Abstract
Recent advances in neuroimaging methods and analysis have led to an expanding body of research that investigates how large-scale brain network organization dynamically adapts to changes in one’s environment, including both internal state changes and external stimulation. It is now possible to detect changes in functional connectivity that occur on the order of seconds, both during an unconstrained resting state and during the performance of constrained cognitive tasks. It is thought that these dynamic, time-varying changes in functional connectivity, often referred to as dynamic functional connectivity (dFC), include features that are relevant to behavior and cognition. This review summarizes four aspects of the nascent literature directly testing that assumption: 1) how changes in functional network organization on the order of task blocks relate to differences in task demands and to cognitive ability; 2) how differences in dFC variability between different contexts relate to cognitive demands and behavioral performance; 3) how ongoing fluctuations in dFC impact perception and attention; and 4) how different patterns of dFC correspond to individual differences in cognition. The review ends by discussing promising directions for future research in this field. First, it comments on how dFC analyses can help to elucidate the mechanisms of healthy cognition. Next, it describes how dFC processes may be disrupted in disease, and how probing such dysfunction can increase understanding of neural etiology, as well as behavioral and cognitive impairments, observed in psychiatric and neurologic populations. Last, it considers the potential for computational models to uncover neuronal mechanisms of dFC, and how both healthy cognition and disease emerge from network dynamics.
Keywords: Dynamic functional connectivity, Network dynamics, Time-varying, Resting state, Cognition, Individual differences
1. Introduction
The brain has an incredible ability to dynamically adjust to a constantly changing environment. This ability enables adaptive changes in cognition and behavior that allow humans and animals to successfully navigate a complex and inconstant world. An appreciation of the role of dynamic neuronal signaling in adaptive cognition and behavior is not new (e.g., Hebb, 1949). What is new is the ability to measure large-scale neural functioning across the entire brain at high enough temporal and spatial resolution to detect these dynamic changes while individuals are engaging in complex cognition. A growing body of research, predominantly–but not exclusively–using functional magnetic resonance imaging (fMRI), indicates that brain network organization dynamically changes when a constrained cognitive context changes. This could be, for example, a change from an intrinsic, resting state to the performance of a cognitive task; or between pairs of tasks that have different cognitive demands (for a review, see: Medaglia et al., 2015). This literature estimates what has been termed the “functional connectome”, or a complete description of the functional connections between regions distributed throughout the entire brain (Bullmore and Bassett, 2011). Pairwise estimates of the functional connectivity (FC) between brain regions are combined to form a description of whole-brain FC patterns, in which distinct functional networks that interact with each other can be detected. This literature largely assumes that FC remains constant across a block of rest or a cognitive task and assesses changes in FC patterns across those blocks, on the order of minutes. As an example, it was recently demonstrated that whole-brain functional network organization changed systematically during both a task probing motor execution and a task probing working memory as compared to rest, with increased network segregation underlying successful motor execution and increased network integration underlying successful working memory (Cohen and D’Esposito, 2016). With recent advances in analysis techniques, it is now possible to detect time-varying changes in FC measurements on the order of seconds. This rapid time-varying FC is often referred to as dynamic FC (dFC). Much dFC research to date has focused on three aspects of time-varying FC patterns. First, on characterizing dFC within resting state scans, both within and across populations (i.e., across development or diagnoses). Second, on assessing the validity of dFC as measured with fMRI and on improving dFC estimation techniques to minimize artifacts and spurious findings. And third, on relating fMRI estimates of dFC to those acquired via electrophysiological methods to determine the neuronal source of these dynamic fluctuations in FC patterns. Informative reviews of this literature have already been written (Calhoun et al., 2014; Hutchison et al., 2013; Preti et al., 2016). The current review takes a novel approach and highlights progress to date regarding how these dFC measurements, during both rest and cognitive tasks, relate to behavior and cognitive ability. With proper methodological implementation, if characteristics of these measurements are reliably related to behavioral and cognitive outcomes it indicates that there are aspects that are likely neural in origin.
1.1. Introduction to common dFC estimation methods
Optimal methods for estimating dFC are still being developed (for reviews, see: Hutchison et al., 2013; Preti et al., 2016). This section summarizes existing methods to provide context for the following discussion of the literature. Pre-processing of raw fMRI data for a dFC analysis requires the same steps as static FC analyses, which have been discussed in detail elsewhere (for a recent review, see: Ciric et al., 2017). Just as with static FC analyses, the brain is then parcellated into regions of interest, or nodes (Figure 1A). This is often executed using structural or functional brain atlases, or via data-driven approaches such as independent component analysis. Once data has been sufficiently processed, there are two main categories of methods that can be used to quantify what is referred to as dFC. First, and more common, are various approaches to estimating short segments of static FC that, when combined, allow for the investigation of time-varying dynamics in FC across those segments. Second are approaches that estimate activity patterns at individual data acquisition timepoints, or changes in activity patterns, and how those fluctuate across time.
Figure 1. Steps to conduct a dFC analysis.
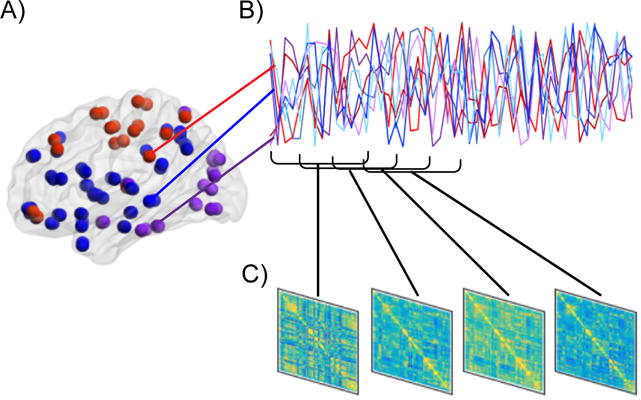
A) First, the brain is parcellated into nodes, which can consist of anatomical or functional regions of interest, or components derived from a data-driven method such as independent component analysis. B) Second, the time-series across all pairs of nodes are related to each other, often by computing correlations or coherence, but other methods such as co-activation patterns or temporal derivatives can be used as well. Commonly, this is repeated within pre-specified and overlapping “windows” of fixed length (as pictured), but novel methods that do not require the assumptions of sliding window approaches can also be utilized, such as dynamical conditional correlations (Lindquist et al., 2014), multiplication of temporal derivatives (Shine et al., 2015) or co-activation patterns (Liu and Duyn, 2013). C) Last, individual connectivity matrices are computed for each window. Once multiple FC matrices are computed for each time-series, dFC analyses quantifying how the matrices differ from each other can be conducted.
The most commonly used method for quantifying dFC is the sliding window approach. Here, FC connectivity matrices are computed over fixed-length segments (“windows”) of the fMRI time-series. There are limits regarding how short that length should be to minimize spurious dynamics. Suggestions range from 40–100 s depending on features of the collected data as well as processing steps implemented (Leonardi and Van De Ville, 2015; Zalesky and Breakspear, 2015). The sliding window approach allows for multiple connectivity matrices to be computed for each fMRI run. Typically, separate windows overlap substantially (Figure 1B). Connectivity matrices can then be compared across windows to assess how FC dynamically varies from one window to the next (Figure 1C). Observations within a window can be given equal weight or, alternatively be down-weighted at the beginning and end of the window. This latter method is termed a tapered sliding window. Within each window, FC is computed as it would be in a standard, static FC analysis. With fMRI data, this is often achieved via calculating the correlation or coherence amongst all pairs of nodes.
An emerging method to calculate a series of FC matrices throughout the scan is the dynamic conditional correlation (DCC) approach (Choe et al., 2017; Lindquist et al., 2014). This is a model-based approach that accounts for certain aspects of fMRI data that traditional sliding window approaches cannot account for. For example, window lengths do not have to be set in advance or equal across the length of the scan, allowing for greater flexibility to detect non-regular changes in FC. Further, past timepoints can be taken into account and appropriately weighted. The DCC method has been show to improve reliability and to better fit fMRI data than sliding window approaches (Choe et al., 2017; Lindquist et al., 2014).
Once multiple FC matrices are constructed, there are two common methods to quantify dynamic changes in FC. First, all connectivity matrices across all windows and participants can be clustered into groups of similar matrices. This can be accomplished using a clustering method such as k-means. With this method, each cluster centroid of a group of individual matrices is then termed a functional “brain state”1. Different brain states can be compared to each other, as can characteristics such as how often one transitions from one brain state to another or how long one remains in a single brain state before changing to another (Calhoun et al., 2014). Second, graph theoretical analyses can be conducted on each FC matrix. In graph theoretical analyses, each region of interest or independent component is considered to be a node of a graph, and each functional connection between pairs of nodes is an edge. Various metrics to summarize brain network organization or the roles of individual nodes can then be calculated, and the variability of those metrics across time can be quantified (Bullmore and Sporns, 2009).
Another method used to characterize dFC is via co-activation patterns (Liu and Duyn, 2013). Here, significant BOLD activation at each data acquisition time point is assessed, and similar patterns of co-activation are clustered together. Co-activation patterns produce networks that look similar to networks created using seed-based FC analysis or independent component analysis, but have higher temporal resolution and require fewer assumptions than sliding window FC approaches. Co-activation patterns can be clustered and their temporal features characterized similarly to FC-derived brain states.
An alternative to using co-activation patterns with similar temporal resolution is via the multiplication of temporal derivatives (Shine et al., 2015). This method calculates the change in BOLD activation from one timepoint to the next. The similarity of the change across two regions of interest is a quantification of the functional coupling between those two regions. Pairwise FC matrices across all regions of interest are populated by functional coupling values. FC matrices are estimated at each timepoint, which can then be treated like any other FC matrices, as described above. Typically in this approach multiple FC matrices are averaged together in a sliding fashion to produce more reliable estimates of functional coupling at each timepoint. This method has been demonstrated to be relatively more robust to head motion and other sources of noise (Shine et al., 2015).
Lastly, psychophysiological interaction (PPI) analyses (Friston et al., 1997) can be used to quantify dFC. PPI analyses are conducted within the context of a general linear model. The key regressor in the model describes the interaction between a behavioral regressor (e.g., response time or block timing) and a physiological regressor (the activity within a seed region of interest at each time point). The interaction term describes whether the FC between the seed region and any other voxels dynamically changes as a function of the behavioral regressor. In this manner a PPI analysis can detect behaviorally meaningful time-varying changes in FC.
More research is needed to confirm which dFC method(s) most accurately reflect true variation in FC across time, as well as appropriate timescales for measuring such fluctuations. It is likely that the optimal method changes depending on timescale and research question. Current research, discussed in more detail below, indicates that sliding window correlations may be particularly susceptible to noise and physiological artifacts (including head motion). Model-based methods such as DCC, as well as methods focusing on patterns and derivatives of co-activation, are promising avenues for future investigations.
1.2. Potential mechanisms of dFC as measured with fMRI
It is critical to establish the validity of observed dynamics in FC measurements, especially in light of recent literature questioning whether variation in FC within a scan reflects relevant neural information (Hindriks et al., 2016; Laumann et al., 2016; Liégeois et al., 2017). Dynamic FC estimations likely include a combination of meaningful neuronal activity, physiological signals (difference in rate or volume of blood flow, respiration, heart rate, spurious effects of motion) and noise of acquired data. It has recently been asserted that observed fluctuations in correlation strength of BOLD signals, in particular using sliding window analytical approaches, may be due to sampling variability and detected even when the underlying FC is stationary (Handwerker et al., 2012; Laumann et al., 2016). Further, the amount of fMRI data typically collected is underpowered to detect dynamics in the data when appropriate statistical comparisons are made, even if such dynamics do exist. It has been demonstrated that with 5 minutes of resting state data there is a 15% chance that true dynamics would be detected (Hindriks et al., 2016). Critically, if artifacts such as motion are not appropriately controlled for, the observation of dynamics in FC data increases spuriously (Laumann et al., 2016), as do relationships between FC and cognitive and behavioral outcome variables (Siegel et al., 2016). It is encouraging that in both cases spurious effects are greatly mitigated if motion and other artifacts are sufficiently removed from the data. These findings underscore the importance of appropriately accounting for motion, including the exclusion of high motion participants, before conducting any FC analyses. Notably, in cases where meaningful dFC is expected because the known arousal or cognitive state of a participant has changed, it can be successfully detected. For example, FC matrices change as a participant shifts between sleep and wake, as well as between a resting state and cognitive tasks (Laumann et al., 2016). It is worth pointing out that one metric of non-stationarity, kurtosis, is increased both during cognitive tasks and during rest as compared to surrogate data that has been constructed as stationary (Laumann et al., 2016). However, whether increased kurtosis during rest is due only to fluctuations in arousal, or to both arousal fluctuations and changes in other ongoing cognitive processes, is still up for debate. Research relating brain states during rest to stages of sleep based on simultaneous EEG recordings has found that much of the variability across brain states can be accounted for by sleep stage (Haimovici et al., 2017). Taken together, these studies emphasize the importance of measuring and controlling for changes in arousal when estimating dFC before attributing dFC measurements to cognition.
Much of the above literature has focused on data using sliding window approaches. As discussed above, alternate methodology to detect dFC has been developed. Evidence exists that some of these methods may more accurately differentiate between static and dynamic FC. As an example, the DCC approach is more likely to result in stable FC estimates as compared to the sliding window approach when the underlying data is designed to be stationary (Lindquist et al., 2014). It is also more reliable and better able to separate signal from noise (Choe et al., 2017). Co-activation patterns (Liu and Duyn, 2013), which recapitulate static FC networks consistent with connectivity approaches, are another promising method to assess dFC. These instantaneous (on the order of data acquisition timing) co-occurring fluctuations of activity level may better explain brain dynamics as assessed via fMRI than fluctuations in FC (Liégeois et al., 2017). While more research remains to be conducted, these investigations imply that it is possible to detect dFC in BOLD data both during cognitive tasks and during rest using either appropriately-modeled correlation approaches (DCC) or changes in activation (co-activation patterns or multiplication of temporal derivatives) – provided that there is sufficient data, appropriate nuisance regression and methodology, and relevant null models to determine significance. While much of the below literature has used the sliding window approach and may not appropriately implement null models, by relating observed dynamics to measurable behavioral and cognitive outcome, as well as by measuring statistical changes across known cognitive contexts, it extends the field by providing important initial investigations into the behavioral and cognitive relevance of observable fluctuations in FC.
Another manner by which to validate BOLD dFC estimates is to use multimodal imaging techniques that relate FC measurements as assessed using fMRI to more direct measures of neuronal functioning. This literature demonstrates relationships between BOLD dFC and electrophysiological measures of neural functioning, including electroencephalography (EEG), magnetoencephalograpy (MEG), electrocorticography (ECoG) and local field potential (LFP; Keilholz, 2014; Tagliazucchi and Laufs, 2015). As an example, time-varying infraslow (<1 Hz) fluctuations have been observed when using fMRI BOLD measurements, ECoG in participants undergoing seizure monitoring and LFP measurements from implanted electrodes in rats (Ko et al., 2011; Majeed et al., 2011; Pan et al., 2013; Thompson et al., 2014). Interestingly, these fluctuations in all modalities occur in a quasiperiodic pattern and include semi-regular alterations within the default mode network (DMN) and between the DMN and task-positive networks (TPNs). Both infraslow electrical activity and DMN-TPN FC as measured with fMRI have been linked to task performance, implying shared underlying neural mechanisms (Keilholz, 2014). Dynamic changes in BOLD FC in the same infraslow range have also been linked with higher frequencies of LFP FC. In sliding window analyses, the change in BOLD FC between left and right primary somatosensory cortices in rats is correlated with the change in LFP FC within theta, high beta and gamma bands (Thompson et al., 2013b). In humans using non-invasive electrophsyiological measurements (EEG), changes in FC using sliding window analyses are associated with changes in EEG power within the alpha, beta and gamma bands (Chang et al., 2013; Tagliazucchi et al., 2012). Finally, whole-brain dFC derived from both sliding windows and co-activation patterns during simultaneous calcium imaging and BOLD measurements in mice is significantly correlated, both within method and across method. More specifically, sliding window BOLD dFC is related to both sliding window and co-activation pattern calcium imaging dFC (Matsui et al., 2017).
It has been proposed that low frequency electrical activity contributes to large-scale coordination across the brain, while higher frequencies organize local activity. Further, it is well established that synchronization across multiple frequencies contributes to cognition and behavior (Canolty and Knight, 2010). Therefore, while dFC as measured with BOLD may not have the temporal or spatial resolution to reflect rapid changes in local neuronal activity, it could reflect large-scale coordination across distinct brain regions that emerges from local changes. It is reasonable, therefore, that this large-scale coordination underlies changes in cognitive state observed with behavioral measures.
In addition to probing the neuronal basis of BOLD dFC estimates and validating estimation techniques, a third manner by which to validate the neural relevance of dFC is to relate dFC measurements to individual differences in cognition and behavior. This line of research aims to determine which aspects of dFC are cognitively meaningful and therefore likely to arise from brain function. It is this category of literature that is the focus of the remainder of the current review.
2. Current Literature
2.1. Early dynamic functional connectivity research: changes in network organization due to changing task demands
While still an emerging body of literature in its own right, a precursor to probing rapid dynamic alterations of FC (on the order of seconds) is measuring changes in FC patterns that occur between entire blocks of cognitive tasks with different demands. These studies have consistently found that there are many similarities in network structure during rest and during the performance of different cognitive tasks (Cole et al., 2014; Krienen et al., 2014). Crucially, there are also meaningful task-specific differences (Cole et al., 2013; Davison et al., 2015; Krienen et al., 2014). Tasks that involve integrating across multiple distinct cognitive processes (e.g., memory, working memory, visuospatial attention) consistently result in increased long-range connections that integrate across distinct intrinsic networks as compared to rest or simpler tasks (e.g., Braun et al., 2015; Cocchi et al., 2013; Cohen and D’Esposito, 2016; Cohen et al., 2014; Davison et al., 2015; Fornito et al., 2012; Kitzbichler et al., 2011; Spadone et al., 2015). Conversely, tasks that require less cognitive integration, such as those probing motor execution, result in increased segregation across distinct intrinsic networks (Bassett et al., 2015; Cohen and D’Esposito, 2016). Studies that have probed behavior have found that these dynamic changes in functional connections across task blocks result in improved behavioral performance (Bassett et al., 2015; Braun et al., 2015; Cohen and D’Esposito, 2016; Cohen et al., 2014; Spadone et al., 2015). A recent review by Medaglia and colleagues (2015) discusses this body of literature more thoroughly.
Much of the above literature calculates average (static) FC over minutes-long task blocks. However, a recent study found that even with short, 15 s blocks it was possible to detect systematic differences in functional network organization during rest as compared to during a choice reaction time task (Monti et al., 2014). Specifically, cognitive-control related nodes of the fronto-parietal network (FPN) became more integrated during the task as compared to rest, while nodes of the DMN and primary sensory regions (visual, motor, auditory) did not. This is consistent with static FC literature indicating greater integration across networks, including cognitive control networks, during complex cognitive task blocks (Medaglia et al., 2015).
A natural extension of the above work is to determine how variable these FC patterns are across tasks. Some studies have concluded that the majority of functional connections do not change between rest and cognitive tasks (Cole et al., 2014; Krienen et al., 2014). An outstanding question is just how distinct are those functional connections that do change. With as little as 22.5 to 30 seconds of data, current task environment was successfully identified using a pattern classifier trained to detect whole-brain patterns of FC within blocks (Gonzalez-Castillo et al., 2015; Shirer et al., 2012). Tasks classified probed cognitive processes as varied as rest, episodic memory, music, math, attention and working memory. These findings confirm the validity of probing time-varying changes in FC across short blocks of functional neuroimaging data. Importantly, each task was characterized by distinct whole-brain FC patterns that were related to specific task demands. As an example, a medial temporal network was strongly connected during the episodic memory task, and a language network during the music task (Leonardi et al., 2014). Interestingly, during the resting state whole-brain FC patterns were better described by a combination of the FC patterns observed during individual tasks than by rapid alternation between the task states (Leonardi et al., 2014). This implies that the ongoing cognitive processes that occur during a resting state are complex and potentially reflect multiple simultaneous cognitive domains. Notably, participants with better behavioral performance were more accurately classified, indicating that task engagement (or, perhaps, ability) was reflected in FC patterns (Gonzalez-Castillo et al., 2015).
Recently, data-driven methods have been developed to detect changes in FC patterns across nodes without requiring explicit assignment of each time point to a specific task (Cribben et al., 2012, 2013). This emphasizes the relevance of specialized FC patterns across brain regions for task performance, once again indicating that understanding dFC in the context of cognition is important both for our understanding of FC dynamics as well as for our understanding of the neural basis of cognition.
The above studies provide strong evidence for the idea that block-wise changes in FC are important for successful cognitive performance on a range of tasks. A natural next step is to understand how more rapid dFC estimates subserve cognition and behavior. Such rapid dynamics may not be directly tied to alterations in external task demands. Instead, they may indicate changes in internally-driven factors such as attention, motivation, fatigue or goals; or, likely, they may reflect a combination of both external and internal factors. An understanding of how these rapid dynamics relate to both cognitive performance and to general cognitive and affective state is necessary to understand the role they play in human cognition and behavior.
2.2. Differences in the variability of functional connections between a resting state and task performance
A relatively consistent finding in healthy young adults is that during a resting state, whole-brain FC patterns (brain states) are quite variable (Calhoun et al., 2014). These brain states are presumed to reflect a combination of reinstantiation of past experience and preparation for future demands on the system. They therefore represent an exploration of possible spaces the brain can occupy (Deco and Corbetta, 2011; Ghosh et al., 2008). A growing body of literature has linked differences in functional brain network dynamics to general changes in arousal, including levels of consciousness. Much of the literature that manipulates drug-induced consciousness is conducted with animals. In both monkeys and rats, reduced consciousness resulted in a dose-dependent decrease of dFC variability during a resting state scan (Barttfeld et al., 2015; Hudetz et al., 2015; Hutchison et al., 2014). In humans, natural fluctuations in arousal as assessed by self-report feelings of fatigue and attention have been related to dFC. Higher levels of fatigue were related to more stable dFC measurements, while higher levels of attention were related to more variable dFC measurements within a single individual across repeated resting state scans (Shine et al., 2016b). Drug-induced arousal, such as that which occurs due to caffeine administration, has also been shown to result in increased variability of dFC measurements (Rack-Gomer and Liu, 2012).
A relevant question that follows from this literature is how dFC alters when a cognitive environment is more constrained, such as when a participant is engaged in a cognitive task with specific demands. Only a few studies to date have addressed this question, and they have consistently found that dFC variability decreases during a cognitive task as compared to rest in healthy young adults (Chen et al., 2015; Elton and Gao, 2015; Hutchison and Morton, 2015). In other words, dFC patterns are more stable during cognitive tasks that purportedly require sustained cognition. This appears to be a general characteristic of cognitive engagement, as dFC variability has been shown to decrease during a stimulus-response compatibility task (Elton and Gao, 2015; Hutchison and Morton, 2015) and during a 2-back task probing working memory (Chen et al., 2015). This finding is also consistent across dFC methods, including seed-based sliding window correlations (Elton and Gao, 2015; Hutchison and Morton, 2015) and co-activation patterns (Chen et al., 2015). Functional brain networks demonstrating more stable dFC during task performance include those relevant for attention and cognitive control, including the DMN, dorsal attention network (DAN), executive control network (ECN) and salience network (SN), as well as connections between these networks and primary sensory networks (Chen et al., 2015; Elton and Gao, 2015; Hutchison and Morton, 2015). Notably, a graded impact of task condition has been observed. When comparing dFC during a speeded task condition of a stimulus-response compatibility task to dFC during a “relaxed” task condition, variability was decreased more for the speeded condition (Elton and Gao, 2015). This indicates that effort related to task difficulty may in part influence the increased stability of dFC during the execution of a cognitive task. Finally, participants who displayed the most stable dFC during task performed the best, as assessed via increased accuracy and more stable response times (Elton and Gao, 2015; Hutchison and Morton, 2015). These findings underscore the relevance of dFC within task blocks for successful cognition, highlighting a need to better understand this relationship.
An important direction for further research is to probe how these network dynamics are different across populations. Hutchison and Morton (2015) quantified the change in dFC between rest and a stimulus-response compatibility task in participants aged 9–32. Notably, they found a different pattern of dFC in children as compared to adults. As stated above, dFC became more stable during the task as compared to rest in adults. In children, however, dFC was more stable during rest and more variable during the task as compared to adults. This increased dFC variability during the task was related to worse behavioral performance as indexed by increased response time variability and increased errors. These different functional network dynamics were not due to differences in network structure, as both children and adults displayed similar whole-brain FC patterns. It has been asserted that network dynamics during rest may allow individuals to “explore” different possible brain states (Deco and Corbetta, 2011; Ghosh et al., 2008). This dynamical complexity may not yet be developed in childhood, a hypothesis supported by the finding that less dynamical exploration during unconstrained resting states was observed. It is also possible that adults can suppress spontaneous transitions between brain states during a task with specific cognitive constraints, but that this ability is not yet fully developed in childhood, leading to more variable dFC during cognitive tasks (Hutchison and Morton, 2015). Future research should directly probe whether the maturation of network dynamics is related to the emergence of cognitive ability throughout childhood and adolescence.
Literature probing how dFC alters when individuals are placed in different temporary states indicates systematic differences across states. This holds whether the states are drug-induced or natural changes in general states such as arousal (Barttfeld et al., 2015; Hudetz et al., 2015; Hutchison et al., 2014; Rack-Gomer and Liu, 2012; Shine et al., 2016b), or specific cognitive states such as during task performance (Chen et al., 2015; Elton and Gao, 2015; Hutchison and Morton, 2015). Across multiple tasks and using multiple methods, the cognitively-relevant literature points to a general decrease in dFC when an individual is in a constrained cognitive environment (Chen et al., 2015; Elton and Gao, 2015; Hutchison and Morton, 2015). Decreases in dFC are also observed when arousal is decreased, whether it is due to fatigue or anesthesia (Barttfeld et al., 2015; Hudetz et al., 2015; Hutchison et al., 2014; Shine et al., 2016b). An important target of future work should be to understand the difference between anesthesia-induced stability and task-induced stability. Some hints exist currently: during anesthesia, the dominant FC brain state is similar to underlying anatomical connectivity (Barttfeld et al., 2015). During sustained task performance, on the other hand, the dominant FC brain states display increased integration across cognitive control-related networks and between cognitive-control related networks and task-relevant networks (Elton and Gao, 2015; Hutchison and Morton, 2015). Further research probing the duration of different dFC patterns, as well as their relation to cognitive performance, will shed light on how these changes in dFC across different contexts contribute to cognition, both in healthy adults as well as across populations.
2.3. Spontaneous functional connectivity dynamics impact concurrent behavior
The literature discussed thus far relating dFC to behavior has measured overall variability of dFC measurements during sustained performance of a cognitive task and how that relates to average measures of task performance, such as accuracy and response time variability. A promising direction for further research that takes advantage of the timescale of dFC is probing how trial-by-trial changes in FC profiles relate to trial-by-trial performance. Some literature to date has explored this relationship, inspired by earlier research measuring differences in BOLD activation patterns preceding detected versus missed stimuli (Sadaghiani and Kleinschmidt, 2013) or errors versus correct trials (Eichele et al., 2008; Weissman et al., 2006).
Much existing literature probing pre-stimulus FC and how it relates to behavior focuses on perception and vigilance tasks, such as detecting rare visual or auditory stimuli, noisy coherent visual characteristics such as color or motion, or faint, near-threshold stimuli. It has been observed that rare visual or auditory stimuli are more likely to be detected, and detected more quickly, when FC just preceding the stimulus is characterized by a stronger anticorrelation between the DMN and TPNs, including those that are related to cognitive control (Thompson et al., 2013a; Wang et al., 2016). Stronger positive correlations amongst cognitive control networks, and between cognitive control and primary sensory networks related to the stimulus domain, have also been associated with faster and more accurate stimulus detection (Ekman et al., 2012; Sadaghiani et al., 2015; Wang et al., 2016). Further, whole-brain modularity, or the degree to which the brain separates into distinct networks with only sparse connections across networks, has been shown to be reduced before misses as compared to hits (Sadaghiani et al., 2015). In other words, greater integration across networks, the DMN and task-irrelevant sensory networks in particular, increases the likelihood that stimuli will be missed. Notably, relationships between pre-stimulus FC and stimulus detection have been shown to be stronger in individuals with faster average response times (Thompson et al., 2013a). An interesting link to the arousal literature discussed above is that performance has been shown to be faster and more accurate when task FC preceding to-be-detected stimuli was more similar to FC during high arousal periods of a resting state scan. Conversely, poorer performance was characterized by pre-stimulus FC that was more similar to FC during low arousal periods of a resting state scan (Wang et al., 2016). Critically, hits and misses can be successfully differentiated by a pattern classifier trained on a combination of pre-stimulus activity and pre-stimulus FC. Including FC measures increased the accuracy of the classifier (Sadaghiani et al., 2015). Taken together, these results indicate that a more connected DMN during a task that requires sustained attention impairs performance. Additionally, dFC of the DMN with other networks can alter on a moment-to-moment basis, influencing observed fluctuations in behavior in systematic ways (Sadaghiani et al., 2015; Thompson et al., 2013a; Wang et al., 2016).
A different approach to probing how spontaneous dFC may impact concurrent behavior was executed by Kucyi and colleagues (2017). To avoid the potential confound of an external stimulus such as a visible color change or auditory tone, participants were trained to press a button every 600 ms in a self-paced task. This design removed external stimulation while still allowing for behavioral assessment by comparing blocks of trials that were characterized by stable response times to blocks of trials that were characterized by more variable response times. Increased response time variability was interpreted as indicating periods of increased fatigue or distraction. Using a PPI analysis, it was found that periods of high response time variability were characterized by higher FC within the DMN, as well as between the DMN and SN. For a thorough review of literature measuring changes in FC due to internal distraction, see Kucyi (2017) in this issue. These findings support theories stating that increased DMN connectivity to task-relevant networks may underlie lapses of attention (Sonuga-Barke and Castellanos, 2007; Weissman et al., 2006).
The studies described in this section took advantage of the relatively fast timescale of measurable dFC in humans using fMRI, which can detect changes in FC on the order of seconds instead of minutes. They moved beyond relating average characteristics of FC to average behavioral performance, and compared transient dFC patterns to performance on a trial-by-trial basis. This technique allows one to better characterize within-participant differences in performance by associating different types of trials with different patterns of functional brain network organization. It also provides external validity to estimates of within-block FC dynamics as they relate to arousal and cognition, although the degree to which dFC measurements relate to general arousal versus specific cognitive processes is still up for debate. Extant literature in this domain is focused on attention and stimulus detection paradigms, but future work could study how dFC relates to remembered versus forgotten events, successful versus failed response control, risky versus safe decisions, and other complex cognitive processes.
2.4. Differences in patterns of dynamic functional connectivity correspond to individual differences in cognition
By directly relating cognitive ability to features of dFC, a greater understanding of the neural mechanisms underlying specific aspects of cognition, including both successes and failures, can be achieved. A small number of studies to date have related time-varying dFC on the order of seconds to both general and specific cognitive abilities. These studies ask similar questions to those described above, but they extend that literature in two critical ways. First, they extend the literature discussed in the Early dynamic functional connectivity research: changes in network organization due to changing task demands section by focusing on rapid changes in dFC as opposed to sustained FC across brief task blocks. Second, they seek to understand how dFC patterns change in reaction to alterations in external cognitive demands, which is distinct from the Spontaneous functional connectivity dynamics impact concurrent behavior section that emphasizes how intrinsic changes in dFC impact responses to future external events.
As an example, Chen and colleagues (2016) took advantage of data from the Human Connectome Project (https://www.humanconnectome.org/) to probe dFC during rest in a large sample of participants. They probed how resting state dFC related to cognitive flexibility assessed behaviorally. Static resting state FC has been associated with general cognitive abilities such as IQ, executive functioning, episodic memory and reading ability, among others (for a review, see: Vaidya and Gordon, 2013). An ability such as cognitive flexibility relies upon the flexible engagement of a range of cognitive processes, including attention, processing speed, response inhibition and general executive functioning. Therefore, it is likely that it requires the flexible engagement of different networks depending on specific current demands. It was found that dFC of the SN, which is hypothesized to underlie the processing and facilitation of goal-relevant stimuli, was most flexible during rest (Chen et al., 2016). In other words, the SN was more likely to flexibly interact with many other networks across time than other networks. Other cognitive control-related networks such as the FPN and cingulo-opercular network (CON) had intermediate levels of flexible interactions with other networks as assessed via dFC. Sensory and motor networks had the most stable dFC and interacted with the fewest number of other networks. Crucially, when relating brain network flexibility to behavior, it was found that higher SN flexibility was related to cognitive flexibility, but that flexibility of the FPN and CON was not. This study demonstrates that the SN may be a critical hub for coordinating complex cognition and that its role in cognition may be distinct from that of other cognitive control networks (Chen et al., 2016). Further, it indicates that dFC measurements assessed during rest have cognitive relevance. This underscores the importance of future research relating these dynamic network characteristics to cognition and behavior. Another study that utilized dFC measurements during rest to better understand the cognitive role of intrinsic functional networks focused its analyses on networks arising from different functional subdivisions of posteromedial cortex (PMC), a key node of the DMN (Yang et al., 2014). Findings from static FC studies indicate that the PMC is a highly integrated hub node that communicates with multiple other networks. The dFC analysis implemented in this study identified five different whole-brain dFC profiles that involved PMC, each of which a was a combination of multiple distinct intrinsic networks. This finding supports the idea of PMC as a brain region that is highly integrated across multiple networks. Further, increased time during a resting state in one of those connectivity profiles, which was characterized by positive correlations between a PMC subregion and cognitive control-related networks, was related to poorer cognitive flexibility (specifically, flexibility of thinking and concept formation; Yang et al., 2014). These findings are consistent with prior literature implicating DMN-cognitive control connections in impaired cognitive performance (Kucyi et al., 2017; Sadaghiani et al., 2015; Thompson et al., 2013a; Wang et al., 2016), and again support the relationship between characteristics of dFC and general cognitive ability.
Shine and colleagues (2016a) measured dFC during both a resting state and a battery of cognitive tasks, also using data from the Human Connectome Project. They found that functional brain network organization dynamically altered between a brain state that was characterized by greater network segregation, and a brain state that was characterized by greater network integration. During task engagement, more time was spent in the integrated brain state as compared to during rest, although specific task demands altered the proportion of integration. During simpler tasks, such as a task probing repetitive movements of various effectors, relatively more time was spent in the segregated brain state. Conversely, during complex tasks with a greater combination of cognitive demands, such as an n-back task probing working memory, relatively more time was spent in the integrated brain state. This is consistent with previous research measuring reconfiguration of static FC on the level of task blocks (Cohen and D’Esposito, 2016), as well as research relating overall dFC during a complex stimulus-response compatibility task to that during rest (Elton and Gao, 2015). Moreover, during a resting state these dynamic fluctuations between a segregated brain state and an integrated brain state were associated with pupil diameter, which is considered to be a proxy for arousal. A greater pupil diameter was observed during brain states characterized by greater network integration. In other words, a transient integrated brain state during rest was associated with greater arousal, and during task was associated with more complex cognitive demands (as well as better performance; Shine et al., 2016a).
Taken together, the results of these studies extend earlier research observing task-specific reconfiguration of functional network organization in direct response to changing cognitive demands (for a review, see: Medaglia et al., 2015). They demonstrate that functional network reconfiguration can occur rapidly and transiently, during both rest and during cognitive tasks, and that it is related to measures of arousal, general cognitive ability and cognitive task performance.
3. Promising Directions
3.1. Elucidating the mechanisms of cognition
There is already a long history of using task-based fMRI to understand the neural underpinnings of human cognition and behavior. Neural measurements can be used to confirm and update cognitive models of behavior (D’Esposito, 2007; Frank and Badre, 2015). Likewise, further investigations into dFC and how it relates to cognition will increase our understanding of how different aspects of cognition operate. For example, it is already well-established that average FC across a block relates to average task performance on a range of cognitive tasks (Bassett et al., 2015; Braun et al., 2015; Cohen and D’Esposito, 2016; Cohen et al., 2014; Spadone et al., 2015), as well as to general cognitive ability (for a review, see: Vaidya and Gordon, 2013). But the same individual may perform differently in different contexts, due to a combination of external stimuli (i.e., task demands) and internal changes (i.e., attention, drowsiness; Finn et al., 2017; Poldrack et al., 2015; Shine et al., 2016b). Initial research has related dFC to arousal (Haimovici et al., 2017; Laumann et al., 2016; Shine et al., 2016b; Wang et al., 2016) and to arousal-related changes in performance (Wang et al., 2016). Interestingly, low arousal states are characterized by decreased network dynamics (Barttfeld et al., 2015; Hudetz et al., 2015; Hutchison et al., 2014; Shine et al., 2016b), as are states of high task engagement, presumably accompanied by relatively high arousal and attention (Chen et al., 2015; Elton and Gao, 2015; Hutchison and Morton, 2015). It is thought that the stability observed during low arousal states may reflect underlying anatomical connections (Barttfeld et al., 2015), while the stability observed during active engagement in complex cognition may reflect greater integration across networks relevant to the specific task and to control processes (Elton and Gao, 2015; Hutchison and Morton, 2015). A highly integrated brain state is more costly metabolically (Bullmore and Sporns, 2012) and, while perhaps necessary for successful cognition, may only be sustainable for brief periods of time. Further research characterizing specific dFC differences during low arousal and high arousal, and how those relate to changes in performance across a range of cognitive tasks, would inform our understanding of different underlying sources of functional network stability, as well as how brain state transitions contribute to cognition above and beyond general arousal levels.
This literature could capitalize on the findings from static FC literature that has characterized network reconfiguration across sustained cognitive task conditions. As an example of an instance of this strategy, using static FC methods it has been found found that functional network organization during the performance of a task probing motor execution was similar to that during rest and characterized by network segregation, whereas network organization during the performance of a task probing working memory was characterized by network integration (Cohen and D’Esposito, 2016). Using complementary dFC methods, Shine and colleagues (2016a) found that during both rest and specific tasks, the brain dynamically alternated between a segregated brain state and an integrated brain state. The most time spent in the segregated brain state was during rest, closely followed by a task probing motor execution. The most time spent in the integrated brain state was during a task probing working memory. The results from these two studies are consistent, but the dFC analysis adds extra information about how dFC underlies cognitive performance that cannot be detected using static FC methods (Figure 2). Further refinement of these methods to relate the occurrence of different dFC brain states to performance on specific trials would expand literature probing the impact of concurrent dFC patterns on within-participant performance differences.
Figure 2. DFC analyses complement and extend static FC analyses.
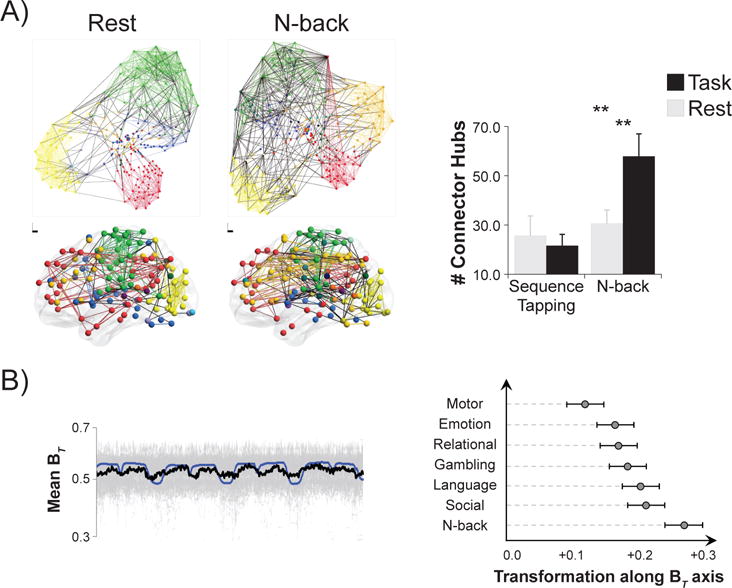
A) Using static FC methods, whole-brain functional network organization was found to reconfigure between rest and an n-back task that probed working memory (Cohen and D’Esposito, 2016). In the left panel each color represents a network, each colored line represents a within-network edge, and each black line represents a between-network edge. On the top, nodes are depicted based on connections; nodes with more shared connections are closer together. On the bottom, nodes are depicted in brain space; each circle corresponds to the coordinates of the center of each node. Note that there is greater integration across distinct networks during the n-back task as compared to rest. In the right panel, the number of connector hub nodes is compared across rest, n-back, and during a sequence tapping task that probed motor execution. Connector hubs are nodes with high inter-network connectivity. The number of connector hubs did not change during sequence tapping as compared to rest, but it increased during the n-back task as compared to rest. Figure adapted with permission from Cohen and D’Esposito (2016). **p < .01. B) Using dFC methods, participation coefficient (BT) was found to fluctuate as current task changed (Shine et al., 2016a). Participation coefficient measures how connected a node is across networks; connector nodes are defined as nodes with high participation coefficients. The left panel demonstrates that using dFC analyses, average participation coefficient (thick black line; individual participant data plotted in gray) varied along with task blocks (task regressors plotted in blue). The right panel demonstrates the extent to which whole-brain FC profiles shifted toward a more integrated brain state (high BT) during different tasks as compared to rest. Consistent with Cohen and D’Esposito (2016), during motor task performance the extent of integration was most similar that during rest, while during n-back performance the extent of integration was much stronger. Figure adapted with permission from Shine et al. (2016a).
To date, there has been some research investigating how dFC impacts trial-by-trial differences in performance. Much of this literature has focused on slow perception tasks with the shortest interval between trials being 4 s, and the longest 480 s (Sadaghiani et al., 2015; Thompson et al., 2013a; Wang et al., 2016). This literature has indicated that specific patterns of FC that are ongoing before a stimulus occurs impact detection of that stimulus. These findings extend literature that has observed that different ongoing patterns of brain activity impact current behavior (for a review, see: Sadaghiani and Kleinschmidt, 2013). Future research can apply these techniques to more complex cognitive tasks in order to learn more about how dynamics of functional brain network organization contribute to various aspects of cognitive performance, such as errors on tasks, improved learning, identification of ambiguous stimuli, or successful remembering as compared to forgetting. Further, these techniques may shed light on features of functional network organization that underlie phenomena such as priming by stimuli that do not reach awareness.
Importantly, the high overlap in overall FC patterns between rest and various task states (Cole et al., 2014; Krienen et al., 2014) implies that cognitively-relevant changes are minimal, while the consistent relationships to performance imply that those changes are meaningful. Better characterizing the functional connections that are task- or cognitive process-specific on the level of task blocks would allow for systematic investigations of the dynamics of those connections across a block in relation to behavioral performance. With precise hypotheses and appropriate methodology, such research would be able to concretely link dynamic changes in FC strength between specific brain regions (or groups of regions) to specific aspects of cognition. As an example, a recent study took advantage of the high temporal resolution of MEG data to examine differences in FC between specific pairs of brain regions within individual trials during a learning task (Fatima et al., 2016). Participants who learned the task quickly displayed strong FC between left inferior parietal cortex and left posterior cingulate cortex during the first 200ms of each trial, while slower learners displayed strong FC between those two regions during the last 200ms of each trial. Inferior parietal-posterior cingulate FC was correlated with visuospatial ability in all participants, but with early FC strength in fast learners and with late FC strength in slow learners.
Crucially, if patterns of dFC linked to specific cognitive processes are identified, and those same patterns are recapitulated during resting state scans, this literature could help inform whether dynamics during rest may be linked in part to ongoing cognitive processes. Additionally, examining the relationship between activation changes and FC changes, which are thought to be distinct and to have additive properties on network organization and on behavior (Gratton et al., 2016; Khambhati et al., 2017 in this issue), would further elucidate the dynamic mechanisms underlying specific aspects of cognition. In this manner, we can inform cognitive theory by implementing novel neuroscience techniques (Frank and Badre, 2015).
3.2. Clinical relevance
An extremely consistent finding across the static FC literature, and that is emerging in the newer dFC literature, is that there are reliable differences in both static FC and dFC between psychiatric and neurologic patients and healthy individuals (for reviews, see: Calhoun et al., 2014; Xia and He, 2011). To date, most of the literature finding differences in dFC across groups has focused on describing differences in the resting state. Interestingly, specific brain states appear to be quite similar between patients and healthy control participants. In other words, it is not the whole-brain patterns of FC that differentiate groups. Instead, it is the number and form of dynamical transitions across brain states, as well as the frequency of brain states. As an example, it was found that throughout the course of a resting state scan patients with schizophrenia dynamically transitioned between five different brain states with similar FC patterns to those observed in healthy control participants (Damaraju et al., 2014). However, patients with schizophrenia spent significantly more time in a disconnected brain state that displayed lower overall FC within and between functional networks. Further, they transitioned less often than control participants to more highly integrated brain states (Damaraju et al., 2014).
It is interesting to note that a striking result of multiple studies is that individual whole-brain patterns of reoccurring functional brain states are similar across conditions or groups. This has been shown to be the case when comparing rest to tasks (Hutchison and Morton, 2015; Shine et al., 2016a), when comparing children to adults (Hutchison and Morton, 2015) and when comparing patients with schizophrenia to healthy individuals (Damaraju et al., 2014). The critical difference, therefore, appears not to be in brain state FC organization itself, but in the dynamics of the brain states. It is thus possible that by improving our understanding of how each of these brain states relates to specific aspects of cognition, we can additionally improve our understanding of ongoing internal processes in patients as compared to healthy individuals. With powerful data-driven methods that can detect brain state transition points without requiring advance knowledge of internal state transitions (Cribben et al., 2012, 2013), future research can clarify the timing of these transitions and how it varies across patient groups. This technique may be particularly useful in populations in which it is hard to collect reliable behavioral data during an MRI scan, such as in young children, patients with dementia or patients with severe cognitive deficits.
DFC methods additionally allow us to test hypotheses related to rapid changes in FC that have previously been difficult to test directly using functional neuroimaging data. As an example, it has been proposed that attention lapses in attention deficit hyperactivity disorder (ADHD) result from intrusions of the DMN into active task states, during which DMN activity and connectivity with other networks is often reduced or anticorrelated in healthy individuals (Sonuga-Barke and Castellanos, 2007). This hypothesis could be directly tested with dFC methods during a cognitive task in which indices of attention lapses, such as prolonged response times or increased errors, can be measured and dFC patterns preceding those periods can be related to behavior. Further, it has already been observed in healthy individuals that increased time during a resting state scan of a particular dFC pattern involving the PMC region of the DMN is related to reduced cognitive flexibility (Yang et al., 2014). If one were to observe more time spent in a brain state related to attention lapses during a task in ADHD than in healthy control participants, this would provide support for the default mode interference hypothesis. This could be a biomarker for ADHD specifically or for any population characterized by greater inattention. As another example, autism spectrum disorder (ASD) is characterized by cognitive and behavioral inflexibility. It has been shown that there are fewer differences in static network connectivity between two different tasks (an arithmetic task and a social attention task) in patients with ASD than in healthy individuals, and that greater network similarity across those two tasks is related to more severe repetitive behaviors (Uddin et al., 2015). Analyses taking advantage of dFC methods could directly test the hypothesis that brain network inflexibility is a characteristic of individuals with ASD.
Research probing dFC differences across cognitive task states in healthy individuals has successfully identified different task conditions using pattern classification (Gonzalez-Castillo et al., 2015; Shirer et al., 2012). These methods could be applied to clinical data to determine whether patients can be successfully differentiated from healthy individuals based on their dFC characteristics, both during a resting state and during specific cognitive tasks. During a resting state, it has been demonstrated that healthy individuals, patients with schizophrenia and patients with bipolar disorder could be differentiated from each other at approximately 84% accuracy by including dFC features in a classifier. A classifier based solely on static FC features was only able to accurately classify the groups at 59% accuracy, which is significantly higher than chance (35%) but significantly lower than that obtained using dFC features (Rashid et al., 2016). This is a promising approach that should be extended to other disorders. It is likely that in patients dFC differs in meaningful ways both during rest and during tasks, therefore combining both resting state and task data may increase our ability to differentiate across diagnoses and to better understand how dFC patterns during rest are related to dFC during externally-driven cognitive processes. Moreover, by identifying the features that contribute most to classification accuracy, a greater understanding of differences across patient groups may be obtained. This is particularly relevant for disorders that are difficult to differentiate clinically (e.g., schizophrenia and bipolar disorder), as well as for disorders that show high comorbidity (e.g., ADHD and ASD). If critical classification features overlap with specific cognitive task states that have been observed in healthy individuals, this line of research could further our understanding of what differentiates patients from control participants.
It is important to acknowledge that much of the research probing dFC in patient populations utilizes sliding window correlations to characterize dynamics. As has been noted, it is possible that many of the group differences are due to various sources of noise, such as differences in motion or respiration rate across groups or to general changes in arousal, which also may be systematically different across groups. Future research could confirm whether the significant differences across populations include a neurally-based component by implementing methodology that is less susceptible to noise, utilizing appropriate null models and tracking general levels of arousal. Additionally, if brain states identified during rest match those identified during cognitive tasks, it may be possible to glean differences in ongoing cognition across populations from resting state dFC.
3.3. Computational modeling
Currently, dFC characteristics can be estimated but the mechanisms underlying these measurements, as well as the degree to which they are of neural origin, are unknown. A critical next step in our understanding of dFC and how it relates to behavior and cognition is to move beyond descriptive measures of dFC, and to probe how these dynamic functional connections evolve, as well as how cognition arises from these dynamics (Bargmann and Marder, 2013; Kopell et al., 2014). As stated earlier, a growing body of literature is using multimodal imaging in an attempt to uncover the neuronal mechanisms that give rise to dFC (Keilholz, 2014; Kopell et al., 2014; Tagliazucchi and Laufs, 2015). Another method that can be used to reveal the mechanisms of network dynamics and how such dynamics underlie cognition is biologically plausible computational modeling. Reviews exist that describe state-of-the-art computational modeling techniques that detail how neuronal firing can give rise to dynamic large-scale network structure as measured during fMRI (Breakspear, 2017; Cabral et al., 2017; Deco and Corbetta, 2011; Deco et al., 2013). While features of existing models differ, they uniformly make two assumptions. First, that underlying anatomical connectivity constrains large-scale functional network connectivity. Second, that observable variations in FC arise from a combination of noise, internal state and external stimulation, among other characteristics. One interesting feature of these models is that there are multiple stable states, termed attractors. Noise introduced to the system can push a model from one relatively stable state to another, just as external stimuli from cognitive tasks, or internal states such as arousal, have been shown to be systematically related to different dFC brain states. Notably, the idea of alternating between more segregated and more integrated brain states depending upon cognitive task demands (Shine et al., 2016a) has been confirmed with computational models (Deco et al., 2015). Further, computational models can successfully predict the functional network dynamics observed during sleep and awake states, in disease (schizophrenia), and in successful treatment of disease (the effects of deep brain stimulation in Parkinson’s Disease; Deco and Kringelbach, 2014).
Not much work has been conducted to date exploring how computational models of dynamic large-scale network functioning predict dFC alterations in response to changes in cognitive demands, so this is a fruitful avenue for further research. For example, these models may explain the decrease in dFC both when under anesthesia as well as when focused on a cognitive task, and how those two brain states with increased stability differ from each other. Further, by introducing the structural and functional changes that occur throughout the lifespan to a computational model, we may be able to better understand how dFC changes and perhaps underlies cognitive development and decline. Last, the models that successfully explain characteristics of certain diseases, such as schizophrenia, may help us understand how both symptoms and cognitive deficits emerge from underlying dysfunctional network dynamics, as well as identify targets for treatment.
4. Conclusion
In conclusion, the observation of dFC patterns using fMRI has opened the door to an entire field of research exploring the mechanisms and the meaning of these functional network dynamics. While this field is still in its infancy, important early work has identified characteristics of dFC that systematically vary with changes in arousal and cognitive demands, and that are related to cognitive ability. Critically, changes in dFC track changes in behavior within an individual, underscoring its potential importance for human cognition. Further work remains to be conducted to uncover the neuronal underpinnings of dFC, to separate relevant neural dynamics from physiological and other noise, to improve methodology and the accuracy of dFC estimates and, critically, to determine how dFC translates to behavior and cognition. A greater understanding of functional network dynamics will contribute to our knowledge of the mechanisms underlying healthy cognition, as well as cognitive impairments and symptoms observed in neurologic and psychiatric disorders.
Acknowledgments
Funding: This work was supported by the National Institute of Mental Health [R00MH102349]. I would additionally like to thank Martin Lindquist and Caterina Gratton for their valuable comments on earlier drafts of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The use of the term “state” to describe a reoccurring pattern of whole-brain connectivity is in parallel to the use of “state” in the human psychology literature to describe brief, temporary aspects of an individual’s arousal or emotional response (as opposed to stable characteristics of an individual, termed “traits”). Similarly, the phrases “resting state” or “cognitive task state” are used to refer to brief, sustained periods with a single set of instructions to a participant that vary across conditions but are presumably maintained within a condition. In each instance, “state” refers to a temporary condition, although the duration of each condition can be quite variable. As an example, “task states” often last between seconds to minutes, while “arousal states” often last between minutes to hours. “Brain states” can be thought of as the neural underpinnings of both task and arousal states (and are likely a combination of both), thus their timescale can range from seconds to hours. As this review refers to each of these uses of the term “state”, throughout I will refer to “brain states” as opposed to “arousal states” or “task states”.
References
- Bargmann CI, Marder E. From the connectome to brain function. Nature Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nature Neuroscience. 2015;18:744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, Meyer-Lindenberg A, Bassett DS. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M. Dynamic models of large-scale brain activity. Nature Neuroscience. 2017;20:340–352. doi: 10.1038/nn.4497. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nature Reviews Neuroscience. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annual Review of Clinical Psychology. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, Deco G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: models and mechanisms. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.045. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adal T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends in Cognitive Sciences. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage. 2013;72:227–236. doi: 10.1016/j.neuroimage.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Chang C, Greicius MD, Glover GH. Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage. 2015;111:476–488. doi: 10.1016/j.neuroimage.2015.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biology. 2016;14:e1002469. doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe AS, Nebel MB, Barber AD, Cohen JR, Xu Y, Pekar JJ, Caffo B, Lindquist MA. Comparing test-retest reliability of dynamic functional connectivity methods. Neuroimage. 2017;158:155–175. doi: 10.1016/j.neuroimage.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, Gur RC, Gur RE, Bassett DS, Satterthwaite TD. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Zalesky A, Fornito A, Mattingley JB. Dynamic cooperation and competition between brain systems during cognitive control. Trends in Cognitive Sciences. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. The Journal of Neuroscience. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D’Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PLoS ONE. 2014;9:e106636. doi: 10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovš G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribben I, Haraldsdottir R, Atlas LY, Wager TD, Lindquist MA. Dynamic connectivity regression: determining state-related changes in brain connectivity. Neuroimage. 2012;61:907–920. doi: 10.1016/j.neuroimage.2012.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribben I, Wager TD, Lindquist MA. Detecting functional connectivity change points for single-subject fMRI data. Frontiers in Computational Neuroscience. 2013;7:143. doi: 10.3389/fncom.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, van Erp TG, Calhoun VD. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage: Clinical. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison EN, Schlesinger KJ, Bassett DS, Lynall ME, Miller MB, Grafton ST, Carlson JM. Brain network adaptability across task states. PLoS Computational Biology. 2015;11:e1004029. doi: 10.1371/journal.pcbi.1004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Corbetta M. The dynamical balance of the brain at rest. The Neuroscientist. 2011;17:107–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Resting brains never rest: computational insights into potential cognitive architectures. Trends in Neurosciences. 2013;36:268–274. doi: 10.1016/j.tins.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84:892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Deco G, Tononi G, Boly M, Kringelbach ML. Rethinking segregation and integration: contributions of whole-brain modelling. Nature Reviews Neuroscience. 2015;16:430–439. doi: 10.1038/nrn3963. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman M, Derrfuss J, Tittgemeyer M, Fiebach CJ. Predicting errors from reconfiguration patterns in human brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16714–16719. doi: 10.1073/pnas.1207523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Gao W. Task-related modulation of functional connectivity variability and its behavioral correlations. Human Brain Mapping. 2015;36:3260–3272. doi: 10.1002/hbm.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima Z, Kovacevic N, Mišić B, McIntosh AR. Dynamic functional connectivity shapes individual differences in associative learning. Human Brain Mapping. 2016;37:3911–3928. doi: 10.1002/hbm.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DM, Shen X, Papademetris X, Constable RT. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.064. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Badre D. How cognitive theory guides neuroscience. Cognition. 2015;135:14–20. doi: 10.1016/j.cognition.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Rho Y, McIntosh AR, Kötter R, Jirsa VK. Noise during rest enables the exploration of the brain’s dynamic repertoire. PLoS Computational Biology. 2008;4:e1000196. doi: 10.1371/journal.pcbi.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Robinson ME, Buchanan LC, Saad ZS, Bandettini PA. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8762–8767. doi: 10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Gordon EM, Adeyemo B, Petersen SE. Evidence for two independent factors that modify brain networks to meet task goals. Cell Reports. 2016;17:1276–1288. doi: 10.1016/j.celrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovici A, Tagliazucchi E, Balenzuela P, Laufs H. On wakefulness fluctuations as a source of BOLD functional connectivity dynamics. Scientific Reports. 2017;7:5908. doi: 10.1038/s41598-017-06389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA. Periodic changes in fMRI connectivity. Neuroimage. 2012;63:1712–1719. doi: 10.1016/j.neuroimage.2012.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: a neuropsychological theory. John Wiley & Sons, Inc; New York, NY: 1949. [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage. 2016;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Liu X, Pillay S. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connectivity. 2015;5:10–22. doi: 10.1089/brain.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Human Brain Mapping. 2014;35:5754–5775. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. Tracking the brain’s functional coupling dynamics over development. The Journal of Neuroscience. 2015;35:6849–6859. doi: 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD. The neural basis of time-varying resting-state functional connectivity. Brain Connectivity. 2014;4:769–779. doi: 10.1089/brain.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambhati AN, Sizemore AE, Betzel RF, Bassett DS. Modeling and interpreting mesoscale network dynamics. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.06.029. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler MG, Henson RNA, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. The Journal of Neuroscience. 2011;31:8259–8270. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. The Journal of Neuroscience. 2011;31:11728–11732. doi: 10.1523/JNEUROSCI.5730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the connectome: the dynome. Neuron. 2014;83:1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BTT, Buckner RL. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130526. doi: 10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A. Just a thought: how mind-wandering is represented in dynamic brain connectivity. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.07.001. in this issue. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hove MJ, Esterman M, Hutchison RM, Valera EM. Dynamic brain network correlates of spontaneous fluctuations in attention. Cerebral Cortex. 2017;27:1831–1840. doi: 10.1093/cercor/bhw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, Gordon EM, Gratton C, Adeyemo B, Gilmore AW, Nelson SM, Berg JJ, Greene DJ, McCarthy JE, Tagliazucchi E, Laufs H, Schlaggar BL, Dosenbach NUF, Petersen SE. On the stability of BOLD fMRI correlations. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw265. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N, Shirer WR, Greicius MD, Van De Ville D. Disentangling dynamic networks: separated and joint expressions of functional connectivity patterns in time. Human Brain Mapping. 2014;35:5984–5995. doi: 10.1002/hbm.22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N, Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Liégeois R, Laumann TO, Snyder AZ, Zhou HJ, Yeo BTT. Interpreting temporal fluctuations in resting-state functional connectivity MRI. bioRxiv. 2017 doi: 10.1101/123456. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Xu Y, Nebel MB, Caffo BS. Evaluating dynamic bivariate correlations in resting-state fMRI: a comparison study and a new approach. Neuroimage. 2014;101:531–546. doi: 10.1016/j.neuroimage.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4392–4397. doi: 10.1073/pnas.1216856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Hasenkamp W, Schwarb H, Schumacher EH, Barsalou L, Keilholz SD. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage. 2011;54:1140–1150. doi: 10.1016/j.neuroimage.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Murakami T, Ohki K. Neuronal origin of the temporal dynamics of spontaneous BOLD activity correlation. bioRxiv. 2017 doi: 10.1101/169698. [DOI] [PubMed] [Google Scholar]
- Medaglia JD, Lynall ME, Bassett DS. Cognitive network neuroscience. Journal of Cognitive Neuroscience. 2015;27:1471–1491. doi: 10.1162/jocn_a_00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti RP, Hellyer P, Sharp D, Leech R, Anagnostopoulos C, Montana G. Estimating time-varying brain connectivity networks from functional MRI time series. Neuroimage. 2014;103:427–443. doi: 10.1016/j.neuroimage.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–297. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen MY, Gorgolewski KJ, Luci J, Joo SJ, Boyd RL, Hunicke-Smith S, Simpson ZB, Caven T, Sochat V, Shine JM, Gordon E, Snyder AZ, Adeyemo B, Petersen SE, Glahn DC, Reese Mckay D, Curran JE, Göring HHH, Carless MA, Blangero J, Dougherty R, Leemans A, Handwerker DA, Frick L, Marcotte EM, Mumford JA. Long-term neural and physiological phenotyping of a single human. Nature Communications. 2015;6:8885. doi: 10.1038/ncomms9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti MG, Bolton TA, Ville DVD. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.12.061. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rack-Gomer AL, Liu TT. Caffeine increases the temporal variability of resting-state BOLD connectivity in the motor cortex. Neuroimage. 2012;59:2994–3002. doi: 10.1016/j.neuroimage.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, Calhoun VD. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Kleinschmidt A. Functional interactions between intrinsic brain activity and behavior. Neuroimage. 2013;80:379–386. doi: 10.1016/j.neuroimage.2013.04.100. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Poline JB, Kleinschmidt A, D’Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8463–8468. doi: 10.1073/pnas.1420687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, Moodie CA, Poldrack RA. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016a;92:544–554. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Koyejo O, Bell PT, Gorgolewski KJ, Gilat M, Poldrack RA. Estimation of dynamic functional connectivity using Multiplication of Temporal Derivatives. Neuroimage. 2015;122:399–407. doi: 10.1016/j.neuroimage.2015.07.064. [DOI] [PubMed] [Google Scholar]
- Shine JM, Koyejo O, Poldrack RA. Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proceedings of the National Academy of Sciences of the United States of America. 2016b;113:9888–9891. doi: 10.1073/pnas.1604898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, Snyder AZ. Data quality influences observed links between functional connectivity and behavior. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw253. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, Romani GL, Corbetta M. Dynamic reorganization of human resting-state networks during visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8112–8117. doi: 10.1073/pnas.1415439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Multimodal imaging of dynamic functional connectivity. Frontiers in Neurology. 2015;6:10. doi: 10.3389/fneur.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Frontiers in Human Neuroscience. 2012;6:339. doi: 10.3389/fnhum.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Human Brain Mapping. 2013a;34:3280–3298. doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Merritt MD, Pan WJ, Magnuson ME, Grooms JK, Jaeger D, Keilholz SD. Neural correlates of time-varying functional connectivity in the rat. Neuroimage. 2013b;83:826–836. doi: 10.1016/j.neuroimage.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD. Quasi-periodic patterns (QPP): large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage. 2014;84:1018–1031. doi: 10.1016/j.neuroimage.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, Phillips J, Feinstein C, Abrams DA, Menon V. Brain state differentiation and behavioral inflexibility in autism. Cerebral Cortex. 2015;25:4740–4747. doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Gordon EM. Phenotypic variability in resting-state functional connectivity: current status. Brain Connectivity. 2013;3:99–120. doi: 10.1089/brain.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ong JL, Patanaik A, Zhou J, Chee MWL. Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:9653–9658. doi: 10.1073/pnas.1523980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Xia M, He Y. Magnetic resonance imaging and graph theoretical analysis of complex brain networks in neuropsychiatric disorders. Brain Connectivity. 2011;1:349–365. doi: 10.1089/brain.2011.0062. [DOI] [PubMed] [Google Scholar]
- Yang Z, Craddock RC, Margulies DS, Yan CG, Milham MP. Common intrinsic connectivity states among posteromedial cortex subdivisions: insights from analysis of temporal dynamics. Neuroimage. 2014;93(Pt 1):124–137. doi: 10.1016/j.neuroimage.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Breakspear M. Towards a statistical test for functional connectivity dynamics. Neuroimage. 2015;114:466–470. doi: 10.1016/j.neuroimage.2015.03.047. [DOI] [PubMed] [Google Scholar]


