Abstract
Objective
To examine how patients’ location of death relates to health care utilization and spending for surviving spouses.
Data Sources/Study Setting
Health and Retirement Study (HRS) 2000–2012 linked to the Dartmouth Atlas and Medicare claims data.
Study Design
This was an observational study. We matched bereaved spouses whose spouses died in a hospital to those whose spouses died outside the hospital using propensity scores based on decedent and spouse demographic and clinical characteristics, care preferences, and regional practice patterns.
Data Collection/Extraction Methods
We identified 1,348 HRS decedents with surviving spouses. We linked HRS data from each dyad with Medicare claims and regional characteristics.
Principal Findings
In multivariable models, bereaved spouses of decedents who died in the hospital had $3,106 higher Medicare spending 12 months postdeath (p = .04) compared to those whose spouses died outside a hospital. Those surviving spouses were also significantly more likely to have an ED visit (OR = 1.5; p < .01) and hospital admission (OR = 1.4; p = .02) in the year after their spouse's in‐hospital death. Increased Medicare spending for surviving spouses persisted through the 24‐month period postdeath ($5,310; p = .02).
Conclusions
Bereaved spouses of decedents who died in the hospital had significantly greater Medicare spending and health care utilization themselves after their spouses’ death.
Keywords: End of life, caregiving, bereavement, treatment intensity, palliative care, Medicare
Caregivers play a vital role in the care of older adults with disabilities and serious illness across all disease stages including the end‐of‐life (EOL) period (Institute of Medicine 2014; National Academies of Sciences 2016; Ornstein et al. 2017b). Yet family caregivers are also at increased risk of poor health. Decades of research suggests that while there may be benefits to providing care (Cohen, Colantonio, and Vernich 2002; Roff et al. 2004; Tarlow et al. 2004), caregivers are also vulnerable to increased depression (Pinquart and Sorensen 2003) and other health problems (Ho et al. 2009; Buyck et al. 2013), and reduced preventive health behaviors (Son et al. 2007; Reeves, Bacon, and Fredman 2012). As a consequence, caregiving is often associated with increased health care costs (Van Houtven, Wilson, and Clipp 2005; Schulz and Cook 2011) and may even lead to increased mortality (Schulz and Beach 1999).
The experience of caring for loved ones at the EOL in particular may impact the health and well‐being of the caregiver. Intensive Care Unit (ICU) admissions, for example, are associated with post‐traumatic stress for caregivers (Anderson et al. 2008; Davidson, Jones, and Bienvenu 2012; Schmidt and Azoulay 2012). Families report worse physical and mental health following deaths when intensive, life‐sustaining treatments were performed at EOL (Wright et al. 2008; Ornstein et al. 2017a). On the other hand, low‐intensity, comfort‐focused treatments such as hospice may mitigate the negative impact of caregiving on families. Beneficial effects of hospice use on caregivers include better bereavement adjustment (Godkin, Krant, and Doster 1983; Seale 1991), increased satisfaction (Kane et al. 1985; Seale 1991; Teno et al. 2005), and decreased depression (Bradley et al. 2004; Wright et al. 2008, 2010; Ornstein et al. 2015a). Similarly, improved outcomes for family members, including adjustment to death postbereavement (Abernethy et al. 2008) and better reported quality of death (Garrido and Prigerson 2014), are associated with use of palliative care services.
Whether the impact of the patient's EOL treatment experience extends to family members’ own health care utilization is largely unknown. Most caregivers increase their own utilization once caregiving ends and they can better attend to their own health needs (Prigerson, Maciejewski, and Rosenheck 2000; Stroebe, Schut, and Stroebe 2007; Guldin et al. 2013). But to date, there is a lack of research on what factors impact utilization of health care by surviving caregivers (Stajduhar et al. 2010). This is a remarkable gap in knowledge given that (1) our social networks play a significant role in our own health behaviors and events (Smith and Christakis 2008) and (2) the negative health effects associated with caregiving are associated with reduced preventive health behaviors (Reeves, Bacon, and Fredman 2012), which may ultimately increase health care utilization. Understanding how the health care experience impacts family members is critical to the development of effective interventions to mitigate negative effects of caregiving. Moreover, advancing current policy initiatives designed to simultaneously improve health outcomes and the cost effectiveness of the health care system requires a better understanding of what the downstream effects of health care and treatment intensity are on the family.
Conceptual Framework
Our conceptual framework for understanding the impact of the patient's EOL care experience on family members’ own health care (Ornstein et al. 2015b) is based on an adapted version of the Aday‐Anderson behavioral model of health care utilization (Aday and Andersen 1974) and incorporates the stress process model of caregiving (Pearlin et al. 1990), the role of patients’ suffering on families (Schulz et al. 2007, 2009), and shared social networks (Christakis 2004). We posit that family health care utilization may be related to patient treatment intensity because of shared values and preferences about health care, financial burdens related directly or indirectly to the patient's care, and the personal experience of witnessing the patient's symptoms, suffering, and challenging incidents within the health care system.
The Aday‐Anderson model proposes that predisposing, enabling, and need characteristics influence access to and use of health care. Predisposing factors are the “immutable” characteristics of the family member (e.g., demographics) as well as their caregiving role both at the end of life and in the long term. Enabling factors include resources individuals have available to them (e.g., insurance, regional service availability). Need factors refer to the conditions that necessitate health care utilization. The stress process model conceptualizes caregiving as a chronic stressor that gives rise to strains in multiple domains and ultimately leads to increased risk for psychiatric distress and physical illness burden. Furthermore, exposure to patient suffering (operationalized as physical, psychological, and existential/spiritual) is an independent source of distress for caregivers. We posit that the intensity of health care treatment received by a patient influences patient symptoms, suffering, and costs, which in turn impact enabling factors for the family caregiver such as financial and time burden and lack of belief in the efficacy of the health care system. Because of this burden, there is a decrease in preventive and self‐care behaviors, which ultimately results in increased urgent care use and health care spending for bereaved family members. Finally, family health care utilization is impacted by patient health care utilization within the context of shared social networks (Christakis 2004). Individuals are linked based on their social relationship within a social network, which are known to impact health behaviors such as smoking, weight gain, and cancer screening (Christakis and Fowler 2007). Behaviors such as use of high‐intensity treatments at EOL may be impacted by shared experiences within these social networks.
Guided by our model, the goal of this study was to test whether the EOL care experience of a person impacts the downstream health care utilization for surviving spouses. We conceptualize treatment intensity at the end of life as a high degree of medical intervention, most often taking place in a hospital setting. Although hospitalizations are the most common marker of treatment intensity, there is no uniform marker or timeframe (Luta et al. 2015). We focused on in‐hospital death as a broad and commonly used marker of high‐intensity EOL treatment relevant to all decedents, not just those with a specific illness or in a specific setting (i.e., not just limited to those with cancer or hospitalized), as compared to death in a nonhospital location.
Methods
Study Population
The Health and Retirement Study (HRS), a nationally representative, longitudinal survey of U.S. adults 51 years of age and older (Health and Retirement Study 2013), interviews participants every two years. If participants are married or partnered, their partners (heretofore referred to as “spouses”) are recruited into the study and surveyed. During each interview cycle, HRS identifies participants who have died since the last interview wave. In these cases, a postdeath interview is conducted with someone who is knowledgeable about the deceased participant. HRS survey data are linked for eligible participants with individual Medicare claims. Study participants provided informed consent upon enrollment and again for linkage to Medicare claims. We identified 3,226 respondents who died between 2000 and 2011 and were survived by a spouse. Because our main outcome was spouse postdeath utilization, we limited our sample to 1,548 decedents whose spouses had completed an HRS interview, were over age 65, and had fee‐for‐service (FFS) Medicare at the time of the decedent's death. Finally, we excluded 173 dyads due to missing data required for propensity score matching.
Measures
Outcome Measures
The primary outcome was the surviving spouse's total Medicare expenditures during the 12 months after his or her spouse's death. This measure includes all Medicare payments for inpatient, outpatient, skilled nursing facility, hospice and home care, and durable medical equipment. Claims spanning the 365th day after death were prorated to include only the expenditures within the one‐year period. We adjusted expenditures for inflation (2012$) based on the consumer price index, and for geographic differences in Medicare price levels using the Centers for Medicare and Medicaid Services (CMS) wage index. We also used Medicare claims to determine whether surviving spouses had any inpatient admissions or emergency department (ED) visits.
Independent Variables
Based on previous work (Kelley et al. 2011), we used HRS postdeath interview data to create a binary indicator of in‐hospital death versus death in any other location. Being in the hospital at the time of death is indicative of a higher level of treatment intensity in the time immediately preceding death and is therefore distinct from death in other settings (home, assisted living, nursing home, hospice facility, and other), which were considered “nonhospital death.”
Other Measures
Based on our group's conceptual frameworks for understanding multilevel predictors of treatment intensity (Kelley et al. 2010) and the impact of patient treatment intensity on family members’ own health care (Ornstein et al. 2015b), we identified factors that could be associated with both likelihood of in‐hospital death and spousal postdeath health care spending. To account for this potential confounding, we used propensity score matching (as described below) based upon the following spousal, decedent, and regional level variables.
Spousal factors drawn from the spouse's last HRS core interview before the patient's death included age, sex, race/ethnicity, education, net worth, self‐reported health, functional status, and level of comorbidity. We also identified whether the spouse was the primary helper with the decedent's activities of daily living (ADLs) or instrumental activities of daily living (IADLs) at the EOL from the postdeath interview.
Decedent factors were extracted from decedents’ final HRS core interview. HRS core interview data included the following: insurance coverage, functional status (whether the participant had difficulty with one or more basic ADLs), residential status (nursing home or community‐dwelling), and self‐reported health. Probable dementia was determined via clinically validated algorithm (Hurd et al. 2013). Self‐report illness data were used to determine cancer diagnosis and level of comorbidity. If the core interview was completed within the month before death, data were drawn from the previous interview. Presence of advance directives and whether or not the family expected the death were extracted from the postdeath interview.
Using the decedent's zip code, each dyad was linked via their hospital referral region (HRR) to the Dartmouth Atlas of Healthcare's End of Life Expenditure Index (EOL‐EI), a measure of physician practice patterns, based upon Medicare beneficiaries’ utilization in the last 6 months of life (Wennberg and Cooper 2013). We created an indicator for those living in the top quartile of EOL‐EI by HRR.
Analysis
Propensity scores were calculated using logistic regression of in‐hospital death. Prior to matching, we checked the balance of covariates across the groups who did and did not die in‐hospital within strata of the propensity score. We then used caliper matching with replacement to match those who died in‐hospital to one or many decedents who died outside of the hospital within 0.02 of the standard deviation of the logit of the propensity score (Stuart 2010). Balance was then verified by examining standardized differences in covariates across treatment groups and variance of covariates before and after matching. Standardized differences < 10 percent indicated adequate balance (Austin 2009; Garrido et al. 2014).
Using the propensity‐score matched sample, we first estimated differences in 12‐month Medicare expenditures and hospitalization and ED visits based on nonparametric equality of medians test and chi‐square test of proportions. In our primary analysis, we estimated a multivariable generalized linear (GLM) model of 12‐month Medicare expenditures postdeath. Due to the skewed distribution of the outcome, we used a gamma distribution with a log link. Regression coefficients were exponentiated into rate ratio estimates, and average marginal effects were calculated to produce average treatment effects on the treated. For our secondary outcomes, incidence of hospitalization, and ED admission 12 months postdeath, we estimated multivariable logistic regression models. In all models, we controlled for all covariates included in the propensity score to adjust for any remaining imbalance between the groups after matching (Stuart 2010). We also included an indicator for year of death to account for secular trends.
We conducted a series of sensitivity analyses. First, we repeated the analyses described above using 24 months postdeath spending as the outcome. We also repeated analyses limiting our sample to those spouses who survived at least 12 months beyond the death of their spouse. To account for individual variation in baseline spending, we limited our sample to those spouses with continuous FFS Medicare 18 months before death. We used the one‐year period from 6 to 18 months before death to determine “baseline spending” levels to avoid any deviation from typical health care utilization patterns due to the spouse's illness and approaching death. We repeated our analyses with only those spouses in highest baseline spending quartile. Finally, we included quartile of predeath spending in our match and reran primary and secondary outcomes. For each additional analysis, we re‐estimated the propensity score and created new matched subsamples with balanced observed covariates.
Results
Surviving spouses were mean age 77.8 years, and 72.6 percent had at least a high school education (Table 1). The majority of surviving spouses (89.0 percent) were independent in all ADLs prior to their spouse's death (mean = 12.7 months). Decedents had a mean age of 80.5 years at death; 69.2 percent were men, 85.8 percent were non‐Hispanic white, and 68.1 percent had at least a high school education. More than one‐third (38.9 percent) of decedents died in the hospital. The remainder died at home (including assisted living facilities) (32.0 percent), in a nursing home (18.4 percent), or in hospice facilities (8.7 percent).
Table 1.
Characteristics of Spousal Dyads by Location of Death before and after Propensity Score Matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| In‐Hospital Death (%) | Nonhospital Death (%) | Standardized Difference | In‐Hospital Death (%) | Nonhospital Death (%) | Standardized Difference | |
| Sample, n | 528 | 831 | 526 | 822 | ||
| Spouse | ||||||
| Age at death, years | 77.6 | 78.0 | 0.06 | 77.6 | 77.7 | 0.01 |
| Female | 69.3 | 69.1 | −0.01 | 69.6 | 69.0 | −0.01 |
| Networth, lowest quartile | 15.9 | 15.9 | −0.00 | 16.0 | 15.7 | −0.01 |
| Race, white, non‐Hispanic | 80.1 | 88.39 | 0.23 | 80.4 | 81.7 | 0.03 |
| Education, high school degree | 69.5 | 74.5 | 0.11 | 69.6 | 71.5 | 0.04 |
| SRH poor or fair | 32.6 | 31.9 | −0.01 | 32.7 | 32.5 | −0.00 |
| No comorbidities | 14.6 | 16.9 | 0.06 | 14.6 | 14.5 | −0.00 |
| Mild comorbidities | 74.1 | 73.0 | −0.03 | 74.0 | 74.1 | 0.00 |
| Moderate/severe comorbidities | 11.4 | 10.4 | −0.03 | 11.4 | 11.5 | 0.00 |
| Dependent in ADLs | 12.1 | 10.1 | −0.06 | 12.0 | 11.6 | −0.01 |
| Primary caregiver EOL | 52.7 | 57.3 | 0.09 | 52.9 | 53.2 | 0.01 |
| Decedent | ||||||
| Age at death, years | 80.8 | 78.0 | 0.12 | 80.0 | 80.1 | 0.01 |
| SRH poor or fair | 58.1 | 63.9 | 0.12 | 58.2 | 56.1 | −0.04 |
| Dependent in ADLs | 31.1 | 42.1 | 0.23 | 31.0 | 29.7 | −0.03 |
| Probable dementia | 23.1 | 31.41 | 0.19 | 23.2 | 22.6 | −0.01 |
| Cancer diagnosis | 22.2 | 33.5 | 0.25 | 22.2 | 21.0 | −0.03 |
| No comorbidities | 7.6 | 5.8 | 0.06 | 7.6 | 7.9 | 0.01 |
| Mild comorbidities | 67.6 | 69.9 | −0.03 | 67.7 | 68.9 | 0.03 |
| Moderate/severe comorbidities | 24.8 | 24.3 | −0.03 | 24.7 | 23.2 | −0.04 |
| Nursing home resident | 4.2 | 12.0 | 0.29 | 4.2 | 4.1 | −0.01 |
| VA Insurance | 5.3 | 7.8 | 0.10 | 5.3 | 5.5 | 0.01 |
| Had advance directive | 55.5 | 62.8 | 0.12 | 55.5 | 56.6 | 0.02 |
| Death expected by family | 48.1 | 67.0 | 0.15 | 48.3 | 47.2 | −0.02 |
| Region | ||||||
| Quartile EOL spending by HRR: Low | 14.6 | 18.7 | 0.11 | 14.6 | 15.3 | 0.02 |
| Quartile EOL spending by HRR: Mid‐low | 18.8 | 24.7 | 0.14 | 18.8 | 19.7 | 0.02 |
| Quartile EOL spending by HRR: Mid‐high | 34.3 | 33.2 | −0.02 | 34.4 | 33.9 | −0.01 |
| Quartile EOL spending by HRR: High | 32.4 | 23.5 | −0.20 | 32.1 | 31.1 | −0.02 |
Mild comorbidities = 1–3; Moderate/severe = 4.
ADL, activities of daily living; EOL, end of life; HRR, Hospital Referral Region; SRH, Self‐reported health; VA, Veteran's Administration.
Prior to matching, there were significant differences among dyads where the decedent died in the hospital compared to those who died elsewhere. Surviving spouses of those who died in the hospital were less likely to be non‐Hispanic white (80.1 percent vs. 88.3 percent) and less likely to have a high school degree (69.5 percent vs. 74.5 percent). The decedents who died in the hospital were less likely to have dementia (23.1 percent vs. 31.4 percent), cancer (22.2 percent vs. 33.5 percent), be ADL dependent (31.1 percent vs. 42.1 percent), live in nursing homes (4.2 percent vs. 12.0 percent), have advance directives (55.5 percent vs. 62.8 percent), or have an expected death (48.1 percent vs. 67.0 percent). Those who died in the hospital were more likely to live in high‐spending EOL‐EI HRRs (32.3 percent vs. 23.5 percent) (Table 1).
In our matched sample (99.2 percent of complete sample), observed confounders were balanced (<10 percent standardized difference) (Table 1) (Austin 2009). Spouses of those who had an in‐hospital death had consistently higher spending up to 24 months after death (see Figure 1). In bivariate analyses, spouses of those who died in the hospital had $4,000 higher Medicare expenditures during the 12 months after death (p < .01) and were more likely to be hospitalized (28.7 percent vs. 22.5 percent; p = .02) and visit the ED in the year after their spouse died than those whose spouses died outside of the hospital (39.2 percent vs. 30.8 percent; p < .01). These significantly higher levels of expenditures and utilization persisted for the 24‐month period after death of a spouse (Table 2).
Figure 1.
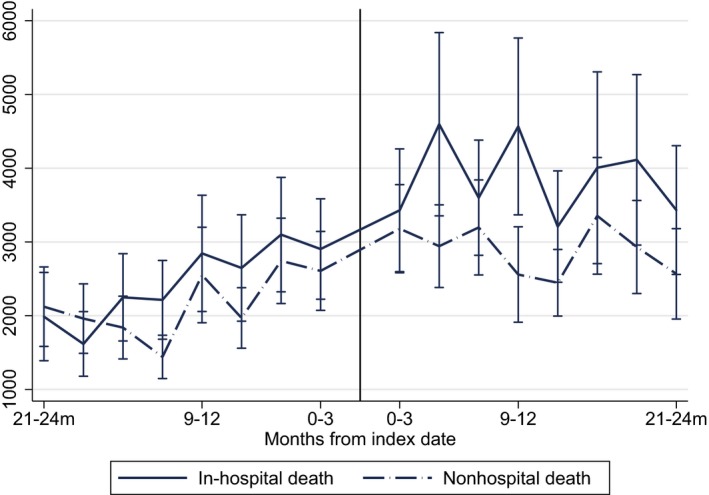
- Notes: Medicare expenditures are wage index and inflation adjusted to 2012$; figure includes mean quarterly expenditures among those spouses with fee‐for‐service Medicare at the time of their spouse's death (n = 1,348; 2418.1 person‐years of follow‐up after death and 2399.8 person‐years of follow‐up after death) adjusted based on propensity for in‐hospital death; CI, confidence Intervals.
Table 2.
Medicare Expenditures and Utilization for Bereaved Spouses Postdeath by Location of Death (Matched Sample)
| Postdeath Follow‐Up Period | n | Mean Medicare Expenditures, 2012$ | Median Expenditures, 2012$ | Hospitalizations (%) | ED Visits (%) |
|---|---|---|---|---|---|
| 12 months | |||||
| In‐hospital death | 526 | 15,959.7 | 3,387.1 | 28.7 | 39.2 |
| Nonhospital death | 822 | 11,775.5 | 3,099.6 | 22.5 | 30.8 |
| p‐value | <.01 | .02 | <.01 | ||
| 24 months | |||||
| In‐hospital death | 485 | 29,500.3 | 9,725.3 | 42.9 | 58.4 |
| Nonhospital death | 753 | 22,307.3 | 8,382.3 | 35.7 | 48.1 |
| p‐value | <.01 | .02 | <.01 | ||
Medicare expenditures are wage index and inflation adjusted to 2012$. Sample propensity score matched based on: spouse age at death, gender, networth, race, education, self‐reported heath, comorbidity level, caregiving status; decedent age, self‐reported health, ADL dependence, dementia status, cancer diagnosis, level of comorbidity, residence, insurance status; presence of advance directive; family expectation of death; and EOL spending by hospital referral region. p‐Values based on chi square test of proportions, nonparametric test of medians. Nonhospital death includes the following locations: home, assisted living, nursing home, hospice facility, and other.
ED, Emergency Department.
In multivariable analysis, in‐hospital death was independently associated with a $3,106 mean increase in spousal Medicare expenditures over the first year (p = .04) and a $5,310 mean increase in spending over 2 years (p = .02) (Table 3). Spouses of those who died in the hospital had a 43 percent increased odds of being hospitalized in the 12 months following the death of their spouse (p = .02). Similarly, spouses of those who died in the hospital had a 51 percent increased odds of visiting the ED in the 12 months following the death of their spouse (p ≤ .01). These differences in spending remained up to 24 months after the death of their spouse (see Table 3).
Table 3.
Association between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Postdeath
| Postdeath Follow‐Up Period | ||
|---|---|---|
| 12 months | 24 months | |
| N | 1,348 | 1,238 |
| Medicare expenditures | ||
| Rate ratio | 1.2 | 1.22 |
| 95% CI | 1.01–1.52 | 1.03–1.46 |
| Average marginal effect | $3,105.81 | $5309.64 |
| Median marginal effect (IQR) | $2,046.67 ($1,358.24–$3,876.56) | $4,049.83 ($2,863.32–$6,432.91) |
| p‐value | .04 | .02 |
| Hospitalizations | ||
| OR | 1.43 | 1.42 |
| 95% CI | 1.07–1.90 | 1.09–1.85 |
| p‐value | .02 | .01 |
| ED visits | ||
| OR | 1.51 | 1.60 |
| 95% CI | 1.16–1.96 | 1.23–2.08 |
| p‐value | <.01 | <.01 |
Medicare expenditures are wage index and inflation adjusted to 2012$. Sample propensity score matched based on spouse age at death, gender, networth, race, education, self‐reported heath, comorbidity level, caregiving status; decedent age, self‐reported health, ADL dependence, dementia status, cancer diagnosis, level of comorbidity, residence, insurance status; presence of advance directive; family expectation of death; and EOL spending by hospital referral region and adjusted for year of death. p‐Values based on GLM and logistic regression models.
ED, Emergency Department; IQR, interquartile range.
In 90 percent of dyads, the surviving spouse lived at least 12 months after the death of their spouse. When limiting our analyses to these dyads, our findings remained unchanged (see Table S1). In‐hospital death was associated with 26 percent increased mean Medicare expenditures (p = .04).
Spouses’ annual median baseline health care spending ranged from $330 (lowest quartile) to $21,082 (highest quartile) (see Table S2). In matched fully adjusted analyses limited to the highest quartile of baseline spenders, there was a $15,223 marginal difference in spending after death among spouses of those who died in the hospital (p < .01) compared to those who experienced a nonhospital death. This group also had a statistically significant twofold increase in hospital and ED utilization 12 months after the death of their spouse. Significantly higher expenditures and likelihood of utilization were evident 24 months postdeath (Table 4). We were unable to achieve adequate balance across all matching variables for the lower three quartiles when we stratified our analysis by quartile of spousal predeath spending.
Table 4.
Association between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Postdeath by High Baseline Spenders Only
| Postdeath Follow‐Up Period | ||
|---|---|---|
| 12 months Postdeath | 24 months Postdeath | |
| N | 256 | 228 |
| Medicare expenditures | ||
| Rate ratio | 1.59 | 1.48 |
| 95% CI | 1.16–2.20 | 1.10–1.98 |
| Average marginal effect | 15,223.47 | 20,761.83 |
| Median marginal effect (IQR) | $12,417.08 ($6,630.23–$19,714.14) | $16,144.51 ($11,619.522–$24,256.16) |
| p‐value | <.01 | <.01 |
| Hospitalizations | ||
| OR | 2.34 | 1.84 |
| 95% CI | 1.23–4.44 | 0.94–3.57 |
| p‐value | <.01 | .07 |
| ED visits | ||
| OR | 2.27 | 1.96 |
| 95% CI | 1.22–4.24 | 0.99–3.90 |
| p‐value | .01 | .06 |
Medicare expenditures are wage index and inflation adjusted to 2012$. Sample propensity score matched based on: spouse age at death, gender, networth, race, education, self‐reported heath, comorbidity level, caregiving status; decedent age, self‐reported health, ADL dependence, dementia status, cancer diagnosis, level of comorbidity, residence, insurance status; presence of advance directive; family expectation of death; and EOL spending by hospital referral region and adjusted for year of death. p‐values based on GLM and logistic regression models.
ED, Emergency Department; IQR, interquartile range.
In matched analyses that include quartiles of predeath spending in the propensity score model (Table S3), in‐hospital death remained significantly associated with both 12‐month hospitalization and ED utilization, although for Medicare spending the effect size was reduced and lost significance.
Discussion
Using a nationally representative sample of older decedents, we found that bereaved spouses of those who died in the hospital had higher levels of Medicare spending and utilization themselves after the death, compared to those whose spouses died outside of a hospital. Specifically, we found that bereaved spouses of decedents who died in the hospital had more than $3,000 increased spending in the 12 months after death and over $5,000 increased spending in the 24 months after death, compared to otherwise similar surviving spouses. In addition, those whose spouses died in the hospital were significantly more likely to have an ED visit and a hospital admission after their spouse's death. Among those individuals in the highest baseline spending quartile, in‐hospital death was significantly associated with an even greater difference: $15,000 higher spending 12 months postdeath.
To our knowledge, this study is one of the first to demonstrate that the experience of the death of one's spouse may have an important impact on subsequent health care utilization and spending. Our work adds to a growing body of literature which finds that high‐intensity treatment including hospitalizations at the EOL may have negative consequences for some patients and their families (Teno et al. 2004; Christakis and Allison 2006; Wright et al. 2010, 2016; Ornstein et al. 2017a). This is of critical importance given that despite surveys indicating a preference among those with serious illness to avoid an in‐hospital death and its associated use of high‐intensity life‐sustaining treatments, in‐hospital death remains common (Barnato et al. 2009; Gomes et al. 2012). The proportion of deaths in acute care hospitals in the United States was 24.6 percent in 2009 (Teno et al. 2013), and the estimates are higher for cancer‐related deaths (Bekelman et al. 2016).
Furthermore, these analyses suggest that assessment of EOL costs may need to account for the downstream costs of health care for spouses. Despite a growing literature demonstrating the evidence to support inclusion of family “health spillovers” in economic evaluation (Bobinac et al. 2011; Brouwer et al. 2013; Al‐Janabi and Van Exel 2016; Al‐Janabi et al. 2016; Fletcher and Marksteiner 2017), current health care cost estimates do not routinely consider potential downstream costs associated with the health care expenditures of family members who care for their seriously ill loved ones (Hurd et al. 2013). A more comprehensive (and less individualistic) perspective on health care and assessment of costs is justified as many caregivers are themselves older, in poor health, and also Medicare beneficiaries (National Academies of Sciences 2016). Future research should examine if the same patterns are found among couples insured by private health plans.
By linking the health care experiences of individuals to their spouses, our work highlights the importance of meeting the needs of family caregivers. Adequate caregiver support throughout the course of serious illness and during the EOL period is essential for high‐quality patient care and for caregiver well‐being, but it may also have important implications for spending patterns for surviving spouses. While access to hospice is a core strategy to improve support for family caregivers at the end of life, supportive services available for caregivers through hospice may arrive too late to benefit caregivers who have already experienced substantial caring‐related difficulties. Early palliative care services including caregiving support should be available at any stage of disease severity that requires caregiving support, and the impact of these services on families’ health and health care outcomes must be evaluated. More generally, our findings highlight the importance of recognizing the impact of serious illness on families, and working to ensure families are fully considered in efforts to improve the quality and experience of care. Some examples of efforts to support families include improving reimbursement mechanisms for advance care planning discussions, expanding family medical leave and other legislation to support working family caregivers, and greater research into interventions designed to support EOL caregivers.
Our findings should be interpreted with a number of potential limitations in mind. Although the mortality follow‐back sampling method has been critiqued (Bach, Schrag, and Begg 2004), this approach is appropriate for our research question that pertains specifically to the experience of decedents and the associated outcomes for surviving spouses. Because we used Medicare claims data to measure costs, we were limited to spouses with FFS Medicare. On average, individuals incur their highest medical costs in the last few months of life, so we did not exclude spouses who died during the 12‐month follow‐up period, which would bias our findings toward the null. In sensitivity analyses, we limited our study to 12‐month survivors and also those with uninterrupted FFS Medicare and found no significant variation in results. Additionally, our use of propensity score matching, although robust, cannot adjust for unmeasured factors and unobserved differences between the groups. Importantly, although we examined a range of patient, spouse, and regional characteristics that may have accounted for variation in both locations of death and spousal postdeath spending, we did not have detailed information on individual patient and family treatment preferences. We were thus unable to determine whether treatment was concordant with preferences. We also could not assess issues of prognostic uncertainty, provider communication, options around care choices, or overall satisfaction with the care received. Although we focus on all spouses regardless of their caregiving role, because provision of care is itself endogenous to the health of caregivers, causal effects are difficult to determine in observational data (Coe and Van Houtven 2009; Do et al. 2015). Finally, our findings regarding changing spending patterns must be interpreted with caution in light of findings on persistence of health spending (Hirth et al. 2015). Although our spending‐related results were no longer significant when we included predeath spending quartile in our match, this could be due to a reduced sample size or to a differential relationship between location and postdeath spending according to level of predeath spending. Unfortunately, lack of balance across quartiles precluded the examination of effects of location of death on spending across all levels of predeath spending.
While our work demonstrates an association between having an in‐hospital death and higher spouse postdeath Medicare expenditures, it raises a number of issues that require further study. First, while we focused on site of death as a marker of treatment intensity, other relevant measures exist, including number of hospital admissions or days, number of physician visits, number of transitions in sites of care, and use of life‐sustaining interventions (Luta et al. 2015). Further examination of specific procedures and experiences may shed light on the association described in our study and could provide an opportunity for improved care quality for both the patient and spouse. Our study lacked detailed clinical data, so we cannot assess many of the specific treatments provided or the quality of communication and shared decision making. Future work could directly assess caregiver experience at the time of in‐hospital death, including possible mediating factors such as exacerbation of symptoms and high out‐of‐pocket expenditures. Similarly, further examination of reasons for spouse hospitalization and ED visits may help to determine how these may be prevented. For example, are these exacerbations of pre‐existing illnesses, or are they due to mental health crises that may be prevented through early increased caregiver support? The vast majority of spouse hospitalizations captured in this study postdeath (80 percent) were categorized as nonelective. In post hoc analyses, we found that in‐hospital death was similarly associated with a statistically significant increase in nonelective hospitalizations (data not shown). Moreover, we did not examine other sources of health care spending, including individual out‐of‐pocket expenditures. A better understanding of these costs and household financial burdens could also reveal opportunities to provide necessary support services.
Conclusions
A patient's family is increasingly recognized as an integral component of the health care system, and a growing number of informal caregivers provide the bulk of long‐term care in the community at huge unpaid costs (Levine et al. 2010). Meeting the needs of caregivers throughout the course of their loved one's serious illness and during the EOL period is a critical component of high‐quality care for patients and promotes caregiver well‐being. Our findings highlight an association between location of death and the surviving spouse's subsequent health care utilization, suggesting that there may be an opportunity to better support the needs of caregiving families while lowering downstream health care costs.
Supporting information
Appendix SA1. Author Matrix.
Table S1. Association between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Post‐death among Twelve‐Month Survivors.
Table S2. Medicare Spending by Location of Death and Baseline Spending Quartile.
Table S3. Association Between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Postdeath after Controlling for Predeath Spending Data.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The Health and Retirement Study (HRS) is funded by the National Institute on Aging (NIA U01 AG009740) and the Social Security Administration, and it was performed at the Institute for Social Research, University of Michigan. The study investigators were supported by the National Institute on Aging K01AG047923 (Dr. Ornstein); the National Institute on Aging K23AG040774 and the American Federation for Aging Research (Dr. Kelley); NIA P30AG024824 and P30AG053760 (Dr. Langa); and VA HSR&D 16‐140 (Dr. Garrido). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosures: None.
Disclaimer: None.
References
- Abernethy, A. P. , Currow D. C., Fazekas B. S., Luszcz M. A., Wheeler J. L., and Kuchibhatla M.. 2008. “Specialized Palliative Care Services Are Associated with Improved Short‐ and Long‐Term Caregiver Outcomes.” Supportive Care in Cancer 16 (6): 585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aday, L. A. , and Andersen R.. 1974. “A Framework for the Study of Access to Medical Care.” Health Services Research 9 (3): 208–20. [PMC free article] [PubMed] [Google Scholar]
- Al‐Janabi, H. , and Van Exel J.. 2016. “Measuring Health Spillovers for Economic Evaluation: A Case Study in Meningitis.” Health Economics 25 (12): 1529–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Janabi, H. , van Exel J., Brouwer W., and J., Coast . 2016. “A Framework for Including Family Health Spillovers in Economic Evaluation” Medical Decision Making 36 (2): 176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. G. , Arnold R. M., Angus D. C., and Bryce C. L.. 2008. “Posttraumatic Stress and Complicated Grief in Family Members of Patients in the Intensive Care Unit.” Journal of General Internal Medicine 23 (11): 1871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, P. C. 2009. “Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity‐Score Matched Samples.” Statistics in Medicine 28 (25): 3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, P. B. , Schrag D., and Begg C. B.. 2004. “Resurrecting Treatment Histories of Dead Patients: A Study Design That Should Be Laid to Rest.” Journal of the American Medical Association 292 (22): 2765–70. [DOI] [PubMed] [Google Scholar]
- Barnato, A. E. , Anthony D. L., Skinner J., Gallagher P. M., and Fisher E. S.. 2009. “Racial and Ethnic Differences in Preferences for End‐of‐Life Treatment.” Journal of General Internal Medicine 24 (6): 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekelman, J. E. , Halpern S. D., Blankart C. R., Bynum J. P., Cohen J., Fowler R., Kaasa S., Kwietniewski L., Melberg H. O., Onwuteaka‐Philipsen B., Oosterveld‐Vlug M., Pring A., Schreyogg J., Ulrich C. M., Verne J., Wunsch H., and Emanuel E. J.. 2016. “Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying with Cancer in Seven Developed Countries.” Journal of the American Medical Association 315 (3): 272–83. [DOI] [PubMed] [Google Scholar]
- Bobinac, A. , van Exel N. J., Rutten F. F., and Brouwer W. B.. 2011. “Health Effects in Significant Others: Separating Family and Care‐Giving Effects.” Medical Decision Making 31 (2): 292–8. [DOI] [PubMed] [Google Scholar]
- Bradley, E. H. , Prigerson H., Carlson M. D., Cherlin E., Johnson‐Hurzeler R., and Kasl S. V.. 2004. “Depression among Surviving Caregivers: Does Length of Hospice Enrollment Matter?” American Journal of Psychiatry 161 (12): 2257–62. [DOI] [PubMed] [Google Scholar]
- Brouwer, W. , Trotter C., Glennie L., Hannigan L., Coast J., Wittenberg E., and Prosser L. A.. 2013. “Disutility of Illness for Caregivers and Families: A Systematic Review of the Literature.” Health Economics 31 (6): 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyck, J. F. , Ankri J., Dugravot A., Bonnaud S., Nabi H., Kivimaki M., and Singh‐Manoux A.. 2013. “Informal Caregiving and the Risk for Coronary Heart Disease: The Whitehall II Study.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 68 (10): 1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis, N. A. 2004. “Social Networks and Collateral Health Effects.” British Medical Journal 329 (7459): 184–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis, N. A. , and Allison P. D.. 2006. “Mortality after the Hospitalization of a Spouse.” New England Journal of Medicine 354 (7): 719–30. [DOI] [PubMed] [Google Scholar]
- Christakis, N. A. , and Fowler J. H.. 2007. “The Spread of Obesity in a Large Social Network over 32 Years.” New England Journal of Medicine 357 (4): 370–9. [DOI] [PubMed] [Google Scholar]
- Coe, N. B. , and Van Houtven C. H.. 2009. “Caring for Mom and Neglecting Yourself? The Health Effects of Caring for an Elderly Parent.” Health Economics 18 (9): 991–1010. [DOI] [PubMed] [Google Scholar]
- Cohen, C. A. , Colantonio A., and Vernich L.. 2002. “Positive Aspects of Caregiving: Rounding out the Caregiver Experience.” International Journal of Geriatric Psychiatry 17 (2): 148. [DOI] [PubMed] [Google Scholar]
- Davidson, J. E. , Jones C., and Bienvenu O. J.. 2012. “Family Response to Critical Illness: Postintensive Care Syndrome‐Family.” Critical Care Medicine 40 (2): 618–24. [DOI] [PubMed] [Google Scholar]
- Do, Y. K. , Norton E. C., Stearns S. C., and Van Houtven C. H.. 2015. “Informal Care and Caregiver's Health.” Health Economics 24 (2): 224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J. , and Marksteiner R.. 2017. “Causal Spousal Health Spillover Effects and Implications for Program Evaluation.” American Economic Journal: Economic Policy 9 (4): 144–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. M. , and Prigerson H. G.. 2014. “The End‐of‐Life Experience: Modifiable Predictors of Caregivers’ Bereavement Adjustment.” Cancer 120 (6): 918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. M. , Kelley A. S., Paris J., Roza K., Meier D. E., Morrison R. S., and Aldridge M. D.. 2014. “Methods for Constructing and Assessing Propensity Scores.” Health Services Research 49 (5): 1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godkin, M. A. , Krant M. J., and Doster N. J.. 1983. “The Impact of Hospice Care on Families.” International Journal of Psychiatry in Medicine 13 (2): 153–65. [DOI] [PubMed] [Google Scholar]
- Gomes, B. , Higginson I. J., Calanzani N., Cohen J., Deliens L., Daveson B. A., Bechinger‐English D., Bausewein C., Ferreira P. L., Toscani F., Menaca A., Gysels M., Ceulemans L., Simon S. T., Pasman H. R., Albers G., Hall S., Murtagh F. E., Haugen D. F., Downing J., Koffman J., Pettenati F., Finetti S., Antunes B., and Harding R.. 2012. “Preferences for Place of Death If Faced with Advanced Cancer: A Population Survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain.” Annals of Oncology 23 (8): 2006–15. [DOI] [PubMed] [Google Scholar]
- Guldin, M. B. , Jensen A. B., Zachariae R., and Vedsted P.. 2013. “Healthcare Utilization of Bereaved Relatives of Patients Who Died from Cancer. A National Population‐Based Study.” Psychooncology 22 (5): 1152–8. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study . 2013. [accessed on September 17, 2013]. Available at http://hrsonline.isr.umich.edu/
- Hirth, R. A. , Gibson T. B., Levy H. G., Smith J. A., Calonico S., and Das A.. 2015. “New Evidence on the Persistence of Health Spending.” Medical Care Research and Review: MCRR 72 (3): 277–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S. C. , Chan A., Woo J., Chong P., and Sham A.. 2009. “Impact of Caregiving on Health and Quality of Life: A Comparative Population‐Based Study of Caregivers for Elderly Persons and Noncaregivers.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 64 (8): 873–9. [DOI] [PubMed] [Google Scholar]
- Hurd, M. D. , Martorell P., Delavande A., Mullen K. J., and Langa K. M.. 2013. “Monetary Costs of Dementia in the United States.” New England Journal of Medicine 368 (14): 1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . 2014. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Kane, R. L. , Klein S. J., Bernstein L., Rothenberg R., and Wales J.. 1985. “Hospice Role in Alleviating the Emotional Stress of Terminal Patients and Their Families.” Medical Care 23 (3): 189–97. [DOI] [PubMed] [Google Scholar]
- Kelley, A. S. , Morrison R. S., Wenger N. S., Ettner S. L., and Sarkisian C. A.. 2010. “Determinants of Treatment Intensity for Patients With Serious Illness: A new Conceptual Framework.” Journal of Palliative Medicine 13 (7): 807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, A. S. , Ettner S. L., Wenger N. S., and Sarkisian C. A.. 2011. “Determinants of Death in the Hospital among Older Adults.” Journal of the American Geriatrics Society 59 (12): 2321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, C. , Halper D., Peist A., and Gould D. A.. 2010. “Bridging Troubled Waters: Family Caregivers, Transitions, and Long‐Term Care.” Health Affairs (Millwood) 29 (1): 116–24. [DOI] [PubMed] [Google Scholar]
- Luta, X. , Maessen M., Egger M., Stuck A. E., Goodman D., and Clough‐Gorr K. M.. 2015. “Measuring Intensity of End of Life Care: A Systematic Review.” PLoS ONE 10 (4): e0123764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . 2016. Families Caring for an Aging America. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Ornstein, K. A. , Aldridge M. D., Garrido M. M., Gorges R., Meier D. E., and Kelley A. S.. 2015a. “Association between Hospice Use and Depressive Symptoms in Surviving Spouses.” Journal of the American Medical Association Internal Medicine 175 (7): 1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein, K. A. , Boerner K., Siu A. L., and Schulz R.. 2015b. “Downstream Effects of End‐of‐Life Care for Older Adults with Serious Illness on Health Care Utilization of Family Caregivers.” Journal of Palliative Medicine 18 (9): 736–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein, K. A. , Aldridge M. D., Garrido M. M., Gorges R., Bollens‐Lund E., Siu A. L., Langa K. M., and Kelley A. S.. 2017a. “The Use of Life‐Sustaining Procedures in the Last Month of Life Is Associated with More Depressive Symptoms in Surviving Spouses.” Journal of Pain and Symptom Management 53(2): 178–87 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein, K. A. , Kelley A. S., Bollens‐Lund E., and Wolff J. L.. 2017b. “A National Profile of End‐of‐Life Caregiving in the United States.” Health Affairs (Millwood) 36 (7): 1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin, L. I. , Mullan J. T., Semple S. J., and Skaff M. M.. 1990. “Caregiving and the Stress Process: An Overview of Concepts and Their Measures.” Gerontologist 30 (5): 583–94. [DOI] [PubMed] [Google Scholar]
- Pinquart, M. , and Sorensen S.. 2003. “Differences Between Caregivers and Noncaregivers in Psychological Health and Physical Health: A Meta‐Analysis.” Psychology and Aging 18 (2): 250–67. [DOI] [PubMed] [Google Scholar]
- Prigerson, H. G. , Maciejewski P. K., and Rosenheck R. A.. 2000. “Preliminary Explorations of the Harmful Interactive Effects of Widowhood and Marital Harmony on Health, Health Service Use, and Health Care Costs.” Gerontologist 40 (3): 349–57. [DOI] [PubMed] [Google Scholar]
- Reeves, K. W. , Bacon K., and Fredman L.. 2012. “Caregiving Associated with Selected Cancer Risk Behaviors and Screening Utilization among Women: Cross‐Sectional Results of the 2009 BRFSS.” BMC Public Health 12: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff, L. L. , Burgio L. D., Gitlin L., Nichols L., Chaplin W., and Hardin J. M.. 2004. “Positive Aspects of Alzheimer's Caregiving: The Role of Race.” Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 59 (4): P185–90. [DOI] [PubMed] [Google Scholar]
- Schmidt, M. , and Azoulay E.. 2012. “Having a Loved One in the ICU: The Forgotten Family.” Current Opinion in Critical Care 18 (5): 540–7. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , and Beach S. R.. 1999. “Caregiving as a Risk Factor for Mortality: The Caregiver Health Effects Study.” Journal of the American Medical Association 282 (23): 2215–19. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , and Cook T.. 2011. “National Alliance for Caregiving. Caregiving Costs: Declining Health in the Alzheimer's Caregiver as Dementia Increases in the Care Recipient” [accessed on April 4, 2011]. Available at http://www.caregiving.org/pdf/research/Alzheimers_Caregiving_Costs_Study_FINAL.pdf
- Schulz, R. , Hebert R. S., Dew M. A., Brown S. L., Scheier M. F., Beach S. R., Czaja S. J., Martire L. M., Coon D., Langa K. M., Gitlin L. N., Stevens A. B., and Nichols L.. 2007. “Patient Suffering and Caregiver Compassion: New Opportunities for Research, Practice, and Policy.” Gerontologist 47 (1): 4–13. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , Beach S. R., Hebert R. S., Martire L. M., Monin J. K., Tompkins C. A., and Albert S. M.. 2009. “Spousal Suffering and Partner's Depression and Cardiovascular Disease: The Cardiovascular Health Study.” American Journal of Geriatric Psychiatry 17 (3): 246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale, C. 1991. “A Comparison of Hospice and Conventional Care.” Social Science and Medicine 32 (2): 147–52. [DOI] [PubMed] [Google Scholar]
- Smith, K. P. , and Christakis N. A.. 2008. “Social Networks and Health.” Annual Review of Sociology 34: 405–29. [Google Scholar]
- Son, J. , Erno A., Shea D. G., Femia E. E., Zarit S. H., and Stephens M. A.. 2007. “The Caregiver Stress Process and Health Outcomes.” Journal of Aging and Health 19 (6): 871–87. [DOI] [PubMed] [Google Scholar]
- Stajduhar, K. , Funk L., Toye C., Grande G., Aoun S., and Todd C.. 2010. “Part 1: Home‐Based Family Caregiving at the End of Life: A Comprehensive Review of Published Quantitative Research (1998‐2008).” Palliative Medicine 24 (6): 573–93. [DOI] [PubMed] [Google Scholar]
- Stroebe, M. , Schut H., and Stroebe W.. 2007. “Health Outcomes of Bereavement.” Lancet 370 (9603): 1960–73. [DOI] [PubMed] [Google Scholar]
- Stuart, E. A. 2010. “Matching Methods for Causal Inference: A Review and a Look Forward.” Statistical Science 25 (1): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow, B. J. , Wisniewski S. R., Belle S. H., Rubert M., Ory M. G., and Gallagher‐Thompson D.. 2004. “Positive Aspects of Caregiving: Contributions of the REACH Project to the Development of New Measures for Alzheimer's Caregiving.” Research on Aging 26 (4): 429–53. [Google Scholar]
- Teno, J. M. , Clarridge B. R., Casey V., Welch L. C., Wetle T., Shield R., and Mor V.. 2004. “Family Perspectives on End‐of‐Life Care at the Last Place of Care.” Journal of the American Medical Association 291 (1): 88–93. [DOI] [PubMed] [Google Scholar]
- Teno, J. M. , Mor V., Ward N., Roy J., Clarridge B., Wennberg J. E., and Fisher E. S.. 2005. “Bereaved Family Member Perceptions of Quality of End‐of‐Life Care in U.S. Regions with High and Low Usage of Intensive Care Unit Care.” Journal of the American Geriatrics Society 53 (11): 1905–11. [DOI] [PubMed] [Google Scholar]
- Teno, J. M. , Gozalo P. L., Bynum J. P., Leland N. E., Miller S. C., Morden N. E., Scupp T., Goodman D. C., and Mor V.. 2013. “Change in End‐of‐Life Care for Medicare Beneficiaries: Site of Death, Place of Care, and Health Care Transitions in 2000, 2005, and 2009.” Journal of the American Medical Association 309 (5): 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtven, C. H. , Wilson M. R., and Clipp E. C.. 2005. “Informal Care Intensity and Caregiver Drug Utilization.” Review of Economics of the Household 3 (4): 415–33. [Google Scholar]
- Wennberg, J. E. , and Cooper M.. 2013. “The Dartmouth Atlas of Health Care” [accessed on September 17, 2013]. Available at http://www.dartmouthatlas.org/
- Wright, A. A. , Zhang B., Ray A., Mack J. W., Trice E., Balboni T., Mitchell S. L., Jackson V. A., Block S. D., Maciejewski P. K., and Prigerson H. G.. 2008. “Associations between End‐of‐Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment.” Journal of the American Medical Association 300 (14): 1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, A. A. , Keating N. L., Balboni T. A., Matulonis U. A., Block S. D., and Prigerson H. G.. 2010. “Place of Death: Correlations with Quality of Life of Patients with Cancer and Predictors of Bereaved Caregivers’ Mental Health.” Journal of Clinical Oncology 28 (29): 4457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, A. A. , Keating N. L., Ayanian J. Z., Chrischilles E. A., Kahn K. L., Ritchie C. S., Weeks J. C., Earle C. C., and Landrum M. B.. 2016. “Family Perspectives on Aggressive Cancer Care Near the End of Life.” Journal of the American Medical Association 315 (3): 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1. Author Matrix.
Table S1. Association between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Post‐death among Twelve‐Month Survivors.
Table S2. Medicare Spending by Location of Death and Baseline Spending Quartile.
Table S3. Association Between In‐Hospital Death and Medicare Expenditures and Utilization for Bereaved Spouses Postdeath after Controlling for Predeath Spending Data.


