ABSTRACT
Despite the potential of Idiopathic Intracranial Hypertension (IIH) to cause visual morbidity, limited literature is available focussing on predictors of visual outcome in IIH. This study was planned to assess visual morbidity in patients of IIH in terms of clinical and neuro-ophthalmo- logical parameters. In this prospective study of 40 patients of IIH, neuro-ophthalmological parameters were noted in the form of visual acuity, visual field, contrast sensitivity, Retinal Nerve Fibre Layer thickness, and visual evoked potential. Visual outcome was defined in using specific criteria. Final visual outcome of patients was compared with clinical and neuro-ophthalmologic para- meters to determine any correlation. The most common presenting clinical symptoms were headache (85%) and Transient visual obscurations (TVOs) (52.5%). In univariate analysis severity of visual loss, Cerebro Spinal Fluid (CSF) pressures and abnormal Visual evoked potential (VEP) were associated with worse visual outcome or need of aggressive management. When adjusted for severity of visual loss no independent clinical/neuroophthalmic predictor could be established. High CSF opening pressure, worsening vision/papilledema, greater Retinal Nerve Fiber Layer (RNFL) thickness and abnormal VEPs may be some of the alarming signs for physicians, but none of these parameters can be used as an independent predictor for visual outcome in isolation. Visual loss at presentation is probably the most important predictor of the final visual outcome in these patients. This may also suggest that patients presenting in an advanced disease course (with worse visual status) fair badly despite best medical/surgical management. Early diagnosis and prompt management is the cornerstone of management.
KEYWORDS: Idiopathic intracranial hypertension, predictors, visual outcome
Introduction
Despite the fact that Idiopathic Intracranial Hypertension (IIH) was described about 125 years ago, a cohesive concept on etio-pathogenesis and approach to management has not emerged. IIH has been viewed in different perspectives and its nomenclature has undergone enumerable changes since its inception as a distinct entity. While some authors referred to the condition as arachnoiditis1, others named it as toxic hydrocephalus2, hypertensive meningeal hydrops3, otitic hydrocephalus, and pseudotumor cerebri.4 In 1937, Dandy5 first documented elevated CSF pressure in IIH along with the spectrum of symptoms of IIH. Years later, Foley6 attempted to simplify the nomenclature by describing this entity as “Benign Intracranial Hypertension”. However, soon it was recognized that this entity is not “Benign” as it can cause devastating loss of vision. In this view, Corbett and Thompson7 referred this condition, more appropriately as “Idiopathic Intracranial Hypertension”. The criteria for diagnosis were modified by Smith JL in.19858 Modified Dandy Criteria8,9 had been widely used for diagnosis of IIH till 2013. The criteria were revised in 2013 to incorporate advances in recent decade.4
Of late, it has been well established that “At stake is vision” and any delay in diagnosis or inappropriate management may cause permanent loss of vision. Despite the potential of this entity to cause significant visual morbidity there is limited literature available focussing on predictors of final visual outcome. Objective clinical and neuro-ophthalmologic predictors may aid in early recognition of patients requiring aggressive medical/surgical intervention.
This study was attempted to determine the outcome of IIH with regards to various clinical and neuro-ophthalmological parameters. A comprehensive attempt was made to analyse visual outcome using well defined strategies and to determine predictors of worse outcome.
Materials and methods
Study design
This was a prospective study carried out in a tertiary care hospital in North India from January 2012 to December 2013. All consecutive patients attending the neurology/ophthalmology clinic and fulfilling the Modified Dandy Criteria8,9 for IIH were included in the study. For patients enrolled in 2013, the revised diagnostic criteria for pseudo tumor cerebri were used.4 Ethical clearance was taken from the Institutional body.
Materials
Visual acuity was done using Snellen’s charts. The baseline Standard Achromatic Perimetry was done in all patients on the Humphrey’s Field Analyser 750 II (Carl Zeiss-Humphrey Systems, Dublin, CA) using the 24–2 testing protocol by SITA-Standard strategy. RNFL measurements were obtained on the Cirrus OCT ® (Carl Zeiss Meditec, Dublin, CA, USA). The RNFL thickness in four different quadrants as well as values for mean RNFL thickness were computed. P 100 latencies were measured on VEP (by pattern reversal study using checker board pattern). Contrast sensitivity was done at five spatial frequencies in all the patients using Functional Acuity Contrast Test (F.A.C.T.) at 3 m distance under normal lighting. The F.A.C.T. chart consists of 45 sine wave gratings arranged in five rows and nine columns. The corresponding spatial frequencies are 1.5,3,6,12 and 18 cycles per degree. The deviation from normal pattern of contrast sensitivity at these frequencies was noted.
All parameters were analysed every month for first 3 months and then at the end of 6 months. All patients underwent Gadolinium enhanced MRI of the Brain with MR Venography to rule out cerebral venous thrombosis.
Grading of the parameters
Visual grading was done based on the visual acuity and visual field (Wall and George).10,11 Some vital points of this grading system are as follows- Grade 0 was given when there was normal visual acuity and visual field. Grade 1/Minimal visual loss; if there was either of the following-a) Visual fields met criteria for deficit but no greater than three contiguous points were abnormal/no points having loss greater than 10 db; b) Enlarged blind spot with no encroachment into the central 10°; c) No defects involving fixation and Visual Acuity 6/6 or better. Grade 2/Mild Visual loss was considered if either– a) Field defects were larger than those in grade 1; b) Blind spot was enlarged and encroaching into central 10° c) Defects involving fixation (less than 10 db loss) and VA of 6/6 or better. Grade 3/Moderate visual loss was considered if either a) Field constriction was present with all points abnormal in one isopter; b) Defects involving fixation and visual acuity was worse than 6/9 but less than 6/36 (For defects not involving fixation: all points loss in greater than one quadrant but less than one hemifield or loss greater than 20 º * 20º in diameter in less than one quadrant was considered.); c) Blind spot encroached fixation (relative defect) with greater than 10 db loss. Grade 4/Marked visual loss was considered when either a) Isopter constriction of <50° but more than 20° to the brightest stimulus was present; b) Defects involving fixation with acuity worse 6/36 to 6/60 (For defects not involving fixation involvement of one hemifield or greater was considered) c)Blind spot encroaching on fixation (relative defect) with greater than 2 log units of loss. Grade 5/Blinding visual loss was considered when visual field constriction–less than 20° to brightest stimulus or visual acuity worse than 6/60 was present.
Papilledema was graded as per the Modified Frisen’s Scale.12 Continuous variables like RNFL- thickness, P100 latency on VEP were analysed accordingly using independent t-test.
Treatment
All patients were treated with acetazolamide in the dose of 500 mg to 2 gm. Topiramate (25 mg to 100 mg) and Furosemide (20–40 mg) was also used in selected patients. In case of worsening of the symptoms on the conventional medical therapy or very poor visual acuity at presentation, patients were offered high dose steroids pulse (Intravenous Methyl prednisone 1 gm daily for a maximum of 5 days) while waiting for a more definitive (surgical) intervention. If there was no/inadequate response, patients were taken up for optic nerve sheath fenestration. The patients with poor vision at baseline or with worsening parameters were offered early optic nerve sheath fenestration. Eight patients had required steroids and one out of the eight had to be subjected to ONSF because of worsening vision. While 40 patients were enrolled for the initial study, one patient had lost to follow-up. So final treatment outcomes were calculated based on 39 patients.
Statistical analysis
Descriptive statistics was used to analyse mean, standard deviation, frequency,and percentage. Inferential statistics for example, t test and Chi Square test (Fischer exact test) were used to estimate the population parameters. Categorical variables were analysed between good outcome (present/absent) as well as need of aggressive treatment(steroids/surgery)(Yes/no) using Chi Square (Fischer exact wherever applicable) test. Independent t-test was applied to compare continuous variables. All calculations were two sided. A value of < 0.05 was considered to be statistically significant. Multiple logistic regression was used after correcting for visual loss at presentation while considering the parameters, which could predict visual outcome in univariate analysis.
Results
Demographic data (Table 1)
Table 1.
Baseline data of patients at presentation.
| |
n |
Percentage (%) |
||
|---|---|---|---|---|
| Gender | Female |
38 |
95 |
|
| Male | 2 | 5 | ||
| Age in years (Mean ± SD) | 32.8 ± 11.06 | |||
| BMI@ in kg/m2 (Mean ± SD) | 26.89 ± 2.74 | |||
| Mean CSF$- Pressure in mm of H2O (Mean ± SD) | 266.9 ± 55.6 | |||
| Mean Retinal fibre Layer thickness on OCT* in µm (Mean ± SD) | 168.5 ± 74.06 | |||
| Mean P100 Latency on VEP^ in m sec (Mean ± SD) | 113.3 ± 10.97 | |||
| Clinical Features | Headache | 34 | 85 | |
| TVOs | 21 | 52.5 | ||
| Diplopia | 9 | 22.5 | ||
| Cranial Nerve Involvement | Six | 9 | 22.5 | |
| Four | 1 | 2.5 | ||
| Five | 1 | 2.5 | ||
| Visual deficits | 10 | 25 | ||
| Tinnitus | 3 | 7.5 | ||
| Papilledema on Fundus examination | Grade 0 | 0 | 0 | |
| (Modified Frisens grade)12 | Grade 1 | 0 | 0 | |
| Grade 2 | 8 | 20 | ||
| Grade 3 | 15 | 37.5 | ||
| Grade 4 | 17 | 42.5 | ||
| Grade 5 | 0 | 2.5 | ||
| Abnormalities on MRI- Brain | Empty sella | 20 | 50 | |
| Scleral flattening | 20 | 50 | ||
| Optic nerve protrusion | 19 | 47.5 | ||
| Optic nerve sheath distention | 12 | 30 | ||
| Optic nerve tortuosity | 17 | 42.5 | ||
| Slit like ventricles | 0 | 0 | ||
@ BMI- Body Mass index; $ CSF- Cerebro Spinal Fluid; * OCT- Optical Coherence Tomography; ^ VEP- Visual Evoked Potential
This prospective study included 40 patients of IIH. The mean age of patients at time of presentation was 32.8 ± 11.06 years (Range 14–56 years). There were 38 women and two men in the study and 70% of the participants were overweight.
The most common symptom at presentation was headache which was seen in 85% patients followed by TVOs in 52.5% patients and visual loss at presentation was present in 25% patients.
The most important finding on the examination was papilledema which was seen in all the patients. However, two patients (5%) had papilledema only in one eye (Unilateral papilledema). Sixth cranial nerve palsy was seen in 22.5% patients. one patient had fourth cranial nerve palsy and one patient had trigeminal (ophthalmic division) palsy as false localising signs. The baseline data of patients at presentation has been detailed in Table 1.
Visual outcome
For the purpose of analysis of visual outcome, the worse eye of each patient was chosen. The eye with the worse visual grade (based on Humphrey Mean deviation and visual acuity) at the entry was chosen. One patient had lost to follow-up hence 39 patients were analysed finally.
Assessment of visual outcome was based upon final visual grade attained by the patient at the end of 6 months
Good outcome was as defined by attainment of visual grade10,11 of zero (no visual loss) or an improvement of ≥ 2 grades from the baseline at 6 months follow-up.
As all the patients were treated, final outcomes could be biased and might not have reflected the true natural history of the disease. All the patients were treated in a stepwise manner. First medical management was administered in the form of acetazolamide(±Topiramate/Furosemide). If they failed this first line treatment, surgical intervention was done. In a few patients, who presented with significant visual loss or the ones who worsened despite best medical management, high dose steroid pulse was administered while waiting for definitive surgical management(n = 8). Thus, need to give steroids suggested failure of routine treatment or in other words more severe disease. The profile of the patients who needed this aggressive management was also noted. One out of these eight patients was subjected to ONSF in view of worsening vision despite best medical management.
Clinical parameters and visual outcomes (Table 2, 3)
Table 2.
Univariate analysis of predictors of good outcome (good outcome defined by visual loss grade = 0 or ≥ 2 grade improvement on 6 month follow-up).
| Clinical/Neuroophthalmic Parameters | Good outcome (no vision loss or > 2 grade improvement on 6 month follow-up) |
p Value | |
|---|---|---|---|
| Yes |
No |
||
| (n = 32) | (n = 7) | ||
| Age in years (Mean ± SD) | 32.28 ± 11.4 | 33.18 ± 11.2 | 0.845 |
| Gender- Female | 30 | 7 | 0.669 |
| Headache | 26 | 7 | 0.568 |
| TVOs | 16 | 4 | 1.0 |
| Diplopia | 7 | 2 | 0.653 |
| Visual Deficit | 9 | 1 | 0.65 |
| High BMI@ (in kg/m2 (Mean ± SD)) | 25 | 6 | 0.383 |
| CSF$ pressure in mm of H2O (Mean±SD) | 260.469 ± 53.4964 | 305.714 ± 50.6153 | 0.048 |
| Baseline RNFL* in µm (Mean±SD) | 161.594 ± 75.5453 | 191.000 ± 68.5176 | 0.350 |
| Baseline P100 latency on VEP^ in msec (Mean±SD) | 111.844 ± 11.3567 | 117.429 ± 5.7404 | 0.217 |
| Abnormal MRI | 27 | 5 | 0.588 |
@ BMI- Body Mass index; $ CSF- Cerebro Spinal Fluid; *RNFL- Retinal Nerve Fiber Layer; ^ VEP- Visual Evoked Potential
Table 3.
Univariate analysis of predictors of need of aggressive treatment (steroids/surgery) on 6 month follow-up.
| Predictors | Need of Steroids |
p Value | |
|---|---|---|---|
| Yes (n = 8) | No (n = 32) | ||
| Age in years (Mean ± SD) | 33.18 ± 11.2 | 32.28 ± 11.4 | 0.849 |
| Gender- Female | 8 | 30 | 0.468 |
| Headache | 5 | 29 | 0.082 |
| TVOs | 2 | 19 | 0.120 |
| Diplopia | 1 | 8 | 0.655 |
| Visual Deficit | 3 | 7 | 0.653 |
| High BMI@(in kg/m2 (Mean ± SD)) | 8 | 24 | 0.102 |
| CSF$ pressure in mm of H2O (Mean±SD) | 242.50 ± 23.14 | 272.96 ± 59.76 | 0.168 |
| Baseline RNFL* in µm (Mean±SD) | 207.87 ± 94.5 | 158.59 ± 66.20 | 0.198 |
| Baseline P100 latency on VEP^ in msec (Mean±SD) | 126.0 ± 6.8 | 110.12 ± 9.43 | 0.00 |
| Abnormal MRI | 8 | 25 | 0.309 |
| Severe visual loss at outset | 8 | 18 | 0.034 |
@ BMI- Body Mass index; $ CSF- Cerebro Spinal Fluid; *RNFL- Retinal Nerve Fiber Layer; ^ VEP- Visual Evoked Potential
Univariate analysis was carried out for all parameters to look for any association with visual outcome. As good vision at the outset is expected to remain better at the follow-up, multivariate analysis was also done after adjusting for vision at the presentation.
The mean CSF opening pressure in current study was 266.9 ± 55.6 mm of H2O.
The mean CSF pressure in the patients with poor outcome was 305.714 ± 50.61 mm of H2O as against CSF pressure of 260.469 ± 53.49 mm of H2O in patients with good outcome. In univariate analysis, the visual outcome showed significant association with CSF opening pressure (p = 0.048). However, when multiple logistic regression was applied, after adjusting for vision at the outset no independent predictor of visual outcome could be established. High CSF opening pressure may be a reflection of more severe disease, however, it is not an independent predictor of visual outcome.
No significant correlation with visual outcome could be established between the gender, age, BMI, or any specific clinical symptom or sign.
Neuro-ophthalmological parameters and visual outcomes (Table 2)
No specific visual field defect correlated well with the final visual outcome. Also no statistically significant correlation of the visual outcome could be established grade of papilledema at presentation.
The average RNFL thickness in µm at presentation in our cohort was 168.5 ± 74.06.No significant association between RNFL thickness and visual outcome was noted.
RNFL thickness (as was calculated by area under the curve) of above 178 µm was found to be inversely correlated with good outcome with a specificity of 66.67% and sensitivity of 56.7%. The positive predictive value of RNFL thickness >178 µm for a poorer outcome at 6 months is 51.52%. No significant association between P100 latency on VEP or with contrast sensitivity at any spatial frequency and visual outcome was noted as well.
Apart from clinical and neuroophthalmic parameters the association between various neuroimaging (Gd MRI- Brain and MR- Venography) findings and visual outcome was also assessed. Though majority of patients had one or another abnormality on Neuroimaging, no definitive association could be established.
Predictors of need of aggressive management (Table 3)
Clinical profile of patients needing aggressive management (steroids/ONSF) was also noted and all clinical and neuroophthalmic parameters were analysed for presence of any association. In univariate analysis severe visual loss and abnormal VEP showed statistically significant correlation with need of aggressive management. However, after adjusting for visual loss at the presentation, no independent predictor could be established.
Discussion
The female to male ratio (19:1) of the patients of IIH was higher in our study as compared to the previous studies.13,14 The current study was a hospital based study which included only newly diagnosed cases of IIH
While various studies have attempted to find predictors of visual outcome in patients of IIH, there are controversies in the available literature.15–18 In the current study, headache was the most common symptom at presentation (85%) followed by transient visual obscurations (52.5%). 22.5% of the patients had diplopia as time of presentation and 25% had significant visual deficits at presentation.
IIH has been associated with obesity, high BMI and also with recent weight gain. Various authors and the recent IIH Treatment Trial13–15 have reported association of obesity in patients of IIH. There are however, controversies as to whether BMI can predict the final visual outcome in these patients.18 In the current study also, 80% patients (32/40) had a BMI of ≥25, 70% were overweight and 10% were frankly obese. In this series, however, Body mass index did not show any correlation with the final visual outcome.
Visual field defects were seen in 42.5% of patients. The most common field defect was enlargement of blind spot seen followed by constriction of peripheral field of vision. None of the visual field defects in particular showed a statistically significant correlation with the visual outcome.
Visual outcome showed significant association with CSF opening pressure when considered in univariate analysis. This result could not be reciprocated, when adjustment for visual loss at the presentation was done; thereby suggesting that CSF pressure can not be used as an independent predictor of visual outcome.
Routinely, papilledema is used along with the visual fields to monitor the treatment.
A prototype regression of papilledema in one of the patients at presentation, at 3 months and at 6 months has been shown in Figure 1. Patients with high grade papilledema along with diminished visual acuity at presentation are more likely to experience treatment failure.16 However, fundus grading can be dubious, is highly dependent on skills of individual and inter-rater variability is high in grading papilledema. The visual field analysis also relies too much on the subject being tested. In addition it is time consuming and subject to procedural errors. Multiple trails need to be given to the patient to eliminate the false negative/positive results. The regression of changes in a prototype patient has been depicted in Figure 2. In our study neither the grade of papilledema or the type of visual defect correlated with worse visual outcome. This might be related to the fact these are not continuous variables and their assessment may be subject to inter-and intra-rater variations.
Figure 1.
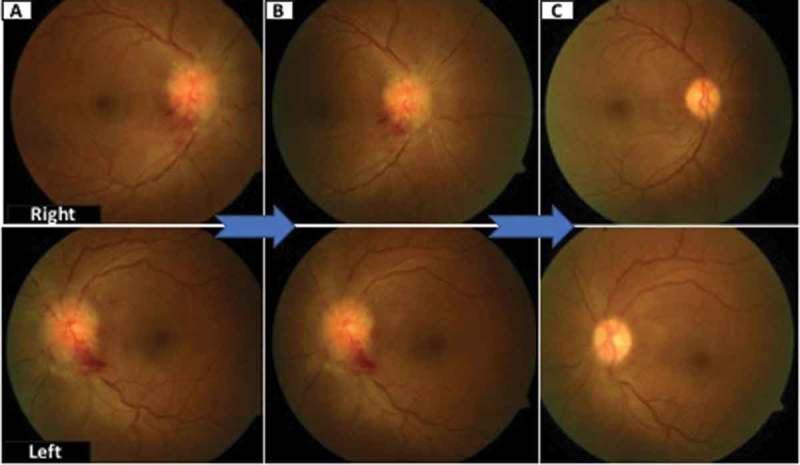
Showing regression of papilledema in a prototype patient. Figure 1A shows papilledema at presentation and its regression at 3 months (Figure 1B) The resolution of papilledema at 6 months has been shown in Figure 1C.
Figure 2.
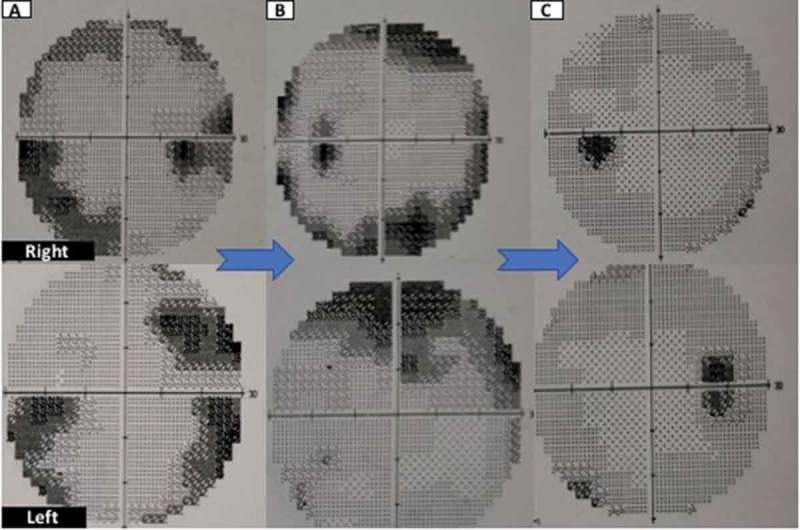
Showing regression of changes in visual fields in a prototype patient. Figure 2A shows peripheral constriction on visual field testing at presentation and its improvement at 3 months (Figure 2B)The resolution of changes at 6 months has been shown in Figure 2C.
In view of these difficulties, we determined role of monitoring of RNFL thickness in IIH for monitoring clinical response and prognosis In the series of Rebolleda and Munoz Negrete19 mean peripapillary RNFL thickness in 22 patients of IIH was 183.3 ± 74.7 µm, while in our series, it was 168.5 ± 74.06 um.
We however could not establish any significant correlation between RNFL thickness and visual outcome.
Figure 3 shows regression in papilledema in coherence with normalisation of retinal nerve fibre layer thickness.
Figure 3.
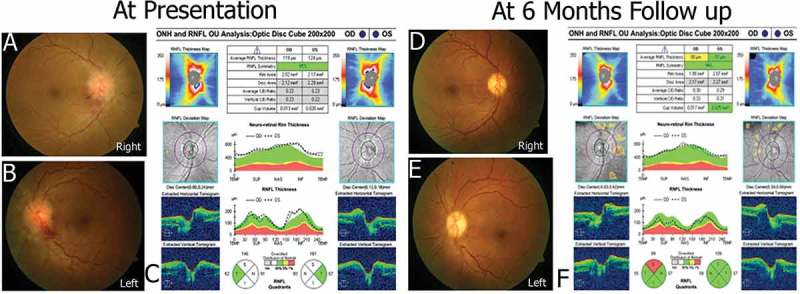
Showing regression of changes in retinal nerve fibre layer thickness in coherence with papilledemain a prototype patient. Figure 3C shows retinal nerve fibre layer thickness at presentation of prototype patient. The findings were consistent with papilledema both eyes(Figure 3A/B) at presentation. Normalisation of retinal nerve fibre layer thickness is seen over 6 months(Figure 3F), consistent with resolution of papilledema(Figure 3D/E).
Abnormal visual evoked potentials were associated with significantly greater need for aggressive treatment in the form of steroids. Abnormalities in contrast sensitivity at any spatial frequency did not correlate to visual outcome. Also, no association between findings on neuroimaging and visual outcome could be established in our study. These findings are in concordance with available literature on the subject.17,18 From the above discussion it is evident that though high CSF opening pressure, worsening vision/papilledema, greater RNFL thickness and abnormal P100 latency on VEP may be some of the alarming signs for physicians, but none of these parameters in isolation can be used as predictor for visual outcome. Visual loss at presentation is probably the most important predictor of the final vision in these patients. This may also suggest that patients presenting in an advanced disease course (with worse visual status) fair badly despite best medical/surgical management. Early diagnosis and prompt management is the cornerstone of management. Early alterations in various neuroophthalmic parameters may help provide a clinician window for early and appropriate management.
Declaration of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the writing and content of the article.
Financial Disclosures/Funding source
None.
References
- 1.Cushing H, Noleson A.. Notes on a series of intracranial tumor and conditions simulating them: tumor suspects; tumors unverified; tumors verified. Arch Neurol Psych. 1923;10:605–668. doi: 10.1001/archneurpsyc.1923.02190300002001. [DOI] [Google Scholar]
- 2.McAlpine D. Toxic hydrocephalus. Brain. 1937;60:180–203. doi: 10.1093/brain/60.2.180. [DOI] [Google Scholar]
- 3.Davidoff LM, Dyke CG. Hypertensive meningeal hydrops: a syndrome frequently following infection in the middle ear or elsewhere in the body. Am J Ophthalmol. 1937;20:908–927. doi: 10.1016/S0002-9394(37)92558-3. [DOI] [Google Scholar]
- 4.Friedman DI, Liu GT, Digre K. Revised diagnostic criteria for the pseudotumorcerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 5.Dandy WE. Intracranial pressure without brain tumor: diagnosis and treatment. Ann Surg. 1937;106:492–513. doi: 10.1097/00000658-193710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley J. Benign forms of intracranial hypertension: “toxic” and “otitic hydrocephalus. Brain. 1955;78:1–41. doi: 10.1093/brain/78.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol. 1989;46:1049–1051. doi: 10.1001/archneur.1989.00520460025008. [DOI] [PubMed] [Google Scholar]
- 8.Smith JL. Whence pseudotumorcerebri? J ClinNeuro-ophthalmol. 1985;5:55–56. [Google Scholar]
- 9.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.WNL.0000029570.69134.1B. [DOI] [PubMed] [Google Scholar]
- 10.Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991;114:155–180. [PubMed] [Google Scholar]
- 11.Wall M. The morphology of visual field damage in idiopathic intracranial hypertension: an anatomic region analysis In: Mills RP, Heijl A, editors. Perimetry Update 1990/1991. Amsterdam: Kugler Publications; 1991:20–27. [Google Scholar]
- 12.Frisen L. Swelling of the optic nerve head: a staging scheme. J NeurolNeurosurg Psychiatry. 1982;45(1):13–18. doi: 10.1136/jnnp.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durcan FJ, Corbett JJ, Wall M. The incidence of PseudotumorCerebri. Arch Neurol. 1988;45:875–877. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 14.Wall M, Kupersmith MJ, Kieburtz KD, et al. NORDIC idiopathic intracranial hypertension study group. the idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014. June;71(6):693–701. doi: 10.1001/jamaneurol.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szewka AJ, Bruce BB, Newman NJ. Biousse V.. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol. 2013. March;33(1):4–8. doi: 10.1097/WNO.0b013e31823f852d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall M, Falardeau J, Fletcher WA, et al. Risk factors for poor visual outcome in patients with idiopathic intracranial hypertension. Neurology. 2015;85(9):799–805. doi: 10.1212/WNL.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saindane AS, Bruce BB, Riggeal BD, Newman NJ, Biousse V. Association of MRI findings and visual outcome in idiopathic intracranial hypertension. AJR Am J Roentgenol. 2013;201:412–418. doi: 10.2214/AJR.12.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afonso CL, Talans A, Monteirio MLR. Factors affecting visual loss and visual recovery in patients with pseudotumor cerebri syndrome. Arq Bras Oftalmol. 2015;78(3):175–179. doi: 10.5935/0004-2749.20150045. [DOI] [PubMed] [Google Scholar]
- 19.Rebolleda G, Munoz Negrete FJ. Follow up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(11):5197–5200. doi: 10.1167/iovs.08-2528. [DOI] [PubMed] [Google Scholar]


