Abstract
Tissue-engineered scaffolds are a powerful means of healing craniofacial bone defects arising from trauma or disease. Murine models of critical-sized bone defects are especially useful in understanding the role of microenvironmental factors such as vascularization on bone regeneration. Here, we demonstrate the capability of a novel multimodality imaging platform capable of acquiring in vivo images of microvascular architecture, microvascular blood flow, and tracer/cell tracking via intrinsic optical signaling (IOS), laser speckle contrast (LSC), and fluorescence (FL) imaging, respectively, in a critical-sized calvarial defect model. Defects that were 4 mm in diameter were made in the calvarial regions of mice followed by the implantation of osteoconductive scaffolds loaded with human adipose-derived stem cells embedded in fibrin gel. Using IOS imaging, we were able to visualize microvascular angiogenesis at the graft site and extracted morphological information such as vessel radius, length, and tortuosity two weeks after scaffold implantation. FL imaging allowed us to assess functional characteristics of the angiogenic vessel bed, such as time-to-peak of a fluorescent tracer, and also allowed us to track the distribution of fluorescently tagged human umbilical vein endothelial cells. Finally, we used LSC to characterize the in vivo hemodynamic response and maturity of the remodeled microvessels in the scaffold microenvironment. In this study, we provide a methodical framework for imaging tissue-engineered scaffolds, processing the images to extract key microenvironmental parameters, and visualizing these data in a manner that enables the characterization of the vascular phenotype and its effect on bone regeneration. Such multimodality imaging platforms can inform optimization and design of tissue-engineered scaffolds and elucidate the factors that promote enhanced vascularization and bone formation.
Keywords: : optical imaging, critical-sized defects, angiogenesis, bone defect
Impact Statement
Multimodal in vivo characterization of microvascular architecture, microvascular blood flow and tracer/cell tracking using intrinsic optical signaling (IOS), laser speckle contrast (LSC) and fluorescence imaging provides invaluable information on the graft microenvironment. The combination of multimodal in vivo imaging and preclinical critical‐sized calvarial defect models can be a powerful new tool for elucidating the factors that promote enhanced vascularization and bone formation at the graft site. Collectively, such tools can provide critical information to help optimize the design, implementation and bench‐to‐bedside translation of future tissue engineering approaches for critical‐sized calvarial defects.
Introduction
Biomaterial-based tissue engineering provides promising therapeutic options for regenerating critical-sized craniofacial bone defects arising from trauma or disease.1 The success of new bone formation is greatly impacted by the host's angiogenic response with vascular networks influencing key microenvironmental factors. Consequently, angiogenesis and osteogenesis are tightly coupled processes.2 Understanding the vascular response to biomaterials is crucial for enabling new bone formation. Recent studies have reported novel histological methods for characterizing different types of vessels and their spatial relationship with native bone.2,3 However, relatively little is known about the in vivo relationship between the microvascular phenotype and new bone formation in critical-sized bone defects. Moreover, to understand and modulate the graft's vascular response, it is necessary to noninvasively characterize angiogenesis, perfusion, vessel maturity, and the distribution of transplanted cells. This study describes a novel imaging platform developed to investigate vascular structure and blood flow in regenerating bone grafts.
Recently, conventional radiological or microscopic imaging methods with the spatial resolution necessary for resolving the microvasculature have been used to characterize the in vivo microenvironment of tissue-engineered bone grafts.4–6 Recently, optical contrast mechanisms such as intrinsic optical signal (IOS), laser speckle contrast (LSC), and fluorescence (FL) imaging have also been used. IOS exploits the distinct wavelength-dependent absorption of oxygenated (HbO2) and deoxygenated (Hb) hemoglobin to characterize changes in their endogenous concentrations and can generate high spatial and temporal resolution “blood volume” maps of the entire field of view (FoV).7 The utility of IOS in functional in vivo imaging of the brain has been demonstrated in animal models.8,9 IOS imaging has also been used in clinical settings to record blood volume changes in functionally responsive brain regions10 and as a tool for presurgical mapping of angiogenic changes in the blood vessels surrounding brain tumors.11 However, to the best of our knowledge, IOS imaging has not been used to image the microenvironment of tissue-engineered bone grafts within defect sites.
LSC exploits the time-dependent interference pattern, or “speckles”, that result when tissue is illuminated by a coherent light source.12 Areas of tissue containing blood vessels show distinct dynamic changes in their speckle statistics due to the motion of red blood cells.13,14 This allows individual blood vessels to be easily identified from surrounding tissue and imaged to produce high-resolution blood flow maps of the region of interest (ROI).12 Furthermore, vessel maturity can be determined with LSC by measuring the in vivo changes in perfusion caused by a vasodilatory agent, such as carbogen (95% oxygen/5% carbon dioxide) gas.15,16 By administering carbogen in controlled increments and imaging the tissue microenvironment with LSC, one can detect changes in blood flow within each vessel. The degree to which each vessel responds to carbogen inhalation is an indicator of its relative maturity, in which the existence of well-developed vascular smooth muscles enables vasodilation of the vessel. LSC has been previously used to monitor in vivo blood flow in cerebral,17 retinal,18,19 and skin20–22 areas of murine models. However, LSC has not been used to map in vivo blood flow changes of tissue-engineered bone grafts in critical-sized defects.
Prior studies have also utilized FL imaging to assess the integration of cranial and calvarial bone allografts and autografts,6,23 as well as for tracking induced pluripotent stem cells (iPSCs).24 In those studies, FL imaging was used to follow the differentiation of iPSCs and 10T1/2 cells, which had been stably transduced to express red fluorescent protein (RFP).25 One can also determine vascular permeability and assess tracer kinetics in the angiogenic vascular bed within the graft site by imaging the transit of an intravascular fluorescent dye.26 When combined with LSC and IOS, FL may be used to corroborate blood flow measurements from LSC and correlate implanted cell fate with vascular phenotype in tissue-engineered bone grafts.
In this study, we used three-dimensional (3D) printed polycaprolactone (PCL) scaffolds seeded with human adipose-derived stem cells (ASCs) to treat critical-sized bone defects in immunocompromised mice. We developed a novel multimodal platform for imaging the graft microenvironment in vivo. We utilized a combination of IOS, LSC, and FL imaging to quantitatively assess the microvascular phenotypes during scaffold engraftment. Here, for the first time, we report the novel methods developed to correlate microvascular morphology (i.e., length, radius, and tortuosity) and hemodynamic parameters (i.e., blood flow and tracer kinetic parameters such as time-to-peak) with microvessel maturity (i.e., vasodilatory response) in a preclinical model of calvarial bone defects.
Materials and Methods
All chemicals were purchased from Sigma Aldrich (St. Louis, MO), unless otherwise noted. For cell culture reagents, high-glucose Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Laboratories (Gaithersburg, MD), and 100 × penicillin–streptomycin (P/S) was purchased from Corning, Inc. (Washington, DC). Fibroblast growth factor-2 (FGF-2) was purchased from PeproTech (Rocky Hill, NJ). For 3D printed scaffold fabrication, 1.75 mm PCL filament was purchased from Intservo (Durham, NC).
Adipose-derived stem cell isolation and culture
Lipoaspirate from a Caucasian female donor (aged 39 years) was obtained from the Johns Hopkins Medical Institutions under an approved institutional review board protocol. To isolate adipose-derived stem cells (ASCs), lipoaspirate was digested with 1 mg/mL Collagenase I (Worthington Biochemical Corp., Lakewood, NJ) at 37°C for 1 h and centrifuged at 300 g for 10 min to obtain a pellet of stromal vascular fraction. Following cell resuspension, RBCs were removed by lysing RBCs in a buffer (155 mM NH4Cl, 10 mM KHCO3, and 1 mM ethylenediaminetetraacetic acid) for 7 min and centrifuged at 300 g for 5 min. The stromal vascular fraction pellet was resuspended and plated onto T-175 flasks to allow for expansion of the adherent ASC population. ASCs were expanded using the expansion medium (DMEM, 10% v/v FBS, 1% v/v P/S, 1 ng/mL FGF-2) for two passages (P2) before use in experiments.
Three-dimensional printed scaffold fabrication
Porous 3D printed PCL scaffolds were printed using a LulzBot TAZ printer with a 375 μm diameter nozzle (Aleph Objectives, Inc., Loveland, CO). Scaffold sheets with 0.5 mm thickness, 30% infill density, and rectilinear infill pattern were designed, sliced, and converted to G-code using Cura software (Ultimaker B.V., Geldermalsen, Netherlands). PCL scaffold sheets were printed according to the G-code using an extrusion and cooling bed temperature of 105°C and 45°C, respectively. Cylindrical scaffolds were punched from the sheets using a 4 mm diameter drill bit.
Before cell culture studies, cylindrical 3D printed scaffolds were treated with 1 M NaOH for 1 h to increase surface hydrophilicity and washed 3 × 10 min in phosphate-buffered saline (PBS). Scaffolds were then sterilized in 100% ethanol for 1 h, followed by 3 × 20 min washes in sterile PBS. One day before seeding, scaffolds were incubated in stromal medium (DMEM, 10% v/v FBS, 1% v/v P/S) at 37°C overnight to allow serum proteins to adsorb to the scaffold surface.
Scaffold seeding
Three-dimensional printed scaffolds were seeded with ASCs encapsulated within a fibrin gel as previously described.27 P2 ASCs were trypsinized and resuspended in 10 mg/mL fibrinogen at a density of 25 × 106 cells/mL. Thrombin (10 U/mL) was added to the ASC-fibrinogen mixture (1:4 v/v) to yield a cell concentration of 20 × 106 cells/mL, and 6 μL of the solution was infused into the pore spaces of 3D printed scaffolds. Scaffolds were incubated at 37°C for 30 min to allow for fibrin to completely cross-link before adding the culture medium. Scaffolds were incubated in stromal medium overnight before in vivo implantation.
In vivo implantation
All animal procedures were approved by the Johns Hopkins Animal Care and Use Committee (JHU ACUC). Male, 11-week-old, homozygous Nu/J mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized using isoflurane and injected subcutaneously with 0.2 mg/kg buprenorphine (Reckitt Benckiser Pharmaceuticals, Inc., Slough, United Kingdom) before surgery. After sterilizing skin at the surgical site with betadine, an incision was made to expose the calvarium and the pericranium was gently removed to allow for drilling of the defect. A 4 mm diameter defect was drilled in the right calvarium using an Ideal Micro-Drill (Harvard Apparatus, Holliston, MA) and a 4 mm circular knife (Xemax Surgical Products, Inc., Napa, CA). Special care was taken to avoid disrupting the underlying dura mater. Scaffolds were implanted by briefly washing them in sterile 0.9% NaCl and press-fitting them into the defect. The incision was then sutured using 6-0 nylon sutures to close the surgical wound. Animals were treated with an intraperitoneal injection of 0.2 mg/kg buprenorphine twice a day for 2 days following surgery for pain management.
Image acquisition
To image microvascular structure and function in the scaffold and tissue microenvironment, we used a minimally invasive multicontrast optical imaging setup, illustrated in Figure 1. This imaging system acquires high temporal (10 frames per second or fps) and spatial resolution (5 μm) images and is capable of three imaging modalities: IOS, LSC, and FL28 imaging. We used a white light source (NI-150; Nikon Instruments, Inc., NY) coupled with 570 ± 5 nm band-pass filter (FB570-10, Thorlabs, NJ) mounted on a filter wheel (FW102C, Thorlabs, NJ). A 632.8 nm He-Ne laser (0.5 mW; Thorlabs) was used to acquire LSC images, and a 473 nm laser (100 mW; Cobalt AB, Sweden) along with a 496 nm long-pass filter (FF01-496/LP-25; Semrock, Inc., NY) were used for acquiring FL images. Mice were first anesthetized using a controlled flow rate of 1.5% isoflurane (Iso Flo, Cat. No. 06-8550-2/R1) in 1 L/min air with a Vapomatic Model: 2 (AM Bickford, Inc., NY) before being placed on an adjustable platform with their heads secured in place using a custom-designed stereotaxic frame. A multi-lens set (AF Micro Nikkor 60 mm 1:2:8D; Nikon Instruments, Inc., NY) was used for image acquisition at ∼2× magnification using a charge coupled device (CCD) camera (Infinity 3; Lumenera, ON, Canada) controlled by a customized MATLAB® (MathWorks, Natick, MA) program.
FIG. 1.
In vivo imaging of the calvarial defect model. (a) Benchtop multicontrast optical imaging system used to acquire in vivo images of the microenvironment of engineered scaffolds implanted in a calvarial defect model. (b) Schematic diagram of the imaging system shown in (a). The illumination sources include: (i) a white light source coupled to a 570 ± 5 nm band-pass filter for IOS imaging, (ii) a 473 nm blue laser for FL excitation, and (iii) a 632 nm He-Ne laser for LSC imaging. The blue and red laser sources were coupled to beam expanders to enable illumination of the entire calvarial defect site. These sources sequentially illuminated the calvarial defect site for each contrast acquisition. The rodent was mounted on a focusing assembly for manual focusing. Light scattered off the calvarial defect is passed through a second set of optics that includes a 496 nm long-pass filter, a ∼2× focusing lens set, and finally onto a CCD image sensor. The long-pass filter eliminates blue fluorescence excitation light without affecting either the IOS or LSC illumination. Images were acquired with the CCD image sensor at 10 frames per second and saved to an external hard drive for subsequent analysis. (c) Diagram of the calvarial defect area location on a mouse skull (adapted from Zhang et al.48). (d) Superior view of a 3D printed PCL scaffold implanted in the calvarial defect. (e) Magnified view of a 4 × 4 mm 3D printed scaffold seeded with ASCs suspended in a fibrin gel. IOS, intrinsic optical signal; FL, fluorescence; LSC, laser speckle contrast; CCD, charge-coupled device; PCL, polycaprolactone; 3D, three-dimensional; ASC, adipose-derived stem cell. Color images are available online at www.liebertpub.com/tec
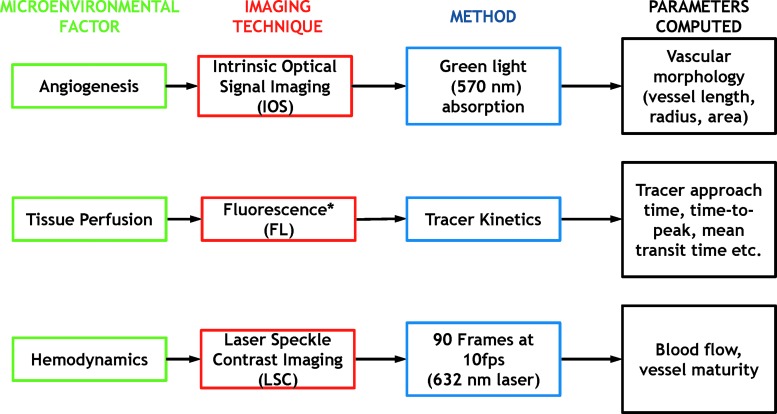
Figure 2 summarizes the microenvironmental parameters obtained from the three imaging techniques used. As shown in the flowchart, IOS images were used to characterize in vivo changes in microvascular morphology, and FL images were used to detect RFP-tagged cells and intravascular fluorescein isothiocyanate (FITC)-conjugated dextran (70 kD). Finally, we used LSC imaging for analyzing perfusion changes and microvessel maturity within the scaffold microenvironment.
FIG. 2.
Multicontrast in vivo imaging of the graft microenvironment. Flowchart illustrating key microenvironmental factors that were interrogated in vivo (green) within the calvarial defect site, the in vivo optical contrast mechanism used (red) to assay these factors, the imaging method used (blue), and the metrics computed for characterizing the in vivo graft microenvironment. *Can also be used to track fluorescently labeled stem cell survival and location. Color images are available online at www.liebertpub.com/tec
IOS imaging
For IOS, the governing equation was developed from a modified version of the Beer–Lambert's Law,7 as shown in Equation (1). Here, the logarithmic ratio of the incident light11 and the reflected light (IR) divided by the length traveled by the light (L) is equal to the concentrations of oxygenated and deoxygenated hemoglobin multiplied by their respective absorption coefficients (mu's) at given wavelengths, 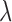 .
.
 |
At the isosbestic point of 570 nm, where the absorption coefficients of oxygenated and deoxygenated hemoglobin are approximately equal, one can reduce Equation (1) to Equation (2). Here, the ratio of incident light to reflected light is proportional to the total hemoglobin concentration in the system, HbT.
 |
Therefore, if we consider background pixels within our image to be points where nearly all light is reflected, then the images we capture show background tissue as bright since they lack hemoglobin, and blood vessels appear dark since they are hemoglobin-rich structures. While there are several isosbestic points between oxygenated and deoxygenated hemoglobin, 570 nm was chosen to maximize light penetration, without compromising signal quality because absorption coefficients decrease at longer wavelengths. Moreover, the derivation of Equation (2) from Equation (1) is based on the assumption that L is constant, although local variations in scattering can change L. Since the variability in the path lengths at different points within the FoV are very small and change with each mouse, this assumption served as a practical simplification. For our experiments, IOS images were acquired at 6 fps with an exposure time of 60 ms.
FL imaging
For FL tracer experiments, 0.2 mL of 10 mg/mL 70 kD dextran-FITC was administered through a tail-vein injection. The transit of FITC through the microvascular bed at the graft site was then imaged under blue laser (i.e., 473 nm) illumination at 6 fps with an exposure time of 150 ms for ∼3 min.
LSC imaging
For LSC, speckle fluctuations or the degree of “blurring” was quantified using Equation (3).29 Acquiring a temporal stack of the speckle pattern with a CCD camera over a given exposure time allows us to quantify the degree of blurring, which is inversely proportional to the relative perfusion. Each pixel in our FoV was assigned a speckle contrast or K value, which was the ratio of the standard deviation, σ, to the mean pixel intensity, <I>, where K2 can be simplified as being inversely proportional to the moving object's flow velocity. For each pixel, a moving average was calculated to obtain a smoothed image.
 |
The variance of the time-averaged pixel intensity was equal to its temporal fluctuations and is given by Equation (4).18 Here, T is the camera exposure time, cτ is the temporal average of the intensity autocorrelation function, and 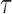 is the decorrelation time.
is the decorrelation time.
 |
Using LSC, we also used a carbogen gas challenge to assess the in vivo hemodynamic response and map vessel maturity. Carbogen gas, a mixture of 95% oxygen and 5% carbon dioxide, increases cerebral blood flow via vasodilation.15 At a microvascular level, increased oxygen delivery causes changes in tissue perfusion, which alters vascular resistance and perfusion pressure.30 Carbon dioxide causes a decrease in pH within the blood, which is detected by receptors in the vessels' walls that then cause the vessel smooth muscle to relax, resulting in vasodilation.31 In our protocol, mice were administered alternate cycles of room air (3 mins or 5.5 mins) and carbogen gas (1 min duration), and LSC image stacks were acquired during the entire experiment. The raw speckle data were smoothed using a 10 × 10 pixel median filter, and a 3 × 3 pixel temporal Gaussian filter was used to reduce noise and increase the signal-to-noise ratio of the LSC images. Images were acquired at 10 fps with an exposure time of 60 ms.
Image processing
After acquiring image stacks using IOS, LSC, and FL imaging, image processing was performed using ImageJ32 and MATLAB. First, image stacks from each imaging modality were co-registered using the ImageJ plugin TurboReg.33 This allowed us to directly compare microvascular parameters across each modality. For IOS images, maximum intensity projection (MIP) images of each image stack were generated and corrected for nonuniform illumination using a rolling ball background subtraction of 50 pixels in radius. Next, blood vessels were segmented using an ImageJ plugin called a “tubeness” filter that distinguishes curvilinear or tubular structures such as blood vessels, bronchi, or neurons by computing the 3D Hessian.34 The plugin uses Gaussian convolution with standard deviation, σ, which we varied to segment blood vessels of different radii. We selected three σ values ranging from 0.5 to 3 for each IOS MIP image to segment every vessel. Subsequently, output images from the tubeness filter were combined using a “maximum” operator and the resulting image thresholded to yield a binary “vessel mask.” Next, morphological operations such as image dilation and erosion were used to restore portions of blood vessels lost during the thresholding step. Finally, any residual noise outside the graft area was removed using a minimum size filter of 10 square pixels to yield the final blood vessel masks. LSC images were processed using a similar approach, but with an average intensity projection of the baseline flow image stack instead of an MIP of the entire experiment. This step was necessary to preserve blood vessel morphology because blood vessels could dilate during administration of the carbogen gas. The result of these image processing steps was a blood vessel mask derived from LSC images. Finally, the IOS- and LSC-derived blood vessel masks were combined to yield an overall mask that allowed us to accurately analyze the morphology of the microvasculature within the FoV.
Next, we generated a tagged skeleton and a Euclidean distance map (EDM) of the overall blood vessel mask using ImageJ. A tagged skeleton is an image wherein the morphology of the segmented blood vessels is represented as single pixel-wide branches along their centerlines. These pixels were then classified based on the number of connected neighbors as “end points” or “junctions.”35 The tagged skeleton image can therefore be used to identify branch points in the microvascular tree, the total number of vessels present, and to assess vessel length. The Euclidean distance map computed from our overall vessel mask replaces vessel pixels with grayscale values corresponding to their distance from the nearest blood vessel boundary.36 Therefore, when logically combined with the tagged skeleton, the EDM can be used to represent radii of vessel segments. This approach yields maps of the blood vessels within the graft site coded by their morphological parameters (i.e., vessel radii, vessel length, and tortuosity) and depicts valuable information that can be used to quantify angiogenesis at the graft site.
For processing of FL images, the acquired image stack was first imported into MATLAB and resampled using linear interpolation to create image stacks with 50 ms temporal resolution. Next, the resampled FL image stacks were subjected to a low-pass filter at a cutoff frequency of 1 Hz to remove high-frequency noise. Images were then analyzed using a custom MATLAB script to determine the time-to-peak (TTP) for each pixel following the administration of FITC. The average TTP for each vessel segment was then computed to create a TTP map. Additionally, our system could acquire FL images of human umbilical vein endothelial cells (HUVEC) labeled with RFP, a week after scaffold implantation.
To assess the relative blood flow within the vessels permeating our scaffold, we began by using the aforementioned methods for determining and mapping vessels within the overall mask. Next, we created average intensity projection images from the baseline blood flow (i.e., LSC) time series as well as the blood flow time series during carbogen gas experiments. By utilizing the vessel segment information from the tagged skeleton image, the blood vessel dimensions from EDM images, and computing the grayscale pixel intensity from the average projection images, we were able to map the relative blood flow per vessel segment. To more easily compare the baseline and carbogen blood flow values, we normalized these data by dividing the grayscale intensity values assigned to each vessel segment by the maximum value that occurred across all LSC trials during the carbogen inhalation experiment, resulting in a relative blood flow value scaled between 0 and 1. To assess the change in blood flow in response to carbogen gas inhalation, the percent increase in the average pixel intensity per vessel segment between baseline air and carbogen inhalation states was computed and mapped to the overall vessel mask. By quantifying carbogen gas-induced changes in blood flow, we could quantify the relative maturity of each vessel segment within the angiogenic graft site. We assume these changes correspond to fluctuations in blood flow caused by vasodilation in mature blood vessels. Finally, we used a k-means clustering approach implemented in MATLAB to classify each vessel segment based on its change in blood flow during carbogen gas inhalation. The input to the k-means clustering was an array containing the flow values of each vessel segment, and the output was a list of four classes that partitioned these flow values based on their nearest means. The index values allocated to these classes were then mapped to the corresponding vessels.
Results
Analysis of vascular morphology in the in vivo graft microenvironment
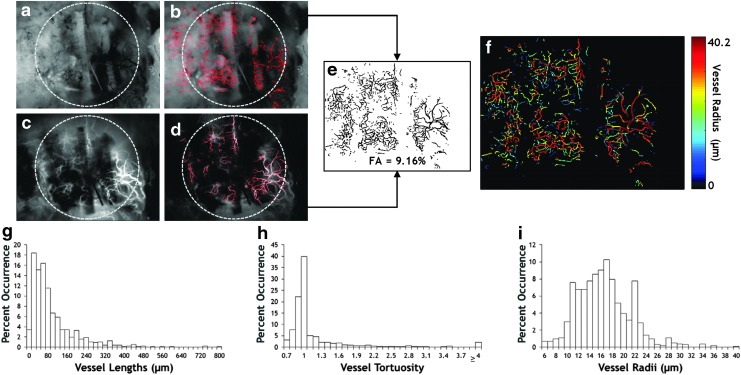
Using IOS and LSC images of the calvarial defect site (Fig. 3a, c), we generated binary vessel masks derived from each modality (Fig. 3b, d), which were combined to create an overall mask (Fig. 3e) that was used to analyze all morphological parameters. With the tagged skeleton and EDM derived from the overall mask, we were able to visually represent characteristics of the vascular network, as shown in Figure 3f. Here, we can see that there was a wide range of vessel radii distributions within the scaffold site. The minimum measureable radius was 5 μm due to the resolution of the camera sensor and optics. Figure 3g–i show the distributions of vessel characteristics assessed from the overall vessel mask (Fig. 3e). For the distribution shown in Figure 3g, the average vessel length was 93.4 μm with the most frequently occurring bin falling between 20 and 40 μm and a maximum vessel length of 793.1 μm. Similarly, from Figure 3h, we see the most frequent range of vessel tortuosity was between 0.7 and 1, with an average of 1.22 and a maximum of ≥4. Additionally from Figure 3i, the most frequent range of vessel radii was from 16 to 17 μm, with an average of 16.6 μm and a maximum of 40.2 μm, which matched the values displayed in the vessel map (Fig. 3f). These morphological data regarding the blood vessels within the graft site provide invaluable information regarding angiogenesis within the scaffold. Furthermore, these measurements can be used to characterize the vessel phenotypes, as described in the section “Discussion.”
FIG. 3.
Analysis of vascular morphology in the in vivo graft microenvironment. (a) IOS image of the calvarial defect site 2 weeks after implantation wherein the vasculature appears dark due to green light absorption. The location of the PCL scaffold is indicated by the gray circle. (c) LSC image of the same FoV wherein perfused blood vessels appear bright due to red laser light being scattered by moving erythrocytes. PCL scaffold is again outlined by a gray circle. (b, d) Images in (a, c) overlaid with their respective binary vessel masks. (e) Combination of the vessel masks in (b, d) to provide an overall mask including all the vessels that were visible using both imaging methods. (f) Map of the average vessel radius corresponding to the vessel segments identified in (e). (g–i) Distributions of blood vessel morphological characteristics corresponding to the sample shown in (e). FoV, field of view. Color images are available online at www.liebertpub.com/tec
Analysis of tracer kinetics and cell distribution in the in vivo graft microenvironment
From the image stacks of the dextran-FITC injections obtained using FL imaging, we were able to clearly observe the transit of the tracer through the vascular bed (Fig. 4a) within the graft site. To illustrate the first-pass of dextran-FITC through the microvasculature, we plotted the mean fluorescent signal intensity from a subregion (Fig. 4b), wherein one can clearly observe the wash-in and wash-out of the tracer and the contribution of individual time points to the overall tracer curve. By importing this image sequence into MATLAB, we could determine the time-to-peak value per vessel segment and map these values to our overall vessel mask (Fig. 4c). Finally, using FL imaging, we were also able to capture the distribution of HUVEC tagged with RFP within the scaffold (Fig. 4d).
FIG. 4.
Analysis of tracer kinetics and cell distribution in the in vivo graft microenvironment. (a) Representative time series of fluorescent images acquired after injection of the intravascular fluorescent tracer dextran-FITC. The location of the PCL scaffold is indicated by the gray circles. (b) Plot of the fluorescence intensity from a region of interest (ROI) (yellow circle) in (a) illustrating the transit of the intravascular tracer through the vasculature within the graft site. Red points correspond to the time points for which snapshots are shown in (a). (c) A TTP map computed from the tracer data for each vessel segment within the FoV. (d) Fluorescent image of a scaffold seeded with a mixture of ASCs and red fluorescent protein–labeled human umbilical vein endothelial cells (red channel) illustrating the utility of the fluorescent channel for cell tracking. FITC, fluorescein isothiocyanate; TTP, time-to-peak. Color images are available online at www.liebertpub.com/tec
Analysis of perfusion and vessel maturity in the in vivo graft microenvironment
From the overall vessel mask and the average intensity projections during air and carbogen breathing blocks, we were able to map changes in blood flow within the microvascular network at the graft site (Fig. 5a, b). To compare measurements, these values were normalized to the highest recorded blood flow value across all experiments. Using these normalized blood flow maps, we calculated the percent change in blood flow due to carbogen inhalation (Fig. 5c). Blood flow changes as high as 25% were observed in some vessels. To more closely analyze the blood flow response, we plotted the percent change in blood flow for a single vessel (Fig. 5d) over the course of the carbogen inhalation experiment, as shown in Figure 5e. Here, we can see that blood flow in the target vessel responded clearly and consistently during the carbogen inhalation blocks. Finally, using a k-means clustering approach, we classified each blood vessel based on its magnitude of blood flow change (Fig. 5f, g) and then applied the same cluster class to additional vessel characteristics to ascertain the relationship between parameters such as vessel radii or TTP and the observed change in blood flow. As shown in Figure 5h and i, vessel radius did not correlate with the blood flow response, whereas the TTP in vessels tended to be longer for vessels that exhibited larger blood flow changes within the graft site.
FIG. 5.
Analysis of perfusion and vessel maturity in the in vivo graft microenvironment. (a) Baseline blood flow map (normalized to the maximum flow across all samples). (b) Normalized blood flow map generated by computing the average blood flow during the carbogen inhalation periods. (c) Map of the percent change in blood flow induced by carbogen inhalation. More mature blood vessels exhibit larger changes in blood flow. Note only positive changes were displayed. (d) LSC image indicating an ROI (yellow circle) for which the blood flow time course is shown in (e). The location of the PCL scaffold is outlined by the gray circle. The baseline of the time series during inhalation of room air (RA) was corrected and only positive perfusion changes were plotted. The robust response to carbogen (CA) inhalation in (e) during the 180–240 s (blue bar) and 570–630 s (blue bar) intervals indicate the degree of maturity of the blood vessels within the yellow circle in (d). (f) Vessel classification based on the magnitude of their carbogen response in (c) was conducted using k-means clustering. Blood vessels labeled red exhibited the largest changes in blood flow, while black indicated those exhibiting the smallest change. (g) Distribution of blood flow changes in response to carbogen for each vessel class identified in (f). (h) Distribution of vessel radii according to the classifications in (f). The lack in variability across vessel sizes indicated that the carbogen-induced blood flow response was independent of blood vessel radius at the graft site. (i) Distribution of the TTP for each vessel class identified in (f). Again, the lack of variability indicated the TTP following injection of the dextran-FITC tracer was independent of vessel class. Color images are available online at www.liebertpub.com/tec
Discussion
Achieving adequate vascularization in tissue-engineered bone grafts is critical to their engraftment, long-term survival, and eventual clinical success. It is well known that blood vessels are essential for graft survival because they deliver O2 and essential nutrients, while also removing cellular waste products. However, vasculature is also essential for bone development and homeostasis, with several studies demonstrating that the coupling of angiogenesis and osteogenesis is necessary for bone growth and remodeling.3,37 Additionally, the blood vessel phenotype plays a critical role in this process. For example, vessels that contribute to bone growth—termed “Type H vessels”—exhibit higher blood flow, CD31 and endomucin expression, and secretion of pro-osteogenic factors28,38 and have a thinner more elongated morphology than other bone vessels.39 Furthermore, osteoprogenitors and osteoblasts are intimately associated with Type H vessels, typically residing within 20 μm of the nearest vessel.2 In light of these findings, some investigation into blood vessel phenotypes within implanted tissue engineering bone grafts has been performed.40 However, most current tissue engineering approaches do not utilize imaging techniques capable of adequately assessing the role of the vascular phenotype and angiogenic–osteogenic coupling in bone regeneration.
Herein lies the value of our imaging system, which combines IOS, FL, and LSC imaging at 5 μm resolution in a way that enables the robust characterization of the vasculature in tissue-engineered bone grafts in vivo while circumventing the shortcomings of other imaging techniques, such as magnetic resonance imaging41 and computed tomography (CT),42 which do not permit interrogation of the graft site at microvessel spatial resolutions. State-of-the-art micro-CT systems permit ex vivo imaging at 2 μm43 and in vivo imaging at 10–20 μm resolution.42,44 However, high-resolution micro-CT systems are considerably more expensive, not well suited for functional in vivo imaging of multiple physiological variables (e.g., blood flow and intravascular oxygenation) and can suffer from reduced contrast-to-noise ratio between contrast-labeled blood vessels and background tissue. This potentially limits the widespread usage of micro-CT for such applications. In contrast, the affordability, flexibility, and ease-of-use of optical imaging systems make them well suited for preclinical in vivo applications. Finally, our system was designed to specifically image microvascular remodeling in vivo and avoids the disadvantages of tissue clearing approaches used for ex vivo imaging such as distortion of the microvascular architecture and a lack of in vivo blood flow information.45
Here, for IOS imaging, we imaged hemoglobin absorption under 570 nm isosbestic illumination to create images of vascular morphology. Typically, at this wavelength, one can acquire images from depths of up to 300–500 μm.46 Although using a longer isosbestic wavelength (e.g., 796–798 nm) could yield information from deeper tissue, this would not permit us to distinguish microvessels because the contrast from hemoglobin absorption is significantly attenuated ( × 50) at this wavelength.46 Since LSC relies on light scattering, one needs to use longer wavelength illumination at which the hemoglobin absorption is minimal (e.g., 632 nm). Consequently, one could potentially interrogate deeper microvascular structures with LSC. Since contrast for LSC relies on flowing blood, microvessels with low or stagnant blood flow may not be visible. Therefore, to overcome these limitations, we merged images from IOS and LSC imaging.
Since the scaffold material in this study was opaque and optically highly scattering, we could only image microvessel formation on the surface and within the pores of the scaffold. Although imaging in the current study was not limited by scaffold thickness, one could envision future studies in which optically transparent scaffolds are utilized to enable imaging of blood vessels below the scaffold. Since the murine calvarium is optically transparent under well-hydrated conditions, we could interrogate microvessels above or below it within a depth of ∼500 μm. Finally, microvessels that grow atop and interspersed with bone can be imaged with this method, irrespective of bone formation as long as the bone is well hydrated. However, imaging underlying microvessels would be limited in instances where newly or excessively formed bone is poorly hydrated, resulting in excessive optical scattering.
Blood vessel maturity can be estimated by quantifying the degree to which blood vessels respond to a vasodilatory challenge, such as carbogen gas inhalation. The underlying premise being that this blood flow response is proportional to the degree of smooth muscle coverage that has developed around blood vessels at the graft site. Here, we chose to evaluate vessel maturity by measuring the blood flow responses during carbogen challenges with LSC imaging because it is less sensitive to inaccuracies, such as motion within the FoV. Although one could derive such estimates from IOS imaging by determining changes in microvessel diameter induced by carbogen, such measurements are challenging since small changes in blood vessel diameter are susceptible to noise resulting from subject motion. LSC imaging is not without its own drawbacks however, as it is difficult to determine whether blood vessels within the graft site are themselves responding to the carbogen or if the increased blood flow is the result of upstream vasodilation. Additionally, while LSC imaging does provide valuable information on the relative blood flow changes, an imaging method that permits absolute blood flow measurements would be preferable for certain applications.
By permitting the evaluation of parameters such as vascular morphology, blood flow, and blood vessel maturity, our imaging system facilitates the characterization of the vascular phenotype. Combining this multimodal imaging platform with clustering and structural/functional blood vessel mapping enables us to quantify changes that accompany the developing or angiogenic vascular bed within a tissue-engineered scaffold. Such observations are critical for quantitatively assessing the impact of bioactive scaffolds on stem cell survival and tissue regeneration as well as for informing their design. Future applications of this imaging platform involve its use to understand the effect of vessel phenotypes on the regenerative microenvironment within the graft. Furthermore, quantitative comparison of these vascular phenotypes across various tissue-engineered scaffolds would enable us to better understand the success or limitations of different approaches for treating critical-sized calvarial defects. The dynamic monitoring of the defect site provided by multimodal imaging platform facilitates insight into the mechanisms governing the regenerative process. These data will inform more effective designs of next-generation biomaterial scaffolds. Future experiments will include the validation of in vivo results with quantitative ex vivo histology-derived metrics of angiogenic status.47 Finally, by tracking the location of implanted stem cells relative to the angiogenic blood vessels, one could follow their differentiation and impact on bone reformation.
Conclusion
We have developed a novel multimodal system for imaging the microenvironment of bioactive scaffolds for tissue engineering applications in vivo. Our system facilitates the use of IOS, FL, and LSC imaging during a single experimental session to provide extensive data on the regrowth of the vascular network and characterization of the vascular phenotypes involved in bone healing. IOS imaging allows us to assess changes in vascular morphology, while FL imaging enables tracking of cell distribution within the scaffolds as well as measurement of tracer kinetic phenomena. LSC imaging provides crucial information on relative blood flow and vessel maturity within a vascular bed that is being dynamically remodeled during osteogenesis. Finally, the multimodal imaging in conjunction with the analyses methods described here make it possible to compare angiogenesis within defect sites across different experimental paradigms. We believe that such tools provide critical information to help optimize the design, implementation, and deployment of future tissue engineering approaches for critical-sized calvarial defects.
Acknowledgments
This work was supported by the National Cancer Institute award number 1R01CA196701-01, the Maryland Stem Cell Research Fund award number 2014-MSCRFI-0699, the National Science Foundation CBET1350554, and a Graduate Research Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Tevlin R., McArdle A., Atashroo D., et al. Biomaterials for craniofacial bone engineering. J Dent Res 93, 1187, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusumbe A.P., Ramasamy S.K., and Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusumbe A.P., Ramasamy S.K., Starsichova A., and Adams R.H. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nat Protoc 10, 1904, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Cook S.D., Salkeld S.L., Brinker M.R., Wolfe M.W., and Rueger D.C. Use of an osteoinductive biomaterial (rhOP-1) in healing large segmental bone defects. J Orthop Trauma 12, 407, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Semyari H., Rajipour M., Sabetkish S., et al. Evaluating the bone regeneration in calvarial defect using osteoblasts differentiated from adipose-derived mesenchymal stem cells on three different scaffolds: an animal study. Cell Tissue Bank 17, 69, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Sheyn D., Cohn Yakubovich D., Kallai I., et al. PTH promotes allograft integration in a calvarial bone defect. Mol Pharm 10, 4462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman E.M. Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt 12, 051402, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juavinett A.L., Nauhaus I., Garrett M.E., Zhuang J., and Callaway E.M. Automated identification of mouse visual areas with intrinsic signal imaging. Nat Protoc 12, 32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H.D., Chen G., Cai J., and Roe A.W. Intrinsic signal optical imaging of visual brain activity: tracking of fast cortical dynamics. Neuroimage 148, 160, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannestra A.F., Pouratian N., Forage J., Bookheimer S.Y., Martin N.A., and Toga A.W. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery 55, 804, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Nariai T., Sato K., Hirakawa K., et al. Imaging of somatotopic representation of sensory cortex with intrinsic optical signals as guides for brain tumor surgery. J Neurosurg 103, 414, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Rege A., Thakor N.V., Rhie K., and Pathak A.P. In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis. Angiogenesis 15, 87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisner J.K., Sumer S., Murrell K.P., Higgins T.J., and Price R.J. Laser speckle flowmetry method for measuring spatial and temporal hemodynamic alterations throughout large microvascular networks. Microcirculation 19, 619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senarathna J., Rege A., Li N., and Thakor N.V. Laser speckle contrast imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng 6, 99, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Ashkanian M., Gjedde A., Mouridsen K., et al. Carbogen inhalation increases oxygen transport to hypoperfused brain tissue in patients with occlusive carotid artery disease: increased oxygen transport to hypoperfused brain. Brain Res 1304, 90, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Macey P.M., Woo M.A., and Harper R.M. Hyperoxic brain effects are normalized by addition of CO2. PLoS Med 4, e173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dragojevic T., Bronzi D., Varma H.M., et al. High-speed multi-exposure laser speckle contrast imaging with a single-photon counting camera. Biomed Opt Express 6, 2865, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H., and Duong T.Q. Simplified laser-speckle-imaging analysis method and its application to retinal blood flow imaging. Opt Lett 32, 2188, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H., Yan Y., and Duong T.Q. Temporal statistical analysis of laser speckle images and its application to retinal blood-flow imaging. Opt Express 16, 10214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrester K.R., Stewart C., Tulip J., Leonard C., and Bray R.C. Comparison of laser speckle and laser Doppler perfusion imaging: measurement in human skin and rabbit articular tissue. Med Biol Eng Comput 40, 687, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Roustit M., Millet C., Blaise S., Dufournet B., and Cracowski J.L. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res 80, 505, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Stewart C.J., Frank R., Forrester K.R., Tulip J., Lindsay R., and Bray R.C. A comparison of two laser-based methods for determination of burn scar perfusion: laser Doppler versus laser speckle imaging. Burns J Int Soc Burn Inj 31, 744, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Cohn Yakubovich D., Tawackoli W., Sheyn D., et al. Computed tomography and optical imaging of osteogenesis-angiogenesis coupling to assess integration of cranial bone autografts and allografts. J Vis Exp e53459, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapoznik E., Niu G., Zhou Y., Murphy S.V., and Soker S. Fluorescent cell imaging in regenerative medicine. Biomed Eng Comp Biol 7, 29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel R., Daheron L., Liao S., et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A 110, 12774, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalchenko V., Israeli D., Kuznetsov Y., and Harmelin A. Transcranial optical vascular imaging (TOVI) of cortical hemodynamics in mouse brain. Sci Rep 4, 5839, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung B.P., Hutton D.L., Kozielski K.L., et al. Platelet-derived growth factor BB enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells. Stem Cells 33, 2773, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramasamy S.K., Kusumbe A.P., Schiller M., et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun 7, 13601, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briers J.D., and Fercher A.F. Retinal blood-flow visualization by means of laser speckle photography. Invest Ophthalmol Vis Sci 22, 255, 1982 [PubMed] [Google Scholar]
- 30.Gal T.J. Nunn's applied respiratory physiology, 5th ed In: Eisenach J.C. (ed.) Anesthesia and Analgesia, Vol. 90 Oxford, UK: Butterworth-Heinemann, 2000, p. 1009 [Google Scholar]
- 31.Yoon S., Zuccarello M., and Rapoport R.M. pCO(2) and pH regulation of cerebral blood flow. Front Physiol 3, 365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider C.A., Rasband W.S., Eliceiri K.W., and N.I.H. Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thevenaz P., Ruttimann U.E., and Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7, 27, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Sato Y., Nakajima S., Shiraga N., et al. Three-dimensional multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. Med Image Anal 2, 143, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Arganda-Carreras I., Fernandez-Gonzalez R., Munoz-Barrutia A., and Ortiz-De-Solorzano C. 3D reconstruction of histological sections: application to mammary gland tissue. Microsc Res Tech 73, 1019, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Leymarie F., and Levine M.D. Fast raster scan distance propagation on the discrete rectangular lattice. CVGIP Image Underst 55, 84, 1992 [Google Scholar]
- 37.Wang Y., Wan C., Deng L., et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117, 1616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie H., Cui Z., Wang L., et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20, 1270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bixel M.G., Kusumbe A.P., Ramasamy S.K., et al. Flow dynamics and HSPC homing in bone marrow microvessels. Cell Rep 18, 1804, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J.-l., Jiao K., Niu L.-N., et al. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 113, 203, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Bible E., Dell'Acqua F., Solanky B., et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials 33, 2858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolland B.J., Kanczler J.M., Dunlop D.G., and Oreffo R.O. Development of in vivo muCT evaluation of neovascularisation in tissue engineered bone constructs. Bone 43, 195, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Rueckel J., Stockmar M., Pfeiffer F., and Herzen J. Spatial resolution characterization of a X-ray microCT system. Appl Radiat Isot 94, 230, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Van Cleynenbreugel T., Schrooten J., Van Oosterwyck H., and Vander Sloten J. Micro-CT-based screening of biomechanical and structural properties of bone tissue engineering scaffolds. Med Biol Eng Comput 44, 517, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Steinman J., Koletar M.M., Stefanovic B., and Sled J.G. 3D morphological analysis of the mouse cerebral vasculature: comparison of in vivo and ex vivo methods. PLoS One 12, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avci P., Gupta A., Sadasivam M., et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32, 41, 2013 [PMC free article] [PubMed] [Google Scholar]
- 47.Pathak A.P., Hochfeld W.E., Goodman S.L., and Pepper M.S. Circulating and imaging markers for angiogenesis. Angiogenesis 11, 321, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., Zhu C., Ye D., et al. Porous silk scaffolds for delivery of growth factors and stem cells to enhance bone regeneration. PLoS One 9, e102371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]