Abstract
The microbiome of a vineyard may play a critical role in fruit development, and consequently, may impact quality properties of grape and wine. Vineyard management approaches that have directly manipulated the microbiome of grape clusters have been studied, but little is known about how vineyard management practices that impact the soil microbial pool can influence this dynamic. We examined three under-vine soil management practices: 1) herbicide application, 2) soil cultivation (vegetation removal), and 3) natural vegetation (no vegetation removal) in a Riesling vineyard in New York over a three-year period. The microbiomes associated with soil and grapes were profiled using high-throughput sequencing of the bacterial 16 S rRNA gene and fungal ITS regions. Our results showed that soil bacterial composition under natural vegetation differs from that seen in glyphosate-maintained bare soil. Soil fungal composition under the natural vegetation treatment was distinct from other treatments. Although our study revealed soil microbiome shifts based on under-vine management, there were no corresponding changes in fruit-associated microbial composition. These results suggested that other vineyard management practices or environmental factors are more influential in shaping the grape-associated microbiome.
Introduction
Vineyard management practices impact fruit and wine composition through many routes1. Widely known effects include the modification of the leaf area to fruit ratio2, alteration of the fruit microclimate3, and changes in nutrient and/or water uptake. However, the role of vineyard microbiology has been largely overlooked until recently, with researchers suggesting microbial composition as a possible driver of wine sensory properties4.
Aside from intentional inoculation, the major sources of yeasts in wine fermentations are derived from the vineyard and winery5,6. The impact of the winery environment on yeast dynamics during fermentation has been extensively studied5,7–9, but vineyard factors have received less attention. Recent studies have found that the microbial assemblages present in grape must and wine fermentation are structured to reflect regional and vineyard site patterns10, which suggests the importance of vineyard factors.
Grape microbiomes can be shaped by climate, region, site, and grape cultivar11–14, and also appear to be associated with the composition of the microbiome involved in wine fermentation, and with wine metabolite profiles and abundances10. Regionally-differentiated yeast genotypes collected from vineyards, forests, and spontaneous fermentations are confirmed to have different impacts on wine chemical composition15. These studies suggest the importance of a biological component to regional wine typicity through vineyard microbiome by indicating the significance of specific vineyard properties on wine characteristics as a function of microbiome composition. To express this role, “microbial terroir” was defined as traits of the land that impart a distinct profile of wine that is specific to the growing region, which suggests that the collection of bacteria and fungi from a region could contribute to regional wine characteristics4,16.
Management practices in the vineyard and winery, such as the use of fungal sprays and sulfiting fermentations, play important roles in the microbial dynamics of grape and wine fermentations that potentially contribute to wine sensory typicity17. Yeast populations in vineyard soil18, grapes, and wine fermentations19–22 have been studied in the context of overall vineyard management approaches, such as organic, conventional, and biodynamical management, where the direct manipulation of grape-associated microbiomes were involved. Differentiated grape microbial management using different phytosanitation sprayers derived predictable results, while masking the effect of other management practices on grape microbial ecology.
Others have investigated vineyard management practices that manipulated the soil environment, which is possibly the vineyard microbial pool23, without direct grape microbial manipulation. A study that was conducted in a hot and arid climate in Spain suggested that soil tillage is related to high diversity in grape-associated yeast24. However, they were unable to statistically test this concept as they relied on culture-dependent techniques to characterize yeast diversity. Another study conducted in California (USA) used high-throughput sequencing methods to demonstrate that vineyard floor management impacted the composition of soil bacteria25. Fungi were not included in their study, nor was the association of soil and grape microbial composition. Thus, whether vineyard management practices that change the vineyard microbiome can impact the grape-associated microbiome remains unclear.
The concept of soil as a source of microorganisms inhabiting grape surfaces is easily understood, but challenging to examine systematically. A study conducted in Long Island, NY (USA) found that bacterial communities associated with grape leaves, flowers, and fruit shared a greater proportion of taxa found in soil compared with each other, which they suggested as evidence of soil serving as a bacterial reservoir in vineyards23. There are several known microbial dispersal mechanisms that transport fungi and bacteria from the ground to crops, including rain26 and wind27. These routes of microbial dispersal are likely to hold in vineyards as well, although there are many other possible routes to be explored. Thus, it is possible that vineyard soil management practices could alter the microbiome in the vineyard - not only at the soil level, but also with aerial parts such as grapes. In one of our previous studies conducted in New York28, we showed that under-vine soil treatments had no impact on vine growth and yield components, but that wine sensory properties differed. We suspected the differences in wine sensory properties might have been a result of changes to the grape associated microbiome that altered secondary metabolite production in grapes or yeast dynamics in the wine fermentation. Under-vine floor management practices might have triggered changes in the grape associated microbiome.
To understand the vineyard management impacts on grape-associated microbiomes, a three-year single-factor (under-vine soil management) study was conducted within an experimental design that corresponds to our previous study28 in a commercial vineyard in the Finger Lakes region of New York. Under-vine soil management was chosen as our vineyard management factor, as we expected that it would directly manipulate the vineyard microbial pool in soil. The objective was to examine how under-vine soil management practices, including herbicide application with glyphosate (GLY), soil cultivation (CULT) using hand weeding, and under-vine natural vegetation (NV) with no cultivation or herbicides, impacted the microbiomes of soil and grapes. The goal of the study is to understand the role of specific vineyard soil management practices on the grape-associated microbiome. We hypothesized that under-vine soil management practices resulting in different types of ground vegetation, through glyphosate application, soil cultivation, or maintaining intact natural vegetation, would result in distinctly different soil and fruit microbial communities.
Results
Fungal communities cluster distinctly between soil and grapes
Fungal community profiles showed distinct clustering of samples derived from grapes, and soil collected under grapevines (Fig. 1). The Bray-Curtis distance metric was used to determine multivariate sample distances, which were visualized through an ordination of a principal coordinates analysis (PCoA). Axes 1 and 2 explained 69% of the variance in the data. The soil samples clustered together distinctly, and separately from grapes along the first PCoA axis, which explained 66% of the variance in the data (Fig. 1). Shannon diversity indices for OTUs differ between soil and grape samples (Supplementary Fig. S1), but no diversity differences were observed among treatments within the sample type in each year.
Figure 1.
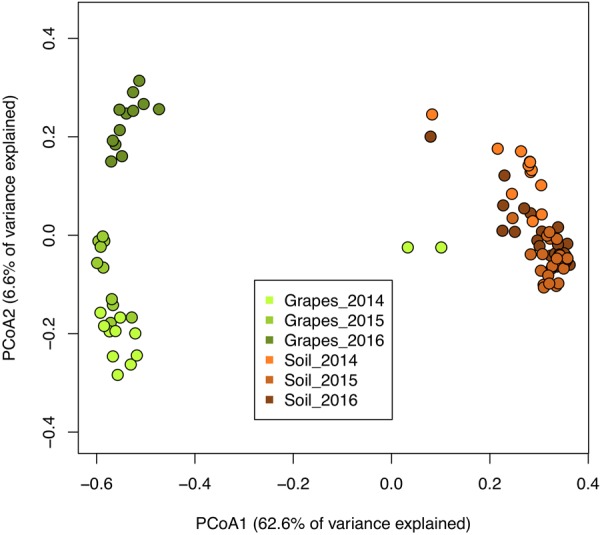
Principal coordinates analysis (PCoA) of fungal communities (ITS region) of soil, and grape from all harvest years and management treatments. The ordination is based on the Bray-Curtis distance metric, with samples clustering by collection type (grape and soil).
Under-vine soil management impacted soil fungal community structure
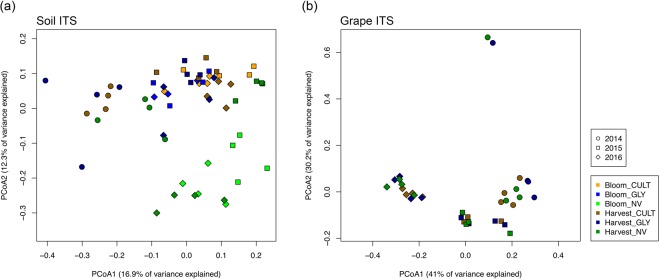
We evaluated the impact of under-vine soil management on microbial community composition. The three-year average under-vine soil vegetation coverage rate for NV was greater than 70%, while coverage rates for CULT and GLY were less than 20% at veraison. PCoA plots with samples from each of the three years of the study (generated using the Bray-Curtis distance metric) showed that NV soil fungal communities differed from those of GLY and CULT treatments (Fig. 2a). Over the three years of the experiment, sample clustering was based primarily on vintage, with each vintage clustered, and then by treatment, where NV separated from GLY and CULT. However, no clustering pattern was detected among the CULT and GLY samples. Notably, the dissimilarities between NV and the other two soil treatments grew with time since groundcover establishment, suggesting possible intensification of the NV treatment effect over time. In 2015 and 2016, the soil samples were taken at two different vine phenological stages - bloom and harvest, which showed separation by PCoA ordination.
Figure 2.
Principal coordinates analysis (PCoA) ordinations of fungal communities (ITS region) derived from (a) soil at grapevine bloom and harvest; and (b) grape at harvest. The three under-vine management treatments include Cultivation (CULT), Glyphosate (GLY) and Natural Vegetation (NV). The PCoA is based on the Bray-Curtis distance metric for three experimental years.
These observations were confirmed by statistical analysis. According to the three-year overall Permutational Multivariate Analysis of Variance (PERMANOVA), vintage and treatment effects were both significant (P < 0.001), while year-to-year climatic differences (R2 = 0.159) explained more variation than treatment (R2 = 0.114). The treatment effect was significant across all three years (p = 0.032 in 2014, p = 0.001 in 2015 and p = 0.001 in 2016) when each year was analyzed individually. The phenological stage effect was significant in both year 2015 (p = 0.008) and 2016 (p = 0.048), while samples were not taken at vine full bloom in 2014 (Table 1).
Table 1.
Comparison of bacterial and fungal community structure dissimilarity in soil and grapes using permutational multivariate analysis of variance (PERMANOVA).
| Factors | Overall | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | P-value | R2 | P-value | R2 | P-value | R2 | P-value | |
| Soil 16 S | ||||||||
| Dispersion test | — | — | — | 0.322 | — | 0.571 | — | 0.044 |
| Treatment | — | — | 0.243 | 0.042 | 0.097 | 0.181 | 0.104 | 0.013 |
| Stage | — | — | — | — | 0.061 | 0.032 | 0.094 | < 0.001 |
| Treatment*Stage | — | — | — | — | 0.083 | 0.757 | 0.085 | 0.176 |
| Soil ITS | ||||||||
| Dispersion test | — | — | — | 0.374 | — | 0.254 | — | 0.181 |
| Treatment | 0.114 | <0.001 | 0.246 | 0.032 | 0.213 | <0.001 | 0.243 | <0.001 |
| Stage | 0.012 | 0.443 | — | — | 0.074 | 0.008 | 0.054 | 0.048 |
| Year | 0.159 | <0.001 | — | — | — | — | — | — |
| Treatment*Stage | — | — | — | — | 0.094 | 0.066 | 0.058 | 0.653 |
| Treatment*Year | 0.082 | <0.001 | — | — | — | — | — | — |
| Grape ITS | ||||||||
| Dispersion test | — | — | — | 0.690 | — | 0.747 | — | 0.765 |
| Treatment | 0.026 | 0.658 | 0.138 | 0.492 | 0.211 | 0.472 | 0.169 | 0.278 |
| Year | 0.498 | <0.001 | — | — | — | — | — | — |
| Treatment*Year | 0.051 | 0.771 | — | — | — | — | — | — |
The p-values of dispersion test were derived from ANOVA.
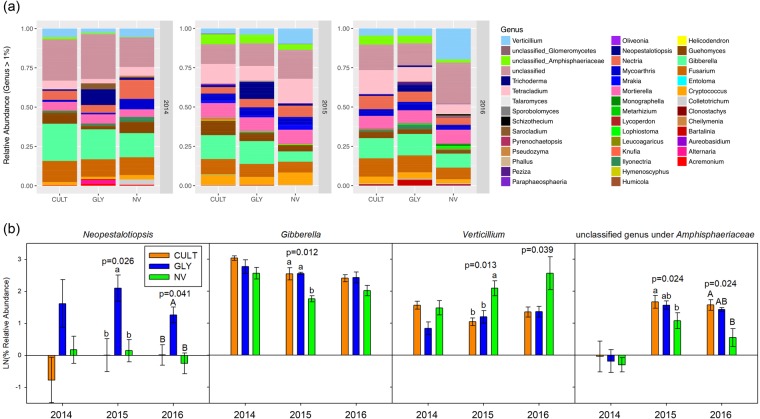
Unclassified fungal genera in soil samples ranged from around 10% to more than 25% relative abundance. However, analyses excluding the unidentified genera did not change the differentiation of NV samples from CULT and GLY samples on the ordination. The top five fungal genera found in the soil (excluding unclassified) were Verticillium, Nectria, Mortierella, Gibberella and Fusarium, based on average relative abundances across all soil samples (Fig. 3a). Fungal genera relative abundance differences were found in Gibberella, Neopestalotiopsis, Verticillium and an unclassified genus under Amphisphaeriaceae family, where NV soils contained fewer Gibberella (P < 0.005 in 2015) and more Verticillium (P < 0.05 in 2015 and 2016) compared to the other two treatments, and less Neopestalotiopsis (P < 0.05 in 2015 and 2016) and unclassified Amphisphaeriaceae (P < 0.05 in 2015 and 2016) relative to GLY soils. CULT soils had less Neopestalotiopsis (P < 0.05 in 2015 and 2016) compared to GLY soils (Fig. 3b). Among these genera, Neopestalotiopsis and Verticillium are found in the top five most important variables along with Monographella, Paraphaeosphaeria and unclassified genera under Nectriaceae in the Random Forest model for soil treatment prediction (Supplementary Fig. S2).
Figure 3.
Mean fungal relative abundance at genus level. (a) Full fungi profile (>1%) in the soil from Cultivation (CULT), Glyphosate (GLY) and Natural vegetation (NV) field treatments (n = 4) and (b) Selective fungi that were different in relative abundance (n = 4) with standard errors. The statistical differences were tested by using one-way analysis of variance (ANOVA) followed with Tukey HSD test comparing log mean relative abundance at α = 0.05. The p-values were derived from ANOVA.
Under-vine soil bacterial community structure was impacted by floor management practice
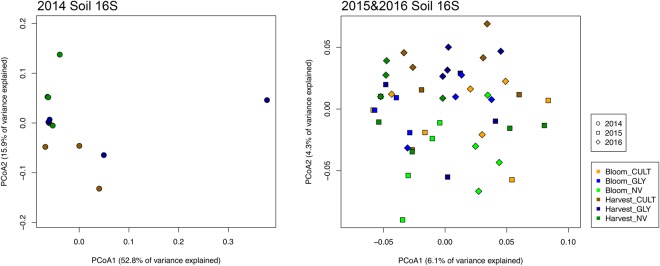
The sequencing reads generated from the 2014 samples contained unexpectedly high amounts of short reads, whereas the sample sequences were comparatively low. Although the quality of the remaining reads was sufficient for a within-year comparison, we decided to present the data year-by-year instead of the three-year overall analysis, due to the dramatic difference in read depth relative to 2015 and 2016 samples. Although the samples did not seem to cluster based on treatments on PCoA plots using UniFrac distance metrics (Fig. 4), the treatment effect was significant in year 2014 (p = 0.042) and 2016 (p = 0.013) according to PERMANOVA (Table 1). In fact, paired-PERMANOVA further revealed that the bacterial community structure among the treatments was different in 2014, where NV differed from GLY (p = 0.026) and CULT (p = 0.033), and 2016, where NV differed from GLY (P = 0.036) (Table 2). Grape-associated bacterial community structure was not further examined due to low yield of bacterial DNA resulting in low PCR amplification.
Figure 4.
Principal coordinates analysis (PCoA) ordinations of soil sample bacterial microbiota derived from Cultivation (CULT), Glyphosate (GLY) and Natural vegetation (NV) field treatments at bloom (B) and harvest(H) based on weighted UniFrac distance metric for 2014, 2015 and 2016 experimental years, where year 2014 was analyzed apart from 2015 and 2016 due to the amount of sequences difference in the samples.
Table 2.
Comparison of bacterial and fungal community structure dissimilarity in soil and grapes using paired-PERMANOVA.
| Pairs | overall | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | p value | R2 | p value | R2 | p value | R2 | p value | |
| Soil 16 S | ||||||||
| CULT vs GLY | — | — | 0.135 | 0.621 | 0.070 | 0.481 | 0.064 | 0.455 |
| CULT vs NV | — | — | 0.186 | 0.033 | 0.072 | 0.209 | 0.088 | 0.064 |
| GLY vs NV | — | — | 0.190 | 0.026 | 0.083 | 0.086 | 0.088 | 0.036 |
| Soil ITS | ||||||||
| CULT vs GLY | 0.061 | 0.005 | 0.157 | 0.238 | 0.110 | 0.027 | 0.103 | 0.022 |
| CULT vs NV | 0.101 | <0.001 | 0.225 | 0.095 | 0.179 | 0.001 | 0.251 | 0.002 |
| GLY vs NV | 0.097 | <0.001 | 0.208 | 0.105 | 0.205 | <0.001 | 0.197 | <0.001 |
| Grape ITS | ||||||||
| CULT vs GLY | 0.022 | 0.860 | 0.186 | 0.103 | 0.089 | 0.809 | 0.135 | 0.608 |
| CULT vs NV | 0.021 | 0.894 | 0.113 | 0.912 | 0.172 | 0.421 | 0.155 | 0.514 |
| GLY vs NV | 0.017 | 0.834 | 0.051 | 0.581 | 0.205 | 0.206 | 0.105 | 0.835 |
Under-vine soil management did not impact fungal communities on grapes
Grape samples were collected at commercial harvest in each year. Over 71% of the variance in grape fungal community structure was explained by the first two PCoA axes, but the grape samples were not structured as a function of under-vine soil treatments (Fig. 2b). PERMANOVA and paired PERMANOVA were used to confirm that no community composition differences were found among treatments. The three-year overall PERMANOVA showed that the year-to-year differences were the only significant effects (Table 1).
Unclassified genera accounted for 5 to more than 30% of the relative abundance in grape samples. The top five fungal genera with the highest average relative abundance of the three years in the grape samples were Sporobolomyces, Aureobasidium, Rhodosporidium, Penicillium, and Entyloma. The fungal genera that differed in relative abundance in soil were not found to differ in relative abundance in grapes. Differences in relative abundance in grape-associated fungal genera were found in Penicillium, Sporobolomyces and unidentified genera across the years. The fungal genus Penicillium was found only in the 2014 grape samples, which was 16.6% in relative abundance, and Sporobolomyces was highest in relative abundance in 2015 (p < 0.05) and lowest in 2016 in grape samples (p < 0.01), and the unidentified genera relative abundance in 2016 was higher than that in 2014 and 2015 (p < 0.0001) (Supplementary Fig. S3). The differences in these fungal genera may account for the separation of the grape samples by vintage on the PCoA plot. The grape-specific (not found in soil) fungal genera detected included Coprinellus, Ischnoderma, Mycosphaerella, Occultifur, Pestalotiopsis, and Tilletiopsis. Many yeast genera commonly found in abundance in grapes, such as Candida, Pichia, Debaryomyces, Lipomyces, Kluyveromyces, and Issatchenkia, were not found or were not abundant (<1% in relative abundance) in this study.
Discussion
The link between soil microbiome composition and regional wine characteristics has been recently studied10,15, leading to greater interest in the role of microbes in fruit and wine composition4. Our multi-year experiment examined whether different under-vine soil management practices could alter grape-associated microbial composition. While a previous study suggested that soil management in the vineyard can impact soil microbial assemblages25 and that grapevine aerial organ-associated bacterial OTUs likely originated from soil23, we hypothesized that implementing different under-vine soil management practices would not only alter soil microbial composition, but that the grape-associated microbiomes would reflect these changes. In our study, changes in the fungal community of the soil, due to adopting different under-vine soil management practices, did not extend to the grapes, leading us to reject our hypothesis for our study site.
Previous studies have shown that vineyard management alters grape and fermentation microbiome composition where systematic vineyard management practices or direct microbial management approaches were applied17,19,20,29. In one study, yeast dynamics during the spontaneous fermentation using grapes obtained from conventionally and non-conventionally managed vineyards differed20. Another study revealed that management practices applied directly onto grapes, such as pesticides, impacted grape-associated yeast diversity, which negatively correlated with the copper residuals found on the grapes19. Unlike these studies, our study did not directly manage the microbes on the grapes, but applied indirect changes to microbial composition in soils. Our study showed a link between under-vine management practices and soil bacterial and fungal composition, which confirmed results from previous studies that focused on soil bacterial25 and fungal composition22. The results of this study further revealed no corresponding changes in the grape fungal microbiome, which does not dispute findings from another study22. The researchers reported that the juice fungal microbiome obtained from conventionally and biodynamically managed vineyards did not differ from each other, despite showing that fungal populations on the grape surface differed by vineyard management approaches.
In our study, under-vine soil treatment impacts on grape fungal composition could also be masked by factors such as climate, geological properties (e.g. soil type), management practices associated with cool climate viticulture (e.g. trellis system, fungal spray use and frequency), vineyard management history, and inter-row vineyard floor management. Among these factors, many are specific to the region, such as large vine size with tall trellis systems, frequent pesticide applications, and hilling soil up over the graft union in winter and down off of the graft union in the spring. In a broader sense, climatic conditions play a significant role in microbiome structure, which is shown in our study, with year-to-year climate differences being the most significant factor explaining variance in soil and grape fungal assemblages, which is consistent with a previous study11.
With weather variability increasing as a function of climate change, there is renewed interest in improving resilience of vines to environmental stress. Cover crops are known to improve soil health by retaining soil moisture, enhancing drainage, raising soil organic matter content, maintaining soil physical structure, and supporting soil microbial properties and processes30–34. Also, cover crops provide a prolific root zone (rhizosphere) that enriches for a diversity of microorganisms that perform many functions, such as mediating soil nutrient cycling, impacting plant growth and development, and influencing pathogen interactions30,31,35–37. This may require long-term assessment, as no soil microbial diversity difference was observed between bare soil and vegetative soil over the course of three years. However, in our study, we did observe a lower relative abundance of Neopestalotiopsis and an unidentified genus under Amphisphaeriaceae in the soil with vegetation, which may possibly relate to grapevine trunk pathogenic species38. Since our study only examined short 16 S rRNA gene and ITS reads, we are not able to determine whether specific organisms we identify are pathogenic or not.
This study aimed to evaluate the role of management practices - specifically vineyard soil management - on the vineyard microbiome. We found that bare soil maintained by soil cultivation and herbicide led to soil bacterial and fungal communities that diverged from the non-cultivation natural vegetation treatment. The results indicate that vineyard microbiome could be susceptible to changes under different soil management practices; however, the spatial gap between soil and the fruiting zone, and the frequent pesticide applications, could impact the level of soil management effects. It also suggests that future studies on the movement of microorganisms from soil to grape would be key to understanding the role of vineyard soil management in shaping the microorganisms associated with grapes.
Despite previous findings on vineyard management effects on vineyard microbiomes, we show that altering soil microbial composition in the vineyard through under-vine management practices did not result in corresponding changes to the grape microbiome at our study site. The concept that soil microbial composition could be impacting fruit and wine composition should be examined in light of vineyard management practices that alter soil biotic components. Regional management practices such as ground cover management, height of the trellis system, and phytosanitation, that respectively modify soil conditions, transportation of microbes from soil to grapes, and grape-associated microbes, could have a significant role in shaping fruit and wine composition in vineyards.
Methods
Vineyard design
The experiment was conducted in a commercial vineyard on Howard gravelly loam soil located in Ovid, NY, USA for three consecutive years from 2014 to 2016. The vines, V. vinifera cultivar Riesling grafted onto 3309 C rootstock, were planted in 2001 with 2.13 m × 2.74 m intra- and inter-row spacing. The trellis system was cane pruned Scott-Henry system with 10 buds per cane on each of four canes. A complete randomized block design was applied to enable four replicates for each treatment, and the treatments were randomly assigned to the experimental units, which are one meter wide under-vine soil strips, within each block. Each experimental unit was across three rows with nine consecutive vines in a row. The grape and soil samples were collected from the middle three vines and the accordance under-vine 1 m × 5.8 m soil strip, in the middle row from each of the experimental unit where the other vines were served as guards for physical and spatial buffering. The vineyard canopy, pest-control and amendments were managed following standard commercial practice in the Finger Lakes region39 by the professional vineyard crew.
Under-vine soil treatments
The experimental units were subjected to three different under-vine soil treatments in a one meter wide strip under vines including spot application of herbicide, in which the active ingredient was glyphosate, cultivation maintained bare soil, and natural vegetation, where weeds grew freely with periodic mowing to keep them out of the fruiting zone. Herbicide and cultivation bare soil strips were established following the commercial standard. In brief, 2% Roundup (Monsanto, MO, USA) was sprayed with electronic pumped spraying nozzle in rate about 3 kg a.i./ha. Cultivation was done by combining mechanical, rototiller to roughly 20 cm depth, and manual tillage, cultivation with hoes. Herbicide was applied on June 23rd, July 9th, July 18th in 2014, June 16th in 2015 and June 15th in 2016. Soil cultivation was applied on June 27th, July 3rd, and July 18th in 2014, June 3rd, July 23rd to July 27th in 2015 and May 25th and June 24th in 2016. A permanent between-row cover crop was maintained separately and was a mix of fescue, white clover and weeds.
Sample collection
At bloom (2015 and 2016) and harvest (2014, 2015 and 2016), ten soil cores per experimental unit were collected using sterile cores (6 cm diameter × 10 cm deep) attached to the slide hammer auger (AMS Inc, American Falls, ID, USA) in a grid pattern. Grape cluster samples were taken at commercial harvest with individual sterilized blazers for each of the experimental unit. Ten clusters from each experimental unit were randomly picked. Sub-samples from each of the experimental units were combined in sterile containers in the field, transported on ice, and stored at −20 °C until further analysis. Sub-samples of five berries per cluster, comprised of two from the top, two from the middle and one from the bottom of the cluster, were detached in the original field sampling container while frozen, and allocated into a new container to make 50 berries per experimental unit for grape DNA extractions. The soil sample of each experimental unit was thawed at room temperature, fully homogenized, and then 0.25 g of soil was taken carefully, avoiding any non-soil particles for downstream DNA extraction.
Sample DNA extraction, amplification and Sequencing
DNA extraction of soil samples followed the protocol for the PowerSoil DNA isolation kit (MO BIO Laboratories, CA, USA). For grape samples, the grapes were thawed and crushed in the zip bag before following the procedures. For each experimental unit, grape must sample was vortexed and homogenized, and transferred into two 2 ml Eppendorf tubes and centrifuged at 11600 × g for 20 minutes. The pellets from the same sample were combined and washed two times with chilled PBS. The pellets were then used for DNA extraction following the protocol from the MoBio PowerPlant DNA isolation kit. The bacterial 16 S rRNA gene V3/V4 regions and fungal ITS barcoded region were amplified with the universal bacterial primers 341 F (5′-CCTACGGGNGGCWGCAG-3′) and 805 R (5′-GACTACHVGGGTATCTAATCC-3′) and fungal primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and 5.8A2R (5′-CTGCGTTCTTCATCGAT-3′), in which the Illumina adaptors at the 5′ end of the primer sequences (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-3′ for the forward primer and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3′ for the reverse primer) were attached40,41. The reaction was conducted in 20 μl containing 9 μl H2O, 8 μl 5prime HotMaster mix (5 PRIME Inc., MD, USA), 1 μl of each primer (forward and reverse) and 1 μl of 1:10 diluted DNA template in thermocycler (Bio-Rad, CA, USA) following the condition of 3 min at 95 °C and then 25 (bacteria) and 30 (fungi) cycles of 30 s at 95 °C, 35 s at 50 °C and 60 s at 72 °C before entering the final step of 10 min at 72 °C. The amplicons were transferred into 96-well plates and cleaned with MagBio HighPrep PCR beads (MagBio Genomics, MD, USA). We then attached unique two-barcode indexes to cleaned amplicons by running PCR with 2.5 μl each of forward and reverse primers (10 µM) carrying designated barcodes, 12.5 μL of Q5 High Fidelity 2× Master Mix (New England Biolabs Inc., MA, USA), 5 μL of template, and 2,5 μl of water, with the following temperature protocol: 8 cycles of 15 s at 98 °C, 30 s at 55 °C and 20 s at 72 °C after 1 min at 98 °C and before 3 min at 72 °C. Sample DNA was normalized with the SequalPrep Normalization Kit (ThermoFisher, Waltham, MA), pooled using equal liquid volumes, and the pool purified with a PureLink QuickGel Extraction Kit (ThermoFisher). Each pool was sent to the Cornell Institute of Biotechnology (Ithaca, NY) for paired-end sequencing, using the 600-cycle MiSeq Reagent Kit v.3 for our 16 S pool, and the 500-cycle MiSeq Reagent Kit v.2 for our ITS pool on the Illumina MiSeq platform (Illumina Inc., CA, USA). The sequencing process generated 4,060,310 ITS and 552,871 16 S rRNA gene reads after downstream processing as described below. All the MiSeq data were uploaded to the NCBI Sequence Read Archive and are public accessible under the project number of SRP132177.
Bioinformatic and statistical analysis
The raw sequences were processed and aligned following the protocol described in the Brazilian Microbiome Project42 with some modifications43. Briefly, paired-end sequence merging, primer trimming, and singleton sequence removal were performed in Mothur v 1.36.1. Operational Taxonomic Units (OTU) were produced at 97% sequence similarity. Taxonomic classification of OTUs was performed in Mothur using the GreenGenes v.13.8 database for 16 S rRNA gene sequences and UNITE v. 7 database for ITS sequences. Suspected non-bacterial and non-fungal OTUs, including chlorophyll and mitochondria, were also removed in Mothur. All downstream data analysis was conducted in R version 3.3.3 with packages Vegan and Phyloseq. The microbial diversity was determined using Shannon Diversity Index using “diversity” function in package vegan. The β-diversity of the assemblage dissimilarity between samples were calculated with the Bray-Curtis distances for fungal community and weighted UniFrac distances for the bacterial community using package vegan. The dissimilarity matrices obtained were also used for Principal Coordinate Analysis (PCoA) plotting against the first two dimensions (highest variables explained). Multivariate dispersion analysis was performed to test the differences in variances among the treatments using command “betadisper” in package vegan where the β-diversities were obtained based on the Bray-Curtis distance metric for fungal community and UniFrac distances for the bacterial community. Permutational Multivariate Analysis of Variance (PERMANOVA) and paired PERMANOVA using “adonis” command in package vegan at 999 permutations and α = 0.05 were performed testing factors including year (for overall analysis only), stage (for soil samples in 2015 and 2016 only), and under-vine soil treatments. When three-year overall analysis was conducted, the year was positioned as a fixed effect with samples within each block in constrained permutation to account for the repeated measures. The overall PERMANOVA was not performed for soil 16 S data due to large differences in sequencing depth between 2014 and the other years (average 450 reads per sample in 2014, and 11648 reads per sample in 2015 and 2016). Paired-PERMANOVA was performed by subsetting the treatments and applying Bonferroni correction to the P-values. The relative abundance of selected fungal genera in the samples were compared using one-way analysis of variance (ANOVA) test followed by Tukey HSD performing in JMP Pro 12.0.1 (SAS Institute, NC, USA), with log transformations when needed under violations of normality.
Data availability
All of the data are provided fully in the result section within and supplementary data accompanying this paper.
Electronic supplementary material
Acknowledgements
We thank Steve Lerch, Justin France, Connor Robertson, and Raquel Kallas for assisting with the field work and Maria Gannett and Liang Cheng for laboratory assistance. We are grateful to Mrs. Francoise Vermeylen and Dr. Erika Mudrak at Cornell Statistics Consulting Unit for their help on statistical modeling. We thank Sheldrake Point Winery and Dave Weimann for letting us conduct the experiment. We also thank Dr. Anna Katharine Mansfield and Dr. Johannes Lehmann for critical and insightful inputs on the study methods and results. The help from members of Cornell vinification and brewing laboratory in Geneva, NY, including Luann Preston-Wisey and Chris Gerling, are deeply appreciated. This research was funded by the Lacroute, Kaplan, and Saltonstall endowments at the Cornell University New York State Agricultural Experiment Station in Geneva, NY, USA.
Author Contributions
J.V. and J.K. developed the project. J.V., J.K. and M.C. contributed to the experimental design. K.P. contributed to the experiment initiation and assisted with the data collection plans. T.B. and M.C. contributed to the laboratory work and data analysis. T.B., J.K., J.V. and M.C. contributed to the data interpretation. M.C. and J.V. led the writing. M.C. and J.V. contributed to the field work. All authors contributed to the manuscript review.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29346-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jackson DI, Lombard PB. Environmental and Management Practices Affecting Grape Composition and Wine Quality - A Review. American Journal of Enology and Viticulture. 1993;44:409–430. [Google Scholar]
- 2.Kliewer WM, Dokoozlian NK. Leaf area/crop weight ratios of grapevines: influence on fruit composition and wine quality. American Journal of Enology and Viticulture. 2005;56:170–181. [Google Scholar]
- 3.Smart, R. & Robinson, M. Sunlight into wine: a handbook for winegrape canopy management. (Winetitles, 1991).
- 4.Gilbert JA, van der Lelie D, Zarraonaindia I. Microbial terroir for wine grapes. Proc Natl Acad Sci USA. 2014;111:5–6. doi: 10.1073/pnas.1320471110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabate J, Cano J, Esteve-Zarzoso B, Guillamon JM. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol Res. 2002;157:267–274. doi: 10.1078/0944-5013-00163. [DOI] [PubMed] [Google Scholar]
- 6.Mortimer R, Polsinelli M. On the origins of wine yeast. Res Microbiol. 1999;150:199–204. doi: 10.1016/S0923-2508(99)80036-9. [DOI] [PubMed] [Google Scholar]
- 7.Ciani M, Mannazzu I, Marinangeli P, Clementi F, Martini A. Contribution of winery-resident Saccharomyces cerevisiae strains to spontaneous grape must fermentation. Antonie Van Leeuwenhoek. 2004;85:159–164. doi: 10.1023/B:ANTO.0000020284.05802.d7. [DOI] [PubMed] [Google Scholar]
- 8.Bokulich NA, Ohta M, Richardson PM, Mills DA. Monitoring Seasonal Changes in Winery-Resident Microbiota. PLoS One. 2013;8:e66437. doi: 10.1371/journal.pone.0066437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Martin F, Sesena S, Fernandez-Gonzalez M, Arevalo M, Palop ML. Microbial communities in air and wine of a winery at two consecutive vintages. Int J Food Microbiol. 2014;190:44–53. doi: 10.1016/j.ijfoodmicro.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Bokulich, N. A. et al. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. MBio7, 10.1128/mBio.00631-16 (2016). [DOI] [PMC free article] [PubMed]
- 11.Bokulich NA, Thorngate JH, Richardson PM, Mills DA. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci USA. 2014;111:E139–148. doi: 10.1073/pnas.1317377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corneo PE, et al. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol Ecol. 2013;84:588–602. doi: 10.1111/1574-6941.12087. [DOI] [PubMed] [Google Scholar]
- 13.Setati ME, Jacobson D, Andong UC, Bauer FF. The vineyard yeast microbiome, a mixed model microbial map. PLoS One. 2012;7:e52609. doi: 10.1371/journal.pone.0052609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns KN, et al. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by geographic features. Soil Biology and Biochemistry. 2015;91:232–247. doi: 10.1016/j.soilbio.2015.09.002. [DOI] [Google Scholar]
- 15.Knight S, Klaere S, Fedrizzi B, Goddard MR. Regional microbial signatures positively correlate with differential wine phenotypes: evidence for a microbial aspect to terroir. Sci Rep. 2015;5:14233. doi: 10.1038/srep14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belda I, Zarraonaindia I, Perisin M, Palacios A, Acedo A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front Microbiol. 2017;8:821. doi: 10.3389/fmicb.2017.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grangeteau C, et al. Wine microbiology is driven by vineyard and winery anthropogenic factors. Microb Biotechnol. 2017;10:354–370. doi: 10.1111/1751-7915.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zehetner F, et al. Soil organic carbon and microbial communities respond to vineyard management. Soil Use and Management. 2015;31:528–533. doi: 10.1111/sum.12204. [DOI] [Google Scholar]
- 19.Martins G, et al. Influence of the farming system on the epiphytic yeasts and yeast-like fungi colonizing grape berries during the ripening process. Int J Food Microbiol. 2014;177:21–28. doi: 10.1016/j.ijfoodmicro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Bagheri B, Bauer F, Setati M. The diversity and dynamics of indigenous yeast communities in grape must from vineyards employing different agronomic practices and their influence on wine fermentation. South African Journal of Enology and Viticulture. 2015;36:243–251. [Google Scholar]
- 21.Patrignani F, et al. Characterisation of yeast microbiota, chemical and sensory properties of organic and biodynamic Sangiovese red wines. Annals of Microbiology. 2016;67:99–109. doi: 10.1007/s13213-016-1241-3. [DOI] [Google Scholar]
- 22.Morrison-Whittle P, Lee SA, Goddard MR. Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agriculture, Ecosystems & Environment. 2017;246:306–313. doi: 10.1016/j.agee.2017.05.022. [DOI] [Google Scholar]
- 23.Zarraonaindia, I. et al. The soil microbiome influences grapevine-associated microbiota. MBio6, 10.1128/mBio.02527-14 (2015). [DOI] [PMC free article] [PubMed]
- 24.Cordero-Bueso G, Arroyo T, Serrano A, Valero E. Influence of different floor management strategies of the vineyard on the natural yeast population associated with grape berries. Int J Food Microbiol. 2011;148:23–29. doi: 10.1016/j.ijfoodmicro.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Burns KN, et al. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by vineyard management. Soil Biology and Biochemistry. 2016;103:337–348. doi: 10.1016/j.soilbio.2016.09.007. [DOI] [Google Scholar]
- 26.Madden L. Effects of rain on splash dispersal of fungal pathogens. Canadian Journal of Plant Pathology. 1997;19:225–230. doi: 10.1080/07060669709500557. [DOI] [Google Scholar]
- 27.Bock C, Cook A, Parker P, Gottwald T, Graham J. Short‐distance dispersal of splashed bacteria of Xanthomonas citri subsp. citri from canker‐infected grapefruit tree canopies in turbulent wind. Plant pathology. 2012;61:829–836. doi: 10.1111/j.1365-3059.2011.02588.x. [DOI] [Google Scholar]
- 28.Jordan LM, Björkman T, Heuvel JEV. Annual under-vine cover crops did not impact vine growth or fruit composition of mature cool-climate ‘Riesling’grapevines. HortTechnology. 2016;26:36–45. [Google Scholar]
- 29.Cordero-Bueso G, et al. Influence of the farming system and vine variety on yeast communities associated with grape berries. Int J Food Microbiol. 2011;145:132–139. doi: 10.1016/j.ijfoodmicro.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Doran JW, Zeiss MR. Soil health and sustainability: managing the biotic component of soil quality. Applied soil ecology. 2000;15:3–11. doi: 10.1016/S0929-1393(00)00067-6. [DOI] [Google Scholar]
- 31.Steenwerth K, Belina KM. Cover crops enhance soil organic matter, carbon dynamics and microbiological function in a vineyard agroecosystem. Applied Soil Ecology. 2008;40:359–369. doi: 10.1016/j.apsoil.2008.06.006. [DOI] [Google Scholar]
- 32.Peregrina, F., Larrieta, C., Ibáñez, S. & García-Escudero, E. Labile Organic Matter, Aggregates, and Stratification Ratios in a Semiarid Vineyard with Cover Crops. Soil Science Society of America Journal74, 10.2136/sssaj2010.0081 (2010).
- 33.Ruiz-Colmenero M, Bienes R, Eldridge DJ, Marques MJ. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena. 2013;104:153–160. doi: 10.1016/j.catena.2012.11.007. [DOI] [Google Scholar]
- 34.Karl AD, Merwin IA, Brown MG, Hervieux RA, Heuvel JEV. Under-vine management impacts soil properties and leachate composition in a New York State Vineyard. HortScience. 2016;51:941–949. [Google Scholar]
- 35.Barrios E. Soil biota, ecosystem services and land productivity. Ecological economics. 2007;64:269–285. doi: 10.1016/j.ecolecon.2007.03.004. [DOI] [Google Scholar]
- 36.Steenwerth K, Belina K. Cover crops and cultivation: Impacts on soil N dynamics and microbiological function in a Mediterranean vineyard agroecosystem. Applied Soil Ecology. 2008;40:370–380. doi: 10.1016/j.apsoil.2008.06.004. [DOI] [Google Scholar]
- 37.Gianinazzi S, et al. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 38.Maharachchikumbura SS, Larignon P, AL-SADI AM, Zuo-Yi L. Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathologia Mediterranea. 2017;55:380–390. [Google Scholar]
- 39.Wolf, T. K. Wine grape production guide for eastern North America. (2008).
- 40.Bell TH, et al. A diverse soil microbiome degrades more crude oil than specialized bacterial assemblages obtained in culture. Applied and environmental microbiology. 2016;82:5530–5541. doi: 10.1128/AEM.01327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yergeau, E. et al. Transplanting soil microbiomes leads to lasting effects on willow growth, but not on the rhizosphere microbiome. Frontiers in microbiology6 (2015). [DOI] [PMC free article] [PubMed]
- 42.Pylro VS, et al. Brazilian microbiome project: revealing the unexplored microbial diversity—challenges and prospects. Microbial ecology. 2014;67:237–241. doi: 10.1007/s00248-013-0302-4. [DOI] [PubMed] [Google Scholar]
- 43.Howard MM, Bell TH, Kao-Kniffin J. Soil microbiome transfer method affects microbiome composition, including dominant microorganisms, in a novel environment. FEMS Microbiology Letters. 2017;364:fnx092–fnx092. doi: 10.1093/femsle/fnx092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data are provided fully in the result section within and supplementary data accompanying this paper.