Abstract
Background
Resuscitation guided by hrombelastography (TEG) improves survival aafter injury. If bleeding is rapid, however, or if no TEG data are available, the optimal strategy remains controversial. Our current practice gives FFP and RBC (1:2) empirically in patients with life-threatening hemorrhage, with subsequent administration based on rapid TEG (rTEG). We identified patients at risk of massive transfusion (MTx) at 1 hour, examined their initial rTEG, and used this value to provide empiric recommendations about transfusions..
Methods
MTx was defined as >4 units of RBC in the first hour. Patients managed by a trauma activation (2014–2017) had an admission rTEG analyzed to determine what proportion met thresholds for administration of cryoprecipitate (Cryo) or platelets (Plt).
Results
Overall, 35 patients received >4 units of RBC in the first hour. Based on the admission rTEG, 37% met criteria for both Plt and Cryo, 35% for either Plt or Cryo and 29% for neither. Kaplan-Meier analysis showed a significant delay in the administration of Cryo and Plt compared to FFP.
Conclusions
Patients who require >4 units of RBCs within the first hour should receive Cryo and Plt if TEG results are not available. Point-of-care devices are needed for optimal care of trauma-induced-coagulopathy, but these data offer guidance in their absence.
Introduction
Preventable deaths after trauma are due predominantly to uncontrolled hemorrhage, which occurs rapidly aterinjury1–3. Trauma-induced coagulopathy (TIC) is independently associated with increased mortality, and methods to treat this disorder effectively are needed4. In patients requiring massive transfusion, resuscitation guided by rapid thrombelastography (rTEG) results in decreased mortality with a decrease in adminitration of blood products compared to conventional clotting assays or ratio-based strategies5, 6. This approach resulted in our current institutional protocol of 1:2 FFP:RBC transfusion in patients in hemorrhagic shock, with simultaneous blood collection for rTEG to guide need for additional blood product use7, 8. There are certain scenarios, however, where rapid hemorrhage outpaces TEG results, and we are left with a dilemma of which products should be given to the bleeding patient.
In order to provide guidance for this group of patients, we needed to identify patients who require a massive transfusion (MTx) at an early time point. Traditional MTx definitions use RBC units over a span of time (from 6–24 hours) to identify patients at greatest risk of death7, 9. Ideally we would identify patients at greatest risk of death in a more rapid time frame, preferably within the first hour. Additionally, while traditional definitions provide a single cut point to identify patients at greatest risk of death, these traditional fefinitions do not identifypopulations at low and intermediate risk 10. Initially, we sought to stratify patients into low, intermediate, and high risk for death based on RBC units transfused within the first 1 to 24 hours. We then hypothesized that if we identified patients at greatest risk of death within 1 hour of admission, we could identify a dominant coagulopathy in this group, and from that, provide empiric recommendations for transfusion.
Methods
Study Design
This is an analysis of prospectively collected data from critically injured trauma patients. Inclusion criteria were patients ≥18 years of age that met the highest-level trauma activation at Denver Health Medical Center, an urban level 1 trauma center. Exclusion criteria were unsalvageable injuries (death ≤30 min from time of injury), prolonged transit time (≥1 h), isolated gunshot wounds to the head, pregnancy, and a documented coagulation disorder (including liver disease). All studies were approved by the Colorado Multiple Institutional Review Board (COMIRB) under a waiver of consent.
Clinical Data and Rapid Thrombelastography
Clinical data were abstracted by trained Profession Research Assistants (PRAs). Data on RBC units transfused 1, 2, 4, 6, 12 and 24 h from injury were collected. PRAs collected blood in citrated vacuum tubes at the time of admission. Blood samples were analyzed using the rTEG assay (exogenous addition of tissue factor and kaolin) on the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL, USA), as described previously 11. The following indices were obtained from the tracings of the TEG: activated clotting time (ACT [s]), angle (°), maximum amplitude (MA [mm]), and lysis 30 min after MA (LY30 [%]). Threshold for fresh frozen plasma (FFP) (ACT>128 s), cryoprecipitate (Cryo) (angle <65°) and platelet (Plt) (MA<55mm) administration were based on prior investigations8. Administration of blood products was in accordance with these thresholds by treating physicians using the clinical lab rTEG8. Physicians were blinded to the research rTEG values.
Massive Transfusion Definition
Receiver operating characteristic (ROC) curves to predict death based on RBC units transfused (within 1, 2, 4, 6, 12 and 24 h of injury) were constructed. Area under the ROC curve (AUROC) of ≥0.7 was the cutoff for a good predictor of death. Interval likelihood ratios are defined as the slope of the ROC curve between any two points on the ROC curve (point A and B)12, calculated as (Sensitivity of Point A – Sensitivity of Point B)/(Specificity of Point B -Specificity of Point A). The positive likelihood ratio (+LHR) in this setting modifies the pretest probability of death, a +LHR>1 increased the post-test probability of death, with a greater +LHR increasing the post-test probability10. We used these +LHR to stratify patients into groups. MTx was defined as the least number of RBC units that corresponded to a +LHR for death of >10x, the high risk group10. An intermediate zone was defined a +LHR for death of 5x (or as close to 5x as could be obtained). The low risk group was the cohort receiving less RBC units than the intermediate risk group. ROC curves were analyzed with and without exclusion of patients with severe TBI (Head AIS>3) to avoid confounders from death due to traumatic brain injury.
Indicated Blood Products and Time to Administration
Patients who received an MTx in the first hour were stratified by their admission rTEG into groups based on the dominant coagulopathy present-a shallow angle indicating the need for Cryo, a low MA indicating the need for Plt, or both. Those without an admission rTEG were excluded from this analysis. Our institutional practice is to give 1:2 FFP:RBC; these patients all receive empiric FFP, and therefore, we did not assess the need for FFP transfusion8. We performed the same analysis on the intermediate and the low risk groups (after exclusion of patients who received no blood products in the first hour) and compared the incidence of any coagulopathy between groups. Subsequently, we sought to identify if our transfusion practices result in a delayed administration of Plt and Cryo compared to FFP in our current protocols. Patients who underwent an MTx within 1 h (>4 units of RBC in the first h, Head (Elsevier have the authors define “AIS”AIS≤3) and had admission coagulopathy underwent Kaplan Meier curve analysis to time to first unit of blood products transfused (FFP, Cryo or Plt).
Statistical Analysis
Non-normally distributed variables were expressed as median and interquartile range (IQR) and the Wilcoxon non-parametric test was used for comparisons. Mantel-Cox test was used to compare survival curves. All tests were two-tailed with significance declared at p<0.05.
Results
Patient Characteristics
In tjis study, 626 patients met criteria and were analyzed (Table 1). There were 86 deaths in this cohort (13.7% aggregate mortality). The cause of death was due to a Traumatic brain injury (TBI) in 55% of patients, hemorrhage in 38% (either due to uncontrolled bleeding or physiologic collapse secondary to massive hemorrhage), organ failure in 5%, and other causes in the remaining 2% of patients. Of patients who died within the first day, 72% died within the first 6 hours, and overall, 63% of deaths occurred within 24 hours from presentation and 80% within 3 days from admission.
Table 1.
Demographics by group
| All Patients (n=626) | Severe TBI (n=106) | No Severe TBI (n=520) | >4 units in 1 hour (n=35) | |
|---|---|---|---|---|
| Age (y) | 33 (26–47) | 31 (26–49) | 33 (26–47) | 32 (26–44) |
| Male Sex (%) | 80.5% | 81.1% | 80.4% | 91% |
| Penetrating Injury (%) | 45.6% | 11.3% | 52.7% | 71% |
| New ISS | 19 (6–34) | 43 (31–58.5) | 17 (6–27) | 41 (31.5–57) |
| ED SBP (mm Hg) | 114 (90–140) | 121 (90–142) | 112 (90–138) | 82 (26–94) |
| ED HR (BPM) | 100 (78–118) | 96 (64–120) | 100 (82–118) | 112 (96–133) |
| Base Deficit (mEq/dL) | 7 (4–10.5) | 7.5 (4–11) | 7 (4–10) | 15 (11–20) |
| Death (%) | 13.7% | 40.6% | 8.3% | 51% |
Ability of RBC transfusions to predict death
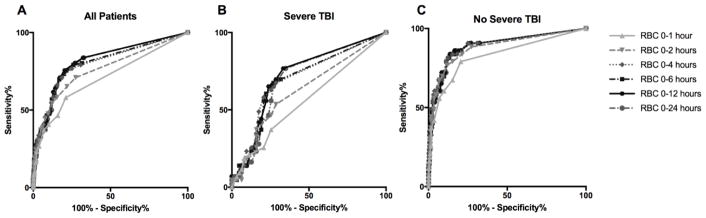
ROC curves were calculated for the ability of RBC transfusions to predict death (Figure 1). The AUROC was 0.71 for RBC units in the first hour indicating that this was a reasonably good predictor of death; however, when patients with severe TBI were excluded from this group, the performance increased with an AUROC for death of 0.83 (Table 2). Performance of RBC units to predict death increased at 2 hours from injury; however, RBC units at later time points did not improve prediction compared to 2 hours. Due to the fact that excluding patients with severe TBI improved the performance of RBC units to predict death, we calculated the intermediate risk and MTx ranges after excluding this group (Table 3). Massive transfusion was defined as >4 units in 1 hour, >6 units in 2 hours, >8 units in hours, >10 units in 6 hours, and >12 hours in 12 or 24 hours. The intermediate risk for death was defined as 4 units of RBC in the first hour or 9–10 units in the first 6 hours (Table 3).
Figure 1.
Receiver operating characteristic (ROC) Curves for Death based on RBC units transfused within 1–24 hours for all patients (Figure 1A), patients with severe TBI (Figure 1B) and patients without severe TBI (Figure 1C).
Table 2.
Area under the ROC curve (AUROC) for death based on RBC units transfused.
| All Patients | Severe TBI | No Severe TBI | ||||
|---|---|---|---|---|---|---|
| AUROC | p value | AUROC | p value | AUROC | p value | |
| RBC 0–1 hours | .71 (.63–.77) | <0.0001 | .56 (.45–.67) | 0.28 | .83 (.75–.90) | <0.0001 |
| RBC 0–2 hours | .76 (.70–.82) | <0.0001 | .62 (.51–.73) | 0.03 | .88 (.82–.94) | <0.0001 |
| RBC 0–4 hours | .79 (.74–.86) | <0.0001 | .70 (.65–.89) | 0.0006 | .89 (.84–.95) | <0.0001 |
| RBC 0–6 hours | .80 (.75–.86) | <0.0001 | .69 (.59–.80) | 0.0007 | .89 (.83–.95) | <0.0001 |
| RBC 0–12 hours | .81 (.76–.87) | <0.0001 | .72 (.62–.82) | 0.0001 | .89 (.83–.95) | <0.0001 |
| RBC 0–24 hours | .81 (.76–.87) | <0.0001 | .70 (.60–.80) | 0.0005 | .89 (.83–.95) | <0.0001 |
Table 3.
Definition of the low, intermediate, and high risk of death (massive transfusions) groups after exclusion of patients without severe TBI
| Low Risk | Intermediate Risk | Massive Transfusion | ||||
|---|---|---|---|---|---|---|
| Cutoff | Mortality | Cutoff (RBC u) | +LHR (Mortality) | Cutoff (RBC u) | +LHR (Mortality) | |
| RBC 0–1 hours | <4 | 4% | 4 | 4.2 (27%) | >4 | 11.8 (51%) |
| RBC 0–2 hours | <6 | 3% | 6 | 5.5 (33%) | >6 | 11.1 (50%) |
| RBC 0–4 hours | <6 | 2% | 6–8 | 2.5 (19%) | >8 | 10.4 (48%) |
| RBC 0–6 hours | <9 | 3% | 9–10 | 4.7 (30%) | >10 | 11.1 (50%) |
| RBC 0–12 hours | <9 | 3% | 9–12 | 4.0 (26%) | >12 | 11.1 (50%) |
| RBC 0–24 hours | <9 | 3% | 9–12 | 4.0 (26%) | >12 | 10.3 (48%) |
Indicated blood products
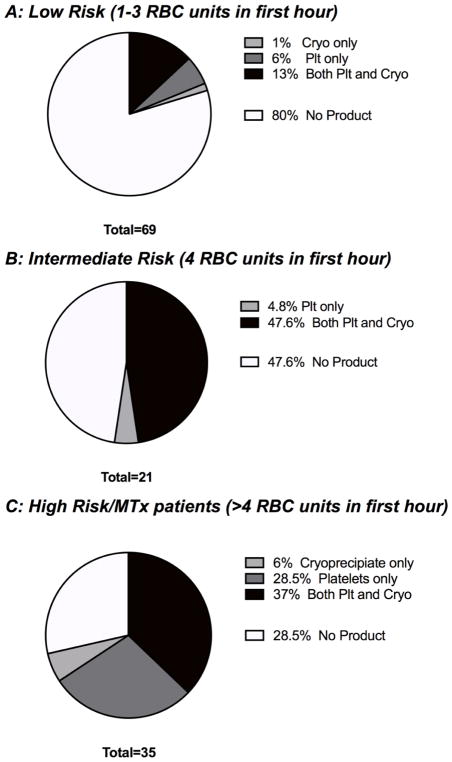
Using the above definitions, we identified those patients who receive >4 units of RBC in the first hour from injury are at high risk of death. We performed an analysis of this group of patients (n=35) to determine the coagulation abnormalities present on admission rTEG to determine if there existed a dominant coagulopathy in this group (Figure 2C). Of these patients, 6% of patients had a coagulopathy requiring Cryo only, 29% had a coagulopathy requiring Plt only, 37% had a coagulopathy requiring both Cryo and Plt, and only 29% had no admission coagulopathy (Figure 2). Empiric treatment with Plt required treating 1.5 patients with platelets to appropriately target 1 patient, for Cryo 2.3 patients would be treated to appropriately target 1 patient. When compared to the low and intermediate risk groups, there was a difference in the presence of any coagulopathy (as assessed by Angle or MA, p<0.0001), with an increase in coagulopathy from the low to intermediate to MTx groups (p<0.0001, Chi squared test for trend). In the low and intermediate risk groups, 80% and 48% of patients had no products indicated, respectively.
Figure 2.
Products indicated on admission rTEG in low (Figure 2A), medium (Figure 2B) and high risk (or MTx cohort) for death (Figure 2C) groups (after exclusion of patients with severe TBI)
Time to product administration
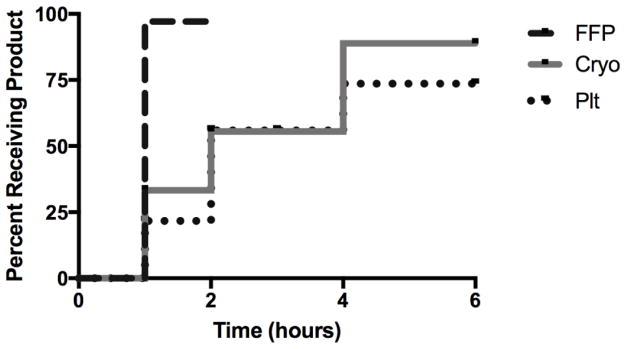
Kaplan-Meier analysis revealed delayed administration of Plt and Cryo compared to FFP, median time to administration of FFP was within 1 hour, within 2 hours for Plt and Cryo (Figure 4). (ELSEVIER EDITOR ASK THE AUTHORS TO MAKE THIS SENTENE COMLETE I CANNOT FIGURE OUT WHAT THEY ARE TRYING TO SAY) Greater than 75% of patients who had Plt or Cryo As indicated by the admission rTEG received these products within 4 hours. Of those patients who did not receive Plt or Cryo within 6 hours, 62% of these patients died within 2 hours from injury.
Discussion
In this study, we have identified thresholds to differentiate patients at high, intermediate, and low risk of death based on RBC units transfused at different time intervals. These thresholds provide us the ability to identify patients at greatest risk of death within the first hour of admission. Analysis of this population revealed that the most common coagulopathy present on admission required treatment with Plt and Cryoprecipitate. Additionally, we showed that under current transfusion protocols, these patients have substantially delayed administration of both Cryo and Plt compared to FFP. Based on this observation, we recommend in this population empiric transfusion of both Plt and Cryo in patients who require >4 units of RBC within the first hour from admission if the rTEG data are not available.
The historic definition of MTx (10 units of RBC in 24 hours) is widely acknowledged to have limitations9, 13–15. Chief among these limitations is that this definition of Mx misses patients who may die before receiving 10 units, it does not include a measure of acuity of hemorrhage, and it only carries value as a retrospective metric and not at the point-of-care. We first suggested this time interval be shortened to 6 hours, but the majority of post-injury deaths due to exsanguination occur within 2 hours in a MTx population7, 16. Our updated definition allows for rapid identification of MTx patients during the first hour after injury. Additionally, the inclusion of TBI into the decision decreases the ability to predict death, particularly at early time points, because hemorrhage is not a predominant cause of death in that population. In this study, 55% of patients died secondary to head injury and substantially less died as a sequelae of hemorrhage. Compared to prior work attempting to identify MTx at more rapid time points (THIS IS REFERENCE 17), which used ≥3 units in any hour period, we reported a greater threshold at 1 hour (>4 units of RBC), but at 2 hours, we have a similar threshold (>6 units)17. Importantly we show that after 2 hours, the ability of RBC units transfused to predict death plateaus and that using a 6 to 24 hour window may not be necessary.
Outside of the ability to predict MTx at rapid time points, these novel definitions offer additional an nuance that is missed in traditional MTx definitions. By stratifying the non-MTx group into an intermediate risk of MTx and low risk of MTx, we can identify an additional endpoint for prospective trials. While an intervention may not decrease the rate of MTx, it may transition an intermediate risk patient into a low risk patient and improve clinical outcomes. Furthermore, when power is an issue for clinical trials due to the low rate of death or MTx, by adding another outcome, we can add patients to increase power and improve the ability of a smaller study to detect small differences.
While many groups advocate fixed-ratio resuscitation, they have not been able to show an improved mortality with this approach18, 19. In contrast, TEG-based resuscitation results in improved mortality compared to conventional clotting assays or fixed ratio protocols5, 6. Our study does not aim to supplant the TEG-driven protocols currently in use and replace it with fixed-ratio strategies but rather fill a knowledge gap in a small subset of patients. In the scenario where the bleeding is rapid and transfusion needs outpace TEG results, we suggest empiric transfusion of both platelets and cryoprecipitate with continued 1:2 FFP:RBC. By using the threshold of >4 units in the first hour, we have identified populations with a high risk of death, but also those patients that are likely to outpace the rapidity of TEG based resuscitation. These data suggest that empiric treatment with Plt will benefit more patients than empiric treatment with Cryo; however, when weighing the risks of under-treatment compared to over-treatment of coagulopathy, we would favor over-treatment in this group. After 4 units of RBC and 2 of FFP, we have begun to resuscitate the patient from shock and provide an optimal environment for Cyro and Plt to counteract any coagulopathy. Extrapolation of empiric, ratio-based transfusion policies to all bleeding patients would result in unnecessary administration of blood products, because 80% of patients who receive 1–3 units of RBC in the first hour have no indication for either Cryo or Plt. We cannot say with certainty why there are delays in the administration of Cryo and Plt compared to FFP, although contributions due to waiting for the TEG results and intentional design of the MTx cooler composition (Cryo and Plt are only present in the second cooler brought to a trauma activation to allow resuscitation from shock) (ELSEVIER THE AUTHORS NEED TO FINISH THIS SENTENCE). We would expect Cryo and Plt, however, to only be delayed until the TEG results-which regardless of rate of bleeding is quicker than the 2 hours median for Plt and Cryo transfusion. We aim to address both of these issues by suggesting empiric transfusion if more than 4 units of RBC have been administered. Given that the majority of patients who did not receive the indicated Plt or Cryo within 6 hours died within the first 2 hours, we anticipate that by moving toward more rapid transfusion, we may be able to improve survival.
The retrospective nature of this study is a limitation, while the small sample size does not allow analysis of whether more rapid administration of Plt and Cryo will improve incidence of ongoing coagulopathy and survival in this cohort. Prospective analysis is needed to ensure that the suggested changes result in improved survival, although retrospective studies suggest a survival benefit of early Plt transfusion20. There is a risk that administration of products to the small cohort of patients who do manifest a coagulopathy will worsen outcomes (particularly with regard to late organ injury), although recent studies have shown that increased blood product administration is not a risk for ARDS21.
This study provides a threshold for MTx of >4 units in the first hour, which is a simple assessment to make in the heat of the moment and requires no lab results or calculation to determine. In this group, although empiric transfusion of both Cryo and Plt is reasonable based on the dominant patterns of coagulopathy observed, our study highlights the need for more rapid point-of-care assessment of coagulopathy to allow individualized correction of coagulopathy. Furthermore, these data suggest that trauma centers review both transfusion policies and practice, because such a review may reveal delays in care and opportunities for quality improvement.
Figure 3.
Kaplan Meier Curve of time to first unit of blood product indicated by rTEG in patients receiving >4 units of RBC in the first hour. Time to administration of FFP differed significantly from Cryoprecipitate and Platelets (p<0.005 for both).
Footnotes
Presented at the Academic Surgical Congress; Jacksonville, FL, January 30th–February 1st 2018
Disclosure: Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart Lung and Blood Institute UM1-HL120877, in addition to the Department of Defense USAMRAA and W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood institute, or the Department of Defense. Additional research support provided by Haemonetics with shared intellectual property.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4–8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 3.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–14. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–85. doi: 10.1097/TA.0b013e31827e20e0. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 8.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82(1):114–9. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, Zaydfudim V, Johnston M, Arbogast P, Young PP. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(Suppl 1):S33–9. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 10.Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology. 2010;112(4):1023–40. doi: 10.1097/ALN.0b013e3181d47604. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36(7):723–37. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MD, Reeves MJ. Interval likelihood ratios: Another advantage for the evidence-based diagnostician. Annals of Emergency Medicine. 2003;42(2):292–7. doi: 10.1067/mem.2003.274. [DOI] [PubMed] [Google Scholar]
- 13.Mitra B, Cameron PA, Gruen RL, Mori A, Fitzgerald M, Street A. The definition of massive transfusion in trauma: a critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18(3):137–42. doi: 10.1097/MEJ.0b013e328342310e. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento B, Rizoli S, Rubenfeld G, Lin Y, Callum J, Tien HC. Design and preliminary results of a pilot randomized controlled trial on a 1:1:1 transfusion strategy: the trauma formula-driven versus laboratory-guided study. J Trauma. 2011;71(5 Suppl 1):S418–26. doi: 10.1097/TA.0b013e318232e591. [DOI] [PubMed] [Google Scholar]
- 15.Rainer TH, Ho AM, Yeung JH, Cheung NK, Wong RS, Tang N, Ng SK, Wong GK, Lai PB, Graham CA. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011;82(6):724–9. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Cripps MW, Kutcher ME, Daley A, McCreery RC, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Cause and timing of death in massively transfused trauma patients. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S255–62. doi: 10.1097/TA.0b013e31829a24b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–400. doi: 10.1097/TA.0b013e31827a3639. discussion -2. [DOI] [PubMed] [Google Scholar]
- 18.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, Rizoli S. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ. 2013;185(12):E583–9. doi: 10.1503/cmaj.121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson BRH, Cohen MJ, Holcomb JB, Pritts TA, Gomaa D, Fox EE, Branson RD, Callcut RA, Cotton BA, Schreiber MA, et al. Risk Factors for the Development of Acute Respiratory Distress Syndrome Following Hemorrhage. Shock. 2017 doi: 10.1097/SHK.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]