ABSTRACT
Objectives: This study aims to explore the effect of bone marrow mesenchymal stem cells (BMSCs) on multiple myeloma (MM) development and the underlying mechanism.
Materials and Methods: BMSCs from C57BL/6 J mice were isolated and the third passage was used for subsequent experiments. Additionally, a series of in vitro transwell coculture assays were performed to explore the effects of BMSCs on the proliferation of MM cells 5TGM1 and CD4+ T cells. Furthermore, a 5TGM1-induced MM mice model was established. Moreover, PD-L1 shRNA was transfected into BMSCs to investigate whether PD-1/PD-L1 pathway involved in BMSCs-mediated regulation of T cells and MM growth.
Results: Data revealed that BMSCs significantly promoted 5TGM1 proliferation in a dose-dependent manner. Furthermore, BMSCs administration exerted stimulatory effects on MM development in terms of shortening the mouse survival rate, promoting tumor growth, and enhancing inflammatory infiltration in the MM model mice. Moreover, BMSCs decreased the percentage of Th1 and Th17 cells, whereas increased that of Th2 and Treg cells. Their corresponding cytokines of these T cell subsets showed similar alteration in the presence of BMSCs. Additionally, BMSCs significantly suppressed CD4+ T cell proliferation. We also found that PD-L1 shRNA inhibited 5TGM1 proliferation likely through activation of CD4+ T cells. Further in vivo experiments confirmed that PD-L1 inhibition attenuated BMSCs-induced MM growth, inflammation infiltration and imbalance of Th1/Th2 and Th17/Treg.
Conclusion: In summary, our findings demonstrated that BMSCs promoted cell proliferation of MM through inhibiting T cell immune responses via PD-1/PD-L1 pathway.
KEYWORDS: Multiple myeloma, bone marrow mesenchymal stem cells, CD4+ T cells, PD-1/PD-L1, 5TGM1
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy and accounts for 13% of all hematological cancers and 1% of all human cancers [1]. One of the major characteristics of MM patients is the occurrence of osteolytic bone lesions [2]. MM patients also suffer from anemia, hypercalcemia, renal failure and repeated infection [3]. MM is closely related to the induction of bone disease and large lytic lesions, which are usually not repaired and are often the sites of relapses [4]. Unfortunately, to date, despite rapid progress in the treatment for MM, death rates are still very high, with no relevant improvement over the past 14 years [5,6]. Therefore, search for effective management of this condition is urgently needed.
Mesenchymal stem cells (MSCs) are derived from the mesoderm and have self-renewal and multi-differentiation potential. MSCs are considered as important seed cells for cell transplantation and tissue engineering, for they can differentiate into various tissue cells, such as osteoblasts, chondrocytes, adipocytes, neurocytes, and muscle cells [7–11]. So far, the study of MSCs has mainly focused on bone marrow mesenchymal stem cells (BMSCs).
The use of MSCs to treat MM bone disease has attracted considerable attention in the field of stem cell-based therapies [10–14]. Some in vitro studies showed that MSCs from MM patients have abnormal genomic, phenotypic, and functional properties [14–17]. This might protected MM cells from spontaneous and drug-induced apoptosis, thereby contributing to impaired bone formation in this disease [18]. Furthermore, recent evidence suggested that subcutaneous injection of MSCs promotes tumor growth and neovascularization in syngeneic mouse models by directly supporting the tumor vasculature and secreting proangiogenic factors [19]. Indeed, a variety of other tumor models have also observed the promotion of cancer growth through MSCs [20]. In contrast, there is evidence supporting the fact that MSCs inhibit tumor growth [20]. In particular, exogenously administrated MSCs can effectively promote bone formation, while suppress bone disease and the growth of highly aggressive MM cells in the bone [4]. Additionally, intrabone-injected MSCs have been shown to act as bystander cells to promote bone formation, suppress osteolysis, and delay MM growth and regrowth [3,4]. In conclusion, the effect of MSCs infusion on cancer growth remains not currently clear. These contradictory results require new insights to explain them.
MSCs have been extensively reported to have immunosuppressive qualities [21,22]. CD4+ T cells will differentiate into different populations, including T helper (Th) 1, Th2, Th9, Th17, T follicular helper (Tfh), regulatory T cells (Tregs) and etc., to mediate different immune responses [23]. Among these subsets, Th1, Th2, Th17, and Tregs are mostly studied subsets of CD4+ T cells [24]. MSCs can alter the status of CD4+ T cells, inhibiting their proliferation and skewing them toward a regulatory phenotype Treg [25,26]. It has been demonstrated that MSCs inhibited proliferation and effector function of T cells via contact-dependent interactions of the programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) [27,28]. Previous studies have reported up-regulation of cell surface PD-L1 on MSCs and subsequent suppression of T cell proliferation [29–31]. The PD-1/PD-L1 pathway is critical to immune homeostasis. The physiological role of PD-1 is to maintain T cell homeostasis by restricting T cell activation and proliferation, thereby preventing autoimmunity [32]. Soluble PD-L1 secreted by MSCs was significantly up-regulated in response to pro-inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), thereby inhibiting the activation status of T cells [33].
MSCs can inhibit the T cell proliferation and exert immune-modulatory effects. So we wondered whether BMSCs inhibited the antitumor immunity of T cells through affecting T cell function, and whether BMSCs regulated the biological behavior of MM via PD-1/PD-L1 pathway. As indicated above, we hypothesized that BMSCs might promote MM cell proliferation through inhibiting T cell immune responses via PD-1/PD-L1 pathway. In this study, we focused on the effects of BMSCs pretreatment on tumor growth of MM and antitumor immunity of T cells, as well as the role of PD-1/PD-L1 in BMSCs-mediated regulation of T cells and MM growth.
Materials and methods
C57BL/6 J mice
Sixty female C57BL/6 J mice (20-25 g) were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Animals used in this study were maintained and used in accordance with guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. This study was approved by the Research Ethic Committee of The first affiliated hospital of Zhengzhou University.
Establishment of 5TGM1 MM model
MM was induced through the intravenous inoculation of 5 × 106 5TGM1 cells in 200 μL PBS in C57BL/6 J mice through the tail vein (5 × 106 BMSCs/10 g body weight). One week after tumor cell inoculation, MM model mice were randomized to receive the injection of either saline (as MM group), BMSCs (once, 1 × 106 BMSCs/10 g body weight), BMSCs transfected with control shRNA, or BMSCs transfected with PD-L1 shRNA via the tail vein. Normal C57BL/6 J mice served as normal group. The 6-week survival rates of the mice in different groups were compared. After 6 weeks of feeding, the cancroid pearls in abdominal cavity of the mice in different groups were analyzed.
Isolation and culture of BMSCs
To isolate BMSCs, bone marrow mononuclear cells were separated from C57BL/6 J mice by density gradient separation technique using Ficoll-Hypaque (GE Lifesciences, USA), washed and re-suspended in alpha minimum essential medium (Invitrogen, USA) supplemented with 20% fetal bovine serum (FBS, Invitrogen) and 2 mM L-glutamine (Invitrogen) in humidified air with 5% CO2 at 37°C for 3 days before coculture [34]. When the attached cells reached 80% confluence, cells were detached with Trypsin-EDTA, and cultured until five passages. The morphological characteristics of BMSCs were examined using an inverted microscope (Nikon E100; Nikon Corp, Japan). The growth curve of the cells cultured for 1–8 days was evaluated by MTT assay. Flow cytometry and differentiation assays were carried out to verify BMSCs according to established criteria [35]. Briefly, a single-cell suspension was stained with fluorescence-labeled monoclonal antibodies (mAbs) specific for phycoerythrin (PE)-conjugated CD29, PE-conjugated CD90, PE-conjugated CD45, PE-conjugated CD34 or the isotype control. All of the antibodies were from eBioscience (eBioscience, USA). After incubation for 40 min at 4°C in the dark, cells were subsequently washed twice in PBS containing 1% FBS, and resuspended for FACS analysis using flow cytometry (FACS Canto II; BD Biosciences, USA). BMSCs were positive for CD29 and CD90, but negative for CD45 and CD34. All the BMSCs used in subsequent experiments were the third generation.
Cell culture
The murine MM cell lines 5TGM1 were cultured in Isocove's modified Dulbecco's medium (IMDM; Gibco, USA) supplemented with 10% FBS, L-glutamine, and penicillin-streptomycin in humidified air with 5% CO2 at 37°C. Media was changed every two to three days.
Transwell coculture conditions
In brief, the upper chamber (0.5 mL culture medium) of Transwell plates (pore size: 8.0 μm; Corning, USA) contained BMSCs with the indicated number or/and 1 × 103 CD4+ T cells, and the bottom chamber (1.5 mL medium) contained 1 × 103 5TGM1 cells.
Cell proliferation assay
Cell proliferation at different time points was measured by MTT assay. In brief, cells were harvested for 24 h and then plated into 96-well plates at a density of 5 × 103 cells/well. After 24 h of incubation, 20 μL MTT (5 mg/mL; Sigma-Aldrich, USA) was added into each well for 4 h of incubation at 37°C. Then the medium was replaced with 150 μL dimethyl sulfoxide (DMSO) for 10 min to solubilize the crystals. Cellular viability was determined by measuring the optical density (OD) at 490 nm with averages from triplicate wells by a spectrophotometer (Multiskan MK3, Thermo, USA). Cellular viability was normalized to control well.
Isolation of mouse CD4+ T cells
CD4+ T cells were isolated from spleens of C57BL/6 mice using Dynal CD4 positive isolation kit (Invitrogen) according to the manufacturer's protocol. The isolated T cells were > 98% CD4+ and were suspended in DMEM culture medium at the concentration of 5 × 106 cells/mL.
Detection of the proportion of Th1, Th2, Th17, and Treg
T-box expressed in T cells (T-bet), Gata binding protein 3 (Gata-3), receptor-related orphan receptor γt (RORγt), and transcription factor forkhead box p3 (Foxp3) are transcription factors of Th1, Th2, Th17, and Treg, respectively [36–38]. For analysis of the proportion of Th1 cells (T-bet+ CD4+ T cells), Th2 cells (Gata-3+ CD4+ T cells), Th17 cells (RORγt+ CD4+ T cells), and Treg cells (Foxp3+ CD4+ T cells) in CD4+ T cells, CD4+ T cells (5 × 105) were intracellularly stained with fluorescence-labeled mAbs specific for fluorescein isothiocyanate (FITC)-T-bet, allophycocyanin (APC)-conjugated Gata-3, FITC-conjugated Foxp3, and APC-conjugated RORγt or the isotype controls. All of the antibodies were from eBioscience. After incubation for 40 min at 4°C in the dark, cells were subsequently washed twice in PBS containing 1% FBS, and resuspended for FACS analysis. Flow cytometry was performed on the FACS Canto II flow cytometer using BD FACSDiva software. Data analysis was performed using FlowJo software.
Real time quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from either BMSCs or CD4+ T cells by using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA was then reverse transcribed into cDNAs using the iScript cDNA synthesis kit (Bio-rad, Germany). The cDNA templates were amplified through qRT-PCR using SYBR Premix Dimmer Eraser kit (TaKaRa, Japan). The relative expression levels of PD-1 and PD-L1 were calculated by the 2−ΔΔCt method and normalized to the internal control GAPDH. The primer sequences were listed as follows: PD-1: 5′-ACCCTGGTCATTCACTTGGG-3′ (forward) and 5′-CATTTGCTCCCTCTGACACTG-3′ (reverse). PD-L1: 5′-GCTCCAAAGGACTTGTACGTG-3′ (forward) and 5′-TGATCTGAAGGGCAGCATTTC-3′ (reverse). GAPDH: 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward) and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse).
Western blot
The proteins were isolated from BMSCs or CD4+ T cells, or tumor tissues from abdominal cavity of mice in lysis buffer. Subsequently, equal proteins were separated by 10% SDS-PAGE gels and transferred onto PVDF membranes (Millipore, USA). After blocking with 5% fat-free milk, primary antibodies against PD-1 and PD-L1 (LifeSpan BioSciences, USA) were added, followed by secondary antibody horseradish peroxidase-conjugated goat anti-rabbit IgG. β-actin was used as the loading control. The protein was detected with an enhanced chemiluminescence kit (Applygen Technologies, China) and the band intensity was quantified with Image-Pro Plus 6.0 software.
Enzyme-linked immunosorbent assay (ELISA)
The levels of various cytokines IFN-γ, interleukin-4 (IL-4), IL-17, and transforming growth factor-β (TGF-β) were measured with an ELISA kit (R&D Systems, USA) according to the manufacturer's instructions.
Cell transfection
The PD-L1 shRNA plasmid pGPU6/PD-L1 has been constructed. PD-L1 shRNA sequence: 5′-CCGGAACCACCCTGTTGTGATAACCCTCGAGGGTTATCACAACAGGGTGGTTTTTTTG-3′ (forward) and 5′-AATTCAAAAAAACCACCCTGTTGTGATAACCCTCGAGGGTTATCACAACAGGGTGGTT-3′ (reverse). For cell transfection, PD-L1 shRNA plasmid pGPU6/PD-L1 was transfected into BMSCs using Lipofectamine 2000™ according to the manufacturer's protocol (Invitrogen). Briefly, the third-passage BMSCs were cultured in at 106 cells per well in 6-well plates (Corning). When the confluence reached 70–80%, BMSCs were transfected with 4 μg plasmid and 10 μL Lipofectamine 2000™ in 0.25 mL Opti-MEM® I Reduced Serum Medium (Invitrogen). 40 h later, the total protein was extracted and the silencing efficiency was determined by Western blot.
Tissue preparation and hematoxylin and eosin (HE) staining
The mice were sacrificed after 6 weeks of feeding. The tumor tissues from abdominal cavity of mice were stained with HE for histopathological analysis. In brief, fresh tissues were fixed in 4% paraformaldehyde solutions for 30 min. Next, tissues were dehydrated and then embedded in paraffin before being cut into 6-μm thick sections. The sections were stained with HE according to the routine staining procedure and analyzed using a microscope (Nikon E100; Nikon Corp).
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software (IBM). The unpaired Student's t test was used to analyze differences between two groups. One-way ANOVA followed by Tukey's test was used to analyze differences among multiple groups. The data are presented as the mean ± standard deviation (SD) from three independent experiments. Values of P less than 0.05 were considered statistically significant.
Results
Culture and identification of BMSCs from C57BL/6 mice
BMSCs from C57BL/6 J mice were isolated and cultured for morphology observations. Cultured BMSCs were observed to be round or oval following inoculation. Then the cell adherence increased, and the cells became multi-fusiform and began to merge into a sheet. Subsequent to culture for 72 h (the third generation), BMSCs were of uniform shape and displayed long-spindle morphology (Figure 1A). After osteogenic induction, alizarin red staining displayed the deposition of densely stained calcium salts in BMSCs culture (red), which was an evidence of the osteogenesis capacity of BMSCs (Figure 1B). Furthermore, oil red O staining revealed that the oil drops were formed after adipogenic induction, suggesting that BMSCs were induced to differentiate into adipocytes (Figure 1C). Moreover, the growth curve of BMSCs demonstrated that BMSCs were expanded well (Figure 1D). In addition, BMSCs were positive for CD29 and CD90, but negative for CD45 and CD34 (Figure 1E–H). Collectively, these results indicated that the obtained BMSCs from C57BL/6 J mice were in accordance with the well-established criteria.
Figure 1.
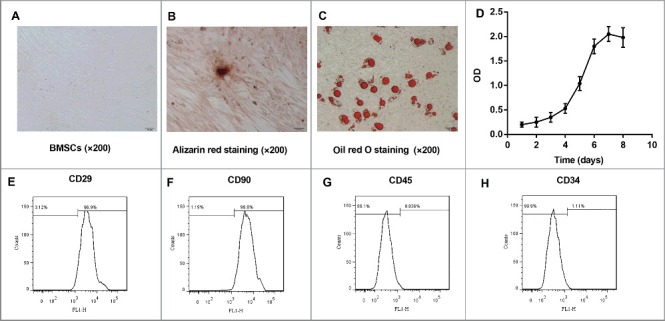
Culture and identification of BMSCs from C57BL/6 J mice. BMSCs were isolated from the bone marrow of mice (n = 10) and expanded in vitro. (A) The morphology of BMSCs subsequent to being passaged for 72 h (P3, ×200; Scale bar: 50 μm). (B) Osteogenic induction of BMSCs at 21 d by alizarin red staining (positive: red, ×200; Scale bar: 50 μm). (C) Adipogenic induction of BMSCs at 21 d by oil red O staining (positive: red, ×200; Scale bar: 50 μm). (D) The cell growth curve from Day 1 to Day 8 was drawn. Cellular viability was determined by measuring the optical density (OD) at 490 nm with averages from triplicate wells. The cell surface markers (E) CD29, (F) CD90, (G) CD45, and (H) CD34 of BMSCs were detected by flow cytometry. Each experiment was performed at least three times. All the BMSCs used in subsequent experiments were the third generation.
BMSCs promote 5TGM1 cell proliferation
To investigate the effects of BMSCs on the proliferation of murine MM tumor cell lines, we explored the viability of 5TGM1 cells when cultured alone or together with BMSCs. We used transwells to separate 5TGM1 cells (bottom) from BMSCs (upper). After 24 h of coculture, a significant increase in cell viability of murine MM cell lines 5TGM1 in the presence of BMSCs was observed compared to cell-line-only control. Furthermore, 1 × 106 BMSCs induced the greatest degree of proliferation of 5TGM1 cells (Figure 2). These results indicated that BMSCs promoted 5TGM1 proliferation in a dose-dependent manner.
Figure 2.
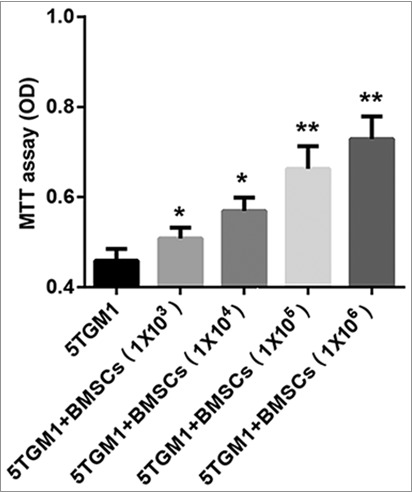
BMSCs increased proliferation of 5TGM1 cells. 5TGM1 cells (1×103, bottom) were cultured alone or transwell cocultured with different number of BMSCs (1×103, 1×104, 1×105, 1×106, upper). After 24 h of transwell coculture, (A) MTT assay was performed at least three times to evaluate the effects of BMSCs on the proliferation of 5TGM1 cells. *P<0.05, **P<0.01 vs. 5TGM1.
PD-L1 expression in BMSCs and PD-1 expression in CD4+ T cells
CD4+ T cells derived from spleens of C57BL/6 mice were induced with lipopolysaccharide (LPS), which could stimulate inflammation and induce innate immune activation. Then these LPS-stimulated CD4+ T cells (bottom) were transwell cocultured with BMSCs (upper) for 24 h. Data revealed that both mRNA and protein expression of PD-L1 was significantly increased in BMSCs transwell cocultured with LPS-stimulated CD4+ T cells compared with that in BMSCs alone (Figure 3A–B). However, CD4+ T cells in transwell coculture with BMSCs exhibited significantly suppressed PD-1 expression in comparison with LPS-stimulated CD4+ T cells alone (Figure 3C–D).
Figure 3.
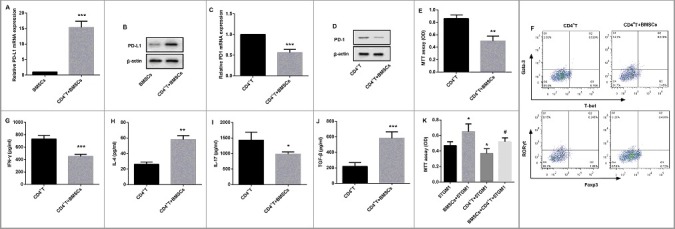
Effects of BMSCs on CD4+ T cells and 5TGM1 proliferation. CD4+ T cells derived from spleens of C57BL/6 mice (n = 10) were isolated, and induced with LPS. Then these CD4+ T cells (1×103, bottom) were transwell cocultured with BMSCs (1×105, upper) for 24 h. (A) qRT-PCR and (B) Western blot were performed to evaluate relative mRNA and protein expression of PD-L1 in BMSCs respectively. ***P<0.001 vs. BMSCs. (C) qRT-PCR and (D) Western blot were performed to evaluate relative mRNA and protein expression of PD-1 in CD4+ T cells respectively. ***P<0.001 vs. CD4+ T cells. β-actin served as the loading control. (E) The proliferation of CD4+ T cells transwell cocultured with BMSCs was evaluated by MTT assay. (F) Flow cytometry was performed to examine the proportion of Th1 cells (T-bet+ CD4+ T cells, Q3), Th2 cells (Gata-3+ CD4+ T cells, Q1), Th17 cells (RORγt+ CD4+ T cells, Q1), and Treg cells (Foxp3+ CD4+ T cells, Q3) in CD4+ T cells with their special fluorescence-labeled mAbs. ELISA was used to detect the cytokine levels of (G) Th1-related IFN-γ, (H) Th2-related IL-4, (I) Th17-related IL-17, and (J) Treg-related TGF-β in the culture medium of CD4+ T cells. *P<0.05, **P<0.01,***P<0.001 vs. CD4+ T. (K) The proliferation of 5TGM1 cells (1×103, bottom) transwell cocultured with either BMSCs (1×105, upper) or CD4+ T cells (1×103, upper), or the combination of 1×105 BMSCs and 1×103 CD4+ T cells (upper) was evaluated by MTT assay. Each experiment was performed at least three times. *P<0.05 vs. 5TGM1. #P<0.05 vs. CD4+ T + 5TGM1.
BMSCs suppress CD4+ T cell proliferation and decrease ratios of Th1/Th2 and Th17/Treg
MTT assay revealed that BMSCs significantly inhibited proliferation of LPS-stimulated CD4+ T cells to a comparable level (Figure 3E). Furthermore, as the imbalance of T cell subsets played important roles in various diseases including MM [24], we examined the effects of BMSCs on the proportion of mostly studied T cell subsets Th1, Th2, Th17, and Treg as well as levels of their corresponding cytokines. As far as Th2 cells are concerned, transwell coculture with BMSCs markedly increased Gata-3 from 2.92% to 14.2% after 24 h culture (Q1) (Figure 3F). Consistent with the stimulation of Th2-specific transcription factor, BMSCs also significantly elevated the levels of Th2-secreted cytokine IL-4 in the culture medium of LPS-induced CD4+ T cells (Figure 3H). Moreover, transwell coculture with BMSCs decreased Th1-specific T-bet (Q3) and Th17-specific RORγt (Q1), and increased Treg-specific Foxp3 (Q3) in LPS-induced CD4+ T cells (Figure 3F). As expected, similar alteration was also observed in their corresponding cytokine levels of Th1-secreted IFN-γ (Figure 3G), Th17-secreted IL-17 (Figure 3I), and Treg-secreted TGF-β (Figure 3J) in the culture medium of LPS-induced CD4+ T cells. These data indicated that BMSCs decreased the ratios of Th1/Th2 and Th17/Treg.
Effects of BMSCs and CD4+ T cells on 5TGM1 cell proliferation
As shown in Figure 3K, 5TGM1 cells (bottom) were transwell cocultured with either CD4+ T cells or BMSCs, or a combination of BMSCs and CD4+ T cells (upper) for 24 h. As expected, an enhanced proliferation of 5TGM1 cells was observed in coculture of BMSCs. In contrast, CD4+ T cells resulted in proliferation inhibition of 5TGM1 cells. However, BMSCs significantly reversed CD4+ T-mediated suppression of 5TGM1 cell proliferation.
PD-L1 shRNA restores BMSCs-mediated inhibition of CD4+ T cells
In order to investigate the role of PD-L1 in BMSCs-mediated inhibition of CD4+ T cells, we knocked down PD-L1 by PD-L1 shRNA in BMSCs to evaluate its effect on CD4+ T cells proliferation. Western blot revealed that PD-L1 protein expression in PD-L1 shRNA-transfected BMSCs was significantly decreased compared with the control BMSCs (Figure 4A). Furthermore, BMSCs significantly decreased both mRNA and protein expression of PD-1 in CD4+ T cells; however, PD-L1 shRNA attenuated the BMSCs-mediated suppression of PD-1 expression in CD4+ T cells (Figure 4B–C). Moreover, MTT assay indicated that PD-L1 shRNA significantly restored BMSCs-mediated inhibition of CD4+ T cells proliferation (Figure 4D). In addition, BMSCs significantly suppressed levels of IFN-γ and IL-17, but promoted levels of IL-4 and TGF-β. Interestingly, although the level of IL-17 showed no significant difference, PD-L1 shRNA effectively reversed BMSCs-mediated inhibition of IFN-γ, and stimulation of IL-4 and TGF-β in CD4+ T cells (Figure 4E–H). Likewise, flow cytometry revealed that PD-L1 shRNA inhibited the BMSCs-mediated reduction of Th1/Th2 and Th17/Treg (Figure 4I). These results indicated that BMSCs affected CD4+ T cells, likely through PD-L1 involvement.
Figure 4.
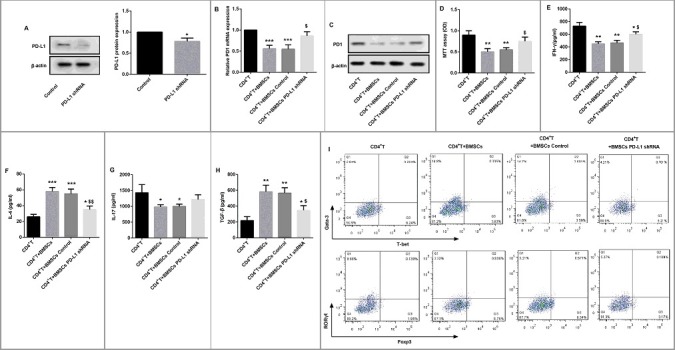
PD-L1 shRNA restored BMSCs-mediated effects on CD4+ T cells. (A) PD-L1 shRNA was transfected into BMSCs to inhibit PD-L1 expression. 40 h later, Western blot was performed to evaluate the knockdown efficiency. β-actin served as the loading control. *P<0.05 vs. Control. CD4+ T cells (1×103, bottom) were cultured alone or transwell cocultured with BMSCs, BMSCs shRNA Control, or BMSCs PD-L1 shRNA (1×105 for each group, upper) for 24 h. (B) qRT-PCR and (C) Western blot were carried out to detect PD-1 mRNA and protein expression in CD4+ T cells respectively. (D) MTT assay was performed to evaluate CD4+ T cell proliferation. ELISA was used to detect the cytokine levels of (E) Th1-related IFN-γ, (F) Th2-related IL-4, (G) Th17-related IL-17 and (H) Treg-related TGF-β in the culture medium of CD4+ T cells. (I) Flow cytometry was performed to examine the proportion of Th1 cells (T-bet+ CD4+ T cells, Q3), Th2 cells (Gata-3+ CD4+ T cells, Q1), Th17 cells (RORγt+ CD4+ T cells, Q1), and Treg cells (Foxp3+ CD4+ T cells, Q3) in CD4+ T cells with their special fluorescence-labeled mAbs. Each experiment was performed at least three times. *P<0.05, **P<0.01,***P<0.001 vs. CD4+ T. $P<0.05, $$P<0.01 vs. CD4+ T + BMSCs control.
PD-L1 shRNA inhibits 5TGM1 proliferation likely through activation of CD4+ T cells
We then explored whether PD-L1 involved in the mechanism by which BMSCs promoted proliferation of 5TGM1 cells. As shown in Figure 5, BMSCs promoted 5TGM1 cell proliferation was significantly suppressed by CD4+ T cells. Furthermore, no matter CD4+ T cells existed or not, BMSCs significantly promoted cell proliferation of 5TGM1 cells. Moreover, PD-L1 shRNA significantly attenuated the BMSCs-induced 5TGM1 proliferation in the presence of CD4+ T cells; however, the BMSCs-induced 5TGM1 proliferation in the absence of CD4+ T cells was not affected by PD-L1 shRNA. These results demonstrated that PD-L1 shRNA inhibited the BMSCs-mediated proliferation promotion of 5TGM1, likely through activation of CD4+ T cells.
Figure 5.
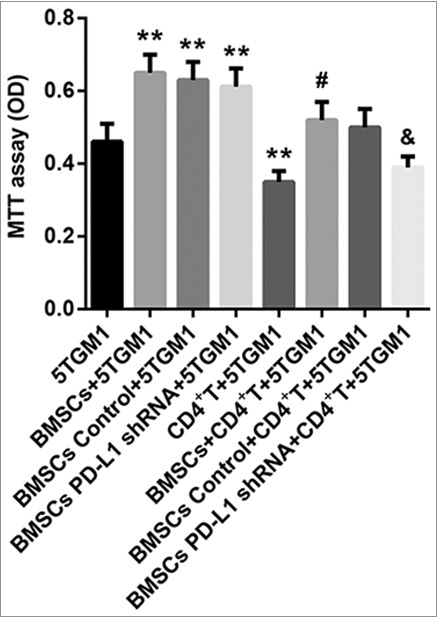
PD-L1 shRNA inhibits 5TGM1 proliferation likely through activation of CD4+ T cells. The proliferation of 5TGM1 cells (1×103, bottom) transwell cocultured with either BMSCs (1×105, upper, transfected or not) or CD4+ T cells (1×103, upper), or the combination of 1×105 BMSCs (transfected or not) and 1×103 CD4+ T cells (upper) was evaluated by MTT assay. Each experiment was performed at least three times. **P<0.01 vs. 5TGM1. #P<0.05 vs. CD4+ T + 5TGM1. &P<0.05 vs. BMSCs Control + CD4+ T+5TGM1.
Effects of BMSCs and PD-L1 shRNA in MM model mice
Finally, we examined the effects of BMSCs and PD-L1 shRNA in MM model mice. As shown in Figure 6A, the 6-week survival rates of mice revealed that no animal in the normal group died but all mice in the other groups died after 6 weeks. Furthermore, MM mice showed dramatically decreased survival rate during the 6-week observation period compared with normal mice. Moreover, BMSCs administration further decreased survival in comparison with the MM group. However, there was no significant difference in the survival rate between mice administered with BMSCs transfected with PD-L1 shRNA and scramble shRNA control.
Figure 6.
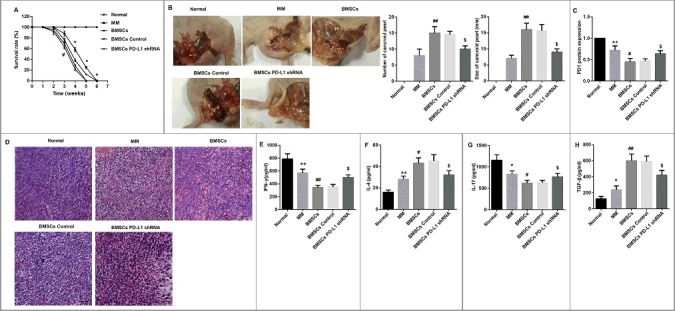
Effects of BMSCs and PD-L1 shRNA in MM model mice. (A) 6-week survival rates of mice in each group was drawn (for each group, n = 12). (B) After 6 weeks of feeding, the cancroid pearls in the abdominal cavity of mice were separated. Representative photographs of typical cancroid pearls in the abdominal cavity of mice were shown. The number and size of cancroid pearls in the abdominal cavity were shown as column graphs. (C) The tumor tissues from abdominal cavity of mice in each group were isolated, partly for protein extraction for PD-1 evaluation by Western blot (for each group, n = 3), (D) partly for HE staining for observation of pathological changes and inflammation infiltration (for each group, n = 4; Scale bar: 20 μm), and partly for tissue homogenate to detect (E) IFN-γ, (F) IL-4, (G) IL-17, and (H) TGF-β by ELISA (for each group, n = 5). *P<0.05, **P<0.01 vs. Normal. #P<0.05, ##P<0.01 vs. MM. &P<0.05 vs. BMSCs Control.
Then the number and size of cancroid pearls in the abdominal cavity were compared among the indicated groups after 6 weeks of feeding. No cancroid pearls were observed in the normal mice, but a certain number of cancroid pearls were identified in MM mice. However, BMSCs administration significantly increased the number and size of the pearls compared with MM model mice. Additionally, administration of BMSCs transfected with PD-L1 shRNA decreased both the number and size of the cancroid pearls compared with administration of scramble shRNA control-transfected BMSCs (Figure 6B).
We then investigated PD-1 protein expression in tumor tissues from abdominal cavity of mice in each group. MM mice showed a significantly decreased PD-1 protein expression compared with normal mice. Moreover, BMSCs administration significantly further reduced PD-1 protein expression. However, the BMSCs-mediated inhibition of PD-1 in MM mice was significantly restored by PD-L1 shRNA (Figure 6C). Furthermore, MM mice showed severe inflammation infiltration in tumor tissues from abdominal cavity as evidenced by HE staining. As expected, MM mice injected by BMSCs displayed enhanced infiltration and a majority of apoptotic cells compared with MM mice without BMSCs injection. Importantly, PD-L1 shRNA ameliorated BMSCs-induced inflammation infiltration in MM mice (Figure 6D). In addition, BMSCs significantly decreased levels of IFN-γ and IL-17, but increased IL-4 and TGF-β in tumor tissues from abdominal cavity of MM mice. However, PD-L1 shRNA significantly reversed the effect of BMSCs on these cytokines (Figure 6E–H). Collectively, these data suggested that PD-L1 shRNA attenuated BMSCs-induced MM growth, inflammation infiltration and imbalance of Th1/Th2 and Th17/Treg in MM model mice.
Discussion
BMSCs have recently assessed as potential therapeutic agents in various clinical applications because of their immune-regulatory properties and trans-differentiation capacity [39]. Some studies suggest that BMSCs inhibited tumor development, However, they are overwhelmed by increasing studies supporting the stimulatory effects exerted by BMSCs on tumor pathogenesis [40]. To date, the use of BMSCs to treat MM has attracted much attention. In this study, we isolated BMSCs from C57BL/6 J mice by density gradient separation. The BMSCs we got have general biological characteristics of mesenchymal stem cells, and also have potentiality of multi-directional differentiation. We found that BMSCs increased proliferation of MM cells 5TGM1 in a dose-dependent manner. Furthermore, in the 5TGM1 MM model mice, BMSCs treatment exerted stimulatory effects on MM development in terms of shortening the mouse survival rate, promoting tumor growth, and enhancing inflammatory infiltration.
Nevertheless, the mechanism by which BMSCs contributed to MM progression requires further investigation. BMSCs seem to promote tumor pathogenesis by supporting tumor microenvironments, increasing tumor growth, and triggering antitumor immune responses [40]. Therefore, we focused on T cells which possess antitumor immunity function. Due to the imperative of human leukocyte antigen (HLA) restriction, T cells are able to kill tumor cells only after their T cell receptor recognizes the peptide-HLA complex on the surface of tumor cells [41]. Negative regulation within tumor microenvironment suppresses antitumor T cell function, resulting in evasion from immune attack in many autoimmune diseases [42]. Zhang et al. [23]. found that depleting CD4+ T cells, but not CD8+ T cells or B cells, significantly enhanced tumor growth and shortened survival of 5TGM1-bearing C57BL/KaLwRij mice. Furthermore, the proportion of CD4+ T cells was decreased in MM group compared with control group [43]. T cells have been reported to exert cytotoxic effects on MM cell lines [44]. Consistent with this, here, we found that CD4+ T cells resulted in proliferation inhibition of 5TGM1 cells.
In addition, the abnormal immune imbalance of T lymphocyte subsets was thought to contribute to the pathogenesis of MM [24]. Th1, Th2, Th17, and Treg cells are mostly studied subsets of CD4+ T cells. Th1 cells produce IFN- γ and regulate the cell-mediated immune response, whereas Th2 cells secrete mainly IL-4 and inhibit Th1 cell-mediated response. Th17 cells produce IL-17 and involved in promoting inflammation in the pathogenesis of many diseases [45–47]. Treg cell suppressed effector T cell proliferation through secreting TGF-β and IL-10 to play its immune-modulatory effects [48]. In this study, we found that BMSCs decreased the percentage of Th1 and Th17 cells, whereas increased that of Th2 and Treg cells. That is to say, BMSCs decreased ratios of Th1/Th2 and Th17/Treg. Moreover, their corresponding cytokines showed similar alteration in the presence of BMSCs. Given that CD4+ T cells resulted in proliferation inhibition of 5TGM1 cells, we speculated that BMSCs promoted proliferation of 5TGM1 cells, likely through inhibition of CD4+ T cells.
Targeting T cell regulatory mechanisms is required to enhance the response to cancer immunotherapy. T cell responses can be suppressed by both intrinsic and extrinsic regulatory mechanisms [49]. One intrinsic suppressive mechanism on antitumor T cell responses is up-regulation of PD-L1 expressed on tumor or stromal cells which binds to PD-1 [42]. The physiological role of PD-1 is to guarantee T cell homeostasis by limiting T cell activation and proliferation. Accordingly, binding of the ligand PD-L1 to PD-1 expressed on the surface of activated T cells delivers an inhibitory signal, reducing cytokine production and proliferation of the T cells. The inhibition of antitumor T cell responses through the PD-1/PD-L1 pathway has been shown in a number of animal models to confer tumor escape from immune control and thus leads to tumor progression [50–53]. Recently, clinical trials have suggested a significant therapeutic impact of PD-1/PD-L inhibition on a variety of solid tumors-for example, by the application of monoclonal antibodies [54]. It has been reported that MSCs inhibited T cell proliferation and effector function via contact-dependent interactions of PD-1/PD-L1 in autoimmune disorders [27,28]. However, little is known regarding involvement of the PD-1/PD-L1 pathway in immune escape by MM. The PD-1 receptor is expressed on the surface of exhausted T and B cells and its ligand PD-L1 is expressed on myeloma cells. Here, we found that PD-L1 was significantly increased in BMSCs cocultured with LPS-stimulated CD4+ T cells compared with that in BMSCs alone. However, CD4+ T cells in transwell coculture with BMSCs exhibited significantly suppressed PD-1 expression in comparison with LPS-stimulated CD4+ T cells alone. Furthermore, MM mice showed a significantly decreased PD-1 protein expression in tumor tissues from abdominal cavity of mice compared with normal mice. The findings are very interesting as discordant results have been reported about PD-1 expression on T cells in MM patients. The majority of studies imply that counts of PD-1+ cells in MM microenvironment or peripheral blood increase and may be associated with worse prognosis [44,55]. Görgün et al. [56] reported an increased expression of PD-1 on CD4+ T cells from newly diagnosed MM and relapsed refractory MM samples compared with healthy donors. Rosenblatt et al. [57] demonstrated that PD-1 expression is upregulated in both CD4+ and CD8+ T cells in patients with advanced MM in comparison with healthy volunteers. Furthermore, Paiva et al. [58] confirmed a significant increase in PD-1 expression on both CD4+ and CD8+ T cells in samples from MRD positive MM and relapsing MM patients. Our discordant results may be due to the different experimental conditions.
We then wondered whether PD-1/PD-L1 pathway involves in BMSCs-mediated inhibition of T cell proliferation. Our results demonstrated that PD-L1 shRNA suppressed BMSCs-mediated effects on CD4+ T cells. This indicated that in MM, PD-L1 involved in BMSCs-mediated inhibition of CD4+ T cells. In addition, we explored whether PD-1/PD-L1 pathway involved in BMSCs-mediated promotion of MM tumor growth. Our research demonstrated that PD-L1 shRNA inhibited BMSCs-mediated proliferation promotion of 5TGM1 cells. The mechanism was likely related to activation of CD4+ T cells. So we can conclude that PD-L1 involves in BMSCs-mediated the inhibition of CD4+ T cells, leading to MM progression. Further in vivo experiments confirmed that PD-L1 inhibition attenuated BMSCs-induced MM growth, inflammation infiltration and imbalance of Th1/Th2 and Th17/Treg.
To date, immunotherapies using anti-PD-1/PD-L1 strategies have been a promising treatment options for MM patients [44]. However, as far as we are concerned, PD-1/PD-L1 pathway may be an important, but in no way the only mechanism underlying the immunosuppression mediated by MSCs. For example, Atsuta et al. [10] demonstrated that Fas/Fas-L pathway plays a crucial role in the MSCs-based inhibition of tumor growth in 5TGM1 MM model mice. Furthermore, despite the progress of immunotherapies using anti-PD-1/PD-L1 strategies in treatment for MM patients, the results of monotherapy with PD-1/PD-L1 inhibitors have been unsatisfactory in MM [41]. Collectively, these observations suggested that a combination approach of anti-PD-1/PD-L1 or investigation of other molecular mechanism is needed.
Conclusion
In summary, our findings demonstrated that BMSCs promoted cell proliferation of MM through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Our study might provide an important basis for further treatment of MM.
Funding Statement
This work was supported by the Hospital Foundation of the First Affiliated Hospital of Zhengzhou University; and National Youth Natural Science Foundation of China [grant number 81400108].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This study was supported by grants from National Youth Natural Science Foundation of China (No. 81400108).
References
- [1].Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol: Official J American Soc Clin Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. PMID:16155016 [DOI] [PubMed] [Google Scholar]
- [2].Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. PMID:16917004 [DOI] [PubMed] [Google Scholar]
- [3].Li X, Ling W, Khan S, et al. Therapeutic effects of intrabone and systemic mesenchymal stem cell cytotherapy on myeloma bone disease and tumor growth. J Bone Miner Res: Official J American Soc Bone Miner Res. 2012;27:1635–1648. doi: 10.1002/jbmr.1620. PMID:22460389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li X, Ling W, Pennisi A, et al. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells (Dayton, Ohio). 2011;29:263–273. doi: 10.1002/stem.572. PMID:21732484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review (CSR) 1975–2011. Bethesda (MD): National Cancer Institute; 2014. [Google Scholar]
- [6].Canitano A, Iessi E, Spugnini EP, et al. Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett. 2016;376:278–283. doi: 10.1016/j.canlet.2016.04.015. PMID:27084522 [DOI] [PubMed] [Google Scholar]
- [7].Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY). 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [8].Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. PMID:11772882 [DOI] [PubMed] [Google Scholar]
- [9].Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells, Mol Dis. 2001;27:632–636. doi: 10.1006/bcmd.2001.0423. [DOI] [PubMed] [Google Scholar]
- [10].Atsuta I, Liu S, Miura Y, et al. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther. 2013;4:111. doi: 10.1186/scrt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonomi A, Steimberg N, Benetti A, et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol Oncol. 2017;35:693–702. doi: 10.1002/hon.2306. PMID:27283119 [DOI] [PubMed] [Google Scholar]
- [12].Ciavarella S, Caselli A, Tamma AV, et al. A peculiar molecular profile of umbilical cord-mesenchymal stromal cells drives their inhibitory effects on multiple myeloma cell growth and tumor progression. Stem Cells Dev. 2015;24:1457–1470. doi: 10.1089/scd.2014.0254. PMID:25758779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang YL, Fu JX, Zhang H, et al. Effects of bone marrow mesenchymal stem cells on the biological characteristics of migrating and homing in multiple myeloma cells. Hematol Oncol. 2016;24:469–473. [DOI] [PubMed] [Google Scholar]
- [14].Garayoa M, Garcia JL, Santamaria C, et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23:1515–1527. doi: 10.1038/leu.2009.65. PMID:19357701 [DOI] [PubMed] [Google Scholar]
- [15].Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. PMID:17344918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garderet L, Mazurier C, Chapel A, et al. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leukemia Lymphoma. 2007;48:2032–2041. doi: 10.1080/10428190701593644. [DOI] [PubMed] [Google Scholar]
- [17].Reagan MR, Ghobrial IM. Multiple myeloma mesenchymal stem cells: characterization, origin, and tumor-promoting effects. Clin Cancer Res: An Official J American Assoc Cancer Res. 2012;18:342–349. doi: 10.1158/1078-0432.CCR-11-2212. PMID:22065077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giuliani N, Mangoni M, Rizzoli V. Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: identification of potential therapeutic targets. Exp Hematol. 2009;37:879–886. doi: 10.1016/j.exphem.2009.04.004. PMID:19446662 [DOI] [PubMed] [Google Scholar]
- [19].Suzuki K, Sun R, Origuchi M, et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med (Cambridge, Mass). 2011;17:579–587. PMID:21424106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Klopp AH, Gupta A, Spaeth E, et al. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells (Dayton, Ohio). 2011;29:11–19. doi: 10.1002/stem.559. PMID:21280155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. PMID:14578755 [DOI] [PubMed] [Google Scholar]
- [22].Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. PMID:12542793 [DOI] [PubMed] [Google Scholar]
- [23].Zhang L, Bi E, Hong S, et al. CD4(+) T cells play a crucial role for lenalidomide in vivo anti-tumor activity in murine multiple myeloma. Oncotarget. 2015;6:36032–36040. PMID:26447613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feng P, Yan R, Dai X, et al. The alteration and clinical significance of Th1/Th2/Th17/Treg cells in patients with multiple myeloma. Inflammation. 2015;38:705–709. doi: 10.1007/s10753-014-9980-4. PMID:25034833 [DOI] [PubMed] [Google Scholar]
- [25].Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells (Dayton, Ohio). 2013;31:1980–1991. doi: 10.1002/stem.1432. PMID:23712682 [DOI] [PubMed] [Google Scholar]
- [26].Mougiakakos D, Jitschin R, Johansson CC, et al. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. PMID:21389316 [DOI] [PubMed] [Google Scholar]
- [27].Chinnadurai R, Copland IB, Patel SR, et al. IDO-independent suppression of T cell effector function by IFN-gamma-licensed human mesenchymal stromal cells. J Immunol (Baltimore, Md: 1950). 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. PMID:24403533 [DOI] [PubMed] [Google Scholar]
- [28].Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. European J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. PMID:15827960 [DOI] [PubMed] [Google Scholar]
- [29].Sheng H, Wang Y, Jin Y, et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. PMID:18607390 [DOI] [PubMed] [Google Scholar]
- [30].Stagg J, Pommey S, Eliopoulos N, et al. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. PMID:16293599 [DOI] [PubMed] [Google Scholar]
- [31].Luz-Crawford P, Noel D, Fernandez X, et al. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PloS One. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. PMID:23028899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ray A, Das DS, Song Y, et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia. 2015;29:1441–1444. doi: 10.1038/leu.2015.11. PMID:25634684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davies LC, Heldring N, Kadri N, et al. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells (Dayton, Ohio). 2017;35:766–776. doi: 10.1002/stem.2509. PMID:27671847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oyajobi BO, Franchin G, Williams PJ, et al. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102:311–319. doi: 10.1182/blood-2002-12-3905. PMID:12649140 [DOI] [PubMed] [Google Scholar]
- [35].Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. PMID:16923606 [DOI] [PubMed] [Google Scholar]
- [36].Anderson AC, Lord GM, Dardalhon V, et al. T-bet, a Th1 transcription factor regulates the expression of Tim-3. European J Immunol. 2010;40:859–866. doi: 10.1002/eji.200939842. PMID:20049876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zheng WP, Flavell RA. Pillars article: The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. J Immunol (Baltimore, Md: 1950). 2016;196: 4426–4435. [PubMed] [Google Scholar]
- [38].Chen Z, Lin F, Gao Y, et al. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11:536–542. doi: 10.1016/j.intimp.2010.11.008. PMID:21081189 [DOI] [PubMed] [Google Scholar]
- [39].Girdlestone J. Mesenchymal stromal cells with enhanced therapeutic properties. Immunotherapy. 2016;8:1405–1416. doi: 10.2217/imt-2016-0098. PMID:28000538 [DOI] [PubMed] [Google Scholar]
- [40].Lee HY, Hong IS. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017;108:1939–1946. doi: 10.1111/cas.13334. PMID:28756624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jelinek T, Hajek R. PD-1/PD-L1 inhibitors in multiple myeloma: The present and the future. Oncoimmunology. 2016;5:e1254856. doi: 10.1080/2162402X.2016.1254856. PMID:28123899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. PMID:19417208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xu ZJ, Zhao D, Li FP, et al. Levels of T-lymphocyte subsets, IL-17, IL-35 and IFN-gamma in peripheral blood and their clinical significance in patients with multiple myeloma. Zhongguo shi yan xue ye xue za zhi. 2017;25:1444–1448. PMID:29070122 [DOI] [PubMed] [Google Scholar]
- [44].Atanackovic D, Luetkens T, Radhakrishnan S, et al. Coinhibitory molecule PD-1 as a therapeutic target in the microenvironment of multiple myeloma. Current Cancer Drug Targets. 2017;17:839–845. doi: 10.2174/1568009617666170906170348. PMID:28875836 [DOI] [PubMed] [Google Scholar]
- [45].Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. PMID:16200068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. PMID:17375096 [DOI] [PubMed] [Google Scholar]
- [47].Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol (Baltimore, Md: 1950). 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. PMID:16785554 [DOI] [PubMed] [Google Scholar]
- [48].Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. PMID:16903903 [DOI] [PubMed] [Google Scholar]
- [49].Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9:89–105. doi: 10.1586/erv.09.144. PMID:20021308 [DOI] [PubMed] [Google Scholar]
- [50].Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. PMID:12091876 [DOI] [PubMed] [Google Scholar]
- [51].Palla AR, Kennedy D, Mosharraf H, et al. Autoimmune hemolytic anemia as a complication of nivolumab therapy. Case Reports Oncol. 2016;9:691–697. doi: 10.1159/000452296. PMID:27920704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. PMID:14559843 [PubMed] [Google Scholar]
- [53].Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. PMID:12218188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Atanackovic D, Luetkens T, Kroger N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia. 2014;28:993–1000. doi: 10.1038/leu.2013.310. PMID:24153012 [DOI] [PubMed] [Google Scholar]
- [55].Jelinek T, Mihalyova J, Kascak M, et al. PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology. 2017;152:357–371. doi: 10.1111/imm.12788. PMID:28685821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gorgun G, Samur MK, Cowens KB, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res: An Official J American Assoc Cancer Res. 2015;21:4607–4618. doi: 10.1158/1078-0432.CCR-15-0200. PMID:25979485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. PMID:21577144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Paiva B, Azpilikueta A, Puig N, et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia. 2015;29:2110–2113. doi: 10.1038/leu.2015.79. PMID:25778100 [DOI] [PubMed] [Google Scholar]


