Abstract
Data concerning the cardiovascular manifestations of primary hyperparathyroidism (PHPT) are inconsistent, which is due, in part, to the decrease in disease severity over the last several decades. In areas where patients tend to be more symptomatic, data support the presence of cardiovascular findings including myocardial and vascular calcification as well as increased cardiovascular mortality. Data from the cohorts in whom the disease is characterized by mild hypercalcemia, suggest that clinically overt cardiovascular manifestations are unusual in PHPT. Recent data, however, support the presence of subtle cardiovascular manifestations in mild disease, such as changes in endothelial function as well as increased vascular stiffness and perhaps diastolic dysfunction. Left ventricular hypertrophy is a more consistent finding across a spectrum of disease severity, though this finding may be related to hypertension, which has long been associated with PHPT.
Keywords: Cardiovascular, mortality, primary hyperparathyroidism
INTRODUCTION
There is considerable debate regarding the cardiovascular (CV) manifestations of primary hyperparathyroidism (PHPT) with conflicting data concerning their extent and clinical significance. Many of these apparent disparities may be related to the evolution of the clinical presentation of PHPT over the last 70 years. Once a symptomatic disorder characterized by significant hypercalcemia (1), modern PHPT is frequently asymptomatic, with mild hypercalcemia discovered incidentally on routine biochemical screening (2). As a result, studies of the CV system in PHPT have enrolled populations with varying disease severity, often leading to discrepant findings. Additionally, investigations have explored different parameters of CV health using disparate modalities in patient populations of varying size. When these factors are considered, many of the ostensible inconsistencies within the literature can be resolved.
One might anticipate CV effects from PHPT. The biochemical features of PHPT include elevations in serum calcium and PTH, which are both known to affect the CV system. Hypercalcemia has been associated with hypertension, left ventricular hypertrophy (LVH), arrhythmias, vasoconstriction, as well as calcification of the myocardium, heart valves, and coronary arteries (3–8). PTH has been shown to have a vasodilatory effect and to exert direct positive chronotropic and indirect inotropic effects on the heart (9). Data suggest that PTH may also act as a “hypertrophic factor” on myocardial muscle cells, thereby inducing LVH (10). Numerous studies have explored many of these potential mechanisms of CV disease in PHPT by assessing mortality, hypertension, cardiac conduction abnormalities and arrhythmias, as well as CV structural and functional changes.
MORTALITY
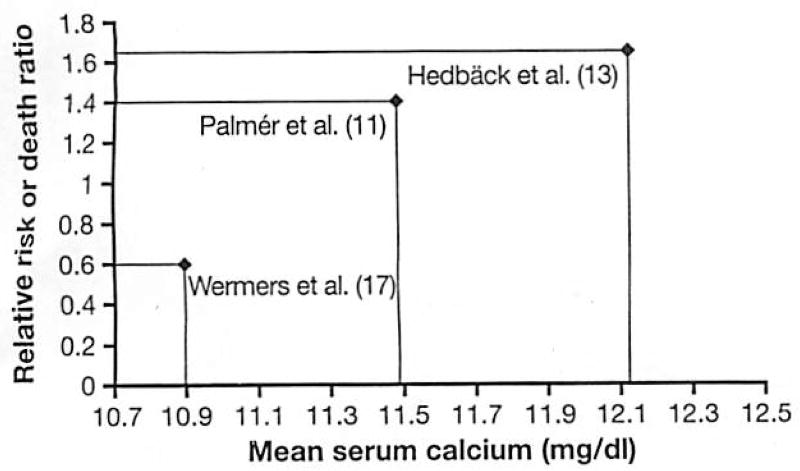
There is ample documentation that CV mortality is increased in patients with severe and moderately severe PHPT (11–14). The higher mortality rate has been shown to decline with time from parathyroidectomy (PTX), but still persists long after surgical cure, suggesting that PHPT might cause enduring damage to the CV system (15). The few North American studies that assessed mortality in patients with PHPT have not found mortality to be adversely impacted (16, 17). In contrast to the European patients described in the studies cited above, PHPT patients diagnosed in Rochester, Minnesota between 1965 and 1992 had lower than expected overall mortality (17). Specifically, CV death rates were strikingly reduced (relative risk 0.6). However, consistent with the European studies, this study did demonstrate that higher maximal serum calcium levels were an independent predictor of mortality. These results are also in accordance with a Swedish investigation indicating that higher serum calcium level, even within the normal range, is an independent predictor of CV mortality (18).
One explanation for these incongruent mortality data is that more patients in the US studies had mild disease, with lower serum calcium levels [mean calcium 2.73 mmol/l (10.9 mg/dl)] and fewer symptoms than patients in the European studies, where average calcium levels were significantly higher. This hypothesis is supported by data from Nilsson et al. (19), who analyzed mortality over a 30 year time period in 10,995 Swedish patients who underwent PTX. While an increased risk of CV mortality was observed in the overall cohort, this risk dissipated in those enrolled later in the study, when the multichannel autoanalyzer allowed diagnosis of the disease in those with no symptoms and lower levels of serum calcium. Hedback et al. (15, 20) also found that survival in Swedish men and women with PHPT who had undergone PTX improved in those with a more recent calendar year of surgery. The decline in death risk paralleled the decrease in mean preoperative serum calcium level over time (15). While these data do lend credence to the idea that the decline in mortality in more recent years is due to lower calcium levels, there are also other potential interpretations. Among these are the possibilities that increased CV mortality in PHPT is reversed by earlier diagnosis and intervention, or that there was a change in the referral pattern for PTX. Alternatively, newly available treatments for CV abnormalities could explain the findings. Several studies that reported mean serum calcium and risk of death are summarized in Figure 1. These data suggest that relative risk of CV death in PHPT varies almost linearly with calcium level and that this is a plausible explanation for disparate findings.
Fig. 1.
Serum calcium and relative risk of cardiovascular death.
HYPERTENSION
Hypertension is frequently seen in association with PHPT (21–23), even among those with mild disease. While a few studies have shown a reduction in blood pressure immediately after PTX (22, 24), which could be due to the non-specific effects of bed rest or anesthesia, the majority of studies indicate that hypertension is not reversible with surgical cure (21, 25–28). The cause of hypertension in PHPT, still unclear, has been hypothesized to be due to hypomagnesemia (29), increases in catecholamines (30), elevations in renin and aldosterone (31) or vasocontriction (22, 32). Because of uncertainties regarding the mechanism and reversibility of hypertension, the causal relationship between PHPT and hypertension has been questioned. One exception to this rule has been in those patients with multiple endocrine neoplasia, in whom hypertension may be due to catecholamine excess and pheochromcytoma resection curative. As there is no expectation that hypertension will improve in those with sporadic disease, the presence of hypertension in patients with PHPT is not currently an indication for PTX.
CARDIAC CONDUCTION ABNORMALITIES AND ARRYTHMIAS
The shortened QT interval, a well-described consequence of hypercalcemia, creates the potential for cardiac arrhythmia in PHPT (33). There is inconsistent data regarding the presence of cardiac rhythm disturbances in PHPT, but again, this appears to be related to the degree of hypercalcemia in the population studied. A study of pre-operative electrocardiograms in 139 Swedish patients with PHPT [mean calcium 3.03 mmol/l (12.1 mg/dl)] found that serum calcium levels correlated positively with T-wave duration and negatively with QT interval (34, 35). In patients with a similar mean calcium level [2.95 mmol/l (11.8 mg/dl)], Nilsson et al. (36) found a small increase in the number of ventricular extrasystolic beats in those with PHPT, which improved with PTX (36).
A small study in patients with more moderate hypercalcemia [mean calcium 2.85 mmol/l (11.4 mg/dl)] confirmed an increase in QT interval after PTX, but found no increased prevalence of supraventricular or ventricular arrhythmias or high-grade AV block (37). QT shortening was not observed in the small study of Barletta et al. [mean calcium 2.88 mmol/l (11.5 mg/dl)], but only 14 patients with PHPT were studied; they did, however, find evidence of increased sympathetic drive to the heart (38). A recent Italian study, that did not report calcium levels, also found QT shortening and higher sympathetic tone in those with PHPT (39).
CARDIAC STRUCTURAL ABNORMALITIES
Left ventricular hypertrophy
LVH, a strong predictor of CV mortality, has been associated with PHPT in most (40–45), but not all (38, 46, 47), studies across a wide range of calcium levels (2.63–3 mmol/l) (10.5–12 mg/dl). The coexistence of hypertension in many patients with PHPT raises the possibility that any LVH observed in PHPT could be attributable to elevations in blood pressure. However, data suggest that LVH is independent of hypertension (43). Instead, several studies support an association of LVH with PTH level (40, 41,43) in PHPT. Only one report noted a correlation between left ventricular mass and calcium (42). The higher incidence of LVH in PHPT may be related to increased CV stiffness (48) (See “Vascular function” below) or a direct effect of PTH (10).
The issue of the reversibility of CV abnormalities is of significant interest in determining the management implications of these findings. LVH has been found to regress following PTX in a number of studies that have followed patients for at least 6 months post surgery (41,43, 44). Studies of shorter duration have not found LVH to improve after PTX (42). Stefenelli et al. reported that regression of LVH was most marked in those without a history of hypertension (44).
Valvular and myocardial calcification
Myocardial and valvular calcifications have clearly been demonstrated in PHPT patients with marked hypercalcemia (44, 49, 50). Stefenelli et al. reported an increased frequency of aortic (63% vs 12.5%), mitral (49% vs 15%) and myocardial calcification (69% vs 17.5%) in those with PHPT [mean calcium 2.98 mmol/l (11.9 mg/dl)], compared to controls (50). There was no change in valvular or myocardial calcification with PTX. Studies in patients with more modest increases in serum calcium [2.78 mmol/l (11.1 mg/dl)] are limited but indicate no increase in valvular or myocardial calcifications (41), suggesting that this phenomenon is related to the level of hypercalcemia and less likely to be seen in those with the milder biochemical phenotype of PHPT so common today.
ANATOMIC VASCULAR ABNORMALITIES
It is logical to expect that excess serum calcium might lead to deposition of calcium in the vascular wall, with resulting stiffening of the vessel. The resulting abnormalities, as in other endocrinopathies, may vary in different parts of the vascular tree.
Coronary artery disease
There are very limited data regarding coronary artery disease in PHPT. The autopsy study of Roberts and Waller (4) concluded that hypercalcemia and PHPT (which affected only half of the patients studied) caused coronary atherosclerosis. The range of calcium in that report was 4.2–6.85 mmol/l (16.8–27.4 mg/dl), making it impossible to generalize these data to patients with mild hyperparathyroidism. More recently, Lind et al. (51) have found serum calcium, even within the normal range, to be an independent, prospective risk factor for Ml in middle-aged Swedish men.
There are some data supporting an increased incidence of coronary artery disease in a cohort of PHPT patient with more moderate hypercalcemia [mean serum calcium 2.96 mmol/l (11.8 mg/dl)] (52). In this study, risk of myocardial infarction was increased before PTX compared to non-hypercalcemic controls and declined to the control level after surgery. Risk of CV death, however, was related to traditional risk factors but not to serum calcium or adenoma size and was decreased in those with kidney stones. This raises uncertainty about the exact nature of the relationship between the hyperparathyroid state and coronary disease.
The recently published Norwegian Tromsø study (53) found serum PTH to be an independent predictor of coronary heart disease in subjects who had normal levels of serum calcium. This report was not intended to specifically study those with primary rather than secondary hyperparathyroidism, and it is likely that the higher PTH levels may have been a surrogate for worse renal function or lower 25-hydroxy vitamin D.
Nilsson et al. investigated reversible signs of myocardial ischemia in those with PHPT [mean calcium 2.97 mmol/l (11.9 mg/dl)]. While there was no difference in ST-segment depression during exercise at baseline between those with PHPT and control subjects, there was improvement in the PHPT group with PTX compared to baseline but no change in controls (36, 47). More data regarding the risk of coronary artery disease are needed in patients with mild PHPT before definitive conclusions can be made.
Carotid vasculature
Carotid intima-medial thickness (IMT) is a strong predictor of systemic atherosclerosis and cerebral vascular events. Recent data from our group extend the previously demonstrated association between serum calcium and mortality. In a large epidemiological study, we have shown that serum calcium levels within the normal range are positively associated with carotid plaque thickness even after adjustment for traditional CV risk factors (54). The carotid vasculature has been incompletely investigated in PHPT. In one study of 20 patients with severe PHPT, carotid IMT was found to be markedly increased compared to controls (46). Other studies showing no effect on carotid IMT of PHPT or its cure are limited by the small sample sizes, and by technical inadequacies (IMT was measured at the common carotid or brachial arteries, rare sites for plaque, rather than at the bifurcation of the carotid and in the internal carotid artery, where the majority of subclinical atherosclerotic disease is found) (38, 55–58).
A recent study by Fallo was evocative but small, including 26 patients with PHPT (59). They reported that in PHPT, only those with traditional CV risk factors had increased IMT compared to controls, while those without risk factors did not. Those individuals with CV risk factors, however, also had higher serum calcium levels, suggesting that increased IMT might be attributable to the high serum calcium, though there was no significant correlation between calcium and IMT. More than anything, these data highlight the need for further study in this area. There are no data on regression of carotid IMT or plaque thickness following PTX.
CARDIAC FUNCTIONAL ABNORMALITIES
As the severity of PHPT has declined, investigators have begun to examine more subtle alterations in the CV system, including abnormalities of CV function. Theoretically, diastolic function may be negatively affected by intracellular calcium overload with decreased compliance from calcium deposition in the myocardium (60), and indeed diastolic dysfunction has been documented in patients with both more severe PHPT [mean calcium 2.9 mmol/l–2.97 (11.6–11.9 mg/dl)] (47, 61) as well as milder forms of disease [mean calcium 2.78–2.79 mmol/l (11.1–11.2 mg/dl)] (41,42), although interpretation of some data (41) are limited due to higher blood pressure in the PHPT group. Contradictory data come from a study that included participants with mild hypercalcemia [(2.81 mmol/l (11.2 mg/dl)]. This study, which stratified patients according to hypertensive status, did not confirm diastolic dysfunction (43). Nor is it clear whether diastolic dysfunction, if present, is secondary to the effects of hypercalcemia or PTH excess. Data on improvement with surgical cure are also conflicting (40–42, 47, 62).
In contrast, systolic cardiac function appears unaffected by PHPT. One study reported a trend toward increased systolic function (ejection fraction), hypothesized to be due to the isotropic effect of elevated serum calcium, which decreased after PTX (47), but most studies have not confirmed this (42, 43, 50, 61).
VASCULAR FUNCTION
To date, different aspects of vascular function have been investigated, making it difficult to make a comprehensive statement concerning the overall effect of the disease on vascular function. Several studies have measured endothelial function. Endothelial cells, which form the inner lining of blood vessels, have a number of functions including mediating vasomotor tone, inflammation and coagulation among others. Endothelial cells synthesize nitric oxide, which causes vasodilation, inhibition of platelet aggregation and monocyte adhesion, as well as a reduction in prothrombotic factors. Endothelial dysfunction is thought to be an early and important step in atherogenesis. In a study of very limited size (12 volunteers), calcium infusion resulted in dose-related impairment in endothelial vasodilatory function and increased systolic blood pressure (32). Nilsson et al. reported an abnormal endothelial vasodilatory response in patients with severe PHPT [3 mmol/l (calcium 12.0 mg/dl)], as demonstrated by the response to infusion of metacholine and nitro-prusside (55) while Neunterfl et al. [(mean calcium 3.0 mmol/l (12.0 mg/dl)] (63) found normal endothelial-dependent dilation (assessed by flow-mediated dilatation). In contrast, Kosch et al. (56) also assessed flow-mediated vasodilation in severely hypercalcemic patients [(calcium 3 mmol/l (12.0 mg/dl)] and found that endothelium-dependent flow mediated vasodilation was impaired in PHPT, but improved with PTX (64). These differences may be due to the fact that the Neunteufl study included controls with a high incidence of other CV risk factors. Baykan et al. also found impaired flow mediated (endothelial) dilation in those with more mild PHPT [mean calcium 2.9 mmol/l (11.6 mg/dl)], which negatively correlated with calcium levels (65). The preponderance of data does seem to suggest that endothelial dysfunction is a feature of PHPT.
Since PTH raises intracellular calcium concentration (66), high calcium in myocytes could cause increased contractility or cell damage that impairs vascular smooth muscle relaxation. Neunterfl did find abnormal vascular smooth muscle reactivity (nitroglycrerin induced changes in arterial media), that was not reversible with PTX (63), though other studies have not confirmed this (55, 56).
Two studies have reported increased vascular stiffness, an independent marker of CV risk (67) in patients with mild PHPT [calcium 2.66–2.74 mmol/l (10.7–10.9 mg/dl)] (48, 68). While in one (48) this finding might have been explained by the inclusion of a younger, less overweight and less hypercholesterolemic control group, the study from our group found that having PHPT was an independent risk factor for increased vascular stiffness (68). Indeed, PHPT was a stronger predictor of increased aortic stiffness than many traditional CV risk factors. Furthermore, vascular stiffness was associated with evidence of more active parathyroid disease. Stiffness was significantly positively correlated with the extent of elevation in PTH levels, and inversely associated with bone density at the distal one-third radius (54).
Hypothetically, arterial stiffness may result from either structural (arterial wall calcification) or functional (i.e. endothelial) abnormalities in large and medium-sized vessels, though there is no evidence for the former. Increased vascular stiffness may be responsible for LVH in PHPT patients without hypertension (48).
SUMMARY AND IMPLICATIONS
In summary, there is a large body of research dealing with various aspects of CV involvement in PHPT. Data on individual abnormalities have been touted to support the presence or absence of such involvement (Table 1). Unfortunately, some of the comparisons that have been made obscure rather than clarify the situation, as they compare studies of patients with varying degrees of disease severity, and look at studies in differing portions of the CV system.
Table 1.
Select studies of cardiac abnormalities in primary hyperparathyroidism.
| Author | Serum calcium | Year | Parameter studied | Results |
|---|---|---|---|---|
| Roberts and Waller (4) | 4.85 mmol/l (19.4 mg/dl) | 1981 | Coronary calcification | Increased |
|
| ||||
| Nuzzo (46) | 3.05 mmol/l (12.2 mg/dl) | 2002 | Blood pressure | Not increased |
| Left ventricular mass index | Not icreased | |||
| Carotid intimal medial thickness | Increased | |||
| Carotid atherosclerotic plaques | Not increased | |||
|
| ||||
| Fallo (59) | 3.05 mmol/l (12.2 mg/dl) | 2003 | Carotid mean and maximum intima-media thickness and carotid plaque number | Increased in those with traditional atherosclerotic risk factors |
|
| ||||
| Stefenelli (44, 50) | 3.03 mmol/l (12.1 mg/dl) | 1993 | Left ventricular hypertrophy | Increased |
| 1997 | ||||
|
| ||||
| Lind (35) | 3.03 mmol/l (12.1 mg/dl) | 1994 | Electrocardiogram | QT interval decreased |
|
| ||||
| Niederle (69) | 3.02 mmol/l (12.1 mg/dl) | 1990 | Valvular and myocardial calcification | Increased |
|
| ||||
| Kosch (64) | 3.0 mmol/l (12.0 mg/dl) | 2000 | Endothelium-independent (nitrogen-induced) vasodilation | Normal |
| Endothelium-dependent (flow-mediated) dilation | Impaired | |||
| Carotid and brachial intima-media thickness | Normal | |||
|
| ||||
| Neunteufl (63) | 3.0 mmol/l (12.0 mg/dl) | 1998 | Endothelium-independent (nitrogen-induced) vasodilation | Impaired |
| Endothelium-dependent (flow-mediated) dilation | Normal | |||
|
| ||||
| Nilsson (47, 55) | 2.97 mmol/l (11.9 mg/dl) | 2000 | Blood pressure | Not increased |
| Heart rate | Normal | |||
| Maximal workload | Normal | |||
| Ventricular premature beats at maximal workload | Increased | |||
| Endothelium-dependent (metacholine and nitroprusside induced) vasodilation | Impaired | |||
|
| ||||
| Baykan (61,65) | 2.9 mmol/l (11.6 mg/dl) | 2007 | Endothelium-dependent (flow-mediated) vasodilation | Impaired |
| Diastolic function (Amax and E/Amax) | Impaired | |||
| Interventricular septum, posterior wall, relative wall thickness | Increased | |||
| Left ventricular mass index | Normal | |||
| Ejection fraction | Normal | |||
|
| ||||
| Barletta (38) | 2.88 mmol/l (11.5 mg/dl) | 2000 | QT Interval | Normal |
| Valvular and myocardial calcification | Not increased | |||
| Left ventricular mass index | Not increased | |||
| Arterial morphology | Normal | |||
| Elastic properties | Normal | |||
| Sympathetic drive | Increased | |||
|
| ||||
| Rosenqvist (37) | 2.85 mmol/l (11.4 mg/dl) | 1992 | Electrocardiogram | No arrhythmia |
|
| ||||
| Piovesan (43) | 2.81 mmol/l (11.2 mg/dl) | 1999 | Left ventricular hypertrophy | Increased |
|
| ||||
| Nappi (42) | 2.79 mmol/l (11.2 mg/dl) | 2000 | Left ventricular mass | Increased |
| Interventricular septal thickness | Increased | |||
| Aortic root and left atrial dimensions | Increased | |||
| Ejection fraction | Decreased | |||
| Diastolic function (Amax and E/Amax) | Impaired | |||
|
| ||||
| Dalberg (41) | 2.78 mmol/l (11.1 mg/dl) | 1996 | Blood pressure | Increased |
| Left atrial diameter | Increased | |||
| Valvular and myocardial calcification | Not increased | |||
|
| ||||
| Rubin (68) | 2.66 mmol/l (10.7 mg/dl) | 2005 | Arterial stiffness (augmentation index) | Increased |
This much is clear. CV mortality is increased in patients with moderate to severe hypercalcemia, but the limited available data have not confirmed this finding in those with mild disease. While PHPT is associated with hypertension, the nature of this association is poorly understood. Hypertension is not reversible with cure of the underlying disease and therefore should not be used as an indication for PTX in patients who do not have multiple endocrine neoplasia. CV structural abnormalities exist, with LVH being the most consistent finding across a spectrum of disease severity, while myocardial and valvular calcifications seem to be present only with severe disease. Even in patients with mild disease, it is essential to dissect out the effects of co-existing hypertension on the left ventricle in this regard. And while coronary and carotid calcifications are an issue in those with severe disease, there is insufficient data from which to draw conclusions in those with mild PHPT. Finally, recent data support the presence of subtle CV manifestations in mild disease, such as changes in endothelial function as well as increased vascular stiffness and perhaps diastolic dysfunction.
Further investigation in this area is clearly necessary. It is not likely that some of the data in this field are “right” and others “wrong”. Instead, many contradictions may well be due to comparisons among very different patient populations and/or different assessment techniques.
In particular, it is important to obtain a clearer picture of the extent and nature of CV involvement in those with very mild PHPT. In patients with very mild levels of hypercalcemia it may be more appropriate to seek subtle functional abnormalities that have been demonstrated to predict deleterious CV outcomes. Finally, longitudinal assessment is needed. Demonstration of significant reversible CV abnormalities in asymptomatic PHPT would likely change recommendation for PTX.
Acknowledgments
This work was supported in part by NIH grants R01DK066329, K24DK74457, K23AR053507, the National Osteoporosis Foundation and the Mary and David Hoar Fellowship Program of the New York Community Trust and The New York Academy of Medicine.
References
- 1.Albright F, Aub J, Bauer W. Hyperparathyroidism: a common and polymorphic condition as illustrated by seventeen proved cases from one clinic. JAMA. 1934;102:1276–87. [Google Scholar]
- 2.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 3.Catellier MJ, Chua GT, Youmans G, Waller BF. Calcific deposits in the heart. Clin Cardiol. 1990;13:287–94. doi: 10.1002/clc.4960130410. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WC, Waller BF. Effect of chronic hypercalcemia on the heart. An analysis of 18 necropsy patients. Am J Med. 1981;71:371–84. doi: 10.1016/0002-9343(81)90163-7. [DOI] [PubMed] [Google Scholar]
- 5.Symons C, Fortune F, Greenbaum RA, Dandona P. Cardiac hypertrophy, hypertrophic cardiomyopathy, and hyperparathyroidism--an association. Br Heart J. 1985;54:539–42. doi: 10.1136/hrt.54.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefenelli T, Wikman-Coffelt J, Wu ST, Parmley WW. Calcium-dependent fluorescence transients during ventricular fibrillation. Am Heart J. 1990;120:590–7. doi: 10.1016/0002-8703(90)90016-q. [DOI] [PubMed] [Google Scholar]
- 7.Campese VM. Calcium, parathyroid hormone, and blood pressure. Am J Hypertens. 1989;2(Pt 2):34S–44S. doi: 10.1093/ajh/2.2.34s. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Aoki K. Hypertensive effects of calcium infusion in subjects with normotension and hypertension. J Hypertens. 1988;6:1003–8. doi: 10.1097/00004872-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ogino K, Burkhoff D, Bilezikian JP. The hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related protein. Endocrinology. 1995;136:3024–30. doi: 10.1210/endo.136.7.7789328. [DOI] [PubMed] [Google Scholar]
- 10.Schlüter KD, Piper HM. Trophic effects of catecholamines an parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992;263(6 Pt 2):H1739–46. doi: 10.1152/ajpheart.1992.263.6.H1739. [DOI] [PubMed] [Google Scholar]
- 11.Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery. 1987;102:1–7. [PubMed] [Google Scholar]
- 12.Ronni-Sivula H. Causes of death in patients previously operated on for primary hyperparathyroidism. Ann Chir Gynaecol. 1985;74:13–8. [PubMed] [Google Scholar]
- 13.Hedbäck G, Tisell LE, Bengtsson BA, Hedman I, Oden A. Premature death in patients operated on for primary hyperparathyroidism. World J Surg. 1990;14:829–35. doi: 10.1007/BF01670531. discussion 836. [DOI] [PubMed] [Google Scholar]
- 14.Ljunghall S, Jakobsson S, Joborn C, Palmér M, Rastad J, Akerström G. Longitudinal studies of mild primary hyperparathyroidism. J Bone Miner Res. 1991;6(Suppl 2):S111–6. doi: 10.1002/jbmr.5650061423. discussion S121-4. [DOI] [PubMed] [Google Scholar]
- 15.Hedbäck G, Odén A, Tisell LE. The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg. 1991;15:399–405. doi: 10.1007/BF01658740. discussion 406-7. [DOI] [PubMed] [Google Scholar]
- 16.Söreide JA, van Heerden JA, Grant CS, Yau Lo C, Schleck C, Ilstrup DM. Survival after surgical treatment for primary hyperparathyroidism. Surgery. 1997;122:1117–23. doi: 10.1016/s0039-6060(97)90216-6. [DOI] [PubMed] [Google Scholar]
- 17.Wermers RA, Khosla S, Atkinson EJ, et al. Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med. 1998;104:115–22. doi: 10.1016/s0002-9343(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 18.Leifsson BG, Ahrén B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab. 1996;81:2149–53. doi: 10.1210/jcem.81.6.8964843. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A. Clinical presentation of primary hyperparathyroidism in Europe--nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res. 2002;17(Suppl 2):N68–74. [PubMed] [Google Scholar]
- 20.Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism--an update. Eur J Clin Invest. 1998;28:271–6. doi: 10.1046/j.1365-2362.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Lafferty FW. Primary hyperparathyroidism. Changing clinical spectrum, prevalence of hypertension, and discriminant analysis of laboratory tests. Arch Intern Med. 1981;141:1761–6. doi: 10.1001/archinte.141.13.1761. [DOI] [PubMed] [Google Scholar]
- 22.Nainby-Luxmoore JC, Langford HG, Nelson NC, Watson RL, Barnes TY. A case-comparison study of hypertension and hyperparathyroidism. J Clin Endocrinol Metab. 1982;55:303–6. doi: 10.1210/jcem-55-2-303. [DOI] [PubMed] [Google Scholar]
- 23.Christensson T, Hellström K, Wengle B. Blood pressure in subjects with hypercalcaemia and primary hyperparathyroidism detected in a health screening programme. Eur J Clin Invest. 1977;7:109–13. doi: 10.1111/j.1365-2362.1977.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 24.Ringe JD. Reversible hypertension in primary hyperparathyroidism pre- and posteroperative blood pressure in 75 cases. Klin Wochenschr. 1984;62:465–9. doi: 10.1007/BF01726908. [DOI] [PubMed] [Google Scholar]
- 25.Rapado A. Arteria l hypertension and primary hyperparathyroidism. Incidence and follow-up after parathyroidectomy. Am J Nephrol. 1986;6(Suppl 1):49–50. doi: 10.1159/000167216. [DOI] [PubMed] [Google Scholar]
- 26.Sancho JJ, Rouco J, Riera-Vidal R, Sitges-Serra A. Long-term effects of parathyroidectomy for primary hyperparathyroidismon arterial hypertension. World J Surg. 1992;16:732–5. doi: 10.1007/BF02067371. discussion 736. [DOI] [PubMed] [Google Scholar]
- 27.Jones DB, Jones JH, Lloyd HJ, Lucas PA, Wilkins WE, Walker DA. Changes in blood pressure and renal function after parathyroidectomy in primary hyperparathyroidism. Postgrad Med J. 1983;59:350–3. doi: 10.1136/pgmj.59.692.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind L, Jacobsson S, Palmér M, Lithell H, Wengle B, Ljunghall S. Cardiovascular risk factors in primary hyperparathyroidism: a 15-year follow-up of operated and unoperated cases. J Intern Med. 1991;230:29–35. doi: 10.1111/j.1365-2796.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 29.Sangal AK, Kevwitch M, Rao DS, Rival J. Hypomagnesemia and hypertension in primary hyperparathyroidism. South Med J. 1989;82:1116–8. doi: 10.1097/00007611-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Marone C, Beretta-Piccoli C, Weidmann P. Role of haemodynamics, catecholamines and renin in acute hypercalcaemic hypertension in man. Clin Sci (Lond) 1980;59(Suppl 6):369s–71s. doi: 10.1042/cs059369s. [DOI] [PubMed] [Google Scholar]
- 31.Gennari C, Nami R, Gonnelli S. Hypertension and primary hyperparathyroidism: the role of adrenergic and renin-angiotensin-aldosterone systems. Miner Electrolyte Metab. 1995;21:77–81. [PubMed] [Google Scholar]
- 32.Nilsson IL, Rastad J, Johansson K, Lind L. Endothelial vasodilatory function and blood pressure response to local and systemic hypercalcemia. Surgery. 2001;130:986–90. doi: 10.1067/msy.2001.118368. [DOI] [PubMed] [Google Scholar]
- 33.Surawicz B. Role of electrolytes in etiology and management of cardiac arrhythmias. Prog Cardiovasc Dis. 1966;8:364–86. doi: 10.1016/s0033-0620(66)80011-7. [DOI] [PubMed] [Google Scholar]
- 34.Lind L, Ljunghall S. Serum calcium and the ECG in patients with primary hyperparathyroidism. J Electrocardiol. 1994;27:99–103. doi: 10.1016/s0022-0736(05)80092-5. [DOI] [PubMed] [Google Scholar]
- 35.Lind L, Ridefelt P, Rastad J, Akerström G, Ljunghall S. Cytoplasmic calcium regulation and the electrocardiogram in patients with primary hyperparathyroidism. Clin Physiol. 1994;14:103–10. doi: 10.1111/j.1475-097x.1994.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson IL, Aberg J, Rastad J, Lind L. Maintained normalization of cardiovascular dysfunction 5 years after parathyroidectomy in primary hyperparathyroidism. Surgery. 2005;137:632–8. doi: 10.1016/j.surg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Rosenqvist M, Nordenström J, Andersson M, Edhag OK. Cardiac conduction in patients with hypercalcaemia due to primary hyperparathyroidism. Clin Endocrinol (Oxf) 1992;37:29–33. doi: 10.1111/j.1365-2265.1992.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 38.Barletta G, De Feo ML, Del Bene R, et al. Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:1815–21. doi: 10.1210/jcem.85.5.6514. [DOI] [PubMed] [Google Scholar]
- 39.Curione M, Letizia C, Amato S, et al. Increased risk of cardiac death in primary hyperparathyroidism: what is a role of electrical instability? Int J Cardiol. 2007;121:200–2. doi: 10.1016/j.ijcard.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 40.Almqvist EG, Bondeson AG, Bondeson L, Nissborg A, Smedgard P, Svensson SE. Cardiac dysfunction in mild primary hyperparathyroidism assessed by radionuclide angiography and echocardiography before and after parathyroidectomy. Surgery. 2002;132:1126–32. doi: 10.1067/msy.2002.128692. discussion 1132. [DOI] [PubMed] [Google Scholar]
- 41.Dalberg K, Brodin LA, Juhlin-Dannfelt A, Famebo LO. Cardiac function in primary hyperparathyroidism before and after operation. An echocardiographic study. Eur J Surg. 1996;162:171–6. [PubMed] [Google Scholar]
- 42.Näppi S, Saha H, Virtanen V, et al. Left ventricular structure and function in primary hyperparathyroidism before and after parathyroidectomy. Cardiology. 2000;93:229–33. doi: 10.1159/000007031. [DOI] [PubMed] [Google Scholar]
- 43.Piovesan A, Molineri N, Casassa F, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999;50:321–8. doi: 10.1046/j.1365-2265.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 44.Stefenelli T, Abela C, Frank H, et al. Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab. 1997;82:106–12. doi: 10.1210/jcem.82.1.3666. [DOI] [PubMed] [Google Scholar]
- 45.Stefenelli T, Globits S, Bergler-Klein J, Woloszczuk W, Längle F, Niederle B. Cardiac changes in patients with hypercalcemia. Wien Klin Wochenschr. 1993;105:339–41. [PubMed] [Google Scholar]
- 46.Nuzzo V, Tauchmanovà L, Fonderico F, et al. Increased intima-media thickness of the carotid artery wall, normal blood pressure profile and normal left ventricular mass in subjects with primary hyperparathyroidism. Eur J Endocrinol. 2002;147:453–9. doi: 10.1530/eje.0.1470453. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson IL, Aberg J, Rastad J, Lind L. Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism-effects of parathyroidectomy. Surgery. 2000;128:895–902. doi: 10.1067/msy.2000.110240. [DOI] [PubMed] [Google Scholar]
- 48.Smith JC, Page MD, John R, et al. Augmentation of central arterial pressure in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:3515–9. doi: 10.1210/jcem.85.10.6880. [DOI] [PubMed] [Google Scholar]
- 49.Längle F, Abela C, Koller-Strametz J, et al. Primary hyperparathyroidism and the heart: cardiac abnormalities correlated to clinical and biochemical data. World J Surg. 1994;18:619–24. doi: 10.1007/BF00353780. [DOI] [PubMed] [Google Scholar]
- 50.Stefenelli T, Mayr H, Bergler-Klein J, Globits S, Woloszczuk W, Niederle B. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med. 1993;95:197–202. doi: 10.1016/0002-9343(93)90260-v. [DOI] [PubMed] [Google Scholar]
- 51.Lind L, Skarfors E, Berglund L, Lithell H, Ljunghall S. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol. 1997;50:967–73. doi: 10.1016/s0895-4356(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 52.Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cardiovascular events before and after surgery for primary hyperparathyroidism. World J Surg. 2003;27:216–22. doi: 10.1007/s00268-002-6541-z. [DOI] [PubMed] [Google Scholar]
- 53.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone levels predict coronary heart disease: the Tromsø study. Eur J Cardiovasc Prev Rehabil. 2004;11:69–74. doi: 10.1097/01.hjr.0000114706.27531.01. [DOI] [PubMed] [Google Scholar]
- 54.Rubin MR, Rundek T, McMahon DJ, Lee HS, Sacco RL, Silverberg SJ. Carotid artery plaque thickness is associated with increased serum calcium levels: The Northern Manhattan study. Atherosclerosis. 2007;194:426–32. doi: 10.1016/j.atherosclerosis.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson IL, Aberg J, Rastad J, Lind L. Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy. Surgery. 1999;126:1049–55. doi: 10.1067/msy.2099.101422. [DOI] [PubMed] [Google Scholar]
- 56.Kosch M, Hausberg M, Vormbrock K, Kisters K, Rahn KH, Barenbrock M. Studies on flow-mediated vasodilation and intima-media thickness of the brachia artery in patients with primary hyperparathyroidism. Am J Hypertens. 2000;13:759–64. doi: 10.1016/s0895-7061(00)00248-x. [DOI] [PubMed] [Google Scholar]
- 57.Kosch M, Hausberg M, Barenbrock M, Posadzy-Malaczynska A, Kisters K, Rahn KH. Arterial distensibility and pulse wave velocity in patients with primary hyperparathyroidism before and after parathyroidectomy. Clin Nephrol. 2001;55:303–8. [PubMed] [Google Scholar]
- 58.Lumachi F, Ermani M, Frego M, et al. lntima-media thickness measurement of the carotid artery in patients with primary hyperparathyroidism. A prospective case-control study and long-term follow-up. In Vivo. 2006;20:887–90. [PubMed] [Google Scholar]
- 59.Fallo F, Camporese G, Capitelli E, Andreozzi GM, Mantero F, Lumachi F. Ultrasound evaluation of carotid artery in primary hyperparathyroidism. J Clin Endocrinol Metab. 2003;88:2096–9. doi: 10.1210/jc.2002-021837. [DOI] [PubMed] [Google Scholar]
- 60.Brutsaert DL, Sys SU, Gillebert TC. Diastolic dysfunction in post-cardiac surgical management. J Cardiothorac Vase Anesth. 1993;7(Suppl 1):18–20. doi: 10.1016/1053-0770(93)90106-u. [DOI] [PubMed] [Google Scholar]
- 61.Baykan M, Erem C, Erdogan T, et al. Assessment of left ventricular diastolic function and the Tei index by tissue Doppler imaging in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf) 2007;66:483–8. doi: 10.1111/j.1365-2265.2007.02756.x. [DOI] [PubMed] [Google Scholar]
- 62.Georgiannos SN, Jenkins BJ, Goode AW. Cardiac output in asymptomatic primary hyperparathyroidism: a stigma of early cardiovascular dysfunction? Int Surg. 1996;81:171–3. [PubMed] [Google Scholar]
- 63.Neunteufl T, Katzenschlager R, Abela C, et al. Impairment of endothelium-independent vasodilation in patients with hypercalcemia. Cardiovasc Res. 1998;40:396–401. doi: 10.1016/s0008-6363(98)00177-1. [DOI] [PubMed] [Google Scholar]
- 64.Kosch M, Hausberg M, Vormbrock K, et al. Impaired flow-mediated vasodilation of the brachia artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res. 2000;47:813–8. doi: 10.1016/s0008-6363(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 65.Baykan M, Erem C, Erdogan T, et al. Impairment of flow mediated vasodilatation of brachia artery in patients with primary hyperparathyroidism. Int J Cardiovasc Imaging. 2007;23:323–8. doi: 10.1007/s10554-006-9166-8. [DOI] [PubMed] [Google Scholar]
- 66.Fardella C, Rodriguez-Portales JA. Intracellular calcium and blood pressure: comparison between primary hyperparathyroidism and essential hypertension. J Endocrinol Invest. 1995;18:827–32. doi: 10.1007/BF03349828. [DOI] [PubMed] [Google Scholar]
- 67.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–82. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 68.Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP, Silverberg SJ. Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:3326–30. doi: 10.1210/jc.2004-1400. [DOI] [PubMed] [Google Scholar]
- 69.Niederle B, Stefenelli T, Glogar D, Woloszczuk W, Roka R, Mayr H. Cardiac calcific deposits in patients with primary hyperparathyroidism: preliminary results of a prospective echocardiographic study. Surgery. 1990;108:1052–6. discussion 1056-7. [PubMed] [Google Scholar]