Abstract
Background
An increase in neutrophil gelatinase-associated lipocalin (NGAL) indicates tubular injury. Diabetic nephropathy causes typical changes in the kidney, characterized by glomerulosclerosis and eventual tubular damage. We validated the usefulness of plasma NGAL (pNGAL) as a biomarker of tubular damage in patients with diabetic nephropathy.
Methods
We included 376 patients with diabetes mellitus (260 patients with chronic renal insufficiency who had not received hemodialysis and 116 hemodialyzed due to diabetic nephropathy) and 24 healthy controls. Patients with chronic renal insufficiency were divided into three groups according to urinary albumin excretion (UAE) levels. pNGAL levels were measured using the Triage NGAL test (Alere, San Diego, CA, USA) and were compared between groups. We also examined whether pNGAL level was related to the degree of albuminuria and cystatin C-based glomerular filtration rate (GFR).
Results
Mean pNGAL levels of the healthy controls, chronic renal insufficiency patients with diabetes mellitus, and hemodialyzed patients were 61.9±5.3 ng/mL, 93.4±71.8 ng/mL, and 1,536.9±554.9 ng/mL, respectively. pNGAL level increased significantly in patients with severe albuminuria (P<0.001) and had a moderate correlation with the degree of albuminuria (r=0.467; P<0.001) and GFR (r=0.519; P<0.001). Multivariate regression analysis showed that the pNGAL level was associated with tubular damage independent of patient age, sex, and GFR.
Conclusions
pNGAL level independently reflects the degree of tubular damage in patients with diabetic nephropathy. Measurement of pNGAL, combined with UAE, would enable simultaneous, highly reliable assessments of tubular damage for such patients.
Keywords: Neutrophil gelatinase-associated lipocalin, Diabetic nephropathy, Hemodialysis, Tubular damage
INTRODUCTION
Recently, neutrophil gelatinase-associated lipocalin (NGAL) has emerged as a useful marker for assessing renal tubular damage in patients with acute and chronic kidney injuries [1]. NGAL is a 22–25 kDa protein of the lipocalin family, which is produced at low levels from the kidney, prostate, lung, and digestive tract epithelial cells. Owing to its low molecular weight, most of the NGAL produced is filtered through the glomerulus and reabsorbed at the proximal tubule [1,2]. If the renal tubule is damaged, NGAL reabsorption decreases while its production from epithelial cells increases, which subsequently leads to increases in NGAL levels in the blood plasma and urine [1,2].
Diabetic nephropathy is caused by damage to the glomerulus, renal tubule, or renal mesenchyme due to hemodynamic factors that accompany a state of continuous hyperglycemia and diabetes [3]. This kidney injury is reversible during the early stage but becomes irreversible once the nephropathy is overt, ultimately developing into end-stage kidney disease. Therefore, diagnosis and treatment of diabetic nephropathy during the early stage is highly relevant to improve the patient's prognosis [3,4,5,6]. Urinary albumin excretion (UAE) is the current standard for categorizing diabetic nephropathy stages, which can accurately reflect the degree of glomerulus damage but cannot fully reflect the degree of renal tubule damage. Plasma NGAL (pNGAL) levels have been shown to increase in patients with severe diabetic nephropathy with continuous macroalbuminuria and a low glomerular filtration rate (GFR) [7,8].
Although data exist on acute renal failures, no study so far has examined the use of pNGAL as a marker of the degree of tubular damage in Korean patients with diabetes mellitus. Therefore, it is necessary to identify a sensitive and reliable marker that can be used to provide specific assessment of the extent of tubular damage in this patient population.
The present study aimed to determine the feasibility of pNGAL as an effective biomarker for assessing tubular damage in diabetic nephropathy, by comparing pNGAL levels among patients with diabetes with different degrees of albuminuria as well as healthy controls.
METHODS
1. Patients and sample collection
This retrospective study was conducted at Asan Medical Center, Seoul, Korea, with 376 patients diagnosed as having type 1 or type 2 diabetes and 24 healthy controls. We included type 1 or type 2 diabetes patients who had undergone urinalysis for assessing renal tubular damage. We excluded patients younger than 18 years and those unable to provide informed consent for the study.
The patients' blood samples were collected and reviewed between June and December 2012. Among these, 260 patients who had not received hemodialysis were divided into three groups based on UAE levels: 100 patients with normoalbuminuria (<20 µg/min), 100 patients with microalbuminuria (20–200 µg/min), and 60 patients with macroalbuminuria (>200 µg/min). A clinical diagnosis of diabetic nephropathy was made for those showing continuous macroalbuminuria (>200 µg/min) with no other underlying renal disease. The remaining 116 patients had chronic renal insufficiency and were under hemodialysis; they formed the hemodialysis group. As controls, 24 healthy individuals who visited the department of family medicine were selected. The controls did not have any history of diabetes, renal disease, hypertension, or cardiovascular disease, and had no drug history. Electronic medical records were reviewed to confirm clinical information and laboratory findings. This study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2012-0815), and informed consent was obtained from both the patients and the controls.
2. Measurement of pNGAL levels
In plasma samples from the hemodialysis patients and the healthy controls, pNGAL levels were measured using a fluorescence immunoassay. The tests were performed with the Triage NGAL test (Alere, San Diego, CA, USA), a point-of-care testing system, according to the manufacturer's guidelines. pNGAL levels were expressed in terms of ng/mL. The NGAL cut-off value was approximately 100 ng/mL, which was measured on a standardized clinical laboratory platform following the manufacturer's recommendation. The assay range was 10–2,631 ng/mL, and the precision CV was 4.3% (within-run 2.3%, between-run 3.0%, and between-day 2.0%). Samples with test results equal to or greater than the analytical measurement range (AMR) were stored below freezing temperature (−20℃) and were measured again within 12 hours after diluting them at 1:1 and 1:2 ratios with a control plasma sample, following the manufacturer's recommendation. Subsequent results were collected and confirmed to show linearity up to 2,631 ng/mL.
3. Estimation of GFR
Serum creatinine (SCr) was measured by a rate-blanked compensated kinetic Jaffe method (Roche Diagnostics, Indianapolis, IN, USA) using an IDMS-traceable calibrator (C.f.a.s calibrator; Roche Diagnostics). The mean within-laboratory imprecision (%CV) of the SCr assay during the study period was 2.8%. Cystatin C (CysC) was determined with the Roche Cobas 8000 modular system using Roche Tina-quant Cystatin C Gen 2 (Roche Diagnostics), a particle enhanced immunonephelometric assay. The measurement range was 0.4–8.0 mg/L, and the mean within-laboratory imprecision was 2.7% during the study period. Estimated GFRs (eGFRs) were calculated using the MDRD Study equation. GFR ≥60 mL/min/1.73 m2 was considered normal.
4. Statistical analysis
Data were checked for normal distribution using the Shapiro-Wilk test and expressed as mean±SD, median and interquartile range, or number and percentage. To compare pNGAL levels between the healthy controls and patients with diabetes mellitus, a one-way ANOVA and the Kruskal Wallis test were used for normally and non-normally distributed data, respectively. SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for these analyses. We also examined whether pNGAL level was related to the degree of albuminuria and CysC-based GFR. Pearson's correlations and multiple regression analysis were used to assess the relationships between pNGAL and other variables. P<0.05 was considered significant. For linearity testing, EP evaluator Release 10 (David G. Rhoads Assoc., Kennett square, PA, USA) was used.
RESULTS
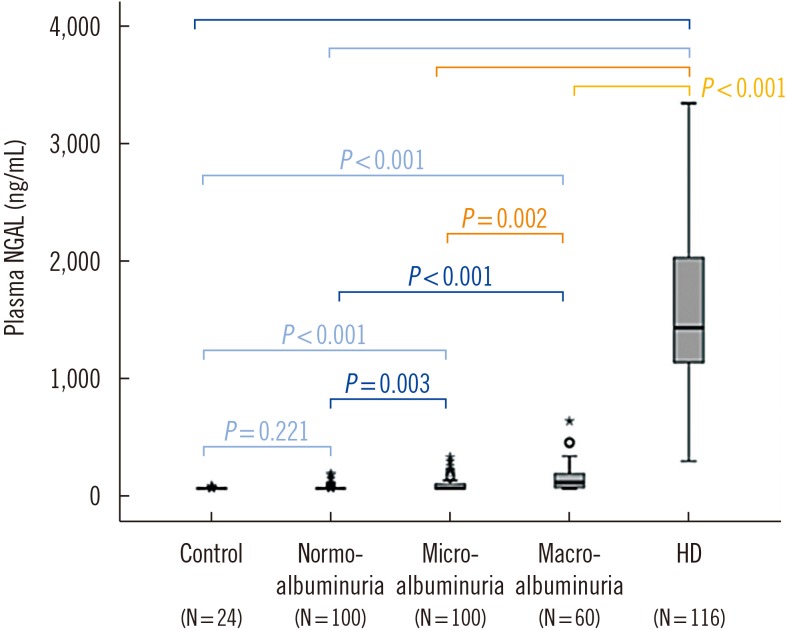
The mean pNGAL levels of the healthy controls, patients with diabetes mellitus, and hemodialyzed patients were 61.9±5.3 ng/mL, 93.4±71.8 ng/mL, and 1,536.9±554.9 ng/mL, respectively. The 260 patients who did not receive hemodialysis had been diagnosed as having diabetes 10±7 years prior to the study period. Their mean pNGAL level (93.4 ng/mL) was significantly lower than that of the hemodialyzed patients (1,536.85 ng/mL). The mean pNGAL level of the healthy controls was substantially lower than that of the patients, at 61.9 ng/mL (Table 1, Fig. 1).
Table 1. Clinical and laboratory data of controls and patients with diabetes mellitus.
| Control | Diabetes mellitus | ||||
|---|---|---|---|---|---|
| Normo-albuminuria | Microalbuminuria | Macroalbuminuria | Hemodialysis | ||
| N | 24 | 100 | 100 | 60 | 116 |
| Age (yr)* | 59 (52–66) | 59 (48–70) | 62 (51–73) | 65 (53–77) | 63 (51–75) |
| pNGAL (ng/mL)† | 61.9 ± 5.3 | 66.8 ± 20.2 | 86.9 ± 49.6 | 148.4 ± 116.0 | 1,536.8 ± 554.9 |
| Cr (mg/dL)† | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.5 | 1.9 ± 1.2 | 10.3 ± 2.4 |
| GFR (mL/min/1.73 m2)† | 118.0 ± 25.1 | 117.7 ± 26.2 | 94.5 ± 12.3 | 51.6 ± 19.6 | 4.8 ± 2.0 |
*Age is expressed as median (range); †Data are presented as mean±SD.
Abbreviations: pNGAL, plasma neutrophil gelatinase-associated lipocalin; Cr, creatinine; GFR, glomerular filtration rate.
Fig. 1. Levels of plasma neutrophil gelatinase-associated lipocalin (pNGAL) among different groups. pNGAL levels are significantly higher in the hemodialysis group.
Abbreviation: HD, hemodialysis.
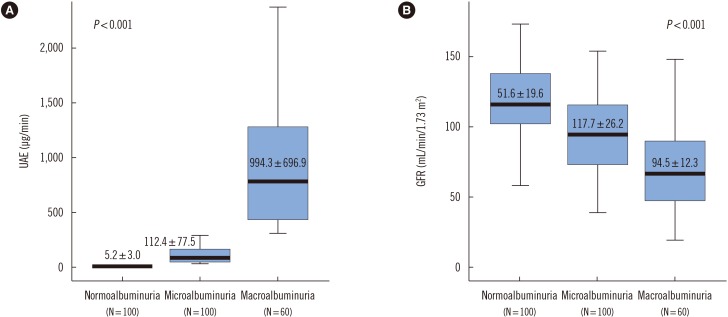
The UAE of normoalbuminuria patients who did not receive hemodialysis was lower than that of the microalbuminuria and macroalbuminuria patients (Fig. 2A). The GFR of the macroalbuminaria patients was lower than that of the normoalbuminuria and microalbuminuria patients (Table 1, Fig. 2B).
Fig. 2. Urinary albumin excretion (UAE) levels (A) and glomerular filtration rate (GFR) (B) of normoalbuminuria, microalbuminuria, and macroalbuminuria patients with diabetes mellitus. The number of subjects in each group is indicated in parentheses.
Overall, pNGAL levels increased (P<0.001) with progression of diabetic nephropathy, although some patients showed higher pNGAL levels within the same stage. In addition, pNGAL levels were significantly higher for the hemodialysis patients (Fig. 1).
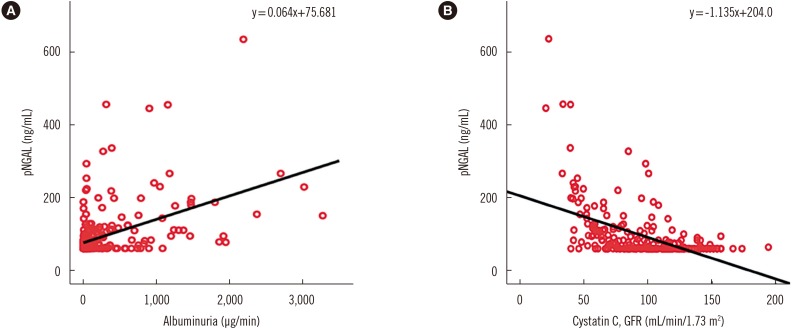
The pNGAL levels of diabetes patients were also moderately correlated with UAE levels and with GFR (Fig. 3). Multivariate regression analysis including patient age and GFR suggested that a 1 ng/mL increase in pNGAL would result in a 3.385 µg increase in UAE, with an odds ratio of 0.338 (P<0.001).
Fig. 3. Correlation of plasma neutrophil gelatinase-associated lipocalin (pNGAL) and albuminuria (A) and cystatin C-based glomerular filtration rate (GFR) in patients with diabetes mellitus. Moderate correlation between plasma neutrophil gelatinase-associated lipocalin (pNGAL) levels and the degree of albuminuria in patients with diabetes mellitus (r=0.467, P<0.001). (B) Moderate correlation between pNGAL levels and cystatin C-based glomerular filtration rate (GFR) in patients with diabetes mellitus (r=0.519, P<0.001).
DISCUSSION
We demonstrated that the pNGAL levels of patients with diabetes mellitus were significantly higher than those of the healthy controls. Moreover, pNGAL levels clearly increased with progression of diabetic nephropathy. Previous studies have suggested tubule-interstitial impairment as a determinant of the rate of kidney function degradation or long-term prognosis for chronic kidney disease (CKD)-associated diseases such as diabetic nephropathy [9,10]. The present study supports these findings by showing an association between higher pNGAL levels and greater progress in diabetic nephropathy. Moreover, some patients within the same sub-group showed significantly higher pNGAL levels; these individuals may be at higher risk of suffering from greater renal tubule damage. Following up on these patients by continuously measuring their pNGAL levels and conducting protein electrophoresis to observe the development of tubular proteinuria would be helpful for improving patient management.
Bolignano et al [9] reported the feasibility of using NGAL as a biomarker for the early detection of diabetic nephropathy by measuring urine and serum NGAL levels of healthy individuals and patients with type 2 diabetes and a normoalbuminuria status, and demonstrated an increase in the NGAL levels of patients with diabetes. In contrast, we did not detect a significant difference in pNGAL levels between the healthy controls and patients with normoalbuminuria. This may be due to the difference in number of patients involved or patients' statements.
Di Somma et al [11] indicated that the assessment of a patient's initial blood NGAL level upon admission to the emergency room improved the diagnosis of acute kidney injury. They considered NGAL a useful marker for diabetic nephropathy. Our study further demonstrated that pNGAL assessment coupled with the judgment of the emergency room physician may be useful in deciding the most appropriate strategy for managing CKD-associated diseases such as diabetic nephropathy. Hur et al [12] and Kim et al [13] had also reviewed data on pNGAL and other biomarkers for sepsis in Korean patients, and our findings support their results.
In the present study, the patients' pNGAL levels were moderately correlated with UAE and GFR in accordance with a previous study [8,9]. The patients with macroalbuminuria showed increased pNGAL levels. We also observed a low GFR in patients with increased pNGAL levels. Based on the finding that pNGAL can reflect kidney injury independent of other factors such as age and GFR, it seems feasible to consider pNGAL a meaningful biomarker for a diagnosis of diabetic nephropathy.
Although the precise mechanisms contributing to the higher pNGAL levels in hemodialysis patients are currently unclear, it seems likely that pNGAL levels could increase due to increased pNGAL production as a result of kidney damage accompanied by a low GFR, which results in clearance obstruction. The present study also supports previous findings that pNGAL further increases due to the continuous upregulation of pNGAL production by renal tubular cells along with the progression of CKD [14,15]. As chronic hemodialysis constitutes an important stimulus of neutrophil degranulation, this could represent another important source of increased pNGAL [15]. Although another study reported a decrease in NGAL levels after even a single round of hemodialysis, the authors noted that patients with end-stage renal insufficiency who required repetitive hemodialysis showed increased pNGAL levels as well [14]. The current results also support this finding.
Since the present study showed a higher mean pNGAL level for hemodialysis patients at 1,536.9 ng/mL, it is necessary to compare these results with those of previous NGAL studies on patients under hemodialysis and those with CKD. Bolignano et al [16] showed that the serum NGAL level of patients with chronic renal disease under hemodialysis was 485.2 ng/mL, whereas Kusaka et al [17] reported a value of 963 ng/mL. In another study, the serum NGAL level of CKD patients was 515.4 ng/mL [7]. Besides the differences in the test kit and samples, the main factor contributing to the difference in pNGAL levels across studies appears to be variation in residual renal functions, considering the significant relationships between pNGAL levels and Scr levels and GFR.
From the multivariate regression analysis, we confirmed that pNGAL level can independently reflect kidney injury in patients with diabetic nephropathy. Thus, measurement of pNGAL level for patients with suspected diabetic nephropathy, in combination with determination of the UAE level, would allow for simultaneous assessment of glomerular damage and tubular damage. Since the multivariate analysis in this study was limited, further confirmatory multivariate analyses are necessary. In particular, pNGAL could be a useful biomarker for early diabetic nephropathy, in which the tubular damage is greater than the glomerular damage. However, it should be noted that patients with end-stage renal insufficiency who have undergone long-term hemodialysis are likely to produce high pNGAL levels, and therefore, pNGAL results should be carefully considered on a case-by-case basis.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: The authors declare no conflict of interest related to this work.
References
- 1.Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis. 2008;52:395–399. doi: 10.1053/j.ajkd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Bolignano D, Coppolino G, Romeo A, De Paola L, Buemi A, Lacquaniti A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) reflects iron status in haemodialysis patients. Nephrol Dial Transplant. 2009;24:3398–3403. doi: 10.1093/ndt/gfp310. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int. 2011;79:1113–1118. doi: 10.1038/ki.2010.554. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144–1150. doi: 10.1111/j.1464-5491.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- 6.Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294:1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 7.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res. 2009;32:91–98. doi: 10.1159/000209379. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P. Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron Clin Pract. 2011;118:c130–c135. doi: 10.1159/000320615. [DOI] [PubMed] [Google Scholar]
- 9.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips AO. The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep. 2003;3:491–496. doi: 10.1007/s11892-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 11.Di Somma S, Magrini L, De Beradinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013;17:R29. doi: 10.1186/cc12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hur M, Kim H, Lee S, Cristofano F, Magrini L, Marino R, et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect Dis. 2014;14:224. doi: 10.1186/1471-2334-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Hur M, Lee S, Marino R, Magrini L, Cardelli P, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med. 2017;37:388–397. doi: 10.3343/alm.2017.37.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malyszko J, Malyszko JS, Koc-Zorawska E, Kozminski P, Mysliwiec M. Neutrophil gelatinase-associated lipocalin in dialyzed patients is related to residual renal function, type of renal replacement therapy and inflammation. Kidney Blood Press Res. 2009;32:464–469. doi: 10.1159/000274048. [DOI] [PubMed] [Google Scholar]
- 15.Musial K, Zwolinska D. Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinases as novel stress markers in children and young adults on chronic dialysis. Cell Stress Chaperones. 2011;16:163–171. doi: 10.1007/s12192-010-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolignano D, Coppolino G, Romeo A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology (Carlton) 2010;15:23–26. doi: 10.1111/j.1440-1797.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 17.Kusaka M, Kuroyanagi Y, Mori T, Nagaoka K, Sasaki H, Maruyama T, et al. Serum neutrophil gelatinase-associated lipocalin as a predictor of organ recovery from delayed graft function after kidney transplantation from donors after cardiac death. Cell Transplant. 2008;17:129–134. doi: 10.3727/000000008783907116. [DOI] [PubMed] [Google Scholar]