Abstract
Background
The pathogenesis driving the formation of abdominal aortic aneurysms (AAA) continues to be poorly understood. Therefore, we systemically define the cytokine and circulating immune cell environment observed in human AAA compared to risk-factor matched (RFM) controls.
Methods
From 2015 to 2017, 274 patients donated blood to the Indiana University Center for Aortic Disease (IUCAD). Absolute concentrations of circulating cytokines were determined using enzyme-linked immunosorbent assays (ELISA) while expression of circulating immune cell phenotypes were assayed via flow cytometric analysis.
Results
Human AAA is characterized by a significant depletion of the antigen-specific, CD4+ Tr1 regulatory lymphocyte which corresponds to an upregulation of the antigen-specific, inflammatory Th17 cell. There were no differences in the incidence of Treg, B10, and myeloid-derived suppressor (MDSC) regulatory cells. Similarly, no disparities were noted in the following inflammatory cytokines: IL-1β, C-reactive protein (CRP), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), and IL-23. However, significant upregulation of the inflammatory cytokines osteopontin (OPN), IL-6, and IL-17 were noted. Additionally, no changes were observed in the regulatory cytokines IL-2, IL-4, IL-13, TNF-stimulated gene 6 protein (TSG-6), and prostaglandin E2 (PGE2), but we did observe a significant decrease in the essential regulatory cytokine IL-10.
Conclusions
In this investigation, we systematically characterize the AAA immune environment and present preliminary evidence that faulty immune regulation may also contribute to aneurysm formation and growth.
Keywords: Cytokines, Abdominal Aortic Aneurysm, Inflammation, Tr1
Background
AAA is a chronic inflammatory condition associated with male gender, advanced age, and tobacco use which continues to be a major source of morbidity and mortality in the Western Hemisphere.1,2 If left untreated, progressive aortic dilation results in increasing wall tension, rupture, hemorrhagic shock, and probable death.3 Currently, the accepted treatment paradigm consists of regimented imaging until a cross-sectional aortic diameter of 5.5 cm is reached – at this point, the risk of rupture and death exceeds the high morbidity associated with surgical reconstruction.4 Recent research has focused heavily on elucidating the pathogenesis and mechanism of AAA formation with hopes of developing a new pharmaceutical option to arrest aneurysm growth and prevent the need for surgical intervention.5–9
Previous studies have established that the histologic environment of the native aorta undergoing the transition to an aneurysmal state is characterized by an infiltration of mononuclear cells such as T and B lymphocytes, neutrophils, macrophages (Mϕs), and mast cells.10–12 Unfortunately, the inciting event and mechanism which drives ectatic transformation remains a mystery. In this manuscript, we present a descriptive analysis of the inflammatory and regulatory circulating immune environments of a large cohort of AAA patients and compare it to risk-factor matched (RFM) controls.
Methods
Separation of Whole Blood
The protocols presented herein are HIPAA compliant and were approved by the Indiana University Institutional Review Board (IRB #1408881234). After written informed consent was obtained, whole blood from 274 subjects over a span of two years was collected to be banked at the IUCAD. Potential subjects were sourced from individuals presenting for their US Preventative Task Force-recommended AAA duplex screening appointments or from previously diagnosed patients presenting for follow-up in vascular surgery clinic.13 Patients who screened negative by ultrasound (diameter <30 mm) at their vascular lab appointments were deemed a RFM control for the purposes of this study.
All whole blood samples were processed within 24 hours of collection using previously described Ficoll (GE Healthcare, Little Chalfont, UK) density centrifugation protocols with the assistance of Accuspin gradient tubes (Sigma, St Louis, MO) to isolate both peripheral blood mononuclear cells (PBMC) and plasma.14 Plasma samples were stored in small aliquots at −80 °C to minimize freeze-thaw cycles; PBMCs were stowed in liquid nitrogen suspended in fetal bovine serum (Sigma) plus 20% dimethyl sulfoxide (Sigma) in units of 106 cells.
Inflammatory and Regulatory Cell Phenotyping
Cell staining for flow cytometric analysis using antibodies specific to identifying cell markers were performed per manufacturer’s instructions (1:10, Miltenyi Biotec, Bergisch Gladbach, Germany) unless otherwise noted. Tr1: CD4-FITC, CD49b-PE (1:20), LAG3-APC (1:20); Treg: CD4-FITC, CD25-PE, FOXP3-APC (alternate: CD4-FITC, CD25-PE, CD127-APC); Th17: CD4-FITC, CD194-PE, CD196-APC; B10: CD1d-APC, CD5-FITC, CD19-PE, IL10-PerCP; MDSC: CD11b-PerCP, CD33-PE, CD66abce-FITC, HLA-DR-APC; Activated Mϕ: CD14-PE, CD16-FITC, CD45-APC; Inflammatory Mϕ: CD14-PE, CD16-FITC, CD45-APC; Resident Mϕ: CD14-PE, CD16-FITC, CD45-APC. Flow cytometric analysis was performed on an Accuri C6 (BD Biosciences, San Jose, CA) flow cytometer and compiled using CellQuest software (BD).
Determination of Circulating Cytokine Concentrations
Circulating inflammatory and regulatory cytokine concentrations were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN) and performed per manufacturer’s instructions. For all protocols, absorbance was measured at a wavelength of 450 nm and absolute concentrations were calculated with the assistance of a 4-parameter standard curve.
Determination of Plasma Antibody Concentrations
Human elastin fragments (ELNf) were generated by in vitro digestion with MMP-2 and MMP-9 (Sigma) per manufacturer’s instructions. Relative antibody concentrations specific to human collagen V (COLV) and ELNf were assayed via a previous described modified ELISA protocol.15 In short, respective antigenic peptides were dissolved in phosphate-buffered saline (PBS) to a stock working solution of 25 ug/mL. This stock solution was used to coat a high protein binding 96-well polystyrene plate (Sigma) for two hours at 37 °C or overnight at 4 °C. Copious washings were performed between all steps with PBS-T (Tween 20, Sigma). The 96-well plate was blocked with 1% bovine serum albumin (BSA, Sigma) for two hours at 37 °C or overnight at 4 °C. Plasma samples were then sequentially diluted up to 1:1000 to determine optimal concentrations and incubated for two hours at 37 °C or overnight at 4 °C. A goat anti-human IgG Fc antibody conjugated to horseradish peroxidase (HRP, Sigma) was utilized as a secondary antibody per manufacturer’s recommended dilution for a duration of one hour at 37 °C. Reactions were performed using a 1-step TMB turbo substrate (Sigma) for 30 minutes before a 1 M sulfuric acid stop solution was added. Absorbance at 450 nm was measured within 30 minutes to calculate relative self-antibody concentrations.
Results
Demographics and Comorbidities
A total of 274 patients with AAA (n=153) or deemed RFM controls (n=121) from January 2015 to September of 2017 donated blood samples to the IUCAD biorepository (Table 1). The mean aneurysm size at the time of sample collection for the AAA cohort was 49.4 mm (median = 50 mm). In the RFM cohort, 52.9% of the patients had an aortic diameter of less than 20 mm at the time of blood collection. AAA patents had more comorbidities as demonstrated by significantly higher incidences of hypertension, active smoking, and coronary artery disease. These findings were corroborated by a higher Framingham risk score (35.6% vs 40.5%, p = 0.02). Of note, a trend towards a decrease in diabetes mellitus was noted in the AAA group compared to the RFM controls. Baseline medications prescribed for the blood donors at the time of collection are noted in Table 2.
Table 1.
Depiction of the comorbidities of the blood donors to the IUCAD biorepository by cohort. RFM, risk-factor matched; HLD, hyperlipidemia; HTN, hypertension; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease; CKD, chronic kidney disease; FHx, family history of AAA.
| RFM (n = 121) | AAA (n = 153) | p-value | |
|---|---|---|---|
| Age | 68.9 ± 4.9 years | 69.4 ± 6.4 years | 0.48 |
| Male | 99.2% | 96.7% | 0.23 |
| HLD | 76.7% | 85.2% | 0.12 |
| HTN | 68.3% | 83.2% | <0.01 |
| BMI>30 | 48.3% | 43.0% | 0.46 |
| DM | 37.5% | 28.2% | 0.12 |
| Active Smoker | 30.0% | 57.0% | <0.01 |
| CAD | 24.2% | 39.6% | <0.01 |
| COPD | 20.0% | 30.2% | 0.07 |
| PAD | 8.3% | 14.8% | 0.10 |
| CKD | 7.5% | 8.7% | 0.82 |
| FHx | 4.2% | 4.7% | 1.0 |
| Framingham Score | 35.6 ± 15.8% | 40.5% ± 18.5% | 0.02 |
Table 2.
Baseline medications of the blood donors to the IUCAD biorepository by cohort. RFM, risk-factor matched; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
| RFM (n = 121) | AAA (n = 153) | p-value | |
|---|---|---|---|
| Statin | 66.7% | 72.5% | 0.29 |
| Aspirin | 43.3% | 55.7% | 0.05 |
| Beta Blocker | 35.0% | 49.0% | 0.02 |
| ACEi | 32.5% | 47.0% | 0.01 |
| Metformin | 24.2% | 16.1% | 0.13 |
| ARB | 15.8% | 10.7% | 0.21 |
| Systemic Steroids | 11.7% | 16.1% | 0.38 |
| Clopidogrel | 6.7% | 6.8% | 1.0 |
| Nitrates | 2.5% | 8.0% | 0.06 |
Cell Phenotype Expression
We observed trends towards increased expression of Th17 (3.2% vs 4.2%, p = 0.22) and inflammatory Mϕs (2.0% vs 3.0%, p = 0.30), but there were no differences in circulating activated and resident Mϕs between RFM and AAA subjects (Figure 1). With respect to the regulatory immune cells, we observed a severe depletion of the Tr1 lymphocyte in the AAA population (6.5% vs 1.4%, p < 0.01). Similarly, while no disparity was observed in the FOXP3 Tregs, a strong trend towards a depletion effect was noted in the alternatively stained CD127lo Tregs (2.6% vs 1.5%, p = 0.06). However, Tr1 and Treg depletion was balanced by an increase in the MDSC population in the AAA patients (2.2% vs 5.8%, p < 0.01). Lastly, no variance was noted with respect to the B10 population between cohorts.
Figure 1.
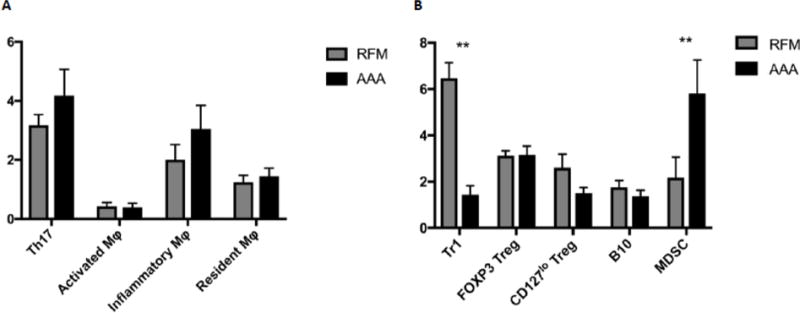
Incidence of varying inflammatory (A) and regulatory (B) immune cell populations in the peripheral blood compared between RFM controls (left, light) and AAA (right, dark) samples. Lymphocytes were expressed as a percentage of CD4+ cells; Mϕs were expressed as a percentage of CD45+ cells; MDSCs were expressed as a percentage of CD14+ cells; B10s were expressed as a percentage of all lymphocytes. (Mean ± SEM; *p < 0.05; **p < 0.01)
Plasma Cytokine Concentration
AAA patients overexpressed the inflammatory cytokines OPN (7.0 vs 9.4 ng/mL, p = 0.05), IL-6 (2.3 vs 4.8 pg/mL, p < 0.01), and IL-17 (11.7 vs 27.7 pg/mL, p < 0.01). Additionally, strong trends towards higher expression of IFN-γ (12.0 vs 20.2 pg/mL, p = 0.14) and IL-23 (64.7 vs 142.6 pg/mL, p = 0.06) were also noted (Figure 2). In contrast, the plasma concentration of the regulatory cytokine TGF-β (Figure 3) was elevated in the AAA condition (111.1 vs 183.2 pg/mL, p = 0.05). Lastly, we observed a depletion of the regulatory cytokine IL-10 in the AAA population compared to the RFM controls (11.8 vs 6.8 pg/mL, p < 0.01) corresponding to the loss of its main secretor, the Tr1 lymphocyte.
Figure 2.
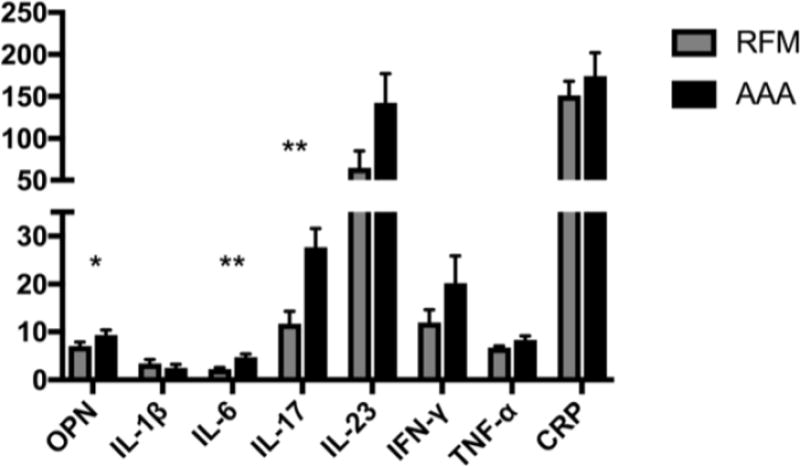
Inflammatory cytokines in the peripheral blood are compared between RFM controls (left, light) and AAA (right, dark) samples. Units for the Y-axis are expressed in pg/mL except for OPN (ng/mL). OPN, osteopontin; IFN, interferon; TNF, tumor necrosis factor; CRP, C-reactive peptide. (Mean ± SEM; *p < 0.05; **p < 0.01)
Figure 3.

Regulatory cytokines in the peripheral blood are compared between RFM controls (left, light) and AAA (right, dark) samples. Units for the Y-axis are expressed in pg/mL. TGF, transforming growth factor; PGE, prostaglandin E; TSG, TNF-inducible gene protein. (Mean ± SEM; *p < 0.05; **p < 0.01)
Antigen and Antibodies Concentrations
There were no changes in the circulating concentrations of ELNf (13.5 vs 14.0 ng/mL, p = 0.86) and COLV (0.65 vs 0.55 ng/mL, p = 0.10) antigens between cohorts (Figure 4). While no disparity was noted when COLV antigen from either the RFM or AAA cohorts were compared to healthy volunteers, ELNf antigen in both cohorts were elevated when compared to the same volunteers (10.0 ng/mL, p < 0.05 respectively). Additionally, no difference in IgG specific to COLV (αCOLV) (0.42 vs 0.40 RUs, p = 0.77) was noted between the plasma of RFM and AAA patients. However, IgG specific to ELNf (αELNf) was significantly increased in the AAA condition (0.19 vs 0.08 RUs, p = 0.02).
Figure 4.
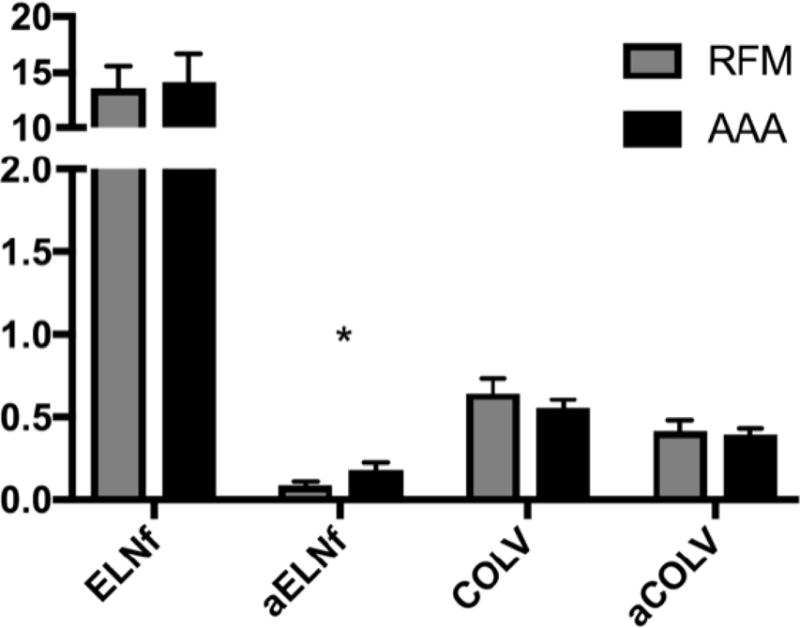
Circulating concentrations of elastin fragments (ELNf) and collagen type V (COLV) antigen and self-recognizing antibodies (αELNf, αCOLV) are compared between RFM controls and AAA samples. (Mean ± SEM; *p < 0.05; **p < 0.01)
Discussion
The early events observed in the pathway to aneurysm formation include the initiation of chronic inflammation, runaway proteolysis, increased oxidative stress, and loss of the extracellular matrix.1 This combination of events leads to aortic ectasia followed by progressive dilation. In this early stage of AAA formation, the aortic wall is characterized by an infiltration of CD4+ T cells, B cells, and macrophages.12 In this setting, B cells and macrophages function as antigen presenting cells (APC) and stimulate heavy depositions of immunoglobulins suggesting an antigen-specific response to the aortic wall.3 Interestingly, AAA shares many distinct characteristics observed in autoimmune diseases such as a predisposition to one organ, genetic susceptibility, and persistent, lingering inflammation. Recently, masses of CD4+ T cells located in the periadventitial vascular associated lymphatic tissue were found to be expressing identical T cell receptors against an unknown antigen present in the aortic wall, and therefore clonally expanded.16
Our current hypothesis is that aneurysm formation can be divided into the 1) sensitization 2) and inflammation stages. In the sensitization phase, elastin is broken down to small fragments most likely by exposure to cigarette smoke and endogenous MMPs, explaining the high risk of AAA formation in those with a significant pack-year history.17 Both MMP-2 and MMP-9 have been shown to be profoundly elevated in the serum and aortic wall of aneurysmal patients compared to controls.18 However, the inhibition of these proteases using doxycycline has largely been unsuccessful at reducing aneurysm growth in large trials.5,19 In our results, we describe the elevation of ELNf in both the RFM and AAA populations, both of which are characterized by heavy tobacco use. However, only the AAA patients generate an inflammatory response in the form of self-recognizing ELNf antibodies. The same elastin fragments have previously been shown to be increased in patients with tobacco-associated COPD.15 Additionally, animal models of COPD have demonstrated an ability of ELNf to perpetuate inflammation, drive disease progression, and polarize naïve monocytes towards the M1 pro-inflammatory Mϕs sensitized to elastin-rich tissues.20 Interestingly, Dale et al inhibited these M1 Mϕs in a murine AAA model and noted decreased aneurysm growth, MMP expression, and loss of elastin from the aortic wall.21 Similarly, COLV autoantibodies have been reported in some cases of COPD.22,23 However, we did not observe any increased sensitivity to this antigen in our AAA subjects.
In the patient without a faulty regulatory reaction, we believe the response characterized by the second, inflammation stage is controlled via the actions of immune suppressing cells such as the antigen-specific CD4+ Tr1 lymphocyte which coexpress lymphocyte activation gene 3 (LAG3) and CD49b while secreting the potent anti-inflammatory cytokine IL-10.24 We report a depletion of Tr1 lymphocytes in AAA corresponding with a loss of their immunosuppressive circulating secretory product, IL-10. Interestingly, there is accumulating evidence that Tr1 and Th17 inflammatory lymphocytes may derive from the same naïve T cell population – progressively gaining IL-10 activity and polarizing towards the Tr1 cell during the resolution stages of inflammatory pathologies.25–27 In the ongoing ARREST phase I clinical trial at our institution, patients with small AAAs are given varying dosages of autologous, anti-inflammatory mesenchymal stem cells and their circulating immune environments are characterized over time.28 Preliminarily, we observe an increase in the Tr1:Th17 ratio without a change in the absolute numbers supporting this polarization phenomenon. We hypothesize that the ability to polarize from the Th17 to the Tr1 in the AAA patient may be compromised. Not surprisingly, a 6-fold increase in the Tr1:Th17 ratio in the RFM controls were seen. A decrease in the Tr1:Th17 ratio also seemed to be correlated to the presence of AAA, regardless of size. Based on this observation, we are currently pursuing the use the Tr1:Th17 ratio as a potential screening tool for the diagnosis of AAA.
We observed an elevation in PGE2 in our AAA patients. While grouped with other anti-inflammatory cytokines in this manuscript, it can also have an inflammatory effect in certain conditions.29 Of particular interest, PGE2 has been demonstrated to inhibit Tr1 polarization through c-Maf suppression which may also contribute to the Tr1 depletion observed in AAA.27 Mechanistic Tr1 studies as a follow-up to these initial findings are underway. Our preliminary in vitro results investigating AAA-derived Tr1 cells suggests that not only is the population depleted, but it is also dysfunctional with decreased ability to migrate toward chemoattractants, secrete IL-10 in response to stimulus, and polarize monocytes to the M2 anti-inflammatory phenotype.
In the inflammation phase, self-sensitized macrophages hone to the infrarenal aorta, a relatively weak section of a large elastic artery prone to excessive mechanical stress.30,31 In this local environment, B and T cells are recruited and activated by Mϕ APCs setting off a cascade of events which results in additional recruitment of inflammatory subtypes.12 We noted a trend towards increased circulating inflammatory Mϕs but were unable to assay them in situ. In circulation, we report an elevation of Th17 CD4+ lymphocytes corresponding to an increased expression of IL-17, suggesting an antigen-specific response. The elevation of IL-17 is a hallmark finding of many autoimmune diseases and its downregulation has been shown to be of benefit in slowing AAA growth in murine models.32–34 The elaboration of cytokines such as IL-2, IFN-γ, and TNF-α by a predominantly Th1 mononuclear response stimulates pro-inflammatory osteopontin (OPN) elaboration from macrophages and vascular smooth muscle cells further propagating inflammation.35,36 IL-6, which plays a role in maturation of lymphocytes, was also significantly elevated in our AAA population. This finding has been corroborated by recent reports;37 additionally, blocking the IL-6R has also shown some efficacy in slowing aneurysm growth in mice.38
There are several limitations to this study and the results reported should be interpreted with these in mind. The first of which is the design of this study, a retrospective query of a prospectively maintained database. Clinical data points from the medical records may be abstracted incorrectly or misrepresented introducing the increased potential for error and bias. Additionally, this study is limited by its largely descriptive nature, but will allow for the transition to further mechanistic investigations. Lastly, while both RFM and AAA patients are derived from the same initial population of individuals greater than 65 years presenting for an AAA screening ultrasound, we observed increased comorbidities in the AAA group suggesting a more overall diseased cohort.
Conclusion
In this investigation, we report the human AAA condition is defined not only by chronic inflammation but also by aberrations in immune regulation – specifically with respect to a depletion of the antigen-specific Tr1 lymphocyte. Therefore, further investigation is required to determine the causes of these irregularities to better define the mechanism of AAA formation.
Acknowledgments
The work described in this article was in part supported by the National Institutes of Health grants 1UM1HL113457 and R01HL128827.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no relevant financial conflicts of interest to the information presented in this manuscript
Author Contributions:
SKW, MPM: writing and reviewing manuscript, data collection, sample collection,
NAD, LAG, ARG, RLM, AKG, AF: sample collection, writing and reviewing manuscript
References
- 1.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. Journal of vascular surgery. 2003;38(3):584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature reviews Cardiology. 2011;8(2):92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 3.Brophy CM, Reilly JM, Smith GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Annals of vascular surgery. 1991;5(3):229–233. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- 4.Participants USAT. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649–1655. [PubMed] [Google Scholar]
- 5.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. Journal of vascular surgery. 2002;36(1):1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 6.Bicknell CD, Kiru G, Falaschetti E, Powell JT, Poulter NR. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK) Eur Heart J. 2016;37(42):3213–3221. doi: 10.1093/eurheartj/ehw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34(45):3491–3500. doi: 10.1093/eurheartj/eht334. [DOI] [PubMed] [Google Scholar]
- 8.Hogh A, Vammen S, Ostergaard L, Joensen JB, Henneberg EW, Lindholt JS. Intermittent roxithromycin for preventing progression of small abdominal aortic aneurysms: long-term results of a small clinical trial. Vascular and endovascular surgery. 2009;43(5):452–456. doi: 10.1177/1538574409335037. [DOI] [PubMed] [Google Scholar]
- 9.Propanolol Aneurysm Trial I. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. Journal of vascular surgery. 2002;35(1):72–79. doi: 10.1067/mva.2002.121308. [DOI] [PubMed] [Google Scholar]
- 10.Bobryshev YV, Lord RS. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154(1):15–21. doi: 10.1016/s0021-9150(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine V, Jacob MP, Houard X, Rossignol P, Plissonnier D, Angles-Cano E, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. The American journal of pathology. 2002;161(5):1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. The American journal of pathology. 1990;137(5):1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 13.LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;161(4):281–290. doi: 10.7326/M14-1204. [DOI] [PubMed] [Google Scholar]
- 14.Corkum CP, Ings DP, Burgess C, Karwowska S, Kroll W, Michalak TI. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT) and standard density gradient. BMC immunology. 2015;16:48. doi: 10.1186/s12865-015-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nature medicine. 2007;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 16.Lu S, White JV, Lin WL, Zhang X, Solomides C, Evans K, et al. Aneurysmal lesions of patients with abdominal aortic aneurysm contain clonally expanded T cells. Journal of immunology (Baltimore, Md: 1950) 2014;192(10):4897–4912. doi: 10.4049/jimmunol.1301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stackelberg O, Wolk A, Eliasson K, Hellberg A, Bersztel A, Larsson SC, et al. Lifestyle and Risk of Screening-Detected Abdominal Aortic Aneurysm in Men. Journal of the American Heart Association. 2017;6(5) doi: 10.1161/JAHA.116.004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Hussien H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, Lindeman JH. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. Journal of vascular surgery. 2009;49(3):741–749. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. Journal of vascular surgery. 2001;34(4):606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 20.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, et al. Elastin fragments drive disease progression in a murine model of emphysema. The Journal of clinical investigation. 2006;116(3):753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, et al. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. Journal of immunology (Baltimore, Md: 1950) 2016;196(11):4536–4543. doi: 10.4049/jimmunol.1502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budding K, van de Graaf EA, Otten HG. Humoral immunity and complement effector mechanisms after lung transplantation. Transplant immunology. 2014;31(4):260–265. doi: 10.1016/j.trim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, et al. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(8):807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19(6):739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 25.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Seminars in immunology. 2011;23(6):438–445. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper KM, Kong W, Ganea D. Prostaglandin E2 inhibits Tr1 cell differentiation through suppression of c-Maf. PLoS One. 2017;12(6):e0179184. doi: 10.1371/journal.pone.0179184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SK, Green LA, Gutwein AR, Drucker NA, Motaganahalli RL, Fajardo A, et al. Rationale and Design of the ARREST Trial Investigating Mesenchymal Stem Cells in the Treatment of Small AAA. Annals of vascular surgery. 2017 doi: 10.1016/j.avsg.2017.08.044. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Luo C, Zhang H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediators of inflammation. 2017;2017:5126048. doi: 10.1155/2017/5126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins MJ, Bersi M, Wilson E, Humphrey JD. Mechanical properties of suprarenal and infrarenal abdominal aorta: implications for mouse models of aneurysms. Medical engineering & physics. 2011;33(10):1262–1269. doi: 10.1016/j.medengphy.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drewe CJ, Parker LP, Kelsey LJ, Norman PE, Powell JT, Doyle BJ. Haemodynamics and stresses in abdominal aortic aneurysms: A fluid-structure interaction study into the effect of proximal neck and iliac bifurcation angle. Journal of biomechanics. 2017;60:150–156. doi: 10.1016/j.jbiomech.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunological reviews. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Z, Wang Y, Zhang K, Liao Y, Ye P, Wu J, et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(11):2429–2438. doi: 10.1161/ATVBAHA.114.304435. [DOI] [PubMed] [Google Scholar]
- 34.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126(11 Suppl 1):S38–45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SK, Green LA, Gutwein AR, Gupta AK, Babbey CC, Motaganahalli RL, et al. Osteopontin May be a Driver of Abdominal Aortic Aneurysm Formation. Journal of vascular surgery. 2017 doi: 10.1016/j.jvs.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 36.Golledge J, Muller J, Shephard N, Clancy P, Smallwood L, Moran C, et al. Association between osteopontin and human abdominal aortic aneurysm. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(3):655–660. doi: 10.1161/01.ATV.0000255560.49503.4e. [DOI] [PubMed] [Google Scholar]
- 37.Kasashima S, Kawashima A, Zen Y, Ozaki S, Kasashima F, Endo M, et al. Upregulated interleukins (IL-6, IL-10, and IL-13) in immunoglobulin G4-related aortic aneurysm patients. Journal of vascular surgery. 2017 doi: 10.1016/j.jvs.2016.12.140. [DOI] [PubMed] [Google Scholar]
- 38.Nishihara M, Aoki H, Ohno S, Furusho A, Hirakata S, Nishida N, et al. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS One. 2017;12(10):e0185923. doi: 10.1371/journal.pone.0185923. [DOI] [PMC free article] [PubMed] [Google Scholar]


