Abstract
Background
Accurate, rapid, and cost-effective screening tests for hepatitis B virus (HBV) and hepatitis C virus (HCV) infection may be useful in laboratories that cannot afford automated chemiluminescent immunoassays (CLIAs). We evaluated the diagnostic performance of a novel rapid automated fluorescent lateral flow immunoassay (LFIA).
Methods
A fluorescent LFIA using a small bench-top fluorescence reader, Automated Fluorescent Immunoassay System (AFIAS; Boditech Med Inc., Chuncheon, Korea), was developed for qualitative detection of hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), and antibody to HCV (anti-HCV) within 20 minutes. We compared the diagnostic performance of AFIAS with that of automated CLIAs—Elecsys (Roche Diagnostics GmbH, Penzberg, Germany) and ARCHITECT (Abbott Laboratories, Abbott Park, IL, USA)—using 20 seroconversion panels and 3,500 clinical serum samples.
Results
Evaluation with the seroconversion panels demonstrated that AFIAS had adequate sensitivity for HBsAg and anti-HCV detection. From the clinical samples, AFIAS sensitivity and specificity were 99.8% and 99.3% for the HBsAg test, 100.0% and 100.0% for the anti-HBs test, and 98.8% and 99.1% for the anti-HCV test, respectively. Its agreement rates with the Elecsys HBsAg, anti-HBs, and anti-HCV detection assays were 99.4%, 100.0%, and 99.0%, respectively. AFIAS detected all samples with HBsAg genotypes A-F and H and anti-HCV genotypes 1, 1a, 1b, 2a, 2b, 4, and 6. Cross-reactivity with other infections was not observed.
Conclusions
The AFIAS HBsAg, anti-HBs, and anti-HCV tests demonstrated diagnostic performance equivalent to current automated CLIAs. AFIAS could be used for a large-scale HBV or HCV screening in low-resource laboratories or low-to middle-income areas.
Keywords: HBsAg, Anti-HBs, Anti-HCV, Lateral flow immunoassay, Diagnostic performance
INTRODUCTION
Hepatitis caused by hepatitis B virus (HBV) or hepatitis C virus (HCV) is a huge health burden worldwide. HBV and HCV infection screening are mainly dependent on tests that detect hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), and antibody to HCV (anti-HCV).
Although highly sensitive automated systems using an enzyme immunoassay (EIA) or chemiluminescence immunoassay (CLIA) are available, many small or emergency laboratories with limited resources prefer to use rapid diagnostic tests (RDTs) utilizing immunochromatographic lateral flow immunoassays (LFIAs) [1,2,3]. RDTs have the advantages of simplicity, no requirement for an expensive analyzer, and rapid detection, and a variety of samples, including whole blood, oral fluid, serum, and plasma, can be used for RDTs [1,2,3].
While LFIAs have been used for rapid detection of hepatitis viruses [4,5,6,7,8], their clinical performance remains limited because of variable sensitivity and non-quantitative results. We developed Automated Fluorescent Immunoassay System (AFIAS) HBsAg, anti-HBs, and anti-HCV test kits (Boditech Med Inc., Chuncheon, Korea) that offer enhanced sensitivity and specificity over previous low-efficiency RDTs. The principle of AFIAS is LFIA using a small bench-top fluorescence reader for qualitative measurement of HBsAg, anti-HBs, and anti-HCV in serum or whole blood samples. We evaluated the diagnostic performance of AFIAS in comparison with that of fully automated CLIAs, using commercially available panels and clinical serum samples.
METHODS
1. Materials
We adapted fluorescent europium chelate [Eu(III)] in LFIA to improve sensitivity and specificity. For the anti-HCV and anti-HBs tests, recombinant HCV and HBV specific antigens were conjugated with europium chelate and used as a detector for target antibodies. The recombinant HCV antigens represent the core, NS3, NS4, and NS5 proteins. For detection of HBsAg, a mouse monoclonal antibody against HBsAg was conjugated with europium chelate and used in a sandwich assay. The large “stokes shift” of these lanthanide-labeled detectors makes it easy to distinguish specific long-wavelength emission (λ em=615 nm) signals from the background fluorescence by time-resolved luminescence enhancing signal-to-noise ratios [9].
2. Preparation of immunoassay strip and cartridge
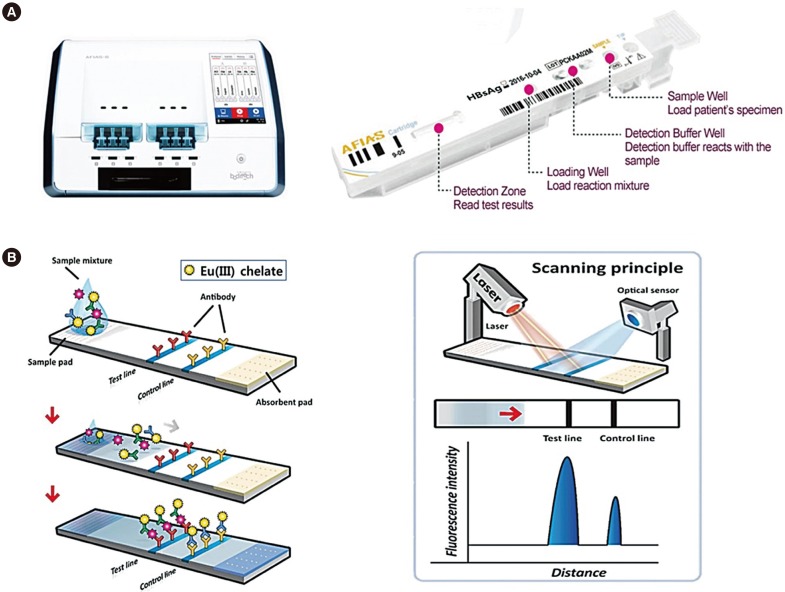
The AFIAS test strip was fabricated in-house to fit into a disposable cartridge and a laser scanner. The sample pad and the absorption pad were cut to a size of 4×20 mm and assembled with nitrocellulose onto a polystyrene-backing card. The capture antigen or antibody was dispensed as 1-mm-wide lines at the test line and the control line using a BioJet dispenser (BioDot, Irvine, CA, USA). The assembled strip was kept in a dry vacuum chamber for 24 hours before being placed into “all-in-one cartridges” designed to optimize the structure and operating principle of the AFIAS fluorescence scanner. The cartridge was then sealed in a foil pouch containing a desiccant and stored under refrigerated conditions (2–8℃). A laser scanner, AFIAS-6 (Boditech Med), was used to measure the fluorescence intensity along the cartridge strip (Fig. 1). The principles of the fluorescence scanner have been previously described [10].
Fig. 1. Schematic illustration of Automated Fluorescent Immunoassay System (AFIAS). (A) AFIAS-6 system and cartridge: AFIAS all-in-one cartridges are designed to optimize the structure and operating principle of the reader. The automated test process enables the performance of multiple simultaneous tests for six different samples. (B) Schematic representation of fluorescent signal detection by LFIA: AFIAS uses a sandwich immunodetection method. The cartridge contains a detection buffer including the dried reagent and monoclonal anti-chicken IgY as an internal control; both dried reagent and monoclonal anti-chicken IgY are labeled with europium chelate [Eu(III)]. When mixed with the sample, the detector binds to target molecules (viral antigens of antibodies) in the sample, forming antigen-antibody complexes. When the complexes migrate onto the nitrocellulose matrix, the other capture (antigen or antibodies) immobilized on the “Test line” forms a sandwich complex. The fluorescence-labeled anti-chicken IgY binds to the chicken IgY fixed to the “Control line.” The AFIAS scanner quantifies the analytes by measuring the fluorescence intensity on the test strip induced by a laser. The fluorescence detector has a fixed absorption wavelength of 333 nm and an emission wavelength of 613 nm, which are the standard wavelengths for the detection of europium [Eu(III)] chelate conjugates. Fluorescence intensity is proportional to the concentration of the target molecules in the samples. The result of the samples is given as “positive,” “negative,” or “indeterminate” in the form of a relative cut-off index (COI).
3. AFIAS test principles and procedure
The AFIAS HBsAg, anti-HBs, and anti-HCV tests can detect targets using an Eu(III) fluorescence immunochromatography-based lateral flow assay. All measurements were performed using a semi-automated AFIAS involving a disposable test strip. First, 100 µL of each sample was added to the sample pad containing a dried fluorescence-labeled detector antigen or antibody, and the sample was then moved onto the test strip by capillary action. If the target antigen or antibodies were present in the sample, they would react with the fluorescence-conjugated antibody or antigen to form an antibody-antigen complex on the nitrocellulose membrane. Fluorescence intensities of the target markers and control materials were detected using a portable fluorescence reader (AFIAS-6 reader). Fluorescence in the control line of the cartridge indicates adequate sample and capillary movement. AFIAS contains lot-specific information and stored calibration statistics based on the values of serially diluted standard materials. The system can test three strips simultaneously within 20 minutes using 100 µL of each sample. Quality control samples were tested daily to verify test results. The sample results are presented as “positive” (HBsAg ≥1.0, anti-HBs ≥15.0, and anti-HCV ≥1.0), “negative” (HBsAg <0.9, anti-HBs <5.0, and anti-HCV <0.9), or “indeterminate” (0.9 ≤HBsAg <1.0, 5.0 ≤anti-HBs <15, and 0.9 ≤anti-HCV <1.0) in the form of a relative cut-off index (COI).
4. Performance evaluation of AFIAS tests
1) Seroconversion panels and genotype specific samples
We used 20 commercially available seroconversion panels to evaluate the sensitivity of the HBsAg (HBV6285, HBV11005, HBV6293, HBV6273, HBV6287, HBV11002, HBV11004, HBV11059, HBV11064, and HBV9072; ZeptoMetrix Co., New York, NY, USA) and anti-HCV (HCV6226, HCV6228, HCV9041, HCV9044, HCV9046, HCV9047, HCV10071, HCV10165, HCV10185, and HCV10235; ZeptoMetrix Co.) tests. The ability of AFIAS HBsAg to detect different genotypes was evaluated using the HBV Worldwide AccuSet performance panel (A–F, H) (0805-0247; SeraCare Life Sciences Inc., Milford, MA, USA). For AFIAS anti-HCV, we used HCV RNA genotype 1 positive plasma (catalog no. 0310-0040), HCV RNA genotype 1a positive plasma (catalog no. 0310-0043), HCV RNA genotype 1b positive plasma (catalog no. 0310-0046), HCV RNA genotype 2a positive plasma (catalog no. 0310-0052), HCV RNA genotype 4 positive plasma (catalog no. 0310-0061), and HCV RNA genotype 6 positive plasma (catalog no. 0310-0067) from SeraCare Life Sciences Inc. and an anti-HCV mixed titer performance panel (HCV/MP) for HCV genotype 2b (Korea Food and Drug Administration [KFDA], Osong, Korea).
2) Clinical serum samples
A total of 3,500 serum samples (1,500 for HBsAg, 500 for anti-HBs, and 1,500 for anti-HCV) were collected from remnants of the samples at Seoul St. Mary's Hospital, Korea. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (KC16DDSE0317, KC16DDSE0318, KC16DDSE0360). Serum samples were routinely requested for diagnosis of hepatitis infection from January 2016 to December 2016 and tested by electrochemiluminescent immunoassay (ELCIA) using the Elecsys HBsAg II, Elecsys anti-HBs, or Elecsys anti-HCV II kits (Roche Diagnostics GmbH, Penzberg, Germany). Some samples were obtained from hospitalized patients with autoimmune diseases, rheumatoid factor, malignancy, or other infections. Collected serum samples were stored at −80℃ for no longer than 12 months, thawed, and centrifuged prior to use. To test cross-reactivity with other viral infections, serum samples with IgG against cytomegalovirus (CMV), Epstein-Barr virus (EBV), hepatitis A virus (HAV), herpes simplex virus (HSV), HBV, HCV, rubella, varicella-zoster virus (VZV), and Treponema pallidum (TP) were tested (See Supplemental Data Table S1). We also tested for interference from icterus (bilirubin <513 µmol/L), hemolysis (Hb <0.12 mmol/L), lipemia (triglyceride <1.69 mmol/L, cholesterol <19.94 mmol/L), and anticoagulants (EDTA, heparin, and sodium citrate).
3) Comparative and confirmatory methods
Samples showing discrepant results for ELCIA and AFIAS to detect HBsAg, anti-HBs, and anti-HCV were additionally tested using the Elecsys HBsAg Confirmatory Test (an independent neutralization test with human anti-HBs; Roche Diagnostics GmbH), ARCHITECT Anti-HBs (Abbott Laboratories, Abbott Park, IL, USA), and Deciscan HCV Plus (Bio-Rad Laboratories, Los Angeles, CA, USA), respectively. The Deciscan HCV PLUS assay is a line immunoassay using specific recombinant and peptide antigens (Core, NS3, NS4) to validate anti-HCV seropositivity. Indeterminate results from confirmatory tests were excluded from statistical analysis. For samples with discrepant results, clinical diagnoses were reviewed to confirm the results. To compare assays using seroconversion panels, Elecsys HBsAg II, ARCHITECT HBsAg (Abbott Laboratories), Elecsys anti-HCV II, and ARCHITECT anti-HCV (Abbott Laboratories) assays were used. All tests were performed and interpreted according to the manufacturer's instructions.
4) Statistics
Statistical analyses were performed using MedCalc version 16. 4.3 (MedCalc Software bvba, Ostend, Belgium). Sensitivity and specificity were calculated with 95% confidence intervals (CI). For qualitative results, the positive, negative and total agreement (% with 95% CI) and Cohen's kappa analysis were performed. For quantitative results, the coefficient of variation (CV) of COI values were analyzed. The limit of blank (LOB) was calculated as 1.645×SD of negative standard materials. The limit of detection (LOD) was calculated as follows: LOD=LOB+1.645 [SD low concentration sample]).
For the AFIAS HBsAg tests, sensitivity was measured using the 3rd International Standard (National Institute for Biological Standards and Control (NIBSC) code:12/226) that was prepared for HBsAg subtype adw1/adw2. The cut-off value of HBsAg test was determined using the serial dilutions of 3rd international standard (47.3 IU/mL, N=3 for each dilution).
The AFIAS anti-HBs test was calibrated using six commercial standard materials (0, 10, 20, 50, 250, and 750 IU/L), Access HBsAb calibrators (0–750 IU/L; catalog no. A24297; Beckman Coulter, Inc., Brea, CA, USA), which were traceable to the 1st international reference for anti-HBs (NIBSC code W1042).
The AFIAS anti-HCV test was standardized with internal calibrators traceable to the reference method with in-house calibration. The cut-off level (0.9 COI) was determined using standard curves by testing 20 replicates of each diluted internal calibrator CV <10%. The LOB (0.216 COI) and LOD (0.436 COI) of anti-HCV were determined as previously described for the AFIAS HBsAg test.
RESULTS
1. AFIAS HBsAg tests
In dilution analysis, the average COI values at different HBsAg concentrations ranging from 0.01 to 47.3 IU/mL were similar to the calculated values, and the CV (%) ranged from 2.0 to 19.5%; the cut-off level was determined to be 0.05 IU/mL at 1.0 COI (See Supplemental Data Table S2) with a CV <10%. The LOB was 0.05 COI, and the LOD was 0.08 COI.
When 10 seroconversion panels were evaluated, comparator CLIAs revealed a higher number of positive samples compared with the AFIAS HBsAg test with five panels, which demonstrated earlier detection (Table 1). The AFIAS HBsAg test detected all seven different genotypes (A–F, H) clearly over the cut-off by testing three replicates of diluted genotype panels (1:1, 1:5, 1:25, and 1:125). The sensitivity and specificity of the AFIAS HBsAg test with 1,500 clinical serum samples were 99.8% (399/400) and 99.3% (1,092/1,100), respectively (Table 2). The agreement with the Elecsys assay was 99.4% (Table 3). Cross-reactivity with other infections and interference were not observed. The samples with discrepant HBsAg test method results are summarized in Supplemental Data Table S3.
Table 1. Comparison of HBsAg assays using commercial seroconversion panels.
| Seroconversion panel | Positive bleeds/Serial bleeds tested (N) | ||
|---|---|---|---|
| Elecsys HBsAg II | ARCHITECT HBsAg | AFIAS HBsAg | |
| HBV6285 (01–16) | 7/16 | 7/16 | 6/16 |
| HBV11005 (01–13) | 3/13 | 4/13 | 3/13 |
| HBV6293 (01–07) | 4/7 | 5/7 | 3/7 |
| HBV6273 (01–06) | 3/6 | 3/6 | 2/6 |
| HBV6287 (01–11) | 2/11 | 2/11 | 2/11 |
| HBV11002 (01–06) | 4/6 | 4/6 | 2/6 |
| HBV11004 (01–08) | 3/8 | 3/8 | 3/8 |
| HBV11059 (01–09) | 5/9 | 5/9 | 5/9 |
| HBV11064 (01–09) | 3/9 | 2/9 | 2/9 |
| HBV9072 (01–17) | 7/17 | 7/17 | 7/17 |
| Total | 41/102 (40.2%) | 42/102 (41.1%) | 35/102 (34.3%) |
Abbreviations: AFIAS, Automated Fluorescent Immunoassay System; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
Table 2. Diagnostic performance of AFIAS for detecting HBsAg, anti-HBs, and anti-HCV.
| Sample N | TP | FN | TN | FP | Sensitivity % (95% CI) | Specificity % (95% CI) | |
|---|---|---|---|---|---|---|---|
| HBsAg | 1,500 | 399 | 1 | 1,092 | 8 | 99.8 (98.6–100.0) | 99.3 (98.6–99.69) |
| Anti-HBs | 500 | 200 | 0 | 300 | 0 | 100.0 (98.2–100.0) | 100.0 (98.8–100.0) |
| Anti-HCV | 1,500 | 395 | 5 | 1,090 | 10 | 98.8 (97.1–99.6) | 99.1 (98.3–99.6) |
Abbreviations: TP, true positive; FN, false negative; TN, true negative; FP, false positive; CI, confidence interval; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to HBsAg; anti-HCV, antibody to hepatitis C virus.
Table 3. Comparison between AFIAS and the Elecsys assays for detecting HBsAg, anti-HBs, and anti-HCV.
| Samples (N) | Positive Agreement (%) (95% CI) | Negative Agreement (%) (95% CI) | Total agreement (%) (95% CI) | Kappa (95% CI) | |
|---|---|---|---|---|---|
| HBsAg | 1,500 | 99.8 (98.6–100.0) | 99.3 (98.6–99.6) | 99.4 (98.9–99.7) | 0.985 (0.975–0.995) |
| Anti-HBs | 500 | 100.0 (97.3–100.0) | 100.0 (98.8–100.0) | 100.0 (98.8–100.0) | 1.000 (1.000–1.000) |
| Anti-HCV | 1,500 | 98.8 (97.1–99.5) | 99.1 (98.3–99.5) | 99.0 (98.4–99.4) | 0.975 (0.962–0.987) |
Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to HBsAg; anti-HCV, antibody to hepatitis C virus.
2. AFIAS anti-HBs tests
The cut-off level was 10.0 IU/L. The LOB was 5.10 IU/L), and the LOD was 5.78 IU/L. The AFIAS anti-HBs test showed linear results up to 5,000 IU/L without a prozone effect (See Supplemental Data Table S4) using a standard material (KFDA 08/026, 95.45 IU/vial) and anti-HBs negative pooled human sera. The sensitivity and specificity of the AFIAS anti-HBs test were both 100% (Table 2), and its agreement with the Elecsys assay was 100% (Table 3). None of the 70 CMV-, EBV-, HAV-, HCV-, rubella-, VZV-, and syphilis-positive serum samples tested showed cross-reactivity with AFIAS anti-HBs (See Supplemental Data Table S1). No interference was found from icterus, hemolysis, lipemia, or anticoagulants.
3. AFIAS anti-HCV tests
The AFIAS anti-HCV test using anti-HCV seroconversion panels showed identical results to the Elecsys and ARCHITECT anti-HCV assays (Table 4). Its sensitivity and specificity were 98.8% and 99.1%, respectively, and its agreement with the Elecsys assay was 99.0% for anti-HCV detection (Table 3). No cross-reactivity with other infections was observed. No interference was found from icterus, hemolysis, lipemia, or anticoagulants.
Table 4. Comparison of anti-HCV assays using commercial seroconversion panels.
| Seroconversion panel | Positive bleeds/Serial bleeds tested (N) | ||
|---|---|---|---|
| Elecsys anti-HCV II | ARCHITECT anti-HCV | AFIAS anti-HCV | |
| HCV6226 (01–12) | 4/12 | 4/12 | 4/12 |
| HCV6228 (01–12) | 4/12 | 4/12 | 4/12 |
| HCV9041 (01–08) | 4/8 | 4/8 | 4/8 |
| HCV9044 (01–06) | 3/6 | 3/6 | 3/6 |
| HCV9046 (01–05) | 4/5 | 4/5 | 4/5 |
| HCV9047 (01–10) | 4/10 | 4/10 | 4/10 |
| HCV10071 (01–07) | 5/7 | 5/7 | 5/7 |
| HCV10165 (01–09) | 4/9 | 4/9 | 4/9 |
| HCV10185 (01–05) | 4/5 | 4/5 | 4/5 |
| HCV10235 (01–05) | 3/5 | 3/5 | 3/5 |
| Total | 39/79 (49.4%) | 39/79 (49.4%) | 39/79 (49.4%) |
Abbreviations: AFIAS, Automated Fluorescent Immunoassay System; anti-HCV, antibody to hepatitis C virus.
DISCUSSION
LFIAs represent an attractive alternative for screening and diagnosis of hepatitis infection, and they have proven popular in clinical laboratories [4,6,11]. However, their performance should be carefully assessed prior to application in clinical practice [12]. Although recent studies have demonstrated the excellent diagnostic performance of HBV or HCV RDTs, their reported sensitivities are variable. The overall sensitivity and specificity of HBV screening tests in Africa have been reported to be 90.0–95.3% and 93.3–100%, respectively [3]. HCV RDTs have also shown variable sensitivity (78.9–99.3%) and specificity (80–100%) [13].
AFIAS is a newly developed point-of-care assay platform. The use of a robust optical signaling probe with a high signal-to-noise ratio is important for the development of high-performance immunoassays. AFIAS utilizes an Eu(III) fluorescent dye to improve sensitivity and an automated fluorescent strip reader to record fluorescence intensity. Although several commercial LFIAs for hepatitis detection are available, assays with an automated fluorescence detection system are limited.
While a recent study reported a highly sensitive LFIA using nanoparticle and assay modifications [14], another study reported that the POC assay for HBsAg has a >300-fold poorer LOD than sensitive commercial immunoassays [15]. However, the AFIAS HBsAg test demonstrates high sensitivity, with a LOD of 0.05 IU/mL for HBsAg. Although AFIAS HBsAg is a qualitative test, we demonstrated that its COI showed similar values to the calculated values of the WHO standard in serial dilution. The AFIAS HBsAg results for 10 HBsAg seroconversion panels were equivalent to those obtained with the Elecsys and ARCHITECT assays. A study assessing several HBsAg RDTs has also reported variable sensitivity and specificity (55.9–100% and 69.4–100%, respectively) [3]. Sensitivity and specificity were 99.8% and 99.3%, respectively, for AFIAS HBsAg and 100% for AFIAS anti-HBs. Studies have reported false-negative results using HBsAg assays in patients with low HBsAg levels, HBsAg mutants, low viral load, or different viral genotypes [15,16,17]. AFIAS HBsAg showed one false-negative result among 400 HBsAg positive sera, and the subject with the false-negative result has inactive disease.
The AFIAS anti-HCV test correctly identified all seroconversion samples detected by CLIAs. Because HCV infection is often asymptomatic until advanced stages [18], early diagnosis is important to prevent and cure HCV infection. The diagnostic performance of AFIAS anti-HCV was acceptable for clinical laboratories, and its agreement with the Elecsys anti-HCV assay result was 99.0%. AFIAS anti-HCV demonstrated a sensitivity of 98.8% and a specificity of 99.1%. Although its sensitivity was the same as that of the Elecsys assay, five sera of the 400 anti-HCV positive clinical samples showed false-negative results. However, no specific anti-HCV profile was observed in the confirmatory tests. The sensitivity and specificity of currently available automated CLIAs for anti-HCV range from 98.0 to 100% and from 96.5 to 100%, respectively [19,20,21,22,23]. In terms of detection of different HBV and HCV genotypes, the AFIAS tests detected all samples consisting of different genotypes and did not exhibit cross-reactivity with other virus infections.
The Elecsys and ARCHITECT assays have an excellent diagnostic performance for a wide range of samples [21,24,25,26,27,28]. AFIAS had good diagnostic performance and agreement with these CLIAs. Thus, AFIAS can be used for large-scale HBV or HCV screening in low-resource laboratories or low-to middle-income areas.
Nevertheless, this study had some limitations. First, we did not collect information such as clinical diagnosis, HBV/HCV genotypes, or coinfection with other viruses, especially HIV. Second, although AFIAS was designed for use with both whole blood and serum samples, we used only stored serum samples as it was not feasible to collect fresh whole blood from a large number of patients. Further studies using whole blood from on-site collection for target populations, including HIV co-infected individuals and those with acute HBV or HCV infection, are needed.
In conclusion, the AFIAS HBsAg, anti-HBs, and anti-HCV tests demonstrated diagnostic performance equivalent to current automated CLIAs. AFIAS could be very useful in emergency or small clinical laboratories to screen HBV or HCV infection because of its simplicity and flexibility.
Acknowledgment
This work was supported by the Technology Innovation Program (No: 10049771, Development of Highly-Specialized Platform for IVD Medical Devices) funded by the Ministry of Trade, Industry & Energy (MI, Korea).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
SUPPLEMENTARY MATERIALS
Cross-reactivity tests of AFIAS HBsAg, anti-HBs, and anti-HCV using highly positive samples for other virus-specific IgGs
Dilution tests for AFIAS HBsAg assay
Samples with discrepant HBsAg results among the test methods
Dilution tests for AFIAS anti-HBs test
References
- 1.Shivkumar S, Peeling R, Jafari Y, Joseph L, Pant Pai N. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558–566. doi: 10.7326/0003-4819-157-8-201210160-00006. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell RJ, Gates RG, Bautista CT, Imbach M, Eggleston JC, Beardsley SG, et al. Laboratory evaluation of rapid test kits to detect hepatitis C antibody for use in predonation screening in emergency settings. Transfusion. 2013;53:505–517. doi: 10.1111/j.1537-2995.2012.03770.x. [DOI] [PubMed] [Google Scholar]
- 3.Njai HF, Shimakawa Y, Sanneh B, Ferguson L, Ndow G, Mendy M, et al. Validation of rapid point-of-care (POC) tests for detection of hepatitis B surface antigen in field and laboratory settings in the Gambia, Western Africa. J Clin Microbiol. 2015;53:1156–1163. doi: 10.1128/JCM.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 5.Xia X, Xu Y, Zhao X, Li Q. Lateral flow immunoassay using europium chelate-loaded silica nanoparticles as labels. Clin Chem. 2009;55:179–182. doi: 10.1373/clinchem.2008.114561. [DOI] [PubMed] [Google Scholar]
- 6.Heiat M, Ranjbar R, Alavian SM. Classical and modern approaches used for viral hepatitis diagnosis. Hepat Mon. 2014;14:e17632. doi: 10.5812/hepatmon.17632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevaliez S, Poiteau L, Rosa I, Soulier A, Roudot-Thoraval F, Laperche S, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect. 2016;22:459.e1–459.e6. doi: 10.1016/j.cmi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Poiteau L, Soulier A, Rosa I, Roudot-Thoraval F, Hézode C, Pawlotsky JM, et al. Performance of rapid diagnostic tests for the detection of antibodies to hepatitis C virus in whole blood collected on dried blood spots. J Viral Hepat. 2016;23:399–401. doi: 10.1111/jvh.12501. [DOI] [PubMed] [Google Scholar]
- 9.Kim KR, Han YD, Chun HJ, Lee KW, Hong DK, Lee KN, et al. Encapsulation-stabilized, europium containing nanoparticle as a probe for time-resolved luminescence detection of cardiac troponin I. Biosensors (Basel) 2017;7:E48. doi: 10.3390/bios7040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BC, Jeong JH, et al. Simplified laser fluorescence scanner for proteomics studies and early cancer diagnosis. In: Chance B, Chen M, et al., editors. Photonics Asia-Optics in health care and biomedical optics: diagnostics and treatment. Shanghai: SPIE; 2002. pp. 103–108. [Google Scholar]
- 11.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect. 2010;16:1062–1069. doi: 10.1111/j.1469-0691.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Jewett A, Drobeniuc J, Kamili S. Rapid diagnostic HCV antibody assays. Antivir Ther. 2012;17:1409–1413. doi: 10.3851/IMP2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DS, Kim YT, Hong SB, Kim J, Huh NS, Lee MK, et al. Development of lateral flow assay based on size-controlled gold nanoparticles for detection of hepatitis B surface antigen. Sensors (Basel) 2016;16:E2154. doi: 10.3390/s16122154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheiblauer H, El-Nageh M, Diaz S, Nick S, Zeichhardt H, Grunert HP, et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang. 2010;98:403–414. doi: 10.1111/j.1423-0410.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YH, Wang Y, Loua A, Day GJ, Qiu Y, Nadala EC, Jr, et al. Evaluation of a new hepatitis B virus surface antigen rapid test with improved sensitivity. J Clin Microbiol. 2008;46:3319–3324. doi: 10.1128/JCM.00498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottero J, Boyd A, Gozlan J, Lemoine M, Carrat F, Collignon A, et al. Performance of rapid tests for detection of HBsAg and anti-HBsAb in a large cohort, France. J Hepatol. 2013;58:473–478. doi: 10.1016/j.jhep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun J, Ko DH, Kang HJ, Whang DH, Cha YJ, Kim HS. Evaluation of the VIDAS anti-HCV assay for detection of hepatitis C virus infection. Ann Lab Med. 2016;36:550–554. doi: 10.3343/alm.2016.36.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y, Park JY, Kim MJ, Kim HS. Comparison of the diagnostic performance of Elecsys Anti-HCV II and Elecsys and Vitros Anti-HCV assays. J Lab Med Qual Assur. 2012;34:51–56. [Google Scholar]
- 21.Esteban JI, van Helden J, Alborino F, Bürgisser P, Cellerai C, Pantaleo G, et al. Multicenter evaluation of the Elecsys® anti-HCV II assay for the diagnosis of hepatitis C virus infection. J Med Virol. 2013;85:1362–1368. doi: 10.1002/jmv.23536. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Kim JH, Yoon S, Park YH, Kim HS. Clinical performance evaluation of four automated chemiluminescence immunoassays for hepatitis C virus antibody detection. J Clin Microbiol. 2008;46:3919–3923. doi: 10.1128/JCM.01603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh EJ, Chang J, Yang JY, Kim Y, Park YJ, Han K. Different signal-to-cut-off ratios from three automated anti-hepatitis C virus chemiluminescence immunoassays in relation to results of recombinant immunoblot assays and nucleic acid testing. Blood Transfus. 2013;11:471–473. doi: 10.2450/2012.0085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacher BJ, Moriconi F, Bowden S, Hammond R, Louisirirotchanakul S, Phisalprapa P, et al. Multicenter evaluation of the Elecsys hepatitis B surface antigen quantitative assay. Clin Vaccine Immunol. 2011;18:1943–1950. doi: 10.1128/CVI.05122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louisirirotchanakul S, Khupulsup K, Akraekthalin S, Chan KP, Saw S, Aw TC, et al. Comparison of the technical and clinical performance of the Elecsys HBsAg II assay with the Architect, AxSym, and Advia Centaur HBsAg screening assays. J Med Virol. 2010;82:755–762. doi: 10.1002/jmv.21706. [DOI] [PubMed] [Google Scholar]
- 26.Yoo SJ, Wang LL, Ning HC, Tao CM, Hirankarn N, Kuakarn S, et al. Evaluation of the Elecsys Anti-HCV II assay for routine hepatitis C virus screening of different Asian Pacific populations and detection of early infection. J Clin Virol. 2015;64:20–27. doi: 10.1016/j.jcv.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Popp C, Krams D, Beckert C, Buenning C, Queirós L, Piro L, et al. HBsAg blood screening and diagnosis: performance evaluation of the ARCHITECT HBsAg qualitative and ARCHITECT HBsAg qualitative confirmatory assays. Diagn Microbiol Infect Dis. 2011;70:479–485. doi: 10.1016/j.diagmicrobio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Tuaillon E, Mondain AM, Nagot N, Ottomani L, Kania D, Nogue E, et al. Comparison of serum HBsAg quantitation by four immunoassays, and relationships of HBsAg level with HBV replication and HBV genotypes. PLoS One. 2012;7:e32143. doi: 10.1371/journal.pone.0032143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cross-reactivity tests of AFIAS HBsAg, anti-HBs, and anti-HCV using highly positive samples for other virus-specific IgGs
Dilution tests for AFIAS HBsAg assay
Samples with discrepant HBsAg results among the test methods
Dilution tests for AFIAS anti-HBs test