Abstract
The dorsomedial striatum, a key site of reward-sensitive motor output, receives extensive afferent input from cortex, thalamus and midbrain. These projections are integrated by striatal microcircuits containing both spiny projection neurons and local circuit interneurons. To explore target-cell specificity of these projections, we compared inputs onto D1-dopamine receptor positive spiny neurons, parvalbumin-positive fast-spiking interneurons and somatostatin-positive low-threshold-spiking interneurons, using cell-type specific rabies virus tracing and optogenetic-mediated projection neuron recruitment in mice. While the relative proportion of retrogradely labeled projection neurons was similar between target cell types, the convergence of inputs was systematically higher for projections onto fast-spiking interneurons. Rabies virus is frequently used to assess cell-specific anatomical connectivity but it is unclear how this correlates to synaptic connectivity and efficacy. To test this, we compared tracing data with target cell-specific measures of synaptic efficacy for anterior cingulate cortex and parafascicular thalamic projections using novel quantitative optogenetic measures. We found that target-specific patterns of convergence were extensively modified according to region of projection neuron origin and postsynaptic cell type. Furthermore, we observed significant divergence between cell type-specific anatomical connectivity and measures of excitatory synaptic strength, particularly for low-threshold-spiking interneurons. Taken together, this suggests a basic uniform connectivity map for striatal afferent inputs upon which presynaptic-postsynaptic interactions impose substantial diversity of physiological connectivity.
Keywords: rabies virus, synapse, striatum, interneuron, electrophysiology
Introduction
The dorsomedial striatum (DMS) receives broad input from cortical, thalamic, and limbic regions and is a critical node for the integration of sensorimotor, motivational, and cognitive information (Balleine et al., 2009; Hunnicutt et al., 2016). The striatum is comprised of D1 and D2 dopamine receptor positive spiny projection neurons (D1R+/D2R+ SPNs), as well as cholinergic (ChINs) and GABAergic interneurons (Kawaguchi et al., 1995; Kawaguchi, 1997; Kreitzer, 2009). Mounting literature points to distinct roles for these neuronal populations in goal-directed behaviors (Tai et al., 2012; Shan et al., 2014; Lee et al., 2017). Given the preponderance of GABAergic connections within the striatum, it seems likely that excitatory projection neurons play a key role in shaping the activity of striatal circuits. Understanding this regulation should provide insight into the striatal contribution to normal and dysfunctional reward-related behavior.
Prior studies have compared the anatomical distribution of inputs to D1R+ and D2R+ SPNs as well as ChINs, noting subtle shifts in the relative contribution of projection neurons (Wall et al., 2013; Guo et al., 2015). However, the afferent connectivity of striatal GABAergic interneurons has yet to be explored. Although sparse, these local interneurons broadly and potently inhibit SPN activity (Kawaguchi et al., 1995; Tepper et al., 2004; Gittis et al., 2010; Tepper et al., 2010; Straub et al., 2016), thereby modulating behavioral output (Xu et al., 2016; Lee et al., 2017; Rapanelli et al., 2017). Two of the most numerous striatal GABAergic interneuron subtypes exhibit highly divergent circuit functions - parvalbumin-expressing fast-spiking interneurons (PV+ FSIs) mediate peri-somatic inhibition while somatostatin-expressing low-threshold spiking interneurons target the distal dendritic compartment of SPNs (SST+ LTSIs; (Kawaguchi et al., 1995; Tepper et al., 2004; Straub et al., 2016). Given these divergent functional roles, it is important to understand whether inputs to the DMS differentially regulate these cell types and how this relates to activation of spiny projection neurons that output to downstream basal ganglia nuclei.
While rabies virus-mediated monosynaptic tracing has been used to explore connectivity onto intermingled neuronal populations (Lammel et al., 2012; Weissbourd et al., 2014; Beier et al., 2015; Schwarz et al., 2015), it is unclear how quantitative analysis of retrogradely labeled neurons relates to synaptic connectivity or strength (Ogawa & Watabe-Uchida, 2017). The ability of specific projection neurons to influence striatal processing depends on multiple factors including the convergence of inputs (number of projections that synapse on a given postsynaptic neuron) and the individual synaptic strength of these connections. It is presently unclear whether target cell-specific rabies virus tracing can provide any information on these variables. Furthermore, cell type-specific mapping of intermixed populations in other brain regions has demonstrated little specificity with regard to the fractional contribution of inputs (Lammel et al., 2012; Weissbourd et al., 2014; Beier et al., 2015; Schwarz et al., 2015), suggesting postsynaptic-driven differences in connectivity may be an essential determinant in creating physiological diversity in mature neural circuits (Reyes et al., 1998).
To probe these issues, we systematically analyzed the anatomical input from frontal cortex and thalamus to three cell types in the DMS – D1R+ SPNs, PV+ FSIs and SST+ LTSIs. We then compared these data with quantitative electrophysiological measures of synaptic efficacy for all six connections. Overall, we found that overarching patterns of cell type-specific anatomical connectivity were not representative of the intrinsic strength or reliability of synaptic transmission for these circuits. Furthermore, comparisons between the cortical and thalamic inputs suggested that a basic framework of projection neuron anatomical connectivity was modified by both region of presynaptic origin and target cell type to generate the full diversity of connectivity within the DMS.
Materials and Methods
Animals
Male mice were housed in groups of 2–5 on a 12-hour light/dark cycle (lights on at 7 am) with ad libitum access to food and water. Drd1a-Cre (Jackson Labs, #37156-Jax), PV-2a-Cre (Jackson Labs, #012358), SST-ires-Cre (Jackson Labs, #013044) mouse lines were maintained on a C57Bl/6J genetic background. Mice were at least 60 days old for stereotaxic injections (Drd1a-Cre, n= 17; PV-2a-Cre, n= 16; SST-ires-Cre, n= 14). All procedures conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Administrative Panel on Laboratory Animal Care (Protocol Number: 805643).
Virus Production
In brief, HEK cells were transiently transfected with helper plasmids (pTIT-N, pTIT-P, pTIT-G, pTIT-L, pCAGGS-T7) and either SAD-ΔG-EGFP or SAD-ΔG-tdTomato constructs (full length cDNA plasmid containing all rabies virus components except the G glycoprotein), and supernatant was transferred to BHK-B19G cells after 3 days. Following two amplification steps, benzonase-treated supernatant was filtered through a 0.22 μm filter, centrifuged at 32,000 × g for 2 hr at 4°C and resuspended in Opti-MEM (Life Technologies) (Lim et al., 2012). SAD-ΔG-EGFP rabies virus was pseudotyped with the EnvA glycoprotein as previously described (Wickersham et al., 2007; Wickersham et al., 2010).
Stereotaxic Surgery
All intracranial injections were conducted in adult mice under general isoflurane (1–2%) anesthesia. In brief, mice were placed in a stereotaxic frame (David Kopf Instruments; Tujunga, CA, USA) and their head was shaved, followed by anti-bacterial scrub with betadine. Small (0.5 mm) holes were drilled above target coordinates, and a pulled glass needle, containing virus was lowered to injection sites. Virus was infused at 0.125 μL/min by using a microinfusion pump (Harvard apparatus; Holliston, MA, USA) and the injection needle was left in place for at least 5 minutes to prevent back-flow. For slice physiology, channelrhodopsin-expressing virus (AAV.DJ-hSyn-ChiEF-2a-Venus) was injected at the following coordinates: ACC: ±0.3 ML, +0.75 AP, −1.3 DV; PFas : ±1.7 ML, −2.2 AP, −3.2 DV. While care was taken to minimize viral spread, there is always some degree of labeling of adjacent structures. For ACC, we noted infection of adjacent M2 and for PFas we noted infection of thalamic structures dorsal to the PFas. In both instances, we labeled our injections according to the predominant projection population. To visualize specific striatal cell types, 500nl of AAV.1-hSyn::DIO-tdTomato (UPenn vector core) was injected into the DMS (+1.75 ML, +0.75 AP, −3.0 DV). All mice were allowed to recover for 3–6 weeks prior to recording.
Cell-type specific retrograde tracing
Mice (D1-Cre n=5, PV-Cre n=4, SST-Cre n=4) were injected with a 1:1 mixture of AAV5-CAG-DIOloxP-TVA66T-2a-mCherry and AAV8-CAG-DIOloxP-G (500nl) into the DMS (+1.75 ML, +0.75 AP, −3.0 DV) and allowed to recover for 7 days prior to rabies virus injection. While this two-part helper system may generate some starter neurons that cannot undergo retrograde labeling, we have no evidence that this phenomenon would be different amongst the target neurons studied. (EnvA)-SAD-ΔG-EGFP rabies virus was subsequently injected under the same conditions and injection volume as initial AAV injection. One week later, mice were deeply anesthetized with i.p. injection of 100 uL Pentobarbital Sodium (Nembutal, 50 mg/mL) and transcardially perfused with phosphate buffered saline followed by 4% formalin. Brains were removed, placed in 4% formalin 12–24 hours, followed by 30% sucrose for 24–48 hours, after which they were stored at −80°C until cryo-sectioning. Coronal 25μm sections were collected, mounted and stitched large-field images were obtained with a 4× objective (Olympus, 4x, 0.16NA) on standard epi-fluorescent microscope (Olympus, BX63). All neuron counts were done manually using ImageJ software. We did not use a criterion for minimum number of starter cells to be included in the data analysis. In support of this choice, we observed a strong correlation between starter cell number and labeled projection neurons across all three lines (D1C R2=0.86, p<0.05; PVC R2=0.9653, p<0.05, SSC R2=0.9823, p<0.01), suggesting animals with lower numbers of starter cells showed consistently proportional retrograde tracing. Starter cells were defined as neurons co-labeled with both GFP (rabies infected) and mCherry (TVA expressed), and projection neurons were those labeled only with GFP. GFP-positive input neurons were manually counted from every 10th 25-μm slice throughout the entire brain (27±1 sections per mouse), except within the DMS itself. Starter cells were counted from all DMS slices (5±0.4 sections per mouse). As each brain had a different number of starter neurons, we normalized inputs by number of starter cells (anatomical convergence). For analysis of transgenic animal specificity, contiguous 50μm slices of dorsomedial striatum were counted in ImageJ, specifically looking for the PV or SST immunoreactivity of neurons labeled by AAV5-CAG-DIOloxP-TVA66T-2a-mCherry virus.
Electrophysiology
Mice were deeply anesthetized trans-cardially perfused with ice-cold aCSF containing (in mM): 124 NaCl, 2.5 KCl, 1.2 HaH2PO4, 24 NaHCO3, 5 HEPES, 13 Glucose, 1.3 MgSO4, 2.5 CaCl2. After perfusion, the brain was quickly removed, submerged and coronally sectioned on a vibratome (VT1200s, Leica) at 250–300 μm thickness in ice-cold aCSF. Slices were transferred to NMDG based recovery solution at 32°C of the following composition (in mM): 92 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 Sodium ascorbate, 2 Thiourea, 3 Sodium pyruvate, 10 MgSO4, 0.5 CaCl2. After 12–15 min. recovery, slices were transferred to room temperature aCSF chamber (20–22°C) and left for at least 1 hour before recording. Following recovery, slices were placed in a recording chamber, fully submerged at a flow rate of 1.4~1.6 mL/min, and maintained at 29–30°C in oxygenated (95% O2, 5% CO2) aCSF containing picrotoxin (100 μM, Sigma).
For voltage-clamp recordings, recording pipettes were pulled from borosilicate glass (World Precision Instruments, TW150–3) that had a tip resistance of 3~5 MΩ when filled with internal solution containing (in mM) 115 CsMeSO4, 20 CsCl, 10 HEPES, 2.5 MgCl, 0.6 EGTA, 1 QX-314, 10 Na-Phosphocreatine, 4 NaATP, 0.3 NaGTP, 0.1 spermine (pH adjusted to 7.3–7.4 with CsOH). Current-clamp recordings were made with electrodes containing (in mM): 140 K-gluconate, 5 KCl, 2 MgCl2, 0.2 EGTA, 10 HEPES, 10 Na-phosphocreatine, 4 Mg-ATP, 0.3 Na-GTP (pH to 7.3 with KOH). For striatal field recordings, pipette was filled with aCSF and their access resistance was fixed 0.8~1.2 MΩ. Field electrode was positioned within 50 um distance from the patched cell. Optical fiber volley was calculated as the peak of the first negative deflection (N1) and field slope was calculated as the first negative peak of the N2 deflection. Every whole cell recording was performed together with field recording to normalize viral expression of excitatory opsin. Voltage-clamp recordings from striatal neurons were obtained under visual control using IR-DIC optics (Olympus, BX51). Visual identification of D1-MSN, PV+ or SST+ neurons was based on expression of tdTomato (Chroma, #49005). Cells were voltage clamped at −70 mV unless otherwise noted. Recordings were performed using a MultiClamp 700B (Molecular Devices), filtered at 2.8 kHz and digitized at 20 kHz. ChIEF expressing axon terminals were stimulated with brief (1 ms) pulses of 473 nm blue light from a collimated LED illuminator (CoolLED, pE-300). We initially tested for the presence of target-field opsin contamination by giving elongated light pulses at the beginning of each recording. If any neuron was found to have a prolonged “somatic” current, we discarded all other recorded neurons from that hemisphere. Input and series resistance were monitored continuously and experiments were discarded if either parameter changed by >20%. AMPAR/NMDAR ratios were determined by comparing peak amplitude of averaged AMPAR EPSCs at −70 mV, with average amplitude of EPSCs recorded at +40 mV, 50 ms after afferent stimulation (NMDAR EPSC). For the input-output plot, 1ms light stimulus of increasing intensity was employed (0.35, 0.78, 1.2, 1.7, 2.1, 3.2, 4.3 mW/mm2). For asynchronous miniature experiments, calcium was substituted by strontium (2.5 mM). Strontium-evoked asynchronous miniature events were analyzed from 40–300 msec. temporal window following optical excitation. All miniature synaptic currents were recorded in the presence of tetrodotoxin (1 μM).
Immunohistochemistry
25-μm cryosectioned brain slices were permeabilized in 0.6% Triton X-100 and blocked in 3% normal goat serum in PBS for 1 hr in free-floating conditions. Primary antibody was incubated overnight in 1% normal goat serum and 0.2% Triton X-100 in PBS (Rat anti-Somatostatin, 1:500, Millipore; Mouse anti-Parvalbumin, 1:500, Sigma-Aldrich). For visualization, slices were incubated with secondary antibody for 1 hour (Goat AMCA-conjugated anti-Rat, 1:500, Jackson immunoResearch laboratory; Goat Alexa350-conjugated anti-Mouse, 1:500, Molecular probes). Immunostained slices were mounted and scanned on a standard epi-fluorescent microscope (Olympus, BX63) under both 10× (Olympus, 0.4NA) & 20× (Olympus, 0.75NA) objectives.
Data analysis
All data are presented as mean ± SEM. Significance was assessed by one-way analysis of variance (ANOVA) or paired Student’s t-test, as appropriate, with Tukey correction for multiple comparisons. Mean differences between groups were considered significant when P<0.05. Electrophysiology data were acquired using custom-built Recording Artist software (Rick Gerkin, Igor Pro6 (Wavemetrics)), and analyzed using Igor Pro7 (Wavemetrics), MATLAB (Mathworks) and Minianalysis (Synaptosoft). Anatomical data was analyzed using ImageJ (NIH). All data were visualized with Graphpad Prism 7 (Graphpad Software). For input-output analyses, multiple data points were collected across a range fiber volleys and subject to linear regression modeling. All slope coefficients for regression lines with R2 >0.7 were used for subsequent analysis.
Results and Statistical Analysis
Mapping Anatomical Connectivity to Local Striatal Interneurons
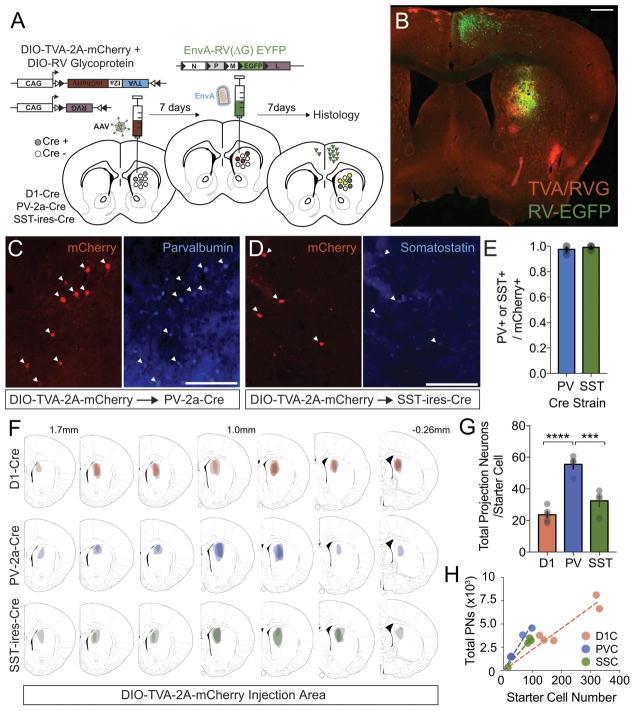
To explore inputs that regulate the local striatal GABAergic network, we employed EnvA-pseudotyped rabies virus tracing, which permits G-protein dependent monosynaptic retrograde labeling from genetically defined starter populations (Fig. 1A,B) (Wickersham et al., 2007; Wall et al., 2010; Wickersham et al., 2010). We examined anatomical connectivity onto parvalbumin-immunoreactive fast-spiking interneurons (PV+FSIs) and somatostatin-immunoreactive low threshold-spiking interneurons (SST+LTSIs), via the Parvalbumin-2a-Cre (PV-2a-Cre) and Somatostatin-ires-Cre (SST-ires-Cre) transgenic lines (Fig. 1C,D). As a comparison point for previously published striatal tracing studies (Wall et al., 2013; Fuccillo et al., 2015; Guo et al., 2015), we employed a D1-Cre BAC transgenic allele to map inputs to direct pathway D1R+ SPNs. To test the fidelity of our mouse strains for labeling striatal interneuron populations, we injected adeno-associated virus (AAV) that expresses mCherry in the presence of Cre recombinase (AAV5-CAG-DIO::TVA-2a-mCherry). Immunohisto-chemical staining of injected brains with either parvalbumin or somatostatin antibodies demonstrated a high degree of specificity for each Cre line within DMS (Fig. 1C,D), with 97% of mCherry+ neurons expressing parvalbumin in the PV-2a-Cre line and >99% of mCherry+ neurons expressing somatostatin in the SST-ires-Cre line (Fig. 1E). Furthermore, we found that the PV-2a-Cre mice labeled a population of weakly immunoreactive PV+ neurons in the DMS that exhibited electrophysiological properties similar to the larger, more strongly PV-immunoreactive FSIs of the dorsolateral striatum (DLS), including brief spike-width, low input resistance and sharp after hyperpolarization (data not shown).
Figure 1.
(A) Experimental workflow for cell type-specific pseudotyped-RV tracing of projection inputs into striatum. (B) Representative section of retrograde labeling from D1-Cre mouse. Diffuse signal on the red channel is background. Scale bar is 500 um. Sections of PV-2a-Cre mice or SST-ires-Cre mice injected with hSyn-DIO::TVA-2a-mCherry, which have undergone immunohistochemical staining for parvalbumin (C) or somatostatin (D). Scale bar is 100 um. (E) Both interneuron lines exhibit high specificity for their respective cell types, as measured by the fraction of mCherry-positive neurons that co-stain for either marker protein. (F) Mapping of the distribution of starter neuron populations (area bounded by starter cells) along the A-P extent of each mouse line. (G) Comparison of the total number of projection neurons per starter cell across D1R+ SPNs, PV+ FSIs and SST+ LTSIs. (H) Relationship between sampled number of starter cells per animal and number of retrogradely labeled projection neurons (each point represents one brain).
The relative contributions of projection neurons to the striatum is strongly linked to the medial-lateral location of striatal target region, with DMS receiving more medial cortical and thalamic populations than DLS (Voorn et al., 2004). We explored these findings by simultaneously injecting rabies virus (RV) expressing tdTomato (SAD-ΔG-tdTOM) in the DMS and RV expressing EGFP (SAD-ΔG-EGFP) in the DLS of adult male C57Bl6J mice (Supp. Fig. 1A). We found well-demarcated boundaries of retrogradely labeled projection neurons along the medio-lateral axis within the cortex, thalamus and substantia nigra (Supp. Fig. 1B–D, not shown). In light of these results, we restricted analyses of rabies tracing to brains where starter cells (defined as mCherry+/EGFP+ double-positive) were exclusively within a defined DMS region (see Fig. 1F). Overall numbers of starter cells (sampled over 250μm intervals) were roughly comparable between both interneuron lines (PV: 53.8±15.2 vs SST: 70.8±16.2), and ~4-fold higher in D1R+ SPNs (217±40; Fig. 1H). The divergence between the reported density of these neuronal subtypes in striatal tissue (Kita & Kitai, 1988) and the relative number of starter cells could reflect differences in the efficiency of Cre expression between transgenic lines or cell type-specific variability in viral transduction. The total number of labeled projection neurons per starter cell was significantly different between target cell types, with a ~2-fold increase in FSIs compared to D1R+ SPNs and LTSIs (D1: 23.6±2.1; PV: 55.5±3.1; SST: 32.4±3.8; ANOVA [F (2,10)=30.96, P<0.0001; Fig. 1G). Importantly, several controls confirmed the fidelity of our pseudotyped RV system: (1) injection of EnvA-RV-EGFP did not label any neurons in wildtype C57Bl6 mice, demonstrating high pseudotyping efficiency (not shown); (2) injection of mixed AAV-CAG-DIO::TVA-2a-mCherry/AAV-CAG-DIO::G-protein viruses into wildtype C57Bl6J mice, followed 10 days later with EnvA-RV-EGFP, labeled a small number of cells locally but no projection populations outside of striatum, suggesting minimal Cre-independent AAV expression (Supp. Fig. 1E; Beier et al., 2015; Ogawa & Watabe-Uchida, 2017).
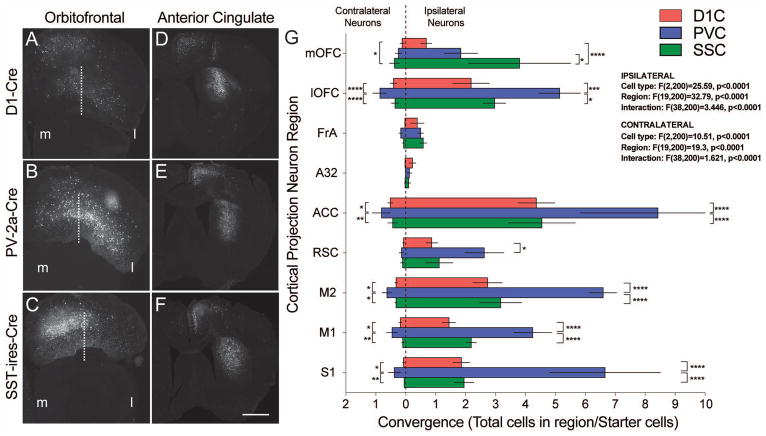
To quantify these data, we counted the number of projection neurons as a fraction of total labeled cells in representative sections spanning the A-P extent of the brain (Supp.Fig. 2–4) (Wall et al., 2013; Weissbourd et al., 2014; Beier et al., 2015). Using this measure, we found bulk connectivity was highest in ACC and secondary motor cortex (M2), followed by orbitofrontal (OFC) and S1 somatosensory cortices, the parafascicular thalamic nucleus (PFas) and the globus pallidus external segment (GPe). Individual statistical analysis of cortical, thalamic and remaining subcortical structures consistently uncovered a main effect of projection neuron origin but no postsynaptic cell type-specificity (Supp.Fig. 2–4). In an attempt to precisely define connectivity at the target cell level, we normalized these counts by the number of initial starter cells to obtain a convergence measurement - an average number of contacts per target neuron (Do et al., 2016). These data revealed a trend whereby PV+ interneurons had higher convergence than both D1R+ SPNs and SST+ cell types from all cortical inputs (Fig. 2), consistent with the differences in brain-wide convergence measures (Fig. 1G). The OFC was the only exception, with the medial OFC (mOFC) demonstrating higher labeling from SST+ cells and the lateral OFC (lOFC) demonstrated higher labeling from PV+ cells (Fig. 2A–C,G). All 3 tracing experiments displayed contralateral cortical labeling, although it was ~10% the number of ipsilateral neurons (Fig. 2G). Furthermore, the basic patterns of cell type connectivity were similar for contralateral and ipsilateral projections (compare left and right bars in Fig. 2G).
Figure 2.
Representative coronal sections of retrogradely labeled cortical projection neuron populations from orbitofrontal (A–C) and anterior cingulate cortex (D–F) onto D1R+ SPNs (A,D), PV+ FSIs (B,E) and SST+ LTSIs (C,F). Note target cell-specific medial/lateral (separated by dashed white line) distribution of orbitofrontal neurons. (G) Graph demonstrating the anatomical convergence by region of cortical origin for projections onto D1R+ SPN (red), PV+ FSI (blue) and SST+ LTSI (green). Convergence values to the left and right represent projections form the side contralateral or ipsilateral to the starter cells, respectively. Scale bar is 500μm.
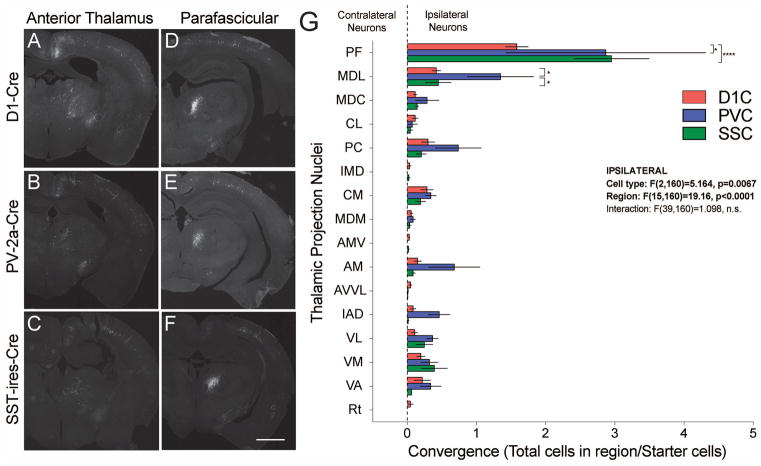
Although cells were present across 16 distinct thalamic nuclei, the majority of labeled neurons were found in the PFas, followed by the mediodorsal and paracentral nuclei (Supp. Fig. 3). There were no contralateral projecting neurons identified in the thalamus of any sample. While the overall convergence measures were lower for thalamus than cortex, the distribution between target populations (biased towards PV+ cells) was similar to cortex (Fig. 3). The only exception was the PFas nucleus, where convergence onto SST+ neurons was similar to that onto PV+ neurons. Together with previous tracing studies, these data suggest only modest anatomical specificity for projection neurons targeting striatal microcircuits (Wall et al., 2013; Guo et al., 2015).
Figure 3.
Representative coronal sections of retrogradely labeled thalamic projection neuron populations from anterior thalamus (A–C) and parafascicular nucleus (D–F) onto D1R+ SPNs (A,D), PV+ FSIs (B,E) and SST+ LTSIs (C,F). (G) Graph demonstrating the anatomical convergence by region of thalamic origin for projections onto D1R+ SPN (red), PV+ FSI (blue) and SST+ LTSI (green). Note the exclusive ipsilateral projections of all thalamic populations. Scale bar is 500μm.
Divergence between Anatomical Tracing and Synaptic Properties of Projections Neurons to Striatum
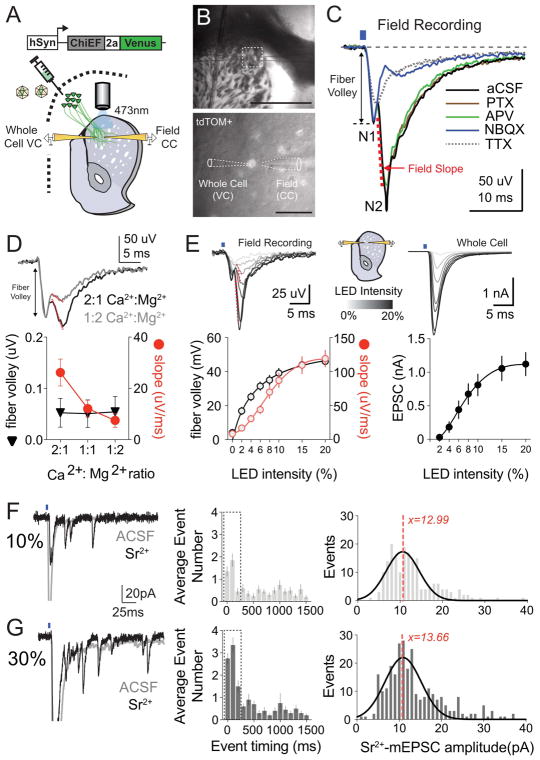
To see how RV-mediated anatomical tracing data correlated with physiological measures of synaptic connectivity and strength, we employed simultaneous striatal field/whole cell recordings to create a normalized input-output assay (Xiong et al., 2015). In this manner, we could describe synaptic strength and neuronal firing as a function of afferent recruitment, one of the main parameters measured by our anatomical studies. To explore the validity of this approach, we performed targeted cortical injection of ChIEF (Lin et al., 2009) and labeled D1R+ SPNs via hSyn-DIO::tdTOM reporter virus injection into D1-Cre BAC transgenic mice. Optical stimulation (473nm LED) of coronal acute slices through a 40x-objective generated a two-component cortico-striatal field (N1,N2, Fig. 4A–C). We considered N1 the presynaptic “optical” fiber volley – a channelrhodopsin dependent waveform representing the total number of cortical axons recruited to a specific patch of striatal tissue (Xiong et al., 2015). Consistent with this idea, N1 was minimally reduced by the sodium channel blocker TTX (Fig. 4C), remained stable across multiple divalent cation concentrations (Fig. 4D) and increased hyperbolically with increasing LED intensity (Fig. 4E). The slope of the N2 component was considered a quantitative measure of the postsynaptic excitatory response, as suggested by its pharmacological sensitivity to the AMPAR blocker NBQX (Fig. 4C), insensitivity to the NMDAR receptor antagonist AP5 and the GABAAR antagonist picrotoxin (Fig. 4C), stepwise decrement in conditions of decreased release probability (Fig. 4D), and sigmoidal increase with higher LED intensities (Fig. 4E). D1R+ SPNs patched in whole cell configuration within 50μm of the field electrode demonstrated a similar sigmoidal increase in synaptic currents (Fig. 4E, right), with both measures plateauing at LED intensities of ~20% power. We attributed increases in fiber volley and synaptic current to the step-wise recruitment of ChiEF-expressing cortical fibers. Consistent with this, whole cell recordings in the presence of strontium (Sr2+), which desynchronizes neurotransmitter release from recently active synapses (Xu-Friedman & Regehr, 1999; 2000), demonstrated an LED-dependent increase in the frequency of asynchronous events without alterations in event amplitude (Fig. 4F,G).
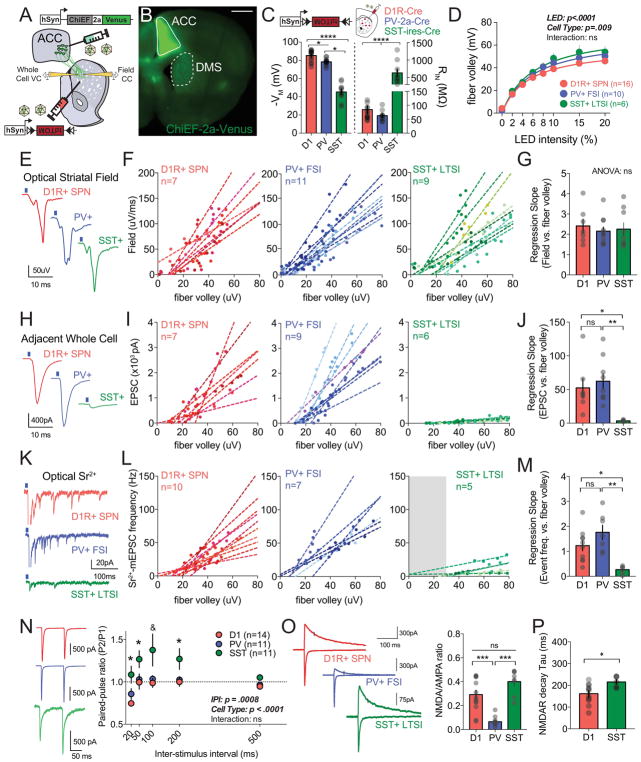
Figure 4.
(A) Experimental scheme of injection and recording sites. (B) Whole-cell and field recording configuration shown in DIC (top, 4x) and under fluorescence (bottom, 40x). Scale bar is 1 mm, 50 um, respectively. (C) Pharmacological dissection of field trace depicting N1 – fiber volley, and N2 – striatal field. Dotted red line indicates fit line of 20–80% peak amplitude, which was used to calculate field slope. Pharmacological manipulations are color-coded at right. (D) Changes in calcium-magnesium ratio affect the N2 component of the field recording (red), but not N1 (black). (E, left) Plot of changes in fiber volley amplitude and field slope across LED intensities. (E, right) EPSC amplitude in nearby voltage clamped neuron similarly increases with higher LED intensity. (F, left) Representative voltage clamp response to cortical optogenetic stimulation in ACSF (grey) or in the presence of strontium (black) at 10% LED intensity. Histograms showing average event number by 100msec bins (middle) and the distribution of strontium mEPSC event amplitudes (right, dotted red line is average Sr2+ mEPSC amplitude). (G) As in F except for 30% LED intensity stimulation of the same recorded neuron.
For electrophysiology analysis, we attempted to express ChiEF primarily in the ACC, the largest cortical input to the DMS (Fig. 2; see Methods). Given the two-fold higher convergence onto PV+ neurons, we hypothesized that ACC inputs would exhibit larger synaptic weights onto PV+ neurons than onto D1R+ SPNs or SST+ cells. Injection of hSyn-ChiEF-2a-Venus into ACC (Fig. 5A,B) was accompanied by striatal injection of hSyn-DIO::tdTOM reporter virus into either D1-Cre, PV-2a-Cre or SST-ires-Cre mice, permitting reliable labeling of these postsynaptic cell types as judged by intrinsic and active membrane properties (Fig. 5C). Increasing LED intensity led to enhancements in fiber volley amplitude, irrespective of the postsynaptic cell type (Fig. 5D). To generate robust, quantitative data on synaptic strength, we measured field slope and whole cell current across a range of optical fiber volley amplitudes, fit these data with a linear regression model, and compared the slope coefficients for each postsynaptic cell type (Fig. 5F,G,I,J). We found that the change of field slope as a function of afferent fiber volley was similar across slices with labeled D1R+ SPNs, PV+ FSIs and SST+ LTSIs (D1: 2.41±.33; PV: 2.16±.2; SST: 2.26±.31; ANOVA [F (2,24)=0.213, P=0.81; Fig. 5E–G). In contrast, whole cell currents recorded from labeled SST+ LTSI neurons near the field electrode diverged significantly from D1R+ SPNs and PV+ FSIs (Fig. 5H–J). While ACC-D1R+ SPNs and ACC-PV+ connections reliably exhibited evoked synaptic currents beginning at 10μV fiber volleys and had comparable slope coefficients, ACC-SST+ connections were not observed until 40μV fiber volley and exhibited a >10× lower rate of synaptic current change per fiber volley unit (D1: 52.42±14.1; PV: 62.27±11.2; SST: 3.46±.47; ANOVA [F (2,19)=7.32, P=0.004; Fig. 5H–J).
Figure 5.
(A) Illustration of the injection scheme for quantitative measurements of anterior cingulate cortical projections onto specific DMS neuronal subtypes. (B) Representative image showing ChiEF-2a-Venus expression in the ACC (injection site, solid white) and DMS (target site, dotted white). Scale bar is 1mm. (C) Passive membrane properties (left, membrane voltage; right, input resistance) of three DMS cell types. (D) Plot of the average fiber volley amplitude across different LED intensities in each transgenic mouse line. (E) Representative field traces recorded in slices from D1-Cre, PV-2a-Cre, SST-ires-Cre mice showing response to optical stimulus. (F) Plot of field slope against fiber volley with individual regression lines corresponding to field response recorded contemporaneously with whole cell (4–5 datapoints/cell are shown in the same color as regression). (G) The slope coefficient for regression analysis of field slope versus fiber volley for each postsynaptic cell type. (H) Representative EPSC traces recorded in each cell type in response to ACC optical stimulation. (I) Plot of individual EPSC amplitudes against fiber volleys with individual regression lines for each recorded neuron. (J) The slope coefficient for regression analysis of EPSC amplitude versus fiber volley. (K) Representative traces of optically evoked strontium-mEPSCs across the different cell types. (L) Plot of the individual strontium-mEPSC frequencies against fiber volley with individual regression lines for each recorded neuron. (M) The slope coefficient for regression analysis of strontium-mEPSC frequencies versus fiber volley. (N) Representative traces of paired-pulse response in each cell type (left) and plot of paired-pulse ratio across multiple ISIs (right). (O) Representative traces of recordings used to extract NMDA/AMPA ratio in each cell type (left, black line marks time point for NMDAR-mediated current measurement). Plot of NMDA/AMPA ratio by postsynaptic target cell (right). (P) Plot of weighted decay value of NMDA currents.
The contrast between these measures and previous anatomical data (Fig. 2) could result from differences in presynaptic release probability, number of release sites or sensitivity of postsynaptic detection, all of which have an unclear relationship to RV-mediated anatomical tracing (Ogawa & Watabe-Uchida, 2017). To explore this, we performed Sr2+-evoked asynchronous release of optically activated fibers, across a range of optical fiber volley amplitudes (Fig. 5K, Supp.Fig. 5A; See Methods). We found that the frequency of Sr2+-evoked asynchronous excitatory postsynaptic currents (aEPSCs), a proxy for presynaptic quantal content, was dramatically reduced in SST+ LTSIs, yielding a ~4× lower regression coefficient as compared to D1R+ SPNs and PV+ FSIs (D1: 1.23±.21; PV: 1.76±.29; SST: 0.27±.07; ANOVA [F (2,19)=8.37, P=0.0025; Fig. 5K–M). We next performed optically evoked paired pulse, a rough measure of presynaptic release probability, and found ACC-SST+ connections had a higher paired-pulse ratio than ACC-D1R+ or ACC-PV+ connections across multiple inter-pulse intervals, suggesting lower release probability at these synapses (Fig. 5N).
To probe whether differential postsynaptic sensitivity to neurotransmitter also contributed to synaptic strength differences, we recorded optically evoked aEPSC amplitudes in Sr2+. We found that the mean aEPSC amplitude from ACC-SST+ synapses was significantly smaller than that of ACC-D1R+ or ACC-PV+ connections (D1: 22.4±.21; PV: 23.8±.29; SST: 12.9±.07), revealing an additional postsynaptic mechanism for the divergence in synaptic strength observed in field-normalized recordings of SST+ LTSIs. Given this difference, we explored other aspects of the postsynaptic compartment, including receptor composition and subtype. We discovered target cell-specific differences in the ratio of AMPA and NMDA glutamate receptors (Fig. 5O), with ACC-PV+ connections having virtually no NMDAR-mediated currents, as well as target-specific differences in NMDAR composition, with ACC-LTSIs exhibiting decay kinetics consistent with a larger proportion of NR2B subunits than ACC-D1+ SPN and ACC-PV+ synapses (Fig. 5P) (Gittis et al., 2010).
Comparing properties of parafascicular-striatal and prefrontal-striatal projections
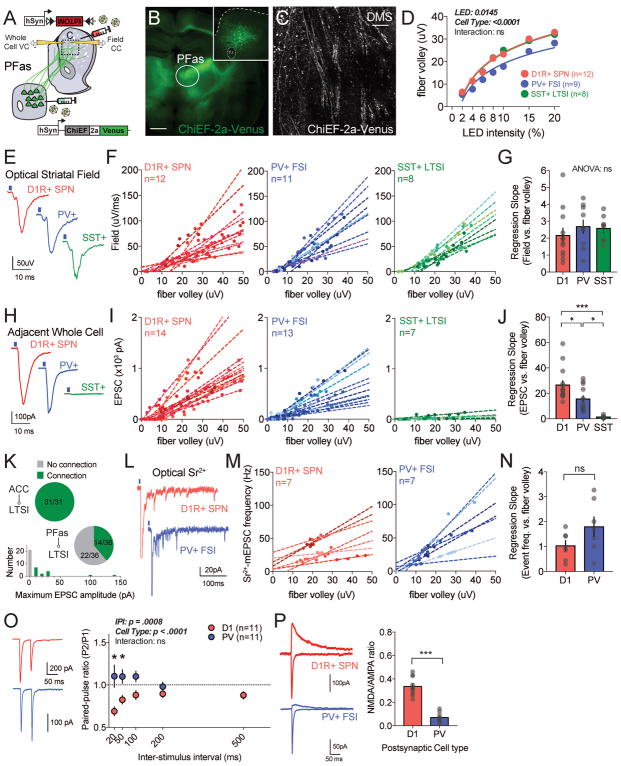
Together these data demonstrate a divergence between RV-tracing anatomical connectivity and the physiological properties of these connections that determine overall synaptic strength. To test the generalizability of these data, we optogenetically isolated thalamo-striatal inputs predominantly from the parafascicular nucleus (PFas; see Methods), the densest thalamo-striatal projection to DMS (Fig. 3). Five-week incubation of hSyn-ChiEF-2a-Venus yielded widespread expression of mVenus in the parafascicular nucleus as well as fiber staining in cortical white matter and neighboring thalamic nuclei (Fig. 6B,C). While increasing optogenetic stimulation caused a more shallow increase in fiber volley amplitude as compared with ACC recordings (compare Fig. 6D & 5D), the slope coefficient of the regression for field strength was similar to ACC projections and did not vary between D1-Cre, PV-2a-Cre and SST-ires-Cre mice (D1: 2.16±.43; PV: 2.68±.38; SST: 2.58±.27; ANOVA [F (2,27)=0.545, P=0.59; Fig. 6E–G). Cell type-specific synaptic strength measures once again diverged from anatomical data, but also could be distinguished from the ACC projections in three ways: (1) overall synaptic efficiency as measured by change in whole cell current per fiber volley increment was reduced for all PFas connections (compare Figs. 5J & 6J); (2) PFas-PV+ FSI connections were significantly less efficient then PFas-D1R+ SPN connections (D1: 26.5±3.5; PV: 15.47±2.6; Bonferroni posthoc, P=0.025; Fig. 6H–J); (3) we could not reliably detect PFas-SST+ LTSI synaptic connections in the majority (~2/3rds) of recorded cells (Fig. 6K, note only 2/36 had a maximum synaptic response >30pA), so we omitted these sparse connections from further analysis.
Figure 6.
(A) Illustration of the injection scheme for quantitative measurements of parafascicular projections onto specific DMS neuronal subtypes. (B) Representative image showing ChiEF-2a-Venus expression in the PFas (upper-right inset shows a short-term injection with AAV-hSyn-EGFP at the PFas coordinates used for the study) and 40× confocal image of ChiEF-2a-Venus axon terminals in DMS (C). (D) Plot of the average fiber volley amplitude across different LED intensities in each transgenic mouse lines. (E) Representative field traces recorded in D1-cre, PV-cre, SST-cre in response to optical stimulus. (F) Plot of field slope against fiber volley with individual regression lines corresponding to field response recorded contemporaneously with whole cell (4–5 datapoints/cell are shown in the same color as regression). (G) The slope coefficient for regression analysis of field slope versus fiber volley for each postsynaptic cell type. (H) Representative EPSC traces recorded in each cell type in response to PFas optical stimulation. (I) Plot of individual EPSC amplitudes against fiber volleys with individual regression lines for each recorded neuron. (J) The slope coefficient for regression analysis of EPSC amplitude versus fiber volley. (K, top) Proportion of LTSI neurons connected to ACC and PFas projection populations. (K, bottom) Histogram of maximal optically-evoked EPSC for PFas-LTSI connections (grey bar represents failures). (L) Representative traces of optically evoked strontium-mEPSCs across the different cell types. (M) Plot of the individual strontium-mEPSC frequencies against fiber volley with individual regression lines for each recorded neuron. (N) The slope coefficient for regression analysis of Sr2+-mEPSC frequencies versus fiber volley. (O) Representative traces of paired-pulse response in each cell type (left) and plot of paired-pulse ratio across multiple ISIs (right). (P) Representative traces of recordings used to extract NMDA/AMPA ratio in each cell type (left, black line marks time point for NMDAR-mediated current measurement). Plot of NMDA/AMPA ratio by postsynaptic target cell (right).
To explore the underpinnings of these target cell-specific difference in synaptic strength, we again generated optogenetic input-output functions in the presence of Sr2+. Similar to ACC projections, we did not note differences between D1R+ SPNs and PV+ LTSIs in the slope coefficient for aEPSC frequency as a function of fiber volley (Fig. 6L–N), and there was no difference between these populations in aEPSC amplitude (Supp.Fig. 5C). However, PFas-PV+ FSI connections exhibited a significantly higher paired-pulse ratio than PFas-D1R+ SPN synapses (Fig. 6O), suggesting that the reduced synaptic strength of PFas-PV+ connections may result from lower probability of release. In contrast to this target cell-specific presynaptic property, we found that postsynaptic receptor composition and subtype were similar for both prefrontal and thalamic inputs (Fig. 6P).
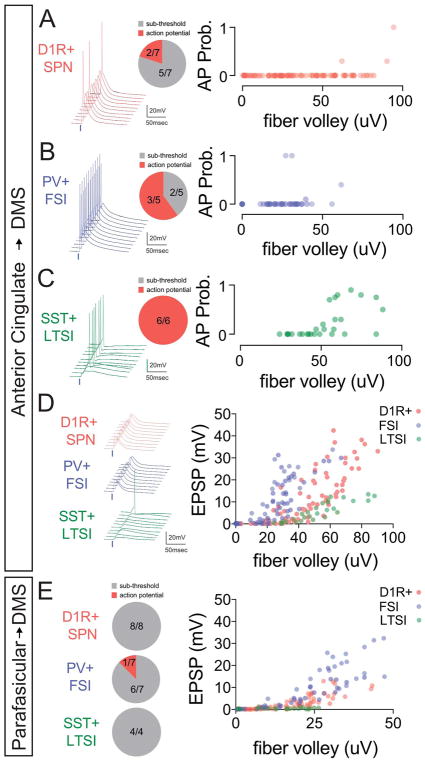
Synaptic current-action potential coupling
Recruitment of striatal neuron firing requires integration of synaptic drive with the electrical properties of the postsynaptic cell, which vary widely across the examined subtypes (Fig. 5C). To see how synaptic connectivity translated to neuronal spiking, we performed similar field-normalized measures while recording nearby neurons in current clamp (Fig. 7). While ACC connections to D1R+ SPNs and PV+ cells occasionally evoked action potentials (APs), the majority of responses were sub-threshold (Fig. 7A,B,D). In contrast, despite the weak synaptic connections of ACC-SST+ neurons, all recorded cells were driven to threshold and the reliability of AP-coupling was dynamic over the range of fiber volleys examined (Fig. 7C). Overall, the trajectory of target cell-specific sub-threshold responses roughly aligned with voltage clamp measures of synaptic strength (compare Fig. 7D with Fig. 5J). Consistent with the reduced density of PFas fibers projecting into the DMS (Fig. 3G & Fig. 6D), we were largely unable to drive spiking in any postsynaptic cell type (Fig. 7E). Notably, while the sub-threshold responses in D1R+ SPNs and FSIs were similar, the LTSIs recorded did not show any synaptic connectivity (Fig. 7E).
Figure 7.
(A–C, left) Representative current clamp traces recorded in each cell type. (A–C, middle) Pie chart depicting the fraction of recorded cells that exhibited AP firing upon optical stimulation of ACC fibers. (A–C, right) Probability of action potential generation for 10 consecutive optical stimuli across a range of fiber volleys (right). Representative traces of sub-threshold EPSP amplitude (D, left) and scatter plot of EPSP amplitude as a function of fiber volley (D, right). (E) The fraction of recorded cells that exhibited AP firing upon optical stimulation of PFas fibers. (left). Scatter plot of fiber volley versus EPSP amplitude for sub-threshold response to PFas stimulation (right).
Discussion
Projection Origin and Striatal Target Neuron are Associated with Specific Anatomical Connectivity Patterns
EnvA-pseudotyped RV tracing of DMS circuits produced distributions of labeled neurons consistent with non-cell type-specific approaches (Pan et al., 2010), including substantial OFC, ACC, M2, PFas and GPe labeling (Supp.Fig. 1). In addition to regional differences in the fractional number of retrogradely labeled cells, postsynaptic cell type was reproducibly associated with specific patterns of anatomical convergence, with PV+ tracings exhibiting 2–3× higher numbers of retrograde neurons per starter cell than D1R+ SPNs and SST+ neurons (compare Fig. 1G, Fig. 2&3). Given the preponderance of D1R+ SPNs compared to interneurons (~40% vs <1% of total striatal neurons), one would expect much higher anatomical convergence values for PV+ neurons if both cell types sampled the same number of ACC inputs. Nevertheless, it is important to note that anatomical convergence, defined here as the total number of retrogradely-labeled inputs per starter cell, should be considered the upper bound of this number. More accurate estimates should consider the number of common inputs to a given postsynaptic cell, which would change the density of inputs at the single-cell level (synaptic convergence) while maintaining a fixed anatomical convergence. In fact, a greater sharing of inputs may explain the similarity in single-neuron synapse strength between ACC-D1R+ and ACC-PV+ connections, despite the higher anatomical convergence onto PV+ interneurons (Fig. 5M).
The only brain regions exhibiting target cell-specific differences in labeling not conforming to PV+ biased convergence patterns were the mOFC and the PFas nucleus. In contrast to the PV+ bias of the lOFC, mOFC had the highest density of retrogradely labeled neurons from SST+ starter populations. This result is particularly intriguing given the proposed functional differences between medial and lateral orbital cortices for value processing and decision-making (Rushworth et al., 2011). Further work is necessary to explore whether different striatal inhibitory cell types may mediate these region-specific differences in reward processing.
Insights from Novel Field-Normalized Synaptic Measures
In order to relate synaptic connectivity with our RV-mediated tracing, we employed novel field-normalized input-output curves that reported synaptic measures as a function of increasing afferent recruitment. The channelrhodopsin-evoked optical waveform (“optical fiber volley”) was used as a proxy for the number of opsin-expressing fibers that were activated in proximity to our recording electrodes. We interpreted the stepwise increase in fiber volley amplitude with increasing LED intensity (Fig. 4E) as activation of afferent fibers with progressively lower levels of ChiEF expression, as opposed to enhancement in the probability of synaptic release (PR) of a fixed number of fibers. While the observed increase in Sr2+-evoked frequency could formally represent either component of quantal content, we favor changes in number of release sites because: (1) paired-pulse ratios within a given cell did not decrease with increasing LED intensity (not shown); (2) we did not observe the increase in event amplitude with increasing LED intensity that should be seen if PR was increasing at these multi-vesicular release synapses (Higley et al., 2009); Supp.Fig. 5); (3) we demonstrated a clear dissociation between paired-pulse ratio and Sr2+-event frequency for cortical and thalamic connections onto D1R+SPNs and PV+ FSIs (compare Fig. 5M,N with Fig. 6N,O).
Our results suggest several interrelated components work together to set the strength of projection synapses onto striatal circuits. First, the density of fibers from a specific projection nucleus will set lower limits on how effectively it can drive striatum. For example, PFas inputs represent ~7% of total inputs irrespective of postsynaptic target, while ACC inputs are closer to 15% of the total retrogradely labeled population (compare Sup.Fig. 2,3) These distinctions manifest physiologically as a reduction in the steepness of the LED intensity-fiber volley plots for PFas versus ACC injections, such that the maximum elicited fiber volley is ~40% lower in PFas slices (compare Fig. 5G,6G). These differences may in part account for the inability of PFas connections to drive postsynaptic firing as compared to ACC projections (Fig. 7D,E). Second, the density of incoming projections is modified by the synaptic convergence of these axons onto individual postsynaptic targets to determine final synaptic strength. To explore this, we used whole cell recorded currents and Sr2+-desynchronized quantal events to estimate how projection density is modified at the individual neuron level. For an equivalent fiber volley, larger postsynaptic currents or higher Sr2+-evoked EPSC frequencies were interpreted as more release sites contacting the recorded neuron. It should be noted that this physiological measure of synaptic convergence integrates both anatomical convergence and the number of shared inputs seen by a given postsynaptic cell (discussed below). Overall, this approach provides a quantitative means of comparing optogenetically recruited projection regions and is ideal for use with viral injection paradigms, where differences in infectivity can create significant between-animal variability.
Divergence between Anatomical and Physiological Connectivity
Our work suggests a substantial divergence between the anatomical connectivity “map” provided by cell type-specific RV tracing and the physiological characteristics of those connections. The most striking examples are seen for ACC and PFas connections to SST+ LTSI (Fig. 5H–J, Fig. 6H–J). In the case of corticostriatal synapses, SST+ neurons display similar fractional distributions and convergence values as D1R+ SPNs, but they exhibit dramatically reduced synaptic strength, which results from multiple factors including reduced PR (Fig. 5N), fewer release sites (Fig. 5M) and reduced postsynaptic sensitivity (Supp.Fig. 5A). Nevertheless, these distinct synaptic connectivity measures are transformed by the intrinsic properties of the target cell, such that small synaptic currents on SST+ cells resulted in consistent postsynaptic spiking (Fig. 6K,7C) whereas the larger D1R+ SPN currents typically did not (Fig. 7A). A similar result was found for PFas projections to SST+ LTSIs, which despite similar fractional distributions and higher convergence than D1R+ SPNs, exhibited dramatically weaker synaptic strength (Fig. 6J). In contrast to prefrontal connections however, the overall synaptic connectivity of PFas-SST+ connections was sparse, with only ~1/3 of neurons showing detectable synaptic currents (Fig. 6K). These results are consistent with recent studies of the excitatory-inhibitory control of LTSI activity by PFas projections (Assous et al., 2017), but conflict with other anatomical and optogenetic data (Kachidian et al., 1996; Ellender et al., 2013). The mechanistic basis of these anatomical-physiological discrepancies is currently unclear but could relate to the presence of silent synapses or a sparse distribution of connectivity due to lower density of striatal-projecting PFas axons or target specificity within patch-matrix compartments (Herkenham & Pert, 1981; Deschenes et al., 1995).
Another interesting discrepancy between anatomical and physiological data can be seen in comparisons between D1R+ SPNs and PV+ FSIs. For both cortical and thalamic projections, the two-fold higher convergence measure of PV+ interneurons as compared to D1R+ SPNs is either accompanied by no change (ACC, Fig. 5J) or a paradoxical decrease in whole cell-recorded evoked currents (PFas, Fig. 6J). One possible explanation is that D1R+ SPNs exhibit greater sharing of projection neuron inputs than PV+ interneurons. In a typical situation with multiple starter cells, sharing of multiple projection neuron inputs would leave the anatomical convergence measure unchanged while dramatically increasing the number of release sites on a given postsynaptic cell. This interesting circuit arrangement suggests that D1R+ SPNs may sample inputs from a wider population of projection neurons than PV+ FSIs, a hypothesis that could be tested via clonal starter cell tracing experiments. For all comparisons between anatomical and synaptic measures, it is important to note that viral expression of channelrhodopsin certainly labeled a larger, less selective cohort of neurons than were retrogradely traced via RV. Nevertheless, we feel that whole cell recordings under conditions of local axonal recruitment, pharmacological blockade of all GABAergic currents and temporally isolated individual pulses, are likely to probe similar projection neuron-postsynaptic neuron connections as those seen with anatomical tracing.
Implications for Striatal Microcircuit Control
This work demonstrates a complex picture of projection neuronal connectivity within the local striatal network. It shows that region of projection neuron origin dictates the density of striatal inputs, and postsynaptic target-cell is associated with characteristic anatomical convergence patterns. Nevertheless, the synaptic connections of striatal circuits are highly heterogeneous with regard to both presynaptic and postsynaptic characteristics, and these differences are not reflected by RV-mediated circuit tracing. By comparing RV anatomical connectivity with physiological connectivity for two major inputs, we observed basic organizational principles for striatal circuits: (1) site of projection neuron origin determines density of projections in striatum irrespective of target-cell; (2) postsynaptic target heavily influences anatomical convergence and postsynaptic receptor subtype content and (3) release site density and release probability are unique to particular pre-post pairings. This combined anatomical-physiological approach further clarifies connectivity into striatum and provides a foundation for work into the assembly and mature function of this region.
Supplementary Material
Acknowledgments
We would like to thank Sarah Seyedroudbari, Ryan McConnell, Vedika Gopal, Andrew Furash and Ning Yue for technical help. We would also like to thank Ethan Goldberg and Patrick Rothwell for comments on the manuscript. This work was supported by grants from the NIMH (R00-MH099243 to M.V.F.; F32-MH-114506 to E.H.), the Whitehall Foundation (M.V.F.), the Intellectual & Developmental Disabilities Research Center (M.V.F.) and the Cognitive & Behavioral Neuroscience Training Grant (T32-MH017168 to F.D.).
Abbreviations
- DMS
Dorsomedial striatum
- D1R+
D1 receptor positive spiny projection neurons
- D2R+
D2 receptor positive spiny projection neurons
- ChINs
Cholinergic interneurons
- GABA
Gamma amino-butyric acid
- SPN
spiny neuron
- PV
Parvalbumin
- FSI
fast-spiking interneurons
- SST
Somatostatin
- LTSI
low-threshold spiking interneurons
- Cre
Cre recombinase
- AAV
Adeno-associated virus
- BAC
Bacterial artificial chromosome
- ACC
Anterior cingulate cortex
- PFas
Parafascicular nucleus
- OFC
Orbitofrontal cortex
- GPe
Globus Pallidus external segment
- aEPSC
asynchronous excitatory post synaptic currents
- NR2B
NMDA receptor subtype B
- AP
Action potential
Footnotes
Competing interest
The authors declare that they have no competing interests.
Author contributions
M.F. conceived and supervised the project and wrote the article. K.C, F.D performed the electrophysiology experiments and analyzed the results. E.H performed the retrograde tracing and analyzed the results. K.C and E.H designed experiments and wrote the article. K.T.B. contributed key reagents. All authors read and approved the final manuscript.
Data Accessibility
The original data for these experiments can be obtained by contacting the corresponding author.
References
- Assous M, Kaminer J, Shah F, Garg A, Koos T, Tepper JM. Differential processing of thalamic information via distinct striatal interneuron circuits. Nat Commun. 2017;8:15860. doi: 10.1038/ncomms15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behavioural Brain Research. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res. 1995;701:288–292. doi: 10.1016/0006-8993(95)01124-3. [DOI] [PubMed] [Google Scholar]
- Do JP, Xu M, Lee SH, Chang WC, Zhang S, Chung S, Yung TJ, Fan JL, Miyamichi K, Luo L, Dan Y. Cell type-specific long-range connections of basal forebrain circuit. Elife. 2016:5. doi: 10.7554/eLife.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender TJ, Harwood J, Kosillo P, Capogna M, Bolam JP. Heterogeneous properties of central lateral and parafascicular thalamic synapses in the striatum. J Physiol. 2013;591:257–272. doi: 10.1113/jphysiol.2012.245233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo MV, Foldy C, Gokce O, Rothwell PE, Sun GL, Malenka RC, Sudhof TC. Single-Cell mRNA Profiling Reveals Cell-Type-Specific Expression of Neurexin Isoforms. Neuron. 2015;87:326–340. doi: 10.1016/j.neuron.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. Journal of Neuroscience. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L, Luo M. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PloS one. 2015;10:e0123381. doi: 10.1371/journal.pone.0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nature neuroscience. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife. 2016:5. doi: 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachidian P, Vuillet J, Nieoullon A, Lafaille G, Kerkerian-Le Goff L. Striatal neuropeptide Y neurones are not a target for thalamic afferent fibres. Neuroreport. 1996;7:1665–1669. doi: 10.1097/00001756-199607080-00028. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neuroscience Research. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends in neurosciences. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: their morphological types and populations. Brain Research. 1988;447:346–352. doi: 10.1016/0006-8993(88)91138-9. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Holley SM, Shobe JL, Chong NC, Cepeda C, Levine MS, Masmanidis SC. Parvalbumin Interneurons Modulate Striatal Output and Enhance Performance during Associative Learning. Neuron. 2017;93:1451–1463. e1454. doi: 10.1016/j.neuron.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa SK, Watabe-Uchida M. Organization of dopamine and serotonin system: Anatomical and functional mapping of monosynaptic inputs using rabies virus. Pharmacol Biochem Behav. 2017 doi: 10.1016/j.pbb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Pan WX, Mao T, Dudman JT. Inputs to the Dorsal Striatum of the Mouse Reflect the Parallel Circuit Architecture of the Forebrain. Frontiers in Neuroanatomy. 2010:4. doi: 10.3389/fnana.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, Tamamaki N, Tanahira C, Taylor JR, Pittenger C. Targeted Interneuron Depletion in the Dorsal Striatum Produces Autism-like Behavioral Abnormalities in Male but Not Female Mice. Biological psychiatry. 2017;82:194–203. doi: 10.1016/j.biopsych.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Ge M, Christie MJ, Balleine BW. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci. 2014;34:9196–9201. doi: 10.1523/JNEUROSCI.0313-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Saulnier JL, Begue A, Feng DD, Huang KW, Sabatini BL. Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron. 2016;92:84–92. doi: 10.1016/j.neuron.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nature neuroscience. 2012;15:1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends in neurosciences. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and Diversity of Striatal GABAergic Interneurons. Frontiers in Neuroanatomy. 2010:4. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends in neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proceedings of the National Academy of Sciences. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Sullivan HA, Seung HS. Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nature Protocols. 2010;5:595–606. doi: 10.1038/nprot.2009.248. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Znamenskiy P, Zador AM. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature. 2015;521:348–351. doi: 10.1038/nature14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Presynaptic strontium dynamics and synaptic transmission. Biophys J. 1999;76:2029–2042. doi: 10.1016/S0006-3495(99)77360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.