Abstract
Objective
To compare alternative strategies for specifying cancer‐free control cohorts for estimating cancer‐attributable costs of care.
Data Source, Study Design, Data Extraction
Secondary data analysis of Surveillance, Epidemiology, and End Results data linked to Medicare claims among patients diagnosed with colorectal, lung, breast, and prostate cancers, 2007–2011. We estimated cancer‐attributable costs using three alternative reference cohorts: (1) noncancer Medicare patients individually matched by demographic characteristics, (2) noncancer patients individually matched on demographic factors and comorbidity score, (3) cancer patients as their own control, using prediagnosis costs.
Principal Findings
Among 44,266 colorectal, 61,584 lung, 55,921 breast, and 67,733 prostate patients, mean total Medicare spending in the first year of diagnosis was $59,496, $54,261, $31,895, and $26,305, respectively. Estimates of cancer‐attributable costs ranged from 79 percent to 82 percent of spending for colorectal, 76 percent–79 percent for lung, 65 percent–74 percent for breast, and 60 percent–75 percent for prostate cancers, depending on the reference cohort used. For all cancers, estimates were higher when patients were used as their own control, compared to demographic and comorbidity‐matched controls.
Conclusions
Choice of reference group can have a substantial impact on proportion of total costs attributed to cancer and should be clearly defined in analyses of the costs of cancer care.
Keywords: Cancer, costs, Medicare
Direct costs of cancer care have been estimated to be ~$100 billion in 2010–2011, comprising over 5 percent of U.S. health care spending (Jemal et al. 2009; Mariotto et al. 2011). Rising health care costs, in turn, have driven the growth of alternative payment models intended to better align providers' financial incentives with the goal of controlling costs. As providers take on a greater burden of risk with alternative payment arrangements, the ability to accurately estimate and assign costs to particular diagnoses will be of increasing importance for allocating resources and for determining reimbursements.
Although many investigators have estimated the costs of medical care attributable to cancer, there has been no consistent and standard methodology used, and methods have varied in terms of the data sources used, patient populations studied, and types of costs included (Brown et al. 2002; Barlow 2009; Riley 2009; Krahn et al. 2010; Tangka et al. 2010; Yabroff et al. 2011; Zheng et al. 2016). Not only does this make critical evaluation of cost estimates challenging, but it also leads to diverging estimates and comparisons among studies difficult. For example, one approach, as used by the Medical Expenditure Panel Survey, categorizes individual costs as cancer versus noncancer based on the service item (Shakespeare et al. 2003). However, this method is resource‐intensive to implement and also limited by the fact that multiple conditions may contribute to the same cost, or a particular condition may influence the costs associated with treating other diagnoses. Therefore, costs are not mutually exclusive, and the same expense could be assigned to multiple conditions.
Another common method, rather than assigning costs based on service item, attempts to calculate “net costs” by comparing costs of a cancer cohort to a noncancer control group (Yabroff et al. 2008). This approach has the advantages of using more readily available claims data, being less time‐intensive to implement, and arriving at an estimate that more closely approximates the incremental cost of having cancer.
However, defining an appropriate noncancer comparison group for calculating net costs is not straightforward, as it is not possible to observe an identical patient over an identical time period in both a cancer and noncancer state. Comparison groups frequently used in the literature include (1) matched noncancer patients and (2) patients as their own control using a pre‐cancer diagnosis period. When using a noncancer comparison group, patients have typically been matched based on readily available demographic characteristics, such as age, gender, race, and place of residence. Less frequently, noncancer patients have also been matched based on clinical characteristics, such as comorbidities, using a variety of matching schemes, such as individual versus frequency matching (Mandrekar and Mandrekar 2015), to identify controls. Although a variety of comparison cohorts have been used in the literature, it is not clear to what extent choice of comparison cohort influences the estimation of cancer‐attributable costs (Barron et al. 2008; Krahn et al. 2010; Tangka et al. 2010; Cipriano et al. 2011; Mariotto et al. 2011; McMahon et al. 2011; Gruber, Stock, and Stollenwerk 2012; Mittmann et al. 2014).
In this study, we examine estimates of cancer‐attributable costs based on the use of comparison cohorts and measure how alternative specifications of cancer‐free control cohorts influence these estimates.
Methods
Data Sources
We used data from the National Cancer Institute's SEER cancer registries linked to Medicare claims data. SEER registries collect data on patient demographics, cancer site, stage, histology, and dates of diagnosis and death. Medicare claims, both inpatient and outpatient, have been linked to SEER for patients over age 65. SEER data for patients diagnosed from January 1, 2007, through December 31, 2011, were linked to data from Medicare Parts A, B, and D, including claims from hospice, durable medical equipment (DME), and home health (HHA) from December 1, 2005, through December 31, 2012.
Study Cohorts
The cancer patient cohort included Medicare‐enrolled patients over age 66 diagnosed with colorectal, lung, breast, or prostate cancer from 2007 to 2011 in a SEER surveillance area. Subjects were continuously enrolled in Medicare Parts A and B and not in an HMO from 13 months prior to diagnosis until death or through 2012 for surviving patients.
Three comparison cohorts were constructed: (1) noncancer patients individually matched based on demographic characteristics, (2) noncancer patients individually matched on demographic characteristics and comorbidity score, and (3) cancer patients in the prediagnosis period as their own control. Individual matching was used over frequency matching, because analyses by stage and between the comparison cohorts required cancer cases to be individually “paired” with controls.
The matched noncancer patient comparison cohorts included Medicare‐enrolled patients drawn from a random 5 percent sample of Medicare beneficiaries residing in the SEER areas and without a SEER cancer diagnosis from 2007 to 2011. The first matched comparison cohort was constructed by selecting noncancer patients that were individually matched in a 3 : 1 ratio to cancer patients based on demographic characteristics only (age group: 66–69, 70–74; 75–79, 80+; gender; race: white, black, other; and SEER region: Northwest, South, Midwest, West). In the second comparison cohort, patients were matched on demographic characteristics in addition to a modified Charlson comorbidity score (0, 1, 2, 3, 4–5, 6–8, 9+) for the year prior to diagnosis using Deyo's implementation (Deyo, Cherkin, and Ciol 1992) of the Charlson score (Charlson et al. 1987) applied to both inpatient and outpatient claims, as suggested by Klabunde for cancer patients (Klabunde et al. 2000).
The third comparison cohort, the “own comparison” cohort, was identical to the cancer patient cohort, but inflation‐adjusted costs in the year prior to diagnosis were used as comparison to estimate costs in the absence of a known cancer. Thus, we required all study patients to be Medicare eligible for at least 13 months prior to their diagnosis date.
All three comparison “control” cohorts were matched on demographic characteristics (Table S1). We summarize the modified Charlson comorbidity score and percent of patients surviving to the end of the comparison period for the cancer and control cohorts in Table 1.
Table 1.
Comorbidity and Survival of Cancer and Comparison Cohorts
| Colorectal | Lung | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer Case | Comparison Cohort | Cancer Case | Comparison Cohort | |||||
| Noncancer Control | Own Control | Noncancer Control | Own Control | |||||
| (3 : 1 Match) | (3 : 1 Match) | |||||||
| Demoa | Demo + Comorbb | Demoa | Demo + Comorbb | |||||
| N | 44,266 | 132,678 | 132,678 | 44,266 | 61,584 | 184,752 | 184,752 | 61,584 |
| Survival to end of year 1e | 75% | 95% | 94% | 100% | 42% | 96% | 95% | 100% |
| Modified Charlson comorbidityd | ||||||||
| 0 | 53% | 56% | Identical to cancer casesc | 43% | 58% | Identical to cancer casesc | ||
| 1 | 23% | 23% | 28% | 22% | ||||
| 2 | 9% | 8% | 13% | 8% | ||||
| 3 | 5% | 5% | 6% | 5% | ||||
| 4–5 | 4% | 4% | 5% | 3% | ||||
| 6–8 | 1% | 1% | 2% | 1% | ||||
| 9+ | 0% | 0% | 0% | 0% | ||||
| Breast | Prostate | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer Case | Comparison Cohort | Cancer Case | Comparison Cohort | |||||
| Noncancer Control | Own Control | Noncancer Control | Own Control | |||||
| (3 : 1 Match) | (3 : 1 Match) | |||||||
| Demoa | Demo + Comorbb | Demoa | Demo + Comorbb | |||||
| N | 55,921 | 167,763 | 167,763 | 55,921 | 67,733 | 203,199 | 203,199 | 67,733 |
| Survival to end of year 1e | 94% | 96% | 96% | 100% | 96% | 96% | 97% | 100% |
| Modified Charlson comorbidityd | ||||||||
| 0 | 61% | 59% | 63% | 59% | ||||
| 1 | 23% | 23% | 21% | 21% | ||||
| 2 | 7% | 8% | 6% | 7% | ||||
| 3 | 4% | 4% | Identical to cancer casesc | 4% | 5% | Identical to cancer casesc | ||
| 4–5 | 2% | 3% | 2% | 4% | ||||
| 6–8 | 0% | 1% | 0% | 1% | ||||
| 9+ | 0% | 0% | 0% | 0% | ||||
*Individually matched three noncancer controls to each cancer case based on demographics (age group: 66–69, 70–74; 75–79, 80+; gender; race: white, black, other; and SEER region: Northwest, South, Midwest, West).
Individually matched three noncancer controls to each cancer case based on demographic (see above) + comorbidity group (0, 1, 2, 3, 4–5, 6–8, 9+).
Due to study design, the distribution of comorbidity and demographics of these comparison cohorts were identical to those of the cancer cases for the specified strata, although, for the cancer patients’ “own control” cohort, controls were exactly one year younger.
Modified Charlson comorbidity score for the year prior to (pseudo) diagnosis using Deyo's implementation of the Charlson score applied to both inpatient and outpatient claims, as suggested by Klabunde for cancer patients.
For cancer cases and matched noncancer controls, the comparison period is defined as one month prior to the (pseudo) diagnosis date through 11 months following diagnosis; for the cancer patients’ “own control” cohort, the comparison period is defined as 13 months through 2 months prior to diagnosis.
Analysis
We calculated total monthly Medicare costs in the first year of diagnosis among patients with each cancer, using Medicare claims data from Parts A, B, and D files, including MedPAR, Carrier/NCH, Outpatient, DME, HHA, Hospice, and Part D files. All cancers reported to the SEER program, including in situ cancers, were included in cost estimates. Monthly costs were calculated irrespective of survival, meaning that patients dying in the first year of diagnosis contributed $0 monthly costs after death and remained in the denominator. The first year of diagnosis was defined as starting from one month prior to the diagnosis date (month −1) recorded by the SEER program until 11 months following diagnosis (month 10). For each noncancer patient, a “pseudodiagnosis” date was assigned in the year of diagnosis, and all costs for the matched noncancer cohorts were calculated relative to that date. For the cancer patients’ “own comparison” cohort, costs were calculated in the prediagnosis period starting from 13 months (month −13) until 2 months prior to diagnosis (month −2). All costs were inflation‐adjusted to 2013 U.S.$ based on the federal hospital insurance (HI) and supplementary medical insurance (SMI) average per beneficiary costs (United States, Board of Trustees of the Federal Hospital Insurance Trust Fund; and United States, Board of Trustees of the Federal Supplementary Medical Insurance Trust Fund).
Cancer‐attributable costs were estimated by subtracting monthly costs for a patient from one of the three comparison cohorts from monthly costs for the cancer patient. Thus, for a given patient, in year 1 (t=months from diagnosis):
Mean cancer‐attributable costs in the first year were calculated by averaging cancer‐attributable costs, as calculated above, among all patients. Median cancer‐attributable costs were calculated by taking the median of the cancer‐attributable costs over the full year among all patients. We then calculated the proportion of cancer patients’ total Medicare costs that were attributable to cancer. Sensitivity analyses were performed in which patients were censored at death and mean monthly cancer‐attributable costs were calculated only among surviving patients.
Mean differences in cancer‐attributable costs across the three comparison cohorts were compared by t‐test for the noncensored analysis (i.e., dying patients remaining in the denominator for all months) and by bootstrapping with 1,000 replicates for the censored analysis (i.e., dying patients removed from the denominator after their month of death).
Analyses were performed separately for each cancer type. Notably, attributable costs are distinct from costs; specifically, it is possible for attributable costs to be negative. Although analyses of costs are typically performed using log‐transformation, we used the nontransformed attributable costs as the dependent variable; due to the large sample size, this analysis is robust to deviations from the normal distribution. p‐values were two‐sided, and values less than .05 were considered statistically significant. SAS software (version 9.4; SAS Institute, Cary, NC, USA) was used for all analyses.
Results
We identified 44,266 colorectal, 61,584 lung, 55,921 breast, and 67,733 prostate cancer patients diagnosed from 2007 to 2011 in the SEER surveillance areas who satisfied our inclusion criteria. All three comparison cohorts were well matched on the demographic characteristics of the cancer patients (Table S1). Modified Charlson comorbidity scores and survival from diagnosis are summarized in Table 1. Compared to noncancer comparison cohorts matched only on demographic characteristics, lung and colorectal cancer patients were less likely to have modified Charlson comorbidity score 0 (43 percent vs. 58 percent and 53 percent vs. 56 percent, respectively), whereas breast and prostate cancer patients were more likely to have comorbidity score 0 (61 percent vs. 59 percent and 63 percent vs. 59 percent, respectively). One‐year survival was similar for the demographic and demographic + comorbidity‐matched cohorts, with the largest differences versus cancer patients being observed for lung and colorectal cancer patients.
Mean total Medicare spending in the first year of diagnosis was $59,496, $54,261, $31,895, and $26,305 for colorectal, lung, breast, and prostate cancer patients, respectively (Figure 1). Median spending was lower than mean spending for all cancers ($46,171, $44,052, $24,283, and $21,475, respectively). By the end of the first year, 25 percent, 58 percent, 6 percent, and 4 percent of colorectal, lung, breast, and prostate patients had died, respectively.
Figure 1.
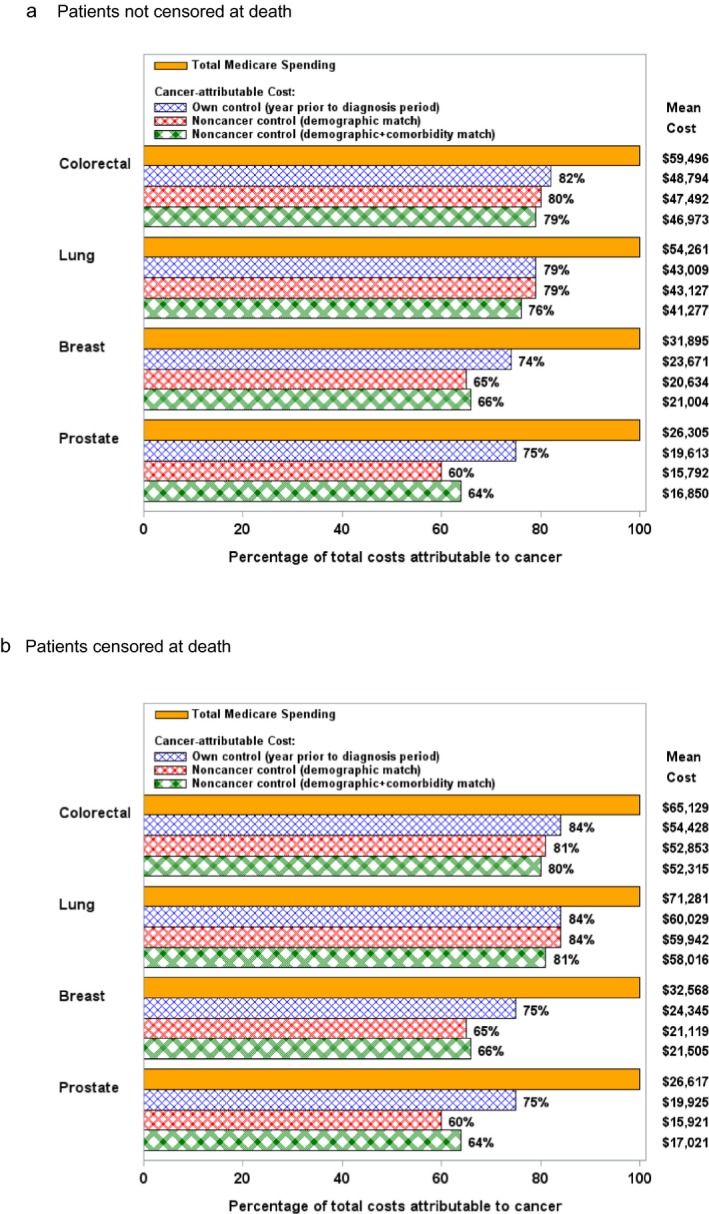
Mean Total Costs Attributed to Cancer, by Comparison Cohort Used [Color figure can be viewed at http://wileyonlinelibrary.com]
Notes. Mean cancer‐attributable costs have been adjusted to 2013 U.S. dollars. Mean difference in cancer‐attributable costs across the three comparison cohorts were compared by t‐test for the noncensored analysis (i.e., dying patients remaining in the denominator for all months) and by bootstrapping with 1,000 replicates for the censored analysis (i.e., dying patients removed from the denominator after their month of death). For the noncensored analysis, all p‐values for comparisons between comparison cohort pairs were ≤.01, except the comparison between own control versus noncancer control by demographic match (p = 0.6) and the comparison between noncancer control by demographic match versus demographic + comorbidity match (p = .02) for lung cancer. For the censored analysis, all p‐values for comparisons between comparison cohort pairs were ≤.01.
Figure 1 presents a comparison of cancer‐attributable costs, calculated using the three comparison cohorts described. When comparing cancer‐attributable costs that were calculated using the noncancer cohort matched on demographic characteristics only versus the noncancer cohort matched on both demographic characteristics and comorbidity score, we found that adjustment for comorbidity increased the percentage of total costs attributable to cancer for prostate (64 percent vs. 60 percent, $1,059 difference in attributable cost, 95 percent CI: $843–$1,274, p < .01) and breast (66 percent vs. 65 percent, $370 difference in attributable cost, 95 percent CI: $280–$462, p < .01) cancer patients, but decreased cancer‐attributable costs for lung (76 percent vs. 79 percent, $1,850 difference in attributable cost, 95 percent CI: $1,520–$2,180, p < .01) and colorectal (79 percent vs. 80 percent, $520 difference in attributable cost, 95 percent CI: $79–$959, p = .02) cancer patients.
When sensitivity analysis was performed with patients removed from the denominator following death, the percentage of total costs attributed to cancer increased for lung and colorectal cancers, but the differences in estimates of cancer‐attributable costs by comparison cohort persisted. Figure 1a,b show results without and with censoring at the time of death, respectively.
When comparing cancer‐attributable costs that were calculated using cancer patients in the year prior to diagnosis as their own control versus the noncancer cohort matched on demographics and comorbidities, we found that the percentage of total costs attributed to cancer was higher when using cancer patients as their own control for all four cancer types, 82 percent vs. 79 percent for colorectal, 79 percent vs. 76 percent for lung, 74 percent vs. 66 percent for breast, and 75 percent vs. 64 percent for prostate cancers (p < .01 for all differences in attributable cost).
Mean cancer‐attributable costs returned close to baseline by the end of the first year. For example, using a demographic and comorbidity‐matched noncancer control, mean cancer‐attributable costs ranged from $357 to $678 in the final month of our analysis period for the four cancers. Mean cancer‐attributable costs by month from diagnosis for the demographic and comorbidity‐matched noncancer control are shown in Figure S1.
Figure 2 presents a comparison of cancer‐attributable costs by stage of cancer. As expected, cancer‐attributable costs were lower for early‐stage disease, likely due to less intensive treatments required. Although the relationships between estimates calculated using the demographic versus demographic and comorbidity‐matched noncancer comparison cohorts varied by stage, estimates for cancer‐attributable costs remained higher when using cancer patients as their own control, compared to a demographic and comorbidity‐matched noncancer comparison group.
Figure 2.
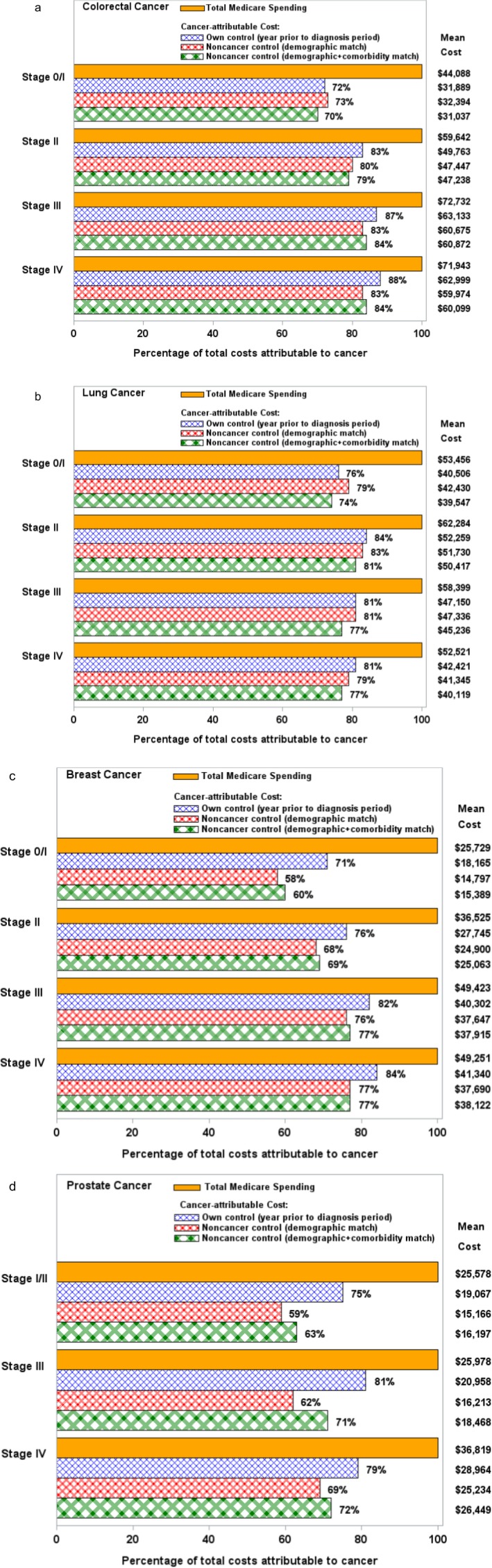
Total Costs Attributed to Cancer by Cancer Stage, by Comparison Cohort Used [Color figure can be viewed at http://wileyonlinelibrary.com]
Notes. (a) Mean cancer‐attributable costs have been adjusted to 2013 U.S. dollars. All p‐values for comparisons between comparison cohort pairs were ≤.01, except as follows: stage 0/I—own control versus noncancer control by demographic match (p = .33), own control versus noncancer control by demographic + comorbidity match (p = .10); stages II, III, and IV—noncancer control by demographic versus demographic + comorbidity match (p = .65, p = .67, p = .82, respectively). (b) Mean cancer‐attributable costs have been adjusted to 2013 U.S. dollars. All p‐values for comparisons between comparison cohort pairs were ≤.01, except as follows: stage 0/I—own control versus noncancer control by demographic + comorbidity match (p = .08); stage II—own control versus noncancer control by demographic match (p = .64), own control versus noncancer control by demographic + comorbidity match (p = .11), noncancer control by demographic versus demographic + comorbidity match (p = .12); stage III—own control versus noncancer control by demographic match (p = .68). (c) Mean cancer‐attributable costs have been adjusted to 2013 U.S. dollars. All p‐values for comparisons between comparison cohort pairs were ≤.01, except as follows: stages II, III, and IV—noncancer control by demographic versus demographic + comorbidity match (p = .52, p = .59, p = .52, respectively). (d) Mean cancer‐attributable costs have been adjusted to 2013 U.S. dollars. All p‐values for comparisons between comparison cohort pairs were ≤.03.
Discussion
Using data from SEER‐Medicare, we calculated cancer‐attributable costs for the four most common cancers in the United States, using three common comparison cohorts: (1) noncancer patients matched on demographic characteristics, (2) noncancer patients matched on demographic characteristics and comorbidity score, and (3) cancer patients in the prediagnosis period. We found that choice of comparison cohort substantially influenced the proportion of patients’ total medical costs attributable to cancer in the first year of diagnosis. When using noncancer patients as the comparison cohort, controlling for comorbidities in addition to demographic characteristics increased estimates of cancer‐attributable costs in prostate and breast cancers and decreased estimates in lung and colorectal cancers. Using cancer patients in the prediagnosis period resulted in higher cancer‐attributable costs for all four cancer types, compared to a demographic and comorbidity‐matched noncancer comparison cohort. Our analysis showed the greatest variation in prostate cancer: between 60 percent and 75 percent of total Medicare costs were attributed to cancer, depending on the comparison group that was selected. As expected, estimates of cancer‐attributable costs were higher in lung and colorectal cancers, when patients’ costs were censored at death. However, the differences in estimates observed when using the three different comparison cohorts remained similar, regardless of whether patients’ costs were censored at death or not.
Our results suggest that the consequences of choosing a comparison cohort in estimating cancer‐attributable costs depend on the population and time horizon being analyzed. For example, attributable cost estimates derived from models that match only on demographic characteristics are likely to overestimate cancer‐attributable costs for patients who typically have more comorbid conditions than the general population, such as lung and colorectal cancer patients. Conversely, this strategy underestimates cancer‐attributable costs for patients who typically have fewer comorbid conditions than the general population, such as prostate and breast cancer patients. This might also reflect a higher propensity for healthier patients to undergo prostate and breast cancer screening, leading to greater rates of cancer diagnosis. As expected, cancer patients whose underlying comorbidity scores were closest to demographic‐matched controls (e.g., breast and colorectal patients) had only minor differences in cancer‐attributable costs.
Additionally, cancer‐attributable costs are more likely to be influenced by choice of comparison cohort when treatment costs are lower. We found the greatest variation in costs by comparison cohort for prostate cancer patients, in whom extended courses of lower‐intensity treatment are common. Conversely, the choice of comparison cohort had a smaller impact in patients with high‐cost cancers, such as lung cancer. Likewise, in the months immediately following diagnosis, estimates of cancer‐attributable costs are likely to be dominated by high‐cost initial treatments and less influenced by choice of comparison cohort.
Thus, although the addition of a comorbidity match to a demographic match may yield more accurate attributable cost estimates when using a noncancer patient control group, for high‐cost cancers or in situations when costs are being estimated over a short interval following diagnosis when cancer costs are likely to predominate, omitting the comorbidity match may yield reasonable estimates.
Another strategy to estimate cancer‐attributable costs is to calculate costs for patients in the prediagnosis period and compare them to costs for the same patients in the postdiagnosis period (McMahon et al. 2011). When we used this method, we found that, for all four cancer types, cancer‐attributable costs were higher than when a demographic and comorbidity‐matched noncancer comparison cohort was used. This was an unexpected result, as one might expect cancer patients have underlying disease even prior to diagnosis that could result in higher control group costs in the year prior to diagnosis. One possible reason for this observation is that, when constructing a comparison group using cancer patients as their own control, cancer patients are not allowed to die until the month of diagnosis. In contrast, patients in the noncancer cohorts can die at any point after their “pseudodiagnosis” date. Consequently, all end‐of‐life costs occurring after diagnosis count as cancer‐attributable when patients are used as their own comparison group, but not necessarily so for a noncancer comparison group. Although one might expect this effect to be much greater in poor‐prognosis cancers, we observed greater cancer‐attributable costs for all four cancer types. Many screening studies have demonstrated a “healthy volunteer effect,” in which participants in cancer screening programs tend to be in higher socioeconomic groups, lead healthier lifestyles, and live longer than nonparticipants (Pinsky et al. 2007; Croswell, Ransohoff, and Kramer 2010). This effect may also explain why some cancer patients might have lower costs in the year prior to diagnosis than matched noncancer patients. Indeed, we observed the most striking difference in cost estimates for early‐stage breast and prostate cancer patients, in whom screen‐detected cancers may be responsible for a higher proportion of the total number of cases.
There are several limitations to our study. We confined our analysis to comparing the effect of commonly used comparison cohorts on the calculation of cancer‐attributable costs. Our analysis was limited to Medicare patients with the four most common cancers, and the availability of information on demographic and comorbid conditions was limited by the claims data. Although our results are relevant for the largest group of patients diagnosed with cancer, it is possible that differences in comparison groups might be smaller or larger for other types of cancers or groups on patients.
Nonetheless, our study demonstrates the need for careful evaluation of comparison cohorts used when calculating attributable costs, including careful consideration of underlying characteristics of the populations being studied and the intent of the analysis. When using noncancer controls, we suggest matching on comorbid characteristics in addition to demographic characteristics, whenever feasible, although a demographic‐only match is a reasonable alternative in cancers that do not have a strong link to other risk factors that are also associated with comorbidities, for example, smoking, or in situations where cancer‐related costs are likely to dominate noncancer‐related costs. Furthermore, we observed that using patients as their own controls led to consistently higher estimates due to the factors discussed above. It is also possible that costs incurred as a result of a not‐yet‐diagnosed cancer could affect costs in the year prior to diagnosis when using cancer patients as their own control.
Estimating and attributing costs from readily available administrative data will become increasingly important for shaping reimbursement and payment reform. The results of our analysis underscore the need for clearly delineating comparison groups in analyses of cost and value so that estimates of cancer costs can be critically evaluated and clearly understood.
Supporting information
Appendix SA1: Author Matrix.
Figure S1. Mean Cancer‐Attributable Costs, by Month from Diagnosis (Demographic and Comorbidity Matched Non‐Cancer Control Cohort).
Table S1. Characteristics of Cancer and Comparison Cohorts.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was supported by grants from the Gloria Spivak Fund and the American Society for Radiation Oncology. This study used the linked SEER‐Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER‐Medicare database. Jennifer Wind provided exceptional project management.
Disclosures: None.
Disclaimer: None.
References
- Barlow, W. E. 2009. “Overview of Methods to Estimate the Medical Costs of Cancer.” Medical Care 47 (7 Suppl 1): S33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron, J. J. , Quimbo R., Nikam P. T., and Amonkar M. M.. 2008. “Assessing the Economic Burden of Breast Cancer in a US Managed Care Population.” Breast Cancer Research and Treatment 109 (2): 367–77. [DOI] [PubMed] [Google Scholar]
- Brown, M. L. , Riley G. F., Schussler N., and Etzioni R.. 2002. “Estimating Health Care Costs Related to Cancer Treatment From SEER‐Medicare Data.” Medical Care 40 (8 Suppl.): IV‐104‐117. [DOI] [PubMed] [Google Scholar]
- Charlson, M. E. , Pompei P., Ales K. L., and MacKenzie C. R.. 1987. “A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation.” Journal of Chronic Diseases 40 (5): 373–83. [DOI] [PubMed] [Google Scholar]
- Cipriano, L. E. , Romanus D., Earle C. C., Neville B. A., Halpern E. F., Gazelle G. S., and McMahon P. M.. 2011. “Lung Cancer Treatment Costs, Including Patient Responsibility, by Disease Stage and Treatment Modality, 1992 to 2003.” Value Health 14 (1): 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell, J. M. , Ransohoff D. F., and Kramer B. S.. 2010. “Principles of Cancer Screening: Lessons from History and Study Design Issues.” Seminars in Oncology 37 (3): 202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo, R. A. , Cherkin D. C., and Ciol M. A.. 1992. “Adapting a Clinical Comorbidity Index for Use with ICD‐9‐CM Administrative Databases.” Journal of Clinical Epidemiology 45 (6): 613–9. [DOI] [PubMed] [Google Scholar]
- Gruber, E. V. , Stock S., and Stollenwerk B.. 2012. “Breast Cancer Attributable Costs in Germany: A Top‐Down Approach Based on Sickness Funds Data.” PLoS ONE 7 (12): e51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal, A. , Siegel R., Ward E., Hao Y., Xu J., and Thun M. J.. 2009. “Cancer Statistics, 2009.” CA: A Cancer Journal for Clinicians 59 (4): 225–49. [DOI] [PubMed] [Google Scholar]
- Klabunde, C. N. , Potosky A. L., Legler J. M., and Warren J. L.. 2000. “Development of a Comorbidity Index Using Physician Claims Data.” Journal of Clinical Epidemiology 53 (12): 1258–67. [DOI] [PubMed] [Google Scholar]
- Krahn, M. D. , Zagorski B., Laporte A., Alibhai S. M., Bremner K. E., Tomlinson G., Warde P., and Naglie G.. 2010. “Healthcare Costs Associated with Prostate Cancer: Estimates from a Population‐Based Study.” BJU International 105 (3): 338–46. [DOI] [PubMed] [Google Scholar]
- Mandrekar, J. N. , and Mandrekar S. J.. 2015. “An Introduction to Matching and Its Application Using SAS” [accessed on December 29, 2015]. Available at http://www2.sas.com/proceedings/sugi29/208-29.pdf
- Mariotto, A. B. , Yabroff K. R., Shao Y., Feuer E. J., and Brown M. L.. 2011. “Projections of the Cost of Cancer Care in the United States: 2010–2020.” Journal of the National Cancer Institute 103 (2): 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, P. M. , Kong C. Y., Bouzan C., Weinstein M. C., Cipriano L. E., Tramontano A. C., Johnson B. E., Weeks J. C., and Gazelle G. S.. 2011. “Cost‐Effectiveness of Computed Tomography Screening for Lung Cancer in the United States.” Journal of Thoracic Oncology 6 (11): 1841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann, N. , Porter J. M., Rangrej J., Seung S. J., Liu N., Saskin R., Cheung M. C., Leighl N. B., Hoch J. S., Trudeau M., Evans W. K., Dainty K. N., DeAngelis C., and Earle C. C.. 2014. “Health System Costs for Stage‐Specific Breast Cancer: A Population‐Based Approach.” Current Oncology (Toronto, Ontario) 21 (6): 281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER)‐Medicare [accessed on September 18, 2015]. Available at http://healthservices.cancer.gov/seermedicare/
- Pinsky, P. F. , Miller A., Kramer B. S., Church T., Reding D., Prorok P., Gelmann E., Schoen R. E., Buys S., Hayes R. B., and Berg C. D.. 2007. “Evidence of a Healthy Volunteer Effect in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.” American Journal of Epidemiology 165 (8): 874–81. [DOI] [PubMed] [Google Scholar]
- Riley, G. F. 2009. “Administrative and Claims Records as Sources of Health Care Cost Data.” Medical Care 47 (7 Suppl 1): S51–5. [DOI] [PubMed] [Google Scholar]
- Shakespeare, T. P. , Lu J. J., Back M. F., Liang S., Mukherjee R. K., and Wynne C. J.. 2003. “Patient Preference for Radiotherapy Fractionation Schedule in the Palliation of Painful Bone Metastases.” Journal of Clinical Oncology 21 (11): 2156–62. [DOI] [PubMed] [Google Scholar]
- Tangka, F. K. , Trogdon J. G., Richardson L. C., Howard D., Sabatino S. A., and Finkelstein E. A.. 2010. “Cancer Treatment Cost in the United States: Has the Burden Shifted over Time?” Cancer 116 (14): 3477–84. [DOI] [PubMed] [Google Scholar]
- United States. Board of Trustees of the Federal Hospital Insurance Trust Fund. and United States . Board of Trustees of the Federal Supplementary Medical Insurance Trust Fund. The … Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds: Communication from the Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. House document. Washington, U.S. G.P.O.: v.
- Yabroff, K. R. , Lund J., Kepka D., and Mariotto A.. 2011. “Economic Burden of Cancer in the United States: Estimates, Projections, and Future Research.” Cancer Epidemiology, Biomarkers & Prevention 20 (10): 2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Yabroff K. R., Guy G. P. Jr, Han X., Li C., Banegas M. P., Ekwueme D. U., and Jemal A.. 2016. “Annual Medical Expenditure and Productivity Loss among Colorectal, Female Breast, and Prostate Cancer Survivors in the United States.” Journal of the National Cancer Institute 108 (5), 10.1093/jnci/djv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Figure S1. Mean Cancer‐Attributable Costs, by Month from Diagnosis (Demographic and Comorbidity Matched Non‐Cancer Control Cohort).
Table S1. Characteristics of Cancer and Comparison Cohorts.


