Abstract
Background
Regulation of coronary vasomotor tone by serotonin is significantly changed after cardioplegic arrest and reperfusion. The current study investigates whether cardiopulmonary bypass (CPB) may also affect peripheral arteriolar response to serotonin in patients with or without diabetes.
Methods
Human peripheral microvessels (90–180 μm diameter) were dissected from harvested skeletal muscle tissues from diabetic (DM) and non-diabetic (ND) patients before and after CPB and cardiac surgery (n = 8/group). In-vitro contractile response to serotonin was assessed by videomicroscopy in the presence or absence of serotonin alone (10−9-10−5M) or combined with the selective serotonin 1B receptor (5-HT1B) antagonist, SB224289 (10−6M). 5-HT1A/1B protein expression in the skeletal muscle was measured by Western-blot and immunohistochemistry.
Results
There were no significant differences in contractile response of peripheral arterioles to serotonin (10−5M) pre-CPB between DM and ND patients. After CPB, contractile response to serotonin was significantly impaired in both DM and ND patients compared to their pre-CPB counterparts (P<0.05). This effect was more pronounced in DM patients than ND patients (P<0.05 vs ND). The contractile response to serotonin was significantly inhibited by the 5-HT1B antagonist in both DM and ND vessels (P<0.05 vs serotonin alone). There were no significant differences in the expression/distribution of 5-HT1A/1B between ND and DM groups or between pre- vs. post-CPB vessels.
Conclusions
CPB is associated with decreased contractile response of peripheral arterioles to serotonin and this effect was exaggerated in the presence of diabetes. Serotonin-induced contractile response of the peripheral arterioles was via 5-HT1B in both DM and ND patients.
INTRODUCTION
Cardiopulmonary bypass (CPB) is known to trigger an inflammatory cascade that may lead to organ dysfunction across multiple organ systems.1 Disturbances in the microvasculature result in reduced myogenic tone and endothelial dysfunction in peripheral arterioles, which may contribute to reduced vascular resistance in the peripheral microcirculation, systemic hypotension, and organ malperfusion.1–5 We and others have shown that CPB is associated with an impaired contractile response of peripheral arterioles to a number of vaso-modulators including phenylephrine, endothelin-1, and thromboxane A2.1–3
Furthermore, diabetes is a major contributor to increased morbidity and mortality in patients undergoing coronary artery bypass grafting (CABG) for coronary artery disease,6–10 and nearly 37% of patients undergoing primary CABG have comorbid diabetes.8 One contributing factor to increased morbidity in patients with diabetes undergoing CABG may be dysregulation of the microvasculature due to multiple factors including hyperglycemia, inflammation, oxidative stress, and marked endothelial dysfunction.11 We have previously demonstrated that in-vitro relaxation and contractile responses to both endothelial dependent and independent vaso-modulators, including ADP, substance P, and sodium nitroprusside, were significantly impaired in tissues from patients with diabetes as compared to those from patients without diabetes.12
One important vaso-modulator in the circulation is serotonin, which acts on several receptors in the vasculature to modulate vascular tone.13 Specifically, the serotonin 1B (5-HT1B) receptor subtype is known to mediate a contractile response in vascular smooth muscle cells.13 We and others have shown that regulation of coronary vasomotor tone by serotonin is significantly changed after cardioplegic arrest and reperfusion,14,15 but the response to serotonin in the peripheral arterioles after CPB is unknown. The goal of this study is to investigate whether CPB may affect the peripheral arteriolar response to serotonin in patients with and without diabetes, and to relate these responses to possible changes in the expression and distribution of serotonin-specific receptors.
METHODS
Case Selection
Hemoglobin A1C (HbA1c) was measured in all patients and the patients were divided into two groups. The non-diabetic (ND) group was defined as those patients with a normal HbA1c (<6.2%) and no history of or treatment for diabetes. The poorly controlled diabetic (DM) group was defined as those patients with diabetes and the most recent HbA1c ≥ 8.5. Exclusion criteria included patients undergoing valve surgery, patients with a cross-clamp time greater than 120 minutes, patients with a CPB time greater than 180 minutes, and a diagnosis of diabetes in a patient with a HbA1c less than 8.5%.
Human Subjects and Tissue Harvesting
Samples of skeletal muscle from the left internal mammary artery bed were harvested before and after CPB from patients undergoing cardiac surgery. The pre-CPB skeletal muscle samples were harvested from the intercostal muscles remote from the internal mammary harvest site with sharp dissection and not using cautery, before CPB was initiated. Generally, the skeletal muscle sample was taken after mobilization of the internal mammary artery and before cannulation. The CPB circuit included a Medtronic Affinity integrated hollow fiber oxygenator/cardiotomy reservoir with trillium coating (Medtronic, Minneapolis, MN), and an arterial 38mg-filter (Medtronic Affinity, Minneapolis, MN) with trillium coating.
After removal of the aortic cross clamp and weaning off of CPB, the post-CPB skeletal muscle samples were harvested from a location remote from the left internal mammary artery bed. Skeletal muscle examined in the study were not directly exposed to papaverine or other vasoactive drugs. Sections of skeletal muscle samples were immediately frozen in liquid nitrogen for immunoblotting, fixed in 10% formalin for 24 hours followed by paraffinization and sectioning into 5μm slices for immunohistochemical staining, or stored in cold Krebs buffer for in-vitro analysis.
All procedures were approved by the Institutional Review Board (IRB) of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients prior to tissue collection and involvement in the study as required by the IRB.
In-vitro Skeletal Muscle Microvascular Studies
Microvessels (90–180μm internal diameters) from the harvested human peripheral skeletal muscle from eight patients per ND and DM groups were dissected using a dissecting microscope. The microvessels were placed in a microvessel chamber, cannulated with dual glass micropipettes (40–80μm in diameter), and secured in place with 10-0 nylon monofilament sutures. Microvessel chambers contained continuously circulating Krebs buffer solution that was oxygenated (95% oxygen and 5% carbon dioxide) and warmed to 37 degrees Celsius. The microvessels were pressurized to 40 mmHg in a no-flow state by using a burette manometer filled with Krebs buffer solution. The microvessel image was projected onto a monitor using an inverted microscope (40–200x, Olympus CK2, Olympus Optical) connected to a video camera. The internal luminal diameter was measured using an electronic dimension analyzer. Microvessels were bathed in the chamber for at least 30 minutes prior to pharmacological intervention. In-vitro contractile response to serotonin was assessed by videomicroscopy in the presence or absence of serotonin alone (109-10−5M) or combined with the selective 5-HT1B antagonist, SB224289 (10−6M).
Immunoblotting
Peripheral skeletal muscle tissue samples from six patients per ND and DM groups were dissected and cleaned of connected tissues and solubilized in SDS-PAGE buffer. Total protein (70μg) was fractionated on an 8–16% SDS-PAGE gel, transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA), and membranes were incubated for an hour at room temperature with 1:1000 dilutions of individual rabbit polyclonal primary antibodies to 5-HT1A and 5-HT1B receptors (ABCAM). The membranes were then washed and incubated with goat polyclonal anti-rabbit secondary antibody at 1:3000 dilutions, washed, and processed for chemiluminescent detection and captured with a digital camera system (G-box, Syngene, Cambridge, England). All membranes were probed with GAPDH (Cell Signaling, Danvers, MA) to correct for loading error. Densitometric analysis of band intensity was performed using NIH Image J software.
Immunohistochemistry
Formalin-fixed skeletal muscle tissue samples from four patients from each group were deparaffinized in xylene and rehydrated in graded ethanol and phosphate-buffered saline solution (PBS). Samples were incubated in Dako Target Retrieval Solution for antigen unmasking, rinsed in PBS, incubated in 3% hydrogen peroxide, washed, and incubated in 5% goat serum in PBS at room temperature for 2 hours for blocking. After washing, samples were incubated in rabbit polyclonal primary antibodies to 5-HT1A and 5-HT1B at 1:500 dilutions each. Immunoreactivity was detected with the appropriate biotinylated secondary antibody at a 1:800 dilution and the enhanced avidin biotinylated peroxidase complex (Vector Laboratories, Burlingame, CA). Color was developed with diaminobenzidine substrate and samples were mounted using Permount SP 15–150. Photomicrographs were taken with a Zeiss LSM510 confocal microscope system (Carl Zeiss MicroImaging, Inc., Thornwood, NY) equipped with a digital camera (Photodoc, Upland, CA).
Data Analysis
Microvessel responses are expressed as percentage of contraction compared to baseline. Microvascular reactivity data were analyzed using repeated measures ANOVA followed by student t test. Western blot data were analyzed using paired t tests to compare densitometry of samples before and after CPB and in ND vs DM patients. Data are reported as mean and standard error of the mean (SEM). Probability values <0.05 were considered significant.
RERULTS
Patient Characteristics
The patient characteristics are listed in Table 1. Tissue samples from 16 patients were studied, with 8 patients in the ND group and 8 patients in the DM group. In the ND group, the mean age was 68±6 years with 6 male and 2 female patients. In the DM group, the mean age was 70±7 years with 7 male and 1 female patients. All patients underwent CABG, and patients in both ND and DM groups had comparable cross clamp times (ND = 90±10 min; DM = 93±11 min) and CPB times (ND = 104±12 min; DM = 106±11 min). All patients received low dose vasopressor support after separation from cardiopulmonary bypass (generally norepinephrine and/or epinephrine) to maintain adequate vascular tone, but no patient suffered from low cardiac output syndrome and all patients had an unremarkable postoperative recovery. The average preoperative blood HbA1c was 5.4±0.4 in the ND group and 8.9±0.5 in the DM group. All patients in the DM group were on pre-operative insulin. Patients with preoperative hypertension included 6 in the ND group and 8 in the DM group, and all of these patients were on appropriate antihypertensive medications (β-blockers, calcium channel blockers, and/or angiotensin-converting enzyme inhibitors) and received perioperative β-blockers.
Table 1.
Baseline characteristics. ND: non-diabetic patients; DM: diabetic patients; HbA1c: hemoglobin A1c; CABG: coronary artery bypass grafting.
| Baseline characteristics | ND (n=8) | DM (n=8) |
|---|---|---|
| HbA1C (%) | 5.4 ± 0.4 | 8.9 ± 0.5 |
| Male/Female | 6/2 | 7/1 |
| Age (y) | 68 ± 6 | 70 ± 7 |
| CABG (n) | 8 | 8 |
| Pre-operative Insulin (n) | 0 | 8 |
| Peri-operative Insulin (n) | 2 | 8 |
| Hypertension (n) | 6 | 8 |
| Hypercholestesterolemia (n) | 7 | 8 |
| Cross Clamp Time (min) | 90 ± 10 | 93 ± 11 |
| CPB Time (min) | 104 ± 12 | 106 ± 11 |
Microvascular Reactivity
There were no significant differences in the baseline diameters of peripheral arterioles in the ND and DM groups or pre-CPB and post-CPB groups. There were no significant differences in the contractile response of peripheral arterioles to serotonin pre-CPB between ND and DM patients (67±8% versus 64±11%; p=0.63; Figure 1).
Figure 1.
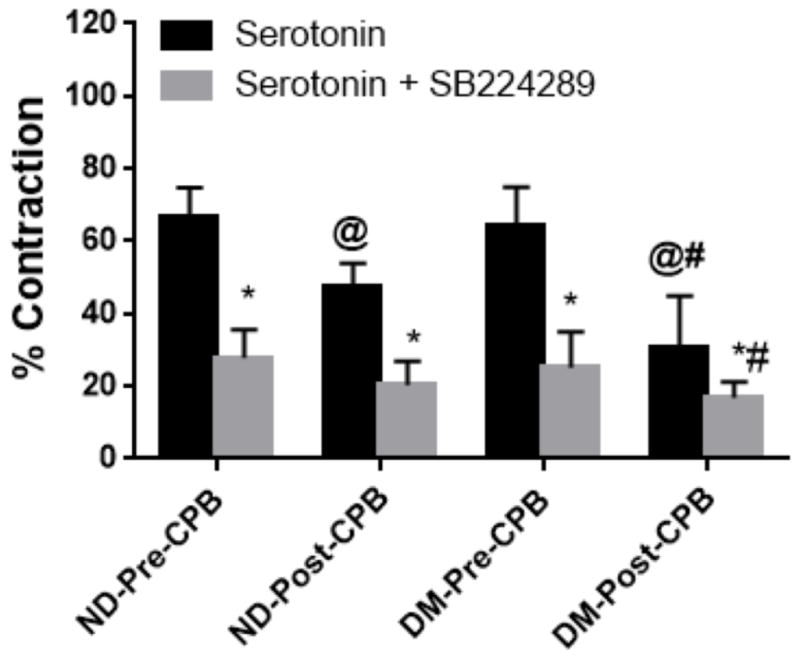
The in-vitro contractile response of peripheral arterioles to serotonin (10−5M) in the presence or absence of the 5-HT1B receptor antagonist SB224289 (10−5M). *P<0.05 vs. ND-Pre-CPB or DM-Pre-CPB. @P<0.05 vs. Pre-CPB, #P<0.05 vs. Post-CPB. n=8/group, ND, non-diabetic patients, DM, diabetic patients.
After CPB, the contractile response to serotonin was significantly impaired in both ND and DM patients compared to their pre-CPB counterparts (ND from 67±8% pre-CPB to 47±7% post-CPB; DM from 64±11% pre-CPB to 31±14% post-CPB; p<0.05; Figure 1). This effect was more pronounced in DM patients than in ND patients (p<0.05; Figure 1). The contractile response to serotonin was significantly inhibited by the 5-HT1B antagonist SB224289 in both DM and ND vessels (ND pre-CPB 28±8%; ND post-CPB 20±7%; DM pre-CPB 25±10%; DM post-CPB 17±4.4%; p<0.05 vs serotonin alone; Figure 1).
Effect of CPB on Expression of Serotonin Receptors
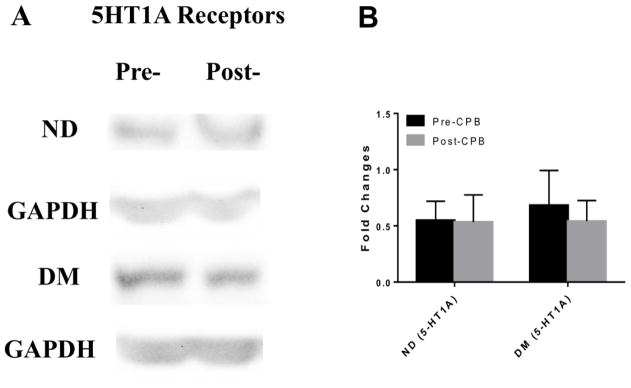
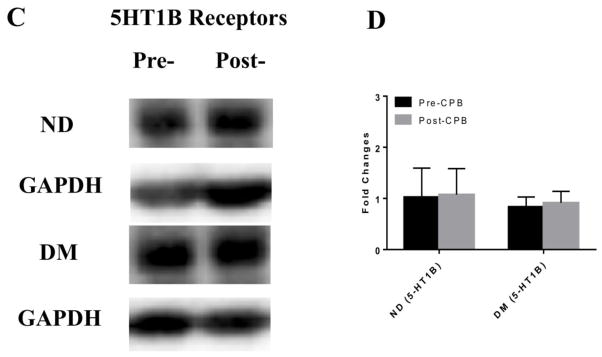
Immunoblot staining of peripheral skeletal muscle showed no significant differences in expression of the 5-HT1A or 5-HT1B receptors between ND and DM patients or between pre-CPB and post-CPB samples (Figure 2).
Figure 2.
A, C Western blots of serotonin receptor proteins in peripheral microvasculature comparing expression of (A) 5-HT1A and (C) 5-HT1B in ND and DM, pre- and post-CP/CPB. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. ND and DM samples were run on separate membranes and the corresponding GAPDH blot is shown below the respective ND or DM group. n=6/group. B, D. Fold changes in optical density from western blot analysis of (B) 5-HT1A and (D) 5-HT1B in ND and DM samples pre- and post- CP/CPB. There was no significant difference in the expression of 5-HT1A or 5-HT1B between ND and DM groups or between pre-and post- CPB vessels.
Effect of CPB on Distribution of Serotonin Receptors
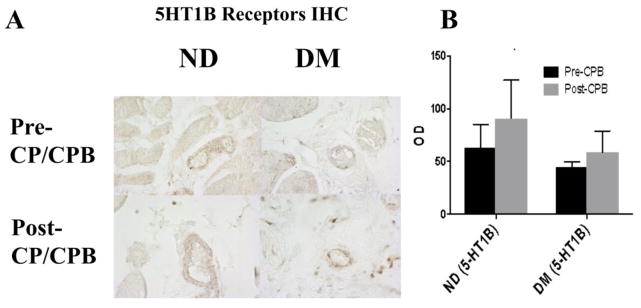
Immunohistochemical staining of peripheral skeletal muscle showed no significant differences in distribution of the 5-HT1A or 5-HT1B receptors between ND or DM patients or between pre-CPB and post-CPB samples (Figure 3. 5-HT1A not shown).
Figure 3.
A. Immunohistochemical staining of peripheral skeletal muscle with 5-HT1B receptor antibodies (brown). B. 5-HT1B receptor distribution optical densities (OD) found using immunoperoxidase staining and image analysis. There were no significant differences between ND and DM groups or between pre- and post- CP/CPB vessels (n=4/group).
DISCUSSION
In this study, we demonstrated using an in-vitro model that the contractile response of human peripheral skeletal muscle arterioles to serotonin as mediated by the 5-HT1B receptor was impaired after CPB in patients undergoing CABG. This contractile response was further impaired in arterioles from patients with poorly controlled diabetes as compared to that from non-diabetic patients. There was no change in the expression or distribution of the 5-HT1B receptor, indicating a change in the functionality of this receptor rather than a change in quantity or localization. The significance of these changes is underscored by the role of the peripheral microcirculation, specifically arterioles with an internal diameter of <200 micrometers, in regulating systemic vascular resistance and ultimately tissue perfusion.1,2,15–17
Serotonin is a biogenic monoamine with a family of receptors that contains at least 15 members, the majority of which belong to the G protein coupled receptor family, and are found in almost all tissues.13 Serotonin release is stimulated by ischemia and contact with artificial surfaces, as occurs in a CPB circuit.13 Further, peripheral serotonin is stored in the blood, specifically in the dense granules of platelets. Platelet activation can cause a marked local concentration of serotonin and other platelet products. Physiological roles of serotonin in the periphery include platelet aggregation for hemostasis and cardiovascular regulation including vasoconstriction and vasodilation.13 Serotonin has been shown to activate phospholipase A2, which results in release of arachidonic acid, an important precursor via the enzymatic action of cyclooxygenase (COX) to a number of inflammatory mediators including thromboxane A2 and prostacyclin.18,19 Furthermore, there is an increase in COX-2 expression/activation in peripheral arterioles of patients post-CPB compared to pre-CPB and this increase is higher in patients with diabetes compared to patients without diabetes.20 These changes may contribute to the altered peripheral microvascular reactivity to serotonin in patients with diabetes following cardiac surgery and CPB.
One specific regulator of vascular tone in the serotonin family of receptors is the 5-HT1B receptor, which is located on both vascular smooth muscle and endothelial cells and mediates a contractile response.13 The 5-HT1A receptor also has a role in vasomotor tone with a vasodilatory effect in the peripheral vasculature.21,22 Our study demonstrated an impairment in the contractile function of the 5-HT1B receptor after cardiopulmonary bypass which was worsened in patients with poorly controlled diabetes. The mechanism of this impairment is likely mediated by multiple factors. First, an inflammatory cascade that is initiated by CPB as blood comes into contact with artificial surfaces leads to an increased release of serotonin.1,13 The sustained increase of serotonin in vivo or prolonged exposure in-vitro may lead to loss of serotonin-mediated vascular smooth muscle cell contraction. Furthermore, CPB leads to the activation and release of prostaglandins, oxygen free radicals, complement, nitric oxide, and inflammatory cytokines, which may contribute to vasomotor dysfunction via increased vascular permeability and changes in vasodilatory and vasoconstrictive responses.1,2,16,23,24
It is known that diabetes may lead to microvascular complications such as retinopathy, nephropathy, and peripheral arterial disease, and that intensive glycemic control significantly reduces these complications.25,26 Furthermore, intensive glycemic control is associated with improved outcomes after CABG.27 The mechanism of worsened impairment of microvessel constriction in patients with diabetes compared to non-diabetic patients may be related to the increased oxidative and nitrosative stress responses that occur in skeletal muscle and microvessels in patients with sub-optimally controlled diabetes.12 In addition, advanced glycosylation end products may affect the microvascular responses. These changes may contribute to the diminished functionality of the 5-HT1B receptor as suggested in our study.
Our findings in this study are consistent with our previous work with other vasomodulators following CPB. We found that CPB leads to an impaired in-vitro contractile response of peripheral arterioles to phenylephrine, endothelin-1, and thromboxane A2,1–3 and an impaired relaxation response to both endothelial dependent and independent vasodilators after CPB.12 The changes in endothelial dependent vasodilatory responses were more pronounced in patients with higher HbA1c levels when compared to non-diabetic patients and patients with well controlled diabetes.12 Extending these findings to the current study, future investigations comparing the microvascular response to serotonin in patients with controlled versus uncontrolled diabetes may yield significant differences.
Limitations
There are several limitations to the present study. First, the sample size is limited with eight patients per group. Also, given that the study involved human samples, one factor that may affect our results is the heterogeneity of the patients enrolled. Differences in medications and incidence of coexisting disease processes may have affected our findings. Finally, this current study lacks extensive clinical outcome data, which would help correlate our findings to clinical outcomes. Future studies with this data may help to correlate microvascular and signaling changes with clinical outcomes. Finally, this study did not examine all of the factors or mechanisms that may contribute to the altered receptor function in the setting of cardiac surgery or diabetes.
Conclusion
In conclusion, CPB is associated with decreased contractile function of human peripheral skeletal muscle microvessels in response to serotonin as mediated by the 5-HT1B receptor subtype. This contractile dysfunction is worsened in patients with poorly controlled diabetes as compared to non-diabetic patients. There was no significant change in the expression or distribution of the 5-HT1B receptor in peripheral microvessels indicating a change in the functional status of the receptor. These results may have implications regarding the cause of vasomotor dysfunction in patients after cardiac surgery.
Acknowledgments
We would like to thank all the nurses, physician assistants and perfusionists working in cardiac surgery at Rhode Island Hospital for collecting the tissue samples and recording patient characteristics. We would also like to thank the nurses and physician assistants in the Division of Cardiac Surgery of Rhode Island Hospital for collecting patient consent forms.
Sources of Funding
This study was supported by the National Heart, Lung, and Blood Institute HL-46716(F.W.S) and HL128831 (F.W.S.). This study was also supported in part by and 1RO1HL127072 (J.F.), 1R01 HL136347-01 (J.F.), AHA-Grant-in-Aid-15GRNT25710105 (J.F.), and by the NIH/NIGMS Training Grant 5T32GM065085-13 (L.A.S).
Footnotes
Presentation: The American Heart Association Scientific Sessions 2017, Nov. 11–15, Anaheim, CA
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004 Nov 1;26(5):1002–14. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, et al. Protein kinase C alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007 Aug;142(2):243–52. doi: 10.1016/j.surg.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Chu LM, Robich MP, Clements RT, Khabbaz KR, Hagberg R, et al. The Effects of Cardiopulmonary Bypass on Endothelin-1-induced Contraction and Signaling in Human Skeletal Muscle Microcirculation. Circulation. 2010 Sep 14;122(11 Suppl):S150–5. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu Y, Singh AK, Dobrilovic N, Feng WC, Chu LM, et al. Impaired contractile response of human peripheral arterioles to thromboxane A-2 after cardiopulmonary bypass. Surgery. 2011 Aug;150(2):263–71. doi: 10.1016/j.surg.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Sellke EW, Feng J, Clements RT, Sodha NR, Khabbaz KR, et al. Calcium-activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass. Surgery. 2008 Aug;144(2):239–44. doi: 10.1016/j.surg.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002 Aug 7;40(3):418–23. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 7.Leavitt BJ, Sheppard L, Maloney C, Clough RA, Braxton JH, Charlesworth DC, et al. Effect of diabetes and associated conditions on long-term survival after coronary artery bypass graft surgery. Circulation. 2004 Sep 14;110(11 Suppl 1):II41–44. doi: 10.1161/01.CIR.0000138197.07051.e7. [DOI] [PubMed] [Google Scholar]
- 8.Raza S, Sabik JF, III, Ainkaran P, Blackstone EH. Coronary artery bypass grafting in diabetics: A growing health care cost crisis. J Thorac Cardiovasc Surg. 2015 Aug;150(2):304–312. e2. doi: 10.1016/j.jtcvs.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999 Apr;67(4):1045–52. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Liu Y, Khabbaz KR, Hagberg R, Robich MP, Clements RT, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011 Feb;149(2):247–52. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picchi A, Capobianco S, Qiu T, Focardi M, Zou X, Cao J-M, et al. Coronary microvascular dysfunction in diabetes mellitus: A review. World J Cardiol. 2010 Nov 26;2(11):377–90. doi: 10.4330/wjc.v2.i11.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Liu Y, Chu LM, Singh AK, Nikola DN, Fingleton JG, et al. Changes in Microvascular Reactivity after Cardiopulmonary Bypass in Patients with Poorly Controlled versus Controlled Diabetes. Circulation. 2012 Sep 11;126(11 Suppl 1):S73–80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Côté F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med. 2004 May;10(5):232–8. doi: 10.1016/j.molmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Robich MP, Araujo EG, Feng J, Osipov RM, Clements RT, Bianchi C, et al. Altered coronary microvascular serotonin receptor expression after coronary artery bypass grafting with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2010 Apr;139(4):1033–40. doi: 10.1016/j.jtcvs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Métais C, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999 Nov 9;100(19 Suppl):II328–334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 16.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Li J, et al. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003 Aug;134(2):247–54. doi: 10.1067/msy.2003.229. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Liu Y, Khabbaz KR, Hagberg R, Sodha NR, Osipov RM, et al. Endothelin-1 Induced Contractile Responses of Human Coronary Arterioles via Endothelin-A Receptors and PKC-α Signaling Pathways. Surgery. 2010 Jun;147(6):798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg KA, Clarke WP. Regulation of 5-HT1A and 5-HT1B receptor systems by phospholipid signaling cascades. Brain Res Bull. 2001 Nov 15;56(5):471–7. doi: 10.1016/s0361-9230(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 19.Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol Baltim Md 1950. 2001 Sep 1;167(5):2831–8. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 20.Feng J, Anderson K, Liu Y, Singh AK, Ehsan A, Sellke FW. Cyclooxygenase 2 contributes to bradykinin-induced microvascular responses in peripheral arterioles after cardiopulmonary bypass. J Surg Res. 2017 Oct;218:246–52. doi: 10.1016/j.jss.2017.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brann M. Molecular Biology of G-Protein-Coupled Receptors: Applications of Molecular Genetics to Pharmacology. Springer Science & Business Media; 2012. p. 343. [Google Scholar]
- 22.Saxena PR, Villalón CM. Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol. 1990;15( Suppl 7):S17–34. [PubMed] [Google Scholar]
- 23.Khan TA, Bianchi C, Ruel M, Voisine P, Li J, Liddicoat JR, et al. Mitogen-Activated Protein Kinase Inhibition and Cardioplegia-Cardiopulmonary Bypass Reduce Coronary Myogenic Tone. Circulation. 2003 Sep 9;108(10 suppl 1):II-348–II-353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- 24.Park KW, Dai HB, Lowenstein E, Sellke FW. Protein kinase C-induced contraction is inhibited by halothane but enhanced by isoflurane in rat coronary arteries. Anesth Analg. 1996 Aug;83(2):286–90. doi: 10.1097/00000539-199608000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet Lond Engl. 2010 Aug 7;376(9739):419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACCORD Study Group, ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010 Jul 15;363(3):233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furnary AP. Rationale for glycemic control in cardiac surgical patients: The portland diabetic project. Insulin. 2006 Sep 1;1(Supplement 1):S24–9. [Google Scholar]