Summary
Our recent study showed that increasing epoxyeicosatrienoic acids (EETs) in kidney by blocking soluble epoxide hydrolase (sEH), an enzyme responsible for EETs degradation, retarded the development of renal dysfunction and progression of aorto-caval (ACF)-induced congestive heart failure (CHF) in Ren-2 transgenic hypertensive rats (TGR). Here we aimed to examine if sEH inhibition, when added to renin-angiotensin system (RAS) blockade, will further enhance protective effect against the development of ACF-induced CHF in TGR. The treatment regimens were started one week after creation of ACF and the follow-up period was 50 weeks. RAS blockade was achieved by administration of angiotensin-converting enzyme inhibitor (ACEi, trandolapril, 6 mg/L) and sEH was blocked with an sEH inhibitor (sEHi, c-AUCB, 3 mg/L). Renal hemodynamics and excretory function were determined two weeks post-ACF, just before the onset of decompensated phase of CHF. After 29 weeks post-ACF, no animal survived. ACEi treatment greatly improved the survival rate (up to 84%) at the end of study. Surprisingly, combined treatment with ACEi and sEHi worsened the rate (53%). Untreated ACF TGR exhibited marked impairment of renal function and the treatment with ACEi alone or combined with sEHi inhibition did not prevent it. In conclusion, we found that addition of sEHi to ACEi treatment did not provide better protection against CHF progression and did not increase the survival rate in ACF TGR: indeed, the rate decreased significantly. Thus, combined treatment with sEHi and ACEi seems no promising approach to further attenuate renal dysfunction and retard progression of CHF.
Keywords: congestive heart failure, renal dysfunction, hypertension, aorto-caval fistula, epoxyeicosatrienoic acids, soluble epoxide hydrolase, renin-angiotensin system
Introduction
Congestive heart failure (CHF) represents a serious public health problem with world-wide prevalence of 1–2% and the yearly increase in the number of new patients estimated at 50%1–4. Despite advances in the treatment, the survival rate of patients with CHF is below that for many common malignancies, especially in cases when CHF is associated with impairment of renal hemodynamics and sodium retention3–7. It is now recognized that renin-angiotensin system (RAS) plays a critical role in the pathophysiology of CHF, but also in the development of renal dysfunction in CHF, and in hypertension in general8–18. Angiotensin-converting enzyme (ACE) blockade has become an integral component of the treatment of CHF and has beneficial long-term effects in experimental and clinical CHF, therefore, it is considered a golden standard therapy. However, its effectiveness in the advanced phase of CHF is limited3,9,19–29. Thus, despite a considerable progress in therapy and many treatment options available, the mortality in CHF remains high, and almost 50% of patients die within 5 years of the diagnosis. Evidently, new treatment strategies are urgently needed3,4,7,9,28,29, however, the prerequisite here is a better understanding of the pathophysiology of CHF. Therefore there is an obvious need for focused experimental studies that would evaluate the effects of new therapeutic approaches.
The rat with aorto-caval fistula (ACF) presents a well-defined model of CHF dependent on volume overload, characterized also by activation of the systemic and intrarenal RAS, signs of cardiac remodeling as well as congestion and impairment of renal function. Thus, in many aspects the model reproduces the course of CHF in untreated human patients21–24,30–35.
The hypertensive rat transgenic for the mouse Ren-2 renin gene [TGR; strain name TGR(mRen2)27] presents a unique angiotensin II (ANG II)-dependent model of hypertension in which its development is attributed to a single gene alteration and to the augmented activation of endogenous RAS36–38. Thus, an experimental model of CHF that combines the well-recognized crucial detrimental factors promoting the progression of CHF is available and we have recently shown ACF TGR exhibit markedly increased CHF-related mortality as compared with the course in ACF Hannover Sprague-Dawley (HanSD) rats (transgene-negative, normotensive controls to TGR)23,35. We found that in addition to the increased RAS activity, ACF TGR also displayed tissue deficiency of biologically active fatty acid epoxides due to increased conversion of epoxyeicosatrienoic acids (EETs) [biologically active metabolites of cytochrome P450 (CYP)-dependent epoxygenase pathway of arachidonic acid (AA)] by soluble epoxide hydrolase (sEH) to biologically inactive dihydroxyeicosatrienoic acids (DHETEs). Indeed, increasing EETs in the kidney by pharmacological blockade of sEH markedly attenuated the development of renal dysfunction and progression of CHF in ACF TGR23. These findings suggest that pharmacological increasing EETs could be a novel tool for the treatment of CHF. However, it is important to recognize that we did not check if addition of sEH inhibition to the standard RAS blockade would exhibit additive beneficial effects on the mortality in TGR with ACF-induced CHF. Considering this limitation, we examined in the present study whether the combined treatment with sEH inhibitor (sEHi) and ACEi will bring enhanced protection against CHF-dependent mortality as compared with results achieved with ACEi treatment alone.
To further elucidate possible mechanisms underlying the expected additive beneficial action of combined sEH and ACE inhibition on the course of ACF-induced CHF, effects of 2-week treatments (i.e. 3 weeks after induction of ACF) on renal function were assessed by clearance methods in a separate groups of animals.
Results
Series 1: Effects of treatment with ACEi alone and combined treatment with sEHi and ACEi on the survival rate
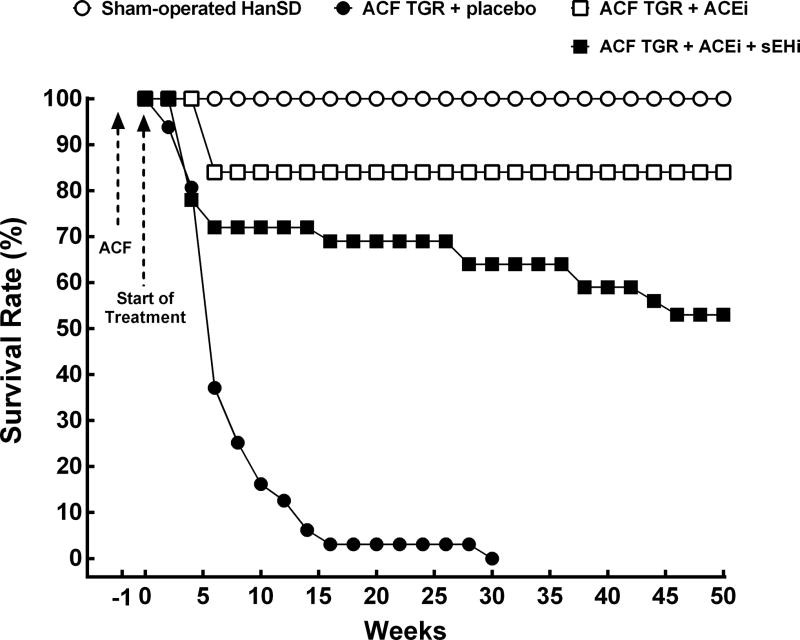
All sham-operated HanSD rats survived until the end of the experiment. One sham-operated TGR unpredictably died 25 weeks after sham-operation, all the others survived until the end of experiment. As shown in Figure 1, untreated ACF TGR began to die by week 2 (i.e. 3 weeks after induction of ACF) and all the animals died by week 29. The treatment with ACEi substantially increased the survival rate, to 84% at the end of study as compared with untreated ACF TGR (p<0.05). The combined treatment with sEHi and ACEi also improved the survival rate throughout the experiment (the final survival rate was 53%) as compared with untreated ACF TGR (p<0.05), however, the actual rate was significantly lower than observed in ACF TGR treated with ACEi alone (p<0.05).
Figure 1.
Survival rates in sham-operated transgene negative Hannover Sprague-Dawley (HanSD) and sham-operated heterozygous Ren-2 transgenic rats (TGR), in untreated TGR with aorto-caval fistula (ACF TGR + placebo), in ACF TGR treated with angiotensin-converting enzyme inhibitor (ACF TGR + ACEi) and in ACF TGR treated with the combination of angiotensin-converting enzyme inhibitor and soluble epoxide hydrolase inhibitor (ACF TGR + ACEi + sEHi). The survival rate curve in ACF TGR + placebo was significantly lower compared to those in the other groups. Both treatments significantly improved the survival rate curve, however the best results were observed in ACF TGR + ACEi + sEHi group (log-rank Mantel-Cox test followed by Gehan-Breslow-Wilcoxon test).
Series 1: Effects of 2-week treatment with ACEi alone and combined treatment with sEHi and ACEi on organ weights, blood pressure and renal hemodynamics and excretory function
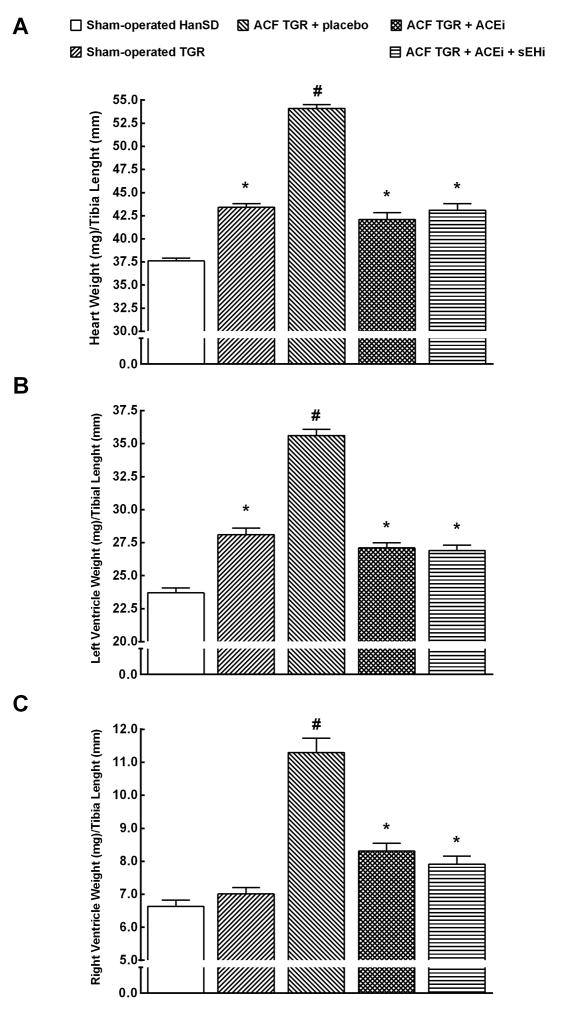
As shown in Figures 2A and 2B, sham-operated TGR exhibited significant cardiac hypertrophy [expressed as whole heart weight (HW) to tibia length (TL)], with marked left ventricle (LV) hypertrophy [expressed as LV weight (LVW) to tibia length (TL)] as compared with sham-operated HanSD rats (p<0.05). Untreated ACF TGR showed marked increases in HW/TL as well as in LVW/TL as compared with sham-operated TGR (p<0.05). The treatment with ACEi alone as well as the combined treatment with sEH inhibitor and ACEi significantly lowered HW/TL as well as LVW/TL to values observed in sham-operated TGR. Figure 2C shows that there were no significant differences in right ventricle (RV) weight [expressed as RV weight (RVW) to TL] between sham-operated HanSD rats and sham-operated TGR. Untreated ACF TGR showed striking increases in RVW/TL as compared with sham-operated TGR (indicating the development of marked RV cardiac hypertrophy) (p<0.05). Both treatment regimes significantly and to a similar degree decreased RVW/TL in ACF TGR.
Figure 2.
Whole heart weight (A), left ventricle weight (B) and right ventricle weight (C) normalized to tibia length in sham-operated transgene negative Hannover Sprague-Dawley (HanSD) and sham-operated heterozygous Ren-2 transgenic rats (TGR), in untreated TGR with aorto-caval fistula (ACF TGR + placebo), in ACF TGR treated with angiotensin-converting enzyme inhibitor (ACF TGR + ACEi) and in ACF TGR treated with the combination of angiotensin-converting enzyme inhibitor and soluble epoxide hydrolase inhibitor (ACF TGR + ACEi + sEHi). * P<0.05 compared with sham-operated HanSD. # P<0.05 compared with all the other groups.
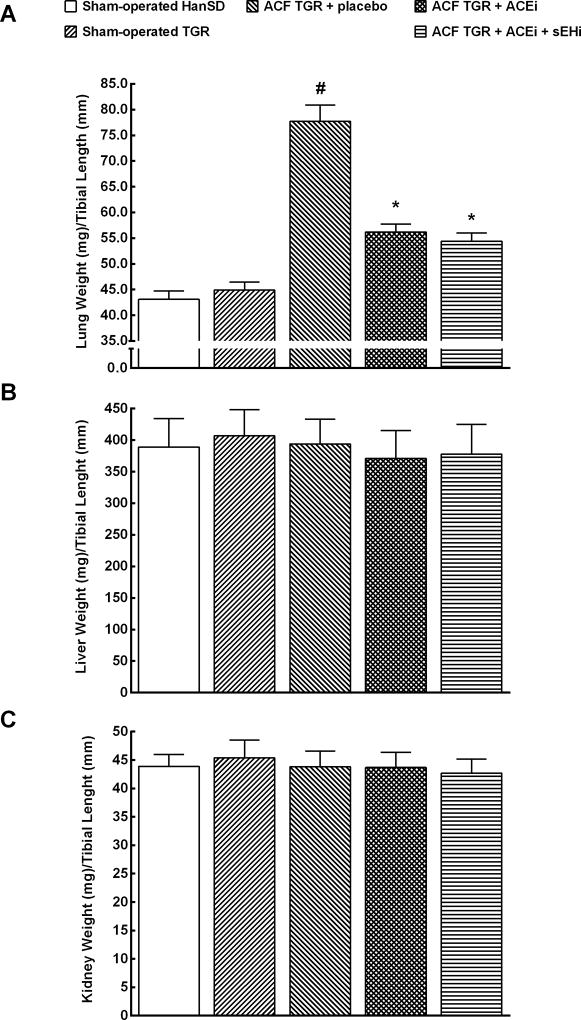
As shown in Figure 3A, untreated ACF TGR displayed significantly higher ratio of lung weight to TL as compared with sham-operated HanSD rats as well as sham-operated TGR (indicating the development of important lung congestion in ACF TGR) (p<0.05 in all cases). The treatment with ACEi alone as well as the combined treatment with sEHi and ACEi significantly lowered this ratio in ACF TGR, but it still remained significantly higher than in sham-operated TGR (p<0.05).
Figure 3.
Lung weight (A), liver weight (B) and kidney weight (C) normalized to tibia length in sham-operated transgene negative Hannover Sprague-Dawley (HanSD) and sham-operated heterozygous Ren-2 transgenic rats (TGR), in untreated TGR with aorto-caval fistula (ACF TGR + placebo), in ACF TGR treated with angiotensin-converting enzyme inhibitor (ACF TGR + ACEi) and in ACF TGR treated with the combination of angiotensin-converting enzyme inhibitor and soluble epoxide hydrolase inhibitor (ACF TGR + ACEi + sEHi). * P<0.05 compared with sham-operated HanSD. # P<0.05 compared with all other groups.
There were no significant differences between experimental groups in liver and kidney weight when normalized to TL (Figures 3B and 3C).
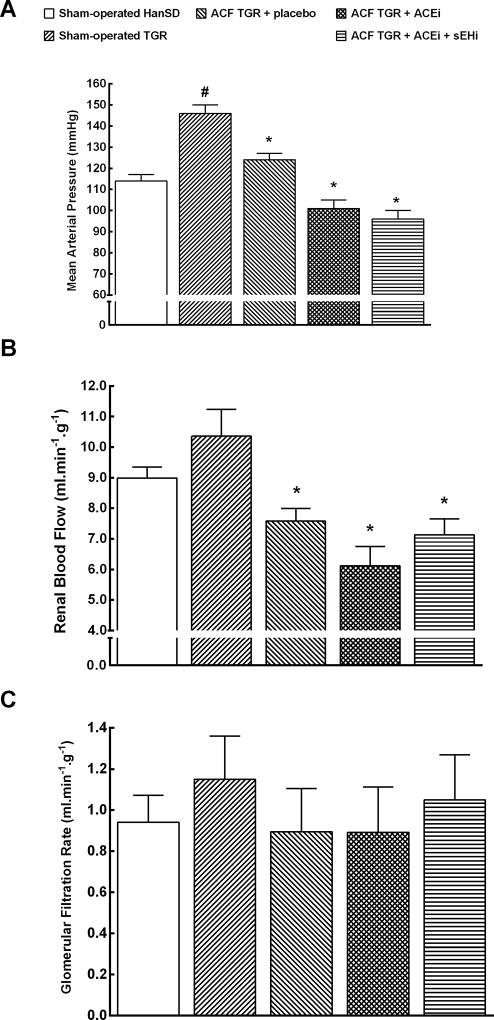
As shown in Figure 4A, sham-operated TGR were markedly hypertensive as compared with sham-operated HanSD rats (p<0.05). Untreated ACF TGR showed significantly lower mean arterial pressure (MAP) as compared with sham-operated TGR, but it still remained higher than MAP values in sham-operated HanSD rats (p<0.05). The treatment with ACEi alone as well as the combined treatment with sEHi and ACEi significantly lowered MAP in ACF TGR as compared with their untreated counterparts and the values of MAP were even significantly lower than in sham-operated HanSD rats (p<0.05).
Figure 4.
Mean arterial pressure (A), renal blood flow (B) and glomerular filtration rate (C) in sham-operated transgene negative Hannover Sprague-Dawley (HanSD) and sham-operated heterozygous Ren-2 transgenic rats (TGR), in untreated TGR with aorto-caval fistula (ACF TGR + placebo), in ACF TGR treated with angiotensin-converting enzyme inhibitor (ACF TGR + ACEi) and in ACF TGR treated with the combination of angiotensin-converting enzyme inhibitor and soluble epoxide hydrolase inhibitor (ACF TGR + ACEi + sEHi). * P<0.05 compared with sham-operated HanSD. # P<0.05 compared with all the other groups.
There were no significant differences in renal blood flow (RBF) between sham-operated HanSD rats and sham-operated TGR (Figure 4B). Untreated ACF TGR exhibited significantly lower RBF as compared with sham-operated TGR (p<0.05). The treatment with ACEi alone as well as the combined treatment with sEHi and ACEi did not significantly change RBF in ACF TGR. There were no significant differences in the glomerular filtration rate (GFR) between experimental groups (Figure 4C).
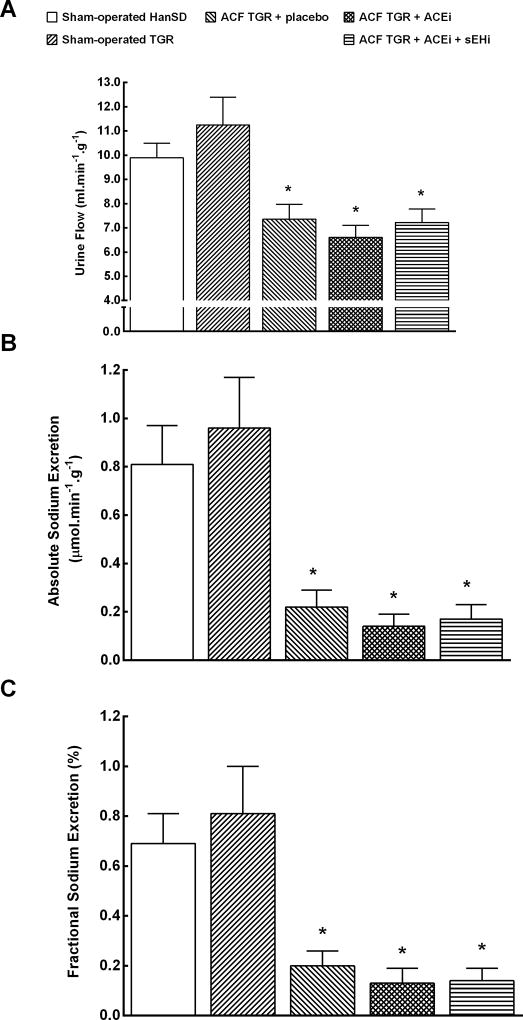
Figures 5A, 5B and 5C show that untreated ACF TGR displayed substantially lower urine flow and absolute and fractional sodium excretion as compared with sham-operated HanSD rats and sham-operated TGR (p<0.05 in all cases). The treatment with ACEi alone or the combined treatment with sEHi and ACEi did not normalize any of these parameters in ACF TGR.
Figure 5.
Urine flow (A), absolute sodium excretion (B) and fractional sodium excretion (C) in sham-operated transgene negative Hannover Sprague-Dawley (HanSD) and sham-operated heterozygous Ren-2 transgenic rats (TGR), in untreated TGR with aorto-caval fistula (ACF TGR + placebo), in ACF TGR treated with angiotensin-converting enzyme inhibitor (ACF TGR + ACEi) and in ACF TGR treated with the combination of angiotensin-converting enzyme inhibitor and soluble epoxide hydrolase inhibitor (ACF TGR + ACEi + sEHi). * P<0.05 compared with sham-operated HanSD.
Discussion
The crucial finding of this study is that addition of sEH blockade to standard ACEi therapy did not improve the survival rate and did not attenuate the development of renal dysfunction in ACF TGR. Surprisingly, the combined treatment worsened the survival rate as compared with ACF TGR treated with ACEi alone. These findings deserve special attention for several reasons.
It is noteworthy that as soon as three weeks after induction of ACF untreated ACF TGR displayed signs of marked cardiac hypertrophy accompanied by lung congestion (indicating LV heart failure) without symptoms of RV failure (no liver congestion). These findings indicate that already by this time untreated ACF TGR were in the transition stage from the compensated to decompensated heart hypertrophy and heart failure, which was also reflected by the onset of CHF-dependent mortality. This is remarkable because we and others have reported that in normotensive animals at least 10 weeks after induction of ACF-induced volume overload are required before the development of CHF symptoms22–24,31–43 and the first deaths occur usually around the 20th week. Moreover, in our original study that characterized the course of CHF-related morbidity and mortality in the same model of normotensive rats we found a median survival of 43 weeks after ACF induction33. Thus, our present results strengthen the evidence that hypertension and markedly activated RAS are two critical features promoting progression of CHF to the fatal end.
Furthermore, it was seen that already three weeks after induction of ACF untreated ACF TGR displayed marked impairment of renal hemodynamics and renal sodium excretion, which agrees with the observation that in patients with CHF the development of renal dysfunction is associated with markedly increased risk of death5,6,10,11. Moreover, the present results further support our earlier findings suggesting that persistent renal dysfunction rather than progressing cardiac remodeling is the determinant of long-term survival rate in ACF-induced model of CHF. Indeed, it appeared that in this model predominantly renal mechanisms determine the beneficial effects of the treatment tools which delay the decompensation of CHF23,24. However, it is emphasized that in ACF TGR the development of renal dysfunction is accelerated compared with normotensive animals, which again supports the notion on the negative effects of hypertension and inappropriately activated RAS on the course of CHF8,9,12–15,28,29.
We found that chronic treatment with ACEi dramatically improved the survival rate in ACF TGR, which again confirms the view on the crucial role of the RAS in the pathophysiology of CHF, and wide agreement on pharmacological blockade of the RAS by ACE inhibition being the first-line treatment in CHF3,9,12–15,22–29. Nevertheless, even though the effectiveness of ACEi treatment was relatively high in the advanced phase of CHF, it did not prevent the development of renal dysfunction and CHF-related mortality was still significant, which further underscores the need to search for new pharmacological strategies that would prevent renal dysfunction and progression CHF to the fatal end.
In this connection, the critically important question is why the combined treatment with sEHi and ACEi did not enhance protection against CHF-dependent mortality but even worsened it as compared to the ACF TGR treated with ACEi alone. To this we have no satisfactory explanation: our hypothesis was that combining the two treatments, each affecting different vasoactive system, should enhance the effectiveness in delaying or even preventing decompensation in CHF. The hypothesis was based on the strong evidence, provided by ourselves and others, indicating that ACF model of CHF is characterized by marked activation of vasoconstrictor/sodium retaining axis and suppression of vasodilatory/natriuretic axis of the intrarenal RAS, and by profound intrarenal deficiency of biologically active epoxy fatty acids (the result of increased sEH-mediated conversion of EETs to DHETEs)23,24,30,32. In addition, we showed recently that treatment with sEHi alone and ACEi alone (same drugs and same doses as employed in the present study) exhibited clear beneficial effects on the course of CHF in ACF TGR, each treatment altering a different vasoactive system23. Importantly, the beneficial effects of chronic sEH inhibition with c-AUCB was accompanied by normalization of tissue availability of EETs, without altering intrarenal concentrations of ANG II and angiotensin-1–7 (ANG 1–7). Dissimilarly, protective effects of chronic treatment with ACEi were associated with significant suppression of the intrarenal vasoconstrictor/sodium retaining axis (reflected by profound decreases in ANG levels) and activation of the intrarenal vasodilatory/natriuretic axis (reflected by increases in ANG 1–7 concentrations) without modifying tissue availability of biologically active epoxy fatty acids (reflected the EETs concentrations). Collectively, these data provided robust evidence which encouraged testing our original hypothesis that the combined treatment with sEHi and ACEi should bring enhanced beneficial effects on the course of CHF. Surprisingly, this was not the case, and for unknown reasons the survival rate even decreased. An appropriately focused study, rather than undue speculation, would be needed to address this issue. We feel that two aspects deserve attention.
First, even in the absence of any evidence on toxic effects of sEHi and ACEi at the doses employed9,23,24,28,29,44,45, some untoward action of their concurrent long-term co-administration cannot be excluded. Thus, appropriate complex toxicological studies are needed.
Second, it will be noticed that both sEHi and ACEi exhibit also important blood pressure (BP)-lowering effects, related to the increased intrarenal EETs bioavailability and their vasodilatory and natriuretic actions (sEHi)44,46–48 or to the intrarenal blockade of the RAS activity (ACEi)16,49. Therefore, the combined treatment could in the long term exhibit additivel BP-lowering effects, perhaps bringing BP below renal autoregulation range50. This could per se impair renal function in ACF TGR and thereby increase CHF-related mortality in this model. Nevertheless, our current data show that the MAP value observed after two weeks of combined treatment (96 ± 3 mmHg) did not significantly differ from that recorded in rats receiving ACEi alone (101 ± 3 mmHg, p > 0.05). In either case MAP remained with the range of effective renal autoregulation. To achieve more meaningful comparative data, comprehensive long-term studies are needed that would evaluate BP measured by radiotelemetry in conscious animals. Unfortunately, relatively short durability of implanted telemetric probes limits at present the plausibility of such prolonged experiments.
In conclusion, we found that addition of sEHi to ACEi treatment did not provide better protection against CHF progression and did not increase the survival rate in ACF TGR, indeed, the rate decreased significantly. Thus, increasing bioavailability of tissue EETs in individuals with pharmacologically-induced suppression of the RAS does not seem to be a promising approach to further attenuate renal dysfunction and progression of CHF, at least in this model of CHF.
Methods
Ethical approval, animals, CHF model, and chronic treatments
The studies were performed in accordance with guidelines and practices established by the Animal Care and Use Committee of the Institute for Clinical and Experimental Medicine, Prague, and of the 2nd Faculty of Medicine, Charles University, Prague, which accord with the European Convention on Animal Protection and Guidelines on Research Animal Use. All animals used in the present study were bred at the Center of Experimental Medicine of this Institute, which is accredited by the Czech Association for Accreditation of Laboratory Animal Care. Heterozygous TGR were generated by breeding male homozygous TGR with female homozygous transgene-negative normotensive Hannover Sprague-Dawley (HanSD) rats and age-matched HanSD rats served as controls. The animals were kept on a 12-hour/12-hour light/dark cycle. Throughout the experiments rats were fed a normal salt, normal protein diet (0.45% NaCl, 19–21% protein) manufactured by SEMED (Prague, Czech Republic) and had free access to tap water.
Male TGR and HanSD rats, at the initial age of 8 weeks, derived from several litters, were randomly assigned to experimental groups to make sure that the animals from a single litter did not prevail in any group. In order to obtain reliable data regarding the effects of two treatment regimes on the survival rate, high initial n values were used (not so for sham-operated animals) to enable valid comparison of the long-term survival rate.
Rats were anesthetized (tiletamine + zolazepam, Virbac SA, Carros Cedex, France, 8 mg/kg; and xylasine, Spofa, Czech Republic, 4 mg/kg intramuscularly) and CHF was induced by volume overload dependent on ACF created using needle technique as originally described by Garcia and Diebold51 and employed and validated by many investigators including our own group21–25,30–35. Briefly, after exposure of the abdominal aorta and inferior vena cava between the renal arteries and iliac bifurcation, the aorta was temporarily occluded at this segment for about 40 seconds. An 18-gauge needle (diameter 1.2 mm) was inserted into the abdominal aorta and advanced across its wall into the inferior vena cava to create ACF. Thereafter the needle was withdrawn and the puncture site was sealed with cyanoacrylate tissue glue. Successful creation of ACF was confirmed by inspection of pulsatile flow of oxygenated blood from the abdominal aorta into the vena cava. Sham-operated rats underwent an identical procedure but without creating ACF.
An inhibitor sEHi cis-4-[4-(3-adamantan-1-yl-ureido) cyclohexyloxy]benzoic acid (c-AUCB) was used, which was prepared freshly and given in drinking water at 3 mg/L. The appropriate amount of c-AUCB was dissolved with gentle warming in polyethyleneglycol and added with rapid stirring to warm drinking water to obtain a 0.1% aqueous solution of polyethylenglycol. The dose of c-AUCB was selected based on our recent studies where it elicited substantial increases in tissue concentration of EETs without altering RAS activity52. Similarly as in previous studies, we chose the c-AUCB dose that blocks sEH activity without altering plasma and tissue ANG II levels with an intention to separate and assess the effect of EETs elevation alone on the course of ACF-induced CHF. Trandolapril (6 mg/L in drinking water; Gopten; Abbot, Prague, Czech Republic), was used to inhibit ACE because in our previous studies and here in preliminary experiments we demonstrated that at this dose the drug provided maximal blockade of RAS and was well tolerated both by rats with ACF-induced CHF and by sham-operated animals23,24.
Series 1: Effects of treatment with ACEi alone and combined treatment with sEHi and ACEi on the survival rate
The rats underwent sham-operation or ACF creation as described above on the week labeled “−1” and were left without treatment during 1 week. At this time point (week 0) the rats were divided in the following experimental groups:
Sham-operated HanSD rats + placebo (initial n = 7)
Sham-operated TGR + placebo (initial n = 9)
ACF TGR + placebo (initial n = 32)
ACF TGR + ACEi (initial n = 32)
ACF TGR + ACEi + sEHi (initial n = 36)
The follow-up period was same as in our previous studies, i.e. 50 weeks.
Series 1: Effects of 2-week treatment with ACEi alone and combined treatment with sEHi and ACEi on organ weights, blood pressure and renal hemodynamics and excretory function
Animals were prepared as described in series 1 and on week 0 the pharmacological treatment was initiated for a period of 2 weeks. At the end of the experimental protocol (on week +2), the rats were anesthetized and acute clearance experiments were performed to evaluate renal hemodynamics and excretory parameters as described in our previous studies23,24,53. The following experimental groups were studied:
Sham-operated HanSD rats + placebo (n = 12)
Sham-operated TGR + placebo (initial n = 10)
ACF TGR + placebo (initial n = 11)
ACF TGR + ACEi (initial n = 10)
ACF TGR + ACEi + sEHi (initial n = 10)
Statistical analysis
Statistical analysis of the data was performed using Graph-Pad Prism software (Graph Pad Software, San Diego, California, USA). Comparison of survival curves was performed by log-rank (Mantel-Cox) test followed by Gehan-Breslow-Wilcoxon test. Statistical comparison of other results was made by Student´s t-test, Wilcoxon´s signed-rank test for unpaired data or one-way ANOVA when appropriate. Values are expressed as mean ± S.E.M. and n represents the number of animals. A p value less than 0.05 was considered statistically significant.
Acknowledgments
This study was primarily supported by the Ministry of Health of the Czech Republic grant no. 17-28220A awarded to M. Táborský. All rights reserved. L.Č. was also supported by Ministry of Health of the Czech Republic within the project for the development of research organization 00023001 (IKEM)- institutional support.
References
- 1.Roger VL. Epidemiology of heart failure. Circ. Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. The war against heart failure. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco C, Haapio M, House AA, Avavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Giamouzis G, Kalogeroupoulos AP, Butler J, et al. Epidemiology and importance of renal dysfunction in heart failure patients. Curr Heart Fail. Rep. 2013;10:411–420. doi: 10.1007/s11897-013-0164-6. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More “malignant” than cancer? Five year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa I, Pfeffer JM, Pfeffer MA, Hostetter TH, Brenner BM. Role of angiotensin II in the altered renal function of congestive heart failure. Circ. Res. 1984;55:669–675. doi: 10.1161/01.res.55.5.669. [DOI] [PubMed] [Google Scholar]
- 9.Moayedi Y, Ross HJ. Advances in heart failure: a review of biomarkers, emerging pharmacological therapies, durable mechanical support and telemonitoring. Clin. Sci. 2017;131:553–566. doi: 10.1042/CS20160196. [DOI] [PubMed] [Google Scholar]
- 10.Braam B, Joles JA, Daniswar AH, Gaillard CA. Cardiorenal syndrome – current understanding and future perspectives. Nat Rev Nephrol. 2014;10:48–55. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 11.Re RN. A reassessment of the pathophysiology of progressive cardiorenal disorders. Med Clin North. Am. 2017;101:103–115. doi: 10.1016/j.mcna.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Dube P, Weber KT. Congestive heart failure: pathophysiologic consequences of neurohormonal activation and the potential for recovery: part I. Am J. Med Sci. 2011;342:348–351. doi: 10.1097/MAJ.0b013e318232750d. [DOI] [PubMed] [Google Scholar]
- 13.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi F, Mascolo A, Mollace V. The pathophysiological role of natriuretic peptide-RAAS cross talk in heart failure. Int J Cardiol. 2017;226:121–125. doi: 10.1016/j.ijcard.2016.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, McMurray JJV. Importance of endogenous compensatory vasoactive peptides in broadening the effects of inhibitors of the renin-angiotensin system for the treatment of heart failure. Lancet. 2017;389:1831–1840. doi: 10.1016/S0140-6736(16)30969-2. [DOI] [PubMed] [Google Scholar]
- 16.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Granger JP, Hall ME. Physiology and pathophysiology of hypertension. In: Albeprn RJ, Caplan MJ, Moe OW, editors. Seldin and Giebisch´s The Kidney physiology and pathophysiology. fifth. Academic Press; 2013. pp. 1319–1352. [Google Scholar]
- 18.Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res. 2017 doi: 10.1016/j.phrs.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Pfeffer JM, Steinberg C, Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;2:406–412. doi: 10.1161/01.cir.72.2.406. [DOI] [PubMed] [Google Scholar]
- 20.Brands MW, Alonso-Galicia M, Mizelle HL, Montani JP, Hildebrandt DA, Hall JE. Chronic angiotensin-converting-enzyme inhibition improves cardiac output and fluid balance during heart failure. Am J Physiol. 1993;264:R414–R422. doi: 10.1152/ajpregu.1993.264.2.R414. [DOI] [PubMed] [Google Scholar]
- 21.Ruzicka M, Yuan B, Leenen FHH. Effects of enalapril versus losartan on regression of volume overload-induced cardiac hypertrophy in rats. Circulation. 1994;90:484–491. doi: 10.1161/01.cir.90.1.484. [DOI] [PubMed] [Google Scholar]
- 22.Brower GL, Levick SP, Janicki JS. Differential effects of prevention and reversal treatment with Lisinopril on left ventricular remodeling in a rat model of heart failure. Heart Lung Circ. 2015;24:919–924. doi: 10.1016/j.hlc.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Červenka L, Melenovský V, Husková Z, Škaroupková P, Nishiyama A, Sadowski J. Inhibition of soluble epoxide hydrolase counteracts the development of renal dysfunction and progression of congestive heart failure in Ren-2 transgenic hypertensive rats with aorto-caval fistula. Clin Exp Pharmacol Physiol. 2015;42:795–807. doi: 10.1111/1440-1681.12419. [DOI] [PubMed] [Google Scholar]
- 24.Červenka L, Melenovský V, Husková Z, et al. Inhibition of soluble epoxide hydrolase does not improve the course of congestive heart failure and the development of renal dysfunction in rats with volume overload induced by aorto-caval fistula. Physiol. Res. 2015;64:857–873. doi: 10.33549/physiolres.932977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J. Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 26.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J. Med. 1992;327:658–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 27.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guideliness for the diagnois and treatment of acute and chronic heart failure: The Task Force of the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardioloy (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–21200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused updatae of the 2013 ACCF/AHA Guideline for the management of heart failure. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Segev R, Francis B, Abu-Saleh N, et al. Cardiac and renal distribution of ACE and ACE-2 in rats with heart failure. Acta Histiochem. 2014;116:1342–1349. doi: 10.1016/j.acthis.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Oliver-Dussault C, Ascah A, Marcil M, et al. Early predictors of cardiac decompensation in experimental volume overload. Mol Cell Biochem. 2010;338:271–281. doi: 10.1007/s11010-009-0361-5. [DOI] [PubMed] [Google Scholar]
- 32.Abassi Z, Goltsmna I, Karram T, Winaver J, Horrman A. Aortocaval fistula in rat: a unique model of volume-overload congestive heart failure and cardiac hypertrophy. J Biomed Biotechnol. 2011:729497. doi: 10.1155/2011/729497. [DOI] [PMC free article] [PubMed]
- 33.Melenovsky V, Skaroupkova P, Benes J, Torresova V, Kopkan L, Cervenka L. The course of heart failure development and mortality in rats with volume overload due to aorto-caval fistula. Kidney Blood Press. Res. 2012;35:167–173. doi: 10.1159/000331562. [DOI] [PubMed] [Google Scholar]
- 34.Melenovský V, Benes J, Skaroupkova P, et al. Metabolic characterization of volume overload heart failure due to aorto-caval fistula in rats. Mol Cell Biochem. 2011;354:83–96. doi: 10.1007/s11010-011-0808-3. [DOI] [PubMed] [Google Scholar]
- 35.Červenka L, Škaroupková P, Kompanowska-Jezierska E, Sadowski J. Sex-linked differences in the course of chronic kidney disease and congestive heart failure: a study in 5/6 nephrectomized Ren-2 transgenic hypertensive rats with volume overload using aorto-caval fistula. Clin Exp Pharmacol Physiol. 2016;43:883–895. doi: 10.1111/1440-1681.12619. [DOI] [PubMed] [Google Scholar]
- 36.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harboring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 37.Lee MA, Böhm M, Paul M, Bader M, Ganten U, Ganten D. Physiological characterization of the hypertensive transgenic rat TGR(mRen2)27. Am J Physiol. 1990;270:E919–E929. doi: 10.1152/ajpendo.1996.270.6.E919. [DOI] [PubMed] [Google Scholar]
- 38.Kujal P, Certíková Chábová V, Vernerová Z, et al. Similar renoprotection after renin-angiotensin-dependent and -independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: are there blood pressure-independent effects? Clin Exp Pharmacol Physiol. 2010;37:1159–1169. doi: 10.1111/j.1440-1681.2010.05453.x. [DOI] [PubMed] [Google Scholar]
- 39.Beneš J, Kazdová L, Drahota Z, et al. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin Sci. 2011;121:29–41. doi: 10.1042/CS20100527. [DOI] [PubMed] [Google Scholar]
- 40.Hutchinson KR, Guggilam A, Cismowski MJ, et al. Temporal pattern of left ventricle structural and functional remodeling following reversal of volume overload heart failure. J App Physiol. 2011;111:1778–1788. doi: 10.1152/japplphysiol.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol. 1996;40:H2071–H2078. doi: 10.1152/ajpheart.1996.271.5.H2071. [DOI] [PubMed] [Google Scholar]
- 42.Wang XI, Ren B, Liu S, et al. Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J App Physiol. 2003;94:752–763. doi: 10.1152/japplphysiol.00248.2002. [DOI] [PubMed] [Google Scholar]
- 43.Brower GL, Janicki JS. Contribution of ventricular remodeling to the pathogenesis of heart failure in rats. Am J Physiol. 2001;280:H674–H683. doi: 10.1152/ajpheart.2001.280.2.H674. [DOI] [PubMed] [Google Scholar]
- 44.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev. 2014;66:1106–1140. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 45.El-Sherbeni AA, Aboutabl ME, Zordoky BNM, Anwar-Mohamed A, El-Kadi AOS. Determination of the dominant arachidonic acid cytochrome P450 monooxygenase in rat heart, lung, kidney, and liver: protein expression and metabolite kinetics. AAPS J. 2013;15:112–122. doi: 10.1208/s12248-012-9425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol. 2012;302:R321–R330. doi: 10.1152/ajpregu.00606.2011. [DOI] [PubMed] [Google Scholar]
- 48.Fan F, Roman RJ. Effect of cytochrome P450 metabolites of arachidonic acid in Nephrology. J Am Soc Nephrol. 2017;28 doi: 10.1681/ASN.2017030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol. Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc. Res. 1990;24:430–432. doi: 10.1093/cvr/24.5.430. [DOI] [PubMed] [Google Scholar]
- 52.Sporková A, Jíchová Š, Husková Z, et al. Different mechanism of acute versus long-term antihypertensive effects of soluble epoxide hydrolase inhibition: studies in Cyp1a1-Ren-2 transgenic rats. Clin Exp Pharmacol Physiol. 2014;41:1003–1013. doi: 10.1111/1440-1681.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honetschlägerová Z, Husková Z, Vaňourková Z, et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J. Physiol. 2011;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]