Abstract
The mechanical properties of the human supraspinatus tendon (SST) are highly heterogeneous and may reflect an important adaptive response to its complex, multiaxial loading environment. However, these functional properties are associated with a location-dependent structure and composition that have not been fully elucidated. Therefore, the objective of this study was to determine the concentrations of types I, II and III collagen in 6 distinct regions of the SST and compare changes in collagen concentration across regions with local changes in mechanical properties. We hypothesized that type I collagen content would be high throughout the tendon, type II collagen would be restricted to regions of compressive loading and type III collagen content would be high in regions associated with damage. We further hypothesized that regions of high type III collagen content would correspond to regions with low tensile modulus and a low degree of collagen alignment. Although type III collagen content was not significantly higher regions that are frequently damaged, all other hypotheses were supported by our results. In particular, type II collagen content was highest near the insertion while type III collagen was inversely correlated with tendon modulus and collagen alignment. The measured increase in type II collagen under the coracoacromial arch provides evidence of adaptation to compressive loading in the SST. Moreover, the structure-function relationship between type III collagen content and tendon mechanics established in this study demonstrates a mechanism for altered mechanical properties in pathological tendons and provides a guideline for identifying therapeutic targets and pathology-specific biomarkers.
Keywords: ELISA, collagen, extracellular matrix, shoulder, biomechanics
INTRODUCTION
Tendon is a structurally and compositionally complex tissue consisting primarily of water, collagen and proteoglycans. Collagen comprises over 80% of the dry weight in tendon, and forms a hierarchically-structured fibrillar network aligned predominantly along the direction of loading [1]. The supraspinatus tendon (SST) is a unique tendon with a high clinical significance as the most commonly injured of the rotator cuff tendons [2–4]. Due to the large range of motion of the shoulder, its oblique enthesis and its proximity to the coracoacromial arch, the SST experiences multi-axial tensile, compressive and shear loads in-vivo [5–8]. As a result, this tendon demonstrates a heterogeneous distribution of collagen fibril orientations and regionally varying anisotropic mechanical properties that have been well-characterized [9–11].
We showed previously that like mechanical properties and collagen orientation, proteoglycan composition in the SST varies from region to region according to local functional demands [12]. Decorin, the most common proteoglycan in tendon, was ubiquitous throughout the SST. Aggrecan, which is generally found in cartilage and other tissues that experience large compressive strains, was concentrated in the anterior and posterior regions of tissue that pass between the coracoacromial arch and humeral head. This area of tissue near the humeral insertion is thought to experience high compressive loads in vivo. Finally, content of biglycan, a proteoglycan typically associated with repair and remodeling, was high on the anterior side of the SST, where tears frequently initiate. Surprisingly, biglycan content was also high in the posterior-joint region of the tendon. However, prior to the current study, it was not known how the major forms of collagen vary by region in the human SST.
The most abundant form of collagen in tendon and throughout the body is type I [13, 14]. Type I collagen fibrils are stiff structures that provide the tendon with its mechanical durability and strength. Like aggrecan, type II collagen is typically associated with tissues that experience compressive loads including growth plate and articular cartilage, where type II collagen comprises nearly 80% of total collagen [15, 16]. Type II collagen also forms strong fibrils, but these fibrils are typically smaller in diameter than collagen I fibrils in tendon and ligament [17]. Type II collagen is typically present in tendon only in small amounts, and is generally concentrated near the bone insertion [18]. Collagen III is another fibrillar collagen that is always associated with type I collagen [13, 14]. Type III collagen fibrils are thinner than type I fibrils [19] and are present in high concentrations in skin, blood vessels and other tissues with large proportions of elastic fibers. During the healing process in tendon, a randomly-oriented initial network of mostly type III collagen is formed at the wound site [20]. Over time, this granulation tissue is replaced by a stronger, better-aligned network of type I collagen [21]. Thus, collagen type III is generally associated with scar tissue and injury. Furthermore, the ratio of type III/type I collagen in tendon has been shown to increase with aging [22] and increased pathology [23, 24].
While the total content of type I and III collagen in the human SST has been investigated [24], the spatial variations of types I, II and III collagen throughout the distinct and unique regions of this tendon are not known. In tendon, it is particularly difficult to extract proteins and when successful, only a representative portion is typically obtained. Therefore, the objective of this study was to establish an efficient protocol for extracting collagen from tendon, measure the regional distributions of types I, II and III collagen in a sufficiently large number of human SST using immunochemical assays and associate these compositional measurements with structural and mechanical measurements performed on the same specimens. We hypothesized that type I collagen content would be ubiquitous throughout the tendon, type II collagen would be highest near the insertion and type III collagen content would be highest in the anterior portion of the tendon, a region commonly associated with damage [2]. Finally, we hypothesized that type III collagen would inversely correlate with tensile modulus and collagen alignment.
METHODS
Sample Preparation
A total of 101 rectangular (~3 × 4 mm), full-thickness samples were cut from 6 regions (Figure 1) of the SSTs of 27 human cadaver shoulders (not every region of every tendon was available for this study). All SST samples analyzed in this study were taken from locations adjacent to the samples tested mechanically [9, 10] and analyzed biochemically for content of specific proteoglycans [12]. The mean age of the 27 donors was 56 ±14 years, and each had no reported history of shoulder injury. A total of 5 additional full-thickness samples were cut from the long head of the biceps tendons of 5 of these donors. Tendons with visible tears (partial or full thickness) were excluded from the study.
Figure 1.
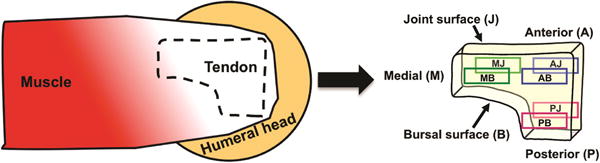
During dissection, all surrounding soft tissues were removed from the SST, including any attached muscle fibers. The humerus was then carefully detached at the enthesis. Phosphate buffered saline (PBS) was used to prevent drying of the tissue throughout harvest and sample preparation. Full-thickness samples were cut from the anterior, medial and posterior regions prior to bisection along their thickness to yield specimens from the anterior-bursal (AB, n=20), anterior-joint (AJ, n=16), posterior-bursal (PB, n=19), posterior-joint (PJ, n=15), medial-bursal (MB, n=17), and medial-joint (MJ, n=14) regions. A similar procedure was followed for dissection of biceps tendons, but each of these specimens was cut from the tendon mid-substance. As our study was focused on determining the relative amounts of the three collagens in the 6 distinct regions of the SST we chose to use as a calibrator the amount of each collagen found in a commonly studied tendon, the biceps.
Using liquid nitrogen, each sample was flash-frozen and cut into 0.5 × 0.5 × 0.5mm cubes using a scalpel. Tissue disruption was carried out using a Spex Freezer Mill (Metuchen, NJ) with the following settings: 5 min pre-cool, 3 impaction cycles at 10 Hz for 2 mins with 1 min pause intervals. Powder was either maintained frozen and portioned for different extractions and studies with precise wet weight determinations. Extractions followed as below.
Collagen Extraction
Collagen extraction was performed in a solution of 1 mg/mL pepsin in 0.5 M acetic acid. A volume of 1 mL of extraction solution was used for every 25 mg (wet weight) of tendon. Pepsin digestion was carried out at either 4⁰ C or 30⁰ C for 15 hours. To visually confirm adequate extraction, a white, opaque appearance in the digested samples was verified. After digestion, an equal volume of 2X-concentrated protease inhibitor (#04693132001, Roche) in Tris-buffered saline (TBS) was added. The sample was then cooled to 4⁰ C and centrifuged at 15,000 × g for 20 minutes. The retrieved supernatant was lyophilized overnight and rehydrated in de-ionized water before adjusting the pH to 8.0 using a small volume (~2 μL) of sodium hydroxide. Finally, 100 μL of 1 mg/mL elastase (#LS006363, Worthington) per 900 μL of sample was added, the sample was incubated for 15 hours at 4° C on a rocker and the supernatant was recovered after centrifugation for 5 minutes at 4° C and 15,000 g.
Using this protocol, the fraction of collagen extracted was found to have a mean value of 0.23 across all tested specimens based on measurements of hydroxyproline content in 50 μL aliquots extracted before and after pepsin digestion as described previously [25]. However, since pepsin digestion could affect the antigenicity of the extracted collagen, the fraction of collagen detectable by ELISA may in fact be lower.
Collagen ELISAs
Content of type I, II and III collagen were determined by ELISA. Type I and II collagen were measured using the Human Collagen Type I ELISA kit (#M036007, MDBioproducts) and the Collagen Type II ELISA kit (#M03600, MDBioproducts). These assays were performed as described in the manufacturer’s protocol, and the provided standards and recommended standard dilutions were used. Samples were diluted by 10-20x in the buffer provided and analyzed in duplicate. While all the antibodies were obtained from commercial sources and were well characterized for specificity (details and references found in respective data sheets), additional specificity testing was performed to determine the of antibodies for each of the three collagen tested (type I capture; type II capture; type III detection) by performing a Western blot using aliquots of the respective collagen before and after bacterial collagenase digestion. This resulted in a loss of immunoreaction in each case after collagenase digestion (data not shown).
Measurement of type III collagen was performed using a custom sandwich ELISA. Standards were prepared from human type III collagen (#1230-01S, Southern Biotech) and diluted in assay buffer (TBS with 0.05% Tween) at concentrations of 2.5, 1.25, 0.625, 0.3125 and 0.15625 μg/mL, while samples were diluted by 5-300x in assay buffer and analyzed in duplicate. A volume of 100 μL of assay buffer containing 0.1 g of a monoclonal IgG1 anti-type III capture antibody (#MAB3392, EMD Millipore [26]) was transferred to each well of a clear polystyrene microplate (#DY990, R&D Systems) and incubated for 15 hours at room temperature. The plate was then aspirated and washed 3 times in assay buffer before blocking with 200 μL of 1% bovine serum albumin (BSA) for 1 hour. After another three washing steps, samples and standards (100 μL) were added to the capture antibody-coated wells and incubated 2 hours. Sample and standard incubation was followed by an additional three washes and 100 μL of a biotinylated polyclonal detection antibody (#1330-08, Southern Biotech) at a stock concentration 0.4 mg/mL diluted by 1:800, 1:1600 or 1:3200 was added to each well. After an hour, the plate was washed another 3 times before transferring 100 μL of 1:5000 diluted streptavidin-HRP solution (#7100-05, Southern Biotech) to each well. Incubation of streptavidin-HRP was followed by the final three washing steps, and 100 μL of substrate solution (#DY999, R&D Systems) was transferred to each well for 20 minutes before a stop solution was added (#DY994, R&D Systems). Optical density was measured at 450-nm using a BioTek Synergy HT Multi-Mode Microplate Reader (BioTek), and the standard curve was fit using a 4-parameter logistic curve fit. Values outside the range of standards were ignored. To standardize the acquired data, for all ELISAs, results were normalized by data obtained from the biceps tendon. Thus, the measured content of each collagen type reflects a relative value compared to a tendon where complex, multiaxial loads that lead to structural and mechanical heterogeneities are not expected to occur. Note that the reported measurements of types I, II and III collagen represent the content of these molecules in the pepsin-soluble fraction of collagen in the SST.
Statistics
Type I collagen yield for the two pepsin digestion conditions (4° C and 30° C) were compared using a Student’s t-test. Comparisons of each collagen type across regions were performed using a Kruskal-Wallis test with Dunn’s post-hoc analysis to account for multiple comparisons. Spearman rank correlations of median content of types I, II and III collagen with median longitudinal linear modulus and circular variance (VAR), a structural parameter that decreases with increasing collagen alignment, were also assessed. Longitudinal linear modulus and VAR were defined and measured as described previously [9]. Significance was set at p < 0.05.
RESULTS
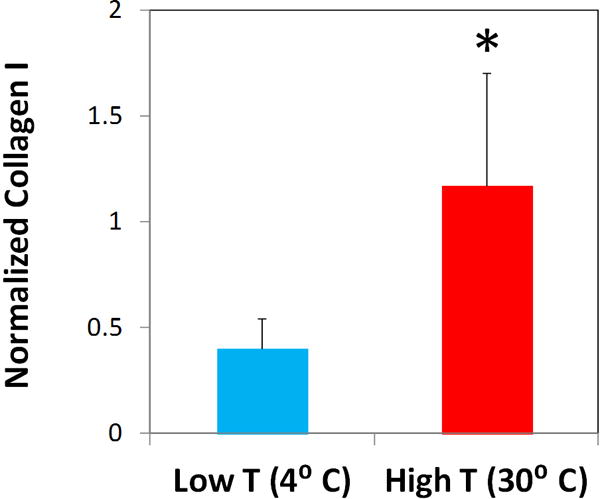
Although extraction at both 4° C and 30° C yielded samples with visible signs of successful digestion (i.e., a milky appearance), digestion at 30° C was found to yield nearly 3 times more type I collagen as measured by ELISA (Figure 2). Previous tests at a range of temperatures supported preservation of collagen alpha chain at the higher temperature but not at those raised above 34° C (data not shown). Therefore, all samples used in the study were extracted at 30° C under careful temperature control. A strikingly higher amount of collagen (~25%) was extracted using the two step protocol combining freeze-milling and higher temperature pepsin digestion.
Figure 2.
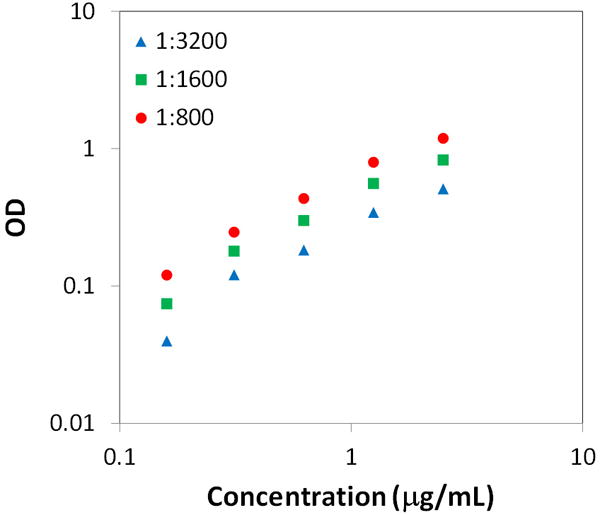
As part of the development of the type II collagen ELISA we verified the reliability of the assay for a wide range of collagen concentrations. The standard curve for the custom sandwich ELISA for type III collagen did not exhibit signs of saturation at any of the tested detection antibody concentrations (Figure 3). Thus, in order to avoid antibody waste while ensuring sufficient assay sensitivity, all tendon samples were analyzed using a 1:1600 dilution (0.25 μg/mL) of the antibody concentrate. Sensitivity of the assay was linear through the range of 0.1 and 10.0 μg/ml.
Figure 3.
Across regions of the human SST, type II collagen content was 18-104 times greater than in the biceps and type III collagen content was 49-113 times greater than in the biceps (Table 1). Both type I and type III collagen did not exhibit statistically significant spatial variations. However, type II collagen content was higher in the anterior-joint and posterior-joint regions than in the medial-bursal region.
Table 1. Normalized Collagen Content of the Human Suprasinatus Tendon (by Region).
Content of types I, II and III content in each region of the human supraspinatus tendon.
| AB | MB | PB | AJ | MJ | PJ | |
|---|---|---|---|---|---|---|
| Collagen I | 0.54 (0.32, 1.1) |
0.62 (0.31, 1.2) |
0.89 (0.46, 1.1) |
0.53 (0.41, 0.97) |
0.82 (0.41, 1.1) |
0.90 (0.58, 1.9) |
| Collagen II | 22 (14, 53) |
18 (13, 27) |
31 (17, 46) |
104* (25, 251) |
20 (14, 39) |
79* (41, 307) |
| Collagen III | 111 (11, 281) |
73 (21, 230) |
159 (53, 345) |
113 (22, 160) |
49 (32, 126) |
120 (60, 317) |
All values are normalized by data obtained from the biceps tendon. In all regions, type II collagen content was at least 18 times greater than in the biceps and type III collagen content was at least 49 times greater than in the biceps. Data are represented as median (Q1, Q3).
p<0.05/15 versus MB.
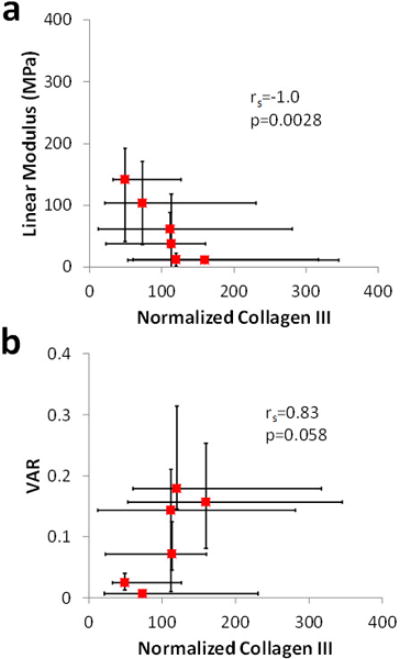
Content of type I and II collagen in each region did not correlate significantly with either linear modulus or circular variance (Figure 4). On the other hand, a significant inverse correlation of type III collagen content with linear modulus was found, while the correlation of type III collagen with circular variance was strong (Spearman rank correlation coefficient rs = 0.83) and approached significance (p = 0.058) (Figure 5).
Figure 4.
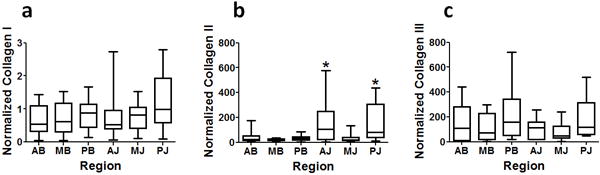
Figure 5.
DISCUSSION
Although prior investigations have determined the total content of types I and III collagen in the human supraspinatus [24] and regional variations in type I, II and III collagen at the humeral insertion of the rabbit shoulder [27], to our knowledge, this study presents the first quantitative measurements of regional variations in types I, II and III collagen across six separate regions of the human supraspinatus tendon. To perform these measurements, a specialized protocol that allowed for improved extraction of collagen from tendon was developed and validated. By incubating samples at a higher temperature (T = 30° C) that is safely below the melting point of intact collagen (Tm = 39° C) and the collagen subunits resulting from pepsin digestion (Tm = 32° C), we were able to successfully extract nearly 3 times more type I collagen than the established lower temperature protocols. While it is possible that this protocol allowed for degradation of a small proportion of collagen into fragments, it is clear from the high yield of immunogenic collagen after 30° C digestion that the added benefit of increased extraction at high temperatures outweighed this potential loss.
As hypothesized, type I collagen content was found to exhibit no regional variations. Like decorin (the most abundant proteoglycan in tendon), type I collagen (the most abundant protein in tendon) is ubiquitous in the SST. Furthermore, levels of type I collagen in the SST were similar to those in the biceps tendon as evidenced by normalized values close to 1 (Figure 4). These findings suggests that type I collagen levels in tendon are not highly sensitive to surrounding the mechanical environment.
On the other hand, content of type II collagen, the predominant form of collagen in tissues that experience high compressive loads, was found to increase in the anterior-joint and posterior-joint regions relative to the medial bursal regions. This finding was consistent with our hypothesis, and is likely due either to the compressive loading environment in the AJ and PJ regions or to their proximity to the articular cartilage coating the humeral head. Interestingly, in all regions of the SST, even the medial portions that experience mostly tensile loads, type II collagen content was many times greater than in the biceps tendon. Thus, all regions of the SST may experience some degree of compressive strain and contribute to this possible adaptation.
Surprisingly, type III collagen was not localized in the anterior portions of the SST where tears are known to initiate. In fact, type III collagen did not exhibit any statistically significant spatial variations. This could be due to the fact that the tested tendons all came from donors with no reported shoulder injuries or visible SST tears. Nevertheless, as hypothesized, type III collagen content did correlate strongly with VAR, a variable that decreases with increased alignment. Furthermore, type III collagen exhibited a significant inverse correlation with linear modulus. These results indicate that type III collagen in the human SST is associated with a low degree of alignment and inferior tensile mechanical properties, providing an important direct structure-function relationship for this tissue. An additional finding of this study was that collagen type III in all regions of the SST was at least 49 times higher than in the biceps tendon midsubstance. This result is consistent with a previous study [24] and suggests that active remodeling in the SST is many times greater than in the biceps tendon.
The primary limitation of this study is that extraction of collagen from the tested tendons, while more efficient than standard protocols (Figure 2), was incomplete. High-yield collagen extraction is an inherent obstacle for any immunochemical assay of collagen from a dense connective tissue. Results from preliminary tests suggest that ~25% of collagen from harvested tissues was present in the supernatant following pepsin digestion and centrifugation (not shown). However, these data were obtained using an assay of hyroxyproline content [28], which is sensitive to collagen fragments and may overestimate the amount of immunogenic extracted collagen. According to a previously published protocol, only 1% of the collagen in the human SSTs of health donors was soluble in pepsin [24]. To provide meaningful data despite the low collagen yield, results were normalized by measurements in the biceps tendon midsubstance. Since collagen yield is not expected to vary by location, this allowed for valid comparisons across regions.
The data obtained in this investigation describe the regional distribution of the three major forms of collagen in the uninjured human SST. These findings can serve as a guideline for understanding and possibly diagnosing biochemical changes occurring in the pathological state. For example, damaged or painful SSTs may exhibit increased type III collagen content on the anterior side where a tear is initiating. In addition, this study established an association of type III collagen content with low modulus and a low degree of alignment. Tendon alterations with aging, injury and pathology, all of which result in increased levels of type III collagen, may be attributable to this structure-function relationship.
CONCLUSIONS
In order to understand compositional variations in the healthy human supraspinatus tendon (SST) associated with known heterogeneities in loading environment, mechanical properties and collagen alignment, regional distributions of type I, II and III collagen in six distinct regions of the tissue were measured immunochemically. Content of types I and III collagen were similar in all tendon regions, while type II collagen was concentrated in regions near the insertion thought to experience large compressive forces in vivo. Furthermore, type III collagen content was inversely correlated with tensile modulus and collagen alignment, providing a direct structure-function relationship for the SST. These findings help establish the baseline compositional properties of the healthy SST and provide a guideline for understanding and assessing pathological changes. With this knowledge we can move towards identifying therapeutic targets and perhaps identify specific collagen epitopes which are related to SST pathology.
Acknowledgments
The authors thank Akash Kumar, Spencer Szczesny and Drs. Charles Clark, Spencer Lake, Jennifer Kadlowec and Daniel Cortes for technical contributions, helpful suggestions and engaging discussions.
This study was supported by NIH/NIAMS AR055598.
Footnotes
DECLARATION OF INTEREST
The authors report no conflict of interest and are alone responsible for the content and writing of this paper.
References
- 1.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Kwon YW, Zuckerman JD. The rotator interval: anatomy, pathology, and strategies for treatment. J Am Acad Orthop Surg. 2007;15(4):218–27. doi: 10.5435/00124635-200704000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578–84. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima T, Rokuuma N, Hamada K, Tomatsu T, Fukuda H. Histologic and biomechanical characteristics of the supraspinatus tendon: Reference to rotator cuff tearing. J Shoulder Elbow Surg. 1994;3(2):79–87. doi: 10.1016/S1058-2746(09)80114-6. [DOI] [PubMed] [Google Scholar]
- 5.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28(1):1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 6.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83-A(1):71–7. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Malcarney HL, Murrell GA. The rotator cuff: biological adaptations to its environment. Sports Med. 2003;33(13):993–1002. doi: 10.2165/00007256-200333130-00004. [DOI] [PubMed] [Google Scholar]
- 8.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994;53(6):367–76. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27(12):1596–602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. J Biomech. 2010;43(4):727–32. doi: 10.1016/j.jbiomech.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004. doi: 10.1115/1.4005852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matuszewski PE, Chen YL, Szczesny SE, Lake S, Elliott DM, Soslowsky LJ, et al. Regional Variation in Human Supraspinatus Tendon Proteoglycans: Decorin, Biglycan, and Aggrecan. Connect Tissue Res. 2012 doi: 10.3109/03008207.2012.654866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ChandraRajan J. Separation of type III collagen from type I collagen and pepsin by differential denaturation and renaturation. Biochem Biophys Res Commun. 1978;83(1):180–6. doi: 10.1016/0006-291x(78)90414-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, et al. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5(1):5–21. [PubMed] [Google Scholar]
- 15.Wardale RJ, Duance VC. Characterisation of articular and growth plate cartilage collagens in porcine osteochondrosis. J Cell Sci. 1994;107(Pt 1):47–59. doi: 10.1242/jcs.107.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124(4):687–93. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- 17.Mow VC, Huiskes R. Basic orthopaedic biomechanics & mechano-biology. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; p. 2005. p. p. [Google Scholar]
- 18.Adamczyk C, Milz S, Tischer T, Putz R, Benjamin M. An immunohistochemical study of the extracellular matrix of entheses associated with the human pisiform bone. J Anat. 2008;212(5):645–53. doi: 10.1111/j.1469-7580.2008.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72(4):352–61. [PubMed] [Google Scholar]
- 20.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 21.Hirose K, Kondo S, Choi HR, Mishima S, Iwata H, Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004;124(6):374–7. doi: 10.1007/s00402-004-0663-8. [DOI] [PubMed] [Google Scholar]
- 22.Smith RK, Birch H, Patterson-Kane J, Firth EC, Williams L, Cherdchutham W, et al. Should equine athletes commence training during skeletal development?: changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet J Suppl. 1999;30:201–9. doi: 10.1111/j.2042-3306.1999.tb05218.x. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves-Neto J, Witzel SS, Teodoro WR, Carvalho-Junior AE, Fernandes TD, Yoshinari HH. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine. 2002;69(2):189–94. doi: 10.1016/s1297-319x(02)00369-x. [DOI] [PubMed] [Google Scholar]
- 24.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53(6):359–66. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dourte LM, Pathmanathan L, Jawad AF, Iozzo RV, Mienaltowski MJ, Birk DE, et al. Influence of Decorin on the Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon. J Biomech Eng-T Asme. 2012;134(3) doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werkmeister JA, Ramshaw JA. Multiple antigenic determinants on type III collagen. Biochem J. 1991;274(Pt 3):895–8. doi: 10.1042/bj2740895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J Anat. 1994;185(Pt 2):279–84. [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards CA, O’Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104(2):161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]


