGCCGTGTT | ENSCAFG00000000524 |
| Reverse primer | ACTCCAGCATCACAGTCTTGGATGT | ||
| NKp44 | Forward primer | ATCGAGTGGCAGGGCAGACA | ENSF00000008847 |
| Reverse primer | TTCCTCCTTCAGACCAATCATGGT | ||
| NKG2D | Forward primer | ACGAAGGCAAAAGAGAAAGCC | XM_849013 |
| Reverse primer | TGATGATTATGGCACCGCAT | ||
| 2B4 | Forward primer | TGGTAACGTGAGCTATGCTTGGTA | ENSF00000008741 |
| Reverse primer | ACGATCACCAGAAAGTACACGAAGT | ||
| CD16 | Forward primer | ACATTCCAGCAGCAACAAGTGA | XM_536141 |
| Reverse primer | AGCAAAATACAGCCCAGTGTCCA | ||
| CD56 | Forward primer | TTGTCCCCAGCCAAGGAGAAAT | NM_001010950 |
| Reverse primer | TAGATGGTGAGCGTGGAGGAAGA | ||
| CD160 | Forward primer | CATCCACATCCACAGCTCAATG | XM_540276 |
| Reverse primer | CGAAGACCTGTCCTTGCACAAA | ||
| Ly49 | Forward primer | TTTCCAGTGCCAAGACATCTCA | AY191818 |
| Reverse primer | CCCAGGTGATGGTTTTCTTGAA | ||
| CD94 | Forward primer | ATTCAGCCAACATTGTCACCAG | DQ228356 |
| Reverse primer | AGTAGCAGTTGCATTGGTGCC | ||
| Granzyme B | Forward primer | GCAGGAACATTCTCAACCCCA | ENSCAFG00000025287 |
| Reverse primer | TTCCAGAGACAGCCTTTTGCC | ||
| Perforin | Forward primer | GCCAACCCTTCATCAGAATTCA | ENSF00000003552 |
| Reverse primer | CACCAATTATCTCGGAAAAGCG |
Statistical analysis
All the results are expressed as the mean±SD and analyzed using a two-tailed Student t-test. Differences were considered statistically significant at P<0.05.
Results
CD3 − cells show natural killer cytotoxicity
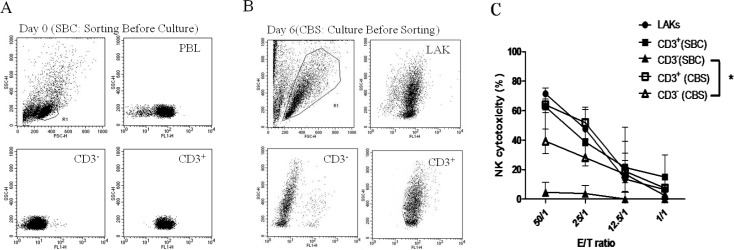
As expected, the NK cytotoxicity assay revealed that CD3+ cells were positive for cytotoxicity when co-cultured with CD5lo cells (Figs. 1A and C). We subsequently changed the conditions by culturing PBLs in an IL-2 medium for 6 days (LAK cells) to perform the NK cytotoxicity assay (Fig. 1B). Notably, both CD3− (46.98% ± 21.09% at an ET ratio of 50:1) and CD3+ (62.29% ± 3.18% at an ET ratio of 50:1) cell fractions showed marked NK cytotoxicity. The NK cytotoxicity of CD3− cells was markedly enhanced in the cell group sorted from LAK cells (P<0.05; Fig. 1C).
Fig. 1.
A: CD3+ (SBC) (■) and CD3– (SBC) (▲) cells sorted from PBLs and cultured separately with IL-2 for 6 days before perfoming the NK killing assay. B: PBLs stimulated with IL-2 (2500 U/ml) for 6 days, and CD3+ (CBS) (□) and CD3− (CBS) (△) cells sorted for a NK killing assay. C: Killing activities are shown in Fig. 1C
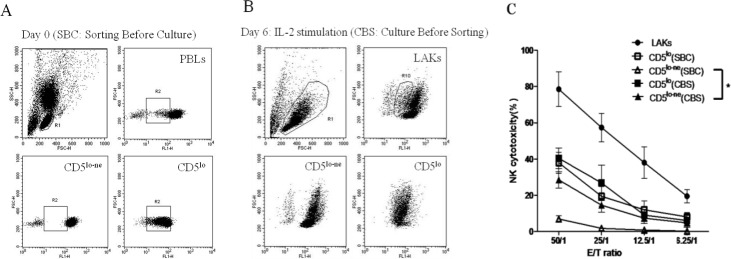
CD5lo-ne cells are natural killer-active
We used the same protocols as shown in Fig. 1 by sorting CD5lo and CD5lo-ne cells to explore the possible cell types involved in the NK activation of CD3− cells. Low surface CD5 density and CD5lo-ne cells were subsequently harvested to evaluate NK cytotoxicity (Fig. 2A, SBC). The results revealed that only CD5lo cells (37.96% ± 11.76% at an ET ratio of 50:1) were NK-cytotoxic, while CD5lo-ne cells showed very low or no cytotoxicity (7.00% ± 3.62% at an ET ratio of 50:1; Fig. 2C). We then sorted the CD5lo and CD5lo-ne cells from the IL-2-stimulated LAK cells. CD5lo cells showed high NK activity (CBS, Fig. 2B). Meanwhile, the CD5lo-ne cell fraction derived from LAK cells (CD5lo-ne, CBS) showed significantly increased NK cytotoxicity compared with PBLs (CD5lo-ne, SBC; Fig. 2C). The killing level of the CD5lo-ne cell (28.52% ± 9.02% at an ET ratio of 50:1) was similar to that of CD5lo cells (37.96% ± 11.76% at an ET ratio of 50:1). Thus, CD5lo-ne cells that were NK-inert became NK-cytotoxic when cocultured with CD5lo cells in the IL-2 medium.
Fig. 2.
A: CD5lo (SBC) (□) and CD5lo-ne (SBC) (△) cells sorted from PBLs and separately cultured with IL-2 (2500 U/ml) for 6 days before perfoming the NK killing assay. B: PBLs cultured with IL-2 for 6 days first, and CD5lo (■) and CD5lo-ne (▲) cells were subsequently sorted for the NK cytotoxic assay. C: Killing activities are shown in Fig. 2C. * P<0.05
CD5lo cells are involved in the natural killer cytotoxic activation of CD5lo-ne cells
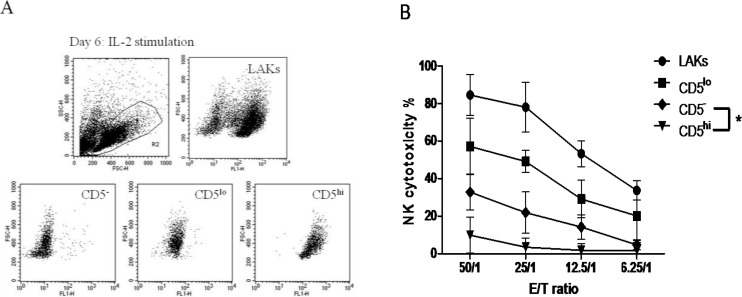
We further determined which subpopulation of CD5lo-ne cells was the actual target of CD5lo cells for activating NK cytotoxicity. PBLs can be classified into three subpopulations: CD5lo, CD5hi, and CD5− cells. As expected, CD5hi cells (9.89% ± 8.87% at an ET ratio of 50:1) and the remaining two populations (32.83% ± 7.18% and 57.11% ± 13.31% for CD5− and CD5lo at an ET ratio of 50:1) were NK-cytotoxic (Figs. 3A-B).
Fig. 3.
CD5− (♦) CD5lo (▪) 5 and CD5hi (▼) cells sorted using a FACSAriaTM flow cytometer (A). NK cytotoxicities of these three types of cell are shown (B). * P<0.05
To validate the findings, we conducted the following experiments. First, CD5hi cells were isolated from PBLs. These isolated cells as well as the CD5− and CD5lo cells were cultured in the IL-2 medium. After a 6-day culture period, CD5− and CD5lo cells were separated using a flow sorter and subjected to the NK cytotoxicity assay (Figs. 4A(i), (ii)). The results revealed that CD5lo cells were NK-active, and CD5− cells had gained killing activity (22.42% ± 7.34% at an ET ratio of 50:1); however, CD5hi cells exerted no NK activity (2.88% ± 2.16%, at an ET ratio of 50:1; Fig. 4C). Similarly, CD5−-depleted PBLs and the isolated CD5− cells were separately cultured with IL-2 for 6 days followed by sorting, to obtain CD5hi and CD5lo cells from the IL-2-cultured CD5−-depleted PBLs, and the NK activity of the three populations was verified (Figs. 4B(i), (ii)).
Fig. 4.
A(i): CD5hi cells (▼) were depleted, and the remaining cells in PBLs (CD5− cells (▲) and CD5lo cells (▪)) were cocultured in IL-2 for 6 days. A(ii): CD5− and CD5lo cells were isolated separately using a FACSAriaTM flow cytometer. B(i) & (ii): CD5−-depleted PBLs and the isolated CD5− cells were separately cultured with IL-2 for 6 days followed by sorting, to obtain CD5hi and CD5lo cells from the IL-2-cultured CD5−-depleted PBLs. C: The killing activities are shown in Fig. 4C. * P<0.05
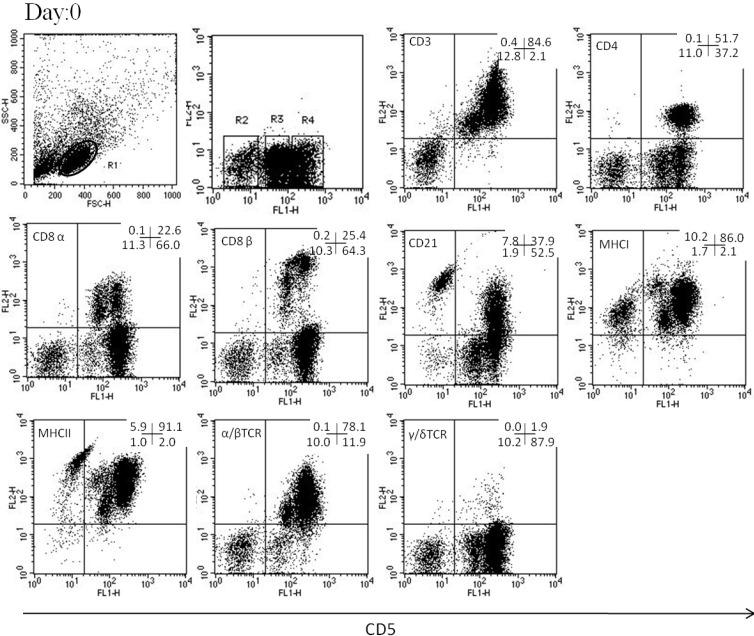
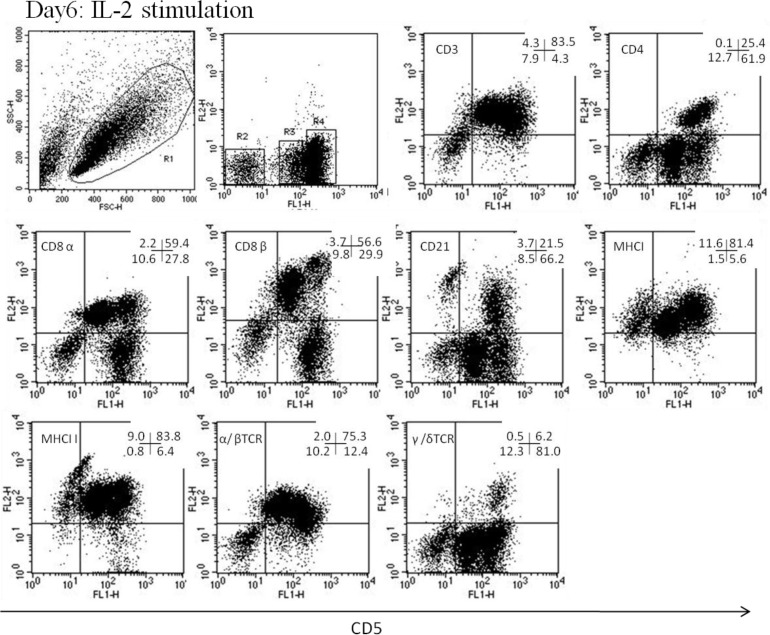
Phenotyping of canine CD5 − cells from peripheral blood lymphocytes and lymphokine-activated killer cells
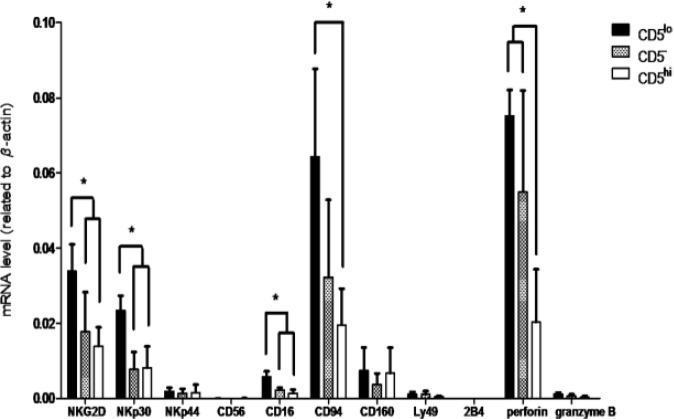
In PBLs, the phenotype of CD5− cells was CD3−, CD4−, CD8α−, CD21+/−, MHCI+/−, MHC II+/−, α/βTCR−, and γ/δTCR− (Fig. 5). In LAK or activated cells, the phenotype was CD3+/−, CD4−, CD8α+/−, CD21+/−, MHCI+/−, MHC II+/−, α/βTCR−, and γ/δTCR− (Fig. 6). The gene expression levels of NK-associated surface markers, NKG2D, NKp30, and CD16, were significantly higher on CD5lo cells than on CD5– and CD5hi cells (P<0.05, Fig. 7). Furthermore, the gene expression levels of CD94 were higher on CD5lo cells than on CD5hi cells, and both CD5− and CD5lo cells showed significantly higher perforin mRNA production than did CD5hi cells (P<0.05, Fig. 7).
Fig. 5.
The analysis was performed after gating on lymphocytes (R1). CD5lo cell population was in the R3 region, CD5− was in the R2 region, and CD5hi was in the R4 region
Fig. 6.
The analysis was performed after gating on lymphocytes (R1). CD5lo cell population was in the R3 region, CD5− cells were in the R2 region, and CD5hi cells were in the R4 region
Fig. 7.
The mRNA expression level was detected on NKG2D, NKp30, NKp44, CD56, CD16, CD94, CD160, Ly49, 2B4, perforin, and granzyme B through real-time RT-qPCR. * P<0.05
CD5lo cells can induce NK toxicity only in CD5 − CD21 − (NTNB) cells
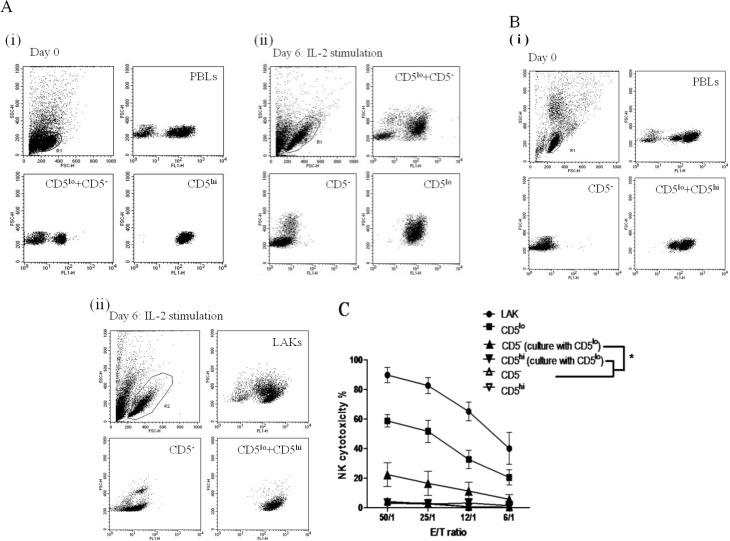
Flow cytometric analysis revealed no apparent change in tested phenotypes between PBLs and LAK cells, except for CD21. CD21+ (B lymphocytes) within the CD5− population decreased from 7.8% in PBLs to 3.7% in LAK cells, whereas that in CD5−CD21− (NTNB) cells increased from 1.9% to 8.5% (Figs. 5 and 6). In the CD5− population, CD5−CD21− cells remarkably in-creased from 27.68% ± 4.32% in PBLs to 74.80% ± 3.43% in LAK cells after IL-2 stimulation (P<0.001). To elucidate the increase, we explored whether CD5−CD21− cells gained NK cytotoxicity in the presence of CD5lo cells. Thus, we sorted PBLs into CD5−CD21+ and CD5−CD21− cell types and cultured them separately with CD5lo cells in the IL-2 medium. After 6 days, CD5lo cells were removed from the culture, and the remaining CD5−CD21+ and CD5−CD21− cells were subjected to the NK cytotoxicity assay. CD5−CD21− cells had a very strong NK killing activity, while CD5−CD21+ cells exhibited only baseline activity (P<0.05, Figs. 8A(i), (ii), B).
Fig. 8.
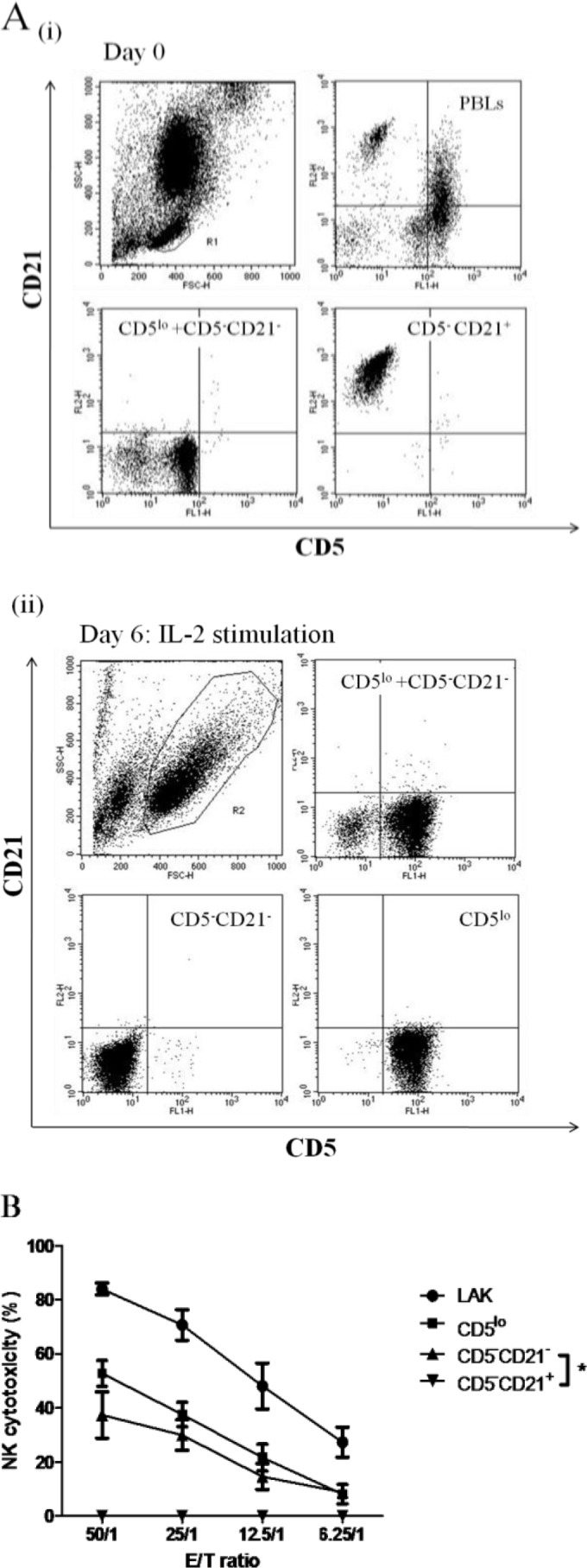
A(i): Canine PBLs separated into CD5−CD21+ (▼) and CD5−CD21− (▲) through FACSAriaTM cell sorting and incubated with purified CD5lo cells in IL-2 for 6 days. A(ii): CD5−CD21− and CD5−CD21+ cells were isolated. B: NK cytotoxic assay was performed (B). * P<0.05
Discussion
In the present study, we observed the novel phenomenon of CD5lo cells being co-cultured with CD5lo-ne (CD5− and CD5−CD21−) populations and assisting CD5lo-ne cells to gain both NK surface molecule expression and cytotoxicity. According to our review of relevant literature, NK cells can regulate a range of different cell types, such as B-cells (Mathew et al., 2014 ▶), dendritic cells (Stojanovic et al., 2014 ▶), and CD4+ helper and CD8+ effector T-cells (Biron, 2010 ▶). However, no study has reported the ability of NK cells to activate another cell type with NK characteristics. This study is the first to reveal and characterize both persistent and inducible canine NK cells.
In the present study, we noticed that canines have two cell populations, CD5lo and CD5− cells, which have NK phenotypes, including the NK marker expression and target cell cytotoxicity. CD5lo and CD5− cells accounted for 14.9% ± 6.68% and 10.1% ± 2.12%, respectively, of the total PBLs. Similar to the NK cells from other species (Gerner et al., 2009 ▶), canine CD5lo cells must be activated by IL-2. Notably, we observed that only CD5lo cells can activate CD5− cells, whereas other populations (i.e., CD5hi, CD21+, and neutrophils) cannot. IL-2 can augment human NK cell functions, leading to increased cytolytic activity (Ribatti, 2017 ▶) and the production of various cytokines, such as IFN-γ, tumor necrosis factor-α (Duggan et al., 2017 ▶), granulocyte-macrophage colony-stimulating factor, IL-3 (Talleur et al., 2017 ▶), IL-10 (Roquilly et al., 2014 ▶), and IL-5 (Biron, 2010 ▶). IL-2 increases both cell numbers and the NK cytotoxic ability of canine PBLs (Denecke et al., 2013 ▶). Huang et al. (2008 ▶) reported that IL-2 activates CD5lo cells to express NK-activating markers (NKp30, NKp44, CD16, and CD94) and execute target cell killing. In most species, IL-2 can activate NK cells, but canine CD5− cells require CD5lo cells to initiate their NK activity. The present study further revealed that CD5− cells had a significant NK killing ability when co-cultured with CD5lo cells in IL-2 medium, although their activity was lower than that of CD5lo cells. The increased NK cytotoxicity of CD5− cells can also be proved by evaluating the expression of the cell cytolytic molecule perforin. Both CD5lo and CD5− expressed significantly higher amounts of perforin than did CD5hi cells.
Phenotyping analysis revealed that CD5lo cells expressed T-cell linage markers (CD3+, CD4+/−, CD8α+/−, CD8β+/−, CD21+/−, MHC I+, MHC II+, α/βTCR+/−, and γ/δTCR−) instead of B-cell lineage markers in PBLs (Huang et al., 2008 ▶). While purifying CD5− cells from LAK cells, the percentage of NTNB cells within the CD5− population increased, whereas that of B-cells decreased. By contrast, the expression levels of NK-cell relative receptors were consistent with the cytotoxicity level of NK cells. No specific markers for canine NK cells have been previously reported. Most studies used a percoll gradient to enrich NK cells, subsequently confirming cell type by morphological analysis and cytotoxic function testing to lyse canine NK-sensitive target cells (CTAC and CL-1 cells) (Knapp et al., 1993 ▶). In a 35%-40% gradient fraction, CD4−CD8− cells showed a higher cytotoxic ability than other cells (CD4−CD8+), and the ultrastructure was different from that of other PBLs but similar to human NK cells (Knapp et al., 1995 ▶). However, canine NK cell-mediated cytotoxicity was the highest in the T-cell subpopulation that was fractionated into a 35%-40% percoll gradient and had also enriched LGLs (Nariai Nakada et al., 1999 ▶). A previous study reported that canine NK cells had a T-cell lineage (Michael et al., 2013 ▶). Similarly, our results show that phenotypes of canine NK cells have two populations that do not express the B-cell surface marker, and only one phenotype expresses the T-cell surface marker.
The mRNA quantities of NKG2D, NKp30, CD16, and CD94 were the highest in CD5lo cells, followed by CD5− cells, and a low mRNA concentration was observed in CD5hi cells. Currently, a canine NK cell-related receptor antibody is commercially unavailable; therefore, we only detected the effects of different expressions of these two subsets on the mRNA level. The expression levels of the activating receptors varied in CD5lo and CD5− cells. CD5lo cells had higher expression on NKG2D, NKp30, and CD16 than on CD5− cells. The expression levels of CD5lo cells on NKG2D, NKp30, CD16, and CD94 were higher than those of CD5hi cells. All these cells were isolated from LAK cells, and their NK cytotoxic ability was confirmed with functional assays. Huang et al. (2008) ▶ reported that CD5lo cells had higher expressions on NKG2D, CD16, CD94, CD160, perforin, and granzyme than on CD5hi cells purified from PBLs, and upon CD5lo and CD5hi cells stimulation with IL-2, CD5lo cells expressed more NKp30, NKp44, CD16, and CD94 mRNA than did CD5hi cells (Huang et al., 2008 ▶). Considering these results and our findings, CD16 and CD94 can be suggested as potential markers for canine NK cells. Moreover, NKp30 mRNA is expressed in high levels in IL-2-activated CD5lo cells but not in resting canine CD5lo NK cells. Therefore, this can be used as a marker of activated NK cells. Shin et al. (2013 ▶) confirmed our finding that expanded canine NK-like CLGLs exhibit NKG2D, NKp30, NKp44, perforin, and granzyme expressions (Shin et al., 2013 ▶). In sum, by observing the levels of NK-associated markers, we showed that CD5− cells can be activated by CD5lo cells to become NK-active. Furthermore, although canine NK markers must still be clearly defined, our results, together with previous publications, have provided valuable evidence suggesting that our tested NK-associated panels can be used to detect canine NK cells. This also emphasizes the relevance of generating antibodies against these canine NK markers.
Although LAKs can be separated into three subpopulations according to their CD5 expression, the interactions among them can be delicate and complicated. In summary, for canine NK cells, we first discovered two cell populations with NK characteristics. One is the CD5lo, which exhibits persistent NK activity, such as IFN-γ production (Huang et al., 2008 ▶), cytotoxicity, and specific surface marker expression, and the other is the CD5−, which has an inducible NK phenotype and only exert functions in the presence of CD5lo cells. The interactions among these cell lineages deserve further investigation in order to find out whether the present findings are only applicable to canine NK cells or to NK cells of other species as well.
References
- Biron, CA More things in heaven and earth: defining innate and adaptive immunity. Nat. Immunol. 2010;11:1080–1082. doi: 10.1038/ni1210-1080. [DOI] [PubMed] [Google Scholar]
- Brudno, JN , Somerville, RP , Shi, V , Rose, JJ , Halverson, DC , Fowler, DH , Gea-Banacloche, JC , Pavletic, SZ , Hickstein, DD , Lu, TL , Feldman, SA , Iwamoto, AT , Hansen, BG , Blacklock-Schuver, B , Hakim, FT , Rosenberg, SA , Gress, RE , Kochenderfer, JN Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, HC , Liao, AT , Jan, TR , Wang, YS , Lei, HJ , Tsai, MH , Chen, MF , Lee, CY , Lin, YC , Chu, RM , Lin, CS Gene-expression profiling to identify genes related to spontaneous tumor regression in a canine cancer model. Vet. Immunol. Immunopathol. 2013;151:207–216. doi: 10.1016/j.vetimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Christaki, E , Diza, E , Giamarellos-Bourboulis, EJ , Papadopoulou, N , Pistiki, A , Droggiti, DI , Georgitsi, M , Machova, A , Lambrelli, D , Malisiovas, N , Nikolaidis, P , Opal, SM NK and NKT cell depletion alters the outcome of experimental pneumococcal pneumonia: relationship with regulation of interferon-γ production. J. Immunol. 2015 doi: 10.1155/2015/532717. Article ID 532717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, C , Yuan, X , Ge, X , Kim, IK , Bedi, D , Boenisch, O , Weiland, A , Jurisch, A , Kotsch, K , Pratschke, J , Reutzel-Selke, A , Tullius, SG Synergistic effects of prolonged warm ischemia and donor age on the immune response following donation after cardiac death kidney transplantation. Surgery. 2013;153:249–261. doi: 10.1016/j.surg.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Duggan, MC , Campbell, AR , McMichael, EL , Opheim, KS , Levine, KM , Bhave, N , Culbertson, MC , Noel, T , Yu, Li , Carson, WE Co-stimulation of the fc receptor and interleukin-12 receptor on human natural killer cells leads to increased expression of cd25. Oncoimmunology. 2017;16(2):e1381813. doi: 10.1080/2162402X.2017.1381813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner, W , Kaser, T , Saalmuller, A Porcine T lymphocytes and NK cells--an update. Dev. Comp. Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Hsiao, YW , Liao, KW , Hung, SW , Chu, RM Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J. Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- Huang, YC , Hung, SW , Jan, TR , Liao, KW , Cheng, CH , Wang, YS , Chu, RM CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukocyte Biol. 2008;84:1501–1510. doi: 10.1189/jlb.0408255. [DOI] [PubMed] [Google Scholar]
- Knapp, DW , Leibnitz, RR , DeNicola, DB , Turek, JJ , Teclaw, R , Shaffer, L , Chan, TC Measurement of NK activity in effector cells purified from canine peripheral lymphocytes. Vet. Immunol. Immunopathol. 1993;35:239–251. doi: 10.1016/0165-2427(93)90037-5. [DOI] [PubMed] [Google Scholar]
- Knapp, DW , Turek, JJ , DeNicola, DB , Chan, TC , Carter, WO , Snyder, PW , Robinson, JP Ultra-structure and cytochemical staining characteristics of canine natural killer cells. Anat. Rec. 1995;243:509–515. doi: 10.1002/ar.1092430413. [DOI] [PubMed] [Google Scholar]
- Macêdo, AA , Marciano, AP , Rocha, LM , Alves-Júnior, JR , Faria, AM , Bittar, JF , Araújo, MS , Santos, RL , Martins-Filho, OA Comparative phenotypic profile of subpopulations of peripheral blood leukocytes in European (Bos taurus taurus) and Zebu cattle (Bos taurus indicus) Genet. Mol. Res. 2013;12:6838–6849. doi: 10.4238/2013.December.19.2. [DOI] [PubMed] [Google Scholar]
- Mathew, S , Kim, J , Mathew, P Characterization of human CS1 gene promoter in NK and B cells. J. Immunol. 2014;126:10. [Google Scholar]
- Michael, HT , Ito, D , Cullar, VC , Zhang, B , Miller, JS , Modiano, JF Isolation and characterization of canine natural killer cells. Vet. Immunol. Immunop. 2013;155:211–217. doi: 10.1016/j.vetimm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai Nakada, Y , Nariai, K , Kosaka, T , Kuwabara, M , Kiuchi, Y Morphological observation of canine natural killer cells mediated cytotoxicity. J. Vet. Med. Sci. 1999;61:835–838. doi: 10.1292/jvms.61.835. [DOI] [PubMed] [Google Scholar]
- Odeberg, J , Browne, H , Metkar, S , Froelich, CJ , Branden, L , Cosman, D , Soderberg-Naucler, C The human cytomegalovirus protein UL16 mediates increased resistance to natural killer cell cytotoxicity through resistance to cytolytic proteins. J. Virol. 2003;77:4539–4545. doi: 10.1128/JVI.77.8.4539-4545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, JS Human natural killer cell deficiencies. Curr. Opin Allergy Clin. Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- Ribatti, D Historical overview on the morphological characterization of large granular lymphocytes/natural killer cells. Immunol. Lett. 2017;190:58–63. doi: 10.1016/j.imlet.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Roquilly, A , Broquet, A , Jacqueline, C , Masson, DP , Segain, JP , Braudeau, C , Vourc’h, M , Caillon, JP , Altare, F , Josien, R , Retière, C , Villadangos, J , Asehnoune, K Hydrocortisone prevents immunosuppression by interleukin-10+ natural killer cells after trauma-hemorrhage. Crit. Care Med. 2014;42:e752–e761. doi: 10.1097/CCM.0000000000000658. [DOI] [PubMed] [Google Scholar]
- Shin, DJ , Park, JY , Jang, YY , Lee, JJ , Lee, YK , Shin, MG , Jung, JY , Carson, WE 3rd , Cho, D , Kim, SK Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet. Immunol. Immunopathol. 2013;153:249–259. doi: 10.1016/j.vetimm.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic, A , Fiegler, N , Brunner-Weinzierl, M , Cerwenka, A CTLA-4 is expressed by activated mouse NK cells and inhibits NK cell IFN-γ production in response to mature dendritic cells. J. Immunol. 2014;192:4184–4191. doi: 10.4049/jimmunol.1302091. [DOI] [PubMed] [Google Scholar]
- Talleur, AC , Triplett, BM , Federico, S , Mamcarz, E , Janssen, W , Wu, W , Shook, D , Leung, W , Furman, WL Consolidation therapy for newly diagnosed pediatric patients with high-risk neuroblastoma using busulfan/melphalan, autologous hematopoietic cell transplantation, anti-GD2 antibody, granulocyte-macrophage colony-stimulating factor, interleukin-2, and haploidentical natural killer cells. Biol. Blood Marrow Tr. 2017;23:1910–1917. doi: 10.1016/j.bbmt.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankayalapati, R , Klucar, P , Wizel, B , Weis, SE , Samten, B , Safi, H , Shams, H , Barnes, PF NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J. Immunol. 2004;172:130–137. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]