Correction to: Scientific Reports 10.1038/s41598-018-26577-0, published online 24 May 2018
This Article and the accompanying Supplementary Information file contain errors.
In Figure 2, the wrong µM symbol was used. The correct Figure 2 appears below as Figure 1.
Figure 1.
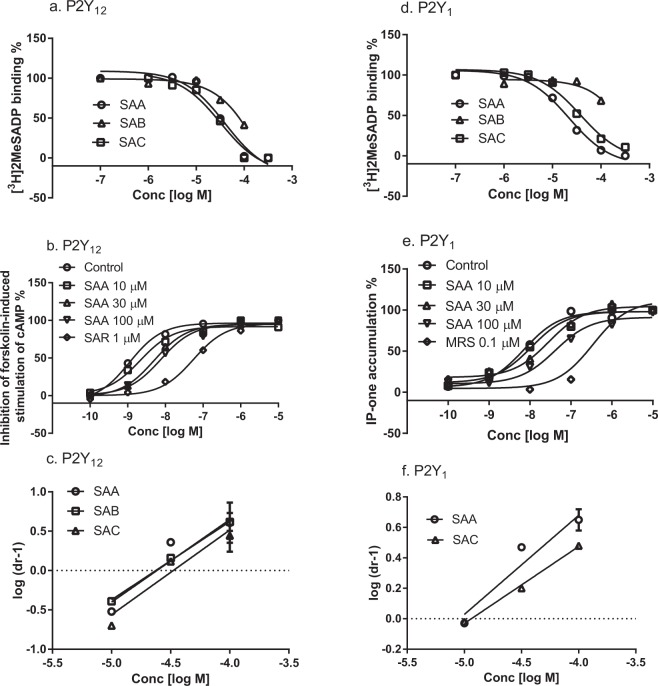
Radioligand binding assays and functional assays showed that SAA and SAC can bind and antagonize both P2Y1 and P2Y12 receptors, while SAB can bind and antagonize only the P2Y12 receptor. (a) Displacement curves of SAA, SAB and SAC against [3H]2MeSADP binding to the P2Y12 receptor. (b) Functional antagonism by SAA of 2MeSADP-induced inhibition of foskolin-stimulated cAMP accumulation in U2OS cells expressing the P2Y12 receptor. (c) Schild plots of the antagonism of SAA, SAB and SAC at the P2Y12 receptors. (d) Displacement curves of SAA, SAB and SAC against [3H]2MeSADP binding to the P2Y1 receptor. (e) 2MeSADPinduced IP-1 accumulation in U2OS cells expressing the P2Y1 receptor (compared to MRS2500). (f) Schild plots of antagonism by SAA and SAC at the P2Y1 receptor. Results are expressed as mean ± SEM. The Ki values from binding experiments and KB values from Schild analyses of functional antagonism by SAA, SAB and SAC are listed in the text and are from at least three independent experiments. MRS, MRS2500; SAR, SAR216471.
In the Supplementary Information file, the title of Table S1 is incorrectly given as ‘SAA, SAB and SAC are not promiscuously binding compounds’. The correct Table S1 appears below as Table 1.
Table 1.
Targets used in the off-target activities tested by the PDSP.
| # | Protein name | # | Protein name |
|---|---|---|---|
| 1 | 5-HT1A | 24 | D3 |
| 2 | 5-HT1B | 25 | D4 |
| 3 | 5-HT1D | 26 | D5 |
| 4 | 5-HT1E | 27 | GABAA |
| 5 | 5-HT2A | 28 | H1 |
| 6 | 5-HT2B | 29 | H2 |
| 7 | 5-HT2C | 30 | H3 |
| 8 | 5-HT3 | 31 | H4 |
| 9 | 5-HT5A | 32 | M1 |
| 10 | 5-HT6 | 33 | M2 |
| 11 | 5-HT7 | 34 | M3 |
| 12 | α1A | 35 | M4 |
| 13 | α1B | 36 | M5 |
| 14 | α1D | 37 | δ-opioid |
| 15 | α2A | 38 | κ-opioid |
| 16 | α2B | 39 | μ-opioid |
| 17 | α2C | 40 | σ1 |
| 18 | β1 | 41 | σ2 |
| 19 | β2 | 42 | DAT |
| 20 | β3 | 43 | NET |
| 21 | BZP rat brain site | 44 | SERT |
| 22 | D1 | 45 | TSPO |
| 23 | D2 |
Finally, in the Supplementary Information file, there is a typographical error in Figure S4 and its accompanying legend where ‘Epinenohrineon’ should read ‘Epinephrine’. The correct Figure S4 appears below as Figure 2.
Figure 2.
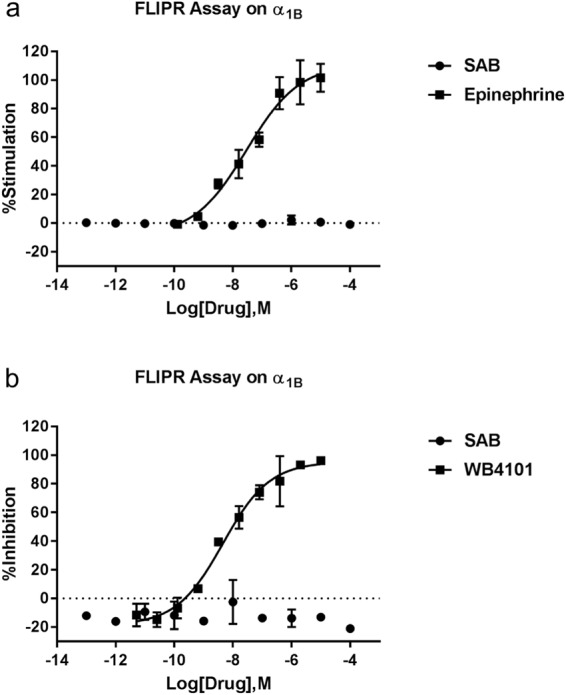
The functional activity of SAB on the adrenergic α1B receptor were tested by FLIPR assays. SAB did not show agonist activity on α1B, epinephrine is a control agonist (EC50 29.8 nM) (a). SAB did not show antagonist activity on α1B, WB4104 is a control antagonist (IC50 4.51 nM) (b).