Abstract
Recent structural and functional studies of the synaptic vesicle fusion machinery suggest an inhibited tripartite complex consisting of neuronal SNAREs, synaptotagmin, and complexin prior to Ca2+-triggered synaptic vesicle fusion. We speculate that Ca2+-triggered fusion commences with the release of inhibition by Ca2+ binding to synaptotagmin C2 domains. Subsequently, fusion is assisted by SNARE complex zippering and by active membrane remodeling properties of synaptotagmin. This additional, inhibitory role of synaptotagmin may be a general principle since other recent studies suggest that Ca2+ binding to extended synaptotagmin C2 domains enables lipid transport by releasing an inhibited state of the system, and that Munc13 may nominally be in an inhibited state which is released upon Ca2+ binding to one of its C2 domains.
Keywords: synaptic vesicle fusion, fusion protein, action potential, synaptic vesicle priming, Ca2+-triggering
Synaptic Transmission and Calcium Triggering
Synaptic transmission between pre- and post-synaptic neurons occurs when the pre-synaptic neuron terminal is temporarily depolarized upon an action potential, opening Ca2+ channels near the active zones of synapses. Since the extracellular Ca2+ concentration is much higher than the cytoplasmic concentration, Ca2+ will flow into the cytoplasm. In turn, Ca2+ will trigger fusion of neurotransmitter-filled synaptic vesicles with the presynaptic membrane in less than a millisecond [1,2]. Upon fusion, neurotransmitter molecules are released into the synaptic cleft, and then bind to receptors that are located in the postsynaptic membrane.
Many, if not most, of the key factors of the core synaptic fusion machinery have been identified, including fusogenic SNAREs (Soluble N-ethylmaleimide sensitive factor Attachment protein REceptor), the Ca2+-sensor synaptotagmin, the activator/regulator complexin, the assembly factors Munc18 (mammalian uncoordinated-18), Munc13 (mammalian uncoordinated-13), and the disassembly factors NSF (N-ethylmaleimide-sensitive factor) and SNAP (soluble NSF adaptor protein). Yet, the molecular mechanisms of Ca2+-triggering, regulation, and membrane fusion are still unclear. Central to these questions is the role of synaptotagmin, which in the past has been primarily viewed as an activating factor upon Ca2+-binding, for example, by bending membranes [3–6] or bridging membranes [7–9]. However, such an activating role does not explain the effect of certain dominant negative mutants of synaptotagmin-1 that abolish evoked release in the background of endogenous wildtype synaptotagmin-1 [10–12]. We note that genetic deletion of synaptotagmin increased the frequency of spontaneous release in flies [13,14], and a similar phenotype was observed upon deletion of synaptotagmin-1 in mouse neurons [15]. However, expression of a dominant negative synaptotagmin-1 mutant also increased spontaneous release in mouse neurons in a Ca2+-dependent fashion [16], suggesting that a Ca2+-sensor other than synaptotagmin-1 is important for spontaneous release. Since the molecular mechanisms of spontaneous release are less certain at this time, we primarily discuss Ca2+-triggered neurotransmitter release in this review. We lay out the rationale for a new model where Ca2+-triggered synaptic vesicle fusion begins with release of inhibition upon Ca2+ binding to synaptotagmin-1 [16]. After release of inhibition, the activating properties of synaptotagmin-1 likely further assist the fusion process.
Key players for synaptic vesicle fusion
Below, we briefly summarize the key players in synaptic vesicle fusion. For more details the reader is referred to recent in-depth reviews [17,18]. Prior to membrane fusion, synaptobrevin-2 (also called VAMP2 – Vesicle Associated Membrane Protein 2) on the synaptic vesicle, and syntaxin-1A and SNAP-25A on the plasma membrane initially form a ternary SNARE complex (see Glossary) with the transmembrane domains of synaptobrevin-2 and syntaxin-1A (Figure 1A) in their respective membranes. This complex is also often referred to as the trans SNARE complex (see Glossary). The energy for membrane fusion is likely provided by zippering (see Glossary) the trans SNARE complex into the fully assembled cis SNARE complex (see Glossary), a parallel four α-helix bundle [19,20]. This notion is supported by the relative equivalence of energy estimates derived from pulling SNARE complexes apart [21] and estimates of the energy required in connecting the outer leaflets of the membranes, in other words, formation of so-called lipid membrane stalks [22].
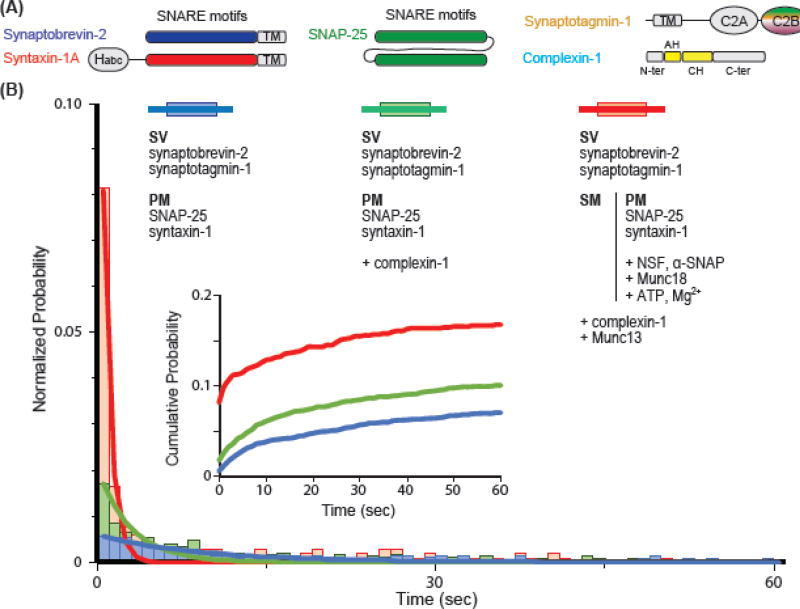
Figure 1. More complete reconstitution improves fusion probability in vitro.
(A) Domain diagrams of SNAREs, synaptotagmin-1, and complexin-1.
(B) Histogram of vesicle fusion time upon entry of 500 µM Ca2+ (at time = 0 sec), as measured in a single-vesicle fusion assay [50,59]. The inset shows the corresponding cumulative probability histogram. Synthetic proteoliposomes that mimic the synaptic vesicle (SV) are reconstituted with synaptobrevin-2 and synaptotagmin-1, while plasma membrane-mimic proteoliposomes (PM) are reconstituted with syntaxin-1 and SNAP-25. The histograms were normalized by the number of associated (docked) vesicle pairs. Alone, this simple reconstitution (blue) produces only ~0.5 % fusing vesicle pairs immediately upon Ca2+ entry. Addition of complexin-1 to this fusion system (green) increases both the number of fusion-competent vesicle pairs as well as the fraction of vesicles that fuse immediately upon Ca2+ entry. In the more complete reconstitution system (red) NSF, α-SNAP and Munc18 are incubated with PM vesicles in the presence of ATP and Mg2+ to disassemble pre-formed binary (syntaxin-1/SNAP-25) SNARE complexes and allowing Munc18 to trap free syntaxin-1 (we refer to these syntaxin-Munc18 vesicles as SM vesicles). Subsequent addition of SNAP-25, Munc13, and complexin-1 with the SV vesicles greatly enhances the total number of fusion competent vesicle pairs as well as the number of vesicles that fuse immediately upon calcium addition. Data are taken from [50,59]. SV: synaptic vesicle mimicking proteoliposome; PM: plasma membrane mimicking proteoliposome; TM: transmembrane domain.
Since SNARE complex assembly is independent of Ca2+, a Ca2+-sensor, such as synaptotagmin, is required for all forms of Ca2+-triggered neurotransmitter release. Synaptotagmins comprise an evolutionary conserved family of Ca2+-sensors composed of an N-terminal single transmembrane-spanning domain, a variable juxtamembrane linker, and two C-terminal cytoplasmic C2 domains, termed C2A and C2B, respectively (Figure 1A) [23]. Genetic deletion of synaptotagmin decreased evoked release and increased spontaneous release [13–15,24]. Moreover, Ca2+-dependent binding to membranes and the Ca2+-sensitivity of neurotransmitter release are correlated, in other words, mutations that reduced Ca2+-dependent binding also reduced the Ca2+-sensitivity and vice versa [25,26]. Synaptotagmin C2 domains may act as electrostatic switches where Ca2+-binding neutralizes the negative charges within both the protein and plasma membrane surface, resulting in membrane binding, insertion or SNARE binding [27–29]. A subset of the synaptotagmin isoforms have been implicated in Ca2+-triggered synaptic vesicle fusion [30]. In this review we focus on synaptotagmin-1, which resides on the synaptic vesicle and is essential for synchronous Ca2+-triggered synaptic vesicle fusion [24,25]. Under certain experimental in vitro conditions in the presence of Ca2+, the synaptotagmin-1 C2B domain or a C2A-C2B fragment may bind to curved membranes or even induce deformation of the membrane [4–6], which may assist the fusion process [3]. Furthermore, they may stimulate membrane juxtaposition or bridging [7–9].
The cytoplasmic protein complexin (we focus on complexin-1 in this review) also plays critical roles in neurotransmitter release [31,32]. Specifically, synchronous evoked neurotransmitter release depends on complexin-1 [33], and this activating role of complexin-1 is conserved across all species and the different types of Ca2+-induced exocytosis studied to date [34–43]. Complexin-1 also regulates spontaneous release, although this effect is less conserved among species and experimental conditions [37,40,41,44].
Additionally, Sec1/Munc18 (SM) proteins are required components of all membrane trafficking pathways, as exemplified by the complete block of synaptic vesicle fusion upon Munc18-1 knockout in mice [45]. At the molecular level, Munc18 captures free syntaxin, locking it into a heterodimeric complex that kinetically prevents formation of the ternary SNARE complex [46]. To enable ternary SNARE complex formation, another factor is required. This protein, Munc13, is a primarily brain-specific, cytoplasmic protein in the presynaptic terminal that is implicated in synaptic vesicle priming and short-term synaptic plasticity [1,47]. At the molecular level, Munc13 accomplishes two tasks: (1) catalyzing the transit of syntaxin from the syntaxin/Munc18 complex into the ternary SNARE complex [46,48,49] and (2) promoting proper assembly of the SNARE complex in conjunction with Munc18, i.e., ensuring the parallel configuration of all components of the SNARE complex [50]. In vivo, Munc13 and Munc18 are required to promote proper SNARE complex assembly since genetic deletion of Munc13 can only be partially rescued with a mutant of syntaxin that bypasses the Munc13 requirement in vitro [50,51]. In addition, Munc18 and Munc13 have been implicated in preventing disassembly of the primed trans SNARE complex by NSF and αSNAP [52] and Munc13 may be involved in vesicle tethering [53] although the molecular mechanisms of these potential additional roles remain to be elucidated.
After fusion, in concert with the adaptor protein, SNAP, the ATPase NSF disassembles the ternary cis SNARE complex into individual proteins upon ATP hydrolysis [54–56]. NSF is a member of the so-called AAA+ (ATPases associated with diverse cellular activities) family consisting of two ATPase rings, and an N-terminal domain. For more structural details of NSF and SNAPs we refer to a recent review [17].
While SNAREs and synaptotagmin-1 can promote full fusion of synthetic liposomes with reconstituted proteins (called proteoliposomes) upon Ca2+-triggering [57,58] with a ratio of Ca2+-dependent fusion to Ca2+-independent fusion of ~ 10 [59], Ca2+-triggered fusion with this minimal system is relatively inefficient (for a review of this and other in vitro fusion assays see [60]). Moreover, Ca2+-triggered fusion is inefficient between docked proteoliposomes and supported bilayers when only synaptotagmin-1 and SNAREs are reconstituted [61]. The situation is greatly improved when additional synaptic fusion proteins are included (Figure 1B). For example, in conjunction with neuronal SNAREs and synaptotagmin-1, complexin-1 increases the Ca2+-triggered amplitude (2.5-fold increase) and synchrony of proteoliposome fusion, and it also suppresses Ca2+-independent single-vesicle fusion (content mixing) [59], resulting in a 50–100-fold enhancement of the probability ratio of Ca2+-triggered fusion to Ca2+-independent fusion. Consistent with their molecular functions, inclusion of both Munc18 and Munc13 further increases the Ca2+-triggered amplitude of single-vesicle content mixing (Figure 1B), the Ca2+-triggered to Ca2+-independent fusion ratio to ~ 400, and the Ca2+ sensitivity to 20 µM in a more complete reconstituted system consisting of SNAREs, synaptotagmin-1, complexin-1, NSF, and αSNAP [50]. Interestingly, inclusion of Munc13 in a reconstitution of single dense-core vesicle fusion with a planar supported bilayer also greatly improved the Ca2+-triggered fusion probability in that system [62].
Together, Munc18, Munc13, NSF, and αSNAP, can be viewed as an assembly and quality control system that ensures proper assembly of fusogenic trans SNARE complexes [46,50]. Such complexes may represent the fulcrum of the primed state of the system as discussed below. Although this system is absolutely essential for efficient neurotransmitter release, it may primarily act upstream from the fusion process; this observation is supported by reconstitution experiments in which Munc13 was removed after Munc18/Munc13 assisted assembly of the synaptic fusion complex and no effect on the Ca2+-triggered fusion amplitude was found [50]. Clearly, this hypothesis needs further testing, but for simplicity we focus on SNAREs, synaptotagmin-1, and complexin-1 below.
Pairwise SNARE/synaptotagmin-1 and SNARE/complexin-1 interactions
Structural studies have proven invaluable in revealing interactions between SNAREs, synaptotagmin-1, and complexin-1. An X-ray crystal structure (see Glossary) of the complex between the neuronal SNARE complex and complexin-1 identified a primary pairwise interaction between these molecules [63] (Figure 2A). Crystal structures of the complex of the neuronal SNARE complex and synaptotagmin-1 [3] revealed pairwise interactions between synaptotagmin-1 and the SNARE complex (of these, the “primary” interface is shown in Figure 2B).
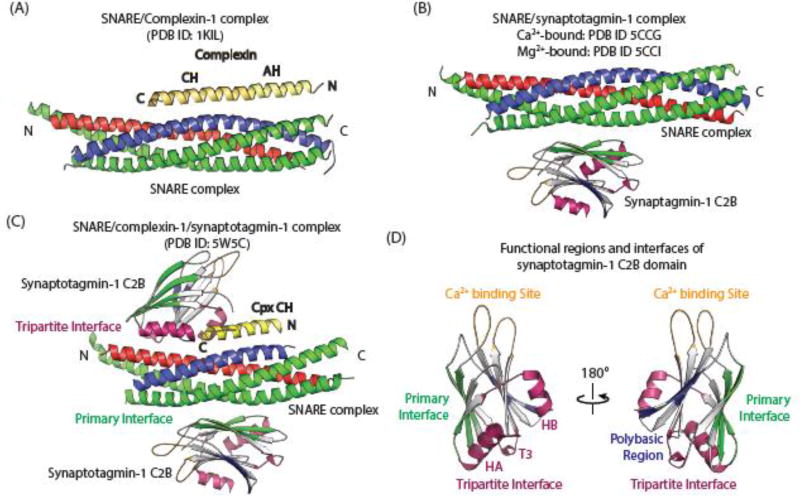
Figure 2. Atomic-resolution structures of SNARE/complexin-1, SNARE/synaptotagmin-1, and SNARE/complexin-1/synaptotagmin-1 complexes.
(A) Crystal structure of the pairwise complex between complexin-1 (yellow) and the SNARE complex (synaptobrevin-2, blue; SNAP-25, green, syntaxin-1A, red) [63] (PBD ID 1KIL). (B) Superposition of the Ca2+ and Mg2+-bound crystal structures of the pairwise complex between the SNARE complex (synaptobrevin-2, blue; SNAP-25, green, syntaxin-1A, red), and synaptotagmin-1 C2B (gray, green, purple, blue, and gold) [3] (PDB IDs 5CCG and 5CCI). For clarity, only the primary C2B-SNARE interface is shown. (C) Crystal structure of the Ca2+-free tripartite complex between the half-zippered SNARE complex (synaptobrevin-2, blue; SNAP-25, green, syntaxin-1A, red), complexin-1 (yellow), and synaptotagmin-1 C2B (gray, green, purple, blue, and gold) [16] (PDB ID 5W5C). (D) Functional regions and interfaces of the synaptotagmin-1 C2B domain. The colors indicate the loops involved in Ca2+-binding (gold), the primary SNARE-synaptotagmin-1 interface (green), the tripartite SNARE/complexin-1/synaptotagmin-1 interface (purple), and the polybasic region (blue).
In general, interactions between macromolecules observed in crystal structures represent probable interactions between molecules, as crystallization selects from a subset of the energetically most probable interactions of the molecules in the crystallization solution. Nevertheless, intra- or inter-molecular interactions observed in the crystal structure may or may not be physiologically relevant [64–66]. A reasonably large interface area, relatively low B-factors (see Glossary) for the interacting residues, and sequence conservation of the interacting residues are indicative of—but not necessarily sufficient to define—a functionally important interface. All these three criteria are satisfied for the SNARE/complexin-1 interface that primarily involves the central α-helix of complexin-1 (Figure 2A) [63] and for the “primary” SNARE/synaptotagmin-1 interface (i.e., the one with the largest interface area, Figure 2B) [3]. In addition, both pairwise interfaces have been observed in very different crystal packing environments [3,16,63,67].
Most importantly, selected residues involved in both these particular pairwise interfaces have been functionally tested. For example, a mutant of complexin-1 that disrupts binding of the complexin-1 central α-helix to the ternary SNARE complex neither rescued deletion of wildtype complexin-1 in neuronal cultures [37], nor increased the Ca2+-triggered amplitude in single-vesicle fusion experiments with reconstituted SNAREs and synaptotagmin-1 [59]. Disruption of the primary SNARE/synaptotagmin-1 complex interface by mutation abolished fast synchronous release, reduced the size of the readily releasable pool (RRP, see Glossary), increased the frequency of spontaneous release in cultured neurons, and also greatly reduced the Ca2+-triggered amplitude in single-vesicle fusion experiments [3,68]. The primary interface is conserved among synaptotagmins (synaptotagmin-1, −2, −9) that are known to be involved in fast synchronous release and among neuronal SNAREs (see sequence alignments in ref. [3]).
In addition to the primary SNARE/synaptotagmin-1 interface [3], other pairwise interactions between the SNARE complex and synaptotagmin-1 C2B have been observed in vitro by single molecule Förster resonance energy transfer (FRET, see Glossary) and solution nuclear magnetic resonance spectroscopy (NMR, see Glossary) studies [69,70]. However, the data from either method were insufficient to reveal a high-resolution structure of these other interfaces, as single molecule FRET data were too sparse to derive high-resolution structures, and the highly dynamic character of the NMR data also prevented the determination of high-resolution structures of the most populated states. In addition, other small pairwise interfaces between the SNARE complexes and synaptotagmin-1 C2 domains have been observed in crystal structures [3,16], but their potential functional importance has not been tested.
Synaptotagmins also interact with anionic membranes in both the presence and absence of Ca2+ [71]. In the absence of Ca2+, the polybasic region (Figure 2D) of synaptotagmin-1 C2B primarily interacts with the membrane, and this membrane interaction stabilizes the synaptotagmin-1/SNARE primary interface [72]. However, polyvalent ions, such as ATP and Mg2+, may interfere with certain interactions between synaptotagmin-1 and the SNARE complex [73]. Specifically, polyvalent ions disrupt interactions between the polybasic region of synaptotagmin-1 C2B, but they do not disrupt the primary interface [72]. Moreover, in vitro Ca2+-triggered fusion experiments showed little difference in absence and presence of 3 mM ATP [3]. Thus, while some in vitro interactions between synaptotagmin-1 and SNAREs are disrupted by electrostatic shielding, in particular those involving the polybasic region, the primary interface is unaffected by polyvalent ions. We will therefore not consider interactions between the polybasic region of synaptotagmin-1 C2B and SNAREs in models of primed synaptic complexes that are presented below.
In addition to the interaction between the central α-helix of complexin-1 and the SNARE complex, an additional SNARE/complexin-1 interface was observed in a crystal structure between a truncated SNARE complex and complexin-1 with a mutation in the accessory α-helix (Figure 1A) [67]. Although a weak interaction involving the accessory α-helix was observed by isothermal titration calorimetry (ITC, see Glossary) for the wildtype protein [74,75] it may not necessarily correspond to the crystal structure with mutated complexin-1, and it was not observed by NMR [44]. These differences between the NMR and ITC results are probably due to differences in the length of the syntaxin constructs used, as a few residues at the C-terminal end of syntaxin appear to have a substantial effect on binding [74,75]. Functionally, the accessory α-helix of complexin-1 was not required for Ca2+-triggered single-vesicle fusion with reconstituted neuronal SNAREs, complexin-1, and synaptotagmin-1 [76], and mutations of the accessory α-helix had little or no effect on evoked release in neurons [44,77]. However, certain mutations of the complexin-1 accessory α-helix affected spontaneous release [44,77,78], although these studies differed in the effect of some of the mutations of the complexin-1 accessory α-helix. Moreover, elimination of the accessory domain all together increased Ca2+-independent single-vesicle fusion (content mixing) [76] compared to wild-type control. In this context, single-molecule experiments showed that the accessory α-helix weakly interacts with the binary t-SNARE complex (syntaxin-1A/SNAP-25A complex, see Glossary) [79], although it remains to be tested if this particular interaction is relevant for regulation of spontaneous release. In any case, since the accessory α-helix is largely expendable for evoked neurotransmitter release in neurons [44,77,78] and for in vitro Ca2+-triggered synaptic vesicle fusion [76], we will not consider it in models of primed synaptic complexes that are presented below.
Tripartite SNARE/complexin-1/synaptotagmin-1 complex
The pairwise SNARE/synaptotagmin-1 and SNARE/complexin-1 interactions alone do not explain certain experimental results. For example, mutation of the Ca2+-binding region of the C2B domain of synaptotagmin-1 has dominant negative effects on both evoked and spontaneous neurotransmitter release, in other words, expression of mutant synaptotagmin-1 reduces evoked release and up-regulates spontaneous release in the background of endogenous wildtype synaptotagmin-1 [10–12] while complexin knockdown abrogates these dominant negative phenotypes [16], suggesting an important mode of cooperation between SNAREs, complexin-1, and synaptotagmin-1. The recent crystal structure of the tripartite SNARE/complexin-1/synaptotagmin-1 complex (Figure 2C) [16] now provides a possible explanation for these experimental results since it revealed an unprecedented interface between one synaptotagmin-1 C2B domain and both the SNARE complex and complexin-1 [16]. Simultaneously, a second synaptotagmin-1 C2B domain interacts with the other side of the SNARE complex via the above-mentioned SNARE/synaptotagmin-1 primary interface. Structure-guided mutagenesis, solution-binding studies by ITC, and electrophysiological recordings showed that both Ca2+-triggered synaptic release and suppression of spontaneous release depend on synaptotagmin-1 C2B residues involved in both the SNARE/complexin-1/synaptotagmin-1 tripartite and the SNARE/synaptotagmin-1 primary interfaces [16]. Moreover, mutations in the synaptotagmin C2B domain that disrupt binding in vitro (as assessed by ITC) also abolished the readily-releasable pool of synaptic vesicles, i.e., the primed state of synaptic vesicles is sensitive to these interacting residues. Both interfaces map to distinct regions on the surface of the synaptotagmin-1 C2B domain (Figure 2D), and they are separate from the Ca2+-binding site and the polybasic region implied in membrane interactions [71].
For the tripartite interface, the synaptotagmin-1 C2B domain binds to the SNARE/complexin-1 subcomplex via interactions with both the SNARE and complexin-1 components (Figure 2C). Among the most striking structural features of this tripartite interface is the continuation of the complexin-1 central α-helix into the α-helix HA (Figure 2D) of synaptotagmin-1. Since the α-helix HA is structurally conserved in C2B domains of all synaptotagmins, Doc2s, and Rabphilin, but not present in the Munc13-1 C2B domain and synaptotagmin C2A domains (see structural and sequence alignments in ref. [16]), the SNARE/complexin-1/synaptotagmin-1 tripartite interface may be more general, with different types of synaptotagmin-regulated exocytosis being mediated by similar complexin-1-dependent fusion mechanisms (synaptotagmin-1, −2, −7, −9, and −10) [43,80–82]. It is thus conceivable that all these synaptotagmins could participate in a tripartite interface while only the subset of synaptotagmin molecules involved in synchronous neurotransmitter release (synaptotagmin-1, −2, or −9) may utilize the primary interface based on primary sequence conservation.
The tripartite interface involves the central α-helix, but not the accessory α-helix of complexin-1 or any other parts of complexin-1, consistent with the above mentioned observations that the accessory α-helix is largely expendable for evoked neurotransmitter release in neurons [44,77,78] and for Ca2+-triggered vesicle fusion in vitro [76]. On the other hand, the N-terminal domain of complexin-1 is important for activation of synchronous Ca2+-triggered release [37,83–85], and it increases the Ca2+-triggered amplitude in single-vesicle fusion (content mixing) experiments when added as an independent fragment in addition to the complexin-1 central α-helix[76]. The complexin-1 N-terminal domain may thus independently interact with the juxtamembrane SNARE domains of the splayed open trans SNARE complex [79,84]. Note that the membrane proximal parts of the trans SNARE complex were not included in the crystal structure of the SNARE/complexin-1/synaptotagmin-1 complex, possibly explaining why the complexin-1 N-terminal domain was not visible in that crystal structure.
Supramolecular arrangements of synaptic fusion complexes
The structure of the SNARE/complexin-1/synaptotagmin-1 complex likely represents the primed pre-fusion state of this tripartite complex since the RRP of synaptic vesicles depends on residues involved in both interfaces [16]. There are likely two or more synaptic complexes involved in Ca2+-triggered fusion [86,87]. In principle, these complexes could be arranged independently from each other around a contact site between synaptic and plasma membranes in star-like fashion, or they could interact with each other. Moreover, synaptotagmins are capable of forming ring-like homo-oligomeric assemblies on synthetic membranes, although the physiological relevance of such assemblies remains to be established [88].
Cryo-electron tomography (cryo-ET, see Glossary) of slices of unstained, vitrified frozen-hydrated mouse synapses [89], or in cryo-ET thin sections of high-pressure frozen hippocampal neuronal cultures [90] only provided very low resolution images that make it difficult to identify individual synaptic proteins or their complexes. Recent work with reconstituted systems does provide clues as to what kinds of structures might exist between membranes. Cryo-ET images and corresponding tomograms of functionally active proteoliposomes with neuronal SNAREs, synaptotagmin-1, complexin-1, and Munc13 revealed contacts with a variety of morphologies between the vesicle membranes with a preference for relatively compact point contacts [91]. Although the resolution of these tomograms is still relatively low, the observed contacts are likely proteinaceous as connected density spans inter-membrane distances of 40–60 Å [91].
At that separation, lipids alone would be unable to bridge the membranes: for example, the critical distance at which lipid stalks form is < 9 Å [22]. Volumetric analysis suggested that the most compact point contacts can accommodate approximately two SNARE/complexin-1/synaptotagmin-1 complexes while the larger contacts can accommodate even more complexes [91]. The larger contacts may represent higher order oligomeric, but probably asymmetric, assemblies. We speculate that multiple membrane contact morphologies co-exist in the neuron and that they are relevant in different contexts (e.g., fast synchronous release, asynchronous release, and spontaneous release [92]). Taken together, we propose that the SNARE/complexin-1/synaptotagmin-1 complexes form protein “stalks” that juxtapose the membranes, keeping them far enough away to reduce the chances of membrane fusion, in other words, these complexes inhibit fusion and set the stage for Ca2+-triggering.
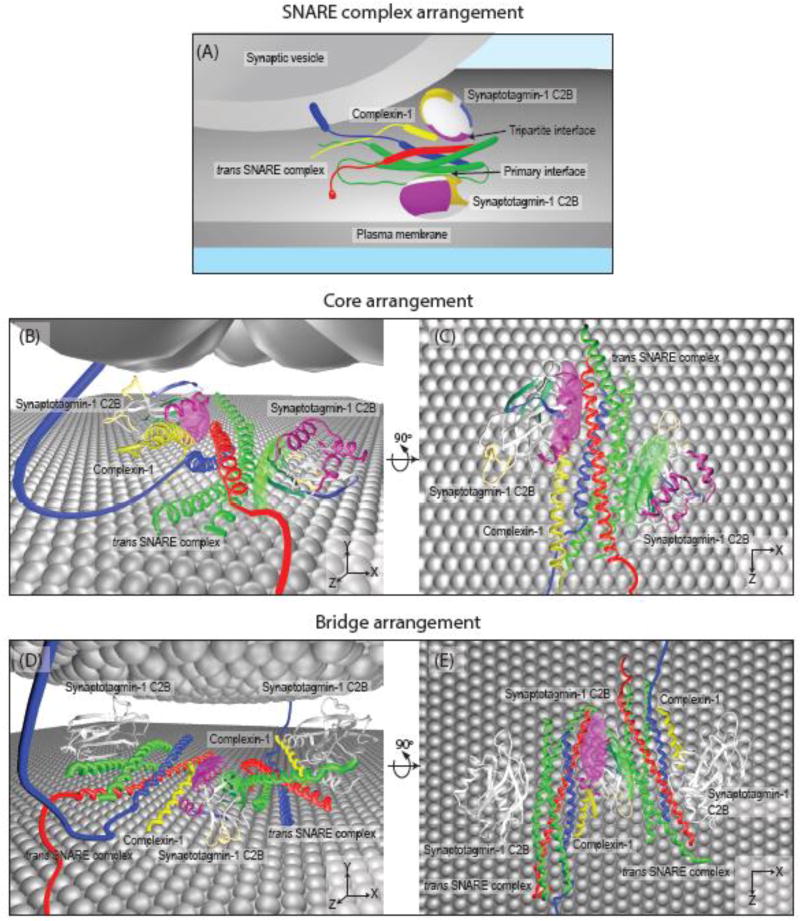
Regardless of the overall organization and number of multiple synaptic complexes, the crystal structure of the SNARE/complexin-1/synaptotagmin-1 complex and the existing cryo-ET studies are compatible with several quaternary arrangements, two of which are shown in Figure 3. In one proposed “core” arrangement, one SNARE complex interacts with two synaptotagmin-1 C2B domains, via the primary and the tripartite interfaces of the two separate C2B domains (Figure 3B,C, and Supplementary Video 1) (corresponding to Figure 1 in ref. [16]). Multiple complexes could be arranged in a starlike fashion. Alternatively, one C2B domain could “bridge” two SNARE complexes, again via the primary and tripartite interfaces of the same C2B domain (Figure 3D,E, and Supplementary Video 2) (corresponding to Extended Data Figure 9 in ref. [16]) and two additional C2B domains may interact with this unit. Again, multiple such bridge SNARE/complexin-1/synaptotagmin-1 quaternary arrangements could be situated around the membrane contact site. Moreover, the presence of the membranes as well as their composition might induce a conformational change of the SNARE/complexin-1/synaptotagmin-1 complex.
Figure 3. Models of quaternary arrangements of the SNARE/complexin-1/synaptotagmin-1 pre-fusion complex.
(A) Schema of the trans SNARE complex interacting with two synaptotagmin-1 C2B domains and the central α-helix of complexin-1 in the core quaternary arrangement. The trans SNARE complex consists of synaptobrevin-2 (blue), syntaxin-1 (red) and SNAP-25 (green). The trans SNARE complex forms two interfaces (referred to as primary and tripartite interfaces) with two synaptotagmin-1 C2B domains (represented as multicolored ellipsoids), one of which also involves the central α-helix of complexin-1 (yellow) [16]. The colors of the C2B ellipsoid indicate the loops involved in Ca2+-binding (gold), the primary SNARE/synaptotagmin-1 interface (green), the tripartite SNARE/complexin-1/synaptotagmin-1 interface (purple), and the polybasic region (blue). For clarity, the rest of synaptotagmin-1 including the C2A domain and the transmembrane domain, has been omitted. For the trans SNARE complex, the primary interface mainly involves SNAP-25 while the tripartite interface involves synaptobrevin-2, syntaxin-1 and complexin-1 (yellow). (B) and (C) Two orthogonal views of the core arrangement consisting of one trans SNARE complex that interacts with one central α-helix of complexin-1, and two synaptotagmin-1 C2B domains. See also Supplementary Video 1. (C) The same core arrangement viewed from above.
(D) and (E) Two orthogonal views of the bridge quaternary arrangement consisting of two trans SNARE/complexin-1 complexes that interact with a single C2B domain via primary and tripartite interfaces, respectively. To exemplify the potentially iterative nature of this interaction, additional synaptotagmin-1 C2B domains are shown in white. See also Supplementary Video 2. (E) A top-down view of (D).
In panels (B)–(E), the primary and tripartite molecular interface areas are indicated by green and purple colored surfaces, respectively, and the known crystal structure of the SNARE/complexin-1/synaptotagmin-1 complex (PDB ID 5W5C) is shown in ribbon representation.
Release of inhibition upon Ca2+ binding?
Regardless of the particular quaternary arrangement (Figure 3), the number of complexes, and how they might interact with each other, we propose that the system is nominally locked or inhibited, preventing synaptic vesicle fusion in the absence of Ca2+, based on the following arguments. First, the dominant negative phenotype of mutations of the synaptotagmin-1 C2B that prevent Ca2+-binding [11,12] as well as the requirement of residues of both interfaces of the SNARE/complexin-1/synaptotagmin-1 complex for maintaining the RRP of synaptic vesicles [16] both suggest that Ca2+-free C2B domains play an essential role to prevent fusion, but that they also play an essential role in establishing the RRP. Second, as mentioned above, cryo-ET studies of contacts between proteoliposomes with reconstituted SNAREs, complexin-1, and synaptotagmin-1 revealed a membrane separation of 40–60 Å [91], i.e., a separation that is too far for lipid stalk formation [22]. Third, for both possible arrangements, the SNARE complex is only partially zippered, and thus provides an “energy store” that becomes available once the inhibition is released.
From a structural perspective, a possible mechanism of fusion inhibition is intuitive for the bridge arrangement (Figure 3D,E) since the transmembrane domains of the two SNARE complexes involved in the bridge arrangement are on opposite sides, placing the structured parts of the SNARE/complexin-1/synaptotagmin-1 complex at the center between the membranes, and providing a large geometric barrier for the SNARE complexes to fully zipper. Starting from the bridge arrangement, the SNARE transmembrane domains would have to move by approximately 5 nm (i.e., half the end-to-end distance of the SNARE complex) if the proximity of the transmembrane domains is a prerequisite for fusion pore formation. If this were an entirely diffusion-driven process, the time it would take for the SNAREs to diffuse around an area of 5 nm2 would be in the range of 3–70 µsec considering the lateral diffusion coefficients of SNARES in membranes (the average diffusion coefficient for synaptobrevin-2 in lipid bilayers is ~ 0.4 µm2 s−1, for syntaxin-1A it is ~ 0.07 µm2 s−1 [93], and for the binary t-SNARE complex it is 1.5-0.5 µm2 s−1 [94] at ambient temperature). Thus, movement of the transmembrane domains starting from the bridge arrangement would be a physically plausible process for synaptic vesicle fusion since it occurs within ~ 0.1 msec [95].
For the core arrangement (Figure 3B,C), the negative charge of the Ca2+ binding region of synaptotagmin-1 C2B might repel negatively charged anionic phospholipids in the membrane, as suggested by membrane penetration and orientation measurements [71,96], effectively increasing the distance between membranes in the absence of Ca2+, thus decreasing the chance of membrane fusion. Distance regulation by synaptotagmin-1 alone had been previously suggested upstream of SNARE complex formation [9], and our models (Figure 3) suggest that it may also apply to the tripartite SNARE/complexin-1/synaptotagmin-1 pre-fusion complex.
At present, there is no uninhibited structure of the SNARE/complexin-1/synaptotagmin-1 complex, i.e., in the presence of Ca2+. We speculate that upon Ca2+-binding to the C2 domains of the primed complex, the synaptotagmin-1 molecule that is involved in the SNARE/complexin-1/synaptotagmin-1 tripartite interface (Figure 3A) is dislodged, for example, by interacting with, or insertion into, one of the membranes [28,71,97–99], by switching to a different interface with the SNARE complex [70], or by membrane bridging [7–9].
This synaptotagmin-1 dislodging may also induce a conformational change of complexin-1 [79]. However, full dislodging of complexin-1 as previously suggested [100] is unlikely considering the tight binding (dissociation constant Kd ≈ 10–100 nM) between complexin-1 and the cis ternary SNARE complex [79]. Moreover, only partial Ca2+-dependent competition between complexin-1 and synaptotagmin-1 in binding to the SNARE complex has been reported [100], and non-competitive binding was observed in both the absence and presence of Ca2+ by another group [101].
The synaptotagmin C2B molecule that forms the primary interface could induce membrane bending in conjunction with the SNARE complex upon Ca2+-binding, and puckering of the membrane near fusion site [3]. We note that synaptotagmin C2A-C2B domains can bend membranes on their own as well [4–6]. Following unlocking of the primed complexes, the SNAREs may then fully zipper [19,20] to trigger fusion.
Autoinhibitory role of extended synaptotagmin C2 domains
Extended synaptotagmins are involved in establishing contacts between endoplasmic reticulum (ER) and plasma membranes, and in promoting lipid transfer between the membranes [102,103]. In mammals, there are three different isoforms, extended synaptotagmin-1, −2, and −3 [104] that comprise an N-terminal hydrophobic hairpin that anchors them to the ER membrane, a SMP (synaptotagmin-like mitochondrial binding protein) domain, and multiple C2 domains. The crystal structure of the SMP-C2A-C2B fragment of extended synaptotagmin-2 in the absence of Ca2+ revealed an intramolecular interface between the C2 and SMP domains [102]. The SMP domain forms a channel that accommodates glycerolphospholipids, and this channel is probably involved in lipid transfer at ER-plasma membrane contact sites. Among mammalian extended synaptotagmins, the C2A-SMP domain interface is highly conserved [103]. We note that the primary sequence analysis published in ref. [102] included the sequence of tricalbins, yeast proteins that also contain a SMP domain and several C2 domains. However, in retrospect, tricalbins are rather different from extended synaptotagmins since only the C2C domains of tricalbin1 and tricalbin2 show Ca2+ dependent membrane binding [103], and, thus, the function of the tricalbins may be different from that of extended synaptotagmins. The functional relevance of the C2A-SMP interactions was tested by mutagenesis, and by disrupting the charge-based interaction under high salt conditions. The available data suggest that upon Ca2+ binding to the extended synaptotagmin-2 C2A domain, the autoinhibitory interaction between the C2A and SMP domains is released, enabling lipid transfer between membranes via the released SMP domain [105], possibly in conjunction with membrane bridging via the C2 domains of extended synaptotagmins [8]. However, as in the case of the SNARE/complexin-1/synaptotagmin-1 complex, the uninhibited conformation of extended synaptotagmins remains to be determined.
Autoinhibitory role of the Munc13 C2B domain
Munc13-1 consists of a C1 domain (C1), a calmodulin-binding domain, two C2 domains (C2A, C2B), a so-called MUN domain, and finally another C2 domain (C2C). The crystal structure of the C1C2BMUN fragment of Munc13-1 revealed inter-domain interactions between the C1, C2B, and MUN domains and a folded linker region between the C1 and C2B domains [106]. Surprisingly, deletion of the Munc13 C2B domain enhanced Ca2+-triggered exocytosis in C. elegans neurons, suggesting an inhibitory function for this domain [107]. The C2B-MUN linker was required for inhibition and Ca2+-binding to C2B domain relieved this inhibition. A cryo-ET study of proteoliposomes with reconstituted syntaxin and SNAP-25 and with bound C1C2BMUN revealed filamentous features that suggested multiple conformations of this fragment in the absence of Ca2+ [91], with the one conformation being consistent with the crystal structure in the absence of Ca2+ [106]. However, no high resolution structures of the bent conformations of C1C2BMUN are available, and a structure of the proposed autoinhibitory conformation of Munc13 in the absence of Ca2+ and presence of the membranes also remains to be determined.
Concluding remarks
In addition to the three examples of C2 domain inhibition presented in this review, there are other examples where C2 domains may inhibit certain functions. For example, the C2 domain of protein kinase C (PKC) βII interacts with its kinase domain in an autoinhibitory conformation [108] and the HECT-type ubiquitin ligase Smurf2 is also autoinhibited by its C2 domain. In both cases, it is possible that Ca2+ binding may release the autoinhibition, although this remains to be experimentally confirmed.
Taken together, synaptotagmin and synaptotagmin-like C2 domains may play a dual role of inhibiting a certain process, such as membrane fusion [16], in the absence of Ca2+, and of activating the same process after release of inhibition by membrane remodeling in the presence of Ca2+ [3–9]. Clearly, there remain key questions regarding the molecular mechanism of Ca2+-triggering (see Outstanding Questions). To begin, the conformation of the trans SNARE/complexin-1/synaptotagmin-1 complex within its native environment (that is between the synaptic and plasma membranes) needs to be determined along with the quaternary arrangement of such complexes. It is still an open question if interactions with other proteins, such as Munc18 and Munc13, play a regulatory role downstream from assembly of the trans SNARE complex, and if so, the structure of such super-complexes remains to be determined. Finally, the molecular steps after Ca2+-binding to the synaptotagmin C2 domains should be visualized, in other words, what happens to the synaptotagmin C2 domains, the SNARE complex, complexin, and the membranes around the emerging fusion pore. Considering the recent advances in imaging methods, in particular in electron microscopy, we predict that these goals are achievable in the not too distant future.
Outstanding Questions.
What is the quaternary arrangement of multiple SNARE/complexin-1/synaptotagmin-1 complex around the fusion site?
Are Munc18 and Munc13 still interacting with these complexes after trans SNARE complexes have formed? Are other factors interacting with these complexes?
What happens to the SNARE/complexin-1/synaptotagmin-1 complex after release of inhibition?
When and how do synaptotagmin C2 domains engage with the membrane after Ca2+-triggering?
Supplementary Material
Highlights.
The recent structure of the pre-fusion complex of neuronal SNAREs, complexin-1, and synaptotagmin-1, along with functional studies, suggest that Ca2+-triggered fusion is initiated by release of inhibition.
Activating properties, such as, full zippering of the SNARE complex, synaptotagmin-induced membrane bending or membrane bridging would act after the release of inhibition.
Inhibitory properties of synaptotagm in C2 domains at resting level of Ca2+ have also been suggested to occur for extended synaptotagm ins and Munc13.
Acknowledgments
We thank Xian Bian, Pietro De Camilli, Josep Rizo, and K. Ian White for stimulating discussions and critical reading of this review, and acknowledge support by the National Institutes of Health (R37MH63105 to A.T.B., K99MH113764 to Q.Z., and a grant by the Stanford Zaffaroni Alzheimer’s Disease Translational Research Program to A.T.B.
Glossary
- Binary t-SNARE complex
subcomplex consisting of the neuronal SNARE proteins syntaxin-1A and SNAP-25A
- B-factor
refers to the thermal factor of atoms or groups of atoms in crystal structures
- cis SNARE complex
fully assembled ternary SNARE complex with both the syntaxin and synaptobrevin transmembrane domains residing in the same membrane
- cryo-ET
cryo-electron tomography, imaging method to visualize large macromolecular complexess and cellular assemblies at up to ~ 40 Å resolution
- FRET
Förster resonance energy transfer, a mechanism of energy transfer between fluorescent dyes
- ITC
isothermal titration calorimetry, method to determine binding constants between macromolecules in solution
- NMR
nuclear magnetic resonance spectroscopy, method to study macromolecular structure and dynamics
- RRP
readily releasable pool, set of synaptic vesicles that are primed and ready to undergo evoked synaptic vesicle fusion
- ternary SNARE complex
complex consisting of the three neuronal SNARE proteins syntaxin-1A, synaptobrevin-2, and SNAP-25A
- trans SNARE complex
partially assembled (zippered) ternary SNARE complex with the transmembrane domains of syntaxin and synaptobrevin residing in the plasma and synaptic vesicle membranes, respectively
- X-ray crystal structure
three-dimensional structure of a molecule or of a complex of molecules obtained by crystallization of the molecule(s), X-ray diffraction data collection, structure solution, and refinement
- Zippering of the SNARE complex
directed assembly of the ternary SNARE complex, starting from the trans SNARE complex, proceeding from the N-terminal to the C-terminal end of the SNARE complex, and resulting in the cis SNARE complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Südhof TC. Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothman JE. The Principle of Membrane Fusion in the Cell (Nobel Lecture) Angew. Chemie Int. Ed. 2014 doi: 10.1002/anie.201402380. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, et al. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 2015;525:62–67. doi: 10.1038/nature14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui E, et al. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–21. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens S, et al. How Synaptotagmin Promotes Membrane Fusion. Science (80-.) 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Schulten K. Synaptotagmin’s Role in Neurotransmitter Release Likely Involves Ca2+-induced Conformational Transition. Biophys. J. 2014;107:1156–1166. doi: 10.1016/j.bpj.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araç D, et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, et al. Single-molecule force spectroscopy of protein-membrane interactions. Elife. 2017;6:1–21. doi: 10.7554/eLife.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bogaart G, et al. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat. Struct. Mol. Biol. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackler JM, et al. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–4. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 11.Wu D, et al. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature. 2017;544:316–321. doi: 10.1038/nature21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, et al. Genetic Analysis of Synaptotagmin C2 Domain Specificity in Regulating Spontaneous and Evoked Neurotransmitter Release. J. Neurosci. 2013;33:187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 14.Littleton JT, et al. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10888–92. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang ZP, et al. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:2039–50. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. doi: 10.1038/nature23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao M, Brunger AT. Recent Advances in Deciphering the Structure and Molecular Mechanism of the AAA + ATPase N-Ethylmaleimide-Sensitive Factor (NSF) J. Mol. Biol. 2016;428:1912–1926. doi: 10.1016/j.jmb.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunger AT, et al. Molecular Mechanisms of Fast Neurotransmitter Release. Annu. Rev. Biophys. 2018;47:22.1–22.29. doi: 10.1146/annurev-biophys-070816-034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton RB, et al. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 20.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science. 2012;337:1340–3. doi: 10.1126/science.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aeffner S, et al. Energetics of stalk intermediates in membrane fusion are controlled by lipid composition. Proc. Natl. Acad. Sci. 2012;109:E1609–E1618. doi: 10.1073/pnas.1119442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perin MS, et al. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J. Biol. Chem. 1991;266:615–22. [PubMed] [Google Scholar]
- 24.Geppert M, et al. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Chacón R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–9. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 26.Rhee J-S, et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18664–9. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brose N, et al. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–5. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 28.Chapman ERER, Davis AFAF. Direct Interaction of a Ca 2 + -binding Loop of Synaptotagmin with Lipid Bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 29.Shao X, et al. Synaptotagmin-syntaxin interaction: The C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Jonas P. Synaptotagmins: That’s Why So Many. Neuron. 2017;94:694–696. doi: 10.1016/j.neuron.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Mohrmann R, et al. Complexins: small but capable. Cell. Mol. Life Sci. 2015 doi: 10.1007/s00018-015-1998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trimbuch T, Rosenmund C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 2016;17:118–125. doi: 10.1038/nrn.2015.16. [DOI] [PubMed] [Google Scholar]
- 33.McMahon HT, et al. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 34.Reim K, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 35.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 2007;10:1235–7. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 36.Xue M, et al. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maximov A, et al. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–21. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobson RJ, et al. Complexin maintains vesicles in the primed state in C. elegans. Curr. Biol. 2011;21:106–13. doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin JA, et al. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol. 2011;21:97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, et al. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc. Natl. Acad. Sci. 2013;110:20777–20782. doi: 10.1073/pnas.1321367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaeser-Woo YJ, et al. C-terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis. J. Neurosci. 2012;32:2877–85. doi: 10.1523/JNEUROSCI.3360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, et al. Evolutionary conservation of complexins: from choanoflagellates to mice. EMBO Rep. 2015;16:1–10. doi: 10.15252/embr.201540305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao P, et al. Complexin Activates Exocytosis of Distinct Secretory Vesicles Controlled by Different Synaptotagmins. J. Neurosci. 2013;33:1714–1727. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trimbuch T, et al. Re-examining how complexin inhibits neurotransmitter release. Elife. 2014;3:e02391. doi: 10.7554/eLife.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Sci. (New York, NY) 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 46.Ma C, et al. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–5. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basu J, et al. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 2005;12:1017–8. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat. Struct. Mol. Biol. 2015;22:547–554. doi: 10.1038/nsmb.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai Y, et al. Molecular mechanisms of synaptic vesicle priming by Munc13 and Munc18. Neuron. 2017;95:591–607. doi: 10.1016/j.neuron.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammarlund M, et al. Open syntaxin docks synaptic vesicles. PLoS Biol. 2007;5:e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He E, et al. Munc13-1 and Munc18-1 together prevent NSF-dependent de-priming of synaptic vesicles. Nat. Commun. 2017;8:15915. doi: 10.1038/ncomms15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, et al. Functional synergy between the Munc13 C-terminal C1 and C2 domains. Elife. 2016;5:1–27. doi: 10.7554/eLife.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Söllner TH, et al. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 55.Mayer A, et al. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 56.Hanson PI, et al. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 57.Kyoung M, et al. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diao J, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. Elife. 2012;1:e00109. doi: 10.7554/eLife.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai Y, et al. Complexin inhibits spontaneous release and synchronizes Ca 2+ -triggered synaptic vesicle fusion by distinct mechanisms. Elife. 2014;3:e03756. doi: 10.7554/eLife.03756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunger AT, et al. Towards reconstitution of membrane fusion mediated by SNAREs and other synaptic proteins. Crit. Rev. Biochem. Mol. Biol. 2015;0:1–11. doi: 10.3109/10409238.2015.1023252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Shin Y-K. Productive and Non-productive Pathways for Synaptotagmin 1 to Support Ca2+-Triggered Fast Exocytosis. Front. Mol. Neurosci. 2017;10:1–11. doi: 10.3389/fnmol.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreutzberger AJB, et al. Reconstitution of calcium-mediated exocytosis of dense-core vesicles. Sci. Adv. 2017;3:e1603208. doi: 10.1126/sciadv.1603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, et al. Three-Dimensional Structure of the Complexin/SNARE Complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 64.Carugo O, Argos P. Protein-protein crystal-packing contacts. Protein Sci. 1997;6:2261–3. doi: 10.1002/pro.5560061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krissinel E, Henrick K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 66.Elcock aH, McCammon Ja. Identification of protein oligomerization states by analysis of interface conservation. Proc. Natl. Acad. Sci. 2001;98:2990–2994. doi: 10.1073/pnas.061411798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kümmel D, et al. Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. 2011;18:927–33. doi: 10.1038/nsmb.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schupp M, et al. Interactions Between SNAP-25 and Synaptotagmin-1 Are Involved in Vesicle Priming, Clamping Spontaneous and Stimulating Evoked Neurotransmission. J. Neurosci. 2016;36:11865–11880. doi: 10.1523/JNEUROSCI.1011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi UB, et al. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 2010;17:318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brewer KD, et al. Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat. Struct. Mol. Biol. 2015;22:555–564. doi: 10.1038/nsmb.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez-Lara Á, et al. PtdInsP 2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. Elife. 2016;5:1–22. doi: 10.7554/eLife.15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S, et al. Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion. Elife. 2016;5:1689–1699. doi: 10.7554/eLife.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park Y, et al. Synaptotagmin-1 binds to PIP2-containing membrane but not to SNAREs at physiological ionic strength. Nat. Struct. Mol. Biol. 2015 doi: 10.1038/nsmb.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prinslow EA, et al. Reconciling isothermal titration calorimetry analyses of interactions between complexin and truncated SNARE complexes. Elife. 2017;6:e30286. doi: 10.7554/eLife.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krishnakumar SS, et al. Re-visiting the trans insertion model for complexin clamping. Elife. 2015;4:1–15. doi: 10.7554/eLife.04463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai Y, et al. N-terminal domain of complexin independently activates calcium-triggered fusion. Proc. Natl. Acad. Sci. 2016;113:E4698–E4707. doi: 10.1073/pnas.1604348113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang X, et al. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory α helix. Neuron. 2010;68:907–20. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho RW, et al. Genetic analysis of the Complexin trans-clamping model for cross-linking SNARE complexes in vivo. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10317–10322. doi: 10.1073/pnas.1409311111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi UB, et al. Complexin induces a conformational change at the membrane-proximal C-terminal end of the SNARE complex. Elife. 2016;5:e16886. doi: 10.7554/eLife.16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gustavsson N, Han W. Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci. Rep. 2009;29:245. doi: 10.1042/BSR20090031. [DOI] [PubMed] [Google Scholar]
- 81.Xu J, et al. Synaptotagmin-1, −2, and −9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–81. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Schonn J-S, et al. Synaptotagmin-1 and −7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc. Natl. Acad. Sci. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue M, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 2007;14:949–58. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xue M, et al. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat. Struct. Mol. Biol. 2010;17:568–75. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dhara M, et al. Complexin synchronizes primed vesicle exocytosis and regulates fusion pore dynamics. J. Cell Biol. 2014;204:1123–40. doi: 10.1083/jcb.201311085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi L, et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–9. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinha R, et al. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14318–23. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rothman JE, et al. Hypothesis: Buttressed Rings Assemble, Clamp , and Release SNAREpins for Synaptic Transmission. FEBS Lett. 2017 doi: 10.1002/1873-3468.12874. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernández-Busnadiego R, et al. Cryo-electron tomography reveals a critical role of RIM1 α in synaptic vesicle tethering. J. Cell Biol. 2013;201:725–740. doi: 10.1083/jcb.201206063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cole AA, et al. A Network of Three Types of Filaments Organizes Synaptic Vesicles for Storage, Mobilization, and Docking. J. Neurosci. 2016;36:3222–3230. doi: 10.1523/JNEUROSCI.2939-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gipson P, et al. Morphologies of synaptic protein membrane fusion interfaces. Proc. Natl. Acad. Sci. 2017;114:9110–9115. doi: 10.1073/pnas.1708492114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaeser PS, Regehr WG. Molecular Mechanisms for Synchronous, Asynchronous, and Spontaneous Neurotransmitter Release. Annu. Rev. Physiol. 2014;76:333–363. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowen ME, et al. Single molecule observation of liposome-bilayer fusion thermally induced by soluble N-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs) Biophys. J. 2004;87:3569–84. doi: 10.1529/biophysj.104.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wölfel M, Schneggenburger R. Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion. J. Neurosci. 2003;23:7059–7068. doi: 10.1523/JNEUROSCI.23-18-07059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hui E, et al. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 2006;91:1767–77. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arunachalam L, et al. Munc18-1 Is Critical for Plasma Membrane Localization of Syntaxin1 but Not of SNAP-25 in PC12 Cells. Mol. Biol. Cell. 2007;19:722–734. doi: 10.1091/mbc.E07-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993;268:26386–90. [PubMed] [Google Scholar]
- 99.Kuo W, et al. The calcium-dependent and calcium-independent membrane binding of synaptotagmin 1: two modes of C2B binding. J. Mol. Biol. 2009;387:284–94. doi: 10.1016/j.jmb.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–87. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 101.Chicka MC, Chapman ER. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry. 2009;48:657–9. doi: 10.1021/bi801962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schauder CM, et al. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014 doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schulz TA, Creutz CE. The Tricalbin C2 Domains: Lipid-Binding Properties of a Novel, Synaptotagmin-Like Yeast Protein Family. Biochemistry. 2004;43:3987–3995. doi: 10.1021/bi036082w. [DOI] [PubMed] [Google Scholar]
- 104.Min S-W, et al. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3823–8. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bian X, et al. Ca 2+ releases E-Syt1 autoinhibition to couple ER-plasma membrane tethering with lipid transport. EMBO J. 2017:e201797359. doi: 10.15252/embj.201797359. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu J, et al. Mechanistic insights into neurotransmitter release and presynaptic plasticity from the crystal structure of Munc13-1 C 1 C 2 BMUN. Elife. 2017:6. doi: 10.7554/eLife.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michelassi F, et al. Article Article A C1-C2 Module in Munc13 Inhibits Calcium-Dependent Neurotransmitter Release. Neuron. 2017;95:577–590. doi: 10.1016/j.neuron.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antal CE, et al. Intramolecular C2 Domain-Mediated Autoinhibition of Protein Kinase C βII. Cell Rep. 2015;12:1252–1260. doi: 10.1016/j.celrep.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.