Abstract
Multiple myeloma (MM) presents a poor prognosis and high lethality of patients due to development of drug resistance. P-glycoprotein (P-gp), a drug-efflux transporter, is upregulated in MM patients post-chemotherapy and is involved in the development of drug resistance since many anti-myeloma drugs (including proteasome inhibitors) are P-gp substrates. Hypoxia develops in the bone marrow niche during MM progression and has long been linked to chemoresistance. Additionally, hypoxia-inducible transcription factor (HIF-1α) was demonstrated to directly regulate P-gp expression. We found that in MM patients P-gp expression positively correlated with the hypoxic marker, HIF-1α. Hypoxia increased P-gp protein expression and its efflux capabilities in MM cells in vitro using flow cytometry. We reported herein that hypoxia-mediated resistance to carfilzomib and bortezomib in MM cells is due to P-gp activity and was reversed by tariquidar, a P-gp inhibitor. These results suggest combining proteasome inhibitors with P-gp inhibition for future clinical studies.
Keywords: multiple myeloma, drug resistance, P-glycoprotein, hypoxia
Introduction
Multiple myeloma (MM) is a hematologic malignancy of the plasma cells that involves mainly the bone marrow. The use of novel therapies and autologous stem cell transplantation improved median survival of MM patients up to 7 years. Although current treatments in MM including carfilzomib and bortezomib are initially effective, over 90% of MM patients eventually relapse and the disease recurs because of the ability of cancerous cells to become drug-resistant to the previously efficacious therapy [1].
An increased expression of the efflux pumps, also called the membrane multi-drug resistance (MDR) transporters, reduce the intracellular concentration of chemotherapies and constitutes one of the major reasons for the development of treatment resistance in myeloma, leukemia and solid tumors [2, 3, 4]. P-glycoprotein (P-gp) is the most studied efflux protein that minimizes the intracellular accumulation of toxic xenobiotics by excretion into bile, urine and the intestinal lumen, and is commonly expressed in several tissues; most notably in kidney, liver, intestines and blood-brain barrier [3]. P-gp overexpression is an adaptive mechanism by which cells, including myeloma cells, combat xenobiotics and other cytotoxic chemotherapies, leading to the development of treatment resistance [2, 3]. P-gp overexpression is rarely detected in newly diagnosed MM patients; however, treatment with high doses of chemotherapeutics such as vincristine, doxorubicin and dexamethasone was associated with P-gp upregulation in more than 80% of MM patients [5, 6, 7, 8, 9]. Since the discovery of P-gp [10], a plethora of P-gp inhibitors were explored in order to overcome MDR and to increase bioavailability of the therapeutic P-gp substrates in cancer treatment [2, 11, 12, 13]. Tariquidar (XR 9576), an anthranilamide derivative, is one of highly potent, selective and non-competitive 3rd generation inhibitor that inhibits ATPase by interacting with a distinct modulatory binding site on the protein; thus tariquidar is believed to be one of the most promising P-gp-mediated drug resistance modulators [14, 15].
Decreased oxygen tension (hypoxia) is another crucial factor contributing to patients’ resistance to chemotherapy as well as to radiotherapy [16, 17, 18]. Hypoxia develops in the bone marrow during MM progression and plays a major role in MM cell dissemination through epithelial-to-mesenchymal transition (EMT)-like mechanisms [19]. Furthermore, hypoxia induces chemo-resistance in MM, particularly to proteasome inhibitors, through the acquisition of stem-like properties [20]. It was demonstrated previously that mdr1 (abcb1), the murine analog of the human MDR1 gene encoding P-gp, is regulated by hypoxia in cancer cells; more specifically that P-gp is a downstream target of hypoxia-inducible factor-1α (HIF-1α) since inhibition of HIF-1α decreased the expression of P-gp [21, 22, 23].
Many drugs used in MM were identified as MDR transporters’ substrates, including bortezomib and carfilzomib [24, 25, 26]. The first ever proteasome inhibitor approved by U.S. Food and Drug Administration to treat MM patients (FDA) was bortezomib (in 2003). Despite relatively high response rates (30–4 0%), there is still a substantial group of patients who get no or little benefit following bortezomib treatment [27]. Carfilzomib received an accelerated approval by the FDA for MM treatment in 2012, and it was shown to illicit a response from a fraction of patients who relapsed from bortezomib. Epoxomicin is a natural substrate of P-gp and since carfilzomib belongs to the epoxyketone class of proteasome inhibitors, it demonstrated substrate specificity to P-gp [24, 26, 28]. It is possible that chronic treatment with epoxomicin/carfilzomib induces P-gp expression and is associated with the acquisition of drug resistance. It was reported by Rumpold et al that bortezomib is a P-gp substrate; however, to a lesser degree than carfilzomib; these two proteasome inhibitors may have different kinetics and P-gp affinity hence their efficiency may differ in P-gp overexpressing cancer cells [26].
In the study described herein, we examined the resistance mechanisms to proteasome inhibitors in MM, focusing on the role of hypoxia and P-gp-induced drug resistance. Here, we tested whether hypoxia-mediated resistance to carfilzomib and bortezomib in MM cells is due to P-gp activity and could be reversed by tariquidar, a specific inhibitor of P-gp efflux activity. In summary, we found that P-gp effluxes drugs extracellularly and compromises therapeutic efficacy, and thus it is believed that P-gp overexpression in hypoxia contributes to drug resistance in MM. We demonstrated that blocking P-gp in hypoxia by combination of tariquidar and proteasome inhibitors in MM cells led to re-sensitization of MM cells to bortezomib and carfilzomib. This strategy minimized the extent of P-gp efflux, which augmented the cytotoxic effects of these drugs in vitro.
Materials and Methods
Gene expression analysis
Two publically accessible datasets of MM patients were used, the MMRF CoMMpass trial [29] and Zhan et al [30]. In the analysis of CoMMpass trial, the gene expression of P-gp (ENSG00000085563) and HIF-1α (ENSG00000100644) from CD-138-selected bone marrow plasma cells from 664 newly diagnosed MM patients were extracted from the E74cDNA Salmon gene count dataset corresponding with the IA9 data release [29]. In the analysis of Zhan et al., gene expression of P-gp (ID probe 209993_at) and HIF-1α (ID probe 200989_at) from CD-138-selected bone marrow plasma cells from 559 newly diagnosed MM patients were extracted from the Gene Expression Omnibus [30].
Cell culture
The MM cell lines (MM.1S, H929, OPM2, U266) were freshly obtained from American Type Culture Collection (ATCC, Rockville, MD) and characterized by ATCC based on the karyotype and expression of cluster of differentiation (CD) markers, receptors and light chain immunoglobulin. Cells were cultured in RPMI-1640 (Corning CellGro) enriched with 10% fetal bovine serum (Gibco, Life Technologies), 2 mmol/L of L-glutamine, 100 U/mL Penicillin and 100 μg/mL Streptomycin (CellGro). Cells were incubated at 37°C (5% CO2) under normoxic (21% O2, NuAire) or hypoxic conditions (1% O2, Coy) followed by in vitro assays.
In vitro assays
Cell viability assay: the cellular sensitivity of MM cells to tariquidar (5μM), bortezomib (5nM) and carfilzomib (5nM) purchased from Selleck Chem was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich), according to manufacturers’ protocol. Briefly, after 24 hours of cell treatment, the MTT solution was added to the cells for 3 hours, then the stop solution was added and the formazan crystals solubilized overnight, followed by the absorbance readout at 570nm using a spectrophotometer.
Protein expression and activity: P-gp protein expression was tested by flow cytometry using an anti-human P-gp antibody (BD Biosciences, catalog # 557003). The basal level of P-gp in MM cells in normoxia and hypoxia were demonstrated as mean fluorescent intensity (MFI). P-gp activity was tested by the intracellular content of rhodamine B (RhoB) and Rho123 (Sigma-Aldrich) previously treated and cultured in normoxia or hypoxia, followed by 1 hour incubation with 0.5 μg/mL of Rho, washed twice with 1 x PBS, the MFI of RhoB (Ex/Em = 540/625 nm) and Rho123 (Ex/Em = 501/529 nm) was measured by flow cytometry, and normalized to unstained.
Statistical analysis
The gene expression data was log transformed to correct for right-shifted distribution. Expression of P-gp was compared between patients with intermediate HIF-1α [HIF-1αint] expression (±1 SD from the mean) to those with high [HIF-1αhigh] (>1 SD above the mean) and low [HIF-1αlow] (>1 SD below the mean) expression using one-way analysis of variance (ANOVA) to demonstrate the correlation of P-gp mRNA expression with this hypoxic marker.
The in vitro experiments were performed in triplicates and replicated independently at least three times. Results are shown as mean ± standard deviation (s.d.) and statistical significance was assessed by student t-test and considered significant for values * p<0.05, ** p<0.01 and *** p<0.001.
Results
P-gp overexpression positively correlates with HIF-1α expression in MM plasma cells
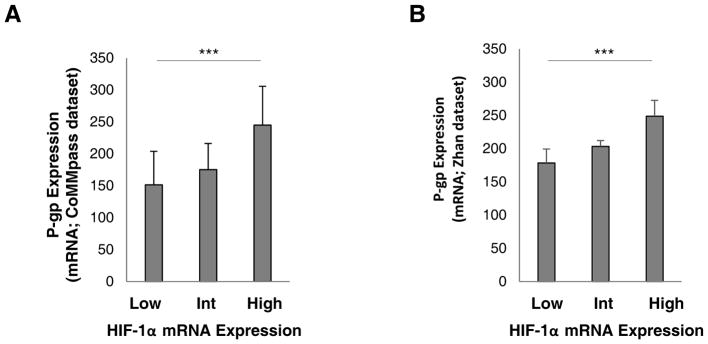
Since P-gp is regulated by hypoxia and is a downstream target of HIF-1α [21, 22, 23], we performed an analysis of P-gp expression in plasma cells collected from MM patients using two datasets, the Multiple Myeloma Research Foundation (MMRF) CoMMpass trial [29] and Zhan et al 2006 [30]. In the CoMMpass trial dataset, all patients expressed HIF-1α and 98% expressed P-gp. The median HIF-1α and P-gp expression was 772.28 and 36.04, respectively. P-gp expression was higher among HIF-1αhigh patients (M= 244.91; SD= 681.42; N= 126) compared to HIF-1αint (M= 175.19; SD= 851.11; N= 430) and HIF-1αlow (M= 151.49; SD= 544.87; N= 108) patients (p <0.0001) (Figure 1A). These results were consistent with the analysis obtained from the Zhan et al dataset where all patients expressed both HIF-1α and P-gp. The median HIF-1α and P-gp expression was 1036.50 and 158.20, respectively. P-gp expression was higher among HIF-1αhigh patients (M= 248.78; SD= 254.39; N= 113) compared to HIF-1αint (M= 203.35; SD= 164.91; N= 348) and HIF-1αlow (M= 178.38; SD= 208.28; N= 98) patients (p <0.0001) (Figure 1B). These results indicate that the magnitude of HIF-1α expression strongly correlates with P-gp mRNA levels.
Figure 1. P-gp overexpression positively correlates with HIF-1α expression in MM plasma cells.
(A) Gene expression analysis of P-gp (ENSG00000085563) and HIF-1α (ENSG00000100644) in MM patients stratified into HIF-1αlow (n=108), HIF-1αint (n=430) and HIF-1αhigh (n=126) based on the CoMMpass trial dataset. (B) Gene expression analysis of P-gp (ID probe 209993_at) and HIF-1α (ID probe 200989_at) in MM patients stratified into HIF-1αlow (n=98), HIF-1αint (n=348) and HIF-1αhigh (n=113) based on published datasets from the Gene Expression Omnibus by Zhan et al. Results are shown as mean ± standard deviation (s.d.); the statistical significance was assessed by one-way ANOVA (*** p<0.0001).
Hypoxia increases P-gp protein expression and activity in MM cells
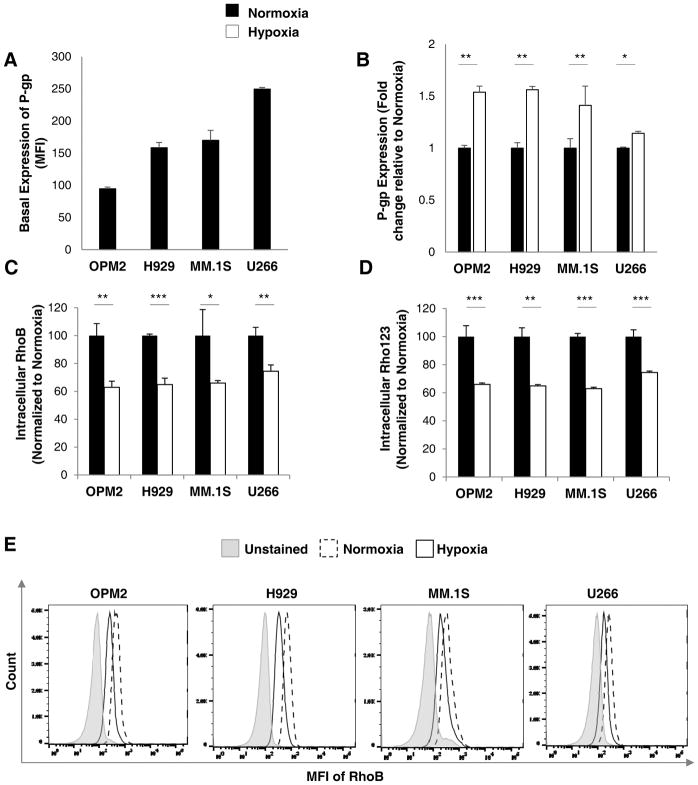
We have tested the basal expression of P-gp in normoxic conditions (21% O2, 24 hours), based on their MFI signal, and we found that U266 expresses higher levels of P-gp protein compared to MM.1S, H929, and OPM2 (Figure 2A). Next, we tested the protein expression and activity of P-gp in cells incubated under hypoxic conditions (1% O2, 24 hours). Indeed, the expression of P-gp protein was found to be increased significantly in hypoxia in MM cell lines when compared to normoxia as demonstrated by flow cytometry analysis (Figure 2B). The functional analysis of P-gp efflux activity was conducted using fluorescent P-gp substrates, rhodamine B (RhoB) and Rho123, via comparing the intracellular content of rhodamine in MM cells previously treated and cultured in normoxia or hypoxia and measured by flow cytometry. There was a statistically significant reduction in the intracellular accumulation of both RhoB (Figure 2C) and Rho123 (Figure 2D) by approximately 30–4 0% in hypoxia when compared to normoxia. Similar findings were reflected in the flow cytometry histograms with distinct differences between RhoB drug accumulation in hypoxia and normoxia (Figure 2E). These results are suggestive of an increased P-gp expression and a subsequent increase in efflux activity in hypoxic conditions.
Figure 2. Hypoxia increases P-gp protein expression and activity in MM cells.
(A) Basal expression of P-gp protein across MM cell lines (OPM2, H929, MM.1S and U266) cultured in normoxia (21% O2; 24 hours) analyzed by flow cytometry and demonstrated as MFI. (B) The expression of P-gp protein in normoxia (21% O2; black bars) and hypoxia (1% O2; white bars) for 24 hours analyzed by flow cytometry demonstrated as fold change and normalized to normoxia. P-gp activity shown as intracellular RhoB (C) and Rho123 (D) content in MM cell lines (OPM2, H929, MM.1S and U266) cultured in normoxia and hypoxia for 24 hours measured by flow cytometry and depicted as MFI normalized to unstained cells, relative to normoxic cells. (E) Histograms demonstrating RhoB in normoxia and hypoxia in MM cell lines. Results are shown as mean ± standard deviation (s.d.); the statistical significance was assessed by student t-test and considered significant for values * p<0.05, ** p<0.01 and *** p<0.001.
P-gp upregulation by hypoxia results in resistance to bortezomib and carfilzomib
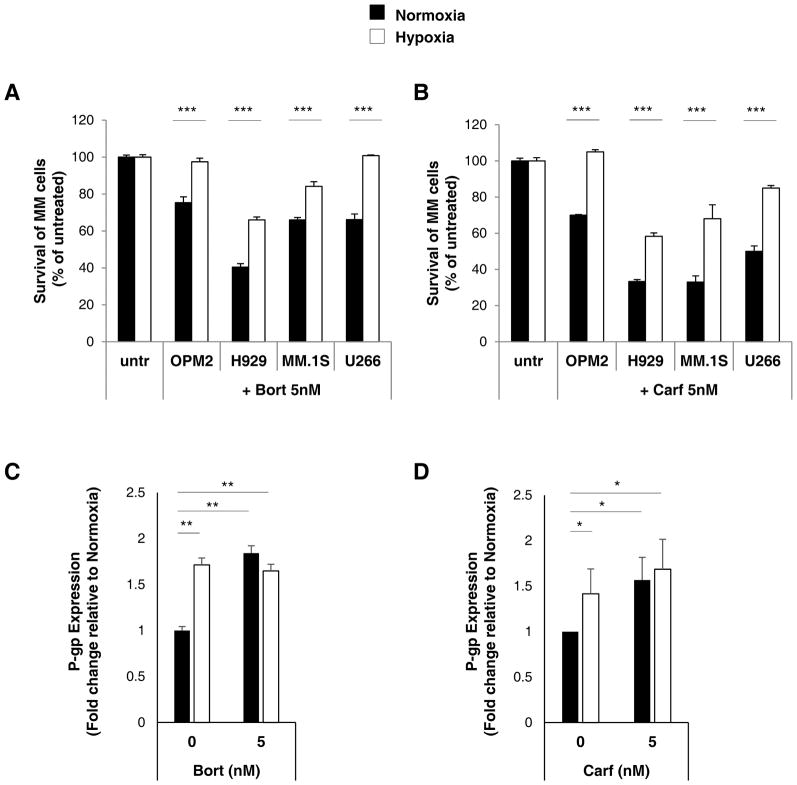
We tested the cellular sensitivity of MM cells to proteasome inhibitors in normoxia and hypoxia which was determined by MTT cytotoxic assay. As depicted in Figure 3A, culturing MM cells in the presence of 5nM of bortezomib in normoxic conditions led to 30–6 0% decrease in survival (depending on the sensitivity of MM cell lines to bortezomib with H929 being the most susceptible to drug cytotoxicity). However, in hypoxic conditions, cell survival following bortezomib treatment was significantly greater in all MM cell lines. Similarly, Figure 3B demonstrates MM cell survival in the presence of 5nM of carfilzomib in normoxic conditions which led to 30–7 0% decrease in survival (depending on the sensitivity of MM cell lines to carfilzomib with H929 and MM.1S being the most susceptible to carfilzomib). Similar to bortezomib observations, in hypoxic conditions, cell survival following carfilzomib treatment was significantly increased suggesting the development of drug resistance in hypoxia. As reflected by flow cytometry staining of P-gp surface protein, in the absence of treatment, P-gp expression was significantly higher under hypoxic conditions compared to normoxia. In the presence of bortezomib (Figure 3C) and carfilzomib (Figure 3D), P-gp expression levels were increased in normoxia; while there were minimal differences in P-gp overexpression in hypoxia in the presence of these proteasome inhibitors. These results indicate drug-induced and hypoxia-induced P-gp overexpression in MM cell lines.
Figure 3. P-gp upregulation by hypoxia results in resistance to bortezomib and carfilzomib.
(A) Cell survival of MM cell lines (OPM2, H929, MM.1S and U266) treated with and without bortezomib (5nM) or (B) carfilzomib (5nM) for 24 hours in normoxic and hypoxic conditions using a MTT assay normalized to untreated (untr) cells. (C) P-gp expression in MM cells treated with bortezomib (5nM) or (D) carfilzomib (5nM) shown as a fold change of P-gp normalized to isotype control and relative to normoxic cells measured by flow cytometry and demonstrated as average from three MM cell lines (H929, MM.1S and U266). Results are shown as mean ± standard deviation (s.d.); the statistical significance was assessed by student t-test and considered significant for values * p<0.05, ** p<0.01 and *** p<0.001.
P-gp inhibition using tariquidar decreases P-gp activity and restores drug sensitivity
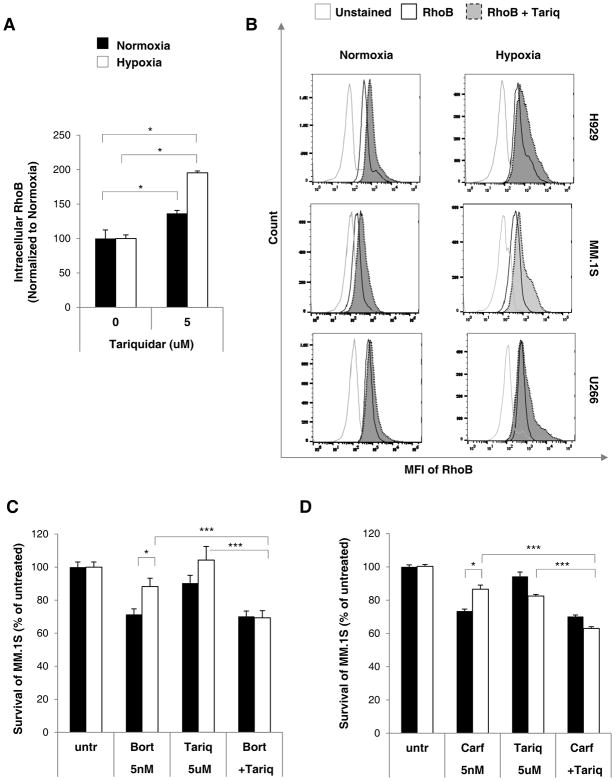
We tested whether blocking P-gp activity with a P-gp-specific inhibitor, tariquidar (5μM), will re-sensitize MM cells to bortezomib and carfilzomib. Tariquidar binds specifically to P-gp with high affinity and was shown to inhibit P-gp activity for 22 hours in vitro after it was removed from the culture medium [15]. It was shown that tariquidar enhances cytotoxicity of drugs [15, 31], and when administered orally, it potentiated the anti-tumor activity of doxorubicin, paclitaxel, etoposide and vincristine in highly resistant tumor mouse models in vivo [15] as well as clinical trial [14]. In this study, upon treatment of the MM cells with tariquidar, the fluorescent substrate RhoB accumulated inside the MM cells more prominently (p<0.05) in hypoxic conditions, possibly due to hypoxia-induced P-gp overexpression as shown by mean fluorescent intensity averaged from three MM.1S, H929 and U266 cell lines (Figure 4A) as well as demonstrated individually on histograms (Figure 4B). Based on these histograms; however, it seems that tariquidar shifts RhoB only in a subgroup of cells (visible especially in U266 in hypoxia), suggesting that tariquidar inhibited P-gp only in a subpopulation of MM cells, and possibly that higher concentrations of tariquidar should be used. In the drug cytotoxicity study, the concomitant treatment of MM.1S cells with bortezomib and tariquidar (Figure 4B) (p<0.001) or carfilzomib and tariquidar (Figure 4C) (p<0.001) displayed significantly lower cell survival compared to cells treated with the P-gp inhibitor alone or the proteasome inhibitors alone, suggesting that hypoxia-induced drug resistance to proteasome inhibitors reduced the sensitivity of MM cells to bortezomib and carfilzomib when the efflux activity of P-gp protein was inhibited.
Figure 4. P-gp inhibition using tariquidar decreases P-gp activity and restores drug sensitivity.
(A) Intracellular RhoB content in MM cells treated with tariquidar (5μM) in normoxia and hypoxia for 24 hours measured by flow cytometry and depicted as (A) MFI normalized to unstained cells, relative to normoxic untreated (untr), averaged from 3 cells lines (H929, MM.1S and U266) or (B) histograms demonstrating RhoB efflux in individual cell lines. (C) Cell survival study of MM.1S cells treated with bortezomib (5nM) or (D) carfilzomib (5nM), in the presence or absence of tariquidar (5μM) or their combination, for 24 hours in normoxia and hypoxia using MTT assay normalized to untreated (untr) MM.1S cells. Results are shown as mean ± s.d. and the statistical significance was assessed by student t-test and considered significant for values * p<0.05, ** p<0.01 and *** p<0.001.
Discussion
Current treatments in MM including proteasome inhibitors are initially effective, but the disease often relapses and cancerous cells develop resistance to the original therapy. The treatment of cancer with chemotherapeutic drugs is frequently impaired or ineffective as a result of either de novo or acquired resistance of tumor cells. Although there are multiple mechanisms causing drug resistance, one of the common mechanism is overexpression of P-gp. P-gp effluxes drugs out from the cells and compromises intra-cellular therapeutic effective concentration of drugs. It was shown that P-gp is rarely detectable in newly diagnosed MM patients, but is present in more than 80% of patients heavily treated with chemotherapy [5, 6, 7, 8, 9]. A majority of chemotherapeutics used in MM were identified as substrates of drug efflux transporter including bortezomib and carfilzomib [24, 25, 26]. Thereby, it is suggested that proteasome inhibitors may be ineffective treatment in MM patients overexpressing P-gp [24].
Additionally, hypoxia has long been linked to drug resistance, with hypoxic tumors being the most resistant to radiotherapy [17], chemotherapy [18, 20] and targeted therapies [16]. We have previously shown that regions of hypoxia arise in MM [19], likely due to tumor overgrowth coupled with insufficient angiogenesis, or dysfunctional blood vessels [32]. Hypoxia-inducible factor-1α (HIF-1α), a key transcription factor, plays a major role in cellular hypoxic response by inducing gene expression affecting cancer cell adaptive responses [33], hence it is frequently used as an intrinsic marker of hypoxia.
In this study, we demonstrated that P-gp mRNA expression correlated with the hypoxic status of MM cells derived from the bone marrow of MM patients. We used two gene expression data sets (CoMMpass dataset and data reported by Zhan et al), and we found that the gene expression of P-gp directly correlated with the expression of HIF-1α. This implies that the more hypoxic the tumor, the higher the P-gp expression and hence more prone to drug resistance.
We have previously reported that MM drug resistance to bortezomib and carfilzomib was increased in hypoxia [20]. In this study, we hypothesize that hypoxia-induced resistance to bortezomib and carfilzomib in MM cells is, in part, due to hypoxia-induced overexpression of P-gp. To test our hypothesis, we studied the effect of hypoxia on the expression of P-gp, and we found that hypoxia induced P-gp expression in all MM cell lines tested. Moreover, we tested the effect of hypoxia on the functionality of P-gp, and we demonstrated that hypoxia increased the efflux of P-gp substrates from MM cells. Then, we confirmed hypoxia-induced resistance to bortezomib and carfilzomib in MM cells, and we showed that both bortezomib and carfilzomib induced expression of P-gp in MM cells, indicating that the hypoxia-induced resistance to these drugs was P-gp-mediated.
We also confirmed the role of P-gp in overcoming the hypoxia-induced resistance to carfilzomib and bortezomib in MM cells, by testing the effect of a P-gp inhibitor (tariquidar) on the sensitivity of MM cells to these drugs in hypoxia and normoxia. First, we demonstrated that tariquidar inhibited the activity of P-gp by reducing the efflux of P-gp substrate (RhoB) in MM cells. Furthermore, we confirmed that inhibition of P-gp with tariquidar reversed the hypoxia-induced resistance to bortezomib and carfilzomib in MM cells. These results suggest that P-gp inhibitor is a promising candidate for sensitization of MM cells to proteasome inhibitors, and provide a preclinical basis for future clinical trials by combining tariquidar with bortezomib or carfilzomib, as a therapeutic strategy to re-sensitize MM patients to proteasome inhibitors.
P-gp inhibitors were shown to improve therapeutic efficacy in pre-clinical and clinical studies in multiple cancers. There were numerous inhibitors of P-gp used in clinical trials, including verapamil [34], cyclosporine [35], azithromycin [36] and CBT-1 [37] (considered as 1st and 2nd generations of P-gp inhibitors); however, most of these are not specific to P-gp, but have therapeutic targets other than P-gp, which increases the risk for off-target side effects unrelated to P-gp inhibition. In addition, some pharmacokinetic complications were associated with P-gp blockers due to inhibition of other enzymes involved in drug metabolism, such as CYP45034 or BSEP [38].
Tariquidar (an anthranilic acid derivative) is a 3rd generation inhibitor that binds with high affinity to P-gp [15], with no other known pharmacological targets, and it was shown to have fewer side effects than the previous generations of P-gp inhibitors [14, 39]. However, lack of specificity of P-gp inhibition in the tumor and normal tissues (on target - side effects), is a characteristic disadvantage for all P-gp inhibitors, including tariquidar. A solution which was suggested to improve the specificity to tumor is by targeted drug delivery of the P-gp inhibitor to the tumor site and reducing delivery to healthy tissues; such as co-delivery of tariquidar and chemotherapy in liposomes, which can be used to increase the selectivity of tariquidar activity to the tumor [40].
In summary, we found that the expression of P-gp in primary MM cells derived from the bone marrow of MM patients was correlated to the hypoxic status of these cells, and that hypoxia induced P-gp expression in MM cells in vitro. We further found that the hypoxia-induced resistance to bortezomib and carfilzomib in MM was reversed by pharmacological inhibition of P-gp by tariquidar. These results suggest that P-gp inhibition is a promising strategy for sensitization of MM cells to proteasome inhibitors, and provide a preclinical basis for future clinical trials combining P-gp inhibitors with proteasome inhibitor.
Acknowledgments
BM: Performed research, analyzed data, designed research and wrote the paper.
BM, HDK, FA, PP and CF: Performed research and analyzed data.
MF and RV: Performed the CoMMpass dataset analysis and edited the manuscript.
NNS and AKA: Designed research, performed research, analyzed data, wrote the paper and supervised the study.
Grant Support
This work was supported in part by the Washington University Institute of Clinical and Translational Sciences under Grant (number UL1TR000448); the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and the National Cancer Institute of the NIH under Award Number U54CA199092.
Footnotes
Disclosure of Interest
Dr. Azab receives research support from Verastem, Selexys, Karyopharm, Cell Works, GlycoMimetics, Tioma and Cleave; and is the founder and owner of Targeted Therapeutics LLC and Cellatrix LLC; however none of these companies sponsored this research. Other authors report no potential conflicts of interest.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–7 2. doi: 10.1182/blood-2007-10-078022. Epub 2008/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham J, Salama NN, Azab AK. The role of P-glycoprotein in drug resistance in multiple myeloma. Leukemia & lymphoma. 2015;56:26–33. doi: 10.3109/10428194.2014.907890. Epub 2014/04/01. [DOI] [PubMed] [Google Scholar]
- 3.Kaye SB, Kerr DJ. Multidrug resistance: clinical relevance in haematological malignancies. Blood reviews. 1991;5:38–41. doi: 10.1016/0268-960x(91)90006-x. Epub 1991/03/01. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan SR, Jaiswal R, Brown RD, Luk F, Bebawy M. Multiple myeloma and persistence of drug resistance in the age of novel drugs (Review) International journal of oncology. 2016;49:33–50. doi: 10.3892/ijo.2016.3516. Epub 2016/05/14. [DOI] [PubMed] [Google Scholar]
- 5.Grogan TM, Spier CM, Salmon SE, Matzner M, Rybski J, Weinstein RS, Scheper RJ, Dalton WS. P-glycoprotein expression in human plasma cell myeloma: correlation with prior chemotherapy. Blood. 1993;81:490–5. Epub 1993/01/15. [PubMed] [Google Scholar]
- 6.Linsenmeyer ME, Jefferson S, Wolf M, Matthews JP, Board PG, Woodcock DM. Levels of expression of the mdr1 gene and glutathione S-transferase genes 2 and 3 and response to chemotherapy in multiple myeloma. British journal of cancer. 1992;65:471–5. doi: 10.1038/bjc.1992.95. Epub 1992/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuessler V, Gieseler F, Gullis E, Pelka-Fleischer R, Stotzer O, Zwierzina H, Wilmanns W. Functional P-gp expression in multiple myeloma patients at primary diagnosis and relapse or progressive disease. Leukemia. 1997;11(Suppl 5):S10–4. Epub 1998/01/22. [PubMed] [Google Scholar]
- 8.Sonneveld P, Durie BG, Lokhorst HM, Marie JP, Solbu G, Suciu S, Zittoun R, Lowenberg B, Nooter K. Modulation of multidrug-resistant multiple myeloma by cyclosporin. The Leukaemia Group of the EORTC and the HOVON. Lancet. 1992;340:255–9. doi: 10.1016/0140-6736(92)92353-h. Epub 1992/08/01. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen JJ, Sonneveld P, Schoester M, Raaijmakers HG, Nieuwenhuis HK, Dekker AW, Lokhorst HM. MDR-1 expression and response to vincristine, doxorubicin, and dexamethasone chemotherapy in multiple myeloma refractory to alkylating agents. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12:115–9. doi: 10.1200/JCO.1994.12.1.115. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 10.Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer research. 1970;30:1174–84. Epub 1970/04/01. [PubMed] [Google Scholar]
- 11.Szakacs G, Hall MD, Gottesman MM, Boumendjel A, Kachadourian R, Day BJ, Baubichon-Cortay H, Di Pietro A. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chemical reviews. 2014;114:5753–74. doi: 10.1021/cr4006236. Epub 2014/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klepsch F, Jabeen I, Chiba P, Ecker GF. Pharmacoinformatic approaches to design natural product type ligands of ABC-transporters. Current pharmaceutical design. 2010;16:1742–52. doi: 10.2174/138161210791163992. Epub 2010/03/13. [DOI] [PubMed] [Google Scholar]
- 13.Bikadi Z, Hazai I, Malik D, Jemnitz K, Veres Z, Hari P, Ni Z, Loo TW, Clarke DM, Hazai E, Mao Q. Predicting P-glycoprotein-mediated drug transport based on support vector machine and three-dimensional crystal structure of P-glycoprotein. PloS one. 2011;6:e25815. doi: 10.1371/journal.pone.0025815. Epub 2011/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox E, Widemann BC, Pastakia D, Chen CC, Yang SX, Cole D, Balis FM. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer chemotherapy and pharmacology. 2015;76:1273–83. doi: 10.1007/s00280-015-2845-1. Epub 2015/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer research. 2001;61:749–58. Epub 2001/02/24. [PubMed] [Google Scholar]
- 16.Ellis LM, Hicklin DJ. Resistance to Targeted Therapies: Refining Anticancer Therapy in the Era of Molecular Oncology. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7471–8. doi: 10.1158/1078-0432.CCR-09-1070. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 17.Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer metastasis reviews. 2007;26:241–8. doi: 10.1007/s10555-007-9056-0. Epub 2007/04/19. [DOI] [PubMed] [Google Scholar]
- 18.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer treatment reviews. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. Epub 2003/08/21. [DOI] [PubMed] [Google Scholar]
- 19.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, Roccaro AM, Sacco A, Ngo HT, Lin CP, Kung AL, Carrasco RD, Vanderkerken K, Ghobrial IM. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94. doi: 10.1182/blood-2011-09-380410. Epub 2012/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muz B, de la Puente P, Azab F, Luderer M, Azab AK. Hypoxia promotes stem cell-like phenotype in multiple myeloma cells. Blood cancer journal. 2014;4:e262. doi: 10.1038/bcj.2014.82. Epub 2014/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, Zhang Y, Liang H. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PloS one. 2014;9:e98882. doi: 10.1371/journal.pone.0098882. Epub 2014/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer research. 2002;62:3387–94. Epub 2002/06/18. [PubMed] [Google Scholar]
- 23.Li J, Shi M, Cao Y, Yuan W, Pang T, Li B, Sun Z, Chen L, Zhao RC. Knockdown of hypoxia-inducible factor-1alpha in breast carcinoma MCF-7 cells results in reduced tumor growth and increased sensitivity to methotrexate. Biochemical and biophysical research communications. 2006;342:1341–51. doi: 10.1016/j.bbrc.2006.02.094. Epub 2006/03/07. [DOI] [PubMed] [Google Scholar]
- 24.Gutman D, Morales AA, Boise LH. Acquisition of a multidrug-resistant phenotype with a proteasome inhibitor in multiple myeloma. Leukemia. 2009;23:2181–3. doi: 10.1038/leu.2009.123. Epub 2009/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley TS, Riz I, Yang W, Wakabayashi Y, Depalma L, Chang YT, Peng W, Zhu J, Hawley RG. Identification of an ABCB1 (P-glycoprotein)-positive carfilzomib-resistant myeloma subpopulation by the pluripotent stem cell fluorescent dye CDy1. American journal of hematology. 2013;88:265–72. doi: 10.1002/ajh.23387. Epub 2013/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rumpold H, Salvador C, Wolf AM, Tilg H, Gastl G, Wolf D. Knockdown of PgP resensitizes leukemic cells to proteasome inhibitors. Biochemical and biophysical research communications. 2007;361:549–54. doi: 10.1016/j.bbrc.2007.07.049. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan D, Hideshima T, Mitsiades C, Richardson P, Anderson KC. Proteasome inhibitor therapy in multiple myeloma. Molecular cancer therapeutics. 2005;4:686–92. doi: 10.1158/1535-7163.MCT-04-0338. Epub 2005/04/14. [DOI] [PubMed] [Google Scholar]
- 28.Ao L, Wu Y, Kim D, Jang ER, Kim K, Lee DM, Kim KB, Lee W. Development of peptide-based reversing agents for p-glycoprotein-mediated resistance to carfilzomib. Molecular pharmaceutics. 2012;9:2197–205. doi: 10.1021/mp300044b. Epub 2012/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonial S, Yellapantula VD, Liang W, Kurdoglu A, Aldrich J, Legendre CM, Stephenson K, Adkins J, McDonald J, Helland A, Russell M, Christofferson A, Cuyugan L, Rohrer D, Blanski A, Hodges M, Derome M, Auclair D, Kidd PG, Jewell S, Craig D, Carpten J, Keats JJ Mmrf CoMMpass Network. Interim Analysis of the Mmrf Commpass Trial: Identification of Novel Rearrangements Potentially Associated with Disease Initiation and Progression. Blood. 2014:124. [Google Scholar]
- 30.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD., Jr The molecular classification of multiple myeloma. Blood. 2006;108:2020–8. doi: 10.1182/blood-2005-11-013458. Epub 2006/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker J, Martin C, Callaghan R. Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. European journal of cancer. 2004;40:594–605. doi: 10.1016/j.ejca.2003.09.036. Epub 2004/02/14. [DOI] [PubMed] [Google Scholar]
- 32.Imai T, Muz B, Yeh CH, Yao J, Zhang R, Azab AK, Wang L. Direct measurement of hypoxia in a xenograft multiple myeloma model by optical-resolution photoacoustic microscopy. Cancer biology & therapy. 2017:1–5. doi: 10.1080/15384047.2016.1276137. Epub 2017/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. Epub 2009/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehnert M, Dalton WS, Roe D, Emerson S, Salmon SE. Synergistic inhibition by verapamil and quinine of P-glycoprotein-mediated multidrug resistance in a human myeloma cell line model. Blood. 1991;77:348–54. Epub 1991/01/15. [PubMed] [Google Scholar]
- 35.Aouali N, Eddabra L, Macadre J, Morjani H. Immunosuppressors and reversion of multidrug-resistance. Critical reviews in oncology/hematology. 2005;56:61–70. doi: 10.1016/j.critrevonc.2004.12.010. Epub 2005/06/28. [DOI] [PubMed] [Google Scholar]
- 36.Sugie M, Asakura E, Zhao YL, Torita S, Nadai M, Baba K, Kitaichi K, Takagi K, Takagi K, Hasegawa T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrobial agents and chemotherapy. 2004;48:809–14. doi: 10.1128/AAC.48.3.809-814.2004. Epub 2004/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly RJ, Robey RW, Chen CC, Draper D, Luchenko V, Barnett D, Oldham RK, Caluag Z, Frye AR, Steinberg SM, Fojo T, Bates SE. A pharmacodynamic study of the P-glycoprotein antagonist CBT-1(R) in combination with paclitaxel in solid tumors. The oncologist. 2012;17:512. doi: 10.1634/theoncologist.2012-0080. Epub 2012/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehne G, Sorensen DR, Tjonnfjord GE, Beiske C, Hagve TA, Rugstad HE, Clausen OP. The cyclosporin PSC 833 increases survival and delays engraftment of human multidrug-resistant leukemia cells in xenotransplanted NOD-SCID mice. Leukemia. 2002;16:2388–94. doi: 10.1038/sj.leu.2402663. Epub 2002/11/28. [DOI] [PubMed] [Google Scholar]
- 39.Kelly RJ, Draper D, Chen CC, Robey RW, Figg WD, Piekarz RL, Chen X, Gardner ER, Balis FM, Venkatesan AM, Steinberg SM, Fojo T, Bates SE. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:569–80. doi: 10.1158/1078-0432.CCR-10-1725. Epub 2010/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Sriraman SK, Kenny HA, Luther E, Torchilin V, Lengyel E. Reversal of Chemoresistance in Ovarian Cancer by Co-Delivery of a P-Glycoprotein Inhibitor and Paclitaxel in a Liposomal Platform. Molecular cancer therapeutics. 2016;15:2282–93. doi: 10.1158/1535-7163.MCT-15-0986. Epub 2016/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]