Supplemental digital content is available in the text.
Abstract
Background
Variation in the use of immunosuppression regimens after liver transplant has not been well described.
Methods
Immunosuppression regimens used after liver transplant were identified in a novel database integrating national transplant registry and pharmacy fill records for 24 238 recipients (2006-2014). Bilevel hierarchical models were developed to quantify the effects of transplant program, recipient, and donor characteristics on regimen choice.
Results
In the first 6 months after transplant, triple immunosuppression (tacrolimus, antimetabolite, corticosteroids) was the most common regimen (42.9%). By months 7 to 12, immunosuppression regimens were more commonly antimetabolite sparing (33.7%) or steroid sparing (26.9%), followed by triple (14.4%), mammalian target of rapamycin inhibitor (mTORi)-based (12.1%), or cyclosporine-based (9.2%). Based on intraclass correlation analysis, clinical characteristics explained less than 10% of the variation in immunosuppression choice, whereas program preference/practice explained 23% of steroid sparing, 26% of antimetabolite sparing, 28% of mTORi, and 21% of cyclosporine-based regimen use. Although case factors were not dominant practice drivers, triple immunosuppression in months 7 to 12 was more common among retransplant recipients and those with prior acute rejection. Hepatocellular carcinoma as cause of liver failure (adjusted odds ratio [aOR], 2.15; P<0.001), cancer within 6 months (aOR, 6.07; P<0.001), and 6-month estimated glomerular filtration rate less than 30 mL/min per 1.3 m2 (aOR, 1.98; P<0.001) were associated with mTORi use compared with triple immunosuppression in months 7 to 12, whereas acute rejection predicted lower use (aOR, 0.72; P=0.003).
Conclusions
Liver transplant immunosuppression is dominantly driven by program preference, but case factors also affect regimen choice. This variation frames a natural experiment for future evaluations of comparative efficacy.
Liver transplant recipients have traditionally required lower levels of immunosuppression (ISx) than other solid organ transplant recipients. However, inadequate ISx coverage can result in acute rejection (AR) and graft loss, whereas overuse contributes to chronic renal insufficiency, malignancy, and infection.1 A recent Cochrane review of induction and maintenance ISx in liver transplantation concluded that published data were insufficient to identify an optimal ISx, and called for novel strategies to assess variation in ISx-related outcomes.2 The development of novel “big data” linking administrative and clinical data sets offers the potential to assess outcome variation by ISx regimen while controlling for marked differences in patient and donor characteristics which characterize contemporary liver transplant practice.
Calcineurin inhibitor (CNI)-based regimens including tacrolimus (Tac) or cyclosporine (CsA) remain the foundation of postliver transplant maintenance ISx. Calcineurin inhibitor–based ISx results in low rates of AR and prolonged graft survival, and Tac is associated with better outcomes than CsA in head-to-head comparison.3 However, in subpopulations, alternative regimens may be superior, because Tac has been associated with an increase in de novo malignancies, a 40% increased risk of posttransplant diabetes, and more aggressive recurrence of autoimmune disease.4 In addition, CNIs contribute to the development of chronic kidney disease (glomerular filtration rate [GFR] <30 mL/min per 1.73 m2) in nearly 20% of liver transplant recipients at 5 years.5 Strategies to decrease the nephrotoxicity associated with CNIs include the use of induction therapy to delay CNI introduction and conversion to mammalian target of rapamycin inhibitor (mTORi) maintenance to allow lower CNI therapeutic levels or avoid CNIs altogether.6,7 Unfortunately, mTORis have been associated with hepatic artery thrombosis and increased AR rates, which could mitigate the advantages of mTORi on kidney function.8,9
Patients with hepatocellular cancer (HCC) represent a growing proportion of liver transplant recipients who may benefit from a tailored ISx strategy. Hepatocellular cancer recurrence is believed to be influenced by degree of ISx, is difficult to treat, and leads to high mortality.10 The mTORi-based regimens may have some antineoplastic benefits for patients with HCC.11 Although the use of sirolimus (SRL) is particularly challenging early posttransplant due to risk of vascular complications and poor wound healing, selected trials have suggested benefit if therapy is delayed for at least 30 days.8,9
The evaluation of customized ISx strategies in liver transplantation has thus far been limited to trends in ISx use after liver transplant have been reported using national registry data at time of discharge.2,12 To characterize contemporary practices in liver transplant ISx, we examined a novel database integrating national registry and pharmacy fill records for a national, contemporary sample of US liver transplant recipients using a multilevel analytic framework in the early (0-6 months) and later (7-12 months) posttransplant periods as distinguish between clinically driven differences in ISx regimen and those based simply on established practice.
MATERIALS AND METHODS
Data Sources
We conducted a retrospective cohort study using linked healthcare databases in the US to ascertain patient characteristics, pharmacy fill records, and outcome events for liver transplant recipients. This study used transplant data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. Baseline demographic information ascertained for liver transplant recipients from OPTN included age, sex, and race as reported by the transplant centers.
Pharmacy fill data were assembled by linking SRTR records for liver transplant recipients with billing claims from Symphony Health (SH), a large US pharmaceutical claims data warehouse that collects prescription drug fill records including self-paid fills and those reimbursed by private and public payers.13 Symphony Health comprises National Council for Prescription Drug Program format prescription claims aggregated from multiple sources including claims warehouses, retail pharmacies, and prescription benefit managers for approximately 60% of US retail pharmacy transactions. Individual claim records include the pharmacy fill date with the national drug code identifying agent and dosage. After institutional review board and Health Resources and Services Administration approvals, SH records were linked with SRTR records for liver transplant recipients. We applied a deterministic deidentification strategy wherein patient identifiers (last name, first name, date of birth, sex, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and Health Information Technology for Economic and Clinical Health (HITECH)-certified encryption technology from SH. The patient deidentification software employs multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.
Sample and Clinical Characteristics
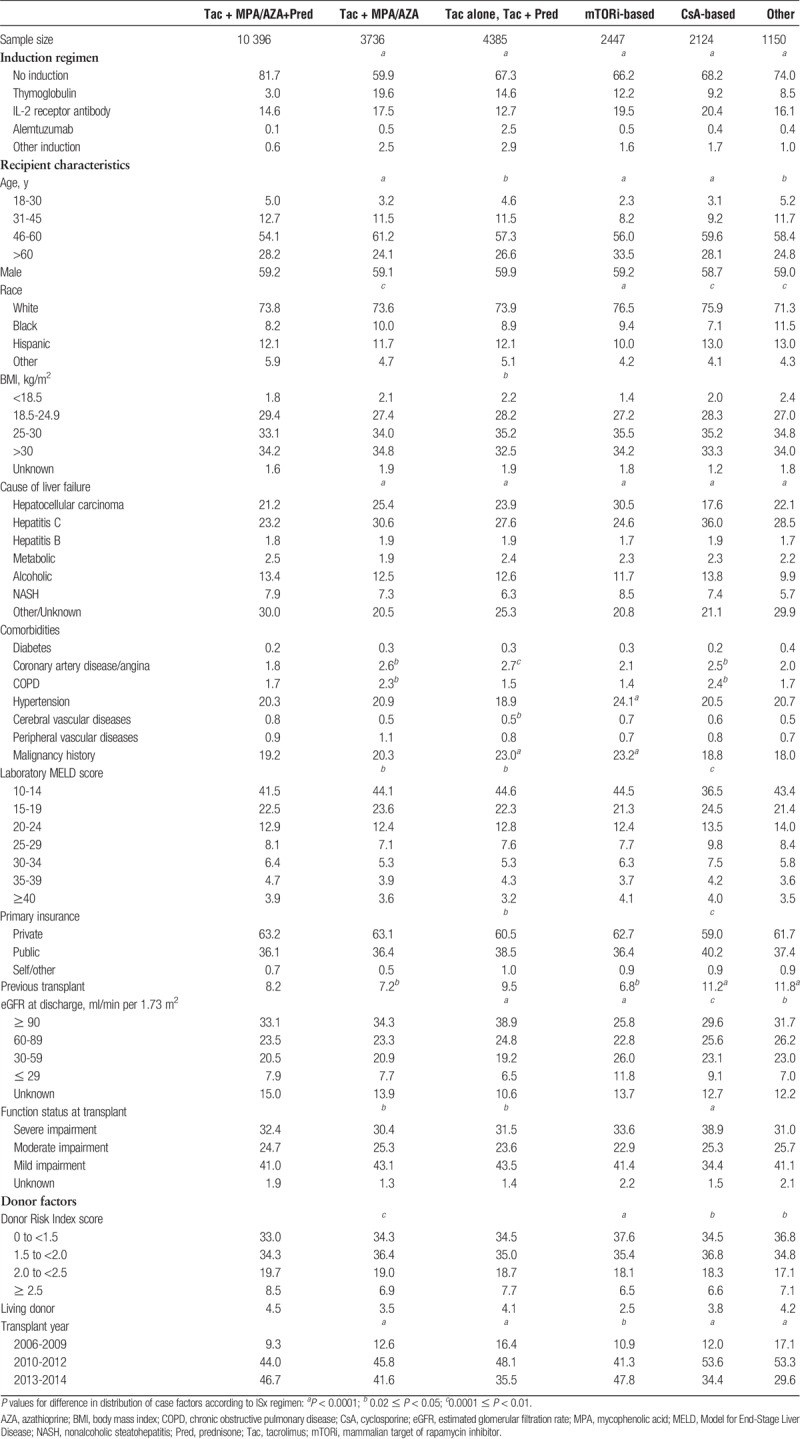
We identified adult liver transplant recipients (age, ≥18 years) with SRTR records of liver transplant in 2006 and 2014 and available pharmaceutical fill records over 12 months posttransplant. Recipient clinical and demographic characteristics, characteristics of the donated organ, and other transplant factors including ischemic time and sharing, were defined by the OPTN Transplant Candidate and Recipient Registration forms (Table 1).
TABLE 1.
Recipient, donor, and transplant characteristics according to liver transplant maintenance ISx regimen, months 0 to 6 posttransplant
Immunosuppression
Using pharmacy fill records, ISx regimens were classified into 6 mutually exclusive groups as per previous methods: group 1 (reference), standard triple therapy, defined as Tac with mycophenolic acid (MPA) (mycophenolate mofetil, mycophenolate sodium), or azathioprine (AZA), and prednisone (Pred), “Tac+MPA/AZA+Pred”; group 2, corticosteroid-sparing, “Tac+MPA/AZA”; group 3, MPA/AZA sparing, “Tac alone, Tac+Pred”; group 4, mTORi-based, defined by any fill for SRL as the mTORi available in the study period, with or without other agents including CNI, “mTORi-based”; group 5, CsA-based, defined by CsA without SRL, “CsA-based”; group 6, “other regimens” including CsA withdrawal, or other trial medications.13 Patients in groups 1 to 3 did not receive SRL or CsA.
Analyses
Observed Variation in Regimen Use Across Centers
To visually assess unadjusted variation in maintenance ISx use at the center level across the United States, the observed proportion of patients receiving each induction regimen was computed for each center and displayed as stacked bar plots.
Combined Center and Case-level Modeling
Bilevel hierarchical models were constructed to adjust for clustering effects. Level 1 comprised patient/donor and transplant (clinical) factors, and level 2 represented the center, wherein use of each alternative regimen was compared individually to the reference regimen (pairwise). Empirical Bayes estimates (EBE) provided the adjusted proportion (with 95% confidence intervals [CI]) of use of a regimen of interest compared with the reference regimen, incorporating case-mix adjustment from the hierarchical model. A 95% CI for a given center's EBE of use of a regimen of interest that did not include the median national rate of use indicated a prescribing pattern statistically significantly different from the expected rate of use for that regimen.
Heterogeneity in ISx prescribing across centers was quantified using intraclass correlation (ICC) and median odds ratios (MOR). The ICC is defined as the ratio of cluster variance (center impact) to the total observed variance in ISx use, with contributions in our study framework defined as center-related, clinically related, and other unmeasured effects. In this context, the ICC quantifies the proportion of total variance in ISx use that is accounted for by center. The MOR provides the median of the odds that patients with identical characteristics will receive the ISx regimen of interest when 2 centers are drawn at random (performed for all possible pairs of centers). For example, a MOR of 2.0 means that if centers are selected at random across all centers, a patient with a given set of characteristics is, on an average, twice as likely to receive the ISx regimen of interest at one of the randomly selected centers as at the other.14 The adjusted odd ratio (95% LCL aOR95% UCL) of receiving a maintenance regimen other than the triple-therapy reference was determined for patient and donor factors, after accounting for the effect of center using the hierarchical model.
Data were analyzed using Stata 13, College Station, TX. Hierarchical logistic regression modeling was in Stata using the “xtmelogit” command with center as a random intercept. The ICC and the MOR were calculated using “xtmrho” (third-party suite) command.
Contributions of Case-level Factors to Variation in ISx Use
To quantify the degree of variance in ISx regimen use explained by recipient and donor characteristics, we performed multivariate logistic regression modeling with maintenance regimen as the dependent variable and case factors as the predictors. Pairwise models were constructed to assess the relative likelihood of using each specific regimen (as outlined above) compared with triple ISx.
Regimen Change Analysis
We examined the frequencies of liver transplant recipients who remained on the same regimen after the 0- to 6-month posttransplant period and those who switched to new regimens in the 7- to 12-month period. To examine the correlates of change in ISx, we performed multivariate logistic regression modeling adjusted for recipient, donor, and transplant risk factors with “change in ISx regimen” as the dependent variable.
RESULTS
Between 2006 and 2014, 24 238 liver transplants were performed in the US; 51 011 linked pharmacy fill records were available. In the first 6 months after transplant, a plurality (42.9%) of transplant recipients received triple therapy; 18.1% received MPA/AZA-sparing (Tac alone, Tac+Pred), 15.4 steroid-sparing, 10.1% mTORi-based, and 8.8% CsA-based. Most patients in the mTORi-based group also received CNI (Tac, 72.5%; CsA, 12.4%). Induction therapy at liver transplant varied with maintenance ISx regimen (Table 1). Patients receiving triple ISx therapy were unlikely to receive induction (81.7% did not), and those who did most frequently received IL-2 receptor blocking antibodies (IL2rAb, 14.6%). Conversely, induction was used in 40.1% of patients on steroid-free regimens (TMG, 19.6%; IL2rAb, 17.5%) and in 32.7% of patients managed with MPA avoidance (TMG, 14.6%; IL2rAb, 12.7%).
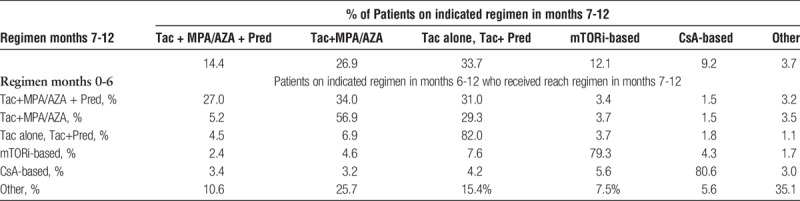
By 7 to 12 months after transplant, the pattern of ISx shifted (Table 2; Figure S1, SDC, http://links.lww.com/TXD/A103). Only 14.4% of patients remained on triple therapy. Mycophenolic acid sparing regimens were the most common (33.7%), followed by steroid-sparing (26.9%). mTORi-based ISx was used in 12.1% and CsA-based in 9.2%. Only 27.0% of patients on triple therapy in the first 6 months remained, so at 6 to 12 months, 34.0% were changed to steroid-sparing and 31.0% to MPA/AZA-sparing regimens. In contrast, most patients on MPA/AZA-sparing (82.0%), mTORi-based (79.3%), and CsA-based (80.6%) ISx remained on their initial regimen.
TABLE 2.
ISx in months 7 to 12 compared with initial regimen months 0 to 6 posttransplant
Patient Characteristics and ISx Regimen Use
Some patient characteristics were associated with maintenance ISx regimens at 0 to 6 months posttransplant (Table 3). Younger patients (ages, 19-30 years) were less likely than patients aged 31 to 45 years to receive steroid sparing versus triple therapy (aOR 0.50 0.670.90). Older patients (age >45 years) were more likely to receive MPA/AZA-sparing, mTORi-based, or CsA-based regimens than to receive triple therapy (P<0.05 for all). Black patients were less likely to receive CsA-based regimens (aOR 0.590.720.89) and Hispanics were less likely to receive mTORi-based regimens (aOR 0.620.740.88) versus triple therapy. Recipients with low body mass index (BMI) were more frequently given steroid-sparing and MPA/AZA-sparing regimens. Compared with recipients with alcoholic liver failure, those with HCC were 81% more likely to receive mTORi-based regimens (aOR 1.48 1.812.21) than triple therapy. Diabetic patients were more commonly given MPA/AZA-sparing regimens than triple therapy (aOR 1.112.445.39). The lower the estimated GFR (eGFR), the higher the odds of receiving mTORi-based ISx (eGFR, 60-89: aOR 1.091.271.47; eGFR 30-59: aOR 1.381.601.86; eGFR 0-29 ml/min/1.73 m2: aOR 1.732.112.58). Over time, use of triple therapy was more likely compared with the other ISx regimens, shown by aOR <1 for all nontriple therapy regimens in 2010 to 2012 versus 2006 to 2009.
TABLE 3.
Associations of recipient, donor, and transplant case characteristics with liver transplant maintenance ISx regimen use in months 0 to 6 posttransplant compared with triple therapy (reference regimen)
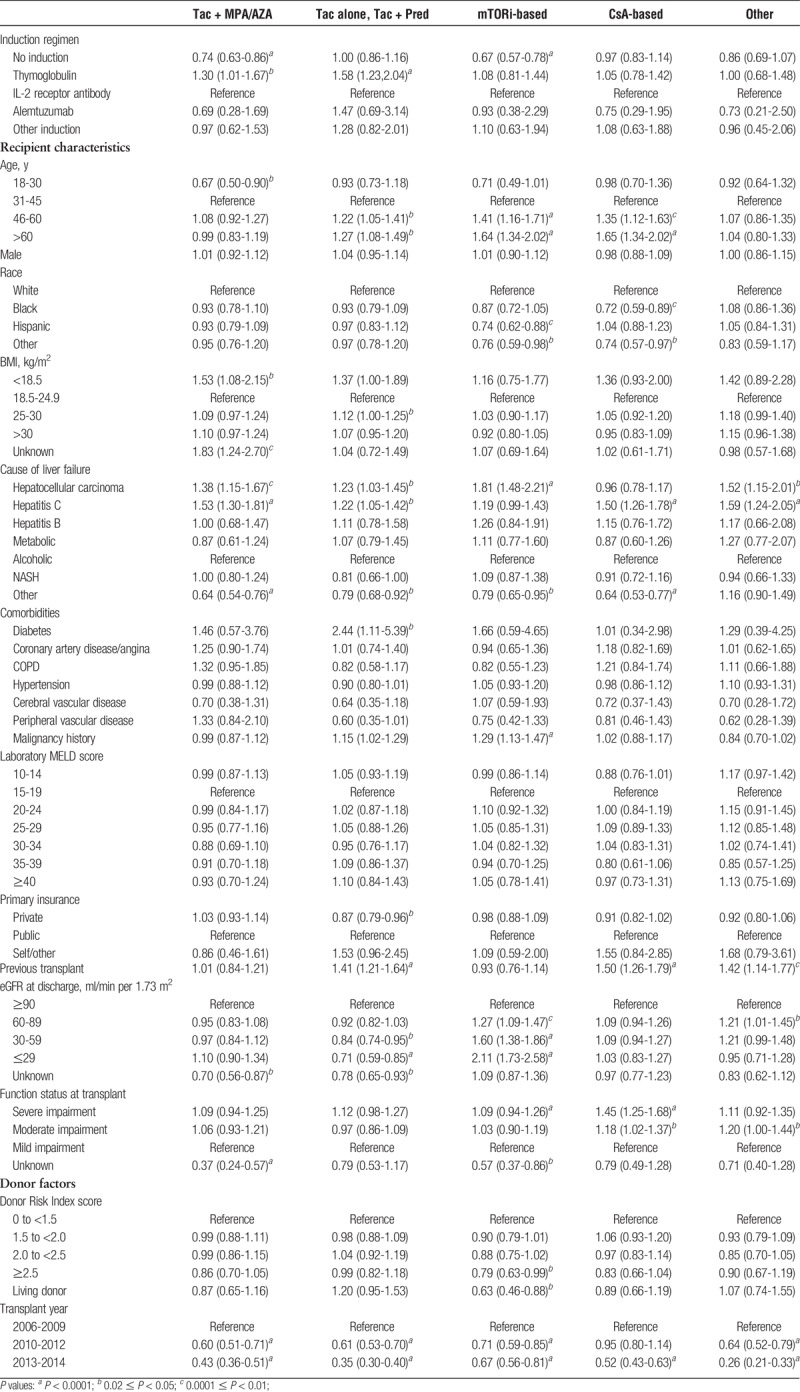
Associations of many case factors with ISx in months 7 to 12 were similar to those in the first 6 months (Table S1, http://links.lww.com/TXD/A106). However, at 7 to 12 months, recipients with BMI >30 kg/m2 were significantly more likely to receive a steroid-sparing regimen (aOR 1.101.161.33). Recipients with AR within the first 6 months were more likely to receive triple therapy in the later period. In addition, recipients with incident malignancy in first 6 months were 6 times more likely (aOR 3.266.0711.27) to receive an mTORi-based regimen than to receive triple therapy. Among patients with severely impaired functional status, CsA-based regimens were more likely to be used (1.251.96 3.09).
Donor Characteristics and ISx Regimen Choice
Recipients of high Donor Risk Index score (≥2.5) (aOR 0.630.79 0.99) or living donor (aOR 0.460.630.88) allografts were less likely to receive mTORi-based regimens than to receive triple therapy (Table 3). Recipients who had undergone previous transplant were more commonly given MPA/AZA-sparing (aOR 1.211.411.64), CsA-based (aOR 1.261.501.79), or other ISx maintenance (aOR 1.14 1.421.77) versus triple therapy.
Center Variation in ISx Use
Choice of ISx regimens differed substantially across centers in the first 6 months after transplant (Figure 1). Regimen use by center varied from 0% to 94.4% for triple therapy, 0% to 58.5% for steroid-sparing, 0% to 100% for MPA/AZA-sparing, 0% to 50.0% for mTORi-based, and 0% to 45.6% for CsA-based ISx. This variation across centers persisted at 7 to 12 months posttransplant (Figure S2, SDC, http://links.lww.com/TXD/A104).
FIGURE 1.
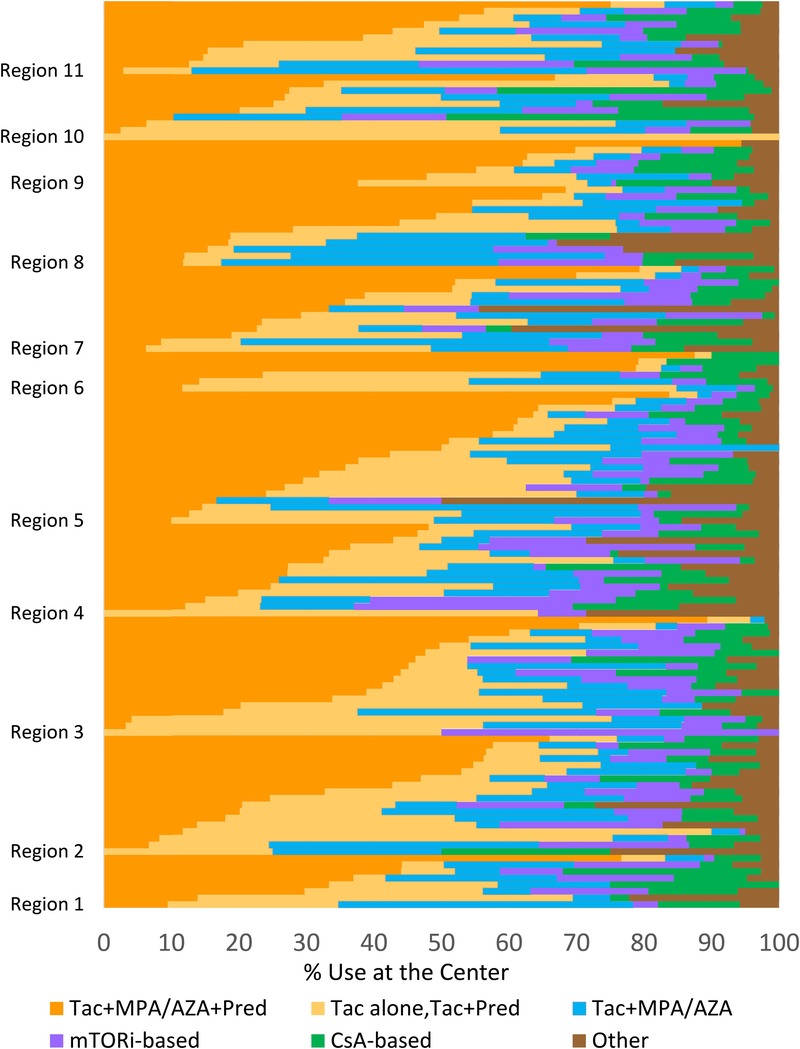
Proportion of patients receiving each maintenance ISx option in months 0 to 6 after liver transplant across US centers (2006-2014). Each horizontal bar represents an individual center within US regions ordered by the proportion of patients receiving each regimen.
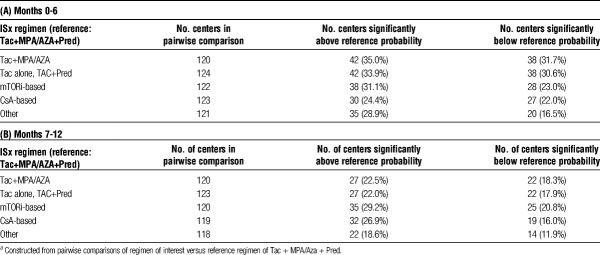
Hierarchical logistic regression models demonstrated that between-center variation in use of specific ISx regimens was significantly greater than expected based on differences in recipient and donor characteristics (P<0.001) (Figure 2). In the first 0 to 6 months posttransplant, 35.0% of centers used steroid-sparing ISx more frequently than expected, 33.9% used MPA/AZA-sparing more than expected; use of mTORi-based and CsA-based ISx was higher than expected at 31.1% and 24.4% of centers, respectively (Table 4A). In the 7- to 12-month period, centers were more uniform in ISx patterns, shown as a reduction in the number of centers with use above or below the reference probability and a lower degree of heterogeneity (Table 4B; Figure S3, SDC, http://links.lww.com/TXD/A105).
FIGURE 2.
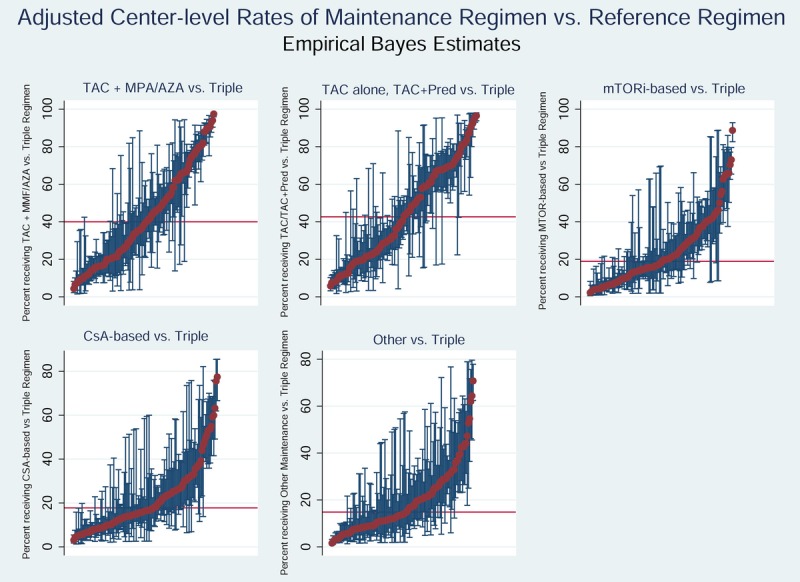
Empirical Bayes estimates for likelihood of use of each maintenance ISx regimen compared with triple therapy in months 0 to 6 after liver transplant. The red bar demonstrates the national average rate of use of each regimen (within pairwise regimen comparisons). Each red dot represents adjusted use at one center, and the blue bars reflect 95% CIs for use at the center determined by EBEs, adjusting for case factors of transplants at the center; exclusion of the national average by a 95% CI reflects adjusted center use significantly above or below the national average.
TABLE 4.
Center-level EBEs adjusted for case-level characteristicsa
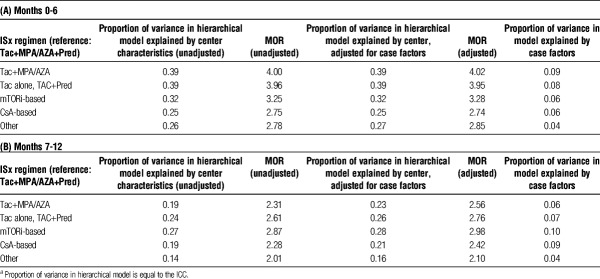
In the first 6 months posttransplant, unadjusted ICCs suggest that 39% of the variation in steroid-sparing and MPA/AZA-sparing ISx use was due to “center effect,” whereas 32% and 25% of the variation in mTORi-based and CsA-based ISx, respectively, were driven by center preference (Table 5A). Similar results were noted after adjustment of ICC for recipient, donor, and transplant factors. Patient characteristics explained less than 10% of the variation in use of all regimens. Median odds ratio from case-factor adjusted analysis varied from 2.8 to 4.0. Thus, a recipient with a given set of characteristics was, on average, 4.0 times as likely to receive a steroid- or antimetabolite-sparing regimen as triple therapy, at specific centers. Between 6 and 12 months, unadjusted and adjusted ICCs varied less due to “center effect” across all ISx (Table 5B).
TABLE 5.
Heterogeneity across unadjusted and both adjusted modelsa
DISCUSSION
This study provides the first rigorous assessment of variation in long-term ISx regimen selection in liver transplant recipients in a national cohort with a sample size large enough to distinguish the effects of center practice and clinical case factors. Our results suggest that liver transplant ISx is far from uniform nationally, with selection of some regimens varying from 0% to 100% of recipients across centers. The degree of variation was higher in the first 6 months than in months 7 to 12 after transplant, when minimized regimens were favored. Multivariate analysis confirmed that center protocol was a stronger determinant of ISx selection than patient or donor characteristics.
Overall, we observed a reduction in the intensity of ISx exposure over time after liver transplant. Tac-based triple ISx was the most commonly used maintenance regimen in the first 6 months; however, only 23% of patients remained on this regimen by months 7 to 12. In contrast, patients started on reduced ISx regimens were more likely to remain on them. Only 4.5% patients who initially received MPA/AZA-sparing regimens and 5.2% who initially received steroid-sparing regimens were converted to triple therapy during months 7 to 12 after transplant. This pattern may suggest that triple therapy is not needed after liver transplant in many cases, because AR rates are low and more than 90% of AR events are steroid-responsive.1
The more frequent use of induction ISx in recipients managed with regimens other than triple therapy may also have contributed to the low conversion rate. In a nonrandomized single-center trial, a steroid-free regimen and weaning to single-agent Tac or SRL at 3 months after thymoglobulin induction yielded 1- and 5-year patient and graft survival comparable to national averages in registry data.15 In a meta-analysis of liver transplant recipients, AR was significantly less frequent when any form of T cell–specific antibody induction was used compared with no induction (relative risk, 0.85; P<0.05).16 The meta-analysis did not mirror typical practice patterns in the US, however, as included studies were limited to 19 trials with corticosteroids administered in all and MPA/AZA in 15 of the 19 trials. Thus, further study is needed to compare the benefits and risks of early triple ISx, lower intensity ISx with induction, and lower intensity ISx without induction.2,7,16
The national data demonstrate some expected associations between ISx choice and recipient characteristics. Older recipients, those with HCC or hepatitis C as cause of liver failure, and those with renal insufficiency at discharge were less likely to be managed with Tac-based triple therapy. At 7 to 12 months, use of triple ISx was more likely in retransplant recipients and in recipients with AR in the first 6 months. In contrast, recipients with newly diagnosed malignancy were nearly 6 times more likely to receive mTORi-based ISx.
The role of mTORi in the management of liver transplant recipients with chronic renal insufficiency remains controversial. In a randomized trial of standard dose, Tac versus mTORi/reduced Tac, eGFR was 8 mL/min per 1.73 m2 higher in the reduced Tac group. Although there was no difference in the composite endpoint of death, graft loss, and biopsy-proven rejection at 3 years (P = 0.33), there was a numerically increased risk of AR in the reduced-Tac group (9.2% vs 4.8%, P = 0.07), leading to study termination.7 A meta-analysis reviewing mTORi and CNI-based ISx found that patients converted to mTORi from CNIs had significantly better renal function at 1 year after randomization. Stratifying for conversion within 6 months versus later after transplant suggested a trend toward more favorable eGFR with early conversion. In our national data study, 20% of patients were converted from mTORi-based regimens to CNI-based regimens, though only 2% were converted to triple therapy. Further analysis is needed to define the outcomes of mTORi-based ISx outside the clinical trial setting, and to identify subgroups likely versus unlikely to benefit from the regimen.
Among patients with HCC, we confirmed increased use of mTORi-based ISx, which resonates with prior single-center and meta-analytic data suggesting lower HCC recurrence risk in patients treated with mTORis compared with CNIs.17,18 The benefit of mTORi-based ISx remains questionable, however, given the results of a large randomized trial. In the SiLVER study of 525 liver transplant recipients with HCC, there was no difference in recurrence-free survival and patient survival with mTORi versus CNI at 5 years.19 However, there was significant benefit in recurrence-free and overall survival over the first 3 years after transplant in patients within Milan criteria who were given mTORi. Furthermore, there was substantial heterogeneity in other components of the ISx regimen across centers (eg, use of induction, steroids, and CNIs), which may have affected the trial findings.
Associations of metabolic risk factors with ISx selection also warrant consideration. Among patients who underwent transplant due to nonalcoholic steatohepatitis (NASH), triple therapy may worsen diabetic control and contribute to end-organ disease.20-24 Development of diabetes after transplant was 29% higher in NASH recipients in one study, and may be worsened by Tac and corticosteroids.25 In our study, diabetic patients were initially commonly treated with MPA/AZA-sparing regimens (34.4%); 21.9% were given monotherapy with Tac. We found no association between ISx choice and increased BMI in early regimen choice. However, at 7 to 12 months, recipients with high BMI were significantly more likely to receive a steroid-sparing regimen. We also noted that recipients with severely impaired functional status and low BMI were more likely to receive CsA-based regimens. This association may reflect neurotoxicity associated chronic malnutrition, which may worsen under Tac-based regimens, resulting in early change to a CsA-based ISx.26
The limitations of the current study include its retrospective, nonrandomized design, although this offers benefits with regard to sample size and national representation across US transplant centers. We lacked precise information on ISx exposure as we lacked data on ISx levels. We studied ISx over the first year posttransplant, and ISx management may change after the first posttransplant anniversary. The large sample size and longitudinal follow-up allowed assessment of changes in clinical characteristics over time (eg, development of AR and malignancy). However, other clinical conditions that affect the choice of ISx are not identified in OPTN data or may occur after the time of transplant, and therefore are not included in this analysis. For example, recipients with a history of severe CNI neurotoxicity, CNI-induced thrombotic microangiopathy, or nephrotoxicity may be more likely to receive mTORi-based therapy. Recipients receiving ISx through trials rather than pharmacy fills cannot be identified through our study data, although some recipients in the “other” category were likely managed under study protocols. Finally, the years of data collection in our study ended in 2014, and ongoing research is needed to evaluate use of and trends in recently approved agents, such as everolimus.27
In conclusion, we found that selection of ISx regimen after liver transplant varies widely across US transplant centers and largely reflects center choice rather than patient or donor characteristics, similar to kidney transplant data we previously published.13,28 Whether tailoring ISx therapy to case factors or using standardized protocols for all patients affects posttransplant mortality remains to be defined. The current observation of center-based practice variation frames a natural experiment for future studies to assess the effects of ISx personalization on patient outcomes and costs with sufficient power to identify low-probability events.
Supplementary Material
ACKNOWLEDGMENTS
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for article editing.
Footnotes
Published online 13 June, 2018.
This work was supported by grants from the Saint Louis University Liver Center and the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
M.N. is supported by the Saint Louis University Liver Center. K.L.L., M.A.S., Z.Z., V.R.D., D.L.S., and D.A.A. are supported by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The authors declare no conflicts of interest.
M.N., A.S.N., R.O., H.R., V.R.D., D.L.S., B.L.K., G.P.H., T.A., M.M., and D.A.A. participated in study design, interpretation, and writing of the article. K.L.L. and M.A.S. participated in study design, acquisition of data and regulatory approvals, data analysis, and writing of the article. Z.Z. participated in study design, data analysis, interpretation, and writing of the article.
Institution at which work was performed: Saint Louis University, St. Louis, MO.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Dhanasekaran R. Management of immunosuppression in liver transplantation. Clin Liver Dis. 2017;21:337–353. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Peralvarez M, Guerrero-Misas M, Thorburn D, et al. Maintenance immunosuppression for adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muduma G, Saunders R, Odeyemi I, et al. Systematic review and meta-analysis of tacrolimus versus ciclosporin as primary immunosuppression after liver transplant. PLoS One. 2016;11:e0160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimmer CD, Angele MK, Schwarz B, et al. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int. 2013;26:999–1006. [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. New Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 6.Saliba F, Duvoux C, Gugenheim J, et al. Efficacy and safety of everolimus and mycophenolic acid with early tacrolimus withdrawal after liver transplantation: a multicenter randomized trial. Am J Transplant. 2017;17:1843–1852. [DOI] [PubMed] [Google Scholar]
- 7.Glover TE, Watson CJ, Gibbs P, et al. Conversion from calcineurin to mammalian target of rapamycin inhibitors in liver transplantation: a meta-analysis of randomized controlled trials. Transplantation. 2016;100:621–629. [DOI] [PubMed] [Google Scholar]
- 8.Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15:303–319. [DOI] [PubMed] [Google Scholar]
- 9.Massoud O, Wiesner RH. The use of sirolimus should be restricted in liver transplantation. J Hepatol. 2012;56:288–290. [DOI] [PubMed] [Google Scholar]
- 10.Khorsandi SE, Heaton N. Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence. Transl Gastroenterol Hepatol. 2016;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Fijter JW. Cancer and mTOR inhibitors in transplant recipients. Transplantation. 2017;101:45–55. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018;18(Suppl 1):172–253. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod DA, Naik AS, Schnitzler MA, et al. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. Am J Transplant. 2016;16:2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo MC, Vanatta JM, Modanlou KA, et al. Steroid-free liver transplantation using rabbit antithymocyte globulin induction in 500 consecutive patients. Transplantation. 2015;99:1231–1235. [DOI] [PubMed] [Google Scholar]
- 16.Penninga L, Wettergren A, Wilson CH, et al. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database Syst Rev. 2014:CD010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klintmalm GB, Nashan B. The role of mTOR inhibitors in liver transplantation: reviewing the evidence. J Transplant. 2014;2014:845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lerut J, Iesari S, Foguenne M, et al. Hepatocellular cancer and recurrence after liver transplantation: what about the impact of immunosuppression? Transl Gastroenterol Hepatol. 2017;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissler EK, Schnitzbauer AA, Zulke C, et al. Use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salameh H, Raff E, Erwin A, et al. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am J Gastroenterol. 2015;110:846–856. [DOI] [PubMed] [Google Scholar]
- 23.Germani G, Becchetti C. Liver transplantation for non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol. 2018;64:138–146. [DOI] [PubMed] [Google Scholar]
- 24.Malhi H, Allen AM, Watt KD. Nonalcoholic fatty liver: optimizing pretransplant selection and posttransplant care to maximize survival. Curr Opin Organ Transplant. 2016;21:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stepanova M, Henry L, Garg R, et al. Risk of de novo post-transplant type 2 diabetes in patients undergoing liver transplant for non-alcoholic steatohepatitis. BMC Gastroenterol. 2015;15:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souto-Rodriguez R, Molina-Perez E, Castroagudin JF, et al. Differences in the incidence and clinical evolution of early neurotoxicity after liver transplantation based on tacrolimus formulation used in the immunosuppressive induction protocol. Transplant Proc. 2014;46:3117–3120. [DOI] [PubMed] [Google Scholar]
- 27.Yee ML, Tan HH. Use of everolimus in liver transplantation. World J Hepatol. 2017;9:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharnidharka VR, Naik AS, Axelrod DA, et al. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018;31:198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.