Abstract
Objectives
The purpose of this study was to describe normal characteristics of distortion product otoacoustic emission (DPOAE) signal and noise level in a group of newborns and infants with normal hearing, followed longitudinally from birth to 15 months of age.
Design
Prospective, longitudinal study of 231 infants who passed newborn hearing screening and were verified to have normal hearing. Infants were enrolled from a well-baby nursery (WBN) and two neonatal intensive care units (NICU) in Cincinnati, Ohio. Normal hearing was confirmed with threshold auditory brainstem response (ABR) and visual reinforcement audiometry (VRA). DPOAEs were measured in up to four study visits over the first year after birth. Stimulus frequencies f1 and f2 were used with f2/f1=1.22, and the DPOAE was recorded at frequency 2f1– f2. A longitudinal repeated measure linear mixed model design was used to study changes in DPOAE level and noise level as related to age, middle ear transfer, race, and NICU history.
Results
Significant changes in the DPOAE and noise levels occurred from birth to 12 months of age. DPOAE levels were the highest at 1 month. The largest decrease in DPOAE level occurred between 1 to 5 months in the mid to high frequencies (2–8 kHz) with minimal changes occurring between the 6, 9 and 12 month ages. The decrease in DPOAE level was significantly related to a decrease in wideband absorbance at the same F2 frequencies. DPOAE noise level increased only slightly with age over the first year with the highest noise levels in the 12 month-old age range. Minor, non-systematic effects for NICU history, race, and gestational age at birth were found, thus these results were generalizable to commonly seen clinical populations.
Conclusions
DPOAE levels were related to wideband middle ear absorbance changes in this large sample of infants confirmed to have normal hearing at ABR and VRA testing. This normative database can be used to evaluate clinical results from birth to age 1 year. The distributions of DPOAE level and SNR data reported herein across frequency and age in normal-hearing infants who were healthy or had NICU histories may be helpful to detect the presence of hearing loss in infants.
INTRODUCTION
Distortion product otoacoustic emissions (DPOAEs) recorded in the ear canal are a rapid, non-invasive, pre-neural measure that provides ear- and frequency-specific information about the integrity of outer hair cell (OHC) function. DPOAEs are able to reliably screen for hearing loss greater than 30 dB HL (Gorga et al., 2000a; Gorga et al., 2005). Due to these advantages, DPOAEs are commonly employed for screening and diagnosis of hearing status in young infants referred from newborn screening, yet important gaps remain in our understanding of changes in these measures over the first year of life. OHCs are one of the most vulnerable components of the peripheral auditory system and if damaged, can result in hearing loss and a reduction or absence of DPOAE levels. The amount of damage incurred to the OHC is directly related to the degree of DPOAE amplitude reduction (Gorga et al., 2000b). Thus, understanding the normal amplitude of DPOAEs across a range of frequencies in newborns and infants is important for clinical assessment.
The DPOAE level (forward transmission) and the response (reverse transmission) depend upon ear canal and middle ear acoustic transfer properties. Therefore, immaturities of the external and middle ear can affect both forward transmission of the DPOAE stimulus and reverse measurement of the DPOAE response. While DPOAE suppression tuning curves have revealed generally adult-like tuning and presumably mature OHC function in humans by full term birth (Abdala and Sininger, 1996; Abdala et al., 1996), premature infants have narrower tuning curves at 6 kHz with steeper low-frequency slopes (Abdala, 1996). These immature tuning curves in premature infants have been interpreted to reflect developmental changes that occur in the outer and middle ear that affect DPOAE responses (Abdala and Keefe, 2006; Keefe and Abdala, 2007). In contrast to relatively mature cochlear tuning properties, the amplitude of DPOAEs across frequencies is much higher in newborns and infants than in older children and adults, despite the use of in-situ calibration to adjust the sound pressure level of the stimuli for the smaller ear canal volume of infants. This observation is consistent with multiple reports reviewed by (Gorga et al., 2000b), wherein DPOAE amplitude in the pediatric population is significantly larger than the amplitude in adults. To explore underlying reasons for these differences, Keefe and Abdala (2007) modeled forward and reverse transmission of DPOAE input-output functions in infants aged full term to 6 months. Predictions of forward transmission showed increased attenuation produced by the immature infant ear canal and middle ear relative to the adult ear canal and middle ear. In contrast, predictions for reverse transmission showed an increased level gain by as much as 13 dB in the ear canal and middle ear of infants relative to adults.
From infancy to adolescence, the external and middle ear structures undergo many complex anatomical and physiological developmental changes that could affect DPOAE transmission. In contrast, cochlear anatomy and structure are mature at 26 to 28 gestational weeks, thus post-natal changes in the external and middle ear structures are more likely to affect forward and reverse transmission of OAEs, although the precise functional effects of these anatomical changes are poorly understood (Abdala and Keefe, 2006).
Several studies have employed a cross-sectional study design to evaluate DPOAE levels in newborns, infants, children, and adults (Prieve et al., 1997; Lasky, 1998; Kon et al., 2000; Abdala and Dhar, 2012). Overall, results from these studies indicate that DPOAE levels from infants are greater than those measured in adults and the results are frequency dependent. DPOAE levels across f2 frequencies from 0.5 to 4 kHz are the most robust in newborns and infants, and peak at 6 to 8 months of age. DPOAE levels are 5–15 dB lower in adults than in infants.
Limited longitudinal data have been obtained to understand development of DPOAE responses during the important period from birth to one year. Zang and Jiang (2007) examined DPOAEs at f2 frequencies of 0.5 to 10 kHz in 35 infants at 3 to 5 days after birth, 6 months, and 12 months of age. Unfortunately, it is unclear whether DPOAE level or SNR was measured. They reported “DPOAE levels” that have a similar configuration to SNR i.e., lower levels in the low frequencies, with DPOAE values increasing as the f2 test frequency increased. The DP levels were much higher than those reported in any other study, but were similar to SNR levels reported in other studies. Abdala et al. (2008) conducted a retrospective study of DP-grams, defined as “DP level plotted as a function of f2 frequency” during the preterm and early post-natal period in a series of 3 experiments that used a combination of cross-sectional and longitudinal design. For the cross-sectional portion of the study, DP-grams were recorded in 125 preterm infants categorized based on post-conceptual age (31–33 weeks, 34–36 weeks, 37–40 weeks), in 118 term infants at birth, and 18 term infants at 4.5 weeks of age. They found that DPOAE levels generally increased as infant age increased with the highest responses recorded at 4.5 weeks of age. DPOAE level generally decreased as f2 frequency increased.
There are limitations to clinical application of published reports on infant DPOAEs thus far. Most studies used a cross-sectional study design with small sample sizes and either focused on preterm/term newborns, or combined infants across birth to 1 year, a time during which many developmental changes occur. The method of DPOAE collection varied among the studies with some employing fixed level primary tones (Lasky, 1998; Kon et al., 2000; Zang and Jiang, 2007; Abdala et al., 2008; Abdala and Dhar, 2012) and others using DPOAE input-output functions (Prieve et al., 1997). While all studies used an f2/f1 ratio of 1.22, there were a variety of primary levels ranging from 40–65 dB SPL and primary SPL differences ranging from 0 dB (L1 = L2) to 10 dB (L1 > L2).
In addition, few studies have verified normal middle ear function or normal hearing related to DPOAE results. Diagnostic air- and bone- conduction toneburst threshold ABR is the accepted standard for determination of hearing in infants and newborns, but has not been measured relative to DPOAE in these previous studies. Prieve et al. (1997) recorded 226 Hz tympanograms, however for neonates, 226 Hz tympanograms are not able to reliably differentiate normal ears and those with middle ear fluid (Paradise et al., 1976; Marchant et al., 1986). From birth to 6 months of age, higher probe tone frequencies from 0.66 to 1 kHz are more effective for detection of middle ear fluid (Hunter and Margolis, 1992; Baldwin, 2006; Zhiqi et al., 2010). Zang and Jiang (2007) assessed middle ear function with 226 Hz- tympanometry at the 6 and 12 month-old visit. However, middle-ear status was not evaluated prior to recording DPOAEs 3 – 5 days after birth during which time middle-ear effusion is common (Roberts et al., 1995; Palva et al., 1999). Other studies either did not measure tympanograms (Lasky, 1998; Abdala et al., 2008), did not report tympanometry exclusion criteria (Abdala and Dhar, 2012), or did not report tympanometry recording parameters (Kon et al., 2000). With regard to hearing status, several studies did not verify normal hearing prior to DPOAE recording (Lasky, 1998; Zang and Jiang, 2007). Kon et al. (2000) classified normal hearing as less than 40 dB nHL for infants. Abdala and Dhar (2012) and Abdala et al. (2008) required the infants to pass a click-evoked auditory brainstem response (ABR) screen at 35 dB nHL. Prieve et al. (1997) attempted to verify normal hearing with sound field visual reinforcement audiometry (VRA), but were only able to obtain reliable VRA results in 10% of children less than two years of age.
Wideband absorbance has been applied to assess middle-ear function related to DPOAE screening referrals for newborns (Keefe et al., 2003a; Sanford and Feeney, 2008; Sanford et al., 2009a; Hunter et al., 2010), and conductive hearing loss in infants (Prieve et al., 2013). In a review of published literature on wideband absorbance, Hunter et al. (2013) reported that infants and children with surgically confirmed otitis media with effusion have lower absorbance in the mid-frequencies. Infants that failed DPOAE screenings at birth have lower absorbance from 1 to 3 kHz in the affected ear (Sanford et al., 2009b; Hunter et al., 2010).
In order to accurately assess cochlear function and reliably detect hearing loss in newborns and infants, normative data are needed from infants with verified normal middle-ear function and hearing status using DPOAE test parameters that are currently used in clinical practice. The purpose of the present study was to characterize DPOAE and noise levels in a prospective cohort of newborns and infants with normal hearing followed longitudinally from birth to 15 months of age.
MATERIALS AND METHODS
Subject Enrollment and Demographics
This study was part of a longitudinal, prospective multi-year project to evaluate the use of wideband acoustic absorbance to identify middle-ear, cochlear, and neural hearing loss in infants and children. Infants were enrolled from the well-baby nursery (WBN) and neonatal intensive care unit (NICU) at Good Samaritan Hospital and the NICU at Cincinnati Children’s Hospital Medical Center after they completed Universal Newborn Hearing Screening (UNHS). The standard UNHS test battery in the WBN was a two-stage protocol that consisted of transient evoked otoacoustic emission (TEOAE, clicks at 80 dB SPL) followed by an automated ABR (clicks at 35 dB nHL) if the TEOAEs were not passed. Infants enrolled in the NICU were tested with ABR, and either TEOAE or DPOAE. Infants from the WBN were tested within 24–48 hours after birth and infants from the NICU were tested prior to discharge. The UNHS yielded overall pass/refer results for each ear.
Infants participated in up to four study visits from birth to 15 months of age. Corrected age at test (defined below) was used to classify results in to one of the following recruitment age ranges: Visit 1(Birth – 4 mos.), Visit 2 (4 – 7 mos.), Visit 3 (7 – 11 mos.), and Visit 4 (11 – 15 mos.) of age. At each visit, otoscopy, DPOAE, and wideband absorbance tests were completed, as well as diagnostic tone-burst ABR testing at Visit 1 and VRA testing at Visits 3 and 4. The study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center and Good Samarian Hospital. Informed consent was obtained from the parent(s) of all infants prior to enrollment.
Of the 488 infants enrolled in the study, a total of 371 ears from 231 infants (125 male and 106 female) met the normal hearing inclusion requirements. Normal hearing inclusion criteria across the longitudinal visits were strict, and included a pass result on the UNHS and normal hearing, verified with both ABR and VRA tests. The ABR and VRA test criteria are described below. All data reported used corrected age (when applicable) where 38 weeks post-menstrual date was considered to be full term. The corrected age was calculated by subtracting the difference between gestational age at birth and 38 weeks from the chronological age.
Test Protocol
An otoscopic examination was performed by the audiologist to assure that the ear canal was patent and to determine the appropriate size impedance probe tip. Wideband absorbance data were acquired using custom research software on a personal computer with a Titan probe and modified AT-235 tympanometry hardware (Interacoustics AT-235 and Titan probe, Middlefart, Denmark). See Keefe et al. (2015) and Hunter et al. (2015) for detailed recording methods and analysis information. Since the ABR and VRA results were the primary diagnostic criteria for hearing status, ears were not excluded on the basis of wideband absorbance results. However, normative absorbance data across the same age range and for the same infants in the current study were reported in Hunter et al. (2016), and were also analyzed in the present study relative to the DPOAE results.
DPOAE testing (2f1–f2) was completed with the Vivosonic Integrity system (Version 5.2, Toronto, ON, Canada) using the ILO P40-UG probe. Clinical DPOAE recording parameters that have been shown to produce the most robust DPOAE in infants and adults were used (Gorga et al., 2003). Primary tone stimulus levels were set at SPLs of 65 dB (L1) and 55 dB (L2) with a primary tone f2/f1 frequency ratio of 1.22 (Gaskill and Brown, 1990; Abdala, 1996; Stover et al., 1996). In-situ calibration was performed in each ear using the default program 1-kHz calibration tone prior to testing followed by a DP-gram acquisition. DPOAE f2 test frequency was measured in descending order at seven frequencies (8, 5.5, 4, 3, 2, 1.5, and 1 kHz), since this is the most common clinical test order. Two trials were measured for each ear, and the trial with the overall better SNR was used for analysis. The SNR was calculated by subtracting the mean DPOAE noise level from the mean DPOAE level at each f2 test frequency. This means that the calculated SNR was sometimes negative in some ears, and this convention was used in the analyzed results. As a practical matter, negative SNRs might be interpreted as if the SNR was 0 dB, since a DPOAE level lower than the DPOAE noise level cannot be accurately measured. The averaging time was set at 12 seconds averaging per stimulus pair per trial. The measurement accuracy was set to “accurate” on the Vivosonic device so that the DPOAE level was required to be stable for 0.4 seconds within ± 1 dB from its median SPL, and the DP signal was required to be within 1 dB of the target level. For signal averaging, the default Kalman weighting algorithm was applied (Vivosonic, Inc., Toronto, Canada). In this algorithm, the DPOAE level is estimated for each 10 ms epoch, and a discrete Fourier transform (DFT) at the specific expected DPOAE frequency is calculated. With each subsequent epoch, the frequency specificity of the DFT becomes narrower. The noise is estimated from each epoch and the noise estimate is used to create a “Kalman weight”. The Kalman weight is used to include the response estimate in the total Kalman weighted average. The advantage of Kalman weighting is that artifact rejection is not necessary and the measurement is faster since noisy responses are retained, but weighted less than quiet responses. DPOAEs were included only if data for at least five frequencies were obtained, as occasionally results were not complete at 1 and 1.5 kHz due to excessive infant noise.
At Visit 1, diagnostic ABRs were completed within a double-walled sound booth using the Vivosonic Integrity system (Version 5.2, Vivosonic, Toronto, ON, Canada). Tone burst thresholds were obtained for both air and bone conduction at 1 and 4 kHz, and also at 0.5 and 2 kHz if time and infant state (natural sleep or quietly resting) allowed. Air conduction stimuli were presented with insert earphones (Etymotic Research ER-3A, Elk Grove Village, IL) using pediatric foam ear tips. Bone conduction stimuli were presented with a B-71 bone oscillator (Radioear Corp, New Eagle, PA) hand-held to the temporal bone just above the pinna (Yang et al., 1987; Stuart et al., 1990). Normal hearing was defined as air and bone conduction thresholds of 30 dB nHL or better at 1 and 2 kHz, and 20 dB nHL or better at 4 kHz. In the early stages of the study prior to establishment of a normative sample (Elsayed et al., 2015), 30 dB nHL was set as the normal criterion across all frequencies (Stapells and Oates, 1997), so an additional requirement was added that the latency was required to be within a normal range at 4 kHz if 30 dB nHL was the lowest level tested. See Elsayed et al. (2015) for additional information regarding ABR recording methods and analysis, as well as for normative data from this sample of infants.
At Visit 3, and also at Visit 4 if results were incomplete at Visit 3, VRA testing was performed within a sound booth using the Intelligent Hearing Systems Smart VRA device with insert earphones (Etymotic Research ER-3A, Elk Grove Village, IL) and a Radioear B-71 bone vibrator (Radioear Corp, New Eagle, PA). The protocol used two examiners, and followed the recommended protocol of Widen et al. (2000), except for a 5 dB adjustment in minimum response level, based on normative data using our system and set-up. The protocol included catch trials to assess false-positive responses. The criteria for normal hearing was a minimum response level of 25 dB HL or better for speech syllables and warble tones between 1 to 4 kHz, with no air-bone gap (air conduction level minus the bone conduction level at the same test frequency) exceeding 10 dB.
Statistical Analysis
Analysis of the longitudinal DPOAE and noise levels were conducted using a linear mixed model with study visit (age range) as a repeated measure to evaluate how DPOAEs changed with age. Due to the complexity of varying numbers of visits, which could be biased by subjects who did not complete all visits, the linear mixed model analysis was used to examine age effects using procedures to minimize effects of missing data. The restricted maximum likelihood (REML) estimation method provides more valid and precise results in terms of missing values, unbalanced and non-normal data distributions compared to the more conventional ANOVA or ANCOVA, and uses all available data in the estimation. For statistical analysis, Visit 1 was subdivided into two age ranges (approx.1 and 2 mos.) to examine early development. Therefore, a total of five non-overlapping age ranges, corrected for gestational age at birth, were defined as follows: Visit 1a (−0.9 – 1.4 mos.), Visit 1b (1.5 – 4.2 mos.), Visit 2 (4.7 – 7.4 mos.), Visit 3 (7.5 – 10.4 mos.) and Visit 4 (10.5 – 14.7 mos.) Note that these age ranges vary slightly from those reported in Table 1, since Visit 1 was split into 1 and 2-month analysis age ranges. In addition, data from one ear was randomly selected for the statistical model so that each subject was included only once in the longitudinal sampling. Due to health and scheduling issues, some participants had two visits within the same age range. In such cases, the visit closest to the center of the target age was used.
TABLE 1.
Study Sample
| Visit | Sample Size (n)
|
Adjusted Age at Test (mo)
|
||
|---|---|---|---|---|
| Participants | Ears | Mean (SD) | Range | |
| 1 (1 mo) | 231 | 371 | 1.3 (0.7) | −0.5 to 4.2 |
| 2 (6 mo) | 114 | 174 | 6.1 (0.6) | 4.7 to 7.7 |
| 3 (9 mo) | 201 | 329 | 9.4 (0.7) | 7.8 to 11.2 |
| 4 (12 mo) | 107 | 162 | 12.4 (0.6) | 11.4 to 14.6 |
SD = Standard Deviation; n = Number; mo = Months
All analyses were controlled for corrected age and gestational age at birth, nursery group (NICU or WBN), race (Caucasian or not Caucasian), and wideband ambient absorbance at frequencies paired to the closest DPOAE f2 frequencies. Significant effects were analyzed using least-square means and 95% confidence intervals. A Studentized maximum modulus multiple pairwise comparison adjustment was applied as the post hoc test for significant age range effects. This method is more powerful for the problem of missing data in the longitudinal design. Data were analyzed employing SAS statistical software, version 9.3 (SAS Institute, Cary, N.C.). The two-sided significance level was set at 0.05.
RESULTS
A total of 231 infants met inclusion criteria and were included in the total normative sample; 54% were male and 46% were female. The majority of infants were Caucasian (59%), 29% were African-American, and 12% were mixed or Asian. In terms of ethnicity, 2% were Latino. Risk factors for hearing loss included NICU stay > 5 days (21%), hyperbilirubinemia (16%), ototoxic drugs (13%), low birth weight (7%), family history (8%), and intrauterine infection (1%). As DPOAEs were measured in both ears at each of 4 possible visits, a total of 1036 DPOAE measurements were recorded in total. The primary study outcomes focused on the diagnostic ABR and VRA study visits, thus the majority of the DPOAE measurements were completed at Visit 1 (N = 371, 36%) and Visit 3 (N = 329, 32%), with fewer tests at Visit 2 (N = 174, 17%) and Visit 4 (N = 162, 16%). A subset of infants were invited to attend these additional visits (2 and 4) to more fully characterize developmental changes.
The mixed model results are displayed in Table 2, with the DPOAE and noise levels modeled individually by frequency. The p-values shown in Table 2 are aggregated over the categories included in the model. Wideband absorbance was significantly and positively related to DPOAE levels at 2–8 kHz. That is, higher absorbance was related to higher DPOAE levels. Increased corrected age was related to decreased DPOAE levels at 1.5, 4, and 5.5 kHz. Increased corrected age was related to increased noise level at 1, 2, and 3 kHz. On the contrary, gestational age at birth, nursery, or race were not related to DPOAE level or noise at most frequencies (p > 0.05). An effect of gestational age at birth on DPOAE level reached significance only at 1.5 kHz, and was not related to noise level. With regard to race, DPOAE levels at 5.5 kHz were higher in Caucasian infants, and was related to noise at 1.5 kHz only. There were no significant effects of nursery on DPOAE level, while nursery was related to noise at 2 kHz only. While gestational age at birth, nursery and race were statistically significant at one frequency, overall there were no systematic effects of these demographic variables. Because the main effects on DPOAE level and noise level were significant for wideband absorbance and corrected age at testing, further analyses focused on these main effects across the five age ranges.
TABLE 2.
Model p-values for DPOAE Level and Noise Level
| Variable | Corrected Age | Gestational Age | Nursery | Race | Wideband Absorbance | |
|---|---|---|---|---|---|---|
| DPOAE Level | 1 kHz | 0.0586 | 0.6079 | 0.6526 | 0.1184 | 0.1055 |
| 1.5 kHz | 0.0093** | 0.0203* | 0.9924 | 0.0569 | 0.0796 | |
| 2 kHz | 0.2640 | 0.4411 | 0.7773 | 0.3898 | 0.0001*** | |
| 3 kHz | 0.5273 | 0.1596 | 0.2367 | 0.4595 | 0.0131* | |
| 4 kHz | <.0001**** | 0.3868 | 0.7393 | 0.4383 | 0.0134* | |
| 5.5 kHz | 0.0026** | 0.9493 | 0.7966 | 0.0147* | <.0001**** | |
| 8 kHz | 0.2649 | 0.2043 | 0.8549 | 0.8851 | <.0001**** | |
| Noise Level | 1 kHz | 0.0044** | 0.0648 | 0.2691 | 0.2072 | DNT |
| 1.5 kHz | 0.4597 | 0.1091 | 0.1463 | 0.0338* | DNT | |
| 2 kHz | 0.0001**** | 0.0921 | 0.0055** | 0.3860 | DNT | |
| 3 kHz | 0.0018** | 0.8367 | 0.5509 | 0.1117 | DNT | |
| 4 kHz | 0.0842 | 0.6430 | 0.6210 | 0.2611 | DNT | |
| 5.5 kHz | 0.0544 | 0.9165 | 0.8122 | 0.0530 | DNT | |
| 8 kHz | 0.2918 | 0.1398 | 0.2809 | 0.3182 | DNT |
Significance levels found in the analysis:
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001
DPOAE = Distortion Product Otoacoustic Emission; DNT = Did not test
Post-hoc comparisons showed higher mean DPOAE levels at 4 kHz for Visit 1a and Visit 1b compared to Visits 2, 3 and 4 (Table 3). There were no significant differences in mean DPOAE level between Visit 1a and Visit 1b at any f2 frequency. At 5.5 kHz, there were significant differences in mean DPOAE level between Visit 1 a, b and 6 months. The mean DPOAE level at Visits 2, 3 and 4 were not significantly different at any frequency.
TABLE 3.
Post Hoc Analysis of Age Related Effects
| Variable | Visit Comparisons* | p-value** | |
|---|---|---|---|
| DPOAE Level | 4 kHz | 1a vs. 2 | <0.0001 |
| 1a vs. 3 | <0.0001 | ||
| 1a vs. 4 | <0.0001 | ||
| 1b vs. 2 | 0.0152 | ||
| 1b vs. 3 | 0.0075 | ||
| 1b vs. 4 | 0.0342 | ||
| 5.5 kHz | 1a vs. 2 | <0.0016 | |
| 1b vs. 2 | 0.0328 | ||
| Noise Level | 1 kHz | 1a vs. 2 | 0.0040 |
| 2 kHz | 1a vs. 4 | 0.0003 | |
| 4 kHz | 1a vs. 4 | 0.0038 | |
| 1b vs. 4 | 0.0467 |
DPOAE = Distortion Product Otoacoustic Emission;
Number of infant ears per age group: 1a (N=240), 1b (N=96), 2 (N=141), 3 (N=182), 4 (N=130).
Studentized maximum modulus multiple pairwise comparison adjustment was applied
Post-hoc analysis indicated that the largest change in DPOAE noise level occurred between Visits 1 and 4. The lowest DPOAE noise levels occurred at Visits 1a and 1b at most test frequencies (1, 2, 3, 4, and 5.5 kHz) compared to all other age ranges. There were no significant differences in mean DPOAE noise levels between infants aged 1 and 6 months. At 1 kHz there was a significant difference in mean DPOAE noise levels between 1 and 6 month-olds. At 2 kHz, there was a difference between Visits 1 and 4, and at 4 kHz there was a significant difference between Visits 1a and 4 and between Visits 1b and 4. No other significant differences were found between age ranges, or for the other frequencies (5.5 and 8 kHz) in noise levels.
The internal consistency or reliability for the DPOAE and noise levels across the seven test frequencies within each individual measurement was estimated using the Cronbach α statistic (Cronbach, 1951), which describes how closely related are a set of items as a group. For clinical application, Cronbach α values higher than 0.8 are recommended (Cronbach, 1951). However, for comparing groups, as in the age ranges for this study, Cronbach α values of 0.7 – 0.8 are regarded as satisfactory. The Cronbach α for DPOAE level and noise level were α = 0.76 and α = 0.71 respectively, which indicated satisfactory reliability.
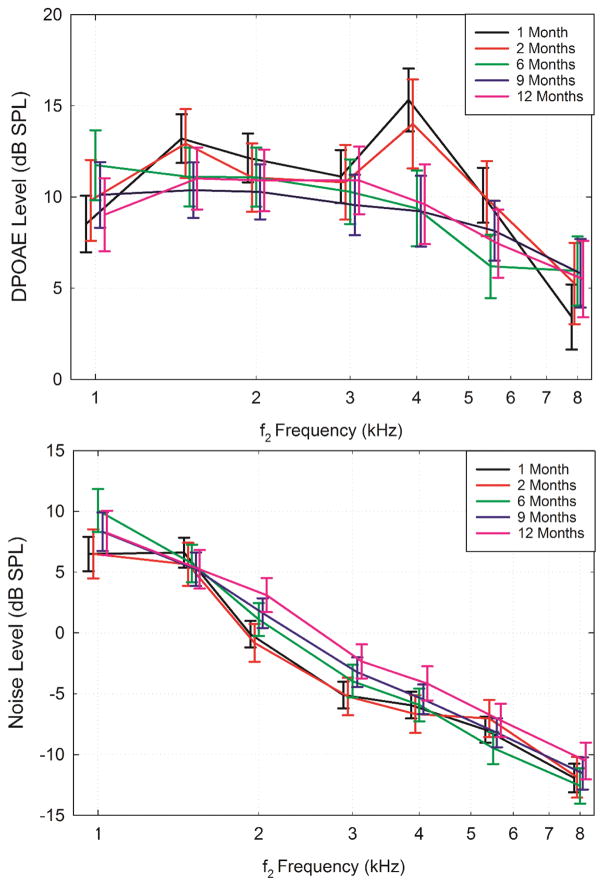
Figure 1 illustrates the model estimated mean DPOAE level (top panel) and noise level (bottom panel) in infants at each Visit as a function of DPOAE f2 frequency. These model estimates take significant confounding factors into account (mainly wideband absorbance) to examine age effects, controlling for absorbance, race, and nursery. The vertical bars represent the 95% confidence intervals, which are offset for visualization purposes. The configuration of the mean DPOAE level across f2 frequency was similar for Visits 1a and 1b, and similar for Visits 2 to 4. At Visits 1a and b, a peak in DPOAE level was apparent at 1.5 and 4 kHz. However, by Visit 2, the peak at 1.5 and 4 kHz disappeared and the mean DPOAE level gradually decreased from 2 – 8 kHz as the f2 frequency increased. The largest change in DPOAE level, about 5 dB, occurred between Visit 1 and 2 in the mid to high frequencies (4, 5.5, 8 kHz). Minimal changes in DPOAE level occurred between Visits 2 – 4. The DPOAE noise level displayed a slight, yet systematic increase with age over the first year with the highest noise levels at Visit 4 across test frequencies. In general, noise levels were much higher at frequencies below 3 kHz for all visits, and decreased with increasing test frequency.
Figure 1.
Model estimated mean DPOAE level (top panel) and noise level (bottom panel) in normal hearing infants at Visits 1–4, denoted by the mean ages of: 1, 2, 6, 9, and 12 months, as a function of DPOAE f2 frequency. Brackets represent the 95% confidence intervals and were offset horizontally for visualization purposes.
As most of the significant differences occurred between Visits 1 and 2, the normative data presented focuses on two re-defined age ranges: younger infants (0–4 months from Visits 1a and 1b) and older infants (≥ 5 months from Visits 2 – 4). Combining into two age ranges is convenient for clinical application, and the results do not support more than two normative age ranges. In addition, since clinical interpretation involves analyzing DPOAE level and SNR, noise levels were not further analyzed.
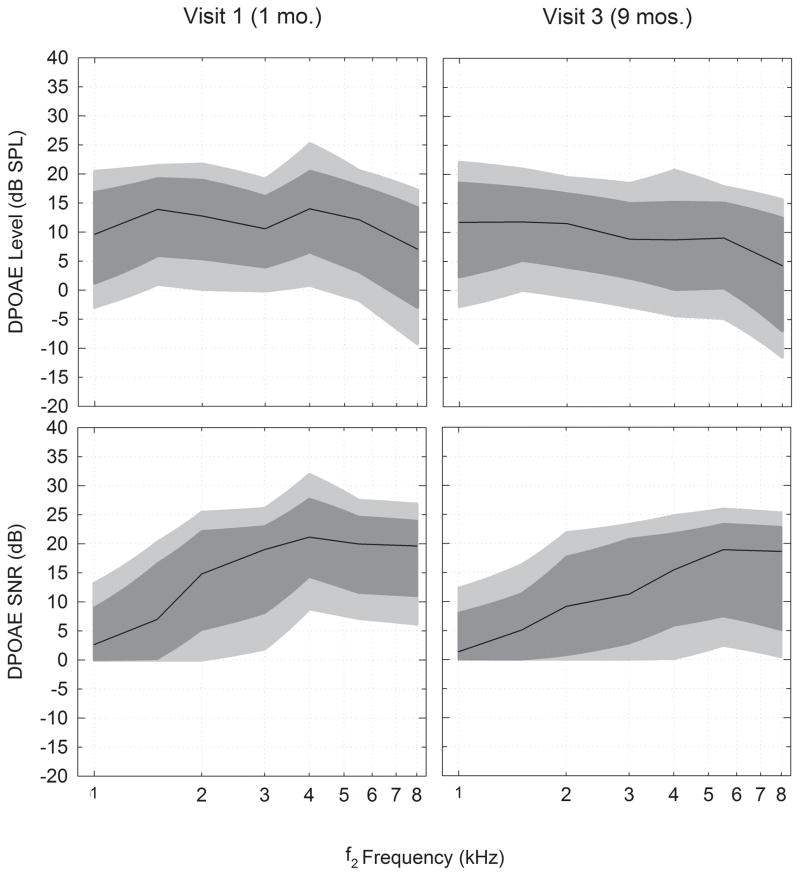
Figure 2 illustrates the raw data for DPOAE level and SNR distributions based on percentile rankings at the younger and older age ranges. The light grey shaded area represents the 10th to 90th percentiles of levels and the dark grey shaded area represents the 20th to 80th percentiles. The dark black line represents the median or 50th percentile. Table 4 provides the raw data in percentiles for the DPOAE level and SNR percentiles (1st, 5th, 10th, 20th, Median, 80th, 90th, 95th, 99th) as well as means and standard deviations for infants with normal hearing in the two combined age ranges. Because the 5th percentiles for SNR did not exceed 0 dB for several frequencies, the 10th and 90th percentiles were plotted instead of the 5th and 95th. Although the lower and higher percentiles are provided in Table 4, due to the noise floor limitations (in which SNR is set to 0 dB), the 10th and 90th percentiles are considered to be more reliable for clinical normative purposes.
Figure 2.
Illustrates the raw data for DPOAE level and SNR distributions based on percentile rankings from normal-hearing infants in younger and older age groups. The light shaded area represents the 10th to the 90th percentile and the dark shaded area represents the 20th to 80th percentile. The dark black line represents the median or 50th percentile. The percentiles used in this figure are reported in Table 4.
Table 4.
DPOAE Level, noise floor and SNR normative database from infants with normal hearing.
| 0–4 mos | Percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 5th | 10th | 20th | Median | 80th | 90th | 95th | 99th | Mean | Std Dev | |
| f2 (kHz) | DP Level (dB) | ||||||||||
| 1.00 | −16.79 | −7.51 | −2.90 | 1.26 | 9.63 | 16.82 | 20.45 | 23.14 | 29.64 | 8.91 | 9.59 |
| 1.50 | −9.64 | −2.51 | 1.04 | 6.00 | 13.90 | 19.18 | 21.45 | 23.05 | 27.65 | 12.61 | 7.89 |
| 2.00 | −9.50 | −4.62 | 0.17 | 5.45 | 12.77 | 18.89 | 21.69 | 23.06 | 31.76 | 11.93 | 8.46 |
| 3.00 | −14.19 | −3.44 | −0.12 | 4.03 | 10.57 | 16.09 | 19.09 | 21.99 | 46.04 | 10.21 | 8.90 |
| 4.00 | −14.17 | −3.98 | 0.90 | 6.61 | 14.01 | 20.43 | 25.20 | 41.12 | 55.38 | 14.65 | 12.32 |
| 5.50 | −15.35 | −7.44 | −1.71 | 3.22 | 12.13 | 17.85 | 20.51 | 22.79 | 26.57 | 10.61 | 9.28 |
| 8.00 | −23.52 | −15.69 | −9.20 | −2.91 | 7.07 | 14.15 | 17.20 | 18.90 | 24.02 | 5.54 | 10.27 |
|
| |||||||||||
| f2 (kHz) | Noise (dB) | ||||||||||
| 1.00 | −16.17 | −9.06 | −6.26 | −2.62 | 6.00 | 14.93 | 18.60 | 21.59 | 29.64 | 6.03 | 9.60 |
| 1.50 | −13.85 | −9.14 | −6.26 | −1.82 | 4.98 | 12.13 | 15.98 | 18.36 | 22.25 | 4.89 | 8.07 |
| 2.00 | −17.90 | −11.77 | −10.15 | −7.83 | −1.89 | 4.54 | 7.96 | 10.90 | 17.39 | −1.65 | 7.21 |
| 3.00 | −17.86 | −15.46 | −13.51 | −10.86 | −6.95 | −0.66 | 2.93 | 5.29 | 11.87 | −5.81 | 6.31 |
| 4.00 | −21.33 | −15.41 | −13.47 | −10.56 | −7.39 | −2.36 | 1.77 | 5.42 | 18.28 | −6.46 | 6.77 |
| 5.50 | −26.08 | −19.68 | −15.33 | −11.57 | −7.68 | −4.04 | −0.02 | 2.59 | 26.57 | −8.00 | 6.32 |
| 8.00 | −28.59 | −24.44 | −21.25 | −17.70 | −11.80 | −7.37 | −5.54 | −0.93 | 24.02 | −12.15 | 7.09 |
|
| |||||||||||
| f2 (kHz) | SNR (dB) | ||||||||||
| 1.00 | <0 | <0 | <0 | <0 | 2.63 | 9.03 | 13.17 | 18.04 | 22.25 | 4.68 | 5.76 |
| 1.50 | <0 | <0 | <0 | 0.16 | 6.96 | 16.59 | 20.23 | 23.22 | 30.97 | 8.80 | 8.01 |
| 2.00 | <0 | <0 | 0.07 | 5.21 | 14.80 | 22.16 | 25.41 | 27.67 | 32.06 | 14.19 | 8.77 |
| 3.00 | <0 | <0 | 1.90 | 8.10 | 18.98 | 22.99 | 26.06 | 29.62 | 37.45 | 16.29 | 8.89 |
| 4.00 | <0 | 2.13 | 8.82 | 14.34 | 21.13 | 27.73 | 31.91 | 38.33 | 52.52 | 21.27 | 9.97 |
| 5.50 | <0 | 3.25 | 7.18 | 11.57 | 19.96 | 24.64 | 27.43 | 29.89 | 34.94 | 18.70 | 7.80 |
| 8.00 | <0 | 0.97 | 6.20 | 11.02 | 19.61 | 23.94 | 26.78 | 28.39 | 34.68 | 17.94 | 7.87 |
| 5–15 Mos | Percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 5th | 10th | 20th | Median | 80th | 90th | 95th | 99th | Mean | Std Dev | |
| f2 (kHz) | DP Level (dB) | ||||||||||
| 1.00 | −12.36 | −6.28 | −2.73 | 2.41 | 11.76 | 18.50 | 22.09 | 25.69 | 51.84 | 11.15 | 10.73 |
| 1.50 | −15.12 | −2.87 | 0.08 | 5.24 | 11.84 | 17.60 | 20.90 | 24.09 | 37.67 | 11.25 | 8.92 |
| 2.00 | −12.82 | −4.45 | −1.02 | 4.07 | 11.54 | 16.65 | 19.44 | 22.53 | 41.50 | 10.74 | 9.17 |
| 3.00 | −18.85 | −6.31 | −2.78 | 2.18 | 8.84 | 14.95 | 18.42 | 23.40 | 46.94 | 8.66 | 9.62 |
| 4.00 | −17.44 | −8.40 | −4.29 | 0.24 | 8.74 | 15.18 | 20.69 | 29.63 | 47.11 | 8.83 | 11.42 |
| 5.50 | −18.08 | −11.02 | −4.78 | 0.49 | 9.04 | 15.03 | 17.84 | 21.91 | 38.85 | 7.88 | 10.04 |
| 8.00 | −27.62 | −15.98 | −11.41 | −6.87 | 4.31 | 12.45 | 15.64 | 19.02 | 22.88 | 3.02 | 10.90 |
|
| |||||||||||
| f2 (kHz) | Noise (dB) | ||||||||||
| 1.00 | −16.82 | −6.83 | −2.65 | 0.83 | 9.42 | 16.83 | 19.94 | 23.33 | 29.17 | 8.96 | 9.35 |
| 1.50 | −14.10 | −8.29 | −5.02 | −0.58 | 6.64 | 13.19 | 17.03 | 20.03 | 22.94 | 6.13 | 8.50 |
| 2.00 | −16.62 | −10.23 | −7.72 | −5.93 | 1.76 | 8.60 | 11.97 | 16.86 | 21.20 | 1.87 | 7.90 |
| 3.00 | −19.12 | −14.81 | −12.46 | −8.95 | −2.94 | 3.35 | 7.04 | 8.87 | 19.62 | −2.88 | 7.56 |
| 4.00 | −22.78 | −18.65 | −15.09 | −11.86 | −6.55 | 0.66 | 4.44 | 9.81 | 18.52 | −5.47 | 8.18 |
| 5.50 | −26.88 | −20.86 | −17.07 | −13.57 | −8.44 | −2.05 | 3.00 | 5.39 | 16.80 | −7.89 | 8.15 |
| 8.00 | −29.44 | −25.83 | −22.66 | −18.26 | −12.11 | −6.55 | −1.85 | 2.57 | 13.77 | −11.96 | 8.47 |
|
| |||||||||||
| f2 (kHz) | SNR (dB) | ||||||||||
| 1.00 | <0 | <0 | <0 | <0 | 1.35 | 8.08 | 12.39 | 17.66 | 34.56 | 4.39 | 6.71 |
| 1.50 | <0 | <0 | <0 | <0 | 5.08 | 11.39 | 16.39 | 21.42 | 31.56 | 6.70 | 7.34 |
| 2.00 | <0 | <0 | <0 | 0.77 | 9.15 | 17.70 | 21.91 | 24.12 | 30.49 | 9.93 | 8.61 |
| 3.00 | <0 | <0 | <0 | 2.81 | 11.24 | 20.76 | 23.33 | 26.89 | 36.09 | 12.20 | 9.14 |
| 4.00 | <0 | <0 | 0.10 | 5.87 | 15.42 | 21.71 | 24.85 | 30.03 | 36.75 | 14.69 | 9.10 |
| 5.50 | <0 | <0 | 2.38 | 7.47 | 18.92 | 23.32 | 25.98 | 29.21 | 34.38 | 16.13 | 8.68 |
| 8.00 | <0 | <0 | 0.41 | 5.10 | 18.62 | 22.79 | 25.31 | 27.37 | 32.30 | 15.43 | 9.01 |
DPOAE levels at Visit 1 were similar in the overall shape to Visit 3, except for a higher peak in the level at 1.5 and 4 kHz. By Visit 3, the median DPOAE levels displayed a more mature (eg. flatter) shape with a gradual decline with increased f2 test frequency (except for a small relative maximum at 4 kHz). At most f2 frequencies (i.e., above 1.5 kHz), the median SNR decreased from Visit 1 to 3. The SNR was smallest in the low frequencies in both age ranges due to increased noise, and the SNR improved as the f2 test frequency increased to 8 kHz.
DISCUSSION
As this is the first large scale longitudinal study to focus on changes in DPOAE responses during the early developmental period from birth to 15 months of age, there are limited data for comparison. Many published studies used different stimulus parameters (L1, L2, f2 test frequencies, and f2/f1) or studied different populations including newborns, older children, and adults (Gorga et al., 1997; Lasky, 1998; Abdala and Dhar, 2012). In addition, several studies combined infants from birth to 1 year of age into a single age range (Prieve et al., 1997; Kon et al., 2000). These combined age ranges may not be appropriate due to the extensive developmental changes in the external and middle ear that occur over the first 4 months, which contribute to maturational changes in DPOAE level and SNR.
Normal Longitudinal Development of DPOAEs
DPOAE Level
The current study found significant changes in mean DPOAE level from birth to 15 months of age. In general, DPOAE levels were the highest at Visit 1a (1 month) and decreased with age. At Visits 1a and 1b (ages 0–4 months), a peak in DPOAE levels occurred at f2 frequencies of 1.5 and 4 kHz. The largest decrease in DPOAE level, about 5 dB, occurred between Visits 1 and 2 in the mid to high frequencies (4, 5.5, 8 kHz). No significant changes in DPOAE level occurred from Visits 2 to 4 (between 5–15 months). These changes are consistent with the time course of major ear-canal and middle-ear development that occur mainly from birth to 6 months.
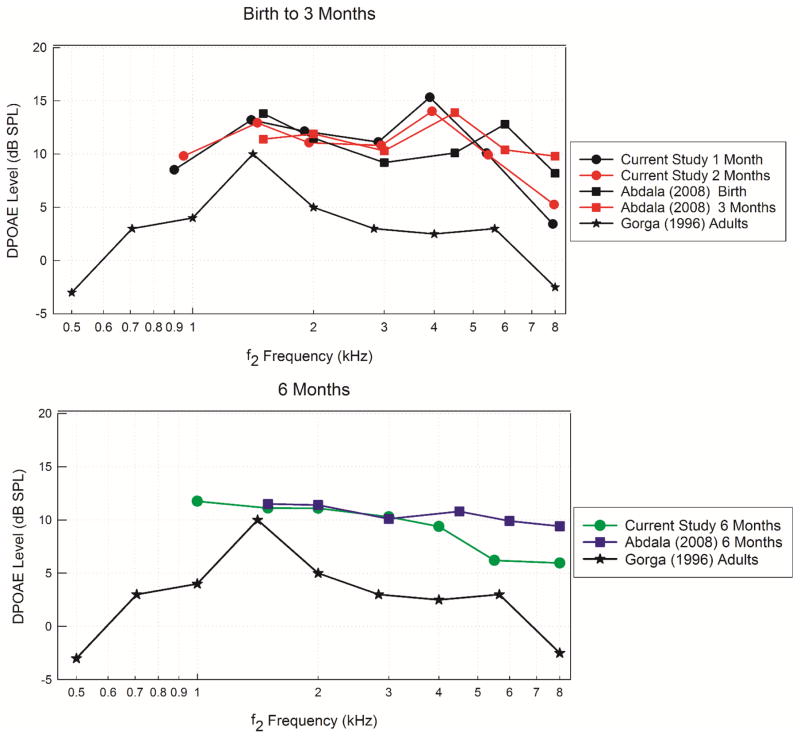
With respect to stimulus parameters and population, the current study is most similar to the report by Abdala et al. (2008), plotted in Figure 3 for mean DPOAE levels in infants aged birth to 3 months (top panel) and 6 months of age (bottom panel). Mean adult DPOAE levels are also plotted in both panels for reference (Gorga et al., 1996). The mean DPOAE levels reported by Abdala et al. (2008) are similar to those obtained in the current study at similar ages. In the top panel, mean DPOAE levels in infants at birth and 1 month of age are within 2 dB at lower (1.5 – 3 kHz) and higher (5.5 and 8 kHz) f2 frequencies. However at 4 kHz, the mean DPOAE level presented in the current study is approximately 5.5 dB SPL higher than the mean DPOAE level reported by Abdala et al. (2008) at 6 kHz. DPOAE levels at 2 months from the current study and 3 months reported by Abdala et al. (2008) are within 1.5 dB SPL with the exception of 8 kHz, where Abdala et al. (2008) reported DPOAE levels 3 dB SPL higher than the current study. At 6 months of age (bottom panel), DPOAE level differences between the current study and Abdala et al. (2008) are less than 1 dB SPL for f2 frequencies from 1.5 to 3 kHz. However for f2 frequencies from 4 to 8 kHz, Abdala et al. (2008) reported mean DPOAE levels that ranged from 1.5 to 4 dB SPL higher than reported in the current study. Given the different test instruments and populations studied, these results show extensive similarities across age group and frequency.
Figure 3.
Mean (raw data) DPOAE levels are plotted as a function of f2 frequency for infants from the current study, Abdala et al. (2008), and Gorga et al. (1997). The top panel displays results from birth to 3 months of age and the bottom panel displays results from infants at 6 months of age. Adult DPOAE levels are displayed in both panels for comparison.
Compared to adult DPOAE levels, infants displayed consistently higher mean DPOAE levels for all f2 frequencies. Mean DPOAE levels at Visit 1 were 3 to 13 dB higher than adults, however by Visit 2, the mean difference ranged from 1 to 7.6 dB. These results are consistent with previous studies that reported that DPOAE levels in preterm (24–37 weeks) and term infants (>37 weeks) at birth were approximately 2 to 10 dB higher than adults (Lafreniere et al., 1991; Bonfils et al., 1992; Lasky et al., 1992; Smurzynski et al., 1993; Abdala et al., 1996).
With respect to the pattern of DPOAE level across frequency, several other published studies have described peaks in infant DPOAE levels at various f2 frequencies. Abdala et al. (2008) reported a peak present at 1.5 to 2 kHz, and another at 4.5 to 6 kHz, in infant ears from birth to 6 months of age. Others have reported dips or reduced DPOAE levels in the mid frequency region from 3.4 to 4 kHz (Lafreniere et al., 1991; Smurzynski et al., 1993). Kon et al. (2000) reported peaks in DPOAE levels at 1.6 and 5.0 kHz with decreased responses in the mid and high frequency range. A peak in DPOAE level is typically seen in adults at lower f2 frequencies (<2 kHz) with a gradual reduction in DPOAE response in the mid to high frequency range (Lasky et al., 1992; Smurzynski et al., 1993; Gorga et al., 1996). Overall, adult DPOAE patterns across frequency are typically flatter with fewer peaks and less variation across f2 frequency than responses obtained from infants (Lasky et al., 1992; Gorga et al., 1996; Gorga et al., 1997). Differences in the in-situ stimulus calibration methods across manufacturers may account for some of the differences observed, but the frequency pattern for DPOAE levels is relatively consistent across the present and other available studies in infants. Thus, these characteristic peaks and valleys likely represent different ear canal and middle-ear transmission effects in the infant ear (Keefe and Abdala, 2007).
DPOAE Noise Level
The DPOAE noise level increased slightly with age over the first year with the highest noise levels seen in the 12 month-old group across test frequencies (see Fig. 1). Mean DPOAE noise levels were higher in the low frequencies and progressively decreased as test frequency increased. At low frequencies (f2 = 1.0 and 1.5 kHz), noise levels were on average 18 dB higher than at the f2 frequency with the lowest noise level (f2 = 8.0 kHz). This pattern was consistent across all five age ranges (1, 2, 6, 9, 12 mos. in Fig. 1). The present results are consistent with previous studies that have shown that noise levels tend to decrease with increasing frequency (Gorga et al., 1993b; Bergman et al., 1995; Prieve et al., 1997) and that noise levels are higher in infants than adults (Gorga et al., 1993a). We observed that older infants were noisier due to body movement and since they were much less likely to sleep during testing than were young infants. Gorga et al. (1993a) reported mean noise levels in adults that ranged from 14 dB SPL (f2 = 0.5 kHz) to −25 dB SPL (f2 = 3.9 kHz). However, in the 1 to 8 kHz frequency range, the mean noise levels in adults ranged from approximately −6 dB SPL (f2 = 1.0 kHz) to −23 dB SPL (f2 = 8.0 kHz), which are much lower than the mean DPOAE noise levels reported in the present study of 6.5 to −11.9 dB SPL, respectively (See Fig. 1). Abdala et al. (2008) reported mean DPOAE noise levels that ranged from 1.5 dB SPL (f2 = 1.5 kHz) to −15 dB SPL (f2 = 9.0 kHz), slightly lower than those reported in the current study. Different manufacturers use different hardware, stimuli and noise averaging algorithms, therefore some quantitative differences in noise levels are to be expected. Notwithstanding that fact, the overall pattern of higher noise levels in infants and for lower frequencies is consistent across studies. The test frequency order we followed (from high to low frequencies) was that most commonly used in clinical practice, to make results more clinically applicable. However, this order of testing could introduce order effects, in which noise in the lower frequencies may be more affected by noise if the infant becomes noisier. In contrast, some infants become less noisy as they settle down after probe insertion, so test order effects could go in either direction. Since we did not randomize or counterbalance test order, this is a limitation of the results.
Differences in noise levels between infants and adults may be attributed to several factors. First, infants tend to breathe more rapidly and noisily than adults. Ear-canal noise generated by blood flow may be larger in infants than adults because the vibratory motion of the blood may be more directly coupled to the middle-ear cavity in the infant than in an adult. Spontaneous OAEs in adult ears are modulated by heartbeat (Long and Talmadge, 1997), and this variability may contribute to the noise levels of other types of OAE measurements. Blood-flow effects have been reported in infants for TEOAE (Rubens et al., 2008) and DPOAE (Keefe et al., 2008). Since the infant ear canal wall is highly compliant, breathing and blood-flow noise may not be attenuated as much as in the adult ear canal. The Eustachian tube is open at rest in infants, while it is closed in adults, so nasopharyngeal noise is likely higher in infants. Secondly, it can be harder to maintain a good probe seal in infants due to movement, crying and yawning. This may result in increased ambient noise entering the ear canal and decreased stimulus levels, which may increase the DPOAE noise levels and decrease the DPOAE level, if the probe is not refit as needed. This issue can be more apparent in older infants since they are more awake and mobile than newborns. Since noise levels are highly frequency dependent and are greater in infants than adults, reliable DPOAE SNR is easier to achieve clinically for higher f2 frequencies, eg., at 2 kHz and higher, consistent with previous studies across a wide age range (Gorga et al., 2000b; Keefe et al., 2003b)
Why are DPOAE levels larger in infants?
The observation of larger DPOAE levels in infants compared to adults can most likely be attributed to the anatomical and related functional immaturities of the ear canal and middle ear (Keefe and Abdala, 2007), and to any differences in the calibration of the sound stimuli in the ear canal. There are substantial anatomical and maturational differences between the infant and adult ear canal and middle ear cavity that can affect the forward transmission of the DPOAE stimulus and reverse measurement of the DPOAE level. In general, the infant ear canal cavity has a smaller cross-sectional area than in adults (Keefe and Abdala, 2007), the tympanic membrane has a more horizontal orientation, and the ear-canal wall is more compliant than in adults (Ikui et al., 1997). These differences may affect stimuli that are delivered through the middle ear and the DPOAE response measured in the ear canal that are not accounted for in the in-situ volume-calibration techniques. One important example is that ear-canal wall mobility effects are most important at frequencies below 1 kHz in infants younger than about 6 months (Hunter et al., 2016).
Keefe and Abdala (2007) described a model of acoustical and mechanical transmission through external, middle, and inner ear to understand why DPOAE suppression responses at an f2 frequency of 6 kHz differed in infants relative to adults. This is the test frequency at which maturational differences in DPOAE suppression tuning curves are largest. Based on the assumption that cochlear function was adult-like at birth, the model decomposed DPOAE response contributions into ear-canal and middle-ear components. Their main findings were that infant middle-ear immaturities result in an attenuation in the forward transmission of the stimulus, and immaturities in the infant ear canal contributes in an overall gain in the DPOAE responses.
Hunter et al. (2016) reported that estimates of ear canal length, which are influenced by ear canal wall mobility and the relative angle of the tympanic membrane, are not adult-like until 6 months of age. Although in-situ calibration of the stimuli generating the DPOAE signal partially accounts for ear-canal volume differences, the calibration assumes that the ear canal is a cylindrical, hard walled cavity. Moreover, the reverse transmission of the DPOAE in the infant ear increases the DPOAE level because it drives into a smaller ear-canal volume. The assumption of a hard-wall ear canal holds in adults, but the walls of the infant ear canal are soft and absorb more sound energy, especially for lower frequencies. Thus, the in-situ calibration is likely insufficient to completely account for infant-adult ear canal differences.
Other potential explanations that have not been thoroughly researched include subtle immaturities of the descending medial efferent fiber innervation of the OHCs (Lavigne-Rebillard and Pujol, 1990), although Abdala et al. (Abdala et al., 2008) suggest that the auditory efferents may be mature at birth. Immaturities of the mass and stiffness properties of the sensory epithelium, and supernumerary hair cells within the cochlea at birth may be a factor (Bredberg et al., 1965; Igarashi, 1980; Lavigne-Rebillard and Pujol, 1986). Perhaps most importantly, infants are less likely to have been exposed to environmental factors, such as noise and ototoxins that could result in damage to the OHCs without a reduction in audiometric thresholds, thus their cochleae are pristine in comparison to older children and adults. All these factors have the potential to affect the generation of DPOAEs.
Limitations and Future Directions
There are some limitations in the current study, including that equipment from only one manufacturer was studied, the need to complete in-situ calibration at individual test frequencies, and need to compare results to infants with defined hearing loss. Differences in algorithms for noise reduction exits among different manufacturers, and these algorithms are proprietary. Thus, differences in methodologies across studies may constrain the generalizability of our results on the noise level, and thus on SNR. These data are useful for determining effects due to age in a population that is similar in age to infants at greater risk for hearing loss, but to determine the effectiveness of DPOAE for detection of hearing loss in this age group, analysis of ears with defined hearing loss is required. A companion study (Blankenship et al., submitted) that includes infants with conductive, sensorineural and mixed hearing loss was completed to address this issue.
CONCLUSION/SUMMARY
Hearing impairment is one of the most common disabilities reported in infants. Early identification of hearing loss combined with an immediate age-appropriate intervention is essential for speech and language development. DPOAEs are a commonly used diagnostic tool to evaluate cochlear function, however in order to maximize test performance, age appropriate normative data are essential. This study reports longitudinal normative data and DPOAE level and SNR ranges that can be used to evaluate the clinical results from birth to age 1 year. DPOAEs may be used to help determine the presence of mild and greater degrees of hearing loss, using clinical cut-off points that are reported in our companion paper in infants with confirmed hearing loss (Blankenship et al., submitted). In addition, threshold tests such as the ABR or a behavioral audiogram is necessary to confirm the degree, type and configuration of hearing loss to guide rehabilitation plans.
Acknowledgments
Source of Funding:
This research was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). Co-author Keefe is involved in commercializing devices to assess middle-ear function in infants.
Portions of this study were presented as poster presentations at the American Academy of Audiology (2014) and American Auditory Society (2016). D.H.K. is involved in commercializing devices to assess middle-ear function. The efforts of Alaaelddin Elsayed, MD, AuD, Leigh Shaid, AuD, several research coordinators, and the families who participated is gratefully acknowledged. We are appreciative of three anonymous reviewers who substantially improved the manuscript.
Abbreviations
- ABR
Auditory Brainstem Response
- DPOAE
Distortion Product Otoacoustic Emission
- NICU
Neonatal Intensive Care Unit
- OHC
Outer Hair Cell
- SNR
Signal-to-Noise Ratio
- SPL
Sound Pressure Level
- TEOAE
Transient Evoked Otoacoustic Emission
- UNHS
Universal Newborn Hearing Screening
- VRA
Visual Reinforcement Audiometry
- WBN
Well-Baby Nursery
Footnotes
Conflicts of Interest
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the United States Government.
AUTHOR CONTRIBUTIONS
L.L.H. designed and performed experiments, co-wrote the paper, and provided interpretive analysis and critical revision to the paper. C.M.B. performed experiments, analyzed data, and co-wrote the paper. D.H.K. and M.P.F. designed experiments, and provided interpretative analysis and critical revision to paper. D.K.B helped design and perform experiments. A.M. assisted in enrollment and data analysis as part of her AuD capstone. D.F.F. designed experiments, and provided interpretative analysis and critical revision to paper. L.L. provided statistical analysis and critical revision to the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
References
- Abdala C. Distortion product otoacoustic emission (2f1–f2) amplitude as a function of f2/f1 frequency ratio and primary tone level separation in human adults and neonates. J Acoustic Soc Am. 1996;100:3726–3740. doi: 10.1121/1.417234. [DOI] [PubMed] [Google Scholar]
- Abdala C, Dhar S. Maturation and aging of the human cochlea: a view through the DPOAE looking glass. J Assoc Res Otolaryngol. 2012;13:403–421. doi: 10.1007/s10162-012-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala C, Keefe DH. Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears. J Acoust Soc Am. 2006;120:3832–3842. doi: 10.1121/1.2359237. [DOI] [PubMed] [Google Scholar]
- Abdala C, Oba SI, Ramanathan R. Changes in the DP-gram during the preterm and early postnatal period. Ear Hear. 2008;29:512–523. doi: 10.1097/AUD.0b013e31816c40bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala C, Sininger YS. The development of cochlear frequency resolution in the human auditory system. Ear Hear. 1996;17:374–385. doi: 10.1097/00003446-199610000-00003. [DOI] [PubMed] [Google Scholar]
- Abdala C, Sininger YS, Ekelid M, Zeng FG. Distortion product otoacoustic emission suppression tuning curves in human adults and neonates. Hear Res. 1996;98:38–53. doi: 10.1016/0378-5955(96)00056-1. [DOI] [PubMed] [Google Scholar]
- Baldwin M. Choice of probe tone and classification of trace patterns in tympanometry undertaken in early infancy. Int J Audiol. 2006;45:417–427. doi: 10.1080/14992020600690951. N7780J87482990GN [pii] [DOI] [PubMed] [Google Scholar]
- Bergman BM, Gorga MP, Neely ST, Kaminski JR, Beauchaine KL, Peters J. Preliminary descriptions of transient-evoked and distortion-product otoacoustic emissions from graduates of an intensive care nursery. J Am Acad Audiol. 1995;6:150–162. [PubMed] [Google Scholar]
- Bonfils P, Avan P, Francois M, Trotoux J, Narcy P. Distortion-product otoacoustic emissions in neonates: normative data. Acta Otolaryngol. 1992;112:739–744. doi: 10.3109/00016489209137468. [DOI] [PubMed] [Google Scholar]
- Bredberg G, Engstrom H, Ades HW. Cellular pattern and nerve supply of the human organ of Corti; a preliminary report. Arch Otolaryngol. 1965;82:462–469. [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Elsayed AM, Hunter LL, Keefe DH, Feeney MP, Brown DK, Meinzen-Derr JK, … Schaid LG. Air and Bone Conduction Click and Tone-Burst Auditory Brainstem Thresholds Using Kalman Adaptive Processing in Nonsedated Normal-Hearing Infants. Ear Hear. 2015;36:471–481. doi: 10.1097/AUD.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill SA, Brown AM. The behavior of the acoustic distortion product, 2f1–f2, from the human ear and its relation to auditory sensitivity. The Journal of the Acoustical Society of America. 1990;88:821–839. doi: 10.1121/1.399732. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinical application of multivariate analyses of distortion-product otoacoustic emission data. Ear Hear. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, Beauchaine KL, Kaminski JR, Peters J, Jesteadt W. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: distortion product responses. J Acoust Soc Am. 1993a;93:2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman BM, Beauchaine KL, Kaminski JR, Peters J, … Jesteadt W. A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. J Acoust Soc Am. 1993b;94:2639–2648. doi: 10.1121/1.407348. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dorn PA, Hoover BM. Further efforts to predict pure-tone thresholds from distortion product otoacoustic emission input/output functions. J Acoust Soc Am. 2003;113:3275. doi: 10.1121/1.1570433. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, Peters J. From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Nelson K, Davis T, Dorn PA, Neely ST. Distortion product otoacoustic emission test performance when both 2f1–f2 and 2f2-f1 are used to predict auditory status. J Acoust Soc Am. 2000a;107:2128–2135. doi: 10.1121/1.428494. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Norton SJ, Sininger YS, Cone-Wesson B, Folsom RC, Vohr BR, … Neely ST. Identification of neonatal hearing impairment: distortion product otoacoustic emissions during the perinatal period. Ear Hear. 2000b;21:400–424. doi: 10.1097/00003446-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Stover L, Neely ST, Montoya D. The use of cumulative distributions to determine critical values and levels of confidence for clinical distortion product otoacoustic emission measurements. J Acoust Soc Am. 1996;100:968–977. doi: 10.1121/1.416208. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Feeney MP, Lapsley Miller JA, Jeng PS, Bohning S. Wideband reflectance in newborns: normative regions and relationship to hearing-screening results. Ear Hear. 2010;31:599–610. doi: 10.1097/AUD.0b013e3181e40ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Keefe DH, Feeney MP, Fitzpatrick DF, Lin L. Longitudinal development of wideband reflectance tympanometry in normal and at-risk infants. Hear Res. 2015;340:3–14. doi: 10.1016/j.heares.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Keefe DH, Feeney MP, Fitzpatrick DF, Lin L. Longitudinal development of wideband reflectance tympanometry in normal and at-risk infants. Hear Res. 2016;340:3–14. doi: 10.1016/j.heares.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Margolis RH. Multifrequency Tympanometry: Current Clinical Application. Am J Audiol. 1992;1:33–43. doi: 10.1044/1059-0889.0103.33. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Prieve BA, Kei J, Sanford CA. Pediatric applications of wideband acoustic immittance measures. Ear Hear. 2013;34(Suppl 1):36s–42s. doi: 10.1097/AUD.0b013e31829d5158. [DOI] [PubMed] [Google Scholar]
- Igarashi Y. Cochlea of the human fetus: a scanning electron microscope study. Archivum histologicum Japonicum = Nihon soshikigaku kiroku. 1980;43:195–209. doi: 10.1679/aohc1950.43.195. [DOI] [PubMed] [Google Scholar]
- Ikui A, Sando I, Sudo M, Fujita S. Postnatal change in angle between the tympanic annulus and surrounding structures. Computer-aided three-dimensional reconstruction study. Ann Otol Rhinol Laryngol. 1997;106:33–36. doi: 10.1177/000348949710600106. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Gorga MP, Jesteadt W, Smith LM. Ear asymmetries in middle-ear, cochlear, and brainstem responses in human infants. J Acoust Soc Am. 2008;123:1504–1512. doi: 10.1121/1.2832615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Gorga MP, Neely ST, Zhao F, Vohr BR. Ear-canal acoustic admittance and reflectance measurements in human neonates. II. Predictions of middle-ear in dysfunction and sensorineural hearing loss. J Acoust Soc Am. 2003a;113:407–422. doi: 10.1121/1.1523388. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Hunter LL, Feeney MP, Fitzpatrick DF. Procedures for ambient-pressure and tympanometric tests of aural acoustic reflectance and admittance in human infants and adults. J Acoust Soc Am. 2015;138:3625–3653. doi: 10.1121/1.4936946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Zhao F, Neely ST, Gorga MP, Vohr BR. Ear-canal acoustic admittance and reflectance effects in human neonates. I. Predictions of otoacoustic emission and auditory brainstem responses. J Acoust Soc Am. 2003b;113:389–406. doi: 10.1121/1.1523387. [DOI] [PubMed] [Google Scholar]
- Kon K, Inagaki M, Kaga M. Developmental changes of distortion product and transient evoked otoacoustic emissions in different age groups. Brain Dev. 2000;22:41–46. doi: 10.1016/s0387-7604(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Lafreniere D, Jung MD, Smurzynski J, Leonard G, Kim DO, Sasek J. Distortion-product and click-evoked otoacoustic emissions in healthy newborns. Arch Otolaryngol Head Neck Surg. 1991;117:1382–1389. doi: 10.1001/archotol.1991.01870240074012. [DOI] [PubMed] [Google Scholar]
- Lasky R, Perlman J, Hecox K. Distortion-product otoacoustic emissions in human newborns and adults. Ear Hear. 1992;13:430–441. doi: 10.1097/00003446-199212000-00009. [DOI] [PubMed] [Google Scholar]
- Lasky RE. Distortion product otoacoustic emissions in human newborns and adults. I. Frequency effects. J Acoust Soc Am. 1998;103:981–991. doi: 10.1121/1.421215. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Development of the auditory hair cell surface in human fetuses. A scanning electron microscopy study. Anat Embryol (Berl) 1986;174:369–377. doi: 10.1007/BF00698787. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Auditory hair cells in human fetuses: synaptogenesis and ciliogenesis. J Electron Microsc Tech. 1990;15:115–122. doi: 10.1002/jemt.1060150204. [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL. Spontaneous otoacoustic emission frequency is modulated by heartbeat. J Acoust Soc Am. 1997;102:2831–2848. doi: 10.1121/1.420339. [DOI] [PubMed] [Google Scholar]
- Marchant CD, McMillan PM, Shurin PA, Johnson CE, Turczyk VA, Feinstein JC, Panek DM. Objective diagnosis of otitis media in early infancy by tympanometry and ipsilateral acoustic reflex thresholds. J Pediatr. 1986;109:590–595. doi: 10.1016/s0022-3476(86)80218-9. [DOI] [PubMed] [Google Scholar]
- Palva T, Northrop C, Ramsay H. Spread of amniotic fluid cellular content within the neonate middle ear. Int J Pediatr Otorhinolaryngol. 1999;48:143–153. doi: 10.1016/s0165-5876(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Paradise JL, Smith CG, Bluestone CD. Tympanometric detection of middle ear effusion in infants and young children. Pediatrics. 1976;58:198–210. [PubMed] [Google Scholar]
- Prieve BA, Fitzgerald TS, Schulte LE, Kemp DT. Basic characteristics of distortion product otoacoustic emissions in infants and children. J Acoust Soc Am. 1997;102:2871–2879. doi: 10.1121/1.420342. [DOI] [PubMed] [Google Scholar]
- Prieve BA, Vander Werff KR, Preston JL, Georgantas L. Identification of conductive hearing loss in young infants using tympanometry and wideband reflectance. Ear Hear. 2013;34:168–178. doi: 10.1097/AUD.0b013e31826fe611. [DOI] [PubMed] [Google Scholar]
- Roberts DG, Johnson CE, Carlin SA, Turczyk V, Karnuta MA, Yaffee K. Resolution of middle ear effusion in newborns. Arch Pediatr Adolesc Med. 1995;149:873–877. doi: 10.1001/archpedi.1995.02170210047008. [DOI] [PubMed] [Google Scholar]
- Rubens DD, Vohr BR, Tucker R, O’Neil CA, Chung W. Newborn oto-acoustic emission hearing screening tests: preliminary evidence for a marker of susceptibility to SIDS. Early Hum Dev. 2008;84:225–229. doi: 10.1016/j.earlhumdev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Feeney MP. Effects of maturation on tympanometric wideband acoustic transfer functions in human infants. J Acoust Soc Am. 2008;124:2106–2122. doi: 10.1121/1.2967864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Keefe DH, Liu YW, Fitzpatrick D, McCreery RW, Lewis DE, Gorga MP. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear Hear. 2009a;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Keefe DH, Liu YW, Fitzpatrick D, McCreery RW, Lewis DE, Gorga MP. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear Hear. 2009b;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurzynski J, Jung MD, Lafreniere D, Kim D, Kamath MV, Rowe JC, … Leonard G. Distortion-product and click-evoked otoacoustic emissions of preterm and full-term infants. Ear Hear. 1993;14:258–274. doi: 10.1097/00003446-199308000-00005. [DOI] [PubMed] [Google Scholar]
- Stapells DR, Oates P. Estimation of the pure-tone audiogram by the auditory brainstem response: a review. Audiol Neurootol. 1997;2:257–280. doi: 10.1159/000259252. [DOI] [PubMed] [Google Scholar]
- Stover L, Gorga MP, Neely ST, Montoya D. Toward optimizing the clinical utility of distortion product otoacoustic emission measurements. The Journal of the Acoustical Society of America. 1996;100:956–967. doi: 10.1121/1.416207. [DOI] [PubMed] [Google Scholar]
- Stuart A, Yang EY, Stenstrom R. Effect of temporal area bone vibrator placement on auditory brain stem response in newborn infants. Ear Hear. 1990;11:363–369. doi: 10.1097/00003446-199010000-00007. [DOI] [PubMed] [Google Scholar]
- Widen JE, Folsom RC, Cone-Wesson B, Carty L, Dunnell JJ, Koebsell K, … Norton SJ. Identification of neonatal hearing impairment: hearing status at 8 to 12 months corrected age using a visual reinforcement audiometry protocol. Ear Hear. 2000;21:471–487. doi: 10.1097/00003446-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Yang EY, Rupert AL, Moushegian G. A developmental study of bone conduction auditory brain stem response in infants. Ear Hear. 1987;8:244–251. doi: 10.1097/00003446-198708000-00009. [DOI] [PubMed] [Google Scholar]
- Zang Z, Jiang ZD. Distortion product otoacoustic emissions during the first year in term infants: a longitudinal study. Brain Dev. 2007;29:346–351. doi: 10.1016/j.braindev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Zhiqi L, Kun Y, Zhiwu H. Tympanometry in infants with middle ear effusion having been identified using spiral computerized tomography. Am J Otolaryngol. 2010;31:96–103. doi: 10.1016/j.amjoto.2008.11.008. [DOI] [PubMed] [Google Scholar]