Abstract
To investigate how additional visual feedback (VFB) affects postural stability we compared 20-sec center-of-pressure (COP) recordings in two conditions: without and with the VFB. Seven healthy adult subjects performed 10 trials lasting 20 seconds in each condition. Simultaneously, during all trials the simple auditory reaction time (RT) was measured. Based on the COP data, the following sway parameters were computed: standard deviation (SD), mean speed (MV), sample entropy (SE), and mean power frequency (MPF). The RT was higher in the VFB condition (p < 0.001) indicating that this condition was attention demanding. The VFB resulted in decreased SD and increased SE in both the medial-lateral (ML) and anterior-posterior (AP) planes (p < .001). These results account for the efficacy of the VFB in stabilizing posture and in producing more irregular COP signals which may be interpreted as higher automaticity and/or larger level of noise in postural control. The MPF was higher during VFB in both planes as was the MV in the AP plane only (p < 0.001). The latter data demonstrate higher activity of postural control system that was caused by the availability of the set-point on the screen and the resulting control error which facilitated and sped up postural control.
Keywords: Body balance, Sway entropy, Biofeedback, Attentional focus
Introduction
The contribution of visual control of balance while standing increases significantly with additional visual feedback (VFB) implemented through the conscious control of the center of pressure displacements under the feet (COP) (Litvinenkova & Hlavacka, 1973).
A positive effect of VFB has been shown in many studies (Litvinenkova & Hlavacka, 1973; Takeya, 1976; Rougier, 2003) indicating a reduction of the centre of gravity motions with associated increase in muscular activity. However, there are also the investigations that do not confirm such influence. Results of Danna-Dos-Santos et al. (2008) indeed demonstrated that participants were unable to decrease sway amplitude when presented with visual feedback, whereas in Boudrahem & Rougier (2009) it has been shown that only 69% of subjects were able to use additional visual feedback to reduce COP displacements. Ambiguous findings are also found in reports of attempts to apply VFB technique for rehabilitation. In some studies, the effects appear quite positive (Shumway-Cook, Anson & Haller, 1988; Sihvonen, Sipilä & Era, 2004; Cheng et al., 2004; Ledebt et al., 2005; Sayenko et al., 2010), whereas others should be considered as scarce (Walker, Brouwer & Culham, 2000; Geiger et al., 2001). The sources of these discrepancies may be inherent in the way of presenting feedback e.g., insufficient scale display of COP on the screen (Vuillerme, Bertrand & Pinsault, 2008) and/or delay of the signal on the screen (Rougier, 2004; Van den Heuvel et al., 2009), but also high cognitive demands associated with learning a new task (Wulf & Shea, 2002; Van Vliet & Wulf, 2006). Also, many studies have provided evidence that there are significant attentional requirements for postural control (Woollacott & Shumway-Cook, 2002). Further, the attentional demand associated with postural control can be modified by the sensory context (Vuillerme, Isableu & Nougier, 2006).
A fuller understanding of postural control mechanisms through the conscious control of the center of pressure displacements under the feet allows for a more in-depth diagnosis of certain pathological conditions, and can also be important in training the balance of both patients and athletes (Szczepańska-Gieracha et al., 2016).
Taken together, there is still no consensus as to how vertical posture is controlled when the participants are presented with visual feedback from the actual position of their COP. The predominant view is that attentional resources must be involved due to the larger complexity of the VFB as compared to quiet stance (Lakhani & Mansfield, 2015), however little is known in what way these resources are used and whether their shifts facilitate or interfere with maintaining balance. To better elucidate the underlying mechanisms, it seems crucial to compare the COP measures during VFB and quiet stance with simultaneously recorded reaction time task. While the traditional sway measures account for postural performance, of special interest is sway entropy which quantifies the attentional investment in postural control (Roerdink, Hlavackova & Vuillerme, 2011).
Therefore, this study aims to answer the question whether and how the postural task with additional visual feedback requires more attentional demands in young adults. Therefore, we examined reaction times during VFB and while changing the amount and structure of COP time series.
Methods
Seven young students participated in the study (mean age (SD): 22.9 (1.1) years; range: 22–25 years, three females, four males). All subjects were healthy and did not undergo any disease that might affect the balance system. They gave their written informed consent to the procedure and were naive as to the purpose of the experiment. The study was approved by the Senate Ethics Committee for Research at the University School of Physical Education in Wroclaw. Written informed consent was obtained from all participants.
Data were collected as previously described in Simoneau, Bégin & Teasdale (2006) and Vuillerme, Isableu & Nougier (2006). Specifically, postural stability was assessed on a force plate (AccuSway, AMTI, Watertown, MA, USA) in front of a computer monitor positioned at eye level at a distance of 1 m. Participants were asked to perform two different dual tasks. In the reference condition (REF), they were asked to sway as little as possible fixating a white sign on the screen and simultaneously perform a probe-reaction time (RT) task. The RT task consisted of responding as rapidly as possible to an unpredictable auditory stimulus by pressing a handheld button. Eight reaction time stimuli were presented within each 20 s trial. During the second condition (VFB), the COP position was displayed as a spot (diameter of about 4 mm) on the monitor and the subjects were instructed to keep the spot inside the circle (diameter of about 5 mm) on the monitor and simultaneously perform the RT task. The ratio between the real displacements of the COP and their display on the 19-inch screen was twofold for both the anterior-posterior (AP) and medial-lateral (ML) planes. The AP and ML displacements of the COP were represented on the screen from top to bottom and from left to right, respectively. Postural tasks were the primary tasks, and the subjects were asked to treat it as a priority. Subjects stood in the position as follows: 17 cm between heel centers, with an angle of 14° between the long axes of the feet (McIlroy & Maki, 1997). Ten trials for each condition (lasting 20-second each) were presented in pseudo-random (balanced) order.
Data were recorded at sampling frequency 50 Hz. The instantaneous center of foot pressure was calculated from the recorded ground reaction forces in the medial-lateral and anterior-posterior plane separately. The raw COP data were not digitally filtered. Postural balance was evaluated by following parameters based on the COP time-series: standard deviation (SD), mean speed (MV), mean power frequency (MPF) (Prieto et al., 1996; Duarte & Freitas, 2010) and sample entropy (SE) (Richman & Randall Moorman, 2000). The SD and MV measure postural performance, with lower values of these parameters indicating better performance and MPF reveals postural strategy. SD, MV and MPF were computed using MATLAB codes available at Duarte & Freitas (2010). SD is the dispersion of COP displacement from the mean position during a trial duration, MV was calculated as the total COP displacement divided by trial duration and MPF is the mean spectral power frequency of the signal estimated up to 25 Hz range. The power spectral density of the detrended COP data was estimated by the Welch periodogram method. SE indexes the regularity or predictability of a time-series. Increased values of sample entropy, which indicate larger irregularity of the COP, has been attributed to a reduced amount of attention invested in posture (Roerdink, Hlavackova & Vuillerme, 2011). Input parameters for estimating the sample entropy were based on the median value of the relative error (Lake et al., 2002) resulting in the selection of pattern length m = 2 and error tolerance r = 0.08 and 0.05 as optimal parameters for ML and AP time series (normalized to unit variance) respectively of all subjects and trials. SE was computed using MATLAB script available at http://www.physionet.org. RT (in milliseconds) helped for determining the attentional demand associated with postural control and was defined as the time interval between the presentation of the auditory stimulus and the subjects’ pressing the handheld button (Abernethy, 1988).
A linear mixed-effects model was used to test the effect of VFB on RT and COP indices. Trial, feedback (no feedback vs. additional visual feedback) and their interaction were subjected as fixed factors. The effect of trial was chosen as fixed factor to account for any potential fatigue and/or learning effects. Participants were included as a random intercept to take dependency (correlation structure) in the data into account (Kuznetsov et al., 2015; Boisgontier et al., 2017). Due to skewed distributions, we used log10-transformed data. The level of significance was set at P < 0.05. Random intercept models take into account the dependence of repeated trials and have substantial advantages over repeated measures ANOVA. All analyses were performed using free and open software JAMOVI 0.8.2.2 with GAMLj module (retrieved from https://www.jamovi.org).
Results
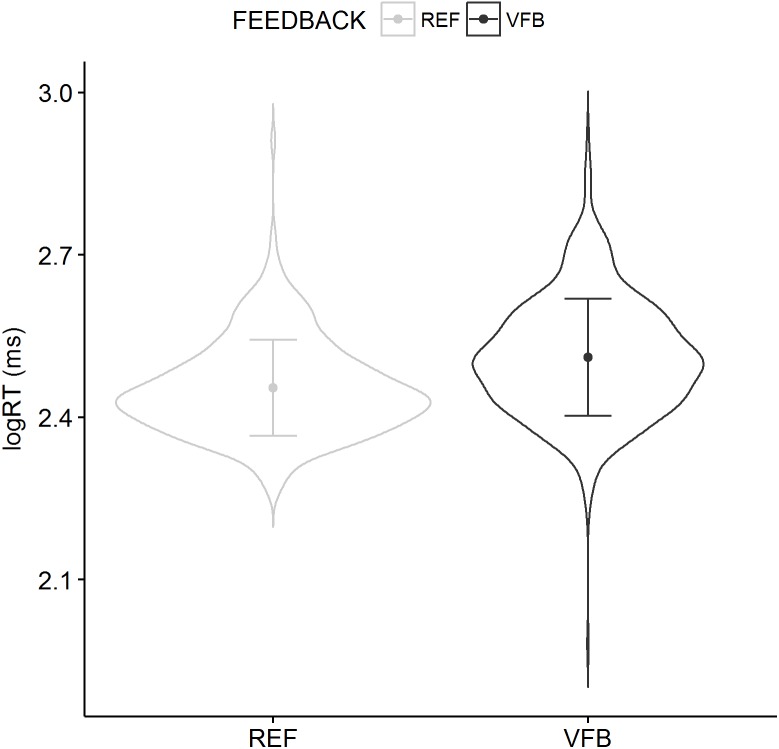
RT was higher in the VFB condition (fixed effects ANOVA, F(1, 1094) = 96.89, p < 0.001). No trial main effect (F(9, 1094) = 1.32, p = 0.219) or feedback × trial interaction (F(9, 1094) = 1.16, p = 0.317) showed statistical significance. The distributions of the RT are shown in Fig. 1.
Figure 1. Violin plots of the RT for the REF and the VFB conditions collapsed over trials with mean and standard deviations superimposed.
These plots show full distribution of the data obtained by kernel density estimation. The dot symbol denotes mean, whisker denotes standard deviation.
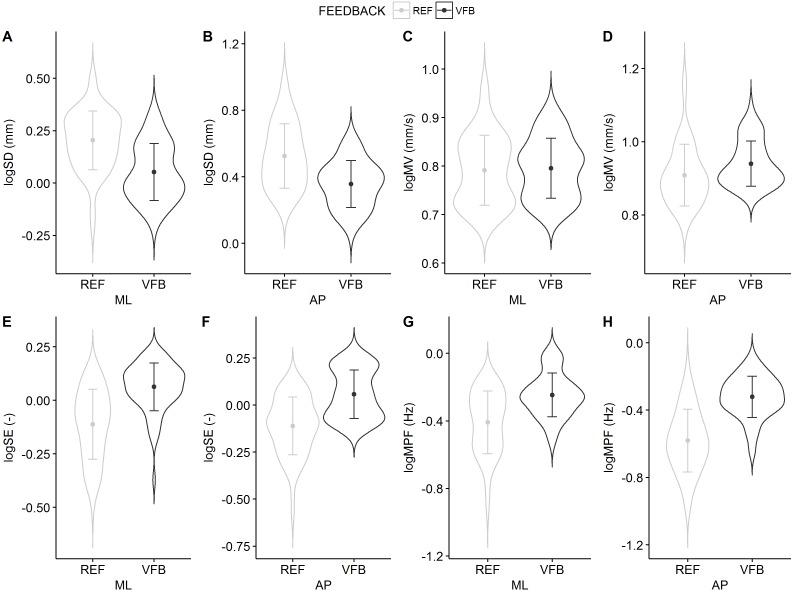
For SD there were no interaction feedback × trial effects for both ML (F(9, 114) = 0.92, p = 0.507) and AP planes (F(9, 114) = 1.82, p = 0.072). SD was lower in the VFB condition for both ML (F(1, 114) = 68.24, p < 0.001) and AP planes (F(1, 114) = 62.47, p < 0.001); there was no significant main effect of trial (ML: F(9, 114) = 1.13, p = 0.347; AP: F(9, 114) = 0.95, p = 0.483).
For MV there were no interaction feedback × trial effects for both ML (F(9, 114) = 0.96, p = 0.479) and AP planes (F(9, 114) = 1.40, p = 0.198). MV was higher in the VFB condition for AP plane (F(1, 114) = 15.08, p < 0.001) and showed no change in the ML plane (F(1, 114) = 0.71, p = 0.402); there was no significant main effect of trial (ML: F(9, 114) = 0.85, p = 0.570; AP: F(9, 114) = 0.74, p = 0.675).
For SE there were no interaction feedback × trial effects for both ML (F(9, 114) = 0.94, p = 0.490) and AP planes (F(9, 114) = 1.07, p = 0.393). SE was higher in the VFB condition for both ML (F(1, 114) = 78.61, p < 0.001) and AP planes (F(1, 114) = 74.83, p < 0.001); there was no significant main effect of trial (ML: F(9, 114) = 1.61, p = 0.120; AP: F(9, 114) = 1.60, p = 0.123).
For MPF there were no interaction feedback × trial effects for both ML (F(9, 114) = 0.98, p = 0.463) and AP planes (F(9,114)=0.846, p = 0.575). MPF was higher in the VFB condition for both ML (F(1, 114) = 68.49, p < 0.001) and AP planes (F(1, 114) = 99.99, p < 0.001); there was no significant main effect of trial (ML: F(9, 114) = 0.813, p = 0.605; AP: F(9, 114) = 0.50, p = 0.827).
The distributions of the COP parameters are shown in Fig. 2.
Figure 2. Violin plots of the COP parameters for the REF and the VFB conditions collapsed over trials with and mean ± standard deviations superimposed: SD, variability; MV, mean speed; SE, sample entropy; MPF, mean power frequency.
These plots show full distribution of the data obtained by kernel density estimation. The dot symbol denotes mean, whisker denotes standard deviation.
All analyses are available for download using the Open Science Framework: https://osf.io/mptkr/.
Discussion
The purpose of this study was to determine whether and how the postural task with additional visual feedback requires additional attentional demands in young adults. The results show that: (1) VFB condition requires additional attentional demands because reaction times were longer, (2) concurrent visual feedback about postural sway shifts focus of attention not directly to postural control because of increase of SE, (3) the implementation of the VFB task has triggered the need for a change in the postural strategy through a reduction in the amount of sway and increase of MV and MPF.
In agreement with our work, the increased COP entropy during visual feedback tasks was reported by Donker et al. (2008) and Lakhani & Mansfield (2015). They attributed these results to the effect of using the external reference system which is thought to facilitate more automatic control of posture (Wulf, McNevin & Shea, 2001). Similarly, the increased reaction times in our experiments account for shifting the attention of participants to the task of keeping their COP inside the target on the screen. Focusing significant attentional resources on the latter task took from the attention which is normally used to maintain postural control which also implies more automaticity in maintaining balance.
All participants were able to effectively use the VFB in reducing their sway amplitude, yet this activity was accompanied by the increase in sway frequency. Higher frequency of postural sway has been often reported during dual tasks that led to reduced amount of attention which is normally involved in postural control. It is argued that increased sway frequency results from increased joint stiffness (Vuillerme & Vincent, 2006; Bieć et al., 2014). Such an interpretation seems justified based on the relationship between the effective postural stiffness and the frequency of the COP signal that was established by Winter et al. (1998). In contrast, Stins, Roerdink & Beek (2011) did not find a direct association between postural stiffness and the level of automaticity in controlling posture. However, it is possible that postural tasks with visual feedback have different effects on the distribution of attentional resources than other supplementary cognitive tasks that are apparently not related to posture.
The difference lies in the final application of the attention diverted from posture and invested into the supplementary task. In the latter tasks a necessary portion of attentional resources is being withdrawn from the normal postural control and this loss requires compensation or some other form of reinforcement. According to several authors, this is usually accomplished by promoting the more automatic control process (Kuczyński, Szymańska & Bieć, 2011; Lakhani & Mansfield, 2015).
However, in the former task, attention transferred to the VFB was indirectly reverted and actually supported the process of postural control. Larger reaction times in the VFB indicate that this task is attention demanding and one can speculate that the diverted attention is necessary for the integration of the ancillary visual input with the remaining sensory information. Again, elevated sway frequency seems to irrevocably contribute to this purpose. In fact, the stiffening of postural strategy was suggested as the means to perform the postural exploratory and/or monitoring function which significantly increases with the difficulty of postural tasks (Latash et al., 2003). This exploratory function of sway is ceaselessly active, even during conscious control of posture, and is thought to have random bearing. A certain level of randomness of spontaneous sway is inherent because of its unconscious origin. However, an additional and quite substantial uncertainty in this signal may develop from the conscious action of participants using the visual error to correct their position inside the target on the screen. Although the purpose of the action is conscious, its timing and magnitude are not, and the two latter factors depend heavily on the sensorimotor abilities and performance of the subjects. In other words, increased sway entropy observed during VFB may not only be the consequence of a more automatic control of posture but also the reflection of increased uncertainty in performance. This would be in agreement with Morrison, Hong & Newell (2007) who found that subjects who voluntarily produced random sway motions exhibited higher COP entropy as compared to standing still. In a similar vein Borg & Laxaback (2010) postulated that higher entropy may be interpreted as an inability in some circumstances to exert effective attentive control.
Conclusions
In conclusion, the VFB is effective in enhancing and improving postural performance. This benefit is associated with increased sensorimotor activity, and its effect on humans, depending on circumstances, may be different. VFB has higher attentional demands as compared to normal stance. VFB increases irregularity and entropy of sway, still presented results seem insufficient to disentangle the role of elevated automaticity or noisiness in these changes.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Krzysztof Kręcisz conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Michał Kuczyński conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Senate Ethics Committee for Research at the University School of Physical Education in Wroclaw granted Ethical approval to carry out the study within its facilities.
Data Availability
The following information was supplied regarding data availability:
Open Science Framework: https://osf.io/mptkr/.
References
- Abernethy (1988).Abernethy B. Dual-task methodology and motor skills research: some applications and methodological constraints. Journal of Human Movement Studies. 1988;14:101–132. [Google Scholar]
- Bieć et al. (2014).Bieć E, Zima J, Wójtowicz D, Wojciechowska-Maszkowska B, Kręcisz K, Kuczyński M. Postural stability in young adults with Down syndrome in challenging conditions. PLOS ONE. 2014;9(4):e94247. doi: 10.1371/journal.pone.0094247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier et al. (2017).Boisgontier MP, Cheval B, Chalavi S, Van Ruitenbeek P, Leunissen I, Levin O, Nieuwboer A, Swinnen SP. Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiology Aging. 2017;50:47–59. doi: 10.1016/j.neurobiolaging.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Borg & Laxaback (2010).Borg FG, Laxaback G. Entropy of balance—some recent results. Journal of NeuroEngineering and Rehabilitation. 2010;7:38. doi: 10.1186/1743-0003-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrahem & Rougier (2009).Boudrahem S, Rougier PR. Relation between postural control assessment with eyes open and centre of pressure visual feedback effects in healthy individuals. Experimental Brain Research. 2009;195:145–152. doi: 10.1007/s00221-009-1761-1. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2004).Cheng PT, Wang CM, Chung CY, Chen CL. Effects of visual feedback rhythmic weight-shift training on hemiplegic stroke patients. Clinical Rehabilitation. 2004;18:747–753. doi: 10.1191/0269215504cr778oa. [DOI] [PubMed] [Google Scholar]
- Danna-Dos-Santos et al. (2008).Danna-Dos-Santos A, Degani AM, Zatsiorsky VM, Latash ML. Is voluntary control of natural postural sway possible? Journal of Motor Behavior. 2008;40:179–185. doi: 10.3200/JMBR.40.3.179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker et al. (2008).Donker SF, Ledebt A, Roerdink M, Savelsbergh GJP, Beek PJ. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Experimental Brain Research. 2008;184:363–370. doi: 10.1007/s00221-007-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte & Freitas (2010).Duarte M, Freitas SMSF. Revision of posturography based on force plate for balance evaluation. Revista Brasileira de Fisioterapia. 2010;14:183–192. doi: 10.1590/S1413-35552010000300003. [DOI] [PubMed] [Google Scholar]
- Geiger et al. (2001).Geiger RA, Allen JB, O’Keefe J, Hicks RR. Balance and mobility following stroke: effects of physical therapy interventions with and without biofeedback/forceplate training. Physical Therapy. 2001;81:995–1005. [PubMed] [Google Scholar]
- Kuczyński, Szymańska & Bieć (2011).Kuczyński M, Szymańska M, Bieć E. Dual-task effect on postural control in high-level competitive dancers. Journal of Sports Sciences. 2011;29:539–45. doi: 10.1080/02640414.2010.544046. [DOI] [PubMed] [Google Scholar]
- Kuznetsov et al. (2015).Kuznetsov NA, Luberto CM, Avallone K, Kraemer K, McLeish AC, Riley MA. Characteristics of postural control among young adults with asthma. Journal of Asthma. 2015;52:191–197. doi: 10.3109/02770903.2014.954290. [DOI] [PubMed] [Google Scholar]
- Lake et al. (2002).Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2002;283:R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lakhani & Mansfield (2015).Lakhani B, Mansfield A. Visual feedback of the centre of gravity to optimize standing balance. Gait and Posture. 2015;41:499–503. doi: 10.1016/j.gaitpost.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Latash et al. (2003).Latash ML, Ferreira SS, Wieczorek SA, Duarte M. Movement sway: changes in postural sway during voluntary shifts of the center of pressure. Experimental Brain Research. 2003;150:314–324. doi: 10.1007/s00221-003-1419-3. [DOI] [PubMed] [Google Scholar]
- Ledebt et al. (2005).Ledebt A, Becher J, Kapper J, Rozendaal RM, Bakker R, Leenders IC, Savelsbergh GJP. Balance training with visual feedback in children with hemiplegic cerebral palsy: effect on stance and gait. Motor Control. 2005;9:459–468. doi: 10.1123/mcj.9.4.459. [DOI] [PubMed] [Google Scholar]
- Litvinenkova & Hlavacka (1973).Litvinenkova V, Hlavacka F. The visual feedback gain influence upon the regulation of the upright posture in man. Agressologie. 1973;14:95–99. [PubMed] [Google Scholar]
- McIlroy & Maki (1997).McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clinical Biomechanics. 1997;12:66–70. doi: 10.1016/S0268-0033(96)00040-X. [DOI] [PubMed] [Google Scholar]
- Morrison, Hong & Newell (2007).Morrison S, Hong SL, Newell KM. Inverse relations in the patterns of muscle and center of pressure dynamics during standing still and movement postures. Experimental Brain Research. 2007;181:347–358. doi: 10.1007/s00221-007-0928-x. [DOI] [PubMed] [Google Scholar]
- Prieto et al. (1996).Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions on Biomedical Engineering. 1996;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- Richman & Randall Moorman (2000).Richman JS, Randall Moorman J. Physiological time-series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Roerdink, Hlavackova & Vuillerme (2011).Roerdink M, Hlavackova P, Vuillerme N. Center-of-pressure regularity as a marker for attentional investment in postural control: a comparison between sitting and standing postures. Human Movement Science. 2011;30:203–212. doi: 10.1016/j.humov.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Rougier (2003).Rougier P. Visual feedback induces opposite effects on elementary centre of gravity and centre of pressure minus centre of gravity motions in undisturbed upright stance. Clinical Biomechanics. 2003;18:341–349. doi: 10.1016/S0268-0033(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Rougier (2004).Rougier P. Optimising the visual feedback technique for improving upright stance maintenance by delaying its display: behavioural effects on healthy adults. Gait and Posture. 2004;19:154–163. doi: 10.1016/S0966-6362(03)00056-0. [DOI] [PubMed] [Google Scholar]
- Sayenko et al. (2010).Sayenko DG, Alekhina MI, Masani K, Vette AH, Obata H, Popovic MR, Nakazawa K. Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord. 2010;48:886–893. doi: 10.1038/sc.2010.41. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook, Anson & Haller (1988).Shumway-Cook A, Anson D, Haller S. Postural sway biofeedback: its effect on reestablishing stance stability in hemiplegic patients. Archives of Physical Medicine and Rehabilitation. 1988;69:395–400. [PubMed] [Google Scholar]
- Sihvonen, Sipilä & Era (2004).Sihvonen SE, Sipilä S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology. 2004;50:87–95. doi: 10.1159/000075559. [DOI] [PubMed] [Google Scholar]
- Simoneau, Bégin & Teasdale (2006).Simoneau M, Bégin F, Teasdale N. The effects of moderate fatigue on dynamic balance control and attentional demands. Journal of NeuroEngineering and Rehabilitation. 2006;3:22. doi: 10.1186/1743-0003-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins, Roerdink & Beek (2011).Stins JF, Roerdink M, Beek PJ. To freeze or not to freeze? Affective and cognitive perturbations have markedly different effects on postural control. Human Movement Science. 2011;30:190–202. doi: 10.1016/j.humov.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Szczepańska-Gieracha et al. (2016).Szczepańska-Gieracha J, Cieślik B, Chamela-Bilińska D, Kuczyński M. Postural stability of elderly people with cognitive impairments. American Journal of Alzheimer’s Disease & Other Dementiasr. 2016;31:241–246. doi: 10.1177/1533317515602547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya (1976).Takeya T. A biofeedback study of postural sway. Psychiatry and Clinical Neurosciences. 1976;30:495–504. doi: 10.1111/j.1440-1819.1976.tb02672.x. [DOI] [Google Scholar]
- Van den Heuvel et al. (2009).Van den Heuvel MRC, Balasubramaniam R, Daffertshofer A, Longtin A, Beek PJ. Delayed visual feedback reveals distinct time scales in balance control. Neuroscience Letters. 2009;452:37–41. doi: 10.1016/j.neulet.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Van Vliet & Wulf (2006).Van Vliet P, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disability and Rehabilitation. 2006;28:831–840. doi: 10.1080/09638280500534937. [DOI] [PubMed] [Google Scholar]
- Vuillerme, Bertrand & Pinsault (2008).Vuillerme N, Bertrand R, Pinsault N. Postural effects of the scaled display of visual foot center of pressure feedback under different somatosensory conditions at the foot and the ankle. Archives of Physical Medicine and Rehabilitation. 2008;89:2034–2036. doi: 10.1016/j.apmr.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Vuillerme, Isableu & Nougier (2006).Vuillerme N, Isableu B, Nougier V. Attentional demands associated with the use of a light fingertip touch for postural control during quiet standing. Experimental Brain Research. 2006;169:232–236. doi: 10.1007/s00221-005-0142-7. [DOI] [PubMed] [Google Scholar]
- Vuillerme & Vincent (2006).Vuillerme N, Vincent H. How performing a mental arithmetic task modify the regulation of centre of foot pressure displacements during bipedal quiet standing. Experimental Brain Research. 2006;169(1):130–134. doi: 10.1007/s00221-005-0124-9. [DOI] [PubMed] [Google Scholar]
- Walker, Brouwer & Culham (2000).Walker C, Brouwer B, Culham E. Use of visual feedback in retraining balance following acute stroke. Physical Therapy. 2000;80:886–895. [PubMed] [Google Scholar]
- Winter et al. (1998).Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. Journal of Neurophysiology. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Woollacott & Shumway-Cook (2002).Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait & Posture. 2002;16:1–14. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Wulf, McNevin & Shea (2001).Wulf G, McNevin N, Shea CH. The automaticity of complex motor skill learning as a function of attentional focus. The Quarterly Journal of Experimental Psychology A. 2001;54:1143–1154. doi: 10.1080/02724980143000118. [DOI] [PubMed] [Google Scholar]
- Wulf & Shea (2002).Wulf G, Shea CH. Principles derived from the study of simple skills do not generalize to complex skill learning. Psychonomic Bulletin and Review. 2002;9:185–211. doi: 10.3758/BF03196276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Open Science Framework: https://osf.io/mptkr/.