Abstract
As early as the 1990’s, chronic critical illness (CCI), a distinct syndrome of persistent high-acuity illness requiring management in the intensive care unit (ICU), was reported under a variety of descriptive terms including the “neuropathy of critical illness,” “myopathy of critical illness,” “ICU acquired weakness,” and most recently “post intensive care unit syndrome”. The widespread implementation of targeted shock resuscitation, improved organ support modalities, and evidence-based protocolized ICU care has resulted in significantly decreased in-hospital mortality within surgical ICUs (SICU), specifically by reducing early multiple organ failure (MOF) deaths. However, a new phenotype of MOF has now emerged with persistent, but manageable organ dysfunction, high resource utilization, and discharge to prolonged care facilities. This new MOF phenotype is now clinically associated with the rapidly increasing incidence of CCI in critically ill surgery patients. While the underlying pathophysiology driving CCI remains incompletely described, the Persistent, Inflammation, Immunosuppression and Catabolism syndrome (PICS) has been proposed as a mechanistic framework in which to explain the increased incidence of CCI in SICUs. The purpose of this review is to provide a historic perspective of the epidemiologic evolution of MOF into PICS, describe the mechanism of PICS that drive and sustain CCI, and review the long-term outcomes of surgical patients who develop CCI.
Keywords: myeloid derived suppressor cell, sepsis, chronic critical illness, persistent inflammation immunosuppression catabolism syndrome, trauma
Introduction
As early as the 1990’s, chronic critical illness (CCI), a distinct syndrome of persistent, high-acuity illness requiring management in the intensive care unit (ICU), was reported under a variety of descriptive terms, including the “neuropathy of critical illness,” “myopathy of critical illness,” “ICU-acquired weakness,” and most recently “post intensive care unit syndrome”. The widespread implementation of targeted shock resuscitation, improved organ support modalities, and evidence-basedprotocolized ICU care has resulted in substantially decreased in-hospital mortality within surgical ICUs (SICU), specifically by decreasing deaths from early multiple organ failure (MOF). Within this setting, a new phenotype of MOF has now emerged with persistent, but manageable organ dysfunction, high resource utilization, and discharge to prolonged care facilities. This new MOF phenotype is now clinically associated with the rapidly increasing incidence of CCI in critically ill surgery patients. While the underlying pathophysiology driving CCI remains incompletely described, the Persistent, Inflammation, Immunosuppression and Catabolism syndrome (PICS) has been proposed as a mechanistic framework in which to explain the increased incidence of CCI in SICUs. The purpose of this review is to provide a historic perspective of the epidemiologic evolution of MOF into PICS, describe the mechanism(s) of PICS that drives and sustains CCI, and review the long-term outcomes of surgical patients who develop CCI.
Evolving Epidemiology of MOF into the Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS)
The advent of ICUs in the early 1970s facilitated survival of patients with single organ failure; concurrently, MOF emerged as a highly lethal syndrome (with mortality greater than 80%). Since then, MOF has plagued ICUs for over four decades, and its epidemiology has evolved as advances in critical care have allowed patients to survive previously lethal insults. Over the years, different predominant clinical presentations of MOF have come and gone, all having consumed tremendous health care resources with associated prolonged ICU stays and prohibitive mortality.(1)
Early case series from the United States (US) concluded that MOF occurred as the result of uncontrolled sepsis (principally intra-abdominal infections), and research efforts were effectively focused on curbing this condition. In the mid-1980s, European studies reported MOF frequently after blunt trauma with no identifiable site of infection.(2) Investigators then recognized that both infectious and noninfectious insults could induce a similar, overwhelming, destructive systemic inflammatory response syndrome (SIRS). Research thus shifted to determining the underlying mechanisms of this phenomenon (e.g. bacterial translocation, ‘cytokine storm’, ischemia-reperfusion, etc.). Simultaneously through the early 1990s, tremendous advances in trauma care substantially decreased early deaths from bleeding, but resulted in an increase either in the recognition or the incidence of the abdominal compartment syndrome that emerged in ICUs worldwide.
While clinical interest focused on understanding abdominal compartment syndrome as a consequence of a prioritization of aggressive volume resuscitation with the goal to restore end organ perfusion and prevent the progression to early MOF and death, epidemiologic studies revealed that MOF was subsequently evolving into a bimodal phenomenon with decreasing early and increasing late mortality.(3–7) Early MOF occurred after either an initial severe insult (one-hit model) or sequential amplifying insults (two-hit model), whereas late MOF was precipitated by secondary nosocomial infections.(3) The compensatory anti-inflammatory response syndrome (CARS) was proposed to follow SIRS and seemed to explain this increased susceptibility to infection and bimodal distribution of MOF.(8) SIRS-induced early MOF was thought to occur because of exaggerated innate immune and inflammatory response, whereas CARS was viewed as progressive depression in adaptive immunity, resulting in secondary infections.
By the late 1990s, fundamental changes in the initial care of patients arriving with severe bleeding were implemented widely, including the use of ultrasonography and the FAST exam, massive transfusion protocols, avoidance of excessive crystalloids, and abandonment of pulmonary artery catheter-directed resuscitation. Subsequently, the incidence of abdominal compartment syndrome decreased.(9) Concordantly, evidence-based medicine became a health care mandate as well as a major driver for improved ICU care. As a result of these initiatives, there has been another striking change in the epidemiology of MOF. Early in-hospital mortality decreased substantially, and the incidence of late-onset MOF-related deaths has largely disappeared.(7)
A substantial portion of high-acuity patients with MOF, however, survive after prolonged ICU stays and progress into a chronic critical illness characterized by “persistent inflammation, immunosuppression and catabolism” (Figure 1).(5) Contrary to the bimodal paradigm, inflammation co-occurs with immunosuppression and anti-inflammation following major acute events, such as severe trauma, burns, pancreatitis, and sepsis.(10–12) For some patients, an initial overly robust and dysfunctional inflammatory response leads to a trajectory of early MOF and fulminant death. Fortunately, advances in modern ICU care and modalities of organ support allow medical practitioners to detect and prevent the trajectory of this previously fatal expression. If patients do not succumb to early MOF, they follow one of two pathways: (A) the patient is rapidly restored to immunologic homeostasis; or (B) immunologic dysfunction persists and leads to chronic critical illness (CCI), characterized by persistent organ dysfunction requiring ICU resources for >14 days.(11, 13, 14)
Figure 1.
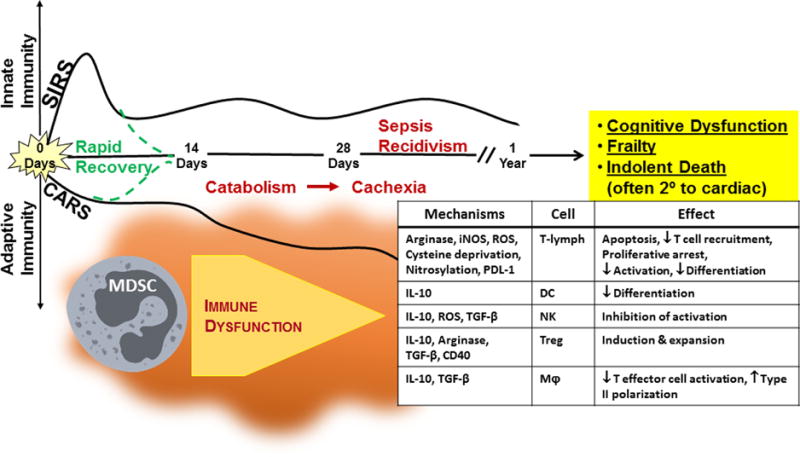
Model of Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) and the role of Myeloid-Derived Suppressor Cells.
After the simultaneous inflammatory and immunosuppressive responses, patients may return to a homeostatic immune state, leading to a rapid recovery, or develop chronic critical illness and subsequently PICS, resulting from protein catabolism, cachexia, organ failure, and secondary infections. A substantial number of these patients fail to ever recover and suffer an indolent death. Key drivers of this persistent inflammation and immunosuppression are myeloid-derived suppressor cells (MDSCs). MDSCs can influence almost every cell of host innate and adaptive immunity. CARS = compensatory anti-inflammatory response syndrome, MOF = multi-organ failure, SIRS = systemic inflammatory response syndrome. Adapted from Mira, et al.(16)
An important subset of these CCI patients progress to PICS, experiencing ongoing immunosuppression (e.g. lymphopenia) and inflammation (e.g. neutrophilia) that is associated with a persistent acute phase response (e.g. high C-reactive protein (CRP) and low pre-albumin levels) with ongoing protein catabolism (Figure 1).(5, 11, 12) Clinically, PICS patients suffer from recurrent nosocomial infections and poor wound healing, as well as frequently developing decubitus ulcers. Despite aggressive nutritional intervention, there is a consistent loss of lean body mass and a proportional decrease in functional status and poor wound healing. They are commonly discharged to long-term acute care facilities (LTACs) where they often face sepsis recidivism requiring re-hospitalization, failure to rehabilitate, and progress to suffer an indolent death (Figure 1).(11, 15)
In summary, improvements in shock resuscitation and critical care support over the past 20 years have led to an evolution in the phenotype of post-shock MOF from one of early fulminant death to one of chronic, but sustainable, organ dysfunctions. This change in MOF phenotype can now be defined clinically as CCI, an ICU duration of >14 days, with ongoing organ dysfunction. CCI is driven by an underlying dysfunctional pathophysiology, including persistent inflammation, immunosuppression and catabolism (PICS).
Long-Term Outcomes of CCI and PICS
Inpatient mortality after CCI secondary to severe trauma or sepsis has markedly declined.(12, 14, 16, 17) Unfortunately, the incidence of CCI continues to increase, and the long-term outcomes of critical illness survivors remain unclear. The majority of published descriptions of the clinical phenotype of patients who survive CCI come from patient cohorts with primary pulmonary failure and the Acute Respiratory Distress Syndrome (ARDS). These studies, appropriately utilizing general descriptive terms such as “Post-Intensive Care Syndrome,” “neuropathy of critical illness”, and “ICU-acquired weakness,” describe a substantial burden and persistence of functional deficits after prolonged respiratory failure and ventilator-dependence.(18, 19)
PICS, as described in this article, endeavors to elucidate the clinical phenotype and characterize the underlying pathophysiology driving CCI morbidities. We offer an operational definition of resource utilization and persistent organ injury, as well as a conceptual framework for the underlying pathophysiologic mechanisms that drive the persistent immunologic dysfunction, lack of organ recovery, and functional deficit. All of these factors contribute to poor long-term outcomes after severe pro-inflammatory insults, such as trauma, burns, or sepsis.(5, 13)
While it is well described that survivors of CCI are at increased risk of death after hospital discharge, the mechanisms driving this mortality risk remain unclear. It has been demonstrated that hospital discharge dispositions that provide high levels of functional support for extended periods of time (i.e. Skilled Nursing Facilities (SNF) and LTACs)) are associated with significantly reater long-term mortality rates after trauma or surgical sepsis.(14, 20) As an example, overall patient mortality has decreased markedlyover the past 15 years in conjunction with the application of evidence-based, standard operating protocols for the care of severely injured trauma patients at a cohort of Level 1 Trauma Centers in the United States.(17) Despite these apparent successes, a population-based, long-term outcomes analysis of trauma patients in the revealed that, while inpatient mortality after severe trauma steadily decreased over a 13-year period to as low as 5 percent, subsequent 3-year mortality was nearly 3-fold greater.(20) Additionally, advancing age and discharge to a SNF (as compared to discharge to home or a rehabilitation facility) were the strongest predictors of long-term mortality.(20) These findings likely reflect the reality of a changing patient phenotype, including people now living with comorbidities as well as the associated increasing age and frailty of patients that now survive severe injury. Finally, it should be noted some believe that changes in how health care payments are now provided, as well as alterations in how ICU patient data are reported, have contributed to creating the ‘appearance’ of improved quality outcomes in ICU settings, but still driving ICU care to induce this new patient phenotype.
While post-trauma, long-term outcomes are poor, long-term outcomes associated with sepsis are dismal. In a combined analysis of two interventional randomized controlled trials after severe sepsis/septic shock, the initial 28-day mortality for this critically ill cohort was approximately 20%. At 6-months, mortality increased to nearly 35 percent.(21) Even more striking than the discrepancy between short and long-term mortality was the marked functional limitations and morbidity burden among sepsis survivors. Of sepsis survivors at 6-months, nearly half reported substantial difficulties with mobility, poor quality of life, and inability to live independently.(21) Another more recent, prospective, longitudinal cohort study of 88 patients with severe sepsis/septic shock revealed that early (within the first week), inpatient mortality from refractory shock and MOF is now less than five percent. Despite this ostensible improvement, 40%of this population subsequently developed CCI and had inpatient discharge dispositions (i.e. LTAC, SNF) known to be associated with poor outcomes and a striking 6-month mortality of nearly 40% (Figure 2).(12) (11)
Figure 2.
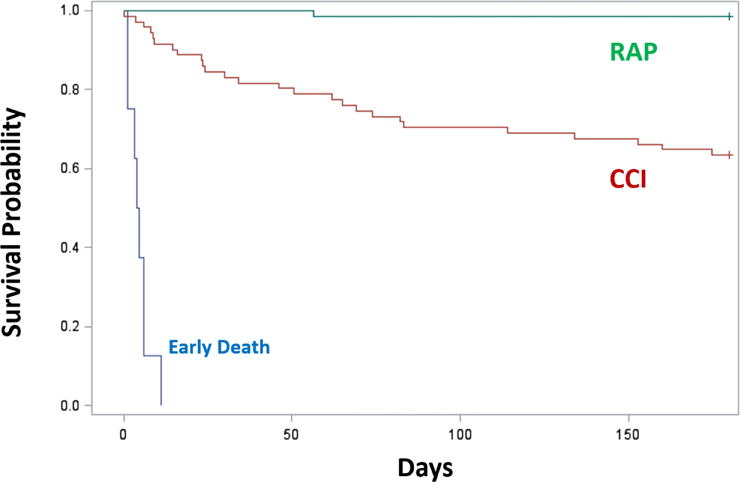
Six-month mortality of sepsis patients with Chronic Critical Illness (CCI) versus those of Rapid Recovery (RAP). We enrolled 145 critically ill surgical sepsis patients in a prospective observational study and classified each as CCI or RAP.(11) CCI was defined as an ICU duration of stay greater than or equal to 14 days with evidence of persistent organ dysfunction, measured using components of the Sequential Organ Failure Assessment (SOFA) score at 14 days (i.e. cardiovascular SOFA ≥ 1, or score in any other organ system ≥ 2). In addition, patients with an ICU duration of stay of less than 14 days would also qualify for CCI if they were discharged to another hospital, a long-term acute care facility, or to a hospice and demonstrated evidence of organ dysfunction at the time of discharge. Those patients experiencing death within 14 days of the onset of sepsis were excluded from the analysis. RAP was defined as any patient who did not meet criteria for CCI or early death. The Kaplan-Meier analysis demonstrates their cumulative survival rate over six months in CCI versus RAP patients (*Log-rank; p<0.0001). Patients who had yet to reach six months after their initial sepsis event were censored and are denoted with tick marks. Adapted from Stortz, et al.(11)
This phenomenon is witnessed in other surgical diseases as well. For example, severe burn patients have been demonstrated to have a much more profound and prolonged hypermetabolic and hyperinflammatory response for up to three years after their injury.(22) Similar to sepsis and trauma, burn survivors have a greater long-term mortality than matched controls, as well as increased mental illness.(23, 24) CCI has been demonstrated after severe acute pancreatitis as well. Yang et al determined that individuals with severe acute pancreatitis with prolonged durations of stay in the ICU had greater morbidity and rates of PICS, which was a risk factor for post-ICU mortality and poor quality of life in these patients.
In summary, critically ill patients with trauma and surgical sepsis are increasingly surviving shock and early MOF, but the burden of disease is being transformed into the development of CCI. Patients who develop CCI have high likelihood of discharge to long-term care facilities, poor functional outcomes, and high post-discharge mortality.
Mechanisms that Induce PICS
A vicious cycle of pathophysiologic alterations is engendered in many CCI patients. This concept is reflected and propagated by chronic low-grade inflammation, such asincreased serum concentrtions of interleukin-6 (IL-6); immunosuppression, such as lymphocyte dysfunction and decreasedantigen-presentation; and catabolism, including defects in carbohydrate, lipid, and protein metabolism. This spectrum of symptoms and findings is defined herein as the Persistent, Inflammation, Immunosuppression and Catabolism Syndrome o(PICS/(Figures 1 and 3).(5, 11, 16, 25) Organ injury, such as acute kidney injury and acute respiratory insufficiency/failure, contributes to the persistence of PICS and vice-versa through propagation of persistent inflammation.(26) This syndrome also includes other organ systems not historically thought of as having systemic effects, such as muscle and intestine, but are now recognized to have significant impact on systemic inflammation and immune suppression.(26)
Figure 3.
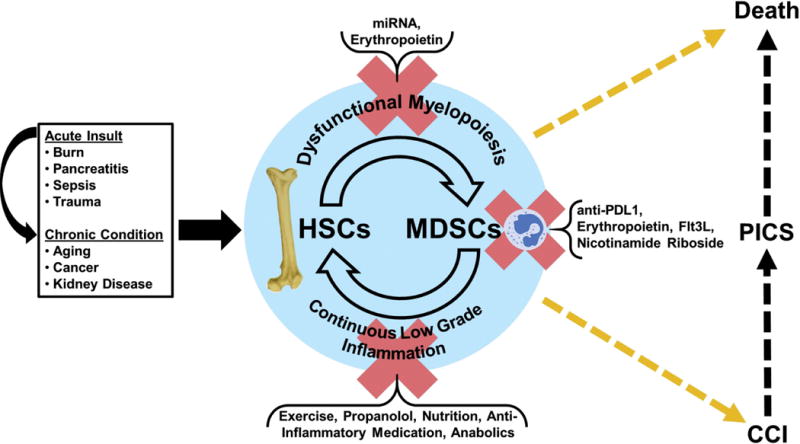
Depiction of the myelodysplasia of PICS.
The vicious cycle and immune dyscrasia of PICS and its subsequent outcomes. PICS is more likely to occur when combined with certain chronic conditions or aging; interventions currently under investigation,however, could potentially disrupt the vicious cycle of PICS.
HSC = hematopoietic stem cells, MDSCs = myeloid-derived suppressor cells.
Most hematopoietic stem cells (HSCs) are relatively quiescent, participating in maintaining immune and hematologic homeostasis in the host. The upregulation of the HSC activity in response to stress, however, is an integral function of innate immunity.(27, 28) After injury or infection, host HSCs become active, entering the cell cycle as well as differentiating. This process, known as ‘emergency myelopoiesis,’ repopulates innate immune effector cells after host stressors stimulate boththe release of mature populations and the creation of bone marrow niches (27, 29). The increased and preferential generation of these myelopoietic cells occurs at the expense of lymphopoiesis and erythropoiesis (Figure 3).(27)
This emergency activation occurs through multiple, redundant pathways and mechanisms, including ligands, such as growth factors (e.g. G/GM-CSF, FltL) and cytokines (e.g. IL-1, IL-6 and IL-17), as well as through mesenchymal or immune cells.(5, 30, 31) This HSC response may result in the creation of immature myeloid populations including myeloid derived suppressor cells (MDSCs), which are a wide range of myeloid cells in various stages of differentiation (Figure 3).(27) Although the exact roles of MDSCs are still being elicited, they are believed to be part of a physiologic response to sepsis and trauma in order to help decreaseinflammation through immunosuppression, while not eliminating all protective innate immunity, such as the toxicity that can occur due to excessive T-cell proliferation and cytokine production.(27, 32, 33) It is clear, however, that their chronic persistence is associated with poor outcomes in sepsis patients,(16, 25) as demonstrated by Mathias et al(34) and Uhel et al.(35)
CCI is associated with several stimuli and mechanisms. In CCI, there is a persistent presence of damage-associated molecular pattern (DAMP) and/or pathogen-associated molecular pattern (PAMP) molecules.(33) This is physiologic in the acute phase of an insult, as the host is programmed to recognize specific ‘danger signals’ or ‘alarmins’ with microbial invasion or tissue damage.(36) These PAMPs and DAMPs can bind to multiple receptors, including toll-like receptors (TLRs), NOD-like receptors (NLRs), complement, retinoic acid-inducible gene (RIG)-like receptors and mannose-binding lectin/scavenger receptors.(33, 36) Thereafter, common and redundant signaling pathways of immunity are activated in various cell types, including immune, epithelial, and endothelial cells.(33, 36) In turn, the production of pro- and anti-inflammatory cytokines, reactive oxygen species,, and reactive nitrogen species increases, as well as there being increased tissue wasting and apoptosis.(33) Myeloid (e.g. neutrophils, macrophages) and lymphoid cells are correspondingly recruited, with direct and indirect effects of infection and injury on endothelium and parenchymal tissue as well as their effects on then neurologic and coagulation systems.(33, 36) Altogether, this scenario leads the host to suffer from common immunosuppressive mechanisms, including, but not limited to: the expansion of MDSCs, T-regulatory cells (Treg), and M2 macrophages; T-cell exhaustion; decreased dendritic cell function; release of immunosuppressive mediator (e.g. IL-10, TGF-β) ; and, expression of inhibitory ligands on parenchymal cells.(33)
As mentioned previously, the immediate and simultaneous SIRS/CARS response, or ‘cytokine storm,’ is an appropriate mammalian reaction to an insult with the intent of addressing injury or infection and subsequently restoring the host to homeostasis.(10) But the perseverance of inflammation, immunosuppression, and catabolism in the host can be highly dysfunctional. The capacity of the medical practitioner to compensate for organ dysfunction in critically ill patients creates a host environment in which damaged organs and tissues maintain a low grade continuous pro-inflammatory state(36) presumably through release of DAMPs and alarmins, many of which are well described in the literature or associated with hyaluronan products, ATP, adenosine, protein S100A, high-mobility group protein B1 (HMGB1), histones, nucleosides and mitochondrial/nuclear DNA. The downstream effects of this chronic inflammation make the host susceptible to opportunistic infections and viral reactivations, alters the host microbiota, and often requires the continuation of intensive care interventions, such as mechanical ventilation and catheters. This, in turn, perpetuates a vicious cycle by preventing the return of the host to homeostasis regarding immune, organ, and metabolic function.(36)
The downstream effects of persistent inflammation are numerous. Of particular interest is a host immune environment similar to that of an elderly individual at baseline – i.e. ‘inflammaging’ (constant low-grade inflammation in the aged) contributing to immunosenescence (the dysfunction of the innate and adaptive immune systems of the aged).(37, 38) Lymphopenia occurs due to both acute apoptosis of effector T and B lymphocytes during sepsis, as well as the HSC shift to myelopoiesis.(16, 36) Lymphocytes also undergo TH2 polarization as well as the expansion of Treg cells. Neutrophilia occurs, but these effector, immature myeloid cells are suboptimal, because they have a decreased capacity for antigen presentation, expression of adhesion molecules, and formation of extracellular traps, as well as an altered pattern of expression of cytokines and chemokines.(36) In addition, there is a shift in macrophages to the M2 phenotype as well as dendritic cell apoptosis(36, 39) and an increase in the number of and suppressive capacity in circulating MDSCs.(34)
Although research regarding the classification of MDSCs is ongoing, MDSCs are generally divided into two forms, monocytic and granulocytic.(34) While these immature myeloid cells have several functions, one of their main functions appears to be to suppress T cell function. The immunosuppression of MDSCs is carried out in several ways, which may be dependent on its subtype.(16) First, MDSCs secrete the anti-inflammatory cytokines IL-10 and TGF-β. Two of the many effects of these cytokines are to polarize macrophages to a type II phenotype and to upregulate Tregs. Second, MDSCs deplete L-arginine via arginase 1 (ARG1) and inducible nitric oxide (NO) synthase (iNOS), which antagonizes clonal expansion, impairs the intracellular signaling, and induces apoptosis in T cells. Third, MDSCs produce increased reactive oxygen species (ROS), which with NO (the byproduct of iNOS), produce peroxynitrites. These then nitrosylate several lymphocyte cell surface proteins and cysteine residents, resulting in decreased T-cell responsiveness and altered IL-2 signaling. Additionally, NO can impede the stability of IL-2 mRNA, while ROS can suppress the function of natural killer (NK) cells. Finally, direct MDSC cell contact via CD40 receptors results in induction of Tregs and upregulation of programmed death ligand-1 (PD-L1), while other checkpoint inhibitors in MDSCs cause T-cell apoptosis (Figure 1).(16) In addition, although its association with MDSCs has not been established, chronic exposure to these factors involved in the PICS can induce HSC defects, including, but not limited to, their ability to repopulate and differentiate.(40–42)
Poor clinical outcomes have been associated with expansion of MDSCs, specifically after sepsis. Mathias et al. demonstrated that MDSCs are persistently increasedin the circulation, predominantly granulocytic, transcriptomically unique, and immunosuppressive to T lymphocytes after severe sepsis or septic shock in the SICU (34). Persistent increased percentages of blood MDSCs in this study were associated with increased nosocomial infections, prolonged intensive care unit stays, increased mortality, and poor functional status at discharge (34). Uhel et al. verified these findings in the medical ICU (MICU) population. Although both monocytic and granulocytic MDSCs were expanded in their MICU population and both of these cell populations inhibited T-cell proliferation, granulocytic MDSCs were more specifically increased in patients with sepsis (35). The granulocytic MDSCs, which demonstrated a high level of ARG1 activity, displayed high levels of degranulation markers, and most importantly, their early expansion predicted the development of nosocomial infections in these patients (35).
Treatment/Therapy
Angus et al recently published the article “Enhanced Recovery from Sepsis” in JAMA which offers suggestions on a broad range of ICU-based and post-discharge strategies to improve long-term outcomes after sepsis.(43) Not surprisingly, data supporting specific strategies are sparse, and the recommendations are limited to optimal ICU treatment, which includes evidence-based protocols to treat pain, sedation, and delirium as well early and aggressive patient mobilization and physical therapy (Table 1).(43) Post-discharge therapy can include rehabilitation programs, adequate case management and/or enhanced primary care, and ICU follow-up clinics.(43).
Table 1.
Recommended in-hospital and post-discharge interventions to prevent PICS after sepsis (modified from (43)).
| In-hospital recommendations |
|
| Post-discharae recommendations |
|
Although evidence for a successful medical or immunomodulation therapy in sepsis is still lacking, many therapies are being investigated currently to improve patient outcomes (Figure 3).(5, 16) Regarding immunopathology, research includes, but is not limited to the following: targeting excessive inflammation; immune stimulation; stem cell administration; and, epigenetic modifications.(44) Currently, clinical trials utilizing immunomodulatory biologics (such as monoclonal antibody blockade of the programmed death ligand [PD-1/PDL-1] pathway) are underway (ClinicalTrials.gov; NCT02960854). These agents have shown success in restoring immunocompetence in several oncologic diseases, including melanoma, renal cell carcinoma, and non-small cell lung cancer.(45–47) Because cancer and sepsis have been demonstrated to have similar immunosuppressive mechanisms (33, 48), translation of these established treatments to sepsis may offer similar therapeutic benefits, but require further study.
Optimal therapeutic options to reverse CCI-associated persistent catabolism and minimize muscle wasting are yet to be determined. Clinical protocols that include early mobilization and exercise, as well as adequate and consistent administration of protein and caloric requirements will likely be required as part of a multi-modality approach to therapy.(49) Dysfunctions in substrate utilization (rather than true deficits) are likely a key component to this catabolic state, meaning that adequate nutrition and/or physical rehabilitation alone are likely not sufficient therapies. Decreases in the metabolic/catabolic burden via administration of agents, such as propranolol and oxandrolone, have substantial therapeutic benefit in pediatric burn patients (50–52) but need further study in a broader, adult population of CCI subjects. Further insight into the mechanisms driving persistent catabolism after CCI are necessary to develop targeted therapies to reverse or limit PICS-associated muscle wasting and improve functional outcomes in any future multi-modality therapeutic approaches.
Conclusion
PICS describes the underlying pathophysiology of chronic critical illness (CCI) that develops after inciting an acute inflammatory response, such as in sepsis, burns, and pancreatitis, when patients fail to achieve/maintain immune and organ homeostasis.(5, 25, 29, 53) Due to the complexity and redundancy of pathways aftera severe pro-inflammatory insult to humans, it is unlikely that a ‘silver bullet’ monotherapy will be identified to improve the outcomes of PICS patients. PICS is, in part, however, a myeloplastic disease, and this aspect of the syndrome, possibly through immunomodulation, will need to be addressed as part of a multi-phased approach to optimize post-trauma and post-sepsis morbidity and mortality – both acute and chronic (Figure 3). Further discovery of the underlying mechanisms will require more studies, including additional genomic, epigenetic, and proteomic analyses, as well as the creation of improved animal models of PICS. Hopefully, ongoing clinical trials testingapproved therapies for other diseases (e.g. PD-L1 inhibitors, IL-7 administration) will provide further direction. Ideally, these therapies will directly influence dysregulated host immunity and prevent the perpetual cycle of PICS (Figure 3).
Acknowledgments
PAE and SCB were supported by P30 AG028740 from the National Institute on Aging and by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) grants 1 R01 GM113945-01 and 1 R03 AG056444-01, respectively. AMM was supported by NIH NIGMS grant R01 GM105893-01A1 and AB was supported by the NIH NIGMS grant R01 GM110240. Finally, PAE, AMM, FAM, SCB, AB, MSS, CL and LLM were all supported by P50 GM111152-01 (NIGMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Financial Disclosure Statement: No conflict of or competing interests has been declared.
References
- 1.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. The Surgical clinics of North America. 1995;75(2):257–77. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 2.Faist E, Baue AE, Dittmer H, Heberer G. Multiple organ failure in polytrauma patients. The Journal of trauma. 1983;23(9):775–87. doi: 10.1097/00005373-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40(4):501–10. doi: 10.1097/00005373-199604000-00001. discussion 10-2. [DOI] [PubMed] [Google Scholar]
- 4.Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg. 2013;74(3):774–9. doi: 10.1097/TA.0b013e31827a6e69. [DOI] [PubMed] [Google Scholar]
- 5.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. The journal of trauma and acute care surgery. 2012;72(6):1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40(4):1129–35. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. The journal of trauma and acute care surgery. 2014;76(3):582–92. doi: 10.1097/TA.0000000000000147. discussion 92-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125(8):680–7. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Balogh Z, McKinley BA, Cox CS, Jr, Allen SJ, Cocanour CS, Kozar RA, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock (Augusta, Ga. 2003;20(6):483–92. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 10.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat Baslanti T, Wang Z, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. Journal of Trauma and Acute Care Surgery. 2017 doi: 10.1097/TA.0000000000001758. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, et al. Evidence for Persistent Immune Suppression in Patients who Develop Chronic Critical Illness After Sepsis. Shock: Injury, Inflammation, and Sepsis: Laboratory and Clinical Approaches. 2017 doi: 10.1097/SHK.0000000000000981. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. doi: 10.1136/bmjopen-2016-015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two-Level One Trauma Centers. Crit Care Med. 2017 doi: 10.1097/CCM.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guirgis FW, Brakenridge S, Sutchu S, Khadpe JD, Robinson T, Westenbarger R, et al. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. The journal of trauma and acute care surgery. 2016;81(3):525–32. doi: 10.1097/TA.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 16.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, et al. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Critical care medicine. 2017;45(2):253–62. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–9. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2006;174(5):538–44. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 19.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. The New England journal of medicine. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 20.Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA. 2011;305(10):1001–7. doi: 10.1001/jama.2011.259. [DOI] [PubMed] [Google Scholar]
- 21.Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T, et al. Long-Term Quality of Life Among Survivors of Severe Sepsis: Analyses of Two International Trials. Crit Care Med. 2016;44(8):1461–7. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason SA, Nathens AB, Byrne JP, Diong C, Fowler RA, Karanicolas PJ, et al. Increased Rate of Long-term Mortality Among Burn Survivors: A Population-based Matched Cohort Study. Annals of surgery. 2018 doi: 10.1097/SLA.0000000000002722. [DOI] [PubMed] [Google Scholar]
- 24.Mason SA, Nathens AB, Byrne JP, Ellis J, Fowler RA, Gonzalez A, et al. Association Between Burn Injury and Mental Illness among Burn Survivors: A Population-Based, Self-Matched, Longitudinal Cohort Study. J Am Coll Surg. 2017;225(4):516–24. doi: 10.1016/j.jamcollsurg.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal MD, Moore FA. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J Adv Nutr Hum Metab. 2015;1(1) doi: 10.14800/janhm.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal MD, Moore FA. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surgical infections. 2016;17(2):167–72. doi: 10.1089/sur.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe. 2014;15(3):295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195(5):709–20. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mira JC, Brakenridge SC, Moldawer LL, Moore FA. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit Care Clin. 2017;33(2):245–58. doi: 10.1016/j.ccc.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, et al. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol. 2012;188(12):5824–8. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- 31.Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57(1):1–8. doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Molecular medicine (Cambridge, Mass. 2011;17(3–4):281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. The New England journal of medicine. 2014;371(4):380–3. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 34.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Annals of surgery. 2017;265(4):827–34. doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhel F, Azzaoui I, Gregoire M, Pangault C, Dulong J, Tadie JM, et al. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. American journal of respiratory and critical care medicine. 2017;196(3):315–27. doi: 10.1164/rccm.201606-1143OC. [DOI] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nature Reviews Disease Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. 2014;192(2):612–22. doi: 10.4049/jimmunol.1302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, et al. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195(5):2396–407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, et al. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol. 2004;173(5):3035–43. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- 40.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Rodriguez S, Wang L, Wang S, Serezani H, Kapur R, et al. Sepsis Induces Hematopoietic Stem Cell Exhaustion and Myelosuppression through Distinct Contributions of TRIF and MYD88. Stem cell reports. 2016;6(6):940–56. doi: 10.1016/j.stemcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skirecki T, Kawiak J, Machaj E, Pojda Z, Wasilewska D, Czubak J, et al. Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther. 2015;6:142. doi: 10.1186/s13287-015-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–20. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 45.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moldawer LL, Hotchkiss R. Immunotherapy: It is not just for cancer anymore. J Leukoc Biol. 2018;103(1):9–11. doi: 10.1002/JLB.4CE1117-447. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic Critical Illness: Application of What We Know. Nutr Clin Pract. 2018;33(1):39–45. doi: 10.1002/ncp.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chao T, Porter C, Herndon DN, Siopi A, Ideker H, Mlcak RP, et al. Propranolol and Oxandrolone Therapy Accelerated Muscle Recovery in Burned Children. Med Sci Sports Exerc. 2018;50(3):427–35. doi: 10.1249/MSS.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256(3):402–11. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herndon DN, Voigt CD, Capek KD, Wurzer P, Guillory A, Kline A, et al. Reversal of Growth Arrest With the Combined Administration of Oxandrolone and Propranolol in Severely Burned Children. Ann Surg. 2016;264(3):421–8. doi: 10.1097/SLA.0000000000001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang N, Li B, Ye B, Ke L, Chen F, Lu G, et al. The long-term quality of life in patients with persistent inflammation-immunosuppression and catabolism syndrome after severe acute pancreatitis: A retrospective cohort study. J Crit Care. 2017;42:101–6. doi: 10.1016/j.jcrc.2017.07.013. [DOI] [PubMed] [Google Scholar]


