Abstract
The unfolded protein response (UPR) improves endoplasmic reticulum (ER) protein folding in order to alleviate stress. Yet it is becoming increasingly clear that the UPR regulates processes well beyond those directly involved in protein folding, in some cases by mechanisms that fall outside the realm of canonical UPR signaling. These pathways are highly specific from one cell type to another, implying that ER stress signaling affects each tissue in a unique way. Perhaps nowhere is this more evident than in the liver, which—beyond being a highly secretory tissue—is a key regulator of peripheral metabolism and a uniquely proliferative organ upon damage. The liver provides a powerful model system for exploring how and why the UPR extends its reach into physiological processes that occur outside the ER, and how ER stress contributes to the many systemic diseases that involve liver dysfunction. This review will highlight the ways in which the study of ER stress in the liver has expanded the view of the UPR to a response that is a key guardian of cellular homeostasis outside of just the narrow realm of ER protein folding.
Keywords: ER stress, unfolded protein response, lipid metabolism, obesity, diabetes, NASH, insulin, inflammation, hepatocellular carcinoma
Graphical Abstract
The unfolded protein response (UPR) restores ER homeostasis during stress. In the liver, the UPR is regulated by—and in turn contributes to—both metabolism and liver damage. This review highlights these connections and what they tell us about both the physiology and pathophysiology of the liver and the cell biology of the UPR.
The canonical unfolded protein response: a primer
The basic mechanisms of UPR signaling were discovered over two decades ago in the yeast Saccharomyces cerevisiae. The central feature of the response is activation of an ER-resident stress sensing protein that initiates a signal transduction cascade upon ER stress, culminating in upregulation of genes encoding ER chaperones and other factors involved in efflux of proteins through the secretory pathway [1]. In yeast, this response is initiated entirely by the IRE1 protein. The vertebrate response includes two other stress-sensing molecules in addition to IRE1—namely PERK and ATF6—but the response is fundamentally conserved, in that upregulation of ER chaperones and related proteins is its central feature. Thus, in all eukaryotes and all tissues, disrupted ER protein folding is remedied by an augmented folding capacity.
Much of what is known about the basic mechanisms of ER stress signaling in vertebrates comes from in vitro studies in mouse embryonic fibroblasts (MEFs), which in the pre-CRISPR era were the only cells in which genetic lesions in the UPR could be readily examined. This response, subsequently confirmed in other cell types, can be considered the canonical UPR. This canonical vertebrate UPR has been reviewed extensively elsewhere [2] and so will be only briefly summarized here. In short, accumulation of unfolded proteins in the ER leads to activation of IRE1 (a ubiquitous α form and a tissue-restricted β form), ATF6 (ubiquitous α and β paralogs, of which α is better understood) and PERK. Activated IRE1 splices Xbp1 mRNA to remove a 26-base intron, allowing for production of an active transcription factor. ATF6 is released to the Golgi during ER stress, where cleavage by Site-1 Protease and Site-2 Protease liberates a cytosolic fragment that is itself a transcription factor. Finally, activated PERK catalyzes phosphorylation of the translation initiation factor eIF2α, which transiently arrests synthesis of most cellular proteins but stimulates translation of at least a subset of mRNAs bearing short open reading frames in their 5′ untranslated regions. Among these is the mRNA encoding ATF4, which is also a transcription factor. XBP1, ATF6, and ATF4 are all bZIP transcription factors—a family comprising several dozen members that form homo- or heterodimers and regulate diverse cellular processes [3]. With considerable overlap among the genes regulated by each of these three transcription factors, together XBP1, ATF6, and ATF4 regulate the expression of ER chaperones, factors involved in ER-associated degradation, and others that impinge directly on the ER protein processing capacity. The UPR also alleviates ER stress by mechanisms independent of transcription: both eIF2α phosphorylation by PERK and a process of degradation of ER-associated mRNAs catalyzed by IRE1α—called regulated IRE1-dependent decay (RIDD)—serve to reduce ER nascent protein influx.
The existence of ER stress is typically inferred from detection of UPR outputs—upregulation of ER chaperones at the RNA and protein level, splicing of Xbp1 mRNA, and phosphorylation of eIF2α are among the more common readouts [4]. Directly detecting protein misfolding is much more challenging; in steady-state, the most common readouts are ER dysmorphogenesis—seen by electron microscopy (e.g. [5, 6])—or ER retention of proteins that would be ordinarily transited out of the secretory pathway (e.g. [7, 8]). Direct detection of stress in living cells by virtue of assessing protein aggregate formation [9] or decreases in ER chaperone mobility [10] is also possible, but in general approaches to monitoring ER function in vivo are lacking. Thus, most studies that assess “ER stress” instead assess UPR activation, which is not necessarily the same thing.
Cells in which ER stress cannot be alleviated (although how exactly “alleviated” is defined from the cell’s point-of-view is not well understood) can succumb to death, as described predominantly in cultured cell studies. This death involves elements of classical apoptotic pathways such as cleavage of caspases and activation of pro-apoptotic BCL2-family members ([11] and references therein). However, there is evidence that necrotic or pyroptotic death pathways might be activated in parallel (see below)—a distinction with important implications for liver pathophysiology. While the mechanisms by which ER stress leads to cell death are still, surprisingly, unclear, the transcription factor CHOP—also a bZIP factor—is known to be important in the process, as cells lacking CHOP are significantly (though not completely) protected from ER stress-induced cell death [12].
At the most basic level, persistent unmitigated ER stress can contribute to disease by eliciting inappropriate cell death, thereby compromising tissue function. This pathway likely contributes to a number of disease states, as seen in the broad protection conferred by CHOP deletion against a fairly wide array of experimental pathologies, including diabetes, atherosclerosis, Charcot-Marie Tooth disease, colitis, spinal cord injury, and exercise-induced muscle damage [13–21]. But is this the only pathway by which ER stress causes disease? Is it possible that interactions between ER stress signaling and other physiological processes disrupt cellular function in other ways? Studying the UPR in MEFs, divorced from their physiological context, offers little insight into this question. It is here that studies in the liver have proven so valuable in highlighting a central physiological role for the UPR.
The liver: a central, complex, tractable organ
The liver offers several advantages for understanding UPR signaling in vivo. Most notably, it is a highly secretory organ, responsible for the production of very low density lipoprotein (VLDL)—a massive, complex lipoprotein particle that is extruded through the secretory pathway—as well as secretion of nascent high density lipoprotein particles, albumin, blood clotting factors, and hormones. The bulk of the liver’s work is performed by hepatocytes, which make up approximately seventy percent of the organ’s cells and eighty percent of its mass. Consistent with this secretory role, hepatocytes display a highly elaborated rough ER; indeed, electron micrographs of the lamellar sheets of rough ER extending throughout the hepatocyte cytoplasm are quite often literally the “textbook picture” of the organelle. XBP1 is required for embryonic liver development [22, 23], as it is for other highly secretory cells such as pancreatic acinar cells or B lymphocytes [22, 24]. (Surprisingly, IRE1α is not required for liver development [25]; this discrepancy has not yet been reconciled.) And while neither PERK nor ATF6 is strictly essential for liver development, deletion of any of the three UPR pathways results in adult liver phenotypes, particularly when dietary or pharmacological challenges are applied (see below). Thus, ER stress is of clear importance to liver function and dysfunction. (In addition to the extensive rough ER network, hepatocytes also contain ample smooth ER, due to the need for drug detoxification and lipid synthesis. How perturbation to the smooth ER does or does not elicit ER stress remains very poorly understood.)
Hepatic metabolic regulation
The liver plays a central role in the regulation of whole-body lipid and carbohydrate metabolism (reviewed in [26]). Along with muscle and white adipose, the liver is one of the major targets of insulin and glucagon, and is thus highly responsive to nutritional flux. Insulin has several effects on hepatocytes: it upregulates glycogen synthesis, whereby excess glucose is polymerized and stored for later glycogenolysis and release for distribution to other tissues when blood glucose levels begin to fall. Insulin also stimulates lipogenesis, i.e., the synthesis of lipids from carbohydrate precursors, which takes place at the ER membrane. As with glycogenesis, lipogenesis is an energy storage mechanism: fats are stored in the liver as triglycerides in lipid droplets, and these triglycerides can be liberated and used for VLDL production, to supply other organs with this dense energy source. Finally, insulin cues protein synthesis through activation of the mTOR pathway. Conversely, fasting and glucagon have opposite effects to insulin: stimulation of glycogenolysis, fatty acid oxidation, ketone body production, and gluconeogenesis—the process whereby acetyl CoA, produced by fatty acid oxidation, is converted into glucose. Importantly, gluconeogenesis contributes to glucose dysregulation in obesity and diabetes. The liver also produces FGF21 in response to fasting, which increases energy expenditure and sensitizes cells to the effects of insulin both in the liver itself and through its effects on distant tissues, particularly adipose (both brown and white) and the brain [27]. Beyond production and secretion of fats, the liver also contributes to their clearance. Dietary fats or fats liberated from adipose by lipolysis are taken up through hepatocyte cell-surface transporters, as is excess plasma LDL.
Based on these processes, the liver is a major regulator of levels of both triglycerides and glucose in the blood and of metabolism in many tissues besides itself. As such, the liver plays a central role in the progression of the Metabolic Syndrome, which refers to the collection of conditions that occur as a consequence of obesity and include elevated blood sugar, triglyceride, and cholesterol, and is often associated with ectopic storage of triglyceride in the liver, known as nonalcoholic fatty liver disease (NAFLD) (reviewed in [28]). The continuing increase in the prevalence of obesity worldwide has provided extra impetus for understanding the factors that disrupt metabolism and insulin signaling in the liver, including ER stress.
Hepatic cell types and their interactions in liver damage
The other major cell types of the liver are cholangiocytes, which line the bile ducts that collect bile produced by hepatocytes and channel it to the gall bladder; Kupffer cells, which are liver-resident macrophages; endothelial cells that line the hepatic blood vessels; and stellate cells, which are fibroblast-like cells that are activated by inflammatory stimuli to deposit collagen and facilitate wound repair—a process that can result in liver scarring, or fibrosis, in the case of chronic inflammation [29]. The liver is also populated by various other immune cells as part of normal surveillance. The liver, unlike many other organs, is readily and efficiently targeted for manipulation of gene expression by several different methods. These include gene delivery by both adenoviruses and adeno-associated viruses (the latter of which, though limited in the size of insert which it can accommodate, has the advantage of eliciting little immune response) and direct DNA delivery by hydrodynamic injection of naked plasmid through the tail vein (reviewed in [30]). Further, the liver’s large size (approximately 1 gram in a 25 gram mouse), relative cellular homogeneity, and comparatively low expression of RNases make downstream molecular and biochemical analysis fairly simple.
The other remarkable feature of the liver is its substantial proliferative capacity, which paradoxically both protects the liver from damage and also contributes to chronic liver disease (reviewed in [31]). Perhaps because of its place in the portal circulation and its role in drug detoxification, the liver undergoes constant low-level cell proliferation and replenishment. The dramatic potential of this capacity can be seen during partial hepatectomy, when removal of up to two-thirds of the liver mass stimulates full regeneration of the organ. Liver damage, either small or grand, activates an inflammatory response dependent upon both resident Kupffer cells and infiltrating immune cells. These cells release inflammatory cytokines that stimulate hepatocellular proliferation and also stellate cell activation. In the context of acute injury, these protective responses help repair and restore the liver to normal function. However, in the case of chronic damage, these pathways can compromise liver function [32].
Chronic insults to the liver are many, the three most prevalent of which are alcoholism, viral hepatitis (principally Hepatitis B or C), and, increasingly, obesity. Though by distinct mechanisms, each of these insults results in liver damage (reviewed in [33]), the earliest manifestation of which is steatosis, or fat accumulation. Though occult and reversible, steatosis can progress to steatohepatitis, which is fatty liver with inflammation, of either the alcoholic sort or nonalcoholic (NASH). Unchecked inflammation can progress to fibrosis and, eventually, cirrhosis, which ultimately requires a liver transplant. Hepatocellular carcinoma (HCC) typically arises in the context of a cirrhotic liver, and its prognosis is grim [34]. Some of the earliest studies to examine the role of ER stress in the liver reported that each of the three major stimuli—alcoholism, viral hepatitis, and obesity—also results in ER stress and UPR activation in the liver (see below). These findings raised the possibility that ER stress might play a role in the dysregulation of liver function that occurs during such stimuli. Moreover, they raised the question of whether ER stress might be more intimately tied to hepatic metabolism and regeneration beyond simply compromising liver function through ER stress-induced cell death—and thus whether the liver could deliver an expanded view of ER stress signaling.
Surprising roles for ER stress in liver function: initial findings
Initial inklings that the UPR might be intimately tied to liver function came from studies showing transcriptional signatures of ER stress in response to expression of selected proteins from the Hepatitis B [35] of Hepatitis C Virus (HCV) [36] genome, or from a subgenomic replicon of HCV (mice are not natural HCV hosts and so at the time expression of these partial HCV genomes was the best mouse model of HCV infection) [37]. At around the same time, a mouse model of alcoholism, using chronic intragastric infusion, was found to both raise plasma levels of homocysteine (as was known to occur in human alcoholics) and to elicit upregulation of ER stress markers in the liver. Treatment of mice with betaine, which is a methyl donor that allows conversion of homocysteine to methionine, reversed both liver damage and ER stress [38]. Together, these studies thus demonstrated a correlation between the two most common causes of fatty liver and ER stress.
The watershed finding, however, appeared in 2004, demonstrating a link between hepatic ER stress and insulin signaling [39]. Insulin resistance—i.e., the inability of peripheral tissues like the liver to respond to a given bolus of insulin—is the defining feature of the pre-diabetic state. ob/ob or db/db mice—which are genetically impaired in appetite control—or mice fed a high fat diet (HFD) display progressive insulin resistance [40]. In both genetic and dietary models of obesity, mice showed activation of ER stress markers including phosphorylation of the mitogen-activated protein kinase JNK (c-JUN N-terminal kinase). JNK had previously been linked to IRE1 activation [41], and was already known to promote inhibitory serine phosphorylation of the insulin receptor substrate [42], which mediates insulin-dependent signaling [43]. Ablation of IRE1 in cultured cells instead promoted activating tyrosine phosphorylation of IRS1, providing evidence that IRE1 activation led directly to insulin resistance. Mice heterozygous for whole-body deletion of XBP1 (Xbp1+/−) showed worsened glucose homeostasis and increased insulin resistance on a HFD, implying that a compromised ability to respond to ER stress could contribute to the pathology of the Metabolic Syndrome. This point was further supported by the demonstration that so-called pharmacological chaperones also improve insulin sensitivity and glucose parameters in ob/ob mice, particularly in the liver [44]. These agents included 4-phenylbutyric acid (PBA) and tauroursodeoxycholic acid (TUDCA), both of which appear to functionally improve ER protein processing. (It should be noted that the mechanisms of action of PBA and TUDCA are not understood, and neither seems to act directly on the protein folding process; both are defined as “pharmacological chaperones” based on their ability to reduce ER stress, and, circularly, presumed to reduce ER stress by virtue of being “pharmacological chaperones” In fact, PBA has been shown to affect hepatic lipid metabolism through induction of autophagy [45]) On the other hand, mice with an inducible deletion of XBP1 (though not liver-specific) showed increased hepatic ER stress and JNK activity, yet also increased insulin sensitivity, meaning that ER stress alone is not sufficient to cause insulin resistance [46].
Pathways from obesity to ER stress
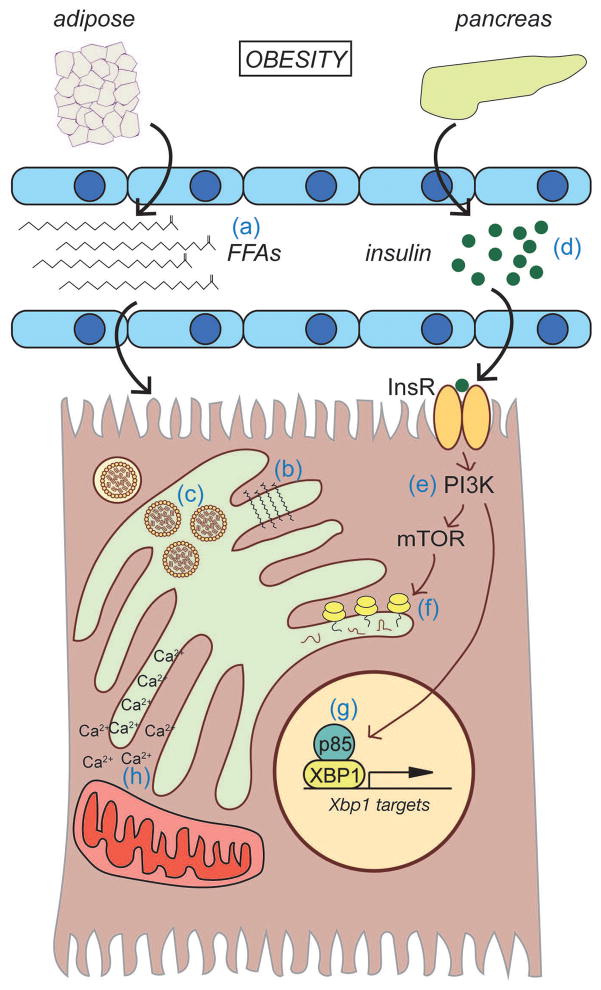
A reporter for IRE1/XBP1 pathway activation confirmed that obesity elicits ER stress predominantly in the liver [47]. Further, insulin sensitivity and glucose regulation could be improved by hepatic overexpression of either of the two ER-resident HSP70-family chaperones, ORP150/HYOU1 [48] or BiP/GRP78 [49], which provided more direct evidence that perturbed ER protein folding in the liver specifically contributes to insulin resistance. How, then, does obesity disrupt ER function (Figure 1)?
Figure 1.
Disruption of ER function during obesity
Obesity results in increased circulating free fatty acids (FFAs) due to overwhelming of adipose capacity (a). Consequences of FFA uptake by hepatocytes can include ER membrane structural distortion (b) or stimulation of VLDL production (c). Obesity is also associated with increased food intake and hyperinsulinemia (d), which activates PI3 Kinase through insulin receptor signaling (e). Downstream of PI3K is mTOR which, when activated, promotes protein biogenesis, including that of ER client proteins (f). The p85 regulatory subunit of PI3K also can interact with XBP1 and enhance its nuclear localization, thus stimulating ER protection from stress that would be compromised when hepatocytes become insulin resistant. Obesity also alters ER calcium homeostasis and interactions with mitochondria (h).
Lipotoxicity and other pathways arising from excess fat
One possible means by which obesity elicits hepatic ER stress is lipotoxicity. As the fat-storage capacity of white adipose becomes exhausted during obesity, increased lipolysis releases free fatty acids into the blood, which then get taken up ectopically by other tissues, most notably the liver [50]. Exposure to saturated fats elicited ER stress in hepatocytes both in vitro and in vivo [51, 52]. Saturated fats can be directly incorporated into the ER membrane, which decreases membrane fluidity due to the tighter packing of unkinked aliphatic hydrocarbon chains [53]. This altered membrane structure might affect ER protein processing indirectly, or might directly activate UPR sensors independent of the organelle’s protein folding status [54]. Given that at least IRE1 is activated by clustering [55], diminished membrane fluidity might favor persistent sensor activation. Both PERK and IRE1 appear to sense ER stress induced by saturated lipids through their transmembrane domains, rather than their ER lumenal domains [56]. That saturated fat in particular is lipotoxic to hepatocytes is seen by the fact that stearic acid (C18:0) elicited ER stress while oleic acid (C18:1) not only failed to induce ER stress, but also blunted the toxicity of stearic acid [57]. Scd1−/− mice, which cannot synthesize monounsaturated fatty acids, likewise showed markers of ER stress in the liver [58]. Similarly, animals kept on a diet high in saturated fat developed ER stress and liver damage, while animals fed a diet high in polyunsaturated fat did not [51]. Finally, the lipid remodeling enzyme LPCAT3 reduced ER stress and liver damage, and this effect was attributed to redistribution of cellular lipids to more highly unsaturated forms [59].
Ectopic lipid accumulation might also contribute to ER stress by stimulating production of VLDL. To generate a VLDL particle, the enzyme microsomal triglyceride transfer protein (MTTP) and its cofactor protein disulfide isomerase (PDI) coalesce triglycerides around nascent apolipoprotien B as this massive protein emerges into the ER during cotranslational translocation [60]. Providing intermediate concentrations of lipid to cultured hepatocytes stimulated VLDL production and also activated the UPR, possibly because of the need to fold and modify ApoB [61]. Induction of VLDL also elicited JNK activation and led to insulin resistance in vitro and in vivo [62]. That it might be VLDL production and not lipid accumulation in the liver per se that leads to ER stress is suggested by the observation that inhibiting MTTP blocked VLDL secretion and led to fatty liver, but not to ER stress [63], thus dissociating steatosis from ER stress. Ultimately, a key test of the lipotoxicity hypothesis will be to block adipose lipolysis or hepatic uptake in obese mice. While adipose triglyceride lipase (ATGL) has been deleted in mice and this has been found to reduce ER stress [64], ATGL is also expressed in the liver; thus, an adipose-specific deletion will be necessary.
Obesity disrupts innumerable cellular pathways, and so ER stress in obese animals might be exacerbated by those pathways that impinge on ER function. A major role for the ER beyond protein folding is intracellular storage and regulated release of calcium (reviewed in [65]). The high calcium concentrations in the ER are maintained in part by the ability of a number of ER lumenal proteins to act as high-capacity low-affinity calcium binders. Consequently, many ER chaperones operate optimally at high calcium concentrations, and uncontrolled efflux of ER calcium is one of the stimuli traditionally used to elicit ER stress in vitro. One consequence of obesity is diminished expression of the SERCA2b (Sarco/endoplasmic reticulum Ca2+-ATPase) protein, which contributes to calcium reuptake in the ER after release. SERCA overexpression in the livers of obese mice reduced steatosis and ER stress markers [66, 67].
Although the mechanism by which obesity dysregulates SERCA2b expression is not yet known, disrupted calcium homeostasis in the ER might contribute not only to ER stress but to altered metabolism as well. The ER makes close physical contacts with mitochondria, and one of the roles of these associations is regulated transfer of calcium between the two organelles [68]. Obesity has been shown to increase these associations, which could potentially overload the mitochondria with calcium and disrupt oxidative phosphorylation [69]. On the other hand, liver-specific knockout of MFN2, which contributes to ER-mitochondria tethering [70], showed impaired insulin sensitivity and glucose homeostasis, along with apparently increased ER stress [71]. Thus, it might be that either too much or too little crosstalk between the two organelles could contribute to metabolic dysregulation during obesity. More broadly, the ER-mitochondria interactions are increasingly being recognized as crucial for numerous cellular processes including mitochondrial fission and dynamics, autophagy, and inflammation (reviewed in [72]). How these pathways of crosstalk contribute to liver dysfunction is an emerging area of interest.
Overfeeding cues
Although obesity elicits ER stress, it might be that overnutrition per se—i.e., taking in too many calories—causes or exacerbates hepatic ER stress independent of the associated obesity. The simple act of eating a meal after a prolonged fast is sufficient to activate the UPR in the liver in mice [73–75]. This finding suggests that feeding itself perturbs hepatic ER function, though presumably this perturbation and its downstream consequences are transient. To whatever extent feeding alters metabolism and glucose homeostasis through an ER stress-dependent mechanism, overfeeding might lead to improper persistence of these mechanisms.
At least some of the effects of feeding on ER function are due to insulin, infusion of which is sufficient to elicit hepatic ER stress [76]. Insulin has myriad anabolic effects on hepatocytes, including stimulation of protein synthesis by activation of the mTOR pathway [77]. The tuberous sclerosis complex (TSC) proteins are negative regulators of mTOR signaling, and deletion of TSC1 or TSC2 in cultured cells caused constitutive ER stress that could be blocked with the mTOR inhibitor rapamycin [78]. Rapamycin also blocked UPR activation elicited by feeding after a fast [75]. Similarly, hepatic overexpression of the deacetylase SIRT1 (Sirtuin 1), which is an mTOR inhibitor [79], reduced ER stress and improved metabolic parameters [80].
The simplest hypothesis to account for the effects of insulin signaling on ER stress is that increased protein translation increases the influx of nascent proteins into the ER; as hepatocytes are highly secretory, presumably a high percentage of the proteome comprises secretory and membrane proteins. However, other insulin-dependent signaling pathways converge on UPR signaling as well, and might enhance the mTOR-dependent response. Among these is regulation of XBP1 by PI3 kinase. p85α and β are regulatory subunits of PI3 kinase, and XBP1 was pulled out from a screen of p85α-interacting proteins [81]. Both p85α and β could in fact interact with XBP1, and they promoted its nuclear translocation. Insulin activated PI3 kinase, and also stimulated the p85-XBP1 interaction. Nuclear translocation of XBP1 was blocked in p85α/β knockout cells, and overexpression of p85α or β improved glucose parameters and stimulated XBP1 nuclear translocation [82]. Nuclear localization of XBP1 and transcriptional activity were also enhanced in vitro and in vivo by phosphorylation by p38 MAP kinase [83] or IKKβ (IκB kinase) [84], and by the BRD7 protein, which interacts with the p85/XBP1 complex [85]. Together, these findings suggest that UPR transcriptional output can be enhanced by signaling through insulin and other cellular pathways. As one of the central features of Metabolic Syndrome associated with obesity is insulin resistance, it could be that obesity-associated ER stress initially arises from insulin signaling, and that later, after insulin resistance develops and hepatocyte protein synthesis rates fall [67], ER stress is propagated by lipotoxicity instead.
When considering the pathways by which obesity leads to ER stress, it is important to distinguish between markers of UPR activation and the actual accumulation of misfolded or unfolded proteins in the ER lumen or the relative occupancy of the chaperones therein. Detecting UPR activation by target gene upregulation is relatively easy, although even then there is significant overlap between genes regulated by a bona fide ER stress and those regulated by other stresses that lead to eIF2α phosphorylation by non-PERK kinases [86]. But, more importantly, these readouts do not speak directly to the protein folding capacity of the ER, which is difficult to measure in vivo. In addition, each UPR pathway is activated with distinct kinetics, and there is evidence that the three pathways need not be activated in tandem at least under certain circumstances, meaning there is no such thing as a single invariant “UPR” [87–89]. The times at which ER stress is examined—not only time since the onset of stress but even circadian time [90]—greatly impact the output that is observed, which presents a confounding factor in comparing one study to another.
The effects of chronic ER stress on liver function
A key question about the relationship between obesity and ER stress is the extent to which ER stress signaling mechanisms become fundamentally altered—such that the UPR no longer signals appropriately and thus fails to adequately protect cellular function—versus simply representing the consequences of a functionally intact UPR responding to stimulation far more frequently than it likely evolved to handle. There is evidence that obesity can alter both the sensitivity of the UPR and its ability to respond appropriately. BiP is a key regulator of ER stress signaling, since it both plays a major role in ER protein folding and binds to and regulates the activation of each of the three UPR stress sensors [91, 92]. Bip mRNA expression was suppressed in the livers of obese NAFLD patients prior to undergoing bariatric surgery compared to patients without NAFLD [93], implying that steatosis associated with obesity might impair Bip transcription and thus presumably render the ER hypersensitive to stress. The gluconeogenic transcription factor FOXO1 (Forkhead Box O1), which is repressible by insulin, can bind to the Bip promoter, and FOXO1 overexpression in the liver was sufficient to stimulate Bip upregulation [94]. Thus, overfeeding and the concomitant insulin persistence might lead to quasi-permanent repression of Bip. In addition, obese mice also appear—by an as-yet unknown mechanism—to lose expression of ATF6α [95], which is a major stress-dependent regulator of Bip and other ER chaperones [96]. Moreover, a recent study demonstrated that obesity leads to S-nitrosylation of ER proteins in the mouse liver, including nitrosylation of IRE1α; liver-specific restoration of nitrosylation-resistant IRE1α then improved insulin sensitivity in ob/ob mice [97]. Together, these findings suggest that obesity might neuter otherwise protective UPR signaling.
Experimental attempts to create chronic ER stress
Obesity—along with other stimuli like alcoholism or viral hepatitis—are widely considered to represent forms of chronic ER stress. But what does “chronic ER stress” actually look like, and how does the cell respond to it? It is not necessarily the case that the cellular response to chronic ER stress is an extended version of the response to acute stress. Even without external influences such as loss of ATF6α or nitrosylation of IRE1α described above, it is possible that the various feedback loops embedded within UPR signaling alter the response’s output when it is chronically activated. Currently, most of what is known about UPR signaling pathways comes from studying acute ER stresses in vitro. While this approach has been instrumental in fleshing out the framework of the UPR, it says very little about how the pathways behave during chronic stress, particularly in vivo. By contrast, the stimuli that are presupposed to elicit chronic ER stress in vivo, such as obesity and others, influence many other cellular pathways, which might themselves impinge on ER function.
Bridging this gap requires good in vivo models of chronic ER stress specifically. One way of accomplishing this is by genetic mutations that compromise ER function. In zebrafish, the foie gras mutant, which disrupts a gene involved in ER-to-Golgi vesicular traffic [98], leads to ER stress and hepatic steatosis [99]. Such a mutant recapitulates a truly chronic stress, although it does not allow the duration of intensity of the stress to be varied. Longer-term ER stress (at least over two days) can also be elicited in fish by growing embryos in water containing various doses of an ER stressor. This approach demonstrated that, while ER stress was elicited at all doses, only the strongest treatments also led to hepatic lipid dysregulation [88]. Similarly, we challenged mice every day for up to two-and-a-half weeks with a very low dose of the ER stressor tunicamycin. Surprisingly, this treatment led to quasi-stable suppression of Bip mRNA expression, reminiscent of Bip suppression seen in obese animals [100]. This suppression was attributed to the ability of the UPR to rapidly deactivate itself after each bout of stress, in part by maintaining activity of the RIDD pathway. Although repeated treatment with tunicamycin is far from physiological, and was carried out over days rather than months or years, the study demonstrated proof-of-principle that chronic ER stress can lead to unexpected UPR outcomes without external factors necessarily impinging on UPR signaling.
Regulation of metabolism by hepatic ER stress
As efforts to model viral hepatitis, alcoholism, and obesity in mice showed that these conditions were associated with ER stress, parallel efforts to determine the physiological consequences of UPR ablation provided hints of mechanistic links between UPR signaling and steatosis and liver damage. A trio of papers in 2008 together linked each UPR pathway to hepatic lipid homeostasis. Liver-specific ablation of XBP1 was found to reduce plasma lipid content without promoting hepatic steatosis. Based on induction of Xbp1 mRNA splicing, upregulation of lipogenic genes, and binding of XBP1 to lipogenic gene promoters in mice fed a high fructose diet (HFrD), XBP1 was proposed as a direct regulator of lipogenesis [101]. Subsequent work has shown that many of the effects of XBP1 deletion are attributable to compensatory hyperactivation of IRE1 [102] (and this may be true for the impaired insulin sensitivity in Xbp1+/− mice as well [39]), and called into question whether XBP1 in fact promotes lipogenesis [103, 104]. Nonetheless, this initial finding demonstrated a link between the IRE1-XBP1 axis and hepatic lipid homeostasis. At around the same time, animals with impaired eIF2α phosphorylation in the liver were shown to have heightened insulin sensitivity and improved glucose homeostasis, and to be resistant to HFD-induced fatty liver [74]. The suppressed induction of lipogenic genes in those animals gave rise to the hypothesis that eIF2α phosphorylation promotes translational regulation of lipogenic regulators. Finally, animals constitutively lacking ATF6α were found to accumulate dramatic amounts of lipid in the liver upon challenge with ER stress inducing agents [105]. This accumulation was accompanied not only by impairment of VLDL secretion—which might be expected in animals subjected to a harsh ER stress and with a compromised ability to respond to that stress—but also by apparent transcriptional suppression in the liver of a host of genes involved in both lipogenesis and fatty acid oxidation. However, there was no direct role for ATF6α in this regulation; rather, the same basic phenotype was elicited in animals with liver-specific ablation of PERK [106] or IRE1 or non-phosphorylatable eIF2α, or of animals with an intact UPR but lacking the ER cochaperone p58IPK [105]. In fact, even wild-type animals suppressed lipid metabolic genes upon challenge with an ER stressor; this regulation was only more profound and persistent in animals with a compromised UPR. Together, these three studies suggested that, not only does lipid accumulation cause ER stress in the liver, but ER stress in the liver can also disrupt lipid metabolism. But toward what end? Why does a response tasked with protecting ER function regulate lipogenesis, which occurs on but outside of the ER, and fatty acid oxidation, which largely takes place in mitochondria? And by what mechanisms are such pathways regulated? The predominant transcription factors of the UPR—XBP1, ATF4, and ATF6—are all activators, so by what mechanisms are metabolic genes regulated?
Indirect control of metabolism by the UPR
Since the initial studies described above, it has become clear that there is an association between stimuli that lead to ER stress and dysregulated hepatic lipid metabolism. These include: knockout of the PA28 regulatory subunit of the proteasome [107]; knockout of SIRT7, which leads to enhanced ribosomal biogenesis and, potentially, increased protein synthesis [108]; knockout of LRH1/NR5A2, which compromises ATF2-dependent transcription [109]; and loss of CPEB4, which is a circadian-regulated poly(A) binding protein that controls mRNA localization [110]. However, from these studies the arrow of causation is not clear: does ER stress dysregulate lipid metabolism, or does altered lipid metabolism cause ER stress?
Mechanistic studies of lipid dysregulation in UPR-compromised animals have pointed to mechanisms by which the UPR controls metabolic pathways; much of this attention has focused on the IRE1/XBP1 pathway in the context of altered diets. Animals with liver-specific loss of IRE1α are sensitive to ER stresses of various sorts, and develop steatosis under such conditions in part due to failure to efficiently secrete VLDL [105, 111]. These animals also became steatotic under the milder stress of a high fructose diet, during which they were found to have diminished levels of plasma triglyceride but unaffected plasma levels of ApoB [103]. This finding suggested that Ire1α−/− livers secrete VLDL with diminished triglyceride content. Indeed, expression of the MTTP cofactor PDI was reduced in Ire1α−/− livers, and inhibition of MTTP phenocopied the VLDL lipidation defect, while overexpression of XBP1 or PDI in cultured hepatocytes rescued. It is worth noting that the best-known function of PDI is not as MTTP’s cofactor, but as an oxidase and isomerase, responsible for disulfide bond formation and rearrangement on ER client proteins [112].
Another XBP1 target gene in the liver is GALE, the role of which is to shunt sugars away from metabolic pathways and toward biosynthetic pathways. These include the formation of dolichol-linked oligosaccharides used during N-linked glycosylation, which is an ER-specific post-translational modification that is critical in proper ER protein folding [113]. The GalE promoter contains an XBP1 binding site, and the diversion of hepatic glucose caused by XBP1 overexpression had systemic effects on plasma glucose [114]. Together, these findings illustrate how the UPR’s attempts to enhance protein processing—via disulfide bond formation or N-linked glycosylation—can have knock-on effects on metabolism.
Not all of the phenotypes of Ire1α−/− livers are attributable to XBP1. Comparing gene expression in animals lacking both IRE1α and XBP1 in the liver against those lacking only XBP1 revealed that regulation of some genes was IRE1-dependent but XBP1-indepenent. These included ANGPTL3, which is secreted by the liver, controls plasma lipoprotein clearance [115], and was elevated when IRE1 was lacking. This and other genes were identified as potential RIDD targets [102]. As one of the major functions of RIDD is to relieve the burden of nascent protein synthesis in the ER, this finding also underscores the indirect impact of the UPR on metabolic pathways. Likewise, liver-specific inhibition of eIF2α phosphorylation impaired insulin sensitivity in muscle and fat, likely due to overproduction of secreted factors such as IGFBP3 that would otherwise be translationally repressed by eIF2α phosphorylation [116].
Transcriptional control of metabolic genes by the UPR
UPR transcription factors can also interact with and alter the activity of transcription factors governing other cellular processes, including metabolism (Figure 2). In some cases, these interactions are direct. Overexpressed XBP1 can promote the degradation of the FOXO1 transcription factor in vitro and in vivo [117]. Since FOXO1 promotes gluconeogenesis, expression of XBP1—even an XBP1 with its DNA binding domain deleted—can improve glucose homeostasis in both ob/ob mice and streptozotocin-treated mice (a model for Type I diabetes). Alternatively, UPR transcription factors can bind to cofactors that would otherwise regulate the activity of metabolic transcription factors, or bind directly to metabolic transcription factors and so influence their activity. This phenomenon appears to account for the ability of ATF6 to inhibit gluconeogenesis [95, 118]. ATF6 binds to the cofactor CRTC2, which ordinarily binds to and stimulates the activity of the gluconeogenic transcription factor CREB [95]. ATF6 has also been shown to interact with the master regulator of fatty acid oxidation, PPARα, and antagonize its activity, potentially accounting for fatty liver development and insulin resistance in mice expressing a dominant negative ATF6 on a high fat/high sucrose diet [119]. (On the other hand, knockdown of ATF6 reduces hepatic steatosis in response to ethanol exposure in zebrafish larvae [120], so how ATF6 interacts with metabolic transcriptional regulators might depend heavily on both environmental and developmental context).
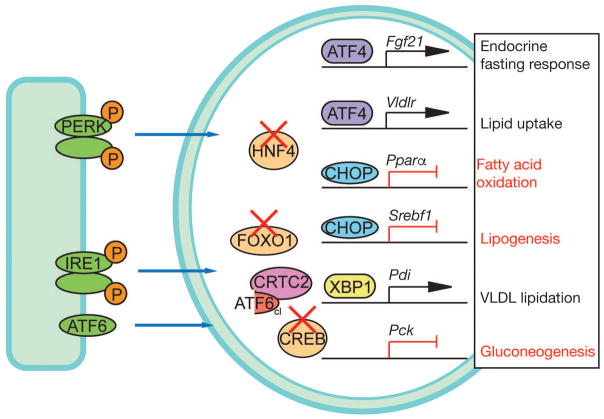
Figure 2.
Regulation of metabolic gene expression by the UPR
The UPR regulates metabolic gene expression via several mechanisms. One is direct activation of metabolic gene transcription, such as of Fgf21 and Vldlr by ATF4, or by direct repression of Pparα and Srebf1 by CHOP. Regulation of metabolism can also be indirect, in the sense that at least PDI, which is a target of XBP1, facilitates VLDL biogenesis in addition to its broader role in maintaining the ER oxidative protein folding capacity. Finally, UPR transcription factors can interfere with the activity of metabolic transcriptional regulators, such as HNF4α, FOXO1, or CREB, with consequences for their downstream processes.
Some metabolic genes are direct targets of UPR factors. In wild-type animals, tunicamycin treatment results in moderate steatosis, part of which arises from failure to secrete triglyceride in the form of VLDL from the dysfunctional ER [105]. However, other mechanisms contribute as well, including increased hepatic uptake of circulating VLDL. ATF4 upregulated the VLDL receptor in hepatocytes (VLDLR is not normally expressed in these cells), and Vldlr−/− mice were partially protected from tunicamycin-induced hepatic steatosis [121]. ER stress also resulted in upregulation of FGF21 via ATF4 and, as a result, increased serum FGF21 [122, 123]. We showed that pharmacological ER stress also inhibits hepatic fatty acid oxidation; this is likely due to genetic suppression of genes that regulate the process [73]. This suppression is partially due to the action of CHOP. Although CHOP is best known as an inducer of cell death during ER stress, it is a member of the C/EBP family of transcriptional regulators, which have diverse roles in regulating cell proliferation and metabolism [124]. As we showed, CHOP binds to C/EBP binding sites in the promoters of key metabolic genes, including the master regulator of fatty acid oxidation PPARα. The suppression of Pparα and other metabolic genes was blunted in Chop−/− mice, although CHOP overexpression alone was not sufficient to suppress such genes, implying the existence of an additional stress-dependent regulator [125]. Paradoxically, CHOP also contributed to suppression of the lipogenic gene Srebf1, which implied that CHOP might be capable of inhibiting either fatty acid oxidation or lipogenesis, depending on context. The ability to also suppress lipogenesis might explain why Chop−/− mice actually have fattier liver than wild-type animals on a HFD [126, 127] although this idea has not been tested. The stress-dependent CHOP-independent signal that regulates metabolic genes during ER stress has also not yet been identified, but might involve translational regulation of the metabolic transcription factor C/EBPβ [128] or inhibition of the metabolic regulator HNF4α [129]. Another potential factor is CREBH, a liver-specific transmembrane transcription factor that, like ATF6, is cleaved in the Golgi upon activation (see Box 1).
Box 1. CREBH: a potential link between ER stress, inflammation, and metabolic regulation.
Canonical UPR signaling proceeds through the three ubiquitously expressed stress sensing molecules, IRE1α, PERK, and ATF6 (α and β). Yet ATF6 is a member of a family of related transmembrane transcription factors that are activated upon ER stress by proteolytic cleavage in the Golgi. These include Luman, BBF2H7, CREB4, OASIS, and CREBH. These factors all seem to serve more specialized roles in linking ER homeostasis to other cellular processes [176]. CREBH is of particular interest here because its expression is restricted to the liver [177, 178], and the protein appears to directly connect ER stress to metabolism.
CREBH is dispensable for liver development [179]; however, the protein is responsive to and also regulates both metabolic and inflammatory pathways. In vivo, both expression and cleavage of CREBH are induced by either ER stress or inflammatory signals, whereas in vitro only ER stress results in significant cleavage [180]. Therefore, cleavage by inflammatory signals in vivo likely requires a non-cell-autonomous secondary signal that in turn elicits ER stress. Activated CREBH then augments the transcriptional upregulation of UPR targets by binding to unfolded protein response elements (UPREs; sites which are also recognized by ATF6 and XBP1). It also binds to UPRE-like sites in the promoters of Crp and Apcs, genes involved in the inflammatory response [180]. Therefore, CREBH likely contributes to the initiation of inflammation by ER stress and the augmentation of UPR signaling by inflammation. CREBH is also required for the upregulation of hepcidin, which is secreted by the liver during inflammation and inhibits iron export from macrophages and enterocytes, thus dropping plasma iron levels [181]. This response is believed to be a defense against pathogens that require iron, but leads to non-productive anemia in the case of sterile inflammation.
Examining the phenotypes of mice lacking CREBH has highlighted a metabolic role for this protein as well. CREBH is a transcriptional target of HNF4α [179], which plays integral roles in both liver development and hepatic lipid metabolism. Fasting upregulates CREBH expression, and CREBH stimulates hepatic gluconeogenic gene transcription in concert with the coregulator CRTC2 [182]. Crebh−/− mice on an atherogenic diet (high in fat, cholesterol, and cholate) develop steatosis, and CREBH binds to promoters of genes involved in both lipogenesis and fatty acid oxidation [183]. As these are competing processes, it seems likely that the effects of CREBH on metabolism depend highly on context and the cofactors that are present. Recent evidence suggests that CREBH activity displays a circadian periodicity [184, 185], and so diurnal cycles might be one factor influencing CREBH output.
While CREBH serves as a potential bridge between ER stress and both inflammation and metabolic regulation, it remains an open question to what extent CREBH activity truly depends on ER stress and to what extent UPR output in the liver during physiological stresses truly depends on CREBH. CREBH appears to undergo a fairly significant amount of cleavage basally, meaning that its activity could be regulated solely by changes in its expression independent of ER stress. Likewise, although CREBH clearly enhances UPR output during pharmacological stress, it might not affect expression of ER chaperones during normal metabolic flux [182].
Influence of metabolic flux on ER protein folding
Some of the regulatory steps described above—such as upregulation of GALE or PDI—can be seen as directed toward the improvement of ER function with ancillary consequences for metabolism. However, the logic behind suppression of fatty acid oxidation by the UPR is less clear, since fatty oxidation is largely a mitochondrial process. We showed that inhibition of fatty acid oxidation protects hepatocytes in vitro and in vivo from ER stress, implying that the inhibition of this process also benefits ER function [130]. One of the consequences of fatty acid oxidation is production of acetyl CoA, the oxidation of which by the TCA cycle produces NADH but also, particularly in the liver, NADPH [131]. NADPH is a cofactor for glutathione reductase, which generates reduced glutathione for anti-oxidant defense, at the expense of oxidized glutathione. Oxidized glutathione, however, helps buffer the protein oxidizing capacity of the ER lumen, where disulfide bonds must be formed on nascent proteins [132]. Thus, one hypothesis is that inhibiting fatty acid oxidation spares oxidized glutathione for the purposes of maintaining the ER oxidative folding capacity, at the expense of defense against reactive oxygen species. In support of this idea, inhibiting fatty acid oxidation made the ER more oxidizing, and inhibiting NADPH production or glutathione reduction also protected against ER stress [130]. However, whether inhibition of fatty acid oxidation occurs during more physiological stresses has not been tested, and whether that inhibition would be protective under such circumstances has yielded conflicting results [133–136]. The direct regulation of metabolism by the UPR also raises the more general possibility that steatosis elicited by ER stress is a protective, rather than maladaptive, response.
ER stress in liver damage and inflammation
Steatosis is typically the first step along the pathway of liver damage. The fact that ER stress in the liver can regulate lipid metabolism through both direct and indirect mechanisms already positions ER stress as an instigating factor in liver disease. However, the progression of liver disease beyond steatosis requires inflammation, usually incited by signals released from damaged, dying, or dead cells [137]. The process involves both immune cells and stellate cells. Does ER stress contribute to hepatic inflammation and its consequences, including fibrosis and hepatocellular transformation? And if so, is it simply through the execution of cells that are overwhelmed by ER stress, or is the role of the UPR more complex? Recent work has implicated ER stress in liver damage incited by many different causes, and is revealing a unique role for the UPR in the cycles of cell death, immune cell recruitment, and hepatocyte proliferation that characterize liver disease (Figure 3).
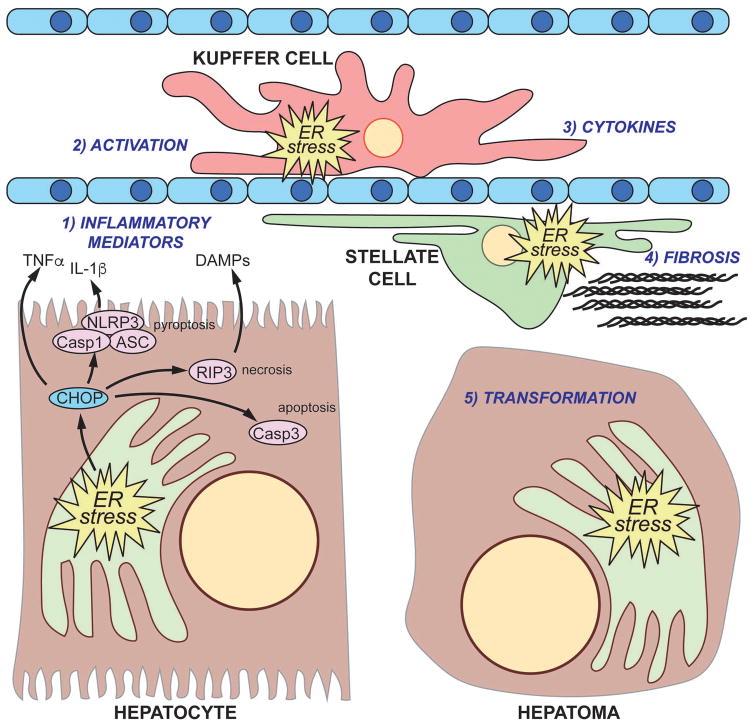
Figure 3.
The complex interplay between ER stress and liver damage and transformation
(1) Unremediable ER stress in hepatocytes can elicit cell death, which might proceed through apoptotic, necrotic, or pyroptotic pathways or a combination thereof. Hepatocytes subjected to ER stress also can secrete pro-inflammatory signals such as TNFα or other factors. Hepatocyte cell death stimulates macrophage activation (2) and secretion of inflammatory cytokines (3), and activation might represent its own ER stress in the macrophage. Likewise, inflammation promotes hepatic stellate cell activation (4), including production and extrusion of collagen through the secretory pathway. The combination of inflammatory cytokines and fibrotic deposition stimulates compensatory hepatocyte proliferation which, if unchecked, can promote tumorigenesis (5).
Inflammation through stress-induced cell death
Among the stimuli that elicit ER stress in the liver besides obesity, alcoholism, and viral hepatitis are acetaminophen [138], which causes oxidative damage due to a hepatic metabolite; methionine/choline-deficient diet (MCD) [139], which elicits fatty liver and inflammation reminiscent of human NASH; and CCl4 [140], which stimulates fibrosis in the absence of steatosis. As with obesity-induced liver dysfunction [44], pharmacological chaperones like TUDCA or PBA reduced hepatic ER stress [141, 142]. More importantly, liver-specific loss of BiP resulted in ER stress, steatosis, liver damage, and exacerbated injury from ethanol, HFD, CCl4, or HIV proteasome inhibitors [143]. Therefore, it can be concluded that intact ER protein folding is required for maintaining basic liver function. Likewise, mice with a liver-specific knockout of XBP1 showed enhanced fibrosis and liver damage on a high fat/high sugar diet [144], as did mice with a liver-specific inability to phosphorylate eIF2α upon a high fructose diet [145], suggesting that the protective capacity of the UPR is required to maintain liver integrity. (On the other hand, mice lacking XBP1 were actually protected from toxicity induced by acetaminophen. This protection was due to compensatory hyperactivation of IRE1 and concomitant RIDD-mediated degradation of mRNAs encoding enzymes responsible for hepatotoxic metabolization of the drug—rendering protective UPR signaling unnecessary [146].)
How then does ER stress promote liver damage? The simplest model would be that some hepatocytes would succumb to over-exuberant UPR signaling during ER stress conditions, initiating cell death processes that not only kill compromised hepatocytes but also activate inflammatory cascades, thereby setting in motion the pathway to steatohepatitis and fibrosis. However, one of the problems with this model is that not all types of cell death are pro-inflammatory; indeed, apoptosis is defined as “silent” in part by its supposed non-inflammatory nature, and although the pathways of ER stress-induced cell death are still not well understood, apoptotic death is almost certainly involved (reviewed in [147]). Animals with a constitutive deletion of CHOP show dampened markers of hepatic inflammation and liver damage under basal conditions [126, 148], seemingly supporting the idea that CHOP-induced cell death is not exclusively apoptotic, or at least not non-inflammatory. Based on this finding, one might predict that Chop−/− animals would be protected from stimuli that incite liver damage. While this is true in some cases—e.g., Chop−/− mice showed diminished fibrosis, necrosis, and apoptosis in animals that have undergone bile duct ligation as a model for cholestatic liver injury [149]—it is not true in others. Surprisingly, Chop−/− mice were sensitized to liver injury induced by HFD or by MCD diet [127]. The reason for this sensitivity likely owes to the interplay between hepatocytes and immune cells in driving liver inflammatory cascades: while hepatocyte cell death can incite these cascades, they rely on activated macrophages and other immune cells for their propagation [150]. Macrophages lacking CHOP were more susceptible to cell death induced by palmitic acid. To the extent that the lipotoxicity that accompanies steatosis represents an ER stress on all the cells of the liver, then the role of CHOP in promoting cell death would be expected to exacerbate liver injury by its effect in hepatocytes but attenuate it by its effect in fibroblasts. Supporting this idea, both transfer of Chop−/− macrophages to wild-type animals and transfer of wild-type macrophages to Chop−/− animals attenuated the liver damage seen by MCD diet in constitutive Chop−/− animals [127]. In other words, inflammation and liver damage are thwarted either if (a) there is no CHOP in hepatocytes to promote hepatocyte cell death, or if (b) CHOP in macrophages, other immune cells, or stellate cells promotes the death of those cells.
Intersection of ER stress and inflammatory signaling pathways
How does ER stress-induced cell death promote inflammation? One possible mechanism is if the cell death in hepatocytes elicited by ER stress is apoptotic but nonetheless results in the activation of macrophages that clear the dead cell remnants. Increased hepatocyte apoptosis is seen in human alcoholic steatohepatitis and NASH arising from various causes [151, 152]. ER stress-induced cell death involves classical markers of apoptotic cell death such as activation of Caspase-3 and formation of the mitochondrial outer membrane pore [153]. If the capacity of the liver to clear apoptotic figures is exceeded, then these dead cells might spontaneously rupture, releasing damage-associated molecular patterns (DAMPs) that stimulate immune cell recruitment and activation [154]. Alternatively, macrophage-mediated clearance of apoptotic bodies in the liver can itself be an activating stimulus [155]. However, the fact that death induced by ER stress is sometimes apoptotic does not exclude that it might also proceed by other mechanisms in some cells. Indeed, in other cell types, ER stress has been shown to activate the NLRP3 inflammasome, which is a molecular complex that results in the production and release of inflammatory cytokines prior to cell rupture [156, 157]. Inflammasome activation in response to ER stress has recently also been demonstrated in the liver, including activation of Caspase-1 and release of IL-1β, and the process was CHOP-dependent [142]. Consistent with this idea, loss of Caspase-1 diminished liver damage in mice challenged with tunicamycin [158]. That ER stress generally elicits inflammatory signaling in hepatocytes is also seen in the coincidence of ER stress and activation of JNK, NF-κB, and IRF3 [159, 160]. Alternatively, or perhaps in addition, ER stress-induced cell death might be necrotic; perpetuating eIF2α signaling in the liver during partial hepatectomy led to cell death that was dependent on the necrotic factor RIP3 [161]. ER stress can also influence the propagation of inflammatory signals generated by other cells. ER stress signaling has been shown to influence the ability of the pro-inflammatory cytokine IL-6 to cause STAT3 activation, with consequences for both glucose metabolism [162] and liver damage and compensatory proliferation [163].
ER stress in hepatocellular carcinoma
The intersection between ER stress, metabolism, liver damage, and inflammation is of its most profound consequence during the pathogenesis of HCC. ER stress is associated with a number of cancers, including breast, colon, prostate, brain, and lung. In these contexts, it is thought that the ability of the UPR to promote adaptation to stress allows tumor cells to survive, and that tipping the balance to stress-induced cell death could prove therapeutically useful [164]. The association of ER stress with viral hepatitis, alcoholism, and obesity, all of which are risk factors for HCC, suggested that it might contribute in some way to HCC initiation and/or progression. Early evidence suggested that ER stress markers are elevated in human HCCs [165]. Subsequently, it has become clear that ER dysfunction can drive HCC formation, as tumors could be generated simply by overexpressing a misfolded ER client protein in the context of a HFD [166] or by liver-specific loss of the ER chaperones BiP or GRP94 [167, 168]. What was most notable about HCC induced by loss of BiP or GRP94 is that, in both cases, the tumors themselves were populated by non-deleted cells. How can chaperone deletion stimulate formation of tumors without that same deletion? One hypothesis is that ER stress in some cells can promote transformation non-cell autonomously. These findings are consistent with the model that anti-adaptive ER stress signaling can promote liver damage, inflammation, and compensatory proliferation of other hepatocytes in the process of tumor initiation, while adaptive ER stress signaling then promotes tumor progression. The idea that ER stress-induced hepatocyte death actually drives HCC formation is substantiated by the finding by us and others that animals lacking CHOP are partially protected from chemically-induced HCC [148, 169]. A priori, one might expect animals lacking the pro-apoptotic CHOP protein to be sensitized to HCC, as impaired stress-induced cell death should promote tumor progression. Indeed, liver tumors of Chop−/− animals displayed reduced cell death. However, Chop−/− livers also showed reduced markers of inflammation and proliferation. Thus, CHOP on balance appears to promote HCC rather than protect against it.
Progression from liver insults to HCC involves not only hepatocytes but also macrophages, which mediate immune and inflammatory responses, and stellate cells, which when activated deposit the collagen that marks the fibrotic milieu in which HCC develops. Beyond a simple cycle of cell death and compensatory proliferation, there is evidence that these other cell types in the liver are involved in propagating ER stress signals. Conditioned media from liver tumor cells subjected to ER stress in vitro activated macrophages in vitro and in vivo, and this effect was dependent on toll-like receptor signaling [170]. Thus, cells experiencing ER stress can release diffusible signals that alter inflammatory progression. In support of this idea, ER stressed hepatocytes have been shown to secrete both the inflammatory cytokine TNFα [171] and ceramide-rich extracellular vesicles [172], both of which can affect macrophage activity. ER stress was also seen in hepatic stellate cells upon liver injury [173], and HSC activation induced by TGFβ required IRE1α signaling [174]. It is possible that the expansion of the secretory apparatus required for activated HSCs to produce collagen fibers is the source of this ER stress. In support of this idea, XBP1 induction by HSC differentiation occurred prior to maximal upregulation of collagen and other differentiation markers, and overexpression of XBP1 was sufficient to enhance collagen expression [175]. Thus, ER stress signaling might anticipate, rather than react to, collagen production. Taken together, these studies suggest that the role of ER stress signaling in HCC is complex, and any therapeutic strategy that targets ER stress signaling will have to account for its different roles in tumor initiation and progression, and in hepatocytes versus macrophages and stellate cells.
Conclusions and Perspectives
The goal of this review was not to document every way in which ER stress is implicated in liver function and dysfunction, but instead to highlight those connections that reveal novel features or consequences of UPR signaling. Broadly, these fall into three categories: (1) how nutritional signals elicit ER stress; (2) how the UPR regulates metabolism and other parallel cellular processes and the consequences of this regulation for liver function; and (3) the role of adaptive and anti-adaptive ER stress signaling in mediating hepatocyte death and liver damage, inflammation, and tumorigenesis.
The groundwork has now been laid to address more sophisticated questions about these relationships, including: (1) How is ER protein processing actually disrupted by insulin signaling and by nutritional flux? Is global protein processing impaired, is ER membrane structure disrupted, or is there some more subtle cue? (2) By what mechanisms does the UPR interact with metabolic pathways? To what extent does UPR-dependent transcription interfere with or otherwise alter the activity of transcription factors that are regulated by metabolic conditions, and is this interference part of normal physiological regulation or does it only come into play during severe stresses? Identifying protein-protein interactions that link the UPR with other pathways, as well as mechanisms of crosstalk between the ER and mitochondria, will be key to answering these questions. (3) How does chronic ER stress impact these responses? Is there a difference between a physiological UPR and a pathological UPR? Does UPR signaling become fundamentally impaired, or do conditions like obesity represent the UPR executing the program that it evolved to execute (i.e., regulation of insulin signaling, VLDL production, metabolic transcription, etc.) under conditions that it was not evolved to handle (i.e., constant nutritional excess). The development of robust animal models that specifically recapitulate chronic ER stress in the liver will be needed to identify the effects of chronic ER stress per se on liver function, divorced from confounding contexts like obesity, alcoholism, etc. And (4), how does ER stress elicit inflammation? How do the various cell types of the liver communicate, and what role does ER stress-induced death play in the process? The creation of mouse models in which ER stress signaling is controlled not only by cell type, but also temporally, will be instrumental in dissecting potentially multiple points of influence in the progression of liver disease. What was first identified as a pathway solely concerned with the detection and restoration of ER protein folding is now being recognized as a central regulator of liver function, with many new discoveries on the horizon.
Acknowledgments
The author wishes to thank L. Yang (University of Iowa) for constructive comments on the manuscript and M. A. Meaux for organizational advice. The author also wishes to thank the anonymous peer reviewers for their suggestions for improvement. The author is supported by NIH grant GM115424.
Abbreviations used
- UPR
unfolded protein response
- ER
endoplasmic reticulum
- IRE
Inositol-requiring enzyme
- PERK
PKR-like endoplasmic reticulum kinase
- ATF
activating transcription factor
- MEFs
mouse embryonic fibroblasts
- eIF
eukaryotic initiation factor
- bZIP
basic leucine zipper
- RIDD
regulated IRE1-dependent decay
- CHOP
C/EBP-homologous protein
- VLDL
very low density lipoprotein
- XBP
X-box binding protein
- mTOR
mammalian target of rapamycin
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- JNK
c-Jun N-terminal kinase
- IRS
insulin receptor substrate
- HFD
high-fat diet
- PBA
4-phenylbutyric acid
- TUDCA
tauroursodeoxycholic acid
- BiP
binding protein
- MTTP
microsomal triglyceride transfer protein
- PDI
protein disulfide isomerase
- HFrD
High fructose diet
- ApoB
apolipoprotein B
- CREBH
CRE-binding and hepatocyte-specific factor
- MCD
methionine-choline deficient diet
References
- 1.Mori K. The unfolded protein response: the dawn of a new field. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91:469–80. doi: 10.2183/pjab.91.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 3.Vinson C, Acharya A, Taparowsky EJ. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta. 2006;1759:4–12. doi: 10.1016/j.bbaexp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Read DE, Gupta S. Assays for induction of the unfolded protein response and selective activation of the three major pathways. Methods Mol Biol. 2015;1292:19–38. doi: 10.1007/978-1-4939-2522-3_2. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nuc Ac Res. 2006;34:e93. doi: 10.1093/nar/gkl515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badr CE, Hewett JW, Breakefield XO, Tannous BA. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One. 2007;2:e571. doi: 10.1371/journal.pone.0000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beriault DR, Werstuck GH. Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound, Thioflavin T. Biochim Biophys Acta. 2013;1833:2293–301. doi: 10.1016/j.bbamcr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Lai CW, Aronson DE, Snapp EL. BiP availability distinguishes states of homeostasis and stress in the endoplasmic reticulum of living cells. Mol Biol Cell. 2010;21:1909–21. doi: 10.1091/mbc.E09-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–52. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 12.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Ishigaki Y, Yamada T, Kondo K, Yamaguchi S, Imai J, Uno K, Hasegawa Y, Sawada S, Ishihara H, Oyadomari S, Mori M, Oka Y, Katagiri H. Involvement of endoplasmic stress protein C/EBP homologous protein in arteriosclerosis acceleration with augmented biological stress responses. Circulation. 2011;124:830–9. doi: 10.1161/CIRCULATIONAHA.110.014050. [DOI] [PubMed] [Google Scholar]
- 14.Namba T, Tanaka K, Ito Y, Ishihara T, Hoshino T, Gotoh T, Endo M, Sato K, Mizushima T. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174:1786–98. doi: 10.2353/ajpath.2009.080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Bostrom P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 2011;13:160–9. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–32. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–80. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14:152–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwawaki T, Akai R, Yamanaka S, Kohno K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci USA. 2009;106:16657–62. doi: 10.1073/pnas.0903775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–97. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markan KR, Potthoff MJ. Metabolic fibroblast growth factors (FGFs): Mediators of energy homeostasis. Semin Cell Dev Biol. 2016;53:85–93. doi: 10.1016/j.semcdb.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 29.Grisham JW. The Liver. John Wiley & Sons, Ltd; 2009. Organizational Principles of the Liver; pp. 1–15. [Google Scholar]
- 30.Kren BT, Steer CJ, Roy Chowdhury N, Roy Chowdhury J. The Liver. John Wiley & Sons, Ltd; 2009. Liver-Directed Gene Therapy; pp. 965–990. [Google Scholar]
- 31.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 32.Stauffer JK, Scarzello AJ, Jiang Q, Wiltrout RH. Chronic inflammation, immune escape, and oncogenesis in the liver: a unique neighborhood for novel intersections. Hepatology. 2012;56:1567–74. doi: 10.1002/hep.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuman MG, French SW, French BA, Seitz HK, Cohen LB, Mueller S, Osna NA, Kharbanda KK, Seth D, Bautista A, Thompson KJ, McKillop IH, Kirpich IA, McClain CJ, Bataller R, Nanau RM, Voiculescu M, Opris M, Shen H, Tillman B, Li J, Liu H, Thomes PG, Ganesan M, Malnick S. Alcoholic and non-alcoholic steatohepatitis. Exp Mol Pathol. 2014;97:492–510. doi: 10.1016/j.yexmp.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol. 1997;71:7387–92. doi: 10.1128/jvi.71.10.7387-7392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberman E, Fong YL, Selby MJ, Choo QL, Cousens L, Houghton M, Yen TS. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol. 1999;73:3718–22. doi: 10.1128/jvi.73.5.3718-3722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76:7453–9. doi: 10.1128/JVI.76.15.7453-7459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 39.Özcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 40.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;Chapter 5(Unit5):61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 42.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein SS, Weigert C, Lehmann R. Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle. Curr Med Chem. 2017;24:1827–1852. doi: 10.2174/0929867324666170426142826. [DOI] [PubMed] [Google Scholar]
- 44.Özcan U, Yilmaz E, Özcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nissar AU, Sharma L, Mudasir MA, Nazir LA, Umar SA, Sharma PR, Vishwakarma RA, Tasduq SA. Chemical chaperone 4-phenyl butyric acid (4-PBA) reduces hepatocellular lipid accumulation and lipotoxicity through induction of autophagy. J Lipid Res. 2017;58:1855–1868. doi: 10.1194/jlr.M077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, Kahn M, Samuel VT, Glimcher LH, Shulman GI. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287:2558–67. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshiuchi K, Kaneto H, Matsuoka TA, Kohno K, Iwawaki T, Nakatani Y, Yamasaki Y, Hori M, Matsuhisa M. Direct monitoring of in vivo ER stress during the development of insulin resistance with ER stress-activated indicator transgenic mice. Biochem Biophys Res Commun. 2008;366:545–50. doi: 10.1016/j.bbrc.2007.11.182. [DOI] [PubMed] [Google Scholar]
- 48.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 49.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 52.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder F, Goh EH. Effect of fatty acids on physical properties of microsomes from isolated perfused rat liver. Chem Phys Lipids. 1980;26:207–24. doi: 10.1016/0009-3084(80)90052-3. [DOI] [PubMed] [Google Scholar]
- 54.Halbleib K, Pesek K, Covino R, Hofbauer HF, Wunnicke D, Hanelt I, Hummer G, Ernst R. Activation of the Unfolded Protein Response by Lipid Bilayer Stress. Mol Cell. 2017;67:673–684e8. doi: 10.1016/j.molcel.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci USA. 2010;107:16113–8. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci USA. 2013;110:4628–33. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantzaris MD, Tsianos EV, Galaris D. Interruption of triacylglycerol synthesis in the endoplasmic reticulum is the initiating event for saturated fatty acid-induced lipotoxicity in liver cells. FEBS J. 2011;278:519–30. doi: 10.1111/j.1742-4658.2010.07972.x. [DOI] [PubMed] [Google Scholar]
- 58.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genom. 2008;33:361–72. doi: 10.1152/physiolgenomics.00139.2007. [DOI] [PubMed] [Google Scholar]
- 59.Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, Jung ME, Ford DA, Tontonoz P. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–97. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gruffat D, Durand D, Graulet B, Bauchart D. Regulation of VLDL synthesis and secretion in the liver. Reprod Nutr Dev. 1996;36:375–89. doi: 10.1051/rnd:19960404. [DOI] [PubMed] [Google Scholar]
- 61.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su Q, Tsai J, Xu E, Qiu W, Bereczki E, Santha M, Adeli K. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 63.Liao W, Hui TY, Young SG, Davis RA. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J Lip Res. 2003;44:978–985. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs CD, Claudel T, Kumari P, Haemmerle G, Pollheimer MJ, Stojakovic T, Scharnagl H, Halilbasic E, Gumhold J, Silbert D, Koefeler H, Trauner M. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270–80. doi: 10.1002/hep.25601. [DOI] [PubMed] [Google Scholar]
- 65.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–78. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 66.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci USA. 2010;107:19320–5. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–35. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 71.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA. 2012;109:5523–8. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta. 2014;1837:461–9. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 73.DeZwaan-McCabe D, Sheldon RD, Gorecki MC, Guo DF, Gansemer ER, Kaufman RJ, Rahmouni K, Gillum MP, Taylor EB, Teesch LM, Rutkowski DT. ER Stress Inhibits Liver Fatty Acid Oxidation while Unmitigated Stress Leads to Anorexia-Induced Lipolysis and Both Liver and Kidney Steatosis. Cell Rep. 2017;19:1794–1806. doi: 10.1016/j.celrep.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfaffenbach KT, Nivala AM, Reese L, Ellis F, Wang D, Wei Y, Pagliassotti MJ. Rapamycin Inhibits Postprandial-Mediated X-Box-Binding Protein-1 Splicing in Rat Liver. J Nutr. 2010;140:879–884. doi: 10.3945/jn.109.119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boden G, Song W, Duan X, Cheung P, Kresge K, Barrero C, Merali S. Infusion of glucose and lipids at physiological rates causes acute endoplasmic reticulum stress in rat liver. Obesity (Silver Spring) 2011;19:1366–73. doi: 10.1038/oby.2011.71. [DOI] [PubMed] [Google Scholar]
- 77.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Özcan U, Özcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–79. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–45. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–37. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J, Sun C, Zhou Y, Lee J, Gokalp D, Herrema H, Park SW, Davis RJ, Ozcan U. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med. 2011;17:1251–60. doi: 10.1038/nm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Ibi D, Taniguchi K, Lee J, Herrema H, Akosman B, Mucka P, Salazar Hernandez MA, Uyar MF, Park SW, Karin M, Ozcan U. Inflammation Improves Glucose Homeostasis through IKKbeta-XBP1s Interaction. Cell. 2016;167:1052–1066e18. doi: 10.1016/j.cell.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SW, Herrema H, Salazar M, Cakir I, Cabi S, Basibuyuk Sahin F, Chiu YH, Cantley LC, Ozcan U. BRD7 regulates XBP1s’ activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014;20:73–84. doi: 10.1016/j.cmet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Shimizu Y, Mann MJ, Jin Y, Hendershot LM. Plasma cell differentiation initiates a limited ER stress response by specifically suppressing the PERK-dependent branch of the unfolded protein response. Cell Stress Chap. 2010;15:281–93. doi: 10.1007/s12192-009-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vacaru AM, Di Narzo AF, Howarth DL, Tsedensodnom O, Imrie D, Cinaroglu A, Amin S, Hao K, Sadler KC. Molecularly defined unfolded protein response subclasses have distinct correlations with fatty liver disease in zebrafish. Dis Model Mech. 2014;7:823–35. doi: 10.1242/dmm.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–80. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cretenet G, Le Clech M, Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 92.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 93.Gawrieh S, Baye TM, Carless M, Wallace J, Komorowski R, Kleiner DE, Andris D, Makladi B, Cole R, Charlton M, Curran J, Dyer TD, Charlesworth J, Wilke R, Blangero J, Kissebah AH, Olivier M. Hepatic gene networks in morbidly obese patients with nonalcoholic fatty liver disease. Obes Surg. 2010;20:1698–709. doi: 10.1007/s11695-010-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamagate A, Kim DH, Zhang T, Slusher S, Gramignoli R, Strom SC, Bertera S, Ringquist S, Dong HH. FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology. 2010;151:3521–35. doi: 10.1210/en.2009-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–7. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, Hotamisligil GS. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015;349:500–6. doi: 10.1126/science.aaa0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–72. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 99.Cinaroglu A, Gao C, Imrie D, Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology. 2011;54:495–508. doi: 10.1002/hep.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomez JA, Rutkowski DT. Experimental reconstitution of chronic ER stress in the liver reveals feedback suppression of BiP mRNA expression. Elife. 2016:5. doi: 10.7554/eLife.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Lichtman AH, Iwawaki T, Glimcher LH, Lee AH. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012;16:487–99. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, Davidson NO, Kaufman RJ. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 2012;16:473–86. doi: 10.1016/j.cmet.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herrema H, Zhou Y, Zhang D, Lee J, Salazar Hernandez MA, Shulman GI, Ozcan U. XBP1s Is an Anti-lipogenic Protein. J Biol Chem. 2016;291:17394–404. doi: 10.1074/jbc.M116.728949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Otoda T, Takamura T, Misu H, Ota T, Murata S, Hayashi H, Takayama H, Kikuchi A, Kanamori T, Shima KR, Lan F, Takeda T, Kurita S, Ishikura K, Kita Y, Iwayama K, Kato K, Uno M, Takeshita Y, Yamamoto M, Tokuyama K, Iseki S, Tanaka K, Kaneko S. Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in the liver. Diabetes. 2013;62:811–24. doi: 10.2337/db11-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K, Li P, Cheng HL, Murphy AJ, Valenzuela DM, Luo H, Kapahi P, Krauss R, Mostoslavsky R, Yancopoulos GD, Alt FW, Chua KF, Chen D. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep. 2013;5:654–665. doi: 10.1016/j.celrep.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mamrosh JL, Lee JM, Wagner M, Stambrook PJ, Whitby RJ, Sifers RN, Wu SP, Tsai MJ, Demayo FJ, Moore DD. Nuclear receptor LRH-1/NR5A2 is required and targetable for liver endoplasmic reticulum stress resolution. Elife. 2014;3:e01694. doi: 10.7554/eLife.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maillo C, Martin J, Sebastian D, Hernandez-Alvarez M, Garcia-Rocha M, Reina O, Zorzano A, Fernandez M, Mendez R. Circadian- and UPR-dependent control of CPEB4 mediates a translational response to counteract hepatic steatosis under ER stress. Nat Cell Biol. 2017;19:94–105. doi: 10.1038/ncb3461. [DOI] [PubMed] [Google Scholar]
- 111.Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, Chen YE, Jackowski S, Kaufman RJ. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 2011;30:1357–75. doi: 10.1038/emboj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10:203–10. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- 113.McCaffrey K, Braakman I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016;60:227–235. doi: 10.1042/EBC20160003. [DOI] [PubMed] [Google Scholar]
- 114.Deng Y, Wang ZV, Tao C, Gao N, Holland WL, Ferdous A, Repa JJ, Liang G, Ye J, Lehrman MA, Hill JA, Horton JD, Scherer PE. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J Clin Invest. 2013;123:455–68. doi: 10.1172/JCI62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, McNutt MC, Banfi S, Levin MG, Holland WL, Gusarova V, Gromada J, Cohen JC, Hobbs HH. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc Natl Acad Sci USA. 2015;112:11630–5. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Birkenfeld AL, Lee HY, Majumdar S, Jurczak MJ, Camporez JP, Jornayvaz FR, Frederick DW, Guigni B, Kahn M, Zhang D, Weismann D, Arafat AM, Pfeiffer AF, Lieske S, Oyadomari S, Ron D, Samuel VT, Shulman GI. Influence of the hepatic eukaryotic initiation factor 2alpha (eIF2alpha) endoplasmic reticulum (ER) stress response pathway on insulin-mediated ER stress and hepatic and peripheral glucose metabolism. J Biol Chem. 2011;286:36163–70. doi: 10.1074/jbc.M111.228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Lee J, Fisher SJ, White MF, Biddinger SB, Ozcan U. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–65. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seo HY, Kim MK, Min AK, Kim HS, Ryu SY, Kim NK, Lee KM, Kim HJ, Choi HS, Lee KU, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases cAMP-stimulated hepatic gluconeogenesis via inhibition of CREB. Endocrinology. 2010;151:561–8. doi: 10.1210/en.2009-0641. [DOI] [PubMed] [Google Scholar]
- 119.Chen X, Zhang F, Gong Q, Cui A, Zhuo S, Hu Z, Han Y, Gao J, Sun Y, Liu Z, Yang Z, Le Y, Gao X, Dong LQ, Gao X, Li Y. Hepatic ATF6 Increases Fatty Acid Oxidation to Attenuate Hepatic Steatosis in Mice Through Peroxisome Proliferator-Activated Receptor alpha. Diabetes. 2016;65:1904–15. doi: 10.2337/db15-1637. [DOI] [PubMed] [Google Scholar]
- 120.Howarth DL, Lindtner C, Vacaru AM, Sachidanandam R, Tsedensodnom O, Vasilkova T, Buettner C, Sadler KC. Activating transcription factor 6 is necessary and sufficient for alcoholic fatty liver disease in zebrafish. PLoS Genet. 2014;10:e1004335. doi: 10.1371/journal.pgen.1004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jo H, Choe SS, Shin KC, Jang H, Lee JH, Seong JK, Back SH, Kim JB. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–77. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 122.Schaap FG, Kremer AE, Lamers WH, Jansen PL, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–9. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 123.Kim SH, Kim KH, Kim HK, Kim MJ, Back SH, Konishi M, Itoh N, Lee MS. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia. 2015;58:809–18. doi: 10.1007/s00125-014-3475-6. [DOI] [PubMed] [Google Scholar]
- 124.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 125.Chikka MR, McCabe DD, Tyra HM, Rutkowski DT. C/EBP homologous protein (CHOP) contributes to suppression of metabolic genes during endoplasmic reticulum stress in the liver. J Biol Chem. 2013;288:4405–15. doi: 10.1074/jbc.M112.432344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maris M, Overbergh L, Gysemans C, Waget A, Cardozo AK, Verdrengh E, Cunha JP, Gotoh T, Cnop M, Eizirik DL, Burcelin R, Mathieu C. Deletion of C/EBP homologous protein (Chop) in C57Bl/6 mice dissociates obesity from insulin resistance. Diabetologia. 2012;55:1167–78. doi: 10.1007/s00125-011-2427-7. [DOI] [PubMed] [Google Scholar]
- 127.Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, Yong J, Kaufman RJ. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem. 2013;288:18624–42. doi: 10.1074/jbc.M112.442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y, Bevilacqua E, Chiribau CB, Majumder M, Wang C, Croniger CM, Snider MD, Johnson PF, Hatzoglou M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J Biol Chem. 2008;283:22443–56. doi: 10.1074/jbc.M801046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arensdorf AM, Dezwaan McCabe D, Kaufman RJ, Rutkowski DT. Temporal clustering of gene expression links the metabolic transcription factor HNF4alpha to the ER stress-dependent gene regulatory network. Front Genet. 2013;4:188. doi: 10.3389/fgene.2013.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tyra HM, Spitz DR, Rutkowski DT. Inhibition of fatty acid oxidation enhances oxidative protein folding and protects hepatocytes from endoplasmic reticulum stress. Mol Biol Cell. 2012;23:811–9. doi: 10.1091/mbc.E11-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jennings GT, Sechi S, Stevenson PM, Tuckey RC, Parmelee D, McAlister-Henn L. Cytosolic NADP(+)-dependent isocitrate dehydrogenase. Isolation of rat cDNA and study of tissue-specific and developmental expression of mRNA. J Biol Chem. 1994;269:23128–34. [PubMed] [Google Scholar]
- 132.Delaunay-Moisan A, Appenzeller-Herzog C. The antioxidant machinery of the endoplasmic reticulum: Protection and signaling. Free Radic Biol Med. 2015;83:341–51. doi: 10.1016/j.freeradbiomed.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 133.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, Zhu Y, Borensztajn J, Reddy JK. Sustained activation of PPAR{alpha} by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2011 doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan SM, Sun RQ, Zeng XY, Choong ZH, Wang H, Watt MJ, Ye JM. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. 2013;62:2095–105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su Q, Baker C, Christian P, Naples M, Tong X, Zhang K, Santha M, Adeli K. Hepatic mitochondrial and ER stress induced by defective PPARalpha signaling in the pathogenesis of hepatic steatosis. Am J Physiol Endocrinol Metab. 2014;306:E1264–73. doi: 10.1152/ajpendo.00438.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L, Ren F, Zhang X, Wang X, Shi H, Zhou L, Zheng S, Chen Y, Chen D, Li L, Zhao C, Duan Z. Peroxisome proliferator-activated receptor alpha acts as a mediator of endoplasmic reticulum stress-induced hepatocyte apoptosis in acute liver failure. Dis Model Mech. 2016;9:799–809. doi: 10.1242/dmm.023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsin I-F, Seki E. Hepatocyte Death in Liver Inflammation, Fibrosis, and Tumorigenesis. In: Ding WX, Win XM, editors. Cellular Injury in Liver Diseases. Springer; Cham: 2017. pp. 219–235. [Google Scholar]
- 138.Nagy G, Kardon T, Wunderlich L, Szarka A, Kiss A, Schaff Z, Banhegyi G, Mandl J. Acetaminophen induces ER dependent signaling in mouse liver. Arch Biochem Biophys. 2007;459:273–9. doi: 10.1016/j.abb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 139.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–17. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 140.Chaveroux C, Carraro V, Canaple L, Averous J, Maurin AC, Jousse C, Muranishi Y, Parry L, Mesclon F, Gatti E, Mallet J, Ravassard P, Pierre P, Fafournoux P, Bruhat A. In vivo imaging of the spatiotemporal activity of the eIF2alpha-ATF4 signaling pathway: Insights into stress and related disorders. Sci Signal. 2015;8:rs5. doi: 10.1126/scisignal.aaa0549. [DOI] [PubMed] [Google Scholar]
- 141.Henkel AS, Dewey AM, Anderson KA, Olivares S, Green RM. Reducing endoplasmic reticulum stress does not improve steatohepatitis in mice fed a methionine- and choline-deficient diet. Am J Physiol Gastrointest Liver Physiol. 2012;303:G54–9. doi: 10.1152/ajpgi.00052.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O, Saint-Paul MC, Anty R, Schneck AS, Iannelli A, Gugenheim J, Tran A, Gual P, Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell death & disease. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–39. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X, Henkel AS, LeCuyer BE, Schipma MJ, Anderson KA, Green RM. Hepatocyte X-box binding protein 1 deficiency increases liver injury in mice fed a high-fat/sugar diet. Am J Physiol Gastrointest Liver Physiol. 2015;309:G965–74. doi: 10.1152/ajpgi.00132.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choi WG, Han J, Kim JH, Kim MJ, Park JW, Song B, Cha HJ, Choi HS, Chung HT, Lee IK, Park TS, Hatzoglou M, Choi HS, Yoo HJ, Kaufman RJ, Back SH. eIF2alpha phosphorylation is required to prevent hepatocyte death and liver fibrosis in mice challenged with a high fructose diet. Nutr Metab (Lond) 2017;14:48. doi: 10.1186/s12986-017-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hur KY, So JS, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Iwawaki T, Glimcher LH, Lee AH. IRE1alpha activation protects mice against acetaminophen-induced hepatotoxicity. J Exp Med. 2012;209:307–18. doi: 10.1084/jem.20111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hetz C, Papa FR. The Unfolded Protein Response and Cell Fate Control. Mol Cell. 2017 doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 148.DeZwaan-McCabe D, Riordan JD, Arensdorf AM, Icardi MS, Dupuy AJ, Rutkowski DT. The stress-regulated transcription factor CHOP promotes hepatic inflammatory gene expression, fibrosis, and oncogenesis. PLoS Genet. 2013;9:e1003937. doi: 10.1371/journal.pgen.1003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498–505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 150.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–8. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 151.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–53. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 152.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 153.Pihan P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 155.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 156.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–64. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–73. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang J, Zhang K, Li Z, Guo B. ER Stress-induced Inflammasome Activation Contributes to Hepatic Inflammation and Steatosis. J Clin Cell Immunol. 2016:7. doi: 10.4172/2155-9899.1000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Sem Liv Dis. 2007;27:367–77. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 160.Iracheta-Vellve A, Petrasek J, Gyongyosi B, Satishchandran A, Lowe P, Kodys K, Catalano D, Calenda CD, Kurt-Jones EA, Fitzgerald KA, Szabo G. Endoplasmic Reticulum Stress-induced Hepatocellular Death Pathways Mediate Liver Injury and Fibrosis via Stimulator of Interferon Genes. J Biol Chem. 2016;291:26794–26805. doi: 10.1074/jbc.M116.736991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Inaba Y, Furutani T, Kimura K, Watanabe H, Haga S, Kido Y, Matsumoto M, Yamamoto Y, Harada K, Kaneko S, Oyadomari S, Ozaki M, Kasuga M, Inoue H. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology. 2015;61:1343–56. doi: 10.1002/hep.27619. [DOI] [PubMed] [Google Scholar]
- 162.Kimura K, Yamada T, Matsumoto M, Kido Y, Hosooka T, Asahara S, Matsuda T, Ota T, Watanabe H, Sai Y, Miyamoto K, Kaneko S, Kasuga M, Inoue H. Endoplasmic reticulum stress inhibits STAT3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes. 2012;61:61–73. doi: 10.2337/db10-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Y, Shao M, Wu Y, Yan C, Jiang S, Liu J, Dai J, Yang L, Li J, Jia W, Rui L, Liu Y. Role for the endoplasmic reticulum stress sensor IRE1alpha in liver regenerative responses. J Hepatol. 2015;62:590–8. doi: 10.1016/j.jhep.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 164.Shen K, Johnson DW, Vesey DA, McGuckin MA, Gobe GC. Role of the unfolded protein response in determining the fate of tumor cells and the promise of multi-targeted therapies. Cell Stress Chap. 2017 doi: 10.1007/s12192-017-0844-3. [DOI] [PMC free article] [PubMed]
- 165.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, Yamamoto N, Yamamoto M. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 166.Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, Koike K, Kaufman RJ, Karin M. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS. GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene. 2014;33:4997–5005. doi: 10.1038/onc.2013.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rachidi S, Sun S, Wu BX, Jones E, Drake RR, Ogretmen B, Cowart LA, Clarke CJ, Hannun YA, Chiosis G, Liu B, Li Z. Endoplasmic reticulum heat shock protein gp96 maintains liver homeostasis and promotes hepatocellular carcinogenesis. J Hepatol. 2015;62:879–88. doi: 10.1016/j.jhep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scaiewicz V, Nahmias A, Chung RT, Mueller T, Tirosh B, Shibolet O. CCAAT/enhancer-binding protein homologous (CHOP) protein promotes carcinogenesis in the DEN-induced hepatocellular carcinoma model. PLoS One. 2013;8:e81065. doi: 10.1371/journal.pone.0081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci USA. 2011;108:6561–6. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Willy JA, Young SK, Stevens JL, Masuoka HC, Wek RC. CHOP links endoplasmic reticulum stress to NF-kappaB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol Biol Cell. 2015;26:2190–204. doi: 10.1091/mbc.E15-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233–45. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology. 2016;150:181–193e8. doi: 10.1053/j.gastro.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 174.Heindryckx F, Binet F, Ponticos M, Rombouts K, Lau J, Kreuger J, Gerwins P. Endoplasmic reticulum stress enhances fibrosis through IRE1alpha-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol Med. 2016;8:729–44. doi: 10.15252/emmm.201505925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim RS, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, Robinson CL, Bhattacharya D, Chou HI, Zhang DY, Fuchs BC, Lee Y, Hoshida Y, Friedman SL. The XBP1 Arm of the Unfolded Protein Response Induces Fibrogenic Activity in Hepatic Stellate Cells Through Autophagy. Sci Rep. 2016;6:39342. doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 2011;149:507–18. doi: 10.1093/jb/mvr041. [DOI] [PubMed] [Google Scholar]
- 177.Omori Y, Imai J, Watanabe M, Komatsu T, Suzuki Y, Kataoka K, Watanabe S, Tanigami A, Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nuc Ac Res. 2001;29:2154–62. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chin KT, Zhou HJ, Wong CM, Lee JM, Chan CP, Qiang BQ, Yuan JG, Ng IO, Jin DY. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nuc Ac Res. 2005;33:1859–73. doi: 10.1093/nar/gki332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luebke-Wheeler J, Zhang K, Battle M, Si-Tayeb K, Garrison W, Chhinder S, Li J, Kaufman RJ, Duncan SA. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48:1242–50. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–99. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 181.Oliveira SJ, Pinto JP, Picarote G, Costa VM, Carvalho F, Rangel M, de Sousa M, de Almeida SF. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS, Hong S, Park KG, Lee IK, Choi CS, Hanson RW, Choi HS, Koo SH. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11:331–9. doi: 10.1016/j.cmet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 183.Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, Zhang K. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology. 2012;55:1070–82. doi: 10.1002/hep.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zheng Z, Kim H, Qiu Y, Chen X, Mendez R, Dandekar A, Zhang X, Zhang C, Liu AC, Yin L, Lin JD, Walker PD, Kapatos G, Zhang K. CREBH Couples Circadian Clock With Hepatic Lipid Metabolism. Diabetes. 2016;65:3369–3383. doi: 10.2337/db16-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim H, Zheng Z, Walker PD, Kapatos G, Zhang K. CREBH Maintains Circadian Glucose Homeostasis by Regulating Hepatic Glycogenolysis and Gluconeogenesis. Molecular and cellular biology. 2017:37. doi: 10.1128/MCB.00048-17. [DOI] [PMC free article] [PubMed] [Google Scholar]