Abstract
Advances in medical imaging technology have led to an increased demand for radiopharmaceuticals for early and accurate diagnosis of cardiac function and diseased states. Myocardial perfusion, metabolism, and hypoxia positron emission tomography (PET) imaging radiotracers for detection of cardiac disease lack specificity for targeting inflammation that can be an early indicator of cardiac disease. Inflammation can occur at all stages of cardiac disease and currently, 18F-fluorodeoxyglucose (FDG), a glucose analog, is the standard for detecting myocardial inflammation. 18F-FDG has many ideal characteristics of a radiotracer but lacks the ability to differentiate between glucose uptake in normal cardiomyocytes and inflammatory cells. Developing a PET radiotracer that differentiates not only between inflammatory cells and normal cardiomyocytes, but between types of immune cells involved in inflammation would be ideal. This article reviews current PET radiotracers used in cardiac imaging, their limitations, and potential radiotracer candidates for imaging cardiac inflammation in early stages of development of acute and chronic cardiac diseases. The select radiotracers reviewed have been tested in animals and/or show potential to be developed as a radiotracer for the detection of cardiac inflammation by targeting the enzymatic activities or subpopulations of macrophages that are recruited to an injured or infected site.
Keywords: Positron emission tomography (PET), radiotracer candidates, myocardial perfusion, metabolism, cardiac disease, cardiac inflammation, inflammatory cells, animal models, macrophages
Introduction
Positron emission tomography (PET) is a nuclear-based modality used for decades as an important clinical tool to non-invasively image blood flow [1,2], cardiac tissue metabolism [3,4] and receptor expression [5,6]. PET requires the injection of a radioactive tracer, labeled with a short-lived radioisotope, that upon decay emits a positron that annihilates with an electron to release coincident photons that are detected by a PET camera. Although single-photon emission computed tomography (SPECT) is less expensive and more commonly used in nuclear medicine departments, a recent expansion in the use of PET for cardiac imaging has resulted from the increasing availability of new PET cameras, PET radiotracers and cyclotron facilities capable of producing the short-lived isotopes needed for labeling chemistry. PET has proven to be a superior technique for molecular imaging due to its accurate attenuation correction, higher spatial and temporal resolution, higher sensitivity, quantitative abilities coupled with lower radiation risk due to the use of short-lived isotopes like carbon-11 (20 min), fluorine-18 (110 min) and rubidium-82 (76 secs) [7,8]. Further, PET appears to have improved diagnostic accuracy compared to SPECT lowering the overall costs of therapy and improved outcomes [9,10].
Advances in medical imaging technology have led to an increased demand for radiopharmaceuticals for early and accurate diagnosis of organ function and diseased states. The use of cyclotron produced PET tracers in clinical cardiac imaging has been historically limited due to the requirement of a local cyclotron and radiopharmacy and the challenges associated with the transportation of radiotracers labeled with short-lived PET isotopes to distant locations. However, the International Atomic Energy Agency published statistics in 2006, documenting 80 cyclotrons present in North America. In the last decade, the number of cyclotrons has doubled improving availability and the ease of production of tracers for PET imaging with an estimated 1200 cyclotron facilities that produce medical imaging isotopes world-wide.
Hybrid systems that combine PET with computed tomography (CT) are now routinely found in most nuclear medicine departments, although systems that have PET combined with magnetic resonance imaging (MRI) are appearing at select research intensive sites. The combination of PET with CT allows for the visualization of biological processes facilitated by PET with simultaneous anatomical imaging to identify and determine localization of the tracer uptake in specific tissues and organs. PET/CT is widely used in clinical oncology but also incorporated into clinical cardiology for the detection of coronary artery disease [11,12]. While there are benefits, including better image resolution than with PET alone and reduced image acquisition time resulting in high patient throughput, the addition of CT significantly increases the amount of radiation exposure to the patient and in most cases, exceeds the measured dose from the injected PET radiotracer [13]. As a consequence, PET/MRI is becoming increasingly available since anatomic detail and soft tissue contrast anatomical images are produced without the additional radiation dose resulting from PET/CT. It important to acknowledge that PET/MRI cannot be used on patients that have implanted devices [14].
PET radiotracers for cardiac imaging
PET uses radiolabeled probes to measure the expression of molecular markers, metabolic and physiological processes at different stages of disease [15]. Many radiotracers have been developed and evaluated in preclinical imaging experiments but few have made it into routine human application for reasons including the cost of animal and clinical trials, company’s unwillingness to purchase diagnostic agents that will be used once or twice, and the tracers may lack one or more characteristics that make an ideal PET imaging probe [15]. A PET radiotracer should have high specificity to a molecular target, for example a specific enzyme, biomarker, or transport protein associated with a particular cell population and should have low non-specific uptake into other tissues. Another ideal characteristic is high metabolic stability to prevent the probe from being degraded by enzymes before imaging can be done. A radiotracer should have high affinity for target tissue to achieve sufficient accumulation of the probe for quantitative imaging and analysis. High and rapid uptake of tracer into target tissues followed by long retention time is ideal for high contrast ratio of target-to-background ensuring appropriate analysis of images. Quality of the images is also dependent on the positron range before annihilation [16]. Shorter positron ranges produce higher resolution images [17]. Low production cost and availability of the probes are also important characteristics of an ideal probe. Finally, safety is a concern and therefore probes should have low toxicity and the shortest feasible half-life to keep radiation exposure to a minimum [18,19]. Table 1 shows the half-lives of some radioisotopes useful for cardiac imaging.
Table 1.
Half-lives of some PET radioisotopes useful for cardiac imaging
| Radioisotope | Half-life (minutes) |
|---|---|
| 18Fluorine | 109.8 |
| 64Copper | 762 |
| 11Carbon | 20 |
| 68Gallium | 68 |
| 82Rubidium | 1.27 |
| 15Oxygen | 2.06 |
| 13Nitrogen | 9.97 |
In some cases, it is important for imaging to be done under normal resting conditions, as well as, following a stress. Stress tests involve cardiac imaging after the patient has performed physical exercise on a treadmill or supine bicycle [20-23]. When exercise-induced stress isn’t feasible because the length of the test exceeds the radiotracer half-life or patients who cannot exercise, pharmacological stress is induced by administering dipyridamole, adenosine, regadenoson or dobutamine when the patient is ready to be imaged [23,24]. Like exercise, dobutamine increases blood pressure, heart rate and contractility whereas dipyridamole, adenosine, and regadenoson induce coronary vasodilation to increase myocardial blood flow (MBF) [25-28]. Ideally, both stress and rest images can be obtained with one administration of tracer and within the same day.
Fluorine-18 (18F) is generally considered the isotope of choice for radiolabeling potential PET radiotracers. It is produced at every medical cyclotron facility in the world making it widely accessible. In addition, the 109.8-minute half-life of 18F means there is only a few hours to prepare, purify, perform quality control and transport the radiotracer to nearby sites and hospitals that have a PET camera. Another advantage of 18F is that it can be conservatively incorporated into small molecule radiotracers by replacing a hydrogen or hydroxyl group intended to mimic small molecule drugs or natural metabolites without significantly disrupting molecular recognition. In addition, small molecules are typically cleared from tissues, thus sufficient levels of the radiotracer can accumulate into cardiac tissue for quantitative imaging experiments an hour or two after injection without exposing the patient to dangerous levels of radiation.
Similar to 18F, carbon-11 (11C) is an excellent choice for metabolic imaging since it is a small atom and can be incorporated into molecules that are ligands to a protein or metabolized through normal metabolic processes. The large radiometals like copper-64 (64Cu) are typically used for labeling antibodies and proteins that have longer serum half-lives and bind to receptors while generator produced gallium-68 (68Ga) is a convenient isotope for labeling peptides that are quickly cleared from serum. While the very short-lived isotopes like rubidium-82 (82Rb), nitrogen-13 (13N) and oxygen-15 (15O) are useful for perfusion imaging. A long list of radiotracers has been developed for PET imaging of hypoxia, metabolism, inflammation and myocardial perfusion with several others in development in attempt to create suitable PET probes for various physiological and disease processes.
Hypoxia
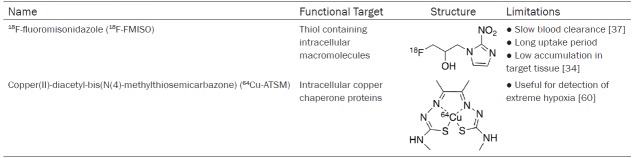
The heart requires oxygen to produce sufficient ATP to maintain function and viability. Hypoxia, or oxygen deficiency, can lead to metabolic and functional abnormalities in the heart [29]. PET radiotracers that can reveal the presence of hypoxic cells have prognostic and diagnostic value and they measure myocardial viability. Brief ischemic events are reversible but chronic hypoxia can cause the myocardium to become necrotic and non-viable [29]. Therefore, early detection of hypoxia is beneficial. For accurate assessment, it is important that hypoxia tracers are specific to hypoxic cells and are not retained in normal or necrotic cells. Therefore, choosing a target unique to hypoxic cells or utilizing a mechanism specific to hypoxic cells is critical. Table 2 summarizes the targets, structures and limitations of two common radiotracers used for detecting hypoxia which are discussed in this section.
Table 2.
Hypoxia radiotracer targets, structures, and limitations
18F-fluoromisonidazole
18F-fluoromisonidazole (FMISO) is the most commonly used PET tracer for measuring hypoxia in neurology, oncology and cardiology. FMISO enters the cells by passive diffusion across the cell membranes. It accumulates in hypoxic cells with low oxygen levels due to the rapid reduction of the radiotracer from the mitochondrial electron transfer system to generate an electrophilic species that reacts with cellular proteins and is retained in the local region [30,31]. Hypoxic cells do not have enough O2 to oxidize FMISO so the reduced radiotracer remains trapped in the hypoxic cell [32,33]. Normoxic cells have enough O2 to oxidize FMISO which allows it to diffuse out of normal cells, thus preventing it from accumulating [33]. Necrotic cells do not have functioning mitochondria for the reduction of the radiotracer and it rapidly diffuses out of the cell. Retention of 18F-FMISO correlates with the severity of hypoxia. This process requires a long uptake period and even then, there is low accumulation of the tracer in target tissue compared to plasma and muscle [34-36].
Tissue oxygenation has been validated in tumors but not in the heart. Use of 18F-FMISO in the heart has been poorly studied and has been limited to animal and in vitro studies. It has not gained the same acceptance as a hypoxia tracer in human nuclear cardiac imaging due to the prolonged blood clearance, tissue uptake time and low accumulation levels which make it difficult to decipher normal tissues and blood from the target tissue affecting image quality [32,37]. Hypoxic cell marker pimonidazole, an immunostain that binds to thiol-containing proteins in hypoxic cells forming intracellular adducts, is used in staining of biopsied tissues and can be a useful validation tool; however, in order to gain information from patients about hypoxia over time, an imaging probe would be useful [38]. Validation of 18F-FMISO accumulating in hypoxic regions have been performed in conjunction with pimonidazole in rabbits with advanced atherosclerosis [39]. Similarly, oncological studies have also used pimonidazole to validate their 18F-FMISO PET imaging results [40]. In comparison to pimonidazole, 18F-FMISO can provide information at a global rather than microscopic level on the distribution of hypoxia [36].
In the heart, 18F-FMISO can detect hypoxic but viable tissue. Its most common use is in characterizing myocardial ischemia and its severity [41,42]. Tested radiotracers have been able to detect the presence of hypoxia but not differentiate the degree of hypoxia [41,43]. A canine study examined the ability of 18F-FMISO as a marker of hypoxia and found accumulation in areas of low blood flow like ischemic myocardium but not in necrotic or normally perfused myocardium [41]. The authors further suggest that 18F-FMISO be used for both hypoxia and assessment of oxygen supply for metabolism. The accuracy of 18F-FMISO is dependent on time allowed for bio-distribution, bio-reduction and wash-out [36,40,41]. Alternative nitroimidazole tracers to 18F-FMISO have been developed for faster clearance and better tumour-to-background ratio for improved image contrast including 18F-fluoroazomycin arabinoside (18F-FAZA), 18F-2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide (18F-EF5), 18F-2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)propan-1-ol (18F-HX4), and 18F-fluoroerythronitroimidazole (18F-FETNIM) [44-48]. These tracers have applications mainly for cancer imaging with few studies investigating uptake by hypoxic cells in the heart [44,49].
64Cu-ATSM
Copper(II)-diacetyl-bis(N(4)-methylthiosemicarbazone) (64Cu-ATSM) is a PET radiotracer used to assess hypoxia in oncology, neurology and cardiology. Bougeois et al. provides an extensive comparison of hypoxia radiopharmaceuticals 18F-FMISO and 64Cu-ATSM that details the labeling process, dosimetry data, mechanisms and image contrast in clinical studies [50]. The use of 64Cu-ATSM for PET imaging in in vivo and in vitro studies is covered in a report by Vãvere et al. (2007) [51]. ATSM can be labelled with isotopes 60/61/62/64Cu based on the physical properties required [50,52]. 64Cu is preferred because it has a sufficient half-life of 12.7 hours and is the most cost effective to produce. Using the cyclotron produced 64Cu, 64Cu-ATSM can be synthesized in a radiopharmacy at high purity and sufficient quantity for clinical purposes [53]. The retention mechanism has been hypothesized by several research groups and is not yet fully known. It is thought that Cu(II)-ATSM diffuses into the cell and is reduced to Cu(I)-ATSM under hypoxic and normoxic conditions [54]. The reduced 64Cu-ATSM is unable to leave the cell and accumulates. However, under normoxic conditions, the reduced complex can be reoxidized and leave the cell.
Two advantages of 64Cu-ATSM are higher cellular uptake and quicker washout from normal tissue, which allows imaging to be done shortly after injection [51,55]. The low redox potential of ATSM is thought to be responsible for its high selectivity [56-58]. Selective retention of the tracer requires intact mitochondria and a significantly high concentration of NADH [59]. Therefore, this tracer is only useful for detection of extreme hypoxia [60]. Researchers have also found that derivatives of ATSM with reduced lipophilicity can increase selectivity to better hypoxic-to-normoxic cell contrast [32].
Metabolism and inflammation
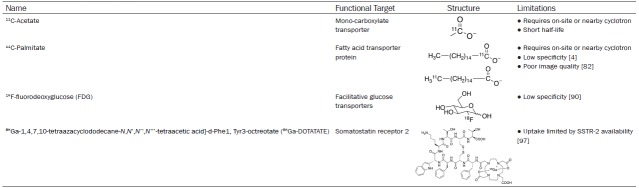
Cardiomyocytes can utilize a number of substrates for energy including glucose, fatty acids, lactate, and ketone bodies to maintain cardiac health under changing environmental conditions. Abnormalities in myocardial metabolism is indicative of pathogenesis of heart disease. For example, glucose metabolism in the heart increases under hypoxic conditions [61,62]. Chronic metabolic adaptations can cause alterations in gene expression, which can lead to the upregulation of proteins and enzymes in metabolic pathways [4]. Table 3 summarizes the functional targets, structures and limitations of select current metabolic PET radiotracers.
Table 3.
Metabolic radiotracers functional targets, structures, and limitations
Inflammation plays a role in heart disease and can be an important imaging target to detect and assess severity of heart disease. Inflammation can be present at all stages of development of cardiovascular disease [63]. Infiltrating immune cells, like macrophages, have higher glucose uptake than normal cells and are found at sites of inflammation [64-66]. Developing a PET radiotracer to detect small amounts of inflammation would be beneficial for early detection.
PET imaging can also detect myocardial viability by examining metabolism and myocardial perfusion imaging (discussed below) in combination. Myocardial tissue can be viable but demonstrate decreased perfusion and this may suggest underlying coronary artery disease (CAD). Assessment of myocardial viability can detect areas where revascularization is possible which improves patient prognosis. If a patient has decreased perfusion but viable myocardium, measured separately using a myocardial perfusion imaging radiotracer like 82Rb (discussed later) for perfusion and 18F-fluorodeoxyglucose to measure viability, they are likely to benefit from revascularization whereas patients with nonviable myocardium will not.
11C-acetate
11C-acetate is a versatile radiotracer since it is rapidly taken up into the cell and converted to acetyl-CoA. From there, it has multiple potential pathways including conversion to amino acids, synthesis of fatty acids or oxidation to CO2 and H2O. In the myocardium, it enters the tricarboxylic acid cycle and is oxidized to CO2 for energy production [67]. PET scans are done immediately after injection because of the tracer’s short half-life of approximately 20 minutes [68]. For the same reason, use of 11C-acetate is limited due to the need for an on-site or nearby cyclotron. Uptake of 11C-acetate into cardiomyocytes is dependent on MBF but clearance of the tracer quantifies myocardial oxidative metabolism [69-71]. This means that high accumulation of the radiotracer in the myocardium indicates low oxidative metabolism, however low accumulation indicates higher oxidative metabolism because the 11C is rapidly released as 11CO2.11C-acetate is the preferred radiotracer for measuring myocardial oxygen consumption with PET.
A review by Grassi et al., thoroughly describes the use of 11C-acetate in oncological imaging [72]. In cardiology, 11C-acetate is used to measure oxidative metabolism and myocardial blood flow [73,74]. Timmer et al., validated the use of 11C-acetate to measure MBF by comparing MBF values to those from 15O-water studies. They tested four methods and determined that 11C-acetate with a single tissue compartment kinetic model and standardized corrections provided the best results for MBF [75]. Other studies using 11C-acetate have shown promising results in evaluating MBF, oxidative metabolic rate, cardiac output and efficiency under stress/rest conditions [72,76].
11C-palmitate
11C-palmitate is a labelled free fatty acid used to measure fatty acid metabolism. In nuclear cardiac imaging, it has been used to measure myocardial triglyceride degradation and oxidation [77,78]. After injection, it binds to serum albumin and is taken up into the myocardium where uptake is dependent on MBF [79]. Slow washout of tracer is indicative of incorporation into the triglyceride pool whereas rapid washout of tracer is associated with oxidative metabolism of fatty acids [80]. Fatty acid catabolism can be directly assessed by the washout kinetics of 11C-palmitate [80]. The accuracy of measuring fatty acid metabolism is reduced because of back-diffusion out of the cell immediately after uptake [81]. Further disadvantages include lack of specificity, the need for an on-site cyclotron for production, and the poor quality of PET images from spillover that require correction [4,82]. The spillover effect is when cross-contamination of radioactivity occurs between different regions because of photon scatter during imaging that result in a false result during image analysis [83]. Similar to 11C-acetate, the short half-life of 11C means that the use of 11C-palmitate is typically restricted to locations with or near a cyclotron facility.
18F-FDG
18F-fluorodeoxyglucose (18F-FDG) has been used as an important research radiotracer in the context of oncological, neurological and cardiologic pathologies. 18F-FDG PET has been used for tracking glucose metabolism to test myocardial viability, and imaging myocardial inflammation. 18F-FDG PET can also monitor progression of disease by assessment of perfusion patterns [84]. FDG is a glucose analog that enters cardiomyocytes via glucose transporters, commonly GLUT1 and GLUT4 [85]. Once inside the cell, hexokinase phosphorylates FDG to FDG-6-phosphate. The phosphorylated metabolite is no longer a substrate for GLUT1 and 4, and cannot be further metabolized and therefore accumulates within the cell. Only tissues with high levels of glucose-6-phosphatase can rapidly dephosphorylate FDG-6-phosphate back to FDG, which then can exit the cell and prevent radiotracer accumulation (e.g., liver) [86,87]. Uptake of 18F-FDG can be influenced by insulin and is therefore dependent on factors related to fed versus fasted state [88,89].
One advantage of 18F-FDG is its longer half-life compared to 11C labeled radiotracers, which allows transport time from the production site to the imaging site. In addition, active transport and metabolic trapping of 18F-FDG in cells means PET imaging studies can be carried out many hours after production. The ideal time between injection of 18F-FDG and imaging is 45-60 minutes to allow for tissue distribution. Unfortunately, because normal cardiomyocytes also use glucose, 18F-FDG has a low specificity for normal cardiomyocytes and can lead to false positives of glucose uptake and perfusion patterns when assessing disease caused by increased FDG uptake by non-specific infectious or inflammatory processes [90,91]. Further development of 18F probes for not only glucose metabolism but for the detection of inflammation are needed.
Somatostatin receptors
Somatostatin receptor (SSTR) PET probes are an alternative to 18F-FDG for targeting inflammation. SSTR PET probes have been developed for oncological imaging but have potential purpose in imaging cardiac inflammation. Somatostatin binding to the receptors exert an immunosuppressive effect that may also help in the development of therapeutic strategies [92]. There are 5 SSTR subtypes. SSTR type 2 (SSTR-2) is over-expressed on the surface of activated macrophages providing a potential target for imaging that is more specific for measuring inflammation caused by macrophage infiltration [93,94]. This type of probe is also capable of detecting proliferating endothelium and angiogenesis [95,96]. 68Ga-1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid]-d-Phe1, Tyr3-octreotate (68Ga-DOTATATE) targets subtype 2 and has been used for investigating the distribution of SSTR-2 and tracking inflammation in the development of atherosclerotic plaque [5,97].
68Ga-DOTATATE binds with high affinity to SSTR-2 [98,99]. However, uptake is limited by the number of occupied SSTR-2 [97]. This tracer has fast blood clearance and has been found to be superior to 18F-FDG in identifying atherosclerotic high-risk versus low-risk lesions and shows greater specificity as an inflammation imaging target [6]. Repeated imaging is possible within the 68-minute half-life of the tracer [100]. The higher energy of the emitted positron from 68Ga is expected to result in lower image resolution however images are comparable to 18F and have sufficiently high resolution [100,101]. A significant benefit of using 68Ga-DOTA-peptides for PET imaging is that a daily supply of the isotope for radiolabeling is available from the 68Ge/68Ga generator that is commercially available and can be shipped to a radiopharmacy thus eliminating the need for an onsite cyclotron [102]. The generator can last up to one year before replacement is required [102]. Banerjee et al., has reviewed several other radiotracers targeting SSTR’s but are not as well studied as 68Ga-DOTATATE for the purpose of identifying cardiac inflammation [100].
Myocardial perfusion imaging
Myocardial perfusion imaging (MPI) tracers are the gold standard for measuring blood flow in the heart with PET. There are several criteria that a tracer must meet to achieve high quality imaging for perfusion analysis. This includes having a high extraction fraction and myocyte retention, unit dose availability from a regional cyclotron, radiotracers labeled with isotopes that have low positron range and high image resolution, possibility of both rest and exercise imaging and absolute quantification of myocardial blood flow [103-105]. Two sets of images are acquired for MPI, one set during rest and one following a stress test. Spillover in the images from the left ventricular blood pool into the myocardium due to the poor spatial resolution of PET is a common problem that requires correction [106]. To correct this problem and improve accuracy of activity measurements, several models of blood pool calculations were introduced [83,107].
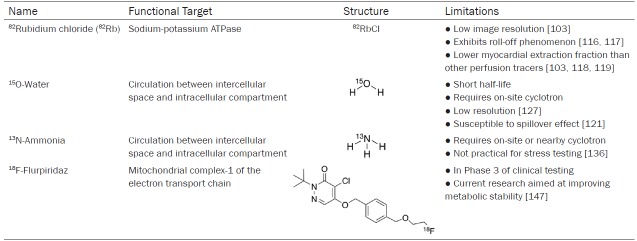
82Rubidium, 14N-ammonia and 15O-water are well established MPI radiotracers for quantitative measurement of MBF that have appropriate dosimetry and safety profiles [18,108]. The 18F labelled tracer, flurpiridaz, is in late stages of FDA approval and has potential for being an ideal PET MPI radiotracer. The functional targets, structures and limitations of these radiotracers are shown in Table 4. The use of PET imaging over other imaging modalities for MPI would likely increase with the development of improved PET radiotracers which minimize short-comings.
Table 4.
Myocardial perfusion tracer targets, structures, and limitations
82Rubidium
82Rubidium (82Rb), a short-lived isotope with a half-life of 76 seconds, is the most widely used radiopharmaceutical in clinical practice for MPI with PET. Rb is an alkali metal that has similar physical and chemical properties to potassium and can therefore be used to image potassium transport in vivo [109-111]. A commercially available strontium-82 (82Sr/82Rb) generator can be used to produce 82Rb allowing PET MPI studies to be performed at locations without nearby access to a cyclotron facility [112]. Shelf-life of the generator is 4 to 5 weeks after which a replacement generator is necessary [112]. After administration by intravenous injection, 82Rb crosses the capillary membrane and is taken up from plasma by myocardial cells through active transport [110]. The short half-life of this tracer usually requires that pharmacological protocols are used instead of the exercise for stress testing [113-115].
82Rb has certain limitations that prevent it from being ideal including having the lowest image resolution of the MPI tracers because of its high positron range (8.6 mm) [103]. Myocardial extraction of 82Rb begins to level off at high blood flow levels and the tracer is no longer able to track blood flow [111,116]. This is called the roll-off phenomenon [116,117]. The myocardial extraction fraction of 82Rb is lower than the other PET perfusion tracers at only 65% resulting in a low signal-to-noise ratio between the heart and other tissues [103,118,119].
15O-water
15O-water was one of the first radiotracers developed for MPI. It displays almost perfect first-pass extraction fraction resulting in a linear relationship between tracer uptake and flow at high blood flow rates [120-122]. 15O-water is freely diffusible across cell membranes and capillaries, which allows for high uptake but prevents tracer from being retained in the myocardium [120,123]. For the same reason, it is difficult to decipher between tracer activity in the blood and myocardial tracer uptake since the tracer can easily diffuse into both regions. 15O-water is susceptible to a spillover effect between the left ventricle blood pool and the myocardial wall because of the limited spatial resolution of PET imaging [121]. Tracer from the blood pool spills into the myocardial regions of interest, falsely elevating tissue tracer concentration. Calculations to correct this problem involve the use of an oxygen-15-labeled carbon monoxide scan, prior to administering 15O-water, to label red blood cells that are confined to the vascular space [121,124-126]. This scan is subtracted from the 15O-water images giving measures for MBF free from intervascular activity. An additional limitation is the need for an on-site cyclotron because of its short half-life of approximately 2 minutes. With 15O-water, images have low resolution because of low myocardium-to-background contrast ratios from tracer freely diffusing between the myocardium and blood pool [127,128]. Another downfall due to half-life is the inability to acquire images with physical stress tests because there is not enough time to inject the tracer, exercise and image [129,130]. Regardless of its strengths, 15O-water has not been FDA approved for MPI with PET.
13N-ammonia
13N-ammonia is another well-known PET radiotracer for measuring MPI. Delivery of this tracer from a cyclotron facility to a PET imaging facility is possible because of the 13N radiolabel half-life of 9.97 minutes; although not ideal. 13N-ammonia has a high first pass myocardial extraction of about 80% and is rapidly cleared from the blood [131]. Ammonia (NH3) can quickly diffuse across membranes or be actively transported by the sodium-potassium pump [131,132]. In the myocyte, NH3 has two potential fates: it can back diffuse into the vascular space and blood or it can be trapped in the myocardium when NH3 converts to glutamine. Ideal linear uptake of tracer is noted at low level of blood flow but at higher blood flow this tracer experiences the roll-off phenomenon like 82Rb [133,134]. A shorter positron range (2.5 mm) of 13N enables good quality imaging but physical stress tests are not practical for routine use because of the time interval between tracer injection and start of imaging as well as the acquisition time [21,135,136]. In comparison to the previously discussed MPI tracers, 13N presents numerous characteristics of an ideal radiotracer including its high extraction fraction, relatively long half-life and low background activity [126,131,137].
18F-flurpiridaz
Over the past several years, 18F labelled analogs have been developed to take advantage of the prolonged half-life and improved image resolution for MPI. 18F-flurpiridaz, previously called 18F-BMS-747158-02, is a structural analog of the insecticide pyridaben that was developed to overcome the need for on-site production and cost of generators as with other MPI radiotracers [138]. It is the only 18F-labeled MPI tracer that is currently in Phase 3 of clinical testing and awaiting FDA approval [138,139].
18F-flurpiridaz targets mitochondria and therefore, accumulates in mitochondria-rich tissues like the myocardium. The tracer accumulates in metabolically active cardiomyocytes by inhibiting the mitochondrial complex-1 (MC-1). MC-1, located in the inner mitochondrial membrane, is part of the electron transport chain responsible for the coupling of NADH oxidation and the reduction of ubiquinone, to the generation of a proton gradient which is then used for ATP synthesis. 18F-flurpiridaz binds specifically to the PSST subunit of MC-1, the same binding site as ubiquinone [140]. 18F-flurpiridaz binds reversibly to the site and does not affect the viability of the cell by binding. No toxic side effects result from 18F-flurpiridaz since the overall activity of MC-1 is not inhibited due to the microdoses used for PET imaging. 18F-flurpiridaz exhibits a rapid uptake in active cardiomyocytes and slow washout [138,140,141]. It shows excellent potential for being an accurate myocardial perfusion probe based on its almost linear relationship between MBF and radiotracer uptake [138,141,142].
18F-flurpiridaz produces higher resolution myocardial perfusion images than SPECT tracers or 82Rb resulting in higher diagnostic confidence in interpretation of the images as shown in Figure 1 [143]. The images produced are excellent due to the short positron range and higher specificity and sensitivity to detect known or suspected CAD compared to SPECT imaging [140,143]. 18F-flurpiridaz has several advantages over the other PET MPI tracers including the longer physical half-life of 18F which allows for administration of the radiotracer at the peak of treadmill exercise followed by imaging. Both rest and stress imaging with this tracer can be done within 20-30 minutes whereas with other tracers this is not possible [144-146]. Another advantage is its ability to detect more subtle perfusion defects while having better image contrast due to high uptake in the target tissue (heart) and lower uptake in other tissues like the liver and lungs compared to 13N-ammonia [142,143,145]. This is not only beneficial for producing excellent images, but additionally, the radioactive dose to other tissues is not an issue. Although, the heart-to-background contrast is better than 13N-ammonia, there is still room for improvement. Other 18F-labelled pyridaben analogues are being examined to improve metabolic stability and improve liver clearance [147,148].
Figure 1.
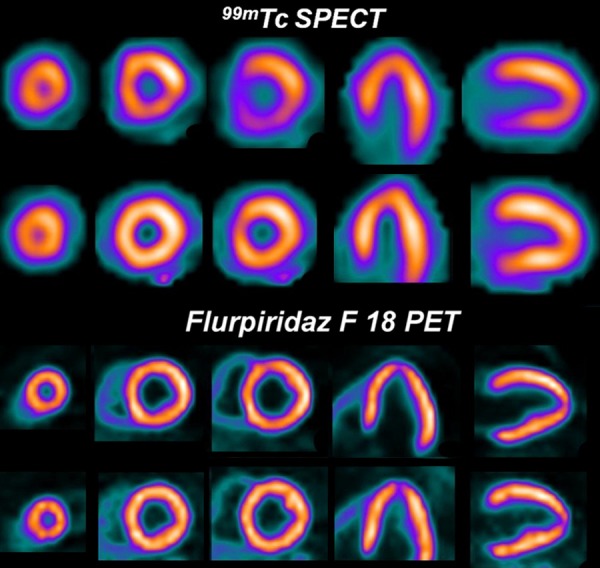
99mTc SPECT images (upper rows) and 18F-flurpiridaz PET images (lower rows) from a patient with normal coronary arteries. A false positive reversible inferior defect is present on the 99mTc SPECT images due to shifting soft-tissue attenuation. The 18F-flurpiridaz PET study, however, provided superior image quality and was normal.
PET imaging of cardiovascular disease
Early detection of both acute and chronic cardiac disease is crucial. Most patients remain undiagnosed as they appear asymptomatic. It is thought to be unnecessary to image asymptomatic patients, which leaves cardiovascular diseases to remain undetected and progress from subclinical to symptomatic disease state. Several metabolic and functional changes occur before structural ones are noticed. However, it would be beneficial to detect subclinical changes prior to development of symptoms for a better prognosis. The following section details a few acute and chronic diseases where PET imaging has been found to be useful for early detection of disease.
Acute disease
Myocarditis
Myocarditis is inflammation of the myocardium caused by viral infections, toxins, drugs, or an activated immune system that attacks host antigens that can affect cardiac muscle function and the cardiac conduction system. There is a wide range of clinical presentations and symptoms that can lead to misdiagnosis [149]. Most patients are asymptomatic and do not seek medical assistance [150]. Myocarditis can progress from acute inflammation to chronic inflammation if the pathogen is not eliminated [150]. If acute myocarditis continues to evolve undetected, it can lead to sudden death, heart failure, or dilated cardiomyopathy [149].
Early metabolic changes associated with myocarditis can be detected with PET. As mentioned earlier, PET scanners frequently come as a hybrid system with CT. Combining PET with CT allows imaging of biological processes with anatomic detail [151]. A study that used 18F-FDG and PET/CT found higher specificity and sensitivity when used within 14 days of onset of suspected acute myocarditis compared to 15-60 days after onset [152]. 18F-FDG focal and diffuse patterns of uptake in the left ventricular posterior wall were indicative of inflammation and correlated with results from endomyocardial biopsy, the gold standard method for detection of inflammation [153-155]. This study was limited by its small sample size and therefore could not conclude the use of 18F-FDG PET alone for diagnosis. 18F-FDG can detect active macrophage and lymphocyte activity indicative of inflammation but its ability to detect myocarditis is not clear. Accuracy of inflammation detection can be improved by combining 18F-FDG PET sensitivity with the high spatial resolution of MRI using a PET/MRI system [155]. However with this, comes the limitations mentioned previously.
68Ga-2-(p-isothiocyanatobenzyl)-1, 4,7-triazacyclononane-1, 4,7-triacetic acid mannosylated human serum albumin (NOTA-MSA) was initially developed as a lymph node PET imaging agent but has potential use for detection of acute myocarditis [156]. Lee et al., used this probe for the detection of myocardial inflammation in a rat model of myocarditis as shown in Figure 2 [157]. This probe targets the mannose receptors on activated infiltrating macrophages. Higher uptake was found in organs with macrophage accumulation including the liver, spleen, bone marrow and myocardium. 68Ga-NOTA-MSA uptake in the left ventricle (LV) was upregulated in myocarditis compared with normal rats. They noted that 68Ga-NOTA-MSA uptake was significantly enhanced earlier in the development of myocarditis before inflammation could be seen on an echocardiograph. The authors suggest 68Ga-NOTA-MSA PET has a potential use for visualizing infiltrating mannose-receptor positive macrophages and early diagnosis of myocarditis. From this study, it is unknown if this tracer specifically targets myocarditis or whether it targets other disease caused by macrophage infiltration like atherosclerosis [157]. It is also unclear if 68Ga-NOTA-MSA binds to mannose receptors on the macrophages only.
Figure 2.
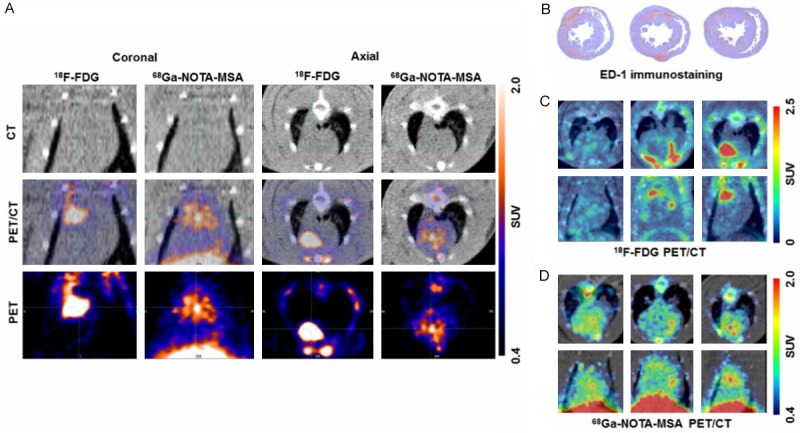
Comparison of 18F-FDG and 68Ga-NOTA-MSA PET for detection of myocardial inflammation in myocarditis. A. The hotspots of myocardial inflammation colocalizes between the 18F-FDG and 68Ga-NOTA-MSA PET scans in some animals. B-D. Different degree of 18F-FDG and 68Ga-NOTA-MSA in spite of the similar degree of inflammatory cell infiltration. B. Similar degree of inflammatory cell infiltration on ED-1 immunostaining. C. Different degree of 18F-FDG uptake between the animals, despite similar degree of inflammatory cell infiltration. D. Similar degree of 68Ga-NOTA-MSA uptake between the animals. SUV, standardized uptake value.
Cardiac sarcoidosis
Cardiac sarcoidosis (CS) is a rare and potentially fatal disease that results from the accumulation of non-caseating granulomas which are a collection of macrophages, lymphocytes and epithelioid cells. The granulomas form around a pathogen or environmental agent to protect the tissue from damage. Sarcoid granulomas can form in the heart and affect the epicardium or pericardium but is most commonly seen in the myocardium. The etiology of CS is still unknown. CS is difficult to detect and underdiagnosed as patients can be asymptomatic [158]. When detected, this disease is associated with poor prognosis but early diagnosis and treatment could improve prognosis.
Known and suspected cases of sarcoidosis are tested for inflammation with 18F-FDG. 18F-FDG PET emerged as a superior technique to 67Ga scintigraphy with higher sensitivity and accuracy for the detection of CS [159-161]. FDG accumulates in sarcoid lesions at sites of macrophage-mediated inflammation where the macrophages are using glucose for energy [90]. Focal or heterogeneous tracer uptake is suggestive of active CS as shown in Figure 3 [162]. Imaging with 18F-FDG has led to many false positives because of the difficulty in deciphering between normal myocyte uptake and inflammatory cell uptake. Patient protocols including prolonged fasting, dietary restrictions and administration of heparin have been put into place in attempt to reduce false positives by suppressing physiological FDG uptake [163,164]. Myocardial 18F-FDG uptake caused by inflammation may not be specific to CS and considered indirect evidence of the presence of CS. Therefore, metabolic PET imaging with FDG is compared to perfusion results.
Figure 3.
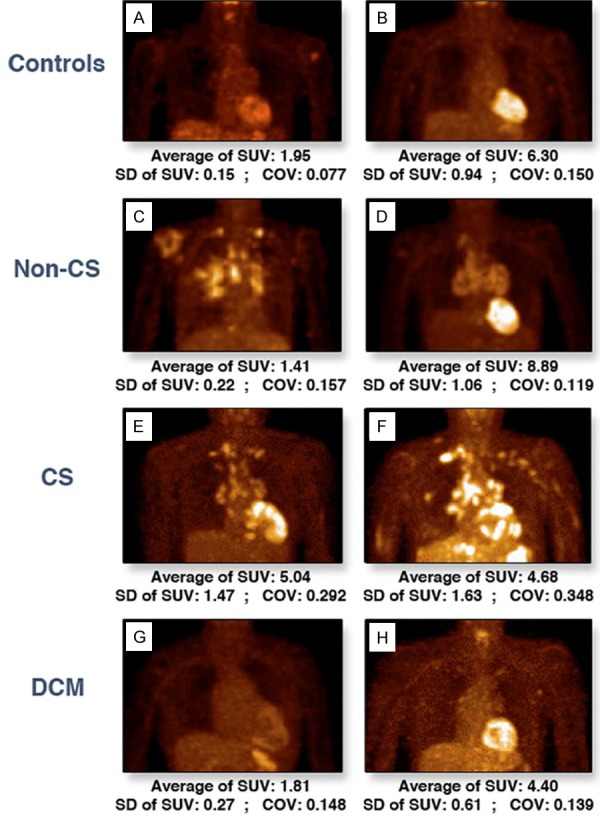
Representative 18F-FDG PET anterior views. The myocardial uptake of 18F-FDG in healthy control subjects (A, B), noncardiac sarcoidosis (C, D), cardiac sarcoidosis (E, F), and dilated cardiomyopathy (G, H) patients. COV = coefficient of variation; CS = cardiac sarcoidosis; DCM = dilated cardiomyopathy; SUV = standardized uptake value.
Perfusion deficits in the myocardium can be detected with 13N-ammonia or 82Rb PET. Comparison of myocardial perfusion images with 18F-FDG PET images allows for differentiation between normal myocardium and scarred myocardium, which can rule out coronary heart disease [165]. Findings can indicate stage of disease progression and if therapy will be useful. Perfusion imaging of tissue viability will detect any damaged tissue by measuring regional MBF at rest and under pharmacological stress.
After detection of CS, steroid therapy is administered but it is only efficient if administered before the development of left ventricular systolic dysfunction and fibrosis [166,167]. Progression of steroid therapy is tracked using 18F-FDG PET [161]. Lower uptake of the tracer is seen when therapy is working to resolve inflammation but also when the disease progresses to fibrosis [90]. Therefore, the images are difficult to analyze and must be used in combination with perfusion imaging.
Chronic disease
CAD
CAD is the obstruction, damage or disease of arteries of the heart. Plaque buildup is characteristic of CAD and can cause narrowing and hardening of the large arteries in the heart slowing down blood flow. The most common cause of blockage is atherosclerosis, which will be discussed later. CAD is also called ischemic heart disease when there is inadequate blood supply to the heart causing ischemia. Prolonged ischemia can result in myocardial infarction or sudden death. PET imaging is used for the diagnosis, prognosis and risk stratification of CAD. A systematic review by Moudi et al. on the diagnosis of CAD by different imaging modalities concluded that PET has high diagnostic value based on sensitivity, specificity and accuracy [168].
PET imaging of MPI is used for detecting obstructive CAD by measuring flow-dependent tracer uptake and tracer accumulation, which is reflective of areas that have reduced blood flow [169]. Regions with decreased tracer uptake may be affected by atherosclerotic lesions or circulatory dysfunction. Visual comparison of stress and rest images may find: (i) normal myocardium which shows uniform radiotracer uptake at stress and rest; (ii) presence of ischemic myocardium where perfusion defects are seen on stress images, but not on rest images; (iii) presence of myocardial scarring where perfusion defects are seen in both stress and rest images [170]. However, there are limitations with visual and semi-quantitative assessment of regional myocardial perfusion defects depending on the radiotracer chosen that may be overcome by absolute quantification with PET [171].
Absolute MBF in milliliters per gram per minute and coronary flow reserve (CFR) can be determined using dynamic imaging and emerge as a superior method to standard stress/rest imaging [172]. Static images alone have high sensitivity and accuracy but dynamic imaging provides additional diagnostic value by expanding the scope of PET to detect subclinical, non-obstructive CAD [11,173-175]. Quantifying MBF or CFR can identify early functional abnormalities in circulation to detect precursors of CAD like early stages of atherosclerosis or microvascular dysfunction [176]. This is advantageous because functional abnormalities occur prior to structural alterations of the arterial wall commonly looked at for diagnosis [177]. MBF responses to vasomotor stress with the use of cold pressor testing or adenosine can be used for the identification of circulatory dysfunction and monitoring pharmacologic interventions or lifestyle modification whereas CFR has prognostic value [171,172,178]. CFR decreases in proportion to the degree of stenosis severity. With low-grade or absence of coronary stenosis, a reduced CFR reflects dysfunction of coronary microcirculation [179]. Patients with preserved CFR have a good prognosis for up to 3 years [176]. When perfusion defects appear, reduced CFR and LV dysfunction are linked to worse outcomes like adverse cardiac events and cardiac death [176,180]. LV ejection fraction reserve has been shown to have prognostic value as well [181]. Cardiac PET with 14N-ammonia or 82Rubidium can assess regional MBF of the LV [171]. In subclinical CAD, there may be heterogeneous relative myocardial uptake of radiotracer or impaired hyperemic blood flow of the left ventricular myocardium [171]. There is great clinical potential when measuring MBF and CFR but there are several limitations that need to be recognized. Cut-off values or reference values for absolute perfusion need to be studied for various tracers and more information is needed about expected MBF and CFR values in different subpopulations.
Metabolic imaging of myocardial ischemia can be used in patients with known or suspected CAD to measure tissue viability. Obstruction of coronary arteries slows down or blocks blood flow in the heart causing ischemia. Under mild to moderate myocardial ischemia, oxygen deprivation leads to a decline in fatty acid oxidation while glucose metabolism increases [182]. This substrate switch ensures the continuation of energy production and cell survival. If there is no metabolic adaptation or ischemia is sustained and severe, cell death occurs by either apoptosis or necrosis [183]. With PET, 18F-FDG uptake reflects metabolically active and viable myocardium capable of glucose utilization. 11C-acetate can determine the amount of oxidative phosphorylation in cardiomyocytes and predict functional recovery after revascularization [184]. Depending on which myocardial metabolic pathways are still intact, viable myocardium can be detected and patients who would benefit from revascularization can be identified.
Atherosclerosis
Atherosclerosis is a disease of the arteries where accumulation of lipids and fibrous elements build up creating plaques (lesions). It begins with vascular endothelial dysfunction and can lead to heart disease and stroke if the plaque ruptures. Atherosclerosis develops over years, with a long subclinical period and can be the primary cause of coronary artery disease. Metabolic disorders also contribute to the development of atherosclerosis (discussed below) [185,186]. As detailed by Evans et al. (2016), PET has several roles in assessing and monitoring atherosclerosis including detecting and quantifying inflammation in plaque by macrophages, detecting destabilizing plaque, and monitoring progression of therapy [5,187,188]. Inflammation, characterized by the recruitment of pro- and anti-inflammatory macrophages, and phagocytic cells to sites of injury or infection, is present at all stages of atherosclerosis from early plaque development to progression and rupture. Atherosclerosis has a long subclinical period where detection of underlying factors including inflammation, hypoxia and apoptosis would be useful before they develop into severe cases of disease.
PET imaging with 18F-FDG has been used to assess inflammation caused by atherosclerosis as areas of inflammation are abundant in macrophages that have increased glycolytic activity and therefore have higher uptake of FDG. This provides improved specificity for determining local inflammation compared to measuring circulating biomarkers. However, 18F-FDG is a non-specific marker for inflammation in atherosclerosis. Several other PET tracers have been evaluated for specific uptake that may be better suited for cardiac imaging of atherosclerosis including SSTR ligands and translocator protein ligands [189,190]. These probes focus on identification of atherosclerosis after the subclinical period targeting biomarkers of vulnerable plaque to identify them prior to rupture.
Microcalcification occurs in response to inflammation and can cause increased mechanical wall stress and microfractures that lead to rupture of plaque. It can also be an indicator of accelerated atherosclerosis [191]. In atherosclerotic plaque, macrophages promote osteogenesis causing vascular calcification [192]. 18F-NaF accumulation may be identifiable in areas of microcalcification in coronary arteries before structural coronary artery calcification in the advanced stages of CAD detected with CT [192-194]. Uptake of this tracer in the heart is low but early detection and quantification of molecular changes in atherosclerotic plaque have been noted in the heart, specifically the aorta [194]. Similarly, 18F-fluoride accumulates in regions of active microcalcification observed during the early stages of plaque formation. It has shown promising characteristics as a probe for detecting vulnerable plaque with highly specific accumulation, low background activity, and easy accessibility [194].
Hypoxia has known involvement in atherogenesis and has led to the development of PET probes for molecular imaging of hypoxia in plaque [195]. Hypoxia may be indicative of vulnerable plaques. There is limited literature on PET imaging of atherosclerotic hypoxia in humans. However, 18F-fluoromisonidazole (FMISO) has been used to identify hypoxia in advanced atherosclerotic plaque in rabbits [39]. Regions with known atherosclerotic plaque had higher uptake than normal regions. Further investigation is needed into the potential usefulness of this probe in humans.
Cardiac myocyte apoptosis is an underlying factor in the development and progression of atherosclerosis [196,197]. Early identification of apoptosis can prevent scar tissue build up and reduce loss of heart function. There are two pathways that lead to apoptosis: extrinsic or intrinsic [198]. Annexin V is a protein in the intrinsic pathway of apoptosis that binds with high affinity to phosphatidylserine present on the surface of apoptotic cells [199,200]. Novel 18F labeled peptides that target protein Annexin V have been continuously developed in an attempt to improve retention and tissue uptake compared to background uptake and blood [200-202]. These tracers that target Annexin V bind not only to apoptotic cells but necrotic cells as well requiring additional testing [200]. There is a need for the development of a radiotracer that can be used with PET for specific, accurate and early identification of apoptosis.
Impaired glucose metabolism
Metabolic abnormalities like impaired glucose metabolism are associated with increased risk of cardiac pathology. Impaired glucose tolerance (IGT) and insulin resistance (IR) can lead to the development of type 2 diabetes mellitus (DM) and metabolic syndrome. IGT is a state of hyperglycemia that can increase the risk of cardiovascular complications [203]. There appears to be a two-way relationship between impaired glucose metabolism and adverse cardiac events [204]. Impaired glucose metabolism is a risk factor for cardiovascular disease while in some cases, heart failure occurs first followed by impaired glucose metabolism that eventually leads to the development of type 2 DM [203,204]. Abnormal glucose metabolism often goes undetected if individuals have no symptoms [204]. PET imaging has been used to detect subclinical metabolic changes and changes in blood flow in the heart before advanced stages of disease.
Patients with IGT or type 2 DM have been found to be at an increased risk for development of atherosclerosis. Mechanisms between atherosclerosis and type 2 DM are unknown but atherosclerosis remains a major cause of morbidity and mortality in the diabetic population [205,206]. As mentioned in the previous section, an early sign of atherosclerosis is vascular inflammation, which can be detected and quantified by 18F-FDG with PET imaging in diabetic and IGT patients [205,207]. Similarly, 18F-FDG has been used to identify inflammation involved in carotid atherosclerosis in patients with metabolic syndrome [208]. 18F-FDG has also been used to track therapeutic benefits of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist [209,210]. Pioglitazone is used as a treatment for type 2 DM to improve IR but may also be useful in reducing atherosclerotic inflammation [209,211,212]. A study by Nitta et al. suggests that pioglitazone may protect against cardiac events in patients with IGT or type 2 DM by suppressing coronary inflammation [210].
Changes in cardiac metabolism towards fatty acid metabolism may also contribute to the development of heart disease. Abnormal fatty acid metabolism plays a role in the development of IR and disruption of glucose homeostasis [213]. PET imaging with 11C-palmitate has been used to assess myocardial fatty acid metabolism in the heart in individuals with type 2 DM and IGT [214,215]. More recently, a positron-emitting fatty acid analog, 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA), was developed and used to image and assess organ-specific partitioning of dietary fatty acids with PET/CT [216]. The tracer is administered orally with a meal and becomes trapped in the heart mitochondria after undergoing the initial steps of beta-oxidation. In rats and humans with IGT, an increase in myocardial dietary fatty acid uptake was noted [217-219]. It has been suggested that increased myocardial uptake of dietary fatty acids may be an early marker or potential contributor to myocardial dysfunction associated with prediabetes and diabetes [217]. Increased myocardial fatty acid partitioning is also associated with early impairment in left ventricular systolic and diastolic functions plus increased cardiac oxidative metabolism suggesting a possible role in the development of metabolic cardiomyopathy [218].
Patients with IGT have significantly reduced left ventricular stroke volume and ejection fraction and tend to display impaired diastolic function, as assessed by PET ventriculography all of which are related to diabetic cardiomyopathy. Early left ventricular function impairment and increased myocardial oxidative metabolism have been found to be associated with excessive myocardial partitioning of dietary fatty acids [218]. PET imaging with 11C-acetate has shown that patients with impaired glucose metabolism have an increase in myocardial oxygen consumption [218]. Additionally, PET has been used to identify coronary dysfunction by measuring MBF in patients with impaired glucose metabolism [214,220,221]. Coronary circulatory function was also found to decrease as severity of IR and carbohydrate metabolism increase [220].
Inflammation tracer development
With any type of molecular imaging, there is importance in identifying and quantifying disease before it advances to the point that therapeutic intervention is no longer effective. This article has touched on a variety of PET tracers used for detecting cardiac diseases. Detecting inflammation is common amongst the diseases mentioned. 18F-FDG is used widely to measure inflammation by tracking macrophage glycolytic activity; however, it has several limitations that hinder its ability to accurately detect activated macrophages. The lack of specificity allows this probe to target other immune cells as well. A recently published article briefly mentions alternative radiotracers and targets to 18F-FDG that are currently in development for specific cardiac diseases [222]. Select radiotracers that have tested in animals and those with potential to be developed to image inflammation in the heart are reviewed here in more detail.
68Ga-NODAGA-RGD is a peptide tracer targeting integrin αvβ3 expressed on macrophages and myofibroblasts involved in extracellular matrix remodeling. It was first used to study αvβ3 expressed on activated endothelial cells involved in tumor growth, invasiveness, and metastasis [223,224]. 68Ga-NODAGA-RGD was tested in pigs with induced flow-limiting coronary stenosis to study the effects of flow-limiting coronary stenosis on expression of integrin αvβ3 [225]. The study compared αvβ3 expression in ischemic versus non-ischemic myocardium and viable versus injured ischemic myocardial areas two weeks after induction of coronary stenosis in pigs. 68Ga-NODAGA-RGD PET imaging was used in combination with 15O-water perfusion PET, and histological evaluation of myocardial injury to compare αvβ3 expression in ischemic versus non-ischemic myocardium and viable versus injured ischemic myocardial areas two weeks after induction of coronary stenosis in pigs. The tracer localized in irreversibly injured myocardium, and not viable myocardium. 68Ga-NODAGA-RGD may be useful for the identification of αvβ3 integrin activation associated with repair of myocardial injury.
18F-Macroflor is polyglucose nanoparticle that has been shrunken in size to allow rapid renal excretion. Nanoparticles are internalized by phagocytic myeloid cells suggesting a promising strategy for imaging cardiac macrophages. 18F-Macroflor, using integrated PET imaging, was tested in mice, rabbits, and a non-human primate and found to have high affinity for macrophages residing in cardiovascular organs [226]. 18F-Macroflor undergoes rapid clearance from blood pool (6.5 minutes in mice, 22 minutes in rabbits, 21.7 minutes in non-human primates) into macrophages with time to be imaged before the tracer is excreted. Uptake was significantly increased in mice with atherosclerosis imaged with PET/CT. When compared to 18F-FDG uptake in rabbits with atherosclerosis, there was an overlap between the tracers. However, 18F-Macroflor had negligible myocyte uptake which is beneficial for cardiac inflammation imaging. Increased specificity for macrophages and rapid pharmacokinetics suggest 18F-Macroflor is a promising cardiac inflammation imaging radiotracer but further research is required in non-human primates prior to human studies.
A promising alternative has been developed that may be more specific in targeting inflammation than 18F-FDG. Phenix et al. developed a method for synthesizing an analogue of 18F-FDG using an enzymatic approach for tagging acid β-glucocerebrosidase (GCase), a recombinant enzyme formulated in commercial Cerezyme which is used to treat Gaucher disease [227]. 18F-Cerezyme was obtained in a total synthesis time of about 2.5 hours and is derived from 18F-FDG. Phenix et al. provides animal testing of this tracer in which they examined the biodistribution of GCase in mice. 18F-Cerezyme has high affinity for mannose receptors on macrophages and showed the highest uptake in macrophage-rich organs (e.g., liver and spleen). Uptake was also seen in the gall bladder, kidneys, intestines, heart, and femur. The biodistribution and PET imaging studies on animals suggest that 18F-Cerezyme is a novel powerful tool for monitoring enzyme distribution. The study concluded that the 18F-labeling method could be adapted for use of alternative enzymes, and create opportunities for application to a variety of enzyme replacement therapies. However, ability to detect macrophage infiltration in cardiac tissue could be utilized as a more specific alternative to 18F-FDG to detect inflammation. Thus, this radiotracer appears promising but requires further investigation.
Developing a PET probe that targets enzyme activity of macrophages or subpopulations of macrophages, like the pro-inflammatory response of M1 or the anti-inflammatory response of M2, will improve the diagnostic capabilities of PET imaging. In reviews by Ali et al. and Quillard et al., the targeting of macrophage enzymatic activity of proteases that participate in extracellular matrix remodeling are discussed. Specifically, metalloproteinases and cysteine cathepsins, as well as targeting myeloperoxidase activity of macrophages [228,229]. Metalloproteinases switch from M1 to M2 macrophages with disease progression leading to difficulties in choosing a target subpopulation unless the time of disease onset is known [230]. Cathepsins K, S, L, and B are suggested to play a role in cardiovascular disease but some cysteine cathepsins like B, L and H are expressed in most immune cells and tissues [231,232]. One study suggests cathepsins Z and F may be exclusive to macrophages after comparison to dendritic cells and splenocytes and are involved in antigen processing and presentation [233]. However, a review by Riese et al., classifies both cathepsin F and Z as having widespread distribution [234].
Both cysteine cathepsins and MMP’s have potential to differentiate between macrophages and other immune cells with PET imaging but no tracer exists that has high specificity for macrophage subpopulations and since myeloperoxidase is secreted by both neutrophils and macrophages it is not the best option if looking for inflammation caused only by macrophages. Protease imaging utilizes small molecules that bind to specific pockets usually within the active site of the protein. Alternatively, radiotracers can be mimics of protease substrates that are hydrolyzed by the enzyme. An enzyme targeting tracer that becomes trapped within the active site of an enzyme be of value for macrophage-specific imaging. Imaging of macrophage enzyme activity holds promise for cardiac inflammation detection but requires further research and refinement before use in the clinical settings for reliable diagnosis and prognosis of cardiac disease.
Disclosure of conflict of interest
None.
References
- 1.Schelbert HR. Positron emission tomography measurements of myocardial blood flow: assessing coronary circulatory function and clinical implications. Heart Br Card Soc. 2012;98:592–600. doi: 10.1136/heartjnl-2011-300790. [DOI] [PubMed] [Google Scholar]
- 2.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 3.Osterholt M, Sen S, Dilsizian V, Taegtmeyer H. Targeted metabolic imaging to improve the management of heart disease. JACC Cardiovasc Imaging. 2012;5:214–226. doi: 10.1016/j.jcmg.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging. 2010;3:211–222. doi: 10.1161/CIRCIMAGING.109.860593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans NR, Tarkin JM, Chowdhury MM, Warburton EA, Rudd JH. PET imaging of atherosclerotic disease: advancing plaque assessment from anatomy to pathophysiology. Curr Atheroscler Rep. 2016;18:30. doi: 10.1007/s11883-016-0584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, Starks LT, Martin-Garrido A, Manavaki R, Yu E, Kuc RE, Grassi L, Kreuzhuber R, Kostadima MA, Frontini M, Kirkpatrick PJ, Coughlin PA, Gopalan D, Fryer TD, Buscombe JR, Groves AM, Ouwehand WH, Bennett MR, Warburton EA, Davenport AP, Rudd JH. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F] FDG PET imaging. J Am Coll Cardiol. 2017;69:1774–1791. doi: 10.1016/j.jacc.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, Van Decker WA, Yakovlevitch M. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol. 2010;17:709–718. doi: 10.1007/s12350-010-9244-0. [DOI] [PubMed] [Google Scholar]
- 8.Timothy M Bateman, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, Hertenstein GK, Moutray KL, Reid K, Cullom SJ. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Blankenstein J, McArdle B, Small G, Guo A, Garrard L, Chow B, Ruddy T, Wells G, Beanlands R, deKemp R. Reduced rate of diagnostic coronary imaging following rubidium PET vs thallium SPECT, as alternatives to technetium SPECT myocardial perfusion imaging. J Nucl Med. 2013;54(Suppl 2):1735. [Google Scholar]
- 10.Merhige ME, Breen WJ, Shelton V, Houston T, D’Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and (82)Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med. 2007;48:1069–1076. doi: 10.2967/jnumed.106.038323. [DOI] [PubMed] [Google Scholar]
- 11.Mylonas I, Kazmi M, Fuller L, deKemp RA, Yam Y, Chen L, Beanlands RS, Chow BJ. Measuring coronary artery calcification using positron emission tomography-computed tomography attenuation correction images. Eur Heart J Cardiovasc Imaging. 2012;13:786–792. doi: 10.1093/ehjci/jes079. [DOI] [PubMed] [Google Scholar]
- 12.Namdar M, Hany TF, Koepfli P, Siegrist PT, Burger C, Wyss CA, Luscher TF, von Schulthess GK, Kaufmann PA. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med. 2005;46:930–935. [PubMed] [Google Scholar]
- 13.Leide-Svegborn S. Radiation exposure of patients and personnel from a PET/CT procedure with 18F-FDG. Radiat Prot Dosimetry. 2010;139:208–213. doi: 10.1093/rpd/ncq026. [DOI] [PubMed] [Google Scholar]
- 14.Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, Heusch P, Nassenstein K, Bockisch A, Forsting M, Schlosser T. Hybrid PET/MR imaging of the heart: feasibility and initial results. Radiology. 2013;268:366–373. doi: 10.1148/radiol.13130231. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Chen X. Design and development of molecular imaging probes. Curr Top Med Chem. 2010;10:1227–1236. doi: 10.2174/156802610791384225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alva-Sánchez H, Quintana-Bautista C, Martínez-Dávalos A, Ávila-Rodríguez MA, Rodríguez-Villafuerte M. Positron range in tissue-equivalent materials: experimental microPET studies. Phys Med Biol. 2016;61:6307–6321. doi: 10.1088/0031-9155/61/17/6307. [DOI] [PubMed] [Google Scholar]
- 17.Phelps ME, Hoffman EJ, Huang SC, Ter-Pogossian MM. Effect of positron range on spatial resolution. J Nucl Med. 1975;16:649–652. [PubMed] [Google Scholar]
- 18.Senthamizhchelvan S, Bravo PE, Esaias C, Lodge MA, Merrill J, Hobbs RF, Sgouros G, Bengel FM. Human biodistribution and radiation dosimetry of 82Rb. J Nucl Med. 2010;51:1592–1599. doi: 10.2967/jnumed.110.077669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaapen P, de Haan S, Hoekstra OS, Halbmeijer R, Appelman YE, Groothuis JG, Comans EF, Meijerink MR, Lammertsma AA, Lubberink M, Götte MJ, van Rossum AC. Cardiac PET-CT: advanced hybrid imaging for the detection of coronary artery disease. Neth Heart J. 2010;18:90–98. doi: 10.1007/BF03091744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krivokapich J, Smith GT, Huang SC, Hoffman EJ, Ratib O, Phelps ME, Schelbert HR. 13N ammonia myocardial imaging at rest and with exercise in normal volunteers. Quantification of absolute myocardial perfusion with dynamic positron emission tomography. Circulation. 1989;80:1328–1337. doi: 10.1161/01.cir.80.5.1328. [DOI] [PubMed] [Google Scholar]
- 21.Chow BJ, Beanlands RS, Lee A, DaSilva JN, deKemp RA, Alkahtani A, Ruddy TD. Treadmill exercise produces larger perfusion defects than dipyridamole stress N-13 ammonia positron emission tomography. J Am Coll Cardiol. 2006;47:411–416. doi: 10.1016/j.jacc.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Abramson BL, Ruddy TD, deKemp RA, Laramee LA, Marquis JF, Beanlands RS. Stress perfusion/metabolism imaging: a pilot study for a potential new approach to the diagnosis of coronary disease in women. J Nucl Cardiol. 2000;7:205–212. doi: 10.1016/s1071-3581(00)70008-0. [DOI] [PubMed] [Google Scholar]
- 23.Wyss CA, Koepfli P, Mikolajczyk K, Burger C, von Schulthess GK, Kaufmann PA. Bicycle exercise stress in PET for assessment of coronary flow reserve: repeatability and comparison with adenosine stress. J Nucl Med. 2003;44:146–154. [PubMed] [Google Scholar]
- 24.Botvinick EH. Current methods of pharmacologic stress testing and the potential advantages of new agents. J Nucl Med Technol. 2009;37:14–25. doi: 10.2967/jnmt.108.057802. [DOI] [PubMed] [Google Scholar]
- 25.Geleijnse ML, Elhendy A, Fioretti PM, Roelandt JR. Dobutamine stress myocardial perfusion imaging. J Am Coll Cardiol. 2000;36:2017–2027. doi: 10.1016/s0735-1097(00)01012-3. [DOI] [PubMed] [Google Scholar]
- 26.Leppo JA. Dipyridamole myocardial perfusion imaging. J Nucl Med. 1994;35:730–733. [PubMed] [Google Scholar]
- 27.Bateman TM. Cardiac positron emission tomography and the role of adenosine pharmacologic stress. Am J Cardiol. 2004;94:19D–24D. doi: 10.1016/j.amjcard.2004.04.013. discussion 24D-25D. [DOI] [PubMed] [Google Scholar]
- 28.Buhr C, Gössl M, Erbel R, Eggebrecht H. Regadenoson in the detection of coronary artery disease. Vasc Health Risk Manag. 2008;4:337–340. doi: 10.2147/vhrm.s1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164:1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med. 2007;37:451–461. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Hirata K, Yamaguchi S, Terasaka S, Shiga T, Houkin K. Usefulness of FMISO-PET for glioma analysis. Neurol Med Chir (Tokyo) 2013;53:773–778. doi: 10.2176/nmc.ra2013-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handley MG, Medina RA, Mariotti E, Kenny GD, Shaw KP, Yan R, Eykyn TR, Blower PJ, Southworth R. Cardiac hypoxia imaging: second-generation analogues of 64Cu-ATSM. J Nucl Med. 2014;55:488–494. doi: 10.2967/jnumed.113.129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitmore GF, Varghese AJ. The biological properties of reduced nitroheterocyclics and possible underlying biochemical mechanisms. Biochem Pharmacol. 1986;35:97–103. doi: 10.1016/0006-2952(86)90565-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, Podoloff DA. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology. 1995;194:795–800. doi: 10.1148/radiology.194.3.7862981. [DOI] [PubMed] [Google Scholar]
- 35.Arabi M, Piert M. Hypoxia PET/CT imaging: implications for radiation oncology. Q J Nucl Med Mol Imaging. 2010;54:500–509. [PubMed] [Google Scholar]
- 36.Troost EG, Laverman P, Kaanders JH, Philippens M, Lok J, Oyen WJ, van der Kogel AJ, Boerman OC, Bussink J. Imaging hypoxia after oxygenation-modification: comparing [18F] FMISO autoradiography with pimonidazole immunohistochemistry in human xenograft tumors. Radiother Oncol. 2006;80:157–164. doi: 10.1016/j.radonc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–280. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 38.Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011;11:167. doi: 10.1186/1471-2407-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using 18F-fluoromisonidazole positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014;7:312–320. doi: 10.1161/CIRCIMAGING.113.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurens E, Yeoh SD, Rigopoulos A, Cao D, Cartwright GA, O’Keefe GJ, Tochon-Danguy HJ, White JM, Scott AM, Ackermann U. Radiolabelling and evaluation of a novel sulfoxide as a PET imaging agent for tumor hypoxia. Nucl Med Biol. 2014;41:419–425. doi: 10.1016/j.nucmedbio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Martin GV, Caldwell JH, Graham MM, Grierson JR, Kroll K, Cowan MJ, Lewellen TK, Rasey JS, Casciari JJ, Krohn KA. Noninvasive detection of hypoxic myocardium using fluorine-18-fluoromisonidazole and positron emission tomography. J Nucl Med. 1992;33:2202–2208. [PubMed] [Google Scholar]
- 42.Caldwell JH, Revenaugh JR, Martin GV, Johnson PM, Rasey JS, Krohn KA. Comparison of fluorine-18-fluorodeoxyglucose and tritiated fluoromisonidazole uptake during low-flow ischemia. J Nucl Med. 1995;36:1633–1638. [PubMed] [Google Scholar]
- 43.Takasawa M, Moustafa RR, Baron JC. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke. 2008;39:1629–1637. doi: 10.1161/STROKEAHA.107.485938. [DOI] [PubMed] [Google Scholar]
- 44.Silvola JM, Saraste A, Forsback S, Laine VJ, Saukko P, Heinonen SE, Ylä-Herttuala S, Roivainen A, Knuuti J. Detection of hypoxia by [18F] EF5 in atherosclerotic plaques in mice. Arterioscler Thromb Vasc Biol. 2011;31:1011–1015. doi: 10.1161/ATVBAHA.110.221440. [DOI] [PubMed] [Google Scholar]
- 45.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, Dubois L, Lambin P. A comparative study of the hypoxia PET tracers [18F] HX4, [18F] FAZA, and [18F] FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys. 2015;91:351–359. doi: 10.1016/j.ijrobp.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Wack LJ, Mönnich D, van Elmpt W, Zegers CM, Troost EG, Zips D, Thorwarth D. Comparison of [18F] -FMISO, [18F] -FAZA and [18F] -HX4 for PET imaging of hypoxia--a simulation study. Acta Oncol Stockh Swed. 2015;54:1370–1377. doi: 10.3109/0284186X.2015.1067721. [DOI] [PubMed] [Google Scholar]
- 47.Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, Hicks RJ. Imaging of tumor hypoxia with [124I] IAZA in comparison with [18F] FMISO and [18F] FAZA--first small animal PET results. J Pharm Pharm Sci. 2007;10:203–211. [PubMed] [Google Scholar]
- 48.Li L, Hu M, Zhu H, Zhao W, Yang G, Yu J. Comparison of 18F-Fluoroerythronitroimidazole and 18F-fluorodeoxyglucose positron emission tomography and prognostic value in locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2010;11:335–340. doi: 10.3816/CLC.2010.n.042. [DOI] [PubMed] [Google Scholar]
- 49.van der Valk FM, Sluimer JC, Vöö SA, Verberne HJ, Nederveen AJ, Windhorst AD, Stroes ES, Lambin P, Daemen MJ. In vivo imaging of hypoxia in atherosclerotic plaques in humans. JACC Cardiovasc Imaging. 2015;8:1340–1341. doi: 10.1016/j.jcmg.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Bourgeois M, Rajerison H, Guerard F, Mougin-Degraef M, Barbet J, Michel N, Cherel M, Faivre-Chauvet A. Contribution of [64Cu] -ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F] -MISO--a selected review. Nucl Med Rev Cent East Eur. 2011;14:90–95. doi: 10.5603/nmr.2011.00022. [DOI] [PubMed] [Google Scholar]
- 51.Vāvere AL, Lewis JS. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans Camb Engl 2003. 2007:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 52.Lewis JS, Herrero P, Sharp TL, Engelbach JA, Fujibayashi Y, Laforest R, Kovacs A, Gropler RJ, Welch MJ. Delineation of hypoxia in canine myocardium using PET and copper(II)-diacetyl-bis(N(4)-methylthiosemicarbazone) J Nucl Med. 2002;43:1557–1569. [PubMed] [Google Scholar]
- 53.Obata A, Kasamatsu S, McCarthy DW, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. Production of therapeutic quantities of (64)Cu using a 12 MeV cyclotron. Nucl Med Biol. 2003;30:535–539. doi: 10.1016/s0969-8051(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 54.Dearling JL, Packard AB. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl Med Biol. 2010;37:237–243. doi: 10.1016/j.nucmedbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Holland JP, Lewis JS, Dehdashti F. Assessing tumor hypoxia by positron emission tomography with Cu-ATSM. Q J Nucl Med Mol Imaging. 2009;53:193–200. [PMC free article] [PubMed] [Google Scholar]
- 56.Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ. Copper bis (thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem. 2002;7:249–259. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 57.Dearling JL, Lewis JS, Mullen GE, Rae MT, Zweit J, Blower PJ. Design of hypoxia-targeting radiopharmaceuticals: selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur J Nucl Med. 1998;25:788–792. doi: 10.1007/s002590050283. [DOI] [PubMed] [Google Scholar]
- 58.Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GE. Studies on the mechanism of hypoxic selectivity in copper bis (thiosemicarbazone) radiopharmaceuticals. J Med Chem. 2002;45:1420–1431. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi N, Fujibayashi Y, Yonekura Y, Welch MJ, Waki A, Tsuchida T, Sadato N, Sugimoto K, Nakano A, Lee JD, Itoh H. Copper-62 ATSM as a hypoxic tissue tracer in myocardial ischemia. Ann Nucl Med. 2001;15:293–296. doi: 10.1007/BF02987849. [DOI] [PubMed] [Google Scholar]
- 60.Medina RA, Mariotti E, Pavlovic D, Shaw KP, Eykyn TR, Blower PJ, Southworth R. 64Cu-CTS: a promising radiopharmaceutical for the identification of low-grade cardiac hypoxia by PET. J Nucl Med. 2015;56:921–926. doi: 10.2967/jnumed.114.148353. [DOI] [PubMed] [Google Scholar]
- 61.Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- 62.Brosius FC, Liu Y, Nguyen N, Sun D, Bartlett J, Schwaiger M. Persistent myocardial ischemia increases GLUT1 glucose transporter expression in both ischemic and non-ischemic heart regions. J Mol Cell Cardiol. 1997;29:1675–1685. doi: 10.1006/jmcc.1997.0405. [DOI] [PubMed] [Google Scholar]
- 63.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 64.Kaim AH, Weber B, Kurrer MO, Gottschalk J, Von Schulthess GK, Buck A. Autoradiographic quantification of 18F-FDG uptake in experimental soft-tissue abscesses in rats. Radiology. 2002;223:446–451. doi: 10.1148/radiol.2232010914. [DOI] [PubMed] [Google Scholar]
- 65.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35:104–112. [PubMed] [Google Scholar]
- 66.Deichen JT, Prante O, Gack M, Schmiedehausen K, Kuwert T. Uptake of [18F] fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging. 2003;30:267–273. doi: 10.1007/s00259-002-1018-8. [DOI] [PubMed] [Google Scholar]
- 67.Randle PJ, England PJ, Denton RM. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970;117:677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein LJ, Visser FC, Nurmohamed SA, Vink A, Peters JH, Knaapen P, Kruijer PS, Herscheid JD, Teule GJ, Visser CA. Feasibility of planar myocardial carbon 11-acetate imaging. J Nucl Cardiol. 2000;7:221–227. doi: 10.1016/s1071-3581(00)70010-9. [DOI] [PubMed] [Google Scholar]
- 69.Gropler RJ, Siegel BA, Geltman EM. Myocardial uptake of carbon-11-acetate as an indirect estimate of regional myocardial blood flow. J Nucl Med. 1991;32:245–251. [PubMed] [Google Scholar]
- 70.Lear JL. Relationship between myocardial clearance rates of carbon-11-acetate-derived radiolabel and oxidative metabolism: physiologic basis and clinical significance. J Nucl Med. 1991;32:1957–1960. [PubMed] [Google Scholar]
- 71.Brown M, Marshall DR, Sobel BE, Bergmann SR. Delineation of myocardial oxygen utilization with carbon-11-labeled acetate. Circulation. 1987;76:687–696. doi: 10.1161/01.cir.76.3.687. [DOI] [PubMed] [Google Scholar]
- 72.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of PET with (11)C-acetate. Am J Nucl Med Mol Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 73.Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, Kofoed KF, Weismueller S, Czernin J, Phelps ME, Schelbert HR. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11] acetate. J Nucl Med. 1998;39:272–280. [PubMed] [Google Scholar]
- 74.Wolpers HG, Burchert W, van den Hoff J, Weinhardt R, Meyer GJ, Lichtlen PR. Assessment of myocardial viability by use of 11C-acetate and positron emission tomography. Threshold criteria of reversible dysfunction. Circulation. 1997;95:1417–1424. doi: 10.1161/01.cir.95.6.1417. [DOI] [PubMed] [Google Scholar]
- 75.Timmer SA, Lubberink M, Germans T, Götte MJ, ten Berg JM, ten Cate FJ, van Rossum AC, Lammertsma AA, Knaapen P. Potential of [11C] acetate for measuring myocardial blood flow: studies in normal subjects and patients with hypertrophic cardiomyopathy. J Nucl Cardiol. 2010;17:264–275. doi: 10.1007/s12350-009-9181-y. [DOI] [PubMed] [Google Scholar]
- 76.Sörensen J, Valind S, Andersson LG. Simultaneous quantification of myocardial perfusion, oxidative metabolism, cardiac efficiency and pump function at rest and during supine bicycle exercise using 1-11C-acetate PET--a pilot study. Clin Physiol Funct Imaging. 2010;30:279–284. doi: 10.1111/j.1475-097X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 77.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 78.Kisrieva-Ware Z, Coggan AR, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial triglyceride oxidation with PET and 11C-palmitate. J Nucl Cardiol. 2009;16:411–421. doi: 10.1007/s12350-009-9051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamaki N, Fujibayashi Y, Magata Y, Yonekura Y, Konishi J. Radionuclide assessment of myocardial fatty acid metabolism by PET and SPECT. J Nucl Cardiol. 1995;2:256–266. doi: 10.1016/s1071-3581(05)80063-7. [DOI] [PubMed] [Google Scholar]
- 80.Tamaki N, Morita K, Kuge Y, Tsukamoto E. The role of fatty acids in cardiac imaging. J Nucl Med. 2000;41:1525–1534. [PubMed] [Google Scholar]
- 81.Fox KA, Abendschein DR, Ambos HD, Sobel BE, Bergmann SR. Efflux of metabolized and nonmetabolized fatty acid from canine myocardium. Implications for quantifying myocardial metabolism tomographically. Circ Res. 1985;57:232–243. doi: 10.1161/01.res.57.2.232. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Huang T, Zhang X, Zhong M, Walker NN, He J, Berr SS, Keller SR, Kundu BK. Determination of fatty acid metabolism with dynamic [11C] Palmitate positron emission tomography of mouse heart in vivo. Mol Imaging. 2015;14:516–525. [PMC free article] [PubMed] [Google Scholar]
- 83.Nuyts H, Maes A, Vrolix M, Schiepers C, Schelbert H, Kuhle W, Bormans G, Poppe G, Buxton D, Suetens P, De Geest H, Mortelmans L. Three-dimensional correction for spillover and recovery of myocardial PET images. J Nucl Med. 1996;37:767–774. [PubMed] [Google Scholar]
- 84.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- 86.Iozzo P, Geisler F, Oikonen V, Mäki M, Takala T, Solin O, Ferrannini E, Knuuti J, Nuutila P 18F-FDG PET Study. Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET study. J Nucl Med. 2003;44:682–689. [PubMed] [Google Scholar]
- 87.Garbarino S, Vivaldi V, Delbary F, Caviglia G, Piana M, Marini C, Capitanio S, Calamia I, Buschiazzo A, Sambuceti G. A new compartmental method for the analysis of liver FDG kinetics in small animal models. EJNMMI Res. 2015;5:107. doi: 10.1186/s13550-015-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iozzo P, Chareonthaitawee P, Di Terlizzi M, Betteridge DJ, Ferrannini E, Camici PG. Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab. 2002;282:E1163–1171. doi: 10.1152/ajpendo.00386.2001. [DOI] [PubMed] [Google Scholar]
- 89.Kumar P, Patel CD, Singla S, Malhotra A. Effect of duration of fasting and diet on the myocardial uptake of F-18-2-fluoro-2-deoxyglucose (F-18 FDG) at rest. Indian J Nucl Med. 2014;29:140–145. doi: 10.4103/0972-3919.136559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skali H, Schulman AR, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013;15:352. [PMC free article] [PubMed] [Google Scholar]
- 91.Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, Im JG. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006;7:57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol. 2007;81:845–855. doi: 10.1189/jlb.0606417. [DOI] [PubMed] [Google Scholar]
- 93.Dalm VA, van Hagen PM, van Koetsveld PM, Achilefu S, Houtsmuller AB, Pols DH, van der Lely AJ, Lamberts SW, Hofland LJ. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab. 2003;285:E344–353. doi: 10.1152/ajpendo.00048.2003. [DOI] [PubMed] [Google Scholar]
- 94.Lichtenauer-Kaligis EG, van Hagen PM, Lamberts SW, Hofland LJ. Somatostatin receptor subtypes in human immune cells. Eur J Endocrinol. 2000;143(Suppl 1):S21–25. doi: 10.1530/eje.0.143s021. [DOI] [PubMed] [Google Scholar]
- 95.Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer. 2005;92:1493–1498. doi: 10.1038/sj.bjc.6602503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarthy MJ, Loftus IM, Thompson MM, Jones L, London NJ, Bell PR, Naylor AR, Brindle NP. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg. 1999;30:261–268. doi: 10.1016/s0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- 97.Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, Bauer W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52. doi: 10.1186/2191-219X-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Mäcke HR. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 99.Velikyan I, Sundin A, Sörensen J, Lubberink M, Sandström M, Garske-Román U, Lundqvist H, Granberg D, Eriksson B. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55:204–210. doi: 10.2967/jnumed.113.126177. [DOI] [PubMed] [Google Scholar]
- 100.Banerjee SR, Pomper MG. Clinical applications of gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Velikyan I. Positron emitting [68Ga] Ga-based imaging agents: chemistry and diversity. Med Chem. 2011;7:345–379. doi: 10.2174/157340611796799195. [DOI] [PubMed] [Google Scholar]
- 102.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rösch F. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 103.Maddahi J. Properties of an ideal PET perfusion tracer: new PET tracer cases and data. J Nucl Cardiol. 2012;19(Suppl 1):S30–37. doi: 10.1007/s12350-011-9491-8. [DOI] [PubMed] [Google Scholar]
- 104.Yu M, Nekolla SG, Schwaiger M, Robinson SP. The next generation of cardiac positron emission tomography imaging agents: discovery of flurpiridaz F-18 for detection of coronary disease. Semin Nucl Med. 2011;41:305–313. doi: 10.1053/j.semnuclmed.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Gaemperli O, Kaufmann PA. PET and PET/CT in cardiovascular disease. Ann N Y Acad Sci. 2011;1228:109–136. doi: 10.1111/j.1749-6632.2011.06030.x. [DOI] [PubMed] [Google Scholar]
- 106.Weinberg IN, Huang SC, Hoffman EJ, Araujo L, Nienaber C, Grover-McKay M, Dahlbom M, Schelbert H. Validation of PET-acquired input functions for cardiac studies. J Nucl Med. 1988;29:241–247. [PubMed] [Google Scholar]
- 107.Lin KP, Huang SC, Choi Y, Brunken RC, Schelbert HR, Phelps ME. Correction of spillover radioactivities for estimation of the blood time-activity curve from the imaged LV chamber in cardiac dynamic FDG PET studies. Phys Med Biol. 1995;40:629–642. doi: 10.1088/0031-9155/40/4/009. [DOI] [PubMed] [Google Scholar]
- 108.Lockwood AH. Absorbed doses of radiation after an intravenous injection of N-13 ammonia in man: concise communication. J Nucl Med. 1980;21:276–278. [PubMed] [Google Scholar]
- 109.Love WD, Burch GE. A comparison of potassium 42, rubidium 86, and cesium 134 as tracers of potassium in the study of cation metabolism of human erythrocytes in vitro. J Lab Clin Med. 1953;41:351–362. [PubMed] [Google Scholar]
- 110.Love WD, Romney RB, Burch GE. A comparison of the distribution of potassium and exchangeable rubidium in the organs of the dog, using rubidium. Circ Res. 1954;2:112–122. doi: 10.1161/01.res.2.2.112. [DOI] [PubMed] [Google Scholar]
- 111.Selwyn AP, Allan RM, L’Abbate A, Horlock P, Camici P, Clark J, O’Brien HA, Grant PM. Relation between regional myocardial uptake of rubidium-82 and perfusion: absolute reduction of cation uptake in ischemia. Am J Cardiol. 1982;50:112–121. doi: 10.1016/0002-9149(82)90016-9. [DOI] [PubMed] [Google Scholar]
- 112.Yoshinaga K, Klein R, Tamaki N. Generator-produced rubidium-82 positron emission tomography myocardial perfusion imaging-From basic aspects to clinical applications. J Cardiol. 2010;55:163–173. doi: 10.1016/j.jjcc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Chow BJ, Ananthasubramaniam K, dekemp RA, Dalipaj MM, Beanlands RS, Ruddy TD. Comparison of treadmill exercise versus dipyridamole stress with myocardial perfusion imaging using rubidium-82 positron emission tomography. J Am Coll Cardiol. 2005;45:1227–1234. doi: 10.1016/j.jacc.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 114.Demer LL, Gould KL, Goldstein RA, Kirkeeide RL, Mullani NA, Smalling RW, Nishikawa A, Merhige ME. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825–835. doi: 10.1161/01.cir.79.4.825. [DOI] [PubMed] [Google Scholar]
- 115.Go RT, Marwick TH, MacIntyre WJ, Saha GB, Neumann DR, Underwood DA, Simpfendorfer CC. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899–1905. [PubMed] [Google Scholar]
- 116.Goldstein RA, Mullani NA, Marani SK, Fisher DJ, Gould KL, O’Brien HA. Myocardial perfusion with rubidium-82. II. Effects of metabolic and pharmacologic interventions. J Nucl Med. 1983;24:907–915. [PubMed] [Google Scholar]
- 117.Donato L, Bartolomei G, Federighi G, Torreggiani G. Measurement of coronary blood flow by external counting with radioactive rubidium. Critical appraisal and validation of the method. Circulation. 1966;33:708–718. doi: 10.1161/01.cir.33.5.708. [DOI] [PubMed] [Google Scholar]
- 118.Goldstein RA. Rubidium-82 kinetics after coronary occlusion: temporal relation of net myocardial accumulation and viability in open-chested dogs. J Nucl Med. 1986;27:1456–1461. [PubMed] [Google Scholar]
- 119.Glover DK, Gropler RJ. Journey to find the ideal PET flow tracer for clinical use: are we there yet? J Nucl Cardiol. 2007;14:765–768. doi: 10.1016/j.nuclcard.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 120.Bergmann SR, Fox KA, Rand AL, McElvany KD, Welch MJ, Markham J, Sobel BE. Quantification of regional myocardial blood flow in vivo with H215O. Circulation. 1984;70:724–733. doi: 10.1161/01.cir.70.4.724. [DOI] [PubMed] [Google Scholar]
- 121.Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14:639–652. doi: 10.1016/0735-1097(89)90105-8. [DOI] [PubMed] [Google Scholar]
- 122.Bol A, Melin JA, Vanoverschelde JL, Baudhuin T, Vogelaers D, De Pauw M, Michel C, Luxen A, Labar D, Cogneau M. Direct comparison of [13N] ammonia and [15O] water estimates of perfusion with quantification of regional myocardial blood flow by microspheres. Circulation. 1993;87:512–525. doi: 10.1161/01.cir.87.2.512. [DOI] [PubMed] [Google Scholar]
- 123.Herrero P, Kim J, Sharp TL, Engelbach JA, Lewis JS, Gropler RJ, Welch MJ. Assessment of myocardial blood flow using 15O-water and 1-11C-acetate in rats with small-animal PET. J Nucl Med. 2006;47:477–485. [PubMed] [Google Scholar]
- 124.Iida H, Kanno I, Takahashi A, Miura S, Murakami M, Takahashi K, Ono Y, Shishido F, Inugami A, Tomura N. Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partial-volume effect. Circulation. 1988;78:104–115. doi: 10.1161/01.cir.78.1.104. [DOI] [PubMed] [Google Scholar]
- 125.Miller TR, Wallis JW, Landy BR, Gropler RJ, Sabharwal CL. Measurement of global and regional left ventricular function by cardiac PET. J Nucl Med. 1994;35:999–1005. [PubMed] [Google Scholar]
- 126.Araujo LI, Lammertsma AA, Rhodes CG, McFalls EO, Iida H, Rechavia E, Galassi A, De Silva R, Jones T, Maseri A. Noninvasive quantification of regional myocardial blood flow in coronary artery disease with oxygen-15-labeled carbon dioxide inhalation and positron emission tomography. Circulation. 1991;83:875–885. doi: 10.1161/01.cir.83.3.875. [DOI] [PubMed] [Google Scholar]
- 127.Hsu B. PET tracers and techniques for measuring myocardial blood flow in patients with coronary artery disease. J Biomed Res. 2013;27:452–459. doi: 10.7555/JBR.27.20130136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Knuuti J, Kajander S, Mäki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of CAD. J Nucl Cardiol. 2009;16:497–506. doi: 10.1007/s12350-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 129.Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med. 1999;40:1848–1856. [PubMed] [Google Scholar]
- 130.Maddahi J, Packard RR. Cardiac PET perfusion tracers: current status and future directions. Semin Nucl Med. 2014;44:333–343. doi: 10.1053/j.semnuclmed.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schelbert HR, Phelps ME, Huang SC, MacDonald NS, Hansen H, Selin C, Kuhl DE. N-13 ammonia as an indicator of myocardial blood flow. Circulation. 1981;63:1259–1272. doi: 10.1161/01.cir.63.6.1259. [DOI] [PubMed] [Google Scholar]
- 132.Walsh WF, Fill HR, Harper PV. Nitrogen-13-labeled ammonia for myocardial imaging. Semin Nucl Med. 1977;7:59–66. doi: 10.1016/s0001-2998(77)80008-1. [DOI] [PubMed] [Google Scholar]
- 133.Bergmann SR, Hack S, Tewson T, Welch MJ, Sobel BE. The dependence of accumulation of 13NH3 by myocardium on metabolic factors and its implications for quantitative assessment of perfusion. Circulation. 1980;61:34–43. doi: 10.1161/01.cir.61.1.34. [DOI] [PubMed] [Google Scholar]
- 134.Shah A, Schelbert HR, Schwaiger M, Henze E, Hansen H, Selin C, Huang SC. Measurement of regional myocardial blood flow with N-13 ammonia and positron-emission tomography in intact dogs. J Am Coll Cardiol. 1985;5:92–100. doi: 10.1016/s0735-1097(85)80089-9. [DOI] [PubMed] [Google Scholar]
- 135.Tamaki N, Yonekura Y, Senda M, Kureshi SA, Saji H, Kodama S, Konishi Y, Ban T, Kambara H, Kawai C. Myocardial positron computed tomography with 13N-ammonia at rest and during exercise. Eur J Nucl Med. 1985;11:246–251. doi: 10.1007/BF00279078. [DOI] [PubMed] [Google Scholar]
- 136.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783–793. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 137.Muzik O, Beanlands RS, Hutchins GD, Mangner TJ, Nguyen N, Schwaiger M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET. J Nucl Med. 1993;34:83–91. [PubMed] [Google Scholar]
- 138.Yu M, Guaraldi MT, Mistry M, Kagan M, McDonald JL, Drew K, Radeke H, Azure M, Purohit A, Casebier DS, Robinson SP. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14:789–798. doi: 10.1016/j.nuclcard.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 139.Maddahi J, Czernin J, Lazewatsky J, Huang SC, Dahlbom M, Schelbert H, Sparks R, Ehlgen A, Crane P, Zhu Q, Devine M, Phelps M. Phase I, first-in-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest. J Nucl Med. 2011;52:1490–1498. doi: 10.2967/jnumed.111.092528. [DOI] [PubMed] [Google Scholar]
- 140.Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, Radeke HS, Azure M, Purohit A, Casebier DS, Robinson SP. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782–788. doi: 10.1016/j.nuclcard.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, Ziegler SI, Casebier DS, Robinson SP, Schwaiger M. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49:630–636. doi: 10.2967/jnumed.107.044727. [DOI] [PubMed] [Google Scholar]
- 142.Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A, Huisman M, Poethko T, Schuster T, Yu M, Robinson S, Casebier D, Henke J, Wester HJ, Schwaiger M. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation. 2009;119:2333–2342. doi: 10.1161/CIRCULATIONAHA.108.797761. [DOI] [PubMed] [Google Scholar]
- 143.Berman DS, Maddahi J, Tamarappoo BK, Czernin J, Taillefer R, Udelson JE, Gibson CM, Devine M, Lazewatsky J, Bhat G, Washburn D. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol. 2013;61:469–477. doi: 10.1016/j.jacc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alpert N, Dean Fang YH, El Fakhri G. Single-scan rest/stress imaging (18)F-labeled flow tracers. Med Phys. 2012;39:6609–6620. doi: 10.1118/1.4754585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Higuchi T, Nekolla SG, Huisman MM, Reder S, Poethko T, Yu M, Wester HJ, Casebier DS, Robinson SP, Botnar RM, Schwaiger M. A new 18F-labeled myocardial PET tracer: myocardial uptake after permanent and transient coronary occlusion in rats. J Nucl Med. 2008;49:1715–1722. doi: 10.2967/jnumed.108.053967. [DOI] [PubMed] [Google Scholar]
- 146.Guehl NJ, Normandin MD, Wooten DW, Rozen G, Sitek A, Ruskin J, Shoup TM, Ptaszek LM, El Fakhri G, Alpert NM. Single-scan rest/stress imaging: validation in a porcine model with 18F-flurpiridaz. Eur J Nucl Med Mol Imaging. 2017;44:1538–1546. doi: 10.1007/s00259-017-3684-6. [DOI] [PubMed] [Google Scholar]
- 147.Mou T, Zhao Z, Fang W, Peng C, Guo F, Liu B, Ma Y, Zhang X. Synthesis and preliminary evaluation of 18F-labeled pyridaben analogues for myocardial perfusion imaging with PET. J Nucl Med. 2012;53:472–479. doi: 10.2967/jnumed.111.088096. [DOI] [PubMed] [Google Scholar]
- 148.Mou T, Zhao Z, Zhang P, Fang W, Peng C, Lu J, Wang Q, Ma Y, Zhang X. Synthesis and bio-evaluation of new (18) F-Labeled pyridaben analogs with improved stability for myocardial perfusion imaging in mice. Chem Biol Drug Des. 2015;86:351–361. doi: 10.1111/cbdd.12499. [DOI] [PubMed] [Google Scholar]
- 149.Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet Lond Engl. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shauer A, Gotsman I, Keren A, Zwas DR, Hellman Y, Durst R, Admon D. Acute viral myocarditis: current concepts in diagnosis and treatment. Isr Med Assoc J. 2013;15:180–185. [PubMed] [Google Scholar]
- 151.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 152.Ozawa K, Funabashi N, Daimon M, Takaoka H, Takano H, Uehara M, Kobayashi Y. Determination of optimum periods between onset of suspected acute myocarditis and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory left ventricular myocardium. Int J Cardiol. 2013;169:196–200. doi: 10.1016/j.ijcard.2013.08.098. [DOI] [PubMed] [Google Scholar]
- 153.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 154.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R American Heart Association; American College of Cardiology; European Society of Cardiology; Heart Failure Society of America; Heart Failure Association of the European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American heart association, the American college of cardiology, and the European society of cardiology. Endorsed by the heart failure society of America and the heart failure association of the European society of cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 155.von Olshausen G, Hyafil F, Langwieser N, Laugwitz KL, Schwaiger M, Ibrahim T. Detection of acute inflammatory myocarditis in epstein barr virus infection using hybrid 18F-fluoro-deoxyglucose-positron emission tomography/magnetic resonance imaging. Circulation. 2014;130:925–926. doi: 10.1161/CIRCULATIONAHA.114.011000. [DOI] [PubMed] [Google Scholar]
- 156.Choi JY, Jeong JM, Yoo BC, Kim K, Kim Y, Yang BY, Lee YS, Lee DS, Chung JK, Lee MC. Development of 68Ga-labeled mannosylated human serum albumin (MSA) as a lymph node imaging agent for positron emission tomography. Nucl Med Biol. 2011;38:371–379. doi: 10.1016/j.nucmedbio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 157.Lee SP, Im HJ, Kang S, Chung SJ, Cho YS, Kang H, Park HS, Hwang DW, Park JB, Paeng JC, Cheon GJ, Lee YS, Jeong JM, Kim YJ. Noninvasive imaging of myocardial inflammation in myocarditis using 68Ga-tagged mannosylated human serum albumin positron emission tomography. Theranostics. 2017;7:413–424. doi: 10.7150/thno.15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 159.Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, Endo K, Yokoyama T, Suzuki T, Kurabayashi M. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 160.Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, Ohkawa M. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–1576. [PubMed] [Google Scholar]
- 161.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 162.Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Baba K, Ishibashi M, Hayabuchi N, Narula J, Imaizumi T. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–1228. doi: 10.1016/j.jcmg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 163.Andrikopoulou E, Bhambhvani P. Optimizing myocardial metabolism for fluorine-18 fluorodeoxyglucose positron emission tomography imaging of cardiac inflammation. J Nucl Cardiol. 2017 doi: 10.1007/s12350-017-0872-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 164.Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med. 2017;42:88–94. doi: 10.1097/RLU.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 165.Mc Ardle BA, Leung E, Ohira H, Cocker MS, deKemp RA, DaSilva J, Birnie D, Beanlands RS, Nery PB. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:297–306. doi: 10.1007/s12350-012-9668-9. [DOI] [PubMed] [Google Scholar]
- 166.Pierre-Louis B, Prasad A, Frishman WH. Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev. 2009;17:153–158. doi: 10.1097/CRD.0b013e3181a1f763. [DOI] [PubMed] [Google Scholar]
- 167.Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–583. doi: 10.1111/j.1540-8167.2008.01417.x. [DOI] [PubMed] [Google Scholar]
- 168.Al Moudi M, Sun Z, Lenzo N. Diagnostic value of SPECT, PET and PET/CT in the diagnosis of coronary artery disease: a systematic review. Biomed Imaging Interv J. 2011;7:e9. doi: 10.2349/biij.7.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 170.Ghosh N, Rimoldi OE, Beanlands RS, Camici PG. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010;31:2984–2995. doi: 10.1093/eurheartj/ehq361. [DOI] [PubMed] [Google Scholar]
- 171.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 172.Gewirtz H. PET measurement of absolute myocardial blood flow and LV function in dilated cardiomyopathy. JACC Cardiovasc Imaging. 2011;4:557–560. doi: 10.1016/j.jcmg.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 173.Fiechter M, Ghadri JR, Gebhard C, Fuchs TA, Pazhenkottil AP, Nkoulou RN, Herzog BA, Wyss CA, Gaemperli O, Kaufmann PA. Diagnostic value of 13N-ammonia myocardial perfusion PET: added value of myocardial flow reserve. J Nucl Med. 2012;53:1230–1234. doi: 10.2967/jnumed.111.101840. [DOI] [PubMed] [Google Scholar]
- 174.Santana CA, Folks RD, Garcia EV, Verdes L, Sanyal R, Hainer J, Di Carli MF, Esteves FP. Quantitative (82)Rb PET/CT: development and validation of myocardial perfusion database. J Nucl Med. 2007;48:1122–1128. doi: 10.2967/jnumed.107.039750. [DOI] [PubMed] [Google Scholar]
- 175.Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging. 2009;2:751–758. doi: 10.1016/j.jcmg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 176.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 177.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 178.Schindler TH, Nitzsche EU, Olschewski M, Brink I, Mix M, Prior J, Facta A, Inubushi M, Just H, Schelbert HR. PET-measured responses of MBF to cold pressor testing correlate with indices of coronary vasomotion on quantitative coronary angiography. J Nucl Med. 2004;45:419–428. doi: 10.1097/00006231-200404000-00092. [DOI] [PubMed] [Google Scholar]
- 179.Rosas EA, Slomka PJ, García-Rojas L, Calleja R, Jácome R, Jiménez-Santos M, Romero E, Meave A, Berman DS. Functional impact of coronary stenosis observed on coronary computed tomography angiography: comparison with 13N-ammonia PET. Arch Med Res. 2010;41:642–648. doi: 10.1016/j.arcmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 180.Tio RA, Dabeshlim A, Siebelink HM, de Sutter J, Hillege HL, Zeebregts CJ, Dierckx RA, van Veldhuisen DJ, Zijlstra F, Slart RH. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 181.Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, Di Carli MF. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2:846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stanley WC, Hall JL, Stone CK, Hacker TA. Acute myocardial ischemia causes a transmural gradient in glucose extraction but not glucose uptake. Am J Physiol. 1992;262:H91–96. doi: 10.1152/ajpheart.1992.262.1.H91. [DOI] [PubMed] [Google Scholar]
- 183.Depre C, Taegtmeyer H. Metabolic aspects of programmed cell survival and cell death in the heart. Cardiovasc Res. 2000;45:538–548. doi: 10.1016/s0008-6363(99)00266-7. [DOI] [PubMed] [Google Scholar]
- 184.Gropler RJ, Geltman EM, Sampathkumaran K, Pérez JE, Schechtman KB, Conversano A, Sobel BE, Bergmann SR, Siegel BA. Comparison of carbon-11-acetate with fluorine-18-fluorodeoxyglucose for delineating viable myocardium by positron emission tomography. J Am Coll Cardiol. 1993;22:1587–1597. doi: 10.1016/0735-1097(93)90582-l. [DOI] [PubMed] [Google Scholar]
- 185.Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O’Donnell CJ, Wang TJ, D’Agostino RB, Wolf PA, Vasan RS. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–1726. doi: 10.2337/db07-0078. [DOI] [PubMed] [Google Scholar]
- 186.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Chen W, Berenson GS, Stein JH. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the bogalusa heart study. J Am Coll Cardiol. 2005;46:457–463. doi: 10.1016/j.jacc.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 187.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 188.Jezovnik MK, Zidar N, Lezaic L, Gersak B, Poredos P. Identification of inflamed atherosclerotic lesions in vivo using PET-CT. Inflammation. 2014;37:426–434. doi: 10.1007/s10753-013-9755-3. [DOI] [PubMed] [Google Scholar]
- 189.Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Højgaard L, Ripa RS, Kjær A. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol. 2015;35:1696–1703. doi: 10.1161/ATVBAHA.114.305067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cuhlmann S, Gsell W, Van der Heiden K, Habib J, Tremoleda JL, Khalil M, Turkheimer F, Meens MJ, Kwak BR, Bird J, Davenport AP, Clark J, Haskard D, Krams R, Jones H, Evans PC. In vivo mapping of vascular inflammation using the translocator protein tracer 18F-FEDAA1106. Mol Imaging. 2014:13. doi: 10.2310/7290.2014.00014. [DOI] [PubMed] [Google Scholar]
- 191.Kataoka Y, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol. 2012;59:1592–1597. doi: 10.1016/j.jacc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 192.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 193.Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, Wilensky R, Newberg AB, Basu S, Langsteger W, Alavi A. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14:114–120. [PubMed] [Google Scholar]
- 194.Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 195.Carlin S, Humm JL. PET of hypoxia: current and future perspectives. J Nucl Med. 2012;53:1171–1174. doi: 10.2967/jnumed.111.099770. [DOI] [PubMed] [Google Scholar]
- 196.Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149:367–380. [PMC free article] [PubMed] [Google Scholar]
- 197.Korngold EC, Jaffer FA, Weissleder R, Sosnovik DE. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev. 2008;13:163–173. doi: 10.1007/s10741-007-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 200.Yagle KJ, Eary JF, Tait JF, Grierson JR, Link JM, Lewellen B, Gibson DF, Krohn KA. Evaluation of 18F-annexin V as a PET imaging agent in an animal model of apoptosis. J Nucl Med. 2005;46:658–666. [PubMed] [Google Scholar]
- 201.Grierson JR, Yagle KJ, Eary JF, Tait JF, Gibson DF, Lewellen B, Link JM, Krohn KA. Production of [F-18] fluoroannexin for imaging apoptosis with PET. Bioconjug Chem. 2004;15:373–379. doi: 10.1021/bc0300394. [DOI] [PubMed] [Google Scholar]
- 202.Murakami Y, Takamatsu H, Taki J, Tatsumi M, Noda A, Ichise R, Tait JF, Nishimura S. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging. 2004;31:469–474. doi: 10.1007/s00259-003-1378-8. [DOI] [PubMed] [Google Scholar]
- 203.Tenenbaum A, Fisman EZ. Impaired glucose metabolism in patients with heart failure: pathophysiology and possible treatment strategies. Am J Cardiovasc Drugs. 2004;4:269–280. doi: 10.2165/00129784-200404050-00001. [DOI] [PubMed] [Google Scholar]
- 204.Deaton C, Mamas MA, Rutter MK, Gibson M, Bowell S, Byrne R, Coezy K, Gow J, Williams S. Glucose and insulin abnormalities in patients with heart failure. Eur J Cardiovasc Nurs. 2011;10:75–87. doi: 10.1016/j.ejcnurse.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 205.Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3:142–148. doi: 10.1161/CIRCIMAGING.109.888909. [DOI] [PubMed] [Google Scholar]
- 206.Gleissner CA, Galkina E, Nadler JL, Ley K. Mechanisms by which diabetes increases cardiovascular disease. Drug Discov Today Dis Mech. 2007;4:131–140. doi: 10.1016/j.ddmec.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tahara N, Kai H, Yamagishi S, Mizoguchi M, Nakaura H, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Vascular inflammation evaluated by [18F] -fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49:1533–1539. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 209.Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, Mawatari K, Yasukawa H, Kaida H, Ishibashi M, Hayabuchi N, Harada H, Ikeda H, Yamagishi SI, Imaizumi T. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4:1110–1118. doi: 10.1016/j.jcmg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 210.Nitta Y, Tahara N, Tahara A, Honda A, Kodama N, Mizoguchi M, Kaida H, Ishibashi M, Hayabuchi N, Ikeda H, Yamagishi S, Imaizumi T. Pioglitazone decreases coronary artery inflammation in impaired glucose tolerance and diabetes mellitus: evaluation by FDG-PET/CT imaging. JACC Cardiovasc Imaging. 2013;6:1172–1182. doi: 10.1016/j.jcmg.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 211.Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7:e013927. doi: 10.1136/bmjopen-2016-013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 213.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–262. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- 214.Knuuti J, Takala TO, Någren K, Sipilä H, Turpeinen AK, Uusitupa MI, Nuutila P. Myocardial fatty acid oxidation in patients with impaired glucose tolerance. Diabetologia. 2001;44:184–187. doi: 10.1007/s001250051597. [DOI] [PubMed] [Google Scholar]
- 215.Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 216.Labbé SM, Grenier-Larouche T, Croteau E, Normand-Lauzière F, Frisch F, Ouellet R, Guérin B, Turcotte EE, Carpentier AC. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to compute tomography. Am J Physiol Endocrinol Metab. 2011;300:E445–453. doi: 10.1152/ajpendo.00579.2010. [DOI] [PubMed] [Google Scholar]
- 217.Labbé SM, Noll C, Grenier-Larouche T, Kunach M, Bouffard L, Phoenix S, Guérin B, Baillargeon JP, Langlois MF, Turcotte EE, Carpentier AC. Improved cardiac function and dietary fatty acid metabolism after modest weight loss in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2014;306:E1388–1396. doi: 10.1152/ajpendo.00638.2013. [DOI] [PubMed] [Google Scholar]
- 218.Labbé SM, Grenier-Larouche T, Noll C, Phoenix S, Guérin B, Turcotte EE, Carpentier AC. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes. 2012;61:2701–2710. doi: 10.2337/db11-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ménard SL, Croteau E, Sarrhini O, Gélinas R, Brassard P, Ouellet R, Bentourkia M ’hamed, van Lier JE, Des Rosiers C, Lecomte R, Carpentier AC. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am J Physiol Endocrinol Metab. 2010;298:E1049–1057. doi: 10.1152/ajpendo.00560.2009. [DOI] [PubMed] [Google Scholar]
- 220.Prior JO, Quiñones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 221.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52:1369–1377. doi: 10.2967/jnumed.110.082883. [DOI] [PubMed] [Google Scholar]
- 222.Kircher M, Lapa C. Novel noninvasive nuclear medicine imaging techniques for cardiac inflammation. Curr Cardiovasc Imaging Rep. 2017;10:6. doi: 10.1007/s12410-017-9400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, von Guggenberg E, Pichler BJ, Virgolini I, Decristoforo C, Haubner R. [68Ga] NODAGA-RGD for imaging αvβ3 integrin expression. Eur J Nucl Med Mol Imaging. 2011;38:1303–1312. doi: 10.1007/s00259-011-1778-0. [DOI] [PubMed] [Google Scholar]
- 224.Haubner R, Finkenstedt A, Stegmayr A, Rangger C, Decristoforo C, Zoller H, Virgolini IJ. [(68)Ga] NODAGA-RGD-metabolic stability, biodistribution, and dosimetry data from patients with hepatocellular carcinoma and liver cirrhosis. Eur J Nucl Med Mol Imaging. 2016;43:2005–2013. doi: 10.1007/s00259-016-3396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grönman M, Tarkia M, Kiviniemi T, Halonen P, Kuivanen A, Savunen T, Tolvanen T, Teuho J, Käkelä M, Metsälä O, Pietilä M, Saukko P, Ylä-Herttuala S, Knuuti J, Roivainen A, Saraste A. Imaging of αvβ3 integrin expression in experimental myocardial ischemia with [68Ga] NODAGA-RGD positron emission tomography. J Transl Med. 2017;15:144. doi: 10.1186/s12967-017-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim HY, Wang C, Chen JW, Swirski FK, Wey HY, Hooker J, Fayad ZA, Mulder WJ, Weissleder R, Nahrendorf M. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun. 2017;8:14064. doi: 10.1038/ncomms14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Phenix CP, Rempel BP, Colobong K, Doudet DJ, Adam MJ, Clarke LA, Withers SG. Imaging of enzyme replacement therapy using PET. Proc Natl Acad Sci U S A. 2010;107:10842–10847. doi: 10.1073/pnas.1003247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ali M, Pulli B, Chen JW. Molecular imaging of macrophage enzyme activity in cardiac inflammation. Curr Cardiovasc Imaging Rep. 2014;7:9258. doi: 10.1007/s12410-014-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb Haemost. 2011;105:828–836. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Perišić Nanut M, Sabotič J, Jewett A, Kos J. Cysteine cathepsins as regulators of the cytotoxicity of NK and T cells. Front Immunol. 2014;5:616. doi: 10.3389/fimmu.2014.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011;58:978–986. doi: 10.1161/HYPERTENSIONAHA.111.180935. [DOI] [PubMed] [Google Scholar]
- 233.Shi GP, Bryant RA, Riese R, Verhelst S, Driessen C, Li Z, Bromme D, Ploegh HL, Chapman HA. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J Exp Med. 2000;191:1177–1186. doi: 10.1084/jem.191.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Riese RJ, Chapman HA. Cathepsins and compartmentalization in antigen presentation. Curr Opin Immunol. 2000;12:107–113. doi: 10.1016/s0952-7915(99)00058-8. [DOI] [PubMed] [Google Scholar]