Abstract
Objective
To investigate the association between older adults’ potentially avoidable hospitalization rates and both a geographic measure of primary care physician (PCP) access and a standard bounded‐area measure of PCP access.
Data Sources
State physician licensure data from the Virginia Board of Medicine. Patient‐level hospital discharge data from Virginia Health Information. Area‐level data from the American Community Survey and the Area Health Resources Files. Virginia Information Technologies Agency road network data. US Census Bureau TIGER/Line boundary files.
Study Design
We use enhanced two‐step floating catchment area methods to calculate geographic PCP accessibility for each ZIP Code Tabulation Area in Virginia. We use spatial regression techniques to model potentially avoidable hospitalization rates.
Data Collection/Extraction
Geographic accessibility was calculated using ArcGIS. Physician locations were geocoded using TAMU GeoServices and ArcGIS.
Principal Findings
Increased geographic access to PCPs is associated with lower rates of potentially avoidable hospitalization among older adults. This association is robust, allowing for spatial spillovers in spatial lag models.
Conclusions
Compared to bounded‐area density measures, unbounded geographic accessibility measures provide more robust evidence that avoidable hospitalization rates are lower in areas with more PCPs per person. Results from our spatial lag models reveal the presence of positive spatial spillovers.
Keywords: Geographic accessibility, potentially avoidable hospitalization, spatial analysis, primary care provider
Access to primary care has long been viewed as an important means of improving population health (IOM 1993). Given recent increases in financial access to care through Medicaid expansions and private insurance subsidies, geographic access to health care stands to receive greater attention from researchers and policy makers (Kulgren and McLaughlin 2010; Kulgren et al. 2012). One potential benefit from increased geographic access to primary care is reduced hospitalizations for ambulatory care sensitive conditions, including diabetes, hypertension, congestive heart failure, and other conditions. Hospitalizations for these conditions are considered potentially avoidable or preventable through improvements in primary care access and/or quality (Weissman, Gatsonis, and Epstein 1992; Billings et al. 1993; IOM 1993; Bindman et al. 1995; Ansari 2007). The relationship between geographic access to care and avoidable hospitalizations among persons aged 65 years and older is of particular interest. Avoidable hospitalizations constitute 12 percent of Medicare inpatient spending and more than 25 percent of all hospitalizations among dual Medicare and Medicaid enrollees (Joynt et al. 2013; Segal et al. 2014). Moreover, because Medicare provides near universal insurance coverage, studies of avoidable hospitalization among older adults can more clearly isolate the role of nonfinancial dimensions of access to care.
Some prior studies of geographic access to care show that older adults who live in areas with more primary care physicians (hereafter, PCPs) per capita have fewer avoidable hospitalizations (Krakuaer et al. 1996; Laditka 2004; Chang et al. 2011; Basu, Mobley, and Thumula 2014; Lin, Eberth, and Probst 2016).1 In contrast, others report either no relationship or a positive relationship between PCPs per capita and avoidable hospitalizations by Medicare beneficiaries (Ricketts et al. 2001; Mobley et al. 2006; Nayar et al. 2012; Joynt et al. 2013). One feature common to all of these studies is the use of a bounded‐area measure of PCPs per capita, such as the ratio of physicians in a county to the county's population. This type of measure has several limitations. First, it more accurately represents primary care availability, as opposed to accessibility, since it does not account for travel costs (Guagliardo 2004). Second, it fails to account for accessible physicians immediately outside the bounded area.
In contrast, unbounded measures of accessibility (i.e., geographic measures) can account for both patient border‐crossing and travel impedance. Geographic measures that calculate travel time across the road network are especially advantageous. A number of studies have used these types of geographic measures to describe how access to health care differs by race (Brown et al. 2016), by rural/urban residence (Rosenthal, Zaslavsky, and Newhouse 2005; Chan, Hart, and Goodman 2006), and over time (Eberth et al. 2014). Especially relevant to our focus are prior studies showing that geographic measures of physician access are directly associated with primary care utilization. Specifically, children living in areas with a greater density of pediatricians, according to a geographic measure of access, have better asthma management, higher vaccine compliance, and fewer ED visits (Teach et al. 2006; Fu et al. 2009; Mathison et al. 2013).
The main objective of this study is to examine the association between avoidable hospitalization rates among older adults and an unbounded or geographic measure of PCP access. The second objective is to compare the predictive power of a geographic access measure in a model of avoidable hospitalization rates to that of a standard physician‐to‐population ratio comparable to one used in the bulk of the prior literature. To the best of our knowledge, no prior work has employed a geographic measure of physician access as spatially explicit as ours to study avoidable hospitalizations among older adults. We also add to the literature by using both spatial lag models and ordinary least squares (OLS) models. Spatial lag models (also called spatial autoregressive models) deal with observations that are dependent on one another due to shared unobserved traits or spillover effects across areas; when present, these forms of spatial dependence create biased and inconsistent OLS estimates (e.g., LeSage and Pace 2009). Prior work by Mobley et al. (2006) estimated a spatial model of avoidable hospitalizations; we build on this using both a spatial model and an unbounded, geographic measure of PCP access.
The context for this study is Virginia. Compared to the United States, Virginia has a similar physician‐to‐population ratio and a somewhat lower rate of avoidable hospitalization among Medicare beneficiaries.2 We use data from the entire state to examine the link between health care use and an unbounded geographic measure of PCP access; in contrast, prior studies employing comparable geographic measures of pediatrician access to model health care use focus on a single city (Teach et al. 2006; Fu et al. 2009; Mathison et al. 2013).
Conceptual Framework
Since avoidable hospitalization is linked to inadequate primary care, our study is informed by established models of health care utilization (Khan and Bhardwaj 1994; Anderson 1995). In Anderson (1995), health care utilization is determined by the individual's need, predisposing factors (i.e., age, sex, education, and ethnicity), and enabling resources (i.e., individual income, insurance status), as well as characteristics of the health care system and the external environment. In addition, health care use is determined by the availability of enabling resources in the community. Such resources increase potential access to care in the community. As Khan and Bhardwaj (1994) describe, potential access to care has both aspatial (such as cultural, political, social, or other nongeographic barriers) as well as spatial dimensions.
Viewed in this framework, our study examines spatial dimensions of access to PCPs in a community. Our key explanatory variables are two spatial measures of PCP access: the standard bounded physician‐to‐population ratio and our preferred unbounded or geographic measure. We examine which of these measures has a more robust relationship with the underuse of ambulatory care, as measured by the avoidable hospitalization rate. As we describe below, our analysis controls for various measures of individual need, predisposing and enabling factors, as well as other community traits.
Data and Methods
Our dependent variable is the rate of avoidable hospitalization among persons age 65 years and older in a ZIP code tabulation area (ZCTA), which is the smallest level of geography we observe in our data. We constructed the rate from data obtained from the Virginia Health Information (VHI) patient‐level database (PLD), a census of inpatient discharges from Virginia hospitals.3 We selected discharges of persons age 65 and older and identified avoidable hospitalizations using the Prevention Quality Indicators (PQIs) defined by the Agency for Healthcare Research and Quality (AHRQ 2001). These include hospitalizations for uncontrolled diabetes without complications, short‐term and long‐term diabetes complications, lower‐extremity amputation among diabetes patients, congestive heart failure, hypertension, angina without a procedure, chronic obstructive pulmonary disease, bacterial pneumonia, dehydration, urinary tract infection, and perforated appendix.
We used information on the discharge record and AHRQ PQI software version 5.0 to flag each avoidable hospitalization in the VHI data, then constructed counts of avoidable hospitalizations for each ZCTA using the patient ZIP code of residence and a 2014 ZIP code‐to‐ZCTA crosswalk (UDS Mapper). For cases in which a ZCTA overlaid more than one ZIP code, we summed the component ZIP code discharges. To increase the stability of the ZCTA‐level counts of avoidable discharges, we combined eight quarters of hospital discharge records from 2013 quarter 3 through 2015 quarter 2, similar to the approach used by Mobley et al. (2006) and Schreiber and Zielinski (1997). We then constructed ZCTA‐level preventable hospitalization rates per 1,000 persons age 65 and older by dividing the 2‐year counts by 2014 ZCTA‐level estimates of the population age 65 years and older.
Our key explanatory variable is an unbounded measure of geographic access to PCPs. We constructed this measure using July 2014 data on PCP practice locations from Virginia's Doctor Profile database (Virginia Board of Medicine). The database contains all physicians licensed in Virginia and is updated on a quarterly basis. We excluded physicians with inactive or expired licenses, practice locations outside Virginia, and physicians who were not involved in primary care. We defined primary care as family or general practice, internal medicine, pediatrics, and obstetrics/gynecology following the primary care definition used by the Health Resources and Services Administration (HRSA). PCP practice locations were geocoded using TAMU GeoServices and ArcGIS 10.2.2 (Texas A&M GeoServices). Geocoded records were aggregated by practice location to create a count of all physicians at each unique practice location. In constructing the count, we weighted each physician by the percentage of his/her time spent at the location, as reported by the physician in the Doctor Profile database. This implicitly assumes that physicians work the same number of total hours as one another, that all time at the location is spent in patient care, and that physician assessments are accurate.
We used the aggregated physician location data to create a measure of PCP access for each population location following the enhanced two‐step floating catchment area (E2SFCA) methodology (Luo and Qi 2009; Dewulf et al. 2013). This methodology has several advantages. Unlike standard bounded‐area density measures, E2SFCA methods allow for border‐crossing between geopolitical units. Furthermore, we use an E2SFCA method that treats more distant providers as less accessible with the use of distance decay coefficients, and where distance is measured in travel time through the actual road network. These techniques produce more realistic measures of geographic access. Finally, E2SFCA methods yield a geographic accessibility measure that can be interpreted similarly to standard bounded‐area physician density measures for ease of comparison.
The first step in the E2SFCA methodology involves calculating a PCP‐to‐population ratio (PPR) by defining a catchment for each physician practice location following a reasonable assumption of travel time. In our case, we define each location's catchment area as the distance that can be traveled through the road network in 30 minutes, which is the same travel time estimate used by HRSA in defining federal shortage areas. In each catchment, we designated three travel time zones corresponding to 0–10 minutes, greater than 10–20 minutes, and greater than 20 minutes, and we used distance decay weights equal to 1.00, 0.68, and 0.22, for the three zones, respectively. These weights were used in a prior analysis employing E2SFCA methods to measure geographic access to physicians (Luo and Qi 2009). We then identified all population locations within each travel time zone from the physician practice location and computed the weighted PPR within the catchment area. In the second step, we identified all physician practice locations within a 30‐minute catchment area around each population point, and we summed the PPRs within each travel time zone. We used the same distance decay weights and corresponding travel time zones in step 2 as in step 1. See Appendix SA2 for additional details on the construction of our geographic measure of PCP access.
We measure predisposing factors and other enabling factors that determine health care use with several demographic and socioeconomic measures. Demographic measures included the shares of the population aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, female, African American, and of Hispanic ethnicity, population density (thousands of persons per square mile), and the share of the population residing in an urban area.4 Socioeconomic measures included the unemployment rate, median household income, the share of the population with incomes below the federal poverty level, the share of the population aged 25 years and older without a high school diploma, the share of the elderly population living alone, and the share of the population speaking English less than “very well.” We constructed the share of each ZCTA's population residing in an urban area using urban classifications from the U.S. Census Bureau (n.d.) and other data (Missouri Census Data Center, 2012). We obtained population density data from Mable/Geocorr. ZCTA‐level data on all other demographic and economic measures were obtained from the U.S. Census Bureau American FactFinder database of 5‐year estimates from 2014.
We also included other community‐level measures of access and health care system resources. For comparison to our unbounded geographic measure of PCP access, we examined a standard bounded‐area measure of PCP access, defined as the number of PCPs per 1,000 persons in a county. To control for hospital access, we included the number of short‐term acute care hospital beds per 1,000 persons in the county. To control for the fact that managed care plans frequently provide additional preventive care services to their members, we included the Medicare Advantage (MA) penetration rate in the county, defined as the share of eligible Medicare participants enrolled in managed care plans among the total Medicare population. In some analysis, we included the density of non‐physician primary care clinicians, defined as the number of nurse practitioners, physician assistants, and advanced practice nurses per 1,000 persons in the county. Because these four variables are not available at the ZCTA level, we used county‐level data from the Area Health Resources Files (AHRF) for the closest year to 2014 available. County‐level data were assigned to ZCTAs using a crosswalk (Missouri Data Center 2012); in cases where a ZCTA overlapped more than one county, we constructed a weighted‐average of the county‐level measures, where the weights were shares of the ZCTA population residing in the relevant counties.
We constructed two additional variables at the ZCTA level using other sources of data. We constructed the number of minutes from the center of the ZCTA to the closest hospital with ArcMAP software version 10.2.2, geocoded locations of ZCTA centroids and short‐term acute care hospitals, and road and speed limit data. We measured the share of each ZCTA's population residing in a Health Professional Shortage Area (HPSA) since PCPs in HPSAs are eligible for Medicare bonuses. This measure was constructed with ArcMap software, Census population estimates, and shapefiles of HPSA boundaries (HRSA, 2016). Table 1 provides the full list of all variables and their sources, and Table S1 reports the pairwise correlation coefficients for all variables.
Table 1.
Characteristics of Virginia ZIP Code Tabulation Areas (ZCTAs)
| Variable | Source and Level | Weighted Means for Virginia ZCTAsa | US |
|---|---|---|---|
| Demographic traits | |||
| Share of the population that is African American | ACS, ZCTA | 0.192 | 0.126 |
| Share of the population that is Hispanic | “ | 0.084 | 0.169 |
| Share of the population that is female | “ | 0.509 | 0.508 |
| Share of the population 65–69 years old | “ | 0.044 | 0.044 |
| Share of the population 70–74 years old | “ | 0.032 | 0.032 |
| Share of the population 75–79 years old | “ | 0.022 | 0.024 |
| Share of the population 80–84 years old | “ | 0.016 | 0.018 |
| Share of the population 85 years and older | “ | 0.016 | 0.019 |
| Population per square mile (per thousand persons) | Mable/Geocorr, ZCTA | 0.190 | 0.087 |
| Share of the population residing in urban areas | Constructed from Census data, ZCTA | 0.759 | 0.807 |
| Socioeconomic traits | |||
| Unemployment rate | ACS, ZCTA | 0.071 | 0.092 |
| Median household income (thousands of dollars) | “ | 72.795 | 53.482 |
| Share of the population with incomes below 100% of the federal poverty level | “ | 0.117 | 0.156 |
| Share of the population 25 years and older without a high school diploma | “ | 0.120 | 0.136 |
| Share of the population 65 years and older living alone | “ | 0.257 | 0.265 |
| Share of the population speaking English less than “very well” | “ | 0.027 | 0.045 |
| Health delivery/system traits | |||
| Medicare Advantage penetration rate | AHRF, county | 0.169 | 0.302 |
| No. of short‐term acute beds per 1,000 population | “ | 2.267 | 2.500 |
| No. of minutes from ZCTA centroid to closest short‐term acute care hospital | Constructed from VHI PLD, ZCTA | 20.703 | NA |
| Share of the population residing in any Health Professional Shortage Area (HPSA) | Constructed from ACS and HRSA data, ZCTA | 0.16 | 0.20b |
| Measures of clinician access | |||
| Geographic accessibility measure (Primary care physician‐to‐population ratio) | VA BOM, ZCTA | 1: 1,149 | NA |
| Geographic accessibility measure (PCPs per 1,000 persons) | “ | 0.870 | NA |
| Bounded‐area accessibility measure (PCPs per to population ratio) | AHRF, county | 1 : 1,724 | NA |
| Bounded‐area accessibility measure (PCPs per 1,000 persons) | “ | 0.580 | NA |
| Bounded‐area accessibility measure (Non‐physician primary care clinicians to population ratio) | “ | 1 : 1,773 | NA |
| Bounded‐area accessibility measure (Non‐physician primary care clinicians per 1,000 persons) | “ | 0.564 | NA |
| Dependent variable | |||
| 2‐year preventable hospitalizations per 1,000 persons age 65 + | Constructed from VHI PLD, ZCTA | 95.066 | NA |
(a) For all but two variables, we report weighted means across the 843 zip code tabulation areas (ZCTAs) in our sample, using the ZCTA population as the weight. For population density, we report the total Virginia population divided by total Virginia land area. For distance to the closest hospital, we report the standard average rather than the weighted average. (b) Reported in Ollove (2013).
NA, not available.
Of the 896 total ZCTAs in Virginia, our sample excludes 9 ZCTAs with zero population, 27 ZCTAs missing estimates of the population aged 65 years and older,5 16 ZCTAs where median income or unemployment rate data were unavailable, and one ZCTA located in Tangier Island, a geographically isolated island accessible only by boat (see Table S2). Because these ZCTAs are sparsely populated or geographically small locations, 99.52 percent of the 2014 Virginia population resided in the remaining 843 ZCTAs in our sample.
We estimated multivariate regression models of the avoidable hospitalization rate in the ZCTA. Since the raw rates are highly skewed, we log transform the variable to normalize its distribution. We estimated OLS models and then spatial lag models to deal with the well‐known concern of spatial autocorrelation. In our context, spatial autocorrelation or dependence can arise if avoidable hospitalization rates in one area depend on avoidable hospitalization rates in another, or if neighboring areas’ rates are affected by some common unobserved factor not contained in our regression models. In any case, spatial autocorrelation can impact the estimation of the explanatory variable coefficients and their standard errors, thus leading to misinterpretation of the regression results (e.g., Anselin and Griffith 1988; Mobley et al. 2006; LeSage and Pace 2009). Since our geographic measure of accessibility does not incorporate counts of physicians in neighboring states, and thus may understate physician accessibility in ZCTAs that share a border with Maryland, North Carolina, West Virginia, Tennessee, and Washington, DC, we estimated models using both the full sample and a sample that excludes “edge‐ZCTAs.” As an additional sensitivity test, we estimated our models controlling for non‐physician primary care providers.
Results
Table 1 shows that, compared to the United States, our Virginia sample has a higher African American population share, a lower Hispanic population share, but a similar age and sex composition. Average socioeconomic status is higher in Virginia than in the United States overall. Our outcome of interest, the 2‐year avoidable hospitalization rate, has a population‐weighted average of 95 hospitalizations per 1,000 persons aged 65 years and over. Although an exact comparison to the United States is not possible with our data, published data cited earlier show that the Virginia 1‐year avoidable hospitalization rate among Medicare beneficiaries is lower than the U.S. rate.
Figure 1 illustrates the geographic variation in the avoidable hospitalization rate. The data display a spatial pattern of small and medium‐sized clusters of ZCTAs with either high or low avoidable hospitalization rates, which suggests weak‐to‐moderate positive spatial autocorrelation. This is confirmed by a positive and significant Moran's I statistic (0.389; p < .001). Figure 2 illustrates the variation in our geographic measure of PCP accessibility. The average ZCTA has a population‐weighted physician‐to‐population ratio of 1 : 1,149, or 0.87 physicians per 1,000 persons (Table 1). Figure 2 shows that geographic accessibility exhibits strong positive spatial autocorrelation, again confirmed by a positive and significant Moran's I statistic (0.74; p < .001). A visual comparison suggests that geographic accessibility of PCPs and avoidable hospitalization rates are inversely associated. Some areas with the lowest rates of avoidable hospitalization also have the highest rates of geographic accessibility (e.g., Northern Virginia), whereas others are marked by very high rates of avoidable hospitalization and fairly low rates of accessibility (e.g., areas along the West Virginia border). These patterns suggest some spatial dependence in preventable hospitalization rates and the potential for spillover effects.
Figure 1.
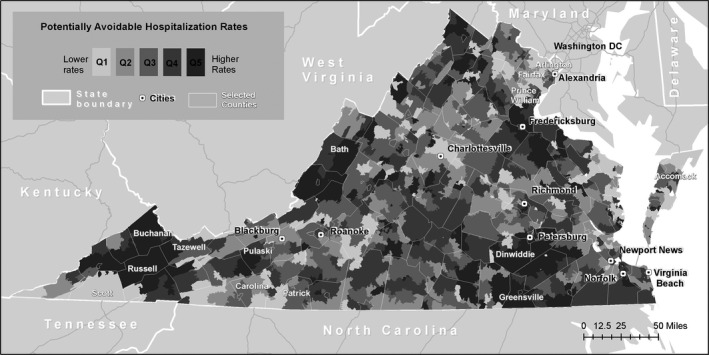
- Notes. This map displays the distribution of the 2‐year avoidable hospitalization rate per 1,000 persons age 65 years and older across Virginia ZCTAs. The quintile ranges are as follows: quintile 1, less than 59.3; quintile 2, greater than or equal to 59.3 and less than 81.0; quintile 3, greater than or equal to 81.0 and less than 102.9; quintile 4, greater than or equal to 102.9 and less than 139.1; and quintile 5, greater than or equal to 139.1.
Figure 2.
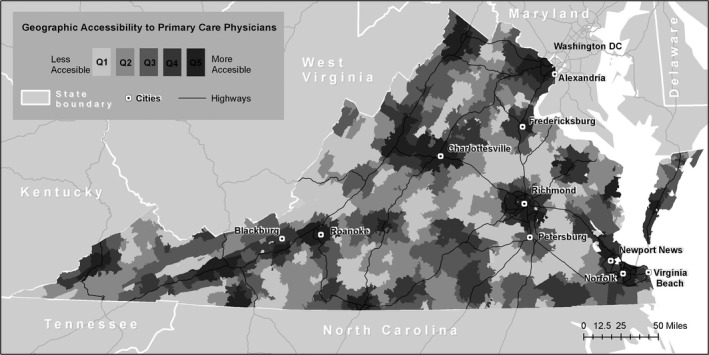
- Notes. This map displays the distribution of the E2SFCA‐based measure of the primary care physician to population ratio across Virginia ZCTAs. The quintile ranges are as follows: quintile 1, less than 0.282; quintile 2, greater than or equal to 0.282 and less than 0.441; quintile 3, greater than or equal to 0.441 and less than 0.641; quintile 4, greater than or equal to 0.641 and less than 0.941; and quintile 5, greater than or equal to 0.941.
Table 2 shows the results from multivariate regressions of the avoidable hospitalization rate among older adults. For brevity, we report the coefficients of the PCP access measures (full regression results are in Table S3). The first column uses the standard bounded‐area physician accessibility measure used in much of the literature, a simple ratio of the number of physicians in the county per 1,000 persons in the county; we estimate the model using OLS. The coefficient estimate is negative and statistically significant.
Table 2.
Associations between Primary Care Physician Access and Potentially Avoidable Hospitalization Rates
| OLS Model All ZCTAs | Spatial Model All ZCTAs | OLS Model All ZCTAs | Spatial Model All ZCTAs | Spatial Model All ZCTAs | Spatial Model Excluding Edge‐ZCTAs | |
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Bounded‐area measure of primary care access | −0.165* (0.088) | −0.042 (0.068) | ||||
| Geographic measure of primary care accessibility | −0.455*** (0.148) | −0.212** (0.088) | −0.201** (0.092) | −0.329*** (0.101) | ||
| Bounded‐area measure of non‐physician clinician access | −0.025 (0.059) | 0.008 (0.065) | ||||
| Spatial lag parameter (lambda) | 0.558*** (0.033) | 0.548*** (0.034) | 0.548*** (0.034) | 0.169*** (0.038) | ||
| Average direct effect of geographic PCP accessibility | −0.045 | −0.228 | −0.217 | −0.331 | ||
| Cumulative indirect effect of geographic PCP accessibility | −0.049 | −0.241 | −0.229 | −0.065 | ||
| Sample size | 843 | 843 | 843 | 843 | 843 | 717 |
The dependent variable is the log of (1 plus the count of avoidable hospitalizations) divided by 1,000 persons aged 65 years and older in the ZCTA. All models include measures of the shares of the population aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, female, African American, and of Hispanic ethnicity, population density, the share of the population residing in an urban area, the unemployment rate, median household income, the share of the population with incomes below 100% of the federal poverty level, the share of the population aged 25 years and older without a high school diploma, the share of the elderly population living alone, and the share of the population speaking English less than “very well,” the number of short‐term acute hospital beds per 1,000 persons, the Medicare Advantage penetration rate, the number of minutes to the closest hospital, and the share the population that resided in any primary care Health Professional Shortage Area or HPSA. Standard errors are reported in parentheses. OLS models report standard errors clustered at the level of the county or county equivalent. Statistical significance is indicated by * (0.1 level), ** (0.05 level), or *** (0.01 level).
We next tested the robustness of the negative association between the standard bounded‐area physician accessibility measure and the avoidable hospitalization rate upon accounting for spatial dependence. We examined two types of spatial models. The first is a spatial error model (SEM), which addresses correlation in the error terms of neighboring ZCTAs. The second is a spatial lag model, which addresses spatial spillovers or cases when outcomes in adjacent areas impact outcomes in the area of interest (Anselin 2002). To ascertain which of these models is appropriate for our data, we perform Lagrange Multiplier (LM) tests on the OLS residuals from the model shown in Table 2 column 1; the results suggest that a spatial lag model provides a better fit to the data than a SEM model.6 Column 2 of Table 2 then reports the results from the spatial lag model. Notably, the inverse relationship between a bounded‐area measure of PCP accessibility and avoidable hospitalizations is not robust to a model specification that accounts for spatial dependence. The coefficient estimate is close to zero and statistically insignificant.
In columns 3 and 4 of Table 2, we estimate the OLS and spatial models using our preferred measure of geographic accessibility. The OLS model shows a statistically significant relationship between geographic accessibility and lower avoidable hospitalization rates. LM tests from the OLS residuals again indicate that a spatial lag model is a good fit to the data. The spatial lag parameter estimate from this model is positive, consistent with positive spatial spillovers. Importantly, the spatial lag model shows that the inverse relationship between geographic accessibility and avoidable hospitalization rates is robust to accounting for spatial dependence.
In column 5, we test whether the inverse relationship between geographic accessibility and avoidable hospitalizations is robust to controlling for a bounded‐area measure of non‐physician primary care provider accessibility. Other research has shown that non‐physician provider accessibility is predictive of avoidable hospitalization rates (Lin, Eberth, and Probst 2016). When we add this control, we continue to find a significant inverse relationship between geographic accessibility and avoidable hospitalizations. In column 6, we exclude ZCTAs that share a border with another state or Washington, DC. Physician accessibility in those ZCTAs may be understated since physicians in neighboring areas outside of Virginia are not included in our data. The significant inverse relationship between geographic accessibility and avoidable hospitalizations is robust to this exclusion. In fact, the association becomes stronger when we exclude the edge‐ZCTAs. The size of the spatial spillover parameter estimate decreases, but it remains positive and significant.
To infer the size of the association between geographic accessibility and avoidable hospitalization rates, we calculate both average direct and cumulative indirect effects (Drukker, Prucha, and Raciborski 2013). The average direct effect is the change in the average area's avoidable hospitalization rate associated with a one‐unit change in geographic accessibility in that area. The cumulative indirect effect is the sum of the spillover changes in all other areas’ avoidable hospitalization rates associated with a one‐unit change in geographic accessibility in one area. According to the model reported in Table 2, column (6), a one standard‐deviation increase (or a 0.36 unit or 60 percent increase) in geographic accessibility is associated with an average direct reduction in the avoidable hospitalization rate of 12.6 percent and a cumulative indirect reduction of 2.5 percent.
Discussion
Millions of Americans have gained insurance coverage since the major provisions of the ACA took effect in 2014 (KFF). These gains in insurance coverage call for a closer examination of other dimensions of access such as the geographic proximity of providers to patients and providers’ willingness to accept patients’ insurance and to offer convenient appointment times. Consistent with this interest in addressing geographic access to care, the Affordable Care Act included temporary Medicare bonus payments to general surgeons providing care in shortage areas (Abrams et al. 2011). This policy built on numerous prior federal and state programs that use financial incentives to address health professional shortages.
As policy makers continue to seek effective tools to address the nonfinancial components of access to care, evidence linking physician accessibility to better health outcomes is needed. Prior studies provide mixed evidence of this connection in the population age 65 years and older, and more important, often rely on physician access measures that suffer from well‐known limitations. In this study, we combine spatial data and spatial methods to make two methodological improvements to prior studies on the link between avoidable hospitalizations and PCP access. We provide the first evidence from a statewide study to show that a geographic measure of primary care access is linked to lower avoidable hospitalization rates. Compared to bounded‐area density measures, unbounded network‐based geographic accessibility measures provide more robust evidence that avoidable hospitalizations are lower in areas with more PCPs per person. This finding represents the best available evidence to date of the inverse association between PCP access and preventable hospitalizations in the age 65 years and older population. As noted earlier, this is a particularly relevant age group to study given that nearly 100 percent of its members have health insurance through Medicare (regardless of a state's decision to expand Medicaid under the ACA). Thus, our results suggest that even in a population with a uniformly high level of financial access to care, there may be important health consequences arising from differences in spatial access.
One limitation of our study is worth noting especially. Because we use a single‐cross section and rely on naturally occurring variation in geographic accessibility, it is possible that there is an omitted factor correlated with both geographic accessibility and preventable hospitalizations, or that physician location is dependent on hospitalization rates. Thus, our work is best viewed as providing evidence of an association between geographic access and avoidable hospitalizations, as opposed to evidence of a causal link between the two. We do, however, provide the best available evidence of this association using a larger study area than previously employed and the only available evidence in the age 65 years and older population. We control for a wide range of covariates in our models, and we explicitly model the spatial relationships in the data. We tested the robustness of our findings to additional controls and changes in the sample (Table 2, columns 5 and 6) and to alternate geographic measures of access, defined from alternate assumptions of catchment time and distance decay (results available upon request). Future work should build on the robustness of our findings by both using a geographic measure of PCP access and leveraging quasi‐experiments that create variation in physician location, such as the aforementioned physician bonuses in the ACA.
Because we examine data from a single state, results may not be generalizable to the United States as a whole; that said, we use a measure of geographic accessibility to primary care defined for a larger geographic area in the United States than prior studies, which have been limited to physicians in large urban areas such as DC, Chicago, and Philadelphia (Teach et al. 2006; Fu et al. 2009; Luo and Qi 2009; Mathison et al. 2013; Brown et al. 2016). Furthermore, our geographic measure of accessibility does not include non‐physician providers of primary care. However, we do include a bounded‐area measure of non‐physician provider density in some models, and we show that our results are robust to this specification change. Lastly, we note that our findings may not necessarily extend to adults under age 65 years; however, most prior studies of younger adults in the United States also find evidence of inverse association between preventable hospitalization and physician access (Rosano et al. 2013).
In addition to showing that geographic measures of PCP accessibility play an important role, our work also shows that spatially adjusted models can make an important contribution. Results from our spatial lag models reveal the presence of positive spatial spillovers. Positive spatial spillovers can arise for various reasons. For example, PCPs in one area may share practice styles, making it such that lower rates of hospitalization in surrounding areas reduce own‐area hospitalization rates. Another explanation is that health behaviors in one area may be shared socially; for example, patients may share information about positive experiences with PCPs in their proximity, providing encouragement to use services more often, thus lowering hospitalization rates in surrounding areas and own areas alike (Mobley et al. 2006; Golgher and Voss 2015). Our results suggest that a significant part of the associations found in the OLS models are the result of spatial autocorrelation, not the correlation between the variables themselves. Methods that account for spatial autocorrelation can also be used to estimate the size of spatial spillovers. The positive spillovers we document suggest that state officials might expect investments in local health care (e.g., innovative practices that encourage patients to visit facilities more often) to have impacts on both targeted and neighboring localities.
More broadly, the findings from this study underline the importance of geographic accessibility for population health. Policy makers should consider investments in geographic accessibility (e.g., improving transportation for the elderly) and other efforts to improve the geographic distribution of PCPs as a potential means of reducing hospitalizations and health care spending and improving population health.
Supporting information
Appendix SA1. Author Matrix.
Appendix SA2. Details on the Construction of the Geographic Measure of Primary Care Physician Access.
Table S1. Correlation Coefficients.
Table S2. Breakdown of ZCTAs Excluded from the Analytic Sample.
Table S3. Full Regression Coefficients.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We are grateful for financial support from the Schroeder Center for Health Policy at the College of William & Mary and for excellent research assistance from Jimmy Cao. This study was reviewed and approved by the Protection of Human Subjects Committee at the College of William & Mary, where the work was performed.
Disclosures: None.
Disclaimer: None.
Notes
There is also an empirical literature on the relationship between PCP access and avoidable hospitalizations among adults of all ages; most studies find evidence of an inverse relationship between avoidable hospitalization and access to primary care (e.g., Saha et al. 2007; Rosano et al. 2013; and Gao et al. 2014).
The bounded‐area physician‐to‐population ratio is 1.28 PCPs per 1,000 persons in Virginia compared to 1.36 PCPs per 1,000 persons in the United States (KFF, 2016; U.S. Census Bureau, 2015). The avoidable hospitalization rate in Virginia is 43.6 per 1,000 Medicare beneficiaries, compared to 49.9 per 1,000 in the United States (United Health Foundation, 2016). Note that Virginia was one of many states that did not expand Medicaid to working‐aged adults (KFF, 2017).
Our license agreement requires us to include this statement: “VHI has provided non‐confidential patient‐level information used in this study which it has compiled in accordance with Virginia law but for which it has no authority to independently verify. By using this study, the user agrees to assume all risks that may be associated with or arise from the use of inaccurate data. VHI cannot and does not represent that the use of VHI's data was appropriate for this study or endorse or support any conclusions of inferences that may be drawn from the use of VHI's data.”
The correlation between population density and share of population residing in an urban area is 0.63. Our results are robust to excluding the share of population residing in an urban area from the set of explanatory variables.
In some cases, estimates are missing because the total population of the ZCTA is very small and the age‐specific estimate has a wide margin of error; in other cases, these ZCTAs are comprised of educational institutions, military facilities, and juvenile detention facilities.
This is because of the larger test statistics associated with the spatial lag model in both standard and robust LM tests and because the robust error test is not significant at the 5 percent level. Model 1 test statistics are as follows: [(Lag: 261.17; p value<.0001), (robust Lag: 20.58; p value<.0001)] versus [(Error: 244.35; p value<.0001), (robust Error: 3.76; p value<.052)]. Model 3 test statistics are as follows: [(Lag: 245.34; p value<.0001), (robust Lag: 24.47; p value<.0001)] versus [(Error: 223.26; p value<.0001), (robust Error: 2.39; p value<.122)].
References
- Abrams, M. , Nuzum R., Mika S., and Lawlor G.. 2011. Realizing Health Reform's Potential: How the Affordable Care Act Will Strengthen Primary Care and Benefit Patients, Providers, and Payers. Washington, DC: The Commonwealth Fund; pub. 1466, Vol. 1. [PubMed] [Google Scholar]
- AHRQ (Agency for Healthcare Research and Quality) . 2001. AHRQ Quality Indicators – Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: AHRQ; Pub. No. 02‐R0203. [Google Scholar]
- Anderson, R. M. 1995. “Revisiting the Behavioral Model and Access to Medical Care: Does It Matter?” Journal of Health and Social Behavior 36 (1): 1–10. [PubMed] [Google Scholar]
- Ansari, Z. 2007. “The Concept and Usefulness of Ambulatory Care Sensitive Conditions as Indicators of Quality and Access to Primary Health Care.” Australian Journal of Primary Health 13 (3): 91–110. [Google Scholar]
- Anselin, L. 2002. “Under the Hood Issues in the Specification and Interpretation of Spatial Regression Models.” Agricultural Economics 27 (3): 247–67. [Google Scholar]
- Anselin, L. , and Griffith D. A.. 1988. “Do Spatial Effects Really Matter in Regression Analysis?” Papers in Regional Science 65 (1): 11–34. [Google Scholar]
- Basu, J. , Mobley L. R., and Thumula V.. 2014. “The Small Area Predictors of Ambulatory Care Sensitive Hospitalizations: A Comparison of Changes over Time.” Social Work in Public Health 29 (2): 176–88. [DOI] [PubMed] [Google Scholar]
- Billings, J. , Zeitel L., Lukomnik J., Carey T. S., Blank A. E., and Newman L.. 1993. “Impact of Socioeconomic Status on Hospital Resource Use in New York City.” Health Affairs 12 (1): 162–73. [DOI] [PubMed] [Google Scholar]
- Bindman, A. B. , Grumbach K., Osmond D., Komaromy M., Vranizan K., Lurie N., J. Billings , and Stewart A.. 1995. “Preventable Hospitalization and Access to Health Care.” Journal of the American Medical Association 274 (4):305–11. [PubMed] [Google Scholar]
- Brown, E. J. , Polsky D., Barbu C. M., Seymour J. W., and Grande D.. 2016. “Racial Disparities in Geographic Access to Primary Care in Philadelphia.” Health Affairs 35 (8): 1374–81. [DOI] [PubMed] [Google Scholar]
- Chan, L. , Hart L. G., and Goodman D. C.. 2006. “Geographic Access to Health Care for Rural Medicare Beneficiaries.” Journal of Rural Health 22 (2): 140–6. [DOI] [PubMed] [Google Scholar]
- Chang, C. , Stukel T. A., Flood A. B., and Goodman D. C.. 2011. “Primary Care Physician Workforce and Medicare Beneficiaries’ Health Outcomes.” Journal of the American Medical Association 305 (20): 2096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf, B. T. , Neutens T., De Weert Y., and Van de Weghe N.. 2013. “Accessibility to Primary Health Care in Belgium: An Evaluation of Policies Awarding Financial Assistance in Shortage Areas.” BMC Family Practice 14: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker, D. M. , Prucha I. R., and Raciborski R.. 2013. “Maximum Likelihood and Generalized Spatial Two‐Stage Least‐Squares Estimators for a Spatial‐Autoregressive Model with Spatial‐Autoregressive Disturbances.” Stata Journal 13 (2): 221–41. [Google Scholar]
- Eberth, J. M. , Eschbach K., Morris J. S., Nguyen H. T., Hossain M. D. M., and Elting L. S.. 2014. “Geographic Disparities in Mammography Capacity in the South: A Longitudinal Assessment of Supply and Demand.” Health Services Research 49 (1): 171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L. Y. , Cowan N., McLaren R., Engstrom R., and Teach S. J.. 2009. “Spatial Accessibility to Providers and Vaccination Compliance among Children with Medicaid.” Pediatrics 124 (6): 1579–86. [DOI] [PubMed] [Google Scholar]
- Gao, J. , Moran E., Li Y., and Almenoff P. L.. 2014. “Predicting Potentially Avoidable Hospitalizations.” Medical Care 52 (2): 164–71. [DOI] [PubMed] [Google Scholar]
- Golgher, A. B. , and Voss P. R.. 2015. How to Interpret the Coefficients of Spatial Models: Spillovers, Direct and Indirect Effects. Spatial Demography 4: 175–205. [Google Scholar]
- Guagliardo, M. F. 2004. “Spatial Accessibility of Primary Care: Concepts, Methods, and Challenges.” International Journal of Health Geographics 3 (1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRSA . 2016. “Data Available for Download” [accessed on September 6, 2016]. Available at https://datawarehouse.hrsa.gov/data/dataDownload.aspx
- HRSA (Health Resources and Services Administration), Bureau of Health Professions . “Primary Medical Care HPSA Designation Criteria” [accessed on October 15, 2015]. Available at http://bhpr.hrsa.gov/shortage/hpsas/designationcriteria/primarycarehpsacriteria.html
- IOM (Institute of Medicine) . 1993. Access to Health Care in America. Edited by Millman M. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Joynt, K. E. , Gawande A. A., Orav E. J., and Jha A. K.. 2013. “Contribution of Preventable Acute Care Spending to Total Spending for High‐Cost Medicare Patients.” Journal of the American Medical Association 309 (24): 2572–78. [DOI] [PubMed] [Google Scholar]
- KFF (Kaiser Family Foundation) . “Status of State Action on the Medicaid Expansion Decision” January 2017 [accessed on February 20, 2017]. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0
- KFF (Kaiser Family Foundation) . “Key Facts about the Uninsured Population” [accessed on August 25, 2016]. Available at http://kff.org/uninsured/fact-sheet/key-facts-about-the-uninsured-population/
- KFF (Kaiser Family Foundation) . Kaiser State Health Facts. Total Professionally Active Physicians, April 2016 [accessed on August 15, 2016]. Available at http://kff.org/other/state-indicator/total-active-physicians/
- Khan, A. A. , and Bhardwaj S. M.. 1994. “Access to Health Care: A Conceptual Framework and its Relevance to Health Care Planning.” Evaluation & the Health Professions 17 (1): 60–76. [DOI] [PubMed] [Google Scholar]
- Krakuaer, H. , Jacoby I., Millman M., and Lukomik J. E.. 1996. “Physician Impact on Hospital Admission and on Mortality Rates in the Medicare Population.” Health Services Research 31 (2): 191–211. [PMC free article] [PubMed] [Google Scholar]
- Kulgren, J. T. , and McLaughlin C. G.. 2010. “Beyond Affordability: The Impact of Nonfinancial Barriers on Access for Uninsured Adults in Three Diverse Communities.” Journal of Community Health 35: 240–48. [DOI] [PubMed] [Google Scholar]
- Kulgren, J. T. , McLaughlin C. G., Mitra N., and Armstrong K.. 2012. “Nonfinancial Barriers and Access to Care for U.S. Adults.” Health Services Research 47 (1): 462–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laditka, J. N. 2004. “Physician Supply, Physician Diversity, and Outcomes of Primary Health Care for Older Persons in the United States.” Health & Place 10: 231–44. [DOI] [PubMed] [Google Scholar]
- LeSage, J. , and Pace R. K.. 2009. Introduction to Spatial Econometrics. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Lin, Y. , Eberth J. M., and Probst J. C.. 2016. “Ambulatory‐Care Sensitive Condition Hospitalizations among Medicare Beneficiaries.” American Journal of Preventive Medicine 51 (4): 493–501. [DOI] [PubMed] [Google Scholar]
- Luo, W. , and Qi Y.. 2009. “An Enhanced two‐Step Floating Catchment Area (E2SFCA) Method for Measuring Spatial Accessibility to Primary Care.” Health & Place 15: 1100–7. [DOI] [PubMed] [Google Scholar]
- Mathison, D. J. , Chamberlain J. M., Cowan N. M., Engstrom R. N., Fu L. Y., Shoo A., and Teach S. J.. 2013. “Primary Care Spatial Density and Nonurgent Emergency Department Utilization: A New Methodology for Evaluating Spatial Access to Care.” Academic Pediatrics 13 (3): 278–85. [DOI] [PubMed] [Google Scholar]
- Missouri Census Data Center . 2012. “Mable/Geocorr12: Geographic Correspondence Engine” [accessed on August 22, 2016]. Available at http://mcdc.missouri.edu/websas/geocorr12.html
- Mobley, L. R. , Root E., Anselin L., Lozano‐Gracia N., and Koschinsky J.. 2006. “Spatial Analysis of Elderly Access to Primary Care Services.” International Journal of Health Geographics 5 (1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar, P. , Nguyen A. T., Appenteng B., and Yu F.. 2012. “Preventable Hospitalizations: Does Rurality or Non‐Physician Clinician Supply Matter?” Journal of Community Health 37: 487–94. [DOI] [PubMed] [Google Scholar]
- Ollove, M . 2013. “Are There Enough Doctors for the Newly Insured? The Pew Charitable Trusts” [accessed on August 22, 2016]. Available at http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2013/12/30/are-there-enough-doctors-for-the-newly-insured
- Ricketts, T. C. , Randolph R., Howard H. A., Pathman D., and Carey T.. 2001. “Hospitalization Rates as Indicators of Access to Primary Care.” Health & Place 7: 27–38. [DOI] [PubMed] [Google Scholar]
- Rosano, A. , Loha C. A., Falvo R., van der Zee J., Ricciardi W., Guasticchi G., and de Belvis A. G.. 2013. “The Relationship between Avoidable Hospitalization and Accessibility to Primary Care: A Systematic Review.” European Journal of Public Health 23 (3): 356–60. [DOI] [PubMed] [Google Scholar]
- Rosenthal, M. B. , Zaslavsky A., and Newhouse J. P.. 2005. “The Geographic Distribution of Physicians Revisited.” Health Services Research 40 (6): 1931–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S. , Solotaroff R., Oster A., and Bindman A. B.. 2007. “Are Preventable Hospitalizations Sensitive to Changes in Access to Primary Care? The Case of the Oregon Health Plan.” Medical Care 45 (8): 712–9. [DOI] [PubMed] [Google Scholar]
- Schreiber, S. , and Zielinski M. S.. 1997. “The Meaning of Ambulatory Care Sensitive Admissions: Urban and Rural Perspectives.” Policy, Practice, and Research Briefs 13 (4): 276–84. [DOI] [PubMed] [Google Scholar]
- Segal, M. , Rollins E., Hodges E., and Roozeboom M.. 2014. “Medicare‐Medicaid Eligible Beneficiaries and Potentially Avoidable Hospitalizations.” Medicare and Medicaid Research and Review 4 (1): E1–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teach, S. J. , Guagliardo M. F., Crain E. F., McCarter R. J., Quint D. M., Shao C., and Joseph J. G.. 2006. “Spatial Accessibility of Primary Care Pediatric Services in an Urban Environment: Association with Asthma Management and Outcome.” Pediatrics 117 (4): S78–S85. [DOI] [PubMed] [Google Scholar]
- Texas A&M Geoservices . “About Our Services” [accessed on September 23, 2016]. Available at http://geoservices.tamu.edu/About/
- UDS Mapper . “HealthLandscape. ZIP Code to ZCTA Crosswalk” [accessed on August 22, 2016]. Available from: http://udsmapper.org/zcta-crosswalk.cfm
- United Health Foundation . 2016. “America's Health Rankings” [accessed on February 1, 2017]. Available at http://www.americashealthrankings.org/ALL
- U.S. Census Bureau . “Urban and Rural Classification” [accessed on August 22, 2016]. Available at https://www.census.gov/geo/reference/urban-rural.html
- U.S. Census Bureau . “Annual Estimates of the Resident Population: April 1, 2010 to July 1, 2015.” Released December 2015 [accessed on August 15, 2016]. Available at http://factfinder.census.gov
- Virginia Board of Medicine . “Practitioner Information, Doctor Profile Data” [accessed on August 1, 2014]. Available at http://www.dhp.virginia.gov/downloads/profiledata.asp
- Weissman, J. S. , Gatsonis C., and Epstein A. M.. 1992. “Rates of Avoidable Hospitalization by Insurance Status in Massachusetts and Maryland.” Journal of the American Medical Association 268 (17): 2388–94. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1. Author Matrix.
Appendix SA2. Details on the Construction of the Geographic Measure of Primary Care Physician Access.
Table S1. Correlation Coefficients.
Table S2. Breakdown of ZCTAs Excluded from the Analytic Sample.
Table S3. Full Regression Coefficients.


