Abstract
Background
Infection with Helicobacter pylori (H. pylori) is a major pathogenic factor for gastroduodenal ulcer disease and gastric carcinoma, as well as for other types of gastric and extragastric disease. As a result of changing epidemiologic conditions (e.g., immigration), changing resistance patterns with therapeutic implications, and new knowledge relating to the indications for pathogen eradication, the medical management of H. pylori is a dynamic process in need of periodic reassessment.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed and the Cochrane Database, with particular attention to three international consensus reports and the updated German S2k guideline.
Results
H. pylori is now dealt with as an infection, whether or not the infected individual has symptoms or suffers from an H.-pylori-induced illness. H.-pylori-associated dyspepsia and functional dyspepsia are distinct entities that can only be diagnosed when competing elements in the differential diagnosis have been ruled out. H. pylori can be detected with noninvasive methods (13C-urea breathing test, stool antigen detection) and with invasive methods (histology, culture, rapid urease test). An important consideration for treatment is that primary clarithromycin resistance is common in many groups of patients; in Germany, its prevalence is now 10.9%. Primary treatment can be with either standard triple therapy (clarithromycin and amoxicillin or metronidazole) or bismuth-containing quadruple therapy. Treatment for 10 to 14 days is more likely to eradicate the pathogen than treatment for 7 days. When H. pylori infection is initially diagnosed in a patient over age 50, gastritis risk stratification should be performed by means of endoscopic biopsy and histologic examination.
Conclusion
The new, clinically relevant developments that are presented and commented upon in this review now enable evidence-based management of H. pylori infection.
The description of Helicobacter pylori in 1984 was an important milestone in the development of gastroenterology all over the world (1). The clinical implications of this infection were only gradually recognized in the years that followed.
Over the past three decades, many national and international expert groups have issued recommendations on the diagnosis and treatment of H. pylori infection based on the best available evidence.
Definition.
Helicobacter pylori (H. pylori) is now considered an infectious disease regardless of whether the affected individual has any symptoms or sequelae.
This was entirely reasonable in view of the changing epidemiologic conditions and resistance patterns, with resulting changes in the therapeutic implications.
Moreover, new knowledge has been gained with respect to the indications for preventive or therapeutic pathogen eradication.
This dynamic process expressed itself recently in the appearance of four new guidelines: three international consensus reports (2– 4) and the updated German S2k guideline (5). In this article, we present the most important recent developments on the basis of a selective review of the literature, with special attention to the new guidelines.
Significance.
H. pylori infection is a major element in the pathogenesis of gastroduodenal ulcer disease and gastric carcinoma.
We devote special consideration to the convergent and divergent assessments and recommendations of these guidelines, focusing on their clinical relevance, with commentary by the authors.
Learning objectives
After reading this article, the reader should know:
that H. pylori induces chronic gastritis, which, in turn, promotes the development of dyspepsia, ulcers, gastric carcinoma, and MALT (mucosa-associated lymphoid tissue) lymphoma;
how to take epidemiologic data into consideration when deciding how to treat asymptomatic persons with an H. pylori infection in Germany;
what diagnostic methods are available, and how to provide appropriate treatment.
Indications for Helicobacter pylori eradication.
Peptic ulcer, gastric MALT lymphoma, functional dyspepsia after esophagogastroduodenoscopy, idiopathic thrombocytopenic purpura, iron-deficiency anemia of unclear cause, and before long-term treatment with ASA or NSAID in a patient with a history of ulcer disease.
Helicobacter pylori is an infectious disease
It was first explicitly formulated in the Kyoto Global Consensus Report that H. pylori gastritis should be considered an infectious disease regardless of whether the affected individual has any symptoms, complications, or consequent illnesses (2). The European Maastricht V/Florence consensus statement accordingly stipulates that any person with H. pylori infection should undergo treatment to eradicate the pathogen (3). The advocates of this concept argue that H. pylori can lead, in an unpredictable way, to ulcer disease, gastric carcinoma, and gastric MALT lymphoma. Successful pathogen eradication heals H.-pylori-induced gastritis and thereby prevents further sequelae. Critics maintain that this concept goes too far, as it leads to eradication treatments that go beyond the established indications and ultimately to a screen-and-treat mentality through which many asymptomatic persons will be unnecessarily treated. They point to the associated high costs, the risk of antibiotic resistance, and other potential adverse consequences of H. pylori eradication.
These adverse consequences, however, are either speculative or inconsistently documented in the literature. For example, in the prospective NHANES study, no association between H. pylori status and overall mortality was detected in a group of 10 000 participants (6).
This study is vulnerable to the criticism that it was carried out in an American population with elevated cardiovascular mortality and a low prevalence of both H. pylori infection and gastric carcinoma. Nevertheless, a significant association was found between H. pylori infection and mortality from gastric carcinoma (hazard ratio 40.95, 95% confidence interval [CI] [4.19; 399], p = 0.0026).
Mass screening for H. pylori.
The European guideline advises a test-and-treat strategy in patients with dyspepsia that has not yet been investigated, but the German guideline advises against noninvasive testing for H. pylori infection (to be followed by pathogen eradication) in persons with upper abdominal symptoms (dyspepsia).
Is mass population-based screening for H. pylori even a realistic scenario? A study from China has shown that mass intervention for the detection and subsequent treatment of H. pylori infection is feasible (7). Further data on the degree to which this lessens the incidence of carcinoma (the ultimate objective of the study) are eagerly awaited. The European guideline advises a test-and-treat strategy for patients with dyspepsia that has not yet been investigated (3), but the German guideline advises against noninvasive testing for H. pylori infection (followed, if positive, by pathogen eradication) in persons with upper abdominal symptoms (dyspepsia), as the prevalence of H. pylori in Germany is low at only 20–40% (5).
This article centers on how best to manage patients with dyspeptic symptoms; we will not discuss disease prevention in asymptomatic persons.
Helicobacter-pylori-associated dyspepsia and functional dyspepsia
The recommendation contained in the German S3 guideline of 2009 (8) to the effect that patients with functional dyspepsia and H. pylori infection can be treated with eradication of the pathogen has been kept unaltered in the updated guideline (5). This recommendation is based on the finding that, in patients with dyspepsia of more than 4–12 weeks’ duration for which organic causes have been excluded by endoscopy, successful H. pylori eradication leads to a 10 to 15% higher rate of lasting symptomatic relief (or at least symptomatic improvement) than can be achieved with placebo or other drugs (9, 10). A recent meta-analysis confirmed that symptomatic improvement is more common after pathogen eradication than in untreated controls (odds ratio [OR] 1.38, 95% CI [1.18; 1.62], p<0.001) (11).
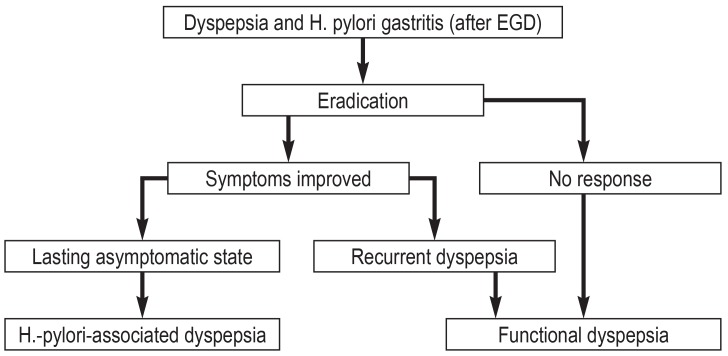
It was first postulated in the Kyoto consensus conference (2) and the Maastricht V/Florence consensus report (3) that H.-pylori-associated dyspepsia and functional dyspepsia should be considered two distinct entities, and that functional dyspepsia should only be diagnosed when H. pylori infection has been excluded. This implies a diagnostic-therapeutic algorithm such as the one shown in Figure 1. In this context, Maastricht V/Florence further specified that an endoscopically-based strategy is a consideration mainly in populations with a low prevalence of H. pylori. The German guideline postulates that testing for H. pylori with ensuing eradication should always be carried out in conjunction with esophagogastroduodenoscopy; it also mentions the low prevalence in the German population (5).
Figure 1.
An algorithm for the diagnosis and treatment of H.-pylori-associated dyspepsia and functional dyspepsia. EGD, esophagogastroduodenoscopy.
Diagnostic evaluation
Eradication in patients with functional dyspepsia.
Successful H. pylori eradication leads to a 10 to 15% higher rate of lasting symptomatic relief, or at least symptomatic improvement, than can be achieved with placebo or other drugs.
The urea breath test and the stool antigen test with monoclonal antibodies are reliable non-invasive methods for the detection of H. pylori infection that are just as sensitive and specific as the invasive tests (12, 13). In a recent Cochrane analysis, data from 99 studies were used to compare the diagnostic accuracy of four non-invasive tests for the detection of H. pylori infection (14). An indirect comparison of these tests did, indeed, yield statistical evidence of differences in diagnostic accuracy. The diagnostic odds ratios were 153 [95% CI 73.7; 316] for the 13C-urea breath test, 105 [74.0; 150] for the 14C-urea breath test, 47.4 [25.2; 88.1] for serology, and 45.1 [24.2; 84.1] for stool antigen detection. The 90% median specificity of testing and the overall 53.7% prevalence of H. pylori in the studies included in the meta-analysis together imply that there will be 46 false positive findings for every 1000 subjects tested for H. pylori. The rates of false negative test results in this hypothetical patient cohort would be 30 [95% CI: 15; 58] for the 13C-urea breath test, 42 [30; 58] for the 14C-urea breath test, 86 [50; 140] for serology, and 89 [52; 146] for the stool antigen test. The authors conclude that, in persons who have not undergone gastrectomy and who have not used antibiotics or protein-pump inhibitors recently, the breath tests are, in fact, more diagnostically accurate than serology or stool antigen detection. Adequate evidence from direct comparison studies was unavailable. It is of practical relevance, too, that specific threshold values for the individual testing methods were not identified.
Diagnostic evaluation.
The urea breath test and the stool antigen test with monoclonal antibodies are reliable non-invasive methods for the detection of H. pylori infection that are just as sensitive and specific as the invasive tests.
In view of the low to moderate prevalence of H. pylori infection in Germany (depending on region and age group), the German guideline demands two positive test findings to establish the diagnosis. In practice, this requirement is met by the histological demonstration of H. pylori combined with chronic active gastritis: the latter serves as additional evidence for bacterial infection of the gastric mucosa. According to the international consensus reports, a single positive non-invasive test is adequate grounds for eradication treatment in patients with dyspepsia who have no alarming symptoms (2, 3). There is, however, universal agreement that patients aged 50 or older who are given an initial diagnosis of H. pylori infection should undergo endoscopy and histologic evaluation so that their gastritis can be accurately classified. For this purpose, two biopsy specimens each are taken from the antrum and the body of the stomach—one each from the lesser and the greater curvature. A further biopsy from the plica angularis is optional; this is the site at which precancerous lesions usually appear. All of the guidelines are in favor of risk stratification by the OLGA or OLGIM scheme, enabling estimation of the risk of gastric carcinoma on the basis of the severity, extent, and location of gastric atrophy. A practical consequence of this is that endoscopic biopsy surveillance is recommended once every three years in patients with advanced, multifocal atrophy, even after H. pylori has been eradicated (15).
Checking on the success of treatment.
Any treatment that is carried out to eradicate the pathogen should be followed up by testing to determine the success of treatment; this can be done with a breath test or stool antigen test, in cases where follow-up endoscopy is not already indicated for other reasons.
Aside from these considerations for primary diagnostic evaluation, it must be explicitly emphasized that a test to detect H. pylori should only be performed if a positive finding would be followed by a therapeutic intervention to eliminate the pathogen. Diagnosis without treatment, i.e., a positive finding that is not followed by treatment, is hard to justify to patients, as well as being economically senseless and medically irresponsible in view of the risks of diagnostic testing. Moreover, any treatment that is carried out to eradicate the pathogen should be followed-up by testing to determine the success of treatment; this can be done with a breath test or stool antigen test, in cases where follow-up endoscopy is not already indicated for other reasons. It should be borne in mind that any check on the success of treatment should be carried out four to six weeks after the end of treatment with antibiotics and/or proton-pump inhibitors (PPI). This requirement is derived from the fact that 3.5 billion defined daily doses (DDD) of PPI are taken in Germany each year (16).
Indications for eradication
Indications for eradication.
An especially important recommendation for clinical practice is that patients with a history of ulcer disease should undergo testing for, and eradication of, H. pylori before the initiation of any long-term treatment with acetylsalicylic acid (ASA) or nonsteroidal anti-inflammatory drugs (NSAID).
The Maastricht V/Florence consensus report (3) and the German guideline contain essentially the same indications with respect to H. pylori eradication, differing only in minor details and recommendation grades. The indications are summarized in the Box. An especially important recommendation for clinical practice is that patients with a history of ulcer disease should undergo testing for, and eradication of, H. pylori before the initiation of any long-term treatment with acetylsalicylic acid (ASA) or nonsteroidal anti-inflammatory drugs (NSAID). It is generally accepted that ASA and NSAID elevate the risk of gastric and duodenal ulcers and ulcer-associated bleeding in persons infected with H. pylori. A meta-analysis has shown that H. pylori increases the already elevated risk of an ulcer in persons taking NSAID by a factor of 3.5 (OR 3.53 [2.16; 5.75]) (17). Conversely, NSAID increase the already elevated risk of an ulcer in a person infected with H. pylori by a factor of 3.5 as well (OR 3.55; [1.26; 9.96]). Helicobacter pylori and NSAID are individually associated with a 1.79-fold and 4.85-fold elevation of the risk of ulcer bleeding, with double the risk in patients with both risk factors (OR 6.13 [3.93; 9.56]), while H. pylori eradication halves the risk of an ulcer (OR 0.43 [0.20; 0.93]) (18). A subgroup analysis has shown, however, that eradication only protects NSAID-naïve patients, not patients being treated with NSAID. The evidence regarding low-dose ASA is not entirely clear, but it is recommended that the pathogen should be eradicated in the subgroup of patients with a history of an ulcer (3, 5). Thus, in general, there seems to be a preventive effect of H. pylori eradication in patients taking either NSAID or ASA. It must nonetheless be stressed that, strictly speaking, this situation necessitates the performance of two non-invasive tests that are not reimbursable in Germany when performed for this purpose.
BOX. Indications for Helicobacter pylori eradication (5).
peptic ulcer disease
gastric MALT lymphoma
functional dyspepsia after esophagogastroduodenoscopy
idiopathic thrombocytopenic purpura (ITP)
iron deficiency of unexplained cause (after adequate diagnostic investigation)
in a patient with a history of peptic ulcer disease before the initiation of long-term treatment with acetylsalicylic acid (ASA) or a nonsteroidal anti-inflammatory drug (NSAID)
upper gastrointestinal hemorrhage under treatment with ASA or NSAID
prophylaxis against gastric carcinoma in a patient at risk
This is no longer true for patients who sustain a hemorrhage while taking ASA or NSAID, as such patients generally undergo endoscopy. If pharmacotherapy is to be reinstated later on, this must be done with caution. The protective effect of successful H. pylori eradication is adequate in patients who resume ASA use and comparable with that of long-term treatment with omeprazole (recurrent ulcer bleeding within six months in 1.9% or 0.9% of patients, respectively) (19). On the other hand, patients who take NSAID must take an accompanying PPI as well (18.8% recurrent ulcer bleeding after H. pylori alone, compared to 4.4% with omeprazole) (20).
Incidence of gastric carcinoma.
The risk of gastric carcinoma in Germany is relatively low (incidence 15.6 per 100 000 persons per year for men and 8.2 per 100 000 persons per year for women in 2012, according to data of the Robert Koch Institute).
H. pylori is the main risk factor for gastric carcinoma, as has been concluded in all consensus reports and guidelines, without exception (2– 5). In parts of the world where the prevalence of gastric carcinoma is high, e.g., the Far East, Maastricht V/Florence recommends a screen-and-treat strategy (3). The risk of gastric carcinoma in Germany is relatively low (incidence 15.6 per 100 000 persons per year for men and 8.2 per 100 000 persons per year for women in 2012, according to data of the Robert Koch Institute). Therefore, according to the German guideline, H. pylori eradication to prevent cancer should only be performed in persons at special risk. Such persons include the first-degree relatives of patients with gastric carcinoma, persons with a type of gastritis that puts them at elevated risk (pangastritis or gastritis mainly affecting the corpus), persons who have undergone the endoscopic or surgical resection of gastric adenomas or early-stage carcinomas, persons with multifocal atrophy, and persons taking PPI over the long term. The last-mentioned risk factor is the least well-documented. A systematic review of the literature that took 16 studies into account, including a total of 1920 patients using PPI, did not reveal any elevated risk of gastric tumors (21). On the other hand, H. pylori eradication has been found to prevent the progression of intestinal metaplasia (a precancerous lesion) is persons taking esomeprazole over the long term (22). A recent study has shown that the risk of gastric carcinoma is elevated in persons who have been taking PPI for a long time even if H. pylori has been eradicated (23).
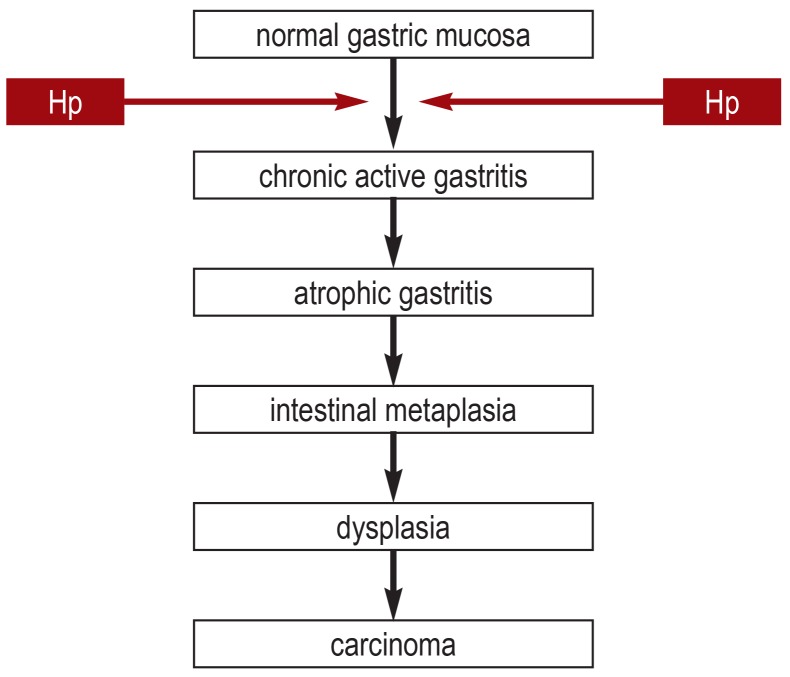
The timing of treatment has a major effect of the efficacy of H. pylori eradication to prevent gastric carcinoma (24). There is a protective effect mainly when no pre-neoplastic changes such as atrophy or intestinal metaplasia have yet arisen (24– 26). This has led to the postulation of a point of no return in the pathogenesis of gastric carcinoma, beyond which malignant changes are inevitable (27) (figure 2). We now know that H. pylori eradication can protect the patient from recurrent carcinoma even in the presence of advanced changes, e.g., after the resection of early gastric carcinoma (28– 34). A recent meta-analysis of 24 studies including a total of 48 064 individuals with 715 cases of gastric carcinoma revealed that the risk of gastric carcinoma was cut in half after successful H. pylori eradication (OR 0.54 [0.46; 0.65]) (35). The same meta-analysis showed that the preventive effect of H. pylori eradication is higher in proportion to the underlying incidence of gastric carcinoma in the population in question, although it was detectable to variable extents in all populations and in all types of patient (patients at elevated risk and asymptomatic persons). It must be borne in mind that H. pylori eradication does not fully eliminate the risk of gastric carcinoma. This was the conclusion of a cohort study of patients with intestinal metaplasia and severe atrophy, in whom the risk of developing gastric carcinoma was still elevated even after successful pathogen eradication (36).
Figure 2.
The pathogenesis of gastric carcinoma (after [27]).
Hp, Helicobacter pylori
The treatment of H. pylori infection
The treatment of Helicobacter pylori infection.
Antibiotic treatment for the eradication of H. pylori should be carefully selected with special attention to the resistance pattern.
An accepted indication and the detection of the pathogen are prerequisites to eradication treatment (5). What is the concrete procedure for H. pylori eradication? A network meta-analysis on the first-line treatment of H. pylori infection has shown that standard triple therapies with clarithromycin and amoxicillin or metronidazole for seven days lead to an eradication rate of only 73% (37), rather than the required quality criterion of 80% (5). This criterion is met, however, if the duration of treatment is extended to 10–14 days of if quadruple therapy is provided. The Toronto consensus report stipulates 14 days of treatment (4), and so does Maastricht V/Florence in somewhat weaker form (3). The German guideline envisions 7 to 14 days of standard triple therapy and 10 days of quadruple therapy including bismuth or triple therapy including fluoroquinolone (5). We find that giving triple therapy for only seven days is no longer acceptable.
A major reason for the failure of standard triple therapy is to be found in primary clarithromycin resistance, which is present in Europe at highly variable prevalence (6–37 %) (38); its prevalence is Germany is 10.9% and rising (39). Any recommendation for treatment must take this state of affairs into account. The first thing to be considered is the probability of primary resistance to clarithromycin and other antibiotics (3– 5), which can only be estimated qualitatively or, at best, semiquantitatively. The rate of clarithromycin resistance is said to be low when it is below 15% and high when it is above 15%.
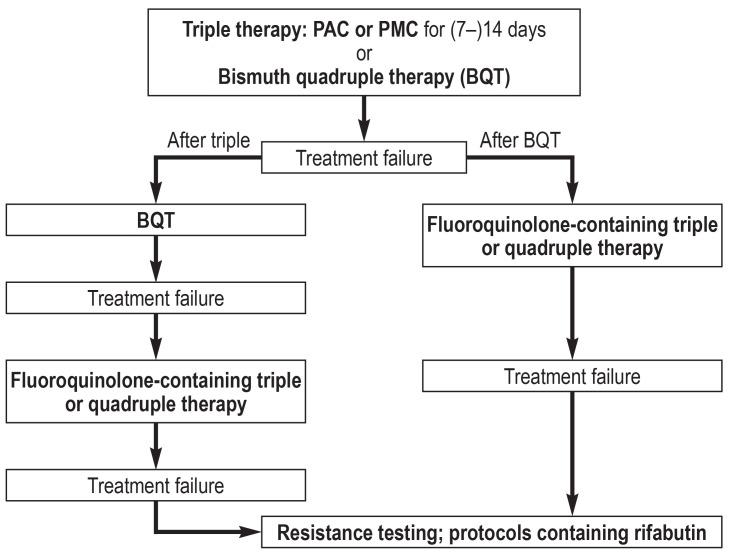
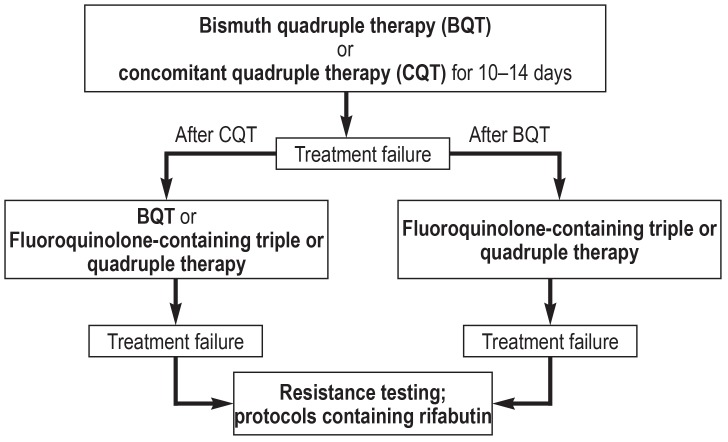
A treatment algorithm for patients with a low rate of primary clarithromycin resistance is shown in Figure 3, while one for patients with a high rate is shown in Figure 4. These two figures contain all of the relevant treatment recommendations found in the guidelines (3– 5). Southern or eastern European ethnic origin and prior treatment with macrolides are risk factors for clarithromycin resistance. The latter, however, can hardly be ascertained by history-taking, but frequent antibiotic use in the past can be taken pragmatically as an indirect index of macrolide use.
Figure 3.
Treatment algorithm for H. pylori eradication for patient groups wih low primary clarithromycin resistance. PAC, proton-pump inhibitor, amoxicillin, and clarithromycin; PMC, proton-pump inhibitor, metronidazole, and clarithromycin.
Figure 4.
Treatment algorithm for H. pylori eradication in patient groups with a high rate of primary clarithromycin resistance.
Important measures to promote the success of treatment include detailed patient education about how to take the medications, including motivation of the patient to adhere to the prescribed course of treatment. There is no unanimous judgment regarding the use of probiotic agents: the German guideline envisions their potential use to improve the tolerability of eradication treatment (5), while the Canadian consensus report rejects their use in routine situations (4).
A meta-analysis of 19 randomized and controlled trials that could not be considered in the creation of the guideline led to the finding that individual types of probiotic agent can prevent side effects of treatment and thereby improve the success of H. pylori eradication (40).
Probiotic agents.
Individual types of probiotic agent can prevent side effects of treatment and thereby improve the success of H. pylori eradication.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 16 September 2018. Submissions by letter, e-mail or fax cannot be considered.
-
The following CME units can still be accessed for credit:
-
“Hints on Diagnosing and Treating Headache”
(issue 17/2018) until 22 July 2018
-
“The Treatment of Gliomas in Adulthood”
(issue 21/2018) until 12 August 2018
-
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 16 September 2018. Only one answer is possible per question. Please choose the most appropriate answer.
Question 1
What treatment regimen is recommended when there is a low rate of primary clarithromycin resistance?
PPI + amoxicillin + clarithromycin
PPI + tinidazole + erythromycin
PPI + tazobactan + piperacillin
PPI + ciprofloxacin + amoxicillin
PPI + fosfomycin + tazobactam
Question 2
What combination of drugs should be given after the failure of bismuth quadruple therapy?
triple therapy containing ciprofloxacin
triple therapy containing fluoroquinolone
triple therapy containing fosfomycin
dual therapy containing amoxicillin
quadruple therapy containing tinidazole
Question 3
How can Helicobacter pylori be reliably detected by noninvasive means?
with an H2 breathing test
with a stool protocol
with bioresonance therapy
with upper abdominal ultrasonography
with a urea breathing test
Question 4
The consumption of what two kinds of drugs elevates the risk of a gastric or duodenal ulcer or of an ulcer bleed in a patient infected with H. pylori?
acetylsalicylic acid and nonsteroidal anti-inflammatory drugs
opioids and sumatriptan
glucocorticosteroids and infliximab
diphenhydramine and lorazepam
trimipramine and St. John’s wort
Question 5
What is the incidence of gastric carcinoma among men in Germany?
7.6/100 000
9.6/100 000
11.6/100 000
13.6/100 000
15.6/100 000
Question 6
According to the Toronto consensus report, what should be the duration of treatment to eradicate Helicobacter pylori infection?
8 days
10 days
12 days
14 days
16 days
Question 7
What is the main reason for the failure of standard triple therapy to eradicate Helicobacter pylori?
primary clarithromycin resistance
poor patient compliance
intolerable side effects of the drugs used
elevated pH tolerance of H. pylori
impaired absorption by the cytochrome P450 system
Question 8
What diagnostic method serves as the basis for the precise classification of gastritis recommended for patients who receive an initial diagnosis of gastritis at age 50 or above?
stool antigen test
14C-urea breathing test
2 biopsies each from antrum and corpus
testing for a CYP2C19 gene defect
colonoscopy for differential diagnosis
Question 9
What does the current German guideline have to say about the role of probiotic agents in the treatment of Helicobacter pylori?
They make eradication less sucessful.
They should not be used in routine practice.
They may make eradication treatment easier to tolerate.
They prevent the development of antibiotic resistance.
They obviate the need for proton-pump inhibitors.
Question 10
What is the threshold level for the rate of clarithromycin resistance to be called “high”?
>5%
>10%
>15%
>30%
>45%
►Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Fischbach has received payment from Aptalis for authorship of a publication. He has also received payment from Aptalis and Allergan for the preparation of continuing medical education events, as well as financial support from Aptalis for a research project that he initiated.
Prof. Malfertheiner has served as a paid advisor for biohit and Allergan. He has received payment from Allergan, Biocodex, and Bayer for the preparation of scientific meetings.
References
- 1.Warren JR, Marshall BL. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;8390:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O`Morain CA, et al. Management of Helicobacter pylori infection - the Maastricht V/Florence Consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 4.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterol. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Fischbach W, Malfertheiner P, Lynen Jansen P, et al. S2k-guideline Helicobacter pylori and gastroduodenal ulcer disease. Z Gastroenterol. 2017;54:167–206. doi: 10.1055/s-0042-119653. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62:1262–1269. doi: 10.1136/gutjnl-2012-303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Kf, Zhang L, Gerhard M, et al. A Large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9–18. doi: 10.1136/gutjnl-2015-309197. [DOI] [PubMed] [Google Scholar]
- 8.Fischbach W, Malfertheiner P, Hoffmann JC, et al. S3-Leitlinie Helicobacter pylori und gastroduodenale Ulkuskrankheit. Z Gastroenterol. 2009;47:68–102. doi: 10.1055/s-0028-1109062. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Mossner J, Fischbach W, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615–625. doi: 10.1046/j.1365-2036.2003.01695.x. [DOI] [PubMed] [Google Scholar]
- 10.Moayyedi P, Soo S, Deeks JJ, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011;16(2) doi: 10.1002/14651858.CD002096.pub5. CD002096. [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Zhao J, Cheng WF, et, et al. J Clin Gastroenterol. 2014;48:241–247. doi: 10.1097/MCG.0b013e31829f2e25. [DOI] [PubMed] [Google Scholar]
- 12.Gisbert JP, Pajares JM. C-urea breath test in the diagnosis of Helicobacter pylori infection - a critical review. Aliment Pharmacol Ther. 2004;20:1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 13.Gisbert JP, de la MF, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and metaanalysis. Am J Gastroenterol. 2006;101:1921–1930. doi: 10.1111/j.1572-0241.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- 14.Best LMJ, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database of Syst Rev. 2018 doi: 10.1002/14651858.CD012080.pub2. CD012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mössner J. The indications, applications, and risks of proton pump inhibitors—a review after 25 years. Dtsch Arztebl Int. 2016;113:477–483. doi: 10.3238/arztebl.2016.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a metaanalysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 18.Vergara M, Catalan M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21:1411–1418. doi: 10.1111/j.1365-2036.2005.02444.x. [DOI] [PubMed] [Google Scholar]
- 19.Chan FKL, Ching JYL, Suen BY, et al. Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterol. 2013;144:528–535. doi: 10.1053/j.gastro.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Lai KC, Lam SK, Chu KM, et al. Lansoprazole reduces ulcer relapse after eradication of Helicobacter pylori in nonsteroidal anti-inflammatory drug users-a randomized trial. Aliment Pharmacol Ther. 2003;18:829–836. doi: 10.1046/j.1365-2036.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 21.Lundell L, Vieth M, Gibson F, et al. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 22.Yang HB, Sheu BS, Wang ST, et al. H pylori eradication prevents the progression of gastric intestinal metaplasia in reflux esophagitis patients using long-term esomeprazole. Am J Gastroenterol. 2009;104:1642–1649. doi: 10.1038/ajg.2009.172. [DOI] [PubMed] [Google Scholar]
- 23.Cheung KS, Chan EW, Wong AYS, et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 24.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348 doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 26.Shiotani A, Uedo N, Iishi H, et al. Predictive factors for metachronous gastric cancer in high- risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113–119. doi: 10.1159/000173719. [DOI] [PubMed] [Google Scholar]
- 27.Correa, P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 28.Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39–46. doi: 10.1016/j.gie.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open- label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 30.Kwon YH, Heo J, Lee HS, et al. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39:609–618. doi: 10.1111/apt.12633. [DOI] [PubMed] [Google Scholar]
- 31.Seo JY, Lee DH, Cho Y, et al. Eradication of Helicobacter pylori reduces metachronous gastric cancer after endoscopic resection of early gastric cancer. Hepatogastroenterology. 2013;60:776–780. doi: 10.5754/hge12929. [DOI] [PubMed] [Google Scholar]
- 32.Toyokawa T, Suwaki K, Miyake Y, et al. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. 2010;25:544–547. doi: 10.1111/j.1440-1746.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- 33.Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst. 20141;06 doi: 10.1093/jnci/dju116. dju116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–1095. doi: 10.1056/NEJMoa1708423. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and metaanalysis. Gastroenterol. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Shichijo S, Hirata Y, Nikura R, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618–624. doi: 10.1016/j.gie.2016.03.791. [DOI] [PubMed] [Google Scholar]
- 37.Li BZ, Threapleton DE, Wang JY, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ. 2015;351 doi: 10.1136/bmj.h4052. h4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Megraud F, Coenen S, Versporten A, et al. (Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 39.Selgrad M, Meile J, Bornschein J, et al. Antibiotic susceptibility of Helicobacter pylori in central Germany and its relationship with the number of eradication therapies. Eur J Gastroenterol Hepatol. 2013;25:1257–1260. doi: 10.1097/MEG.0b013e3283643491. [DOI] [PubMed] [Google Scholar]
- 40.McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4:546–561. doi: 10.1177/2050640615617358. [DOI] [PMC free article] [PubMed] [Google Scholar]