Abstract
Purpose
The objective of the study was to assess the effect of off-label photodynamic therapy (PDT) in combination with intravitreal off-label ziv-aflibercept or off-label aflibercept injection in patients with chronic or repeatedly recurrent acute central serous chorioretinopathy (CSC).
Patients and methods
Changes in best corrected visual acuity (BCVA) and subfoveal subretinal fluid (sSRF) and maximum subretinal fluid (mSRF) were retrospectively analyzed in a single-center cohort study of 17 patients (18 eyes) with persistent subretinal fluid for more than 3 months of duration of CSC. Treatment efficacy was measured between injection and PDT at 30±15 days, 90±15 days and 180±30 days after PDT.
Results
Significant reduction of sSRF and mSRF was shown after therapy with ziv-aflibercept and aflibercept combined with PDT (p<0.001). Course of BCVA showed non-significant improvement within 6 months (p=0.065). One case of allergic reaction after fluorescein angiography and one case of ophthalmic migraine after ziv-aflibercept injection were documented. One case of reversible vision loss occurred during 6 months after combination therapy. No other adverse events or side effects were reported.
Conclusion
Combination therapy of ziv-aflibercept and aflibercept with PDT seems to be beneficial, even in cases of chronic or repeatedly recurrent acute CSC. This includes cases of CSC resistant to or recurrent after medical treatment, PDT alone or therapy with anti-vascular endothelial growth factor alone.
Keywords: therapy-refractory, combination therapy, anti-VEGF, subretinal fluid
Introduction
Central serous chorioretinopathy (CSC) is the fourth most common non-surgical retinopathy, mainly affecting middle-aged patients during their working years.1 It is an idiopathic condition that is characterized by serous neurosensory retinal detachment and/or pigment epithelial detachment, most often confined to the posterior pole and associated with leakage of fluid through the retinal pigment epithelium (RPE) into the subretinal space. A common characteristic is a thickened choroid on optical coherence tomography (OCT).2,3 Choroidal hyperpermeability and RPE changes are the main factors involved in the pathophysiology.4
While acute CSC is typically self-limited within a few months with good visual outcome, about 10% of patients have several recurrences or a chronic course.4,5 Forty percent have bilateral involvement.6
The chronic course is shown to be a progressive chorioretinopathy with significant irreversible vision loss, decreased contrast sensitivity and/or a central scotoma due to photoreceptor damage, RPE atrophy or choroidal neovascularization (CNV).1,7–9 Chronic CSC leads to significantly lower vision-related quality of life and affects mainly working-aged patients. Therefore, it has a significant impact on patients’ personal and professional lives.10
While there is still no international consensus about a gold standard therapy, thanks to relatively high success rates, PDT is the treatment of choice in cases of chronic CSC, especially if the macula is involved and/or it does not respond to medical treatment.11
Several retrospective studies suggest that treatment of CSC patients is effective in 70%–100% patients with full-dose PDT and in 33%–100% with half-dose PDT.12 In cases not responding to or recurring after first PDT, the procedure can be repeated, but not indefinitely due to potential adverse events like RPE atrophy, choriocapillaris hypoperfusion and secondary CNV.13–15 During PDT, verteporfin dye accumulates around hyperpermeable choroidal vessels because of slow blood flow and vascular congestion. Activation of verteporfin by laser application causes photochemical damage to the choroidal epithelium, thereby leading to short-term choriocapillaris hypoperfusion and long-term remodeling. This in turn leads to the upregulation of vascular endothelial growth factor (VEGF).14,16 While early increase of leakage was shown after PDT, VEGF is assumed to be one of the major factors responsible for increased vascular permeability, and can also lead to secondary CNV.17,18 Although these changes are mostly seen with full-dose PDT and decrease with dose reduction, they have also been reported in half-dose PDT.19–23
The upregulation of VEGF after PDT leads to the assumption that patients with CSC benefit from a synergistic effect of PDT combined with anti-VEGF agents.
Anti-VEGF monotherapy is considered to be a safe treatment option with acceptable improvement even in refractory cases, yet there is little known about combination therapy.24–27 Anti-VEGF therapy is assumed to cause a decrease of subretinal fluid (SRF) in CSC by reducing choroidal blood flow and choroidal thickness.28,29 While anti-VEGF monotherapy was not shown to be a promising alternative for PDT in the long run, combination therapy could possibly be a more effective alternative with fewer complications.30,31
Two small retrospective studies investigated the effect of combination therapy with anti-VEGF and PDT in CSC, but used pegaptanib and bevacizumab. Combination therapy seemed to aid in the resolution of SRF and was shown to be a safe treatment option, yet the benefit was not significant.32,33 We investigated the effects of the newer anti-VEGF agent ziv-aflibercept and aflibercept combined with PDT.
Intravitreal aflibercept was shown to be efficient in reducing choroidal thickness and appeared to induce greater reduction of choroidal thickness compared to ranibizumab.34,35
Aflibercept is a fusion protein that binds to VEGF subtypes A and B as well as to placental growth factor and has a greater binding affinity to VEGF than bevacizumab and ranibizumab.36 Aflibercept was also shown to be a safe agent in CSC therapy.37 Ziv-aflibercept contains aflibercept, however, in a higher osmolarity of the buffering solution. Ziv-aflibercept is approved for the intravenous treatment of various malignant tumors. The intravitreal use is off-label, with recent clinical data showing long-term safety and efficacy in retinal diseases.38
At Triemli City Hospital, Zurich, therapy with ziv-aflibercept or aflibercept combined with PDT has been used since 2015 in primarily chronic or repeatedly recurrent acute, therapy-refractory cases of CSC.
We retrospectively analyzed the anatomical and functional outcome of patients with persistent SRF due to CSC treated with a combination of PDT and ziv-aflibercept or aflibercept.
Patients and methods
This retrospective, single-center cohort study of patients with CSC was approved by the Ethics Committee of Zurich, Switzerland, and was conducted in accordance with the tenets of the Declaration of Helsinki.
All included patients signed research consent forms at initial presentation allowing their data to be used for retrospective analysis. In addition, all patients signed an informed consent form for off-label use of PDT and off-label use of ziv-aflibercept and aflibercept.
Primary outcomes were functional and anatomical changes, documented by BCVA, maximum subretinal and subfoveal subretinal fluid (mSRF and sSRF). Treatment effects were measured after intravitreal anti-VEGF injection, before PDT, at 30±15 days, 90±15 days and 180±30 days after PDT. BCVA was measured using Snellen charts and subsequently converted into Early Treatment Diabetic Retinopathy Study (ETDRS) letters for statistical analysis. mSRF and sSRF were evaluated by spectral domain Heidelberg Spectralis OCT® (Heidelberg Engineering, Heidelberg, Germany) with a standard set of 19 B-scans (setting: 512 A-scans, 20°×20°). If mSRF was outside this standard set in some cases, manual scans were performed by medical technical assistants. In cases without additional scans, the mSRF within the standard scan was measured. OCT scans were compared using the “follow-up” mode of the eye-tracking-assisted Heidelberg Spectralis® system (AutoRescan), allowing precise comparison. All SRF measurements were independently performed by two ophthalmologists (JD and FF).
In addition to the OCT examination, all patients underwent a complete ophthalmic examination at each regular visit including BCVA, air-tonometry, slit-lamp examination and fundoscopy.
Eighteen eyes of 17 consecutive patients were evaluated at the Department of Ophthalmology at Triemli City Hospital, Zurich, between 2015 and August 2017, whose treatment consisted of ziv-aflibercept and aflibercept in adjunct with PDT.
Patients who were over 18 years of age, diagnosed with CSC on fluorescein angiography (FA) and indocyanine green angiography, and received intravitreal ziv-aflibercept and aflibercept injection within 30 days prior to PDT were included.
Patients were excluded if other retinal vasculopathies like polypoidal choroidal vasculopathy (PCV) or CNV secondary to CSC as well as other ocular diseases potentially affecting BCVA were diagnosed. In addition, patients with a history of intraocular surgery within the last 3 months before treatment or intraocular pressure >21 mmHg despite therapy were excluded.
Intravitreal injections of 0.05 mL aflibercept (Eylea®; Bayer Schweiz AG, Zurich, Switzerland) or 0.06–0.07 mL ziv-aflibercept (Zaltrap®; Sanofi-Aventis AG, Vernier, Switzerland) were administered within 30 days prior to treatment with PDT with verteporfin (Visudyne®; Novartis AG, Basel, Switzerland). Ziv-aflibercept was filled into sterile syringes (1.0 mL Luer Lock, article no 309628; Becton Dickinson AG, Allschwil, Switzerland) by the pharmacy at Triemli City Hospital using an isolator system and following national guidelines.
The PDT verteporfin dose was 3 mg/m2 (half dose) or 6 mg/m2 (full dose) based on body surface area. Body surface area was calculated from a normogram based on the height and weight of the patient on the day of treatment. The solution was infused through intravenous access over a 10-minute period. Then, 15 minutes after the start of infusion, laser treatment was applied with a 689 nm diode laser (Coherent Opal Photoactivator; Coherent Inc., Dieburg, Germany) on a slit-lamp delivery system (BQ 900 slit lamp; Haag-Streit AG, Köniz, Switzerland). Laser light was delivered for 83 seconds with a full light fluence of 50 J/cm2 and an intensity of 600 mW/cm2. The size of the PDT spot was determined as the greatest linear dimension of the choroidal hyperpermeable lesion. In cases of multifocal lesions, several laser spots were applied consecutively without overlap. Patients were instructed to avoid direct sunlight and to wear sun glasses while outdoors for the next 48 hours.
Statistical analysis was performed using the R programming language (version 3.3.3; https://www.R-project.org/). The exact sign test was used to compare ETDRS BCVA before treatment and 6 months post-PDT. The intraclass correlation coefficient for SRF measurements was computed for a two-way model estimating agreement for single-measures units. As reliability was excellent (ICC >0.98), the mean was computed and used for all analyses for SRF. The dependent sample t-test (for normally distributed differences) or the exact Wilcoxon signed rank test (for not normally distributed differences) was used to compare central and maximum SRF before treatment with PDT and 1, 3 and 6 months post-PDT. For these tests, a last value carried forward approach was used to replace missing values.
Results
Eighteen eyes of 17 patients with chronic or repeatedly recurrent acute CSC received a combination therapy of ziv-aflibercept or aflibercept and PDT. Seventeen eyes were treated with half-dose PDT, while one eye received full-dose PDT due to recurrence after previous half-dose PDT. Fifteen patients received ziv-aflibercept and 3 patients received aflibercept. Fifteen patients had follow-up measurements at 1 month, 16 patients at 3 months and 14 patients at 6 months.
Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Baseline characteristics
|
ETDRS BCVA
|
OCT measurements, sSRF/mSRF (μm)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Gender | Age (years) | Eye | Risk factors | Duration of CSC before ZivA-PDT | Baseline | At 6 months | Baseline | At 6 months |
| 1 | M | 58 | OD | CS | >4 years | 40 | 40 | 193/262 | 0/0 |
| 2 | F | 52 | OD | HT | 9 months | 75 | 85 | 144/149 | 0/48 |
| 3 | M | 42 | OD | S | 9 months | 80 | 85 | 344/366 | 0/5 |
| 4 | M | 42 | OS | 1 year* | 85 | MD | 37/107 | MD | |
| 5 | M | 68 | OD | CS, HT | 4 months | 60 | 80 | 311/327 | 0/0 |
| 6 | M | 53 | OD | HT | 8 months | 75 | MD | 234/324 | MD |
| 7 | M | 40 | OD | S | 1.5 years | 70 | 35 | 115/233 | 84/144 |
| 8 | M | 69 | OD | 6 years | 55 | 70 | 282/282 | 112/112 | |
| 9 | M | 46 | OS | CS, HT | 5.5 years | 85 | 85 | 200/200 | 0/68 |
| 10 | M | 53 | OS | CS | 1.5 years | 65 | 85 | 162/212 | 0/178 |
| 11 | M | 47 | OD | 6 months | 75 | MD | 301/301 | MD | |
| 12 | F | 67 | OD | CS | >3 years | 5 | MD | 184/185 | MD |
| 13 | M | 48 | OS | >4.5 years | 65 | 70 | 199/231 | 231/240 | |
| 14 | M | 51 | OD | 3.5 years | 70 | 70 | 316/322 | 106/107 | |
| 15 | M | 64 | OS | HT | >4 years | 75 | 75 | 251/252 | 177/177 |
| 16 | M | 46 | OS | HT | 1.5 years | 65 | 75 | 75/79 | 14/14 |
| 17 | M | 40 | OS | >2.5 years | 85 | 85 | 100/100 | 0/117 | |
| 18 | F | 61 | OD | >1 years | 80 | 75 | 250/250 | 30/39 | |
| Mean/summary | 15 M/3 F | 52.6 | 11 OD/7 OS | 6× HT/5× CS 2× S | >28.5 months | 67.2 | 75.0 | 205/232 | 54/78 |
| ±SD | 9.8 | >20.0 months | 18.8 | 15.4 | 86/81 | 74/72 | |||
Note:
Bilateral CSC, diagnosis for 1 year, but symptoms of the treated eye for just 1 month.
Abbreviations: BCVA, best corrected visual acuity; CS, corticosteroids; CSC, central serous chorioretinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; F, female; HT, hypertension; M, male; MD, missing data; mSRF, maximum subretinal fluid; OCT, optical coherence tomography; OD, right eye; OS, left eye; PDT, photodynamic therapy; S, stress; SD, standard deviation; sSRF, subfoveal subretinal fluid; ZivA, ziv-aflibercept and aflibercept.
Patients received various other therapies during observation period. Therapies are listed in Table 2.
Table 2.
Treatment
| Patient ID | Treatment before ZivA-PDT | Treatment during 1st month after ZivA-PDT | Treatment till 3rd month after ZivA-PDT | Treatment till 6th month after ZivA-PDT |
|---|---|---|---|---|
| 1 | Acetazolamide, nepafenac | Eplerenone | Eplerenone | Eplerenone, 1× zivafl, 1× HD-PDT |
| 2 | Horse chestnut dry extract, acetazolamide, nepafenac, eplerenone | 1× zivafl | 1× zivafl | 1× FD-PDT |
| 3 | Acetazolamide, eplerenone | Melatonin | Spironolactone, 1× zivafl, FD-PDT | |
| 4 | ||||
| 5 | Eplerenone | |||
| 6 | ||||
| 7 | Acetazolamide, nepafenac, melatonin | 1× zivafl | ||
| 8 | Eplerenone | 1× zivafl | 1× FD-PDT, 2× zivafl | |
| 9 | Eplerenone | |||
| 10 | Acetazolamide, nepafenac, eplerenone | Eplerenone | 1× zivafl | 2× zivafl, 1× HD-PDT |
| 11 | Nepafenac, eplerenone | 1× zivafl | Rifampicin 1× zivafl, 1× HD-PDT | |
| 12 | 3× bevacizumab | |||
| 13 | Acetazolamide, eplerenone, 2× zivafl, 1× HD-PDT | 1× afl | Eplerenone 1× afl | |
| 14 | Acetazolamide, nepafenac, eplerenone 2× HD-PDT | 1× zivafl | ||
| 15 | Acetazolamide, 1× HD-PDT | |||
| 16 | Acetazolamide, 2× zivafl | |||
| 17 | Eplerenone, 1× HD-PDT | 1× zivafl | ||
| 18 | Acetazolamide, eplerenone, nepafenac, 2× zivafl | Spironolactone 1× zivafl | 1× FD-PDT | |
| Summary | 15× medication | 3× medication | 3× medication | 3× medication |
| 9× intravitreal injection | 1× injection | 7× injection | 9× injection | |
| 5× HD-PDT | 1× FD-PDT | 3× HD-PDT, 3× FD-PDT |
Note: Blank spaces in the table indicate either no treatment before ZivA-PDT or data not available.
Abbreviations: afl, aflibercept; FD, full-dose; HD, half-dose; PDT, photodynamic therapy; ZivA, ziv-aflibercept and aflibercept; zivafl, ziv-aflibercept.
Best corrected visual acuity
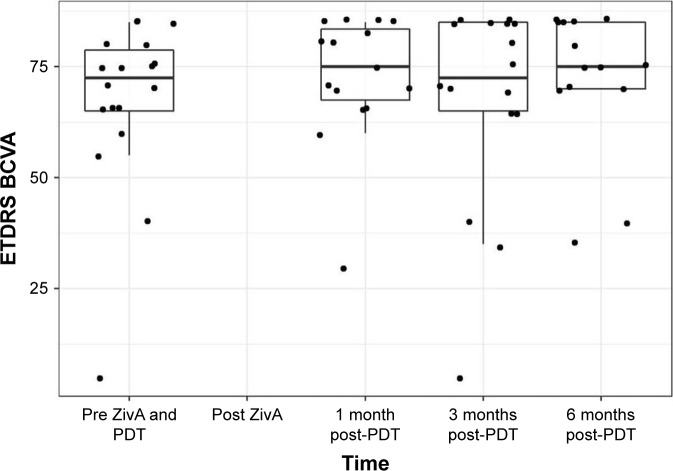
The primary endpoint, best corrected visual acuity (BCVA) change at 6 months follow-up, showed an improvement by a median of 2.5 ETDRS letters from a high median baseline BCVA of 72.5 letters. As shown in Figure 1 and Table 3, this improvement did just not reach statistical significance (p=0.065). Only 1 patient lost more than 5 letters compared to baseline at the 6-month follow-up. Eight patients gained BCVA after 6 months. Five patients showed no change in BCVA from baseline.
Figure 1.
ETDRS BCVA.
Notes: Boxplots show median, first and third quartile, upper/lower whisker extends from the hinge to the largest/smallest value no further than 1.5×IQR from the hinge. Dots indicate individual values.
Abbreviations: BCVA, best corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; IQR, interquartile range; PDT, photodynamic therapy; ZivA, ziv-aflibercept and aflibercept.
Table 3.
ETDRS
| BCVA (by ETDRS) | Median ETDRS | Interquartile range | Significance |
|---|---|---|---|
| Before treatment | 72.50 | 65.00, 78.75 | |
| 1 month after PDT | 75.00 | 67.50, 83.50 | |
| 3 months after PDT | 72.50 | 65.00, 85.00 | |
| 6 months after PDT | 75.00 | 70.00, 85.00 | 0.065# |
Notes: p-value, comparison with before treatment.
Continuity for the distribution of the paired differences was not given, and therefore a sign test was performed.
Abbreviations: BCVA, best corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; PDT, photodynamic therapy.
Subfoveal subretinal fluid height
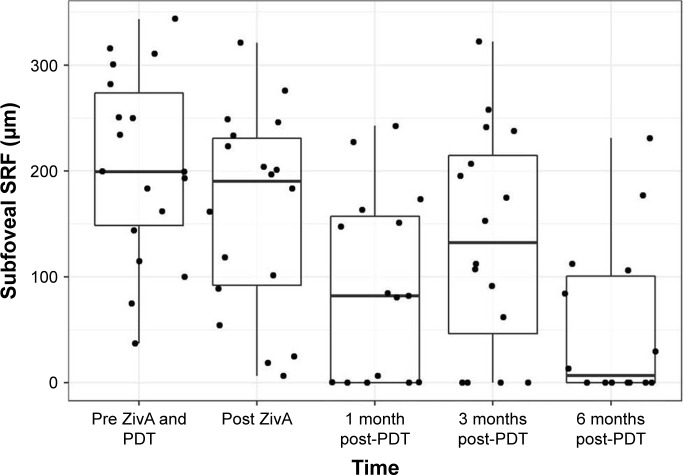
SRF height regressed from a mean of 205.19 μm at baseline to 90.53 μm at month 1 and 53.79 μm at month 6. The mean sSRF height was significantly greater before initiation of treatment compared to 6 months after treatment (p<0.001). At month 6, no patient showed more sSRF than at baseline. At the 6-month follow-up, 13 out of 14 patients showed a reduction of sSRF. Some regression in sSRF (−43.64 μm) was seen after an average of 12 days following the intravitreal injection of ziv-aflibercept and aflibercept alone prior to PDT treatment.
Figure 2 and Table 4 show sSRF height course for the whole group of patients.
Figure 2.
Subfoveal SRF.
Notes: Boxplots show median, first and third quartile, upper/lower whisker extends from the hinge to the largest/smallest value no further than 1.5×IQR from the hinge. Dots indicate individual values.
Abbreviations: IQR, interquartile range; PDT, photodynamic therapy; SRF, subretinal fluid; ZivA, ziv-aflibercept and aflibercept.
Table 4.
sSRF
| Mean sSRF (μm) | SD | Significance | |
|---|---|---|---|
| sSRF before treatment | 205.19 | 88.45 | |
| sSRF after injection, before PDT | 161.56 | 94.77 | 0.002# |
| sSRF 1 month after PDT | 90.53 | 88.24 | <0.001 |
| sSRF 3 months after PDT | 134.97 | 104.55 | 0.008 |
| sSRF 6 months after PDT | 53.79 | 76.50 | <0.001 |
Notes: p-value, comparison with before treatment.
Paired differences were not normally distributed, and therefore an exact Wilcoxon signed-rank test was performed.
Abbreviations: PDT, photodynamic therapy; SD, standard deviation; sSRF, subfoveal subretinal fluid.
Maximum subretinal fluid height
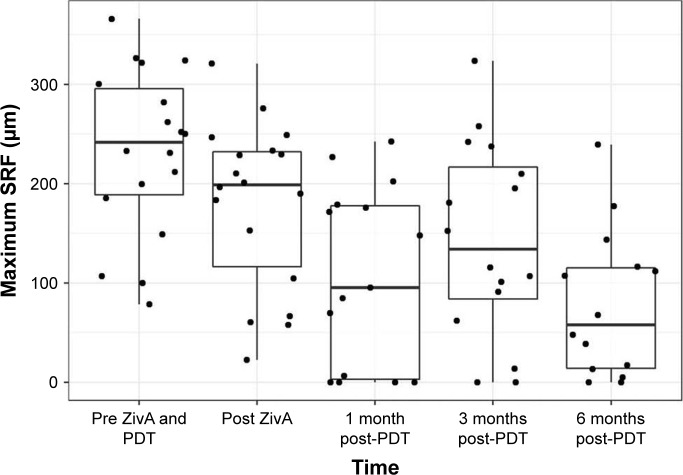
mSRF decreased in two steps. The first improvement was shown between the ziv-aflibercept and aflibercept injection and PDT (−52.78 μm, p=0.002); the second improvement occurred after PDT. The mean mSRF height reduction was −114.47 μm at month 1 (p<0.001), −101.11 μm at month 3 (p=0.008) and −146.75 μm at month 6 (p<0.001). Thirteen patients showed a reduction of mSRF at the 6-month measurement (n=14). One patient had no relevant change in mSRF between initial and final measurement.
Changes in sSRF are shown in Figure 3 and Table 5.
Figure 3.
Maximum SRF.
Notes: Boxplots show median, first and third quartile, upper/lower whisker extends from the hinge to the largest/smallest value no further than 1.5×IQR from the hinge. Dots indicate individual values.
Abbreviations: IQR, interquartile range; PDT, photodynamic therapy; SRF, subretinal fluid; ZivA, ziv-aflibercept and aflibercept.
Table 5.
mSRF
| Mean mSRF (μm) | SD | Significance | |
|---|---|---|---|
| mSRF before treatment | 232.17 | 83.77 | |
| mSRF after injection, before PDT | 179.39 | 84.34 | <0.001# |
| mSRF 1 month after PDT | 106.80 | 90.80 | <0.001 |
| mSRF 3 months after PDT | 143.06 | 97.97 | 0.002 |
| mSRF 6 months after PDT | 77.54 | 74.21 | <0.001 |
Notes: p-value, comparison with before treatment.
Paired differences were not normally distributed, and therefore an exact Wilcoxon signed-rank test was performed.
Abbreviations: mSRF, maximum subretinal fluid; PDT, photodynamic therapy; SD, standard deviation.
Safety
One case of ophthalmic migraine after intravitreal injection and one case of allergic reaction to the FA were documented. None of the eyes experienced adverse events following the intravitreal injection of ziv-aflibercept/aflibercept. One patient had transient vision loss of more than 10 letters in ETDRS BCVA but recovered to baseline ETDRS BCVA after observation period.
Discussion
Our results indicate improved functional and anatomic outcomes in patients with persistent SRF in chronic or repeatedly recurrent acute CSC patients after a combination therapy of ziv-aflibercept and aflibercept and PDT, despite a long history of CSC (28.5 months, standard deviation 20.0 months, range 4 months–6 years) and multiple prior treatments.
Reduction of SRF occurred in two steps. While first reduction of mSRF and sSRF was seen after the intravitreal anti-VEGF treatment with ziv-aflibercept and aflibercept, the second improvement was seen after PDT. BCVA could be stabilized at baseline levels after therapy and during the observation period.
The fact that vision improvement did not quite reach a statistical significance might be attributed to two factors. First, there could be the irreversible retinal and RPE changes in patients with chronic CSC. Second, the high good baseline visual acuity limits the possibility for vision improvement (ceiling effect). In this context, not losing vision could be considered as a good functional outcome.
The good baseline visual acuity, despite the long duration of CSC, might be explained by a relevant number of patients with repeatedly recurrent acute CSC over a long period of time versus patients with chronic persistent CSC over a long time period.
Despite an initial anatomic benefit following ziv-aflibercept and aflibercept only, it remains difficult to judge whether the combined treatment has an additional benefit, especially since there is no control group.
Yet the idea of a synergistic effect is supported by results in other entities with pachychoroid (which were excluded in our study) like PCV where combination therapy seemed to be beneficial.39,40 Mukai et al showed a protective effect of combination therapy of bevacizumab with PDT compared to PDT alone in patients with PCV. Less choriocapillaris occlusions were found in the group that received combination therapy.41 Combination therapy was also shown to be beneficial in the long-term.42
In the present study, although good short-term results were demonstrated at 1st month measurement, sSRF and mSRF showed evidence of recurrence after 3 months of treatment. Twelve of 18 patients required retreatment within 6 months, indicating that in these selected, quite chronic respectively recurrent cases of CSC (high number of various prior treatments), the combination of ziv-aflibercept and aflibercept and PDT is only able to achieve short-term improvements in most patients in this study.
Following treatments were indicated faster, and even within the 1st month (3 of 18 eyes), which is at least partly due to the retrospective study design.32,33 The variety of treatments applied after combination therapy (Table 2) is a limitation of our study, but also indicates the complexity and challenge inherent in this specific patient population. The limited duration of effect in a high number of eyes (12 of 18) following the combination therapy used in this study stands in contrast to the results of the two other studies of Arevalo and Maier, in which combination therapy of bevacizumab and pegaptanib with full-dose PDT in CSC showed good long-term results.32,33 Arevalo and Espinoza showed better visual outcome in combination therapy, although not significantly compared to PDT alone within 12 months. PDT was not repeated in the study group of 8 patients compared to 8 more PDT sessions in PDT group of 10 patients within 12 months of observation group.33 One difference in their study protocol was the time of anti-VEGF injection. While Arevalo and Espinoza and Maier et al injected anti-VEGF immediately after PDT, the injection time of the present study was up to 30 days prior to PDT.32,33 While verteporfin dye accumulates around hyperpermeable areas, the injection of anti-VEGF before PDT could have a negative effect on the efficacy of PDT by reducing hyperpermeability. We, however, selected this treatment strategy to block VEGF, as upregulation of VEGF is a known and potentially unfavorable consequence of PDT. Another difference of our study compared to the two studies already mentioned is the longer mean duration of CSC before treatment, that is, 28.5 months compared to 11.6 months and 15.3 months, as well as the number of pretreatments (15× medical treatment, 9× intravitreal injections, 5× PDT on 18 eyes), which was higher compared to Maier et al (no detailed information on 9 eyes) and Arevalo and Espinoza (2× PDT, 1× intravitreal injection on 8 eyes).32,33 While in the present study, 17 eyes received half-dose PDT and one eye received full-dose PDT, the study protocols of Arevalo and Espinoza and Maier et al used full-dose PDT in all cases.32,33 In our study the duration of treatment effect was less favorable compared to the results of Maier et al and Arevalo and Espinoza.32,33 It remains unclear if this is due to the selected treatment strategy or due to the selected patient population. We consider the higher affinity for VEGF of the VEGF inhibitors used in our study less likely to be the reason for the difference in durability.
At 6 months, sSRF and mSRF decreased again; yet due to the many retreatments during this period, our long-term data cannot be interpreted as a success of combination therapy but rather as a success of persistent search for the optimal individualized treatment regime and in some cases a success of repeating injections.
Although individual cases of chronic or recurrent acute CSC courses must not be over-interpreted due to the common “waxing and waning” in the natural course of chronic CSC, it must be mentioned that overall results appear helpful in the management of these chronic or repeatedly recurrent acute and difficult to manage cases. Three cases had an optimal treatment response (≤15 microns of SRF) without recurrence within the observation period and without any need for further treatment.
Our study was too small to draw conclusions from subgroups but similar predictive factors as in anti-VEGF monotherapy may play a role. Kim et al identified clinical characteristics for good responders to intravitreal bevacizumab monotherapy in CSC, that is, better vision, smaller lesion and thicker choroid at baseline. It was also suggested that successive treatment with anti-VEGF injections is beneficial in the so-called responders to anti-VEGF.43 This seems reasonable in regard to the pharmacokinetics and the limited half-life of available anti-VEGF drugs, whereas aflibercept appears to have the longest duration of effect in the eye among all the currently available VEGF inhibitors.44
Conclusion
Our results suggest that short-term improvements in BCVA and a reduction of SRF can be achieved by combination therapy and that further monotherapy and combination therapies can sustain the beneficial effects. However, it is also obvious that combination therapy with ziv-aflibercept and aflibercept and PDT is far from an ideal treatment strategy in this specific patient population with chronic respectively repeatedly recurrent acute CSC. Further larger and controlled studies are required to obtain definite answers for this specific patient population. Despite these limitations, the combination therapy of ziv-aflibercept and aflibercept with PDT appears to be safe for up to 6 months and is therefore a potential treatment option for this challenging disease.
Acknowledgments
The study was supported by the Werner H Spross Foundation (Zurich, Switzerland).
Footnotes
Author contributions
JMD, FJF, SM and NG developed the conception and design of the study, while MDB contributed substantially to data acquisition and design of the study. JMD, FJF, SM and NG drafted the article, while MDB revised the article critically. All authors approved the final version of the paper to be published and agree to be accountable for all aspects of the work.
Disclosure
The “Stiftung wissenschaftliche Forschung”, Fonds Ophthalmologie, Triemli City Hospital received research grants from Novartis Schweiz AG and Bayer Schweiz AG and payments for invited talks or advisory board participation for MDB and SM from Allergan, Novartis, Alimera, Bayer and Roche. The authors report no other conflicts of interest in this work.
References
- 1.Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33(2):302–308. doi: 10.1097/IAE.0b013e318263d11f. [DOI] [PubMed] [Google Scholar]
- 3.Chung YR, Kim JW, Kim SW, Lee K. Choroidal thickness in patients with central serous chorioretinopathy: assessment of Haller and Sattler Layers. Retina. 2016;36(9):1652–1657. doi: 10.1097/IAE.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 4.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–214. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 5.Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72(11):829–834. doi: 10.1136/bjo.72.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gäckle HC, Lang GE, Freissler KA, Lang GK. Central serous chorioretinopathy. Clinical, fluorescein angiography and demographic aspects. Ophthalmologe. 1998;95(8):529–533. doi: 10.1007/s003470050310. German [with English abstract] [DOI] [PubMed] [Google Scholar]
- 7.Kim YY, Flaxel CJ. Factors influencing the visual acuity of chronic central serous chorioretinopathy. Korean J Ophthalmol. 2011;25(2):90–97. doi: 10.3341/kjo.2011.25.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133(6):787–793. doi: 10.1016/s0002-9394(02)01438-1. [DOI] [PubMed] [Google Scholar]
- 9.Bonini Filho MA, de Carlo TE, Ferrara D, et al. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(8):899–906. doi: 10.1001/jamaophthalmol.2015.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breukink MB, Dingemans AJ, den Hollander AI, et al. Chronic central serous chorioretinopathy: long-term follow-up and vision-related quality of life. Clin Ophthalmol. 2017;11:39–46. doi: 10.2147/OPTH.S115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841. doi: 10.1002/14651858.CD011841.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erikitola OC, Crosby-Nwaobi R, Lotery AJ, Sivaprasad S. Photodynamic therapy for central serous chorioretinopathy. Eye (Lond) 2014;28(8):944–957. doi: 10.1038/eye.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaman A, Arikan G, Saatci AO, Cingil G. Choroidal neovascularization following photodynamic therapy in a patient with chronic central serous chorioretinopathy. Boll Soc Belge Ophthalmol. 2007;303:69–73. [PubMed] [Google Scholar]
- 14.Lee PY, Kim KS, Lee WK. Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2009;53(1):52–56. doi: 10.1007/s10384-008-0613-z. [DOI] [PubMed] [Google Scholar]
- 15.Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006;26(2):239–242. doi: 10.1097/00006982-200602000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, Naumann GO. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44(10):4473–4480. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- 17.Michels S, Schmidt-Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci. 2003;44(5):2147–2154. doi: 10.1167/iovs.02-0604. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 19.Lai TY, Chan WM, Li H, Lai RY, Liu DT, Lam DS. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90(7):869–874. doi: 10.1136/bjo.2006.090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reibaldi M, Cardascia N, Longo A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chori-oretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149(2):307.e2–315.e2. doi: 10.1016/j.ajo.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011;31(1):119–126. doi: 10.1097/IAE.0b013e3181e378f2. [DOI] [PubMed] [Google Scholar]
- 22.Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010;149(3):441, 446.e1–e2. doi: 10.1016/j.ajo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Tseng CC, Chen SN. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol. 2015;99(8):1070–1077. doi: 10.1136/bjophthalmol-2014-305353. [DOI] [PubMed] [Google Scholar]
- 24.Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intra-vitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond) 2013;27(12):1339–1346. doi: 10.1038/eye.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue M, Kadonosono K, Watanabe Y, Kobayashi S, Yamane S, Arakawa A. Results of one-year follow-up examinations after intra-vitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica. 2011;225(1):37–40. doi: 10.1159/000314709. [DOI] [PubMed] [Google Scholar]
- 26.Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010;35(2):91–98. doi: 10.3109/02713680903428306. [DOI] [PubMed] [Google Scholar]
- 27.Entezari M, Ramezani A, Yaseri M. Intravitreal bevacizumab for treatment of refractory central serous choroidoretinopathy. Korean J Ophthalmol. 2012;26(2):139–142. doi: 10.3341/kjo.2012.26.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto M, Matsuura T, Ogata N. Choroidal thickness and choroidal blood flow after intravitreal bevacizumab injection in eyes with central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):25–32. doi: 10.3928/23258160-20150101-04. [DOI] [PubMed] [Google Scholar]
- 29.Kim DY, Joe SG, Yang HS, Lee JY, Kim JG, Yoon YH. Subfoveal choroidal thickness changes in treated idiopathic central serous chorioretinopathy and their association with recurrence. Retina. 2015;35(9):1867–1874. doi: 10.1097/IAE.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 30.Bae SH, Heo JW, Kim C, et al. A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;152(5):784.e2–792.e2. doi: 10.1016/j.ajo.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Bae SH, Heo J, Kim C, et al. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmology. 2014;121(2):558–565. doi: 10.1016/j.ophtha.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Maier M, Valet V, Feucht N, Lohmann CP. Therapy options for chronic central serous chorioretinopathy. Photodynamic therapy combined with bevacizumab – a case series. Ophthalmologe. 2011;108(11):1027–1031. doi: 10.1007/s00347-011-2419-5. German [with English abstract] [DOI] [PubMed] [Google Scholar]
- 33.Arevalo JF, Espinoza JV. Single-session combined photodynamic therapy with verteporfin and intravitreal anti-vascular endothelial growth factor therapy for chronic central serous chorioretinopathy: a pilot study at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1159–1166. doi: 10.1007/s00417-011-1651-7. [DOI] [PubMed] [Google Scholar]
- 34.Mazaraki K, Fassnacht-Riederle H, Blum R, Becker M, Michels S. Change in choroidal thickness after intravitreal aflibercept in pretreated and treatment-naive eyes for neovascular age-related macular degeneration. Br J Ophthalmol. 2015;99(10):1341–1344. doi: 10.1136/bjophthalmol-2015-306636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharbiya M, Cruciani F, Mariotti C, Grandinetti F, Marenco M, Cacace V. Choroidal thickness changes after intravitreal antivascular endothelial growth factor therapy for age-related macular degeneration: ranibizumab versus aflibercept. J Ocul Pharmacol Ther. 2015;31(6):357–362. doi: 10.1089/jop.2014.0160. [DOI] [PubMed] [Google Scholar]
- 36.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitcher JD, 3rd, Witkin AJ, DeCroos FC, Ho AC. A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study. Br J Ophthalmol. 2015;99(6):848–852. doi: 10.1136/bjophthalmol-2014-306018. [DOI] [PubMed] [Google Scholar]
- 38.Mansour AM, Ashraf M, Dedhia CJ, Charbaji A, Souka AAR, Chhablani J. Long-term safety and efficacy of ziv-aflibercept in retinal diseases. Br J Ophthalmol. 2017;101(10):1374–1376. doi: 10.1136/bjophthalmol-2016-309724. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Iida T, Kano M, Itagaki K. Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(9):2099–2110. doi: 10.1007/s00417-013-2323-6. [DOI] [PubMed] [Google Scholar]
- 40.Sakurada Y, Iijima H. Two-year results of photodynamic therapy with or without intravitreal ranibizumab for polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther. 2013;29(9):832–836. doi: 10.1089/jop.2013.0044. [DOI] [PubMed] [Google Scholar]
- 41.Mukai R, Kishi S, Sato T, Watanabe G, Matsumoto H. Protective effect of intravitreal bevacizumab and sub-Tenon triamcinolone acetonide against occlusion of choriocapillaris induced by photodynamic therapy. Ophthalmologica. 2010;224(5):267–273. doi: 10.1159/000287348. [DOI] [PubMed] [Google Scholar]
- 42.Kang HM, Koh HJ. Two-year outcome after combination therapy for polypoidal choroidal vasculopathy: comparison with photodynamic monotherapy and anti-vascular endothelial growth factor monotherapy. Ophthalmologica. 2014;231(2):86–93. doi: 10.1159/000354546. [DOI] [PubMed] [Google Scholar]
- 43.Kim GA, Rim TH, Lee SC, et al. Clinical characteristics of responders to intravitreal bevacizumab in central serous chorioretinopathy patients. Eye (Lond) 2015;29(6):732–740. doi: 10.1038/eye.2015.58. quiz 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balaratnasingam C, Dhrami-Gavazi E, McCann JT, Ghadiali Q, Freund KB. Aflibercept: a review of its use in the treatment of choroidal neovascularization due to age-related macular degeneration. Clin Ophthalmol. 2015;9:2355–2371. doi: 10.2147/OPTH.S80040. [DOI] [PMC free article] [PubMed] [Google Scholar]