Abstract
Mitochondria are the major source of ATP in the cell. Five multi-subunit complexes in the inner membrane of the organelle are involved in the oxidative phosphorylation required for ATP production. Thirteen subunits of these complexes are encoded by the mitochondrial genome often referred to as mtDNA. For this reason, the expression of mtDNA is vital for the assembly and functioning of the oxidative phosphorylation complexes. Defects of the mechanisms regulating mtDNA gene expression have been associated with deficiencies in assembly of these complexes, resulting in mitochondrial diseases. Recently, numerous factors involved in these processes have been identified and characterized leading to a deeper understanding of the mechanisms that underlie mitochondrial diseases.
Keywords: mitochondria, trascription, translation
Introduction
Mitochondrial gene expression is central to maintaining cellular homoeostasis. The control of mitochondrial gene expression is unique in that its components have dual origins in the mitochondria (all RNAs) and nucleus (all protein factors). The regulation of the synthesis and degradation of mitochondrial (mt-) RNAs determines steady-state levels of mitochondrially encoded proteins allowing fine control of the mitochondrial energy metabolism. Therefore, the cell can adapt to changing environmental stresses and satisfy changing cellular energy demands. Defects in the mitochondrial gene expression can lead to respiratory chain dysfunction resulting in a multi-system disease phenotype, predominantly affecting muscular and neuronal tissues.
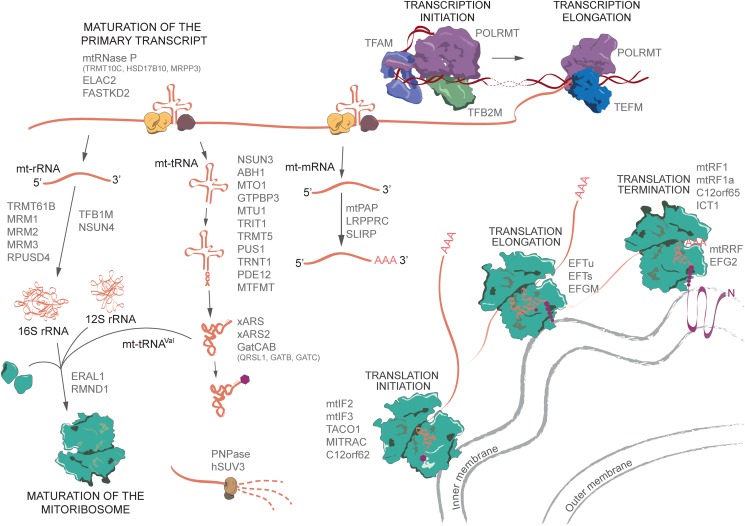
The mammalian mitochondrial genome is highly condensed as far as genetic information is concerned. The mitochondrial genome encodes 2 rRNAs, 22 tRNAs and mRNAs for 13 polypeptides of the oxidative phosphorylation (OxPhos) system. In some cases, the reduction of the mitochondrial genome has led to overlapping genes (MT-ATP8/6, MT-ND4/4L and mt-tRNATyr/mt-tRNACys). The entire mitochondrial genome is transcribed from both the strands as long polycistronic transcripts. These strands are named heavy (H) or light (L) based on their buoyancy in caesium chloride density gradients. The long polycistronic transcripts require multiple processing steps before individual RNA species become functional. After endonucleolytic cleavage of the primary transcript, the ribosomal RNAs undergo chemical modifications before it can function correctly within the mitoribosome, the tRNAs also undergo a large number of chemical modifications, in addition to further polymerization and aminoacylation, and the mRNAs are differentially polyadenylated. Finally, the mRNAs, tRNAs and the assembled mitoribosome come together in the translation apparatus where translational factors direct the progression of translation (Figure 1).
Figure 1. Overview of human mitochondrial transcription, RNA processing and translation.
The list of proteins mentioned in the figure are biased towards those that are associated with mitochondrial diseases, as explained in the article by Boczonadi et al. [1]. Aminoacyl-tRNA synthetases are abbreviated as xARS and xARS2.
In this article, we briefly overview the key stages of mitochondrial gene expression in humans, providing a useful basis for the article by Boczonadi et al. that deals with human diseases resulting from the defects in this pathway [1]. The main goal of our article is to present the basic mitochondrial function of protein factors that have been associated with mitochondrial disease, therefore, some proteins known to be involved in mitochondrial transcription and translation might have not been described in this brief overview.
Mitochondrial transcription
Transcription of the mitochondrial genome originates in the major non-coding region containing the L-strand (LSP) and H-strand (HSP) promoters. The light strand promoter controls the transcription of eight of the tRNAs and the MT-ND6 gene. On the heavy strand, two H-strand two-promoter systems have historically been proposed, where HSP1 transcription produces a transcript containing tRNAPhe, tRNAVal and the two rRNAs (12S and 16S), while transcription from HSP2 generates a transcript that spans almost the entire genome [2–4]. This two-promoter model of H-strand transcription was proposed to explain the high abundance of the two rRNAs. However, more recent animal models [5] and in vitro [6] experiments suggest that heavy strand transcription is under the control of a single promoter and that the difference in rRNA abundance may be a consequence of differential turnover.
Transcription initiation
Transcription in human mitochondria is driven by a DNA-dependant RNA polymerase called POLRMT, which is structurally similar to RNA polymerases in T3 and T7 bacteriophages [7,8]. This includes high sequence homology to the C-terminal catalytic core of the enzyme [9]. At the N-terminal domain, POLRMT also contains two pentatricopeptide repeat (PPR) domains, commonly found in RNA-associated proteins, where they are required for site-specific interactions [7,10]. In contrast with bacteriophage polymerases, which can recognize promoter regions without auxiliary proteins, additional factors are required to perform this function by POLRMT. The initiation of transcription requires the association of POLRMT with mitochondrial transcription factor A (TFAM) and mitochondrial transcription factor B2 (TFB2M). TFAM is a DNA-binding protein, which, in addition to transcription activation, also packages DNA in the nucleoid [11]. TFB2M was produced as a result of a gene duplication event. TFB1M, the other product of this duplication event, is a ribosomal RNA methyltransferase (see below). Although TFB2M also contains a rRNA methyltransferase domain, the key function of this protein is DNA melting during the initiation of transcription [12–16]. Recent evidence shows that in the transcription initiation complex, at both the HSP and LSP, TFAM, bound to DNA, recruits POLRMT to the promoter via its N-terminal extension. TFB2M modifies the structure of POLRMT to induce opening of the promoter [14,17,18].
Transcription elongation
POLRMT requires an additional transcription elongation factor (TEFM) for the elongation stage [19]. Recombinant TEFM strongly promotes POLRMT processivity as it stimulates the formation of longer transcripts in vitro [20]. Also, depletion of TEFM in living cells leads to a reduction in promoter–distal transcription elongation products [19]. Transcription from LSP is often prematurely terminated around the conserved sequence block 2 (CSB2) of the major non-coding region of the mitochondrial genome. The short RNA molecule produced has been suggested to play a key role in priming DNA replication as multiple RNA to DNA transition sites clustering around CSB2 [21,22] (see also the article by Maria Falkenberg [23]). Stimulation of POLRMT processivity by TEFM prevents the formation of G-quadraplexes that inhibit the progression of the elongation complex at CSB2 [20,24]. The capability of TEFM to abolish premature transcription termination has been proposed to function as the switch from replication to the transcription of the LSP-derived primary transcript [24]. Recent structural work showed that TEFM contains a pseudonuclease core that forms a ‘sliding clamp’ around the mtDNA downstream of the transcribing POLRMT, interacting with POLRMT via its C-terminal domain [25].
Transcription termination
The mechanism of termination of HSP transcription is still unclear. It was previously suggested that mitochondrial termination factor 1 (MTERF1) bends the mtDNA connecting the HSP1 promoter site and its apparent tRNALeu(UUR) termination site. MTERF1 would then induce transcription termination through base flipping and DNA unwinding [26–28]. This model was originally proposed to explain the 50-fold higher abundance of mitochondrial rRNAs [27]. However, more recent evidence contradicts this hypothesis. Studies in MTERF1 knockout mice do not show an effect on rRNA steady-state levels [5]. Their increase in abundance is probably a product of increased stability rather than due to the presence of a different promoter. Moreover, it was also recently shown that transcription from the LSP is prematurely terminated by MTERF1 at the 3′-end of the mt-rRNA coding sequence. Binding of MTERF1 to this site prevents the replication fork from progressing into the mt-rRNA genes while they are being transcribed, whilst also preventing transcription of the antisense sequence of the rRNA [5,29,30].
Maturation of the primary transcript
Transcription from the heavy and light strand promoters produces long polycistronic transcripts. The mt-rRNA coding sequences and most of the protein coding sequences are separated by mt-tRNAs. Endonucleolytic excision of these mt-tRNAs releases the mRNAs and rRNAs – a concept known as the ‘tRNA Punctuation Model’ [31,32]. The processing of mt-tRNAs from the primary transcript is performed by RNase P and RNase Z at the 5′- and 3′-end respectively. Unlike previously characterized cytoplasmic and bacterial RNase P enzymes, which contain a catalytic RNA subunit, mammalian mitochondrial RNase P is an entirely proteinaceous heterotrimeric endonuclease. This enzyme is composed of a tRNA m1R9 methyltransferase, TRMT10C (MRPP1), a member of the short-chain dehydrogenase/reductase (SDR) family, SDR5C1 (HSD17B10, MRPP2), and a protein with homology to PiIT N-terminus (PIN) domain-like metallonucleases, PRORP (MRPP3) and cleaves the primary transcript at the 5′-end of tRNAs [33]. ELAC2 is an endonuclease that executes 3′-end maturation of both mitochondrial and nuclear pre-tRNAs [34–36].
The ‘tRNA punctuation model’ does not explain all the primary transcript cleavage events, as not all mRNAs are immediately flanked by tRNAs. Various Fas-activated serine/threonine kinase (FASTK) proteins have been shown to be required for mtRNA stability and the processing of precursors, especially the non-canonical cleavage sites. They all contain a conserved nuclease fold (RAP domain); however, endonucleolytic activity has not been shown for any of the FASTK proteins [37]. For example, depletion or knockout of FASTKD2 leads to the accumulation of various cleavage precursors, especially 16 rRNA and ND6 mRNA [38,39]. Additionally, FASTK has been implicated in MT-ND6 maturation and stability, and FASTKD5, similar to FASTKD4, regulates the maturation of those precursor RNAs that cannot be processed by RNase P and ELAC2 [39,40]. Furthermore, cross-linking immunoprecipitation (CLIP)-based analysis of FADTKD2 binding sites identified 16S rRNA and ND6 as its targets [38]. Recently, FASTKD4 was shown to be required for the stable expression of several mt-mRNAs, whereas FASTKD1 had the opposite effect on the stability of the MT-ND3 mt-mRNA. Interestingly, depletion of both FASTKD1 and FASTKD4 also caused a loss of MT-ND3, suggesting that the loss of FASTKD4 is epistatic [37]. Moreover, FASTKD4 has been suggested to be responsible for the cleavage of the MT-ND5-CYB precursor. A detailed characterization of how the FASTK proteins regulate the mitochondrial transcriptome is likely to be a subject of intense study in the near future.
mRNA maturation and stability
After excision from the primary transcript, all mRNAs, except MT-ND6, undergo 3′ polyadenylation. Polyadenylation in mitochondria is performed by a homodimeric polyadenylic acid RNA polymerase (mtPAP) [41–43]. Seven of thirteen mt-mRNAs do not contain a 3′ stop codon. In these cases, 3′ adenylation completes these stop codons and thus, the open reading frame. Polyadenylation of bacterial transcripts generally mark them for degradation, whereas addition of poly(A) tails to eukaryotic, nuclear-encoded mRNA is necessary for their stability. However, in mammalian mitochondria the effect of polyadenylation on steady-state levels is mRNA-specific. For example, deadenylation decreases complex IV mt-mRNA and increases complex I mt-mRNA levels [41,44–46].
The stability of HSP-derived mitochondrial transcripts is regulated by leucine-rich penticopeptide rich domain containing protein (LRPPRC) [47]. Loss of LRPPRC reduces the steady-state levels of mRNAs whilst not affecting rRNAs and tRNAs, consequently leading to a translation defect and loss of respiratory complexes [48–51]. The presence or absence of LRPPRC in the mitochondria correlates with the level of mt-mRNA polyadenylation [52,53]. As such, LRPPRC mouse knockout models display a loss in HSP-derived transcripts, loss of poly(A) tails and a severe translational defect [50]. More recent data show that LRPPRC is a mt-mRNA chaperone that relaxes secondary structures, therefore, facilitating RNA polyadenylation and coordinated mitochondrial translation [54]. Following translocation into mitochondria, LRPPRC forms a complex with a stem–loop interacting RNA-binding protein (SLIRP) [55]. Within this complex, SLIRP stabilizes LRPPRC by protecting it from degradation [56], whilst being dispensable for polyadenylation of mtDNA-encoded mRNAs [56]. The LRPPRC–SLIRP complex has also been shown to suppress their degradation of mt-mRNAs [57].
Human mitochondrial RNA decay is mediated by a complex of polynucleotide phosphorylase (PNPase) and human Suv3 protein (hSuv3) [58]. PNPase is a 3′–5′ exoribonuclease which has been shown to localize to the intermembrane space [59], and also in distinct foci with hSuv3p and mitochondrial RNA [58]. Knockdown of PNPase leads to the increase in the half-life of mitochondrial transcripts and the accumulation of RNA decay intermediates [58]. hSuv3p is an NTP-dependent helicase. The lack of a functioning hSuv3 helicase leads to the accumulation of aberrant RNA species, polyadenylated molecules and degradation intermediates [60]. Recent evidence shows that exposure to the intercalating agent ethidium bromide (EtBr), which disrupts tRNA secondary structure, causes them to be polyadenylated. Subsequent withdrawal of EtBr causes the polyadenylated tRNAs to be rapidly degraded by the PNPase–hSuv3 degradosome [61]. Knockdown of PNPase leads to lengthening of the poly(A) tails due to inhibited tRNA turnover [61]. Controversially, the localization of PNPase in the intermembrane space has led to implications that it plays a role in the import of endogenous RNA into mitochondria. However, various pieces of evidence suggest that this is not the case and that it primarily functions in the RNA degradosome [62].
tRNA maturation
The mt-tRNAs undergo extensive post-transcriptional maturation including chemical nucleotide modifications and CCA addition at the 3′-end deadenylation. One of the key tRNA positions of chemical modification is the ‘wobble’ base (position 34) at the anticodon of the tRNAs. During translation, the appropriate amino acyl-tRNA is positioned in the mitoribosome through the accurate recognition of a cognate mRNA codon. However, since, many codons code for the same amino acid, the first position of the tRNA anticodon is chemically modified to facilitate non-Watson–Crick base pairing, therefore expanding codon recognition during mitochondrial translation. Some of the enzymes involved in modifying this position include: NSUN3 and ABH1 which are required for the introduction of 5-formylcytosine at the wobble position (f5C34) of mt-tRNAMet [63–66], MTO1 and GTPBP3 are required for the biogenesis of 5-taurinomethyluridine (τm5U) [67,68], and MTU1 (TRMU) which catalyses the 2-thiolation of 5-taurinomethylridine to form τm5s2U of a subset of mt-tRNAs [69,70].
In addition to the modification of the wobble position, position 37 downstream of the anticodon is also frequently chemically modified in order to facilitate stable codon–anticodon interactions and, therefore, increasing accuracy and fidelity of mitochondrial translation. Examples of enzymes that are responsible for modifying mt-tRNA position 37 include TRIT1 responsible for the introduction of an isopentenyl group onto N6 of 37 adenine (i6A37) in a small subset of mt-tRNAs [71] or TRMT5 which introduces N1-methylation of the 37 guanosine (m1G37) [72].
Pseudouridine (Psi), the most common RNA modification, is often referred to as the fifth nucleotide. It is a structural isomer of uridine produced by a rotation around the N3–C6 axis. Psi is generally associated with a stabilizing role, by providing structural rigidity to RNA molecules regardless of sequence or structure, and has been detected in several mt-tRNAs. PUS1 is a pseudouridine synthetase which modifies U27 and U28 on mt-tRNAs [73,74]. Recently, a pseudouridine synthetase, RPUSD4, was characterized as introducing pseudouridine at position 39 of tRNAPhe [75]. Other putative PUSs have been identified as necessary for mitochondrial translation, including RPUSD3 and TRUB2; however, their exact mtRNA targets remain to be further characterized [76,77].
The CCA found at the 3′-end of all mature mt-tRNAs is not encoded by mtDNA and is instead post-transcriptionally synthesized by tRNA-nucleotidyltransferase 1 (TRNT1): TRNT1 does not require a template sequence, instead preferentially selecting CTP and ATP for polymerization [78]. Similarly, a non-encoded 5′ guanine on mt-tRNAHis is post-transcriptionally added by 3′–5′ polymerase activity probably provided by THG1L [79]. The 3′ ends of several mt-tRNAs undergo spurious, mistargeted adenylation precluding correct aminoacylation at the 3′-end (see below). A 3′–5′ exonuclease, PDE12, is required for the removal of these spurious poly(A) tails [45,80].
tRNA aminoacylation
Mitochondrial translation requires that each tRNA is charged with the cognate amino acid. This process is mediated by the mitochondrial aminoacyl-tRNA synthetases (ARS2s), which are encoded by nuclear genes. Of these, 17 ARS2s are unique to the mitochondria, while GARS (Glycyl-tRNA synthetase) and KARS (Lysyl-tRNA synthetase) are encoded by the same loci as the cytoplasmic enzymes, with the mitochondrial isoforms being generated by alternative translation initiation (GARS) [81] or alternative splicing (KARS) [82]. Interestingly, glutaminyl mt-tRNA (mt-tRNAGln) is aminoacylated by an indirect pathway, in which it is first charged with glutamic acid (Glu) by mitochondrial glutamyl-tRNA synthetase (EARS2), after which the Glu-mt-tRNAGln is transamidated into Gln-mt-tRNAGln, using free glutamine as an amide donor [83]. This latter conversion is performed by GatCAB, the glutamyl-tRNAGln amidotransferase protein complex, which consists of three subunits: GatA (QRSL1), GatB (GATB) and GatC (GATC) [84].
Mitochondrial ribosome: structure and assembly
The mitoribosome consists of a large 39S subunit (mtLSU) and a small 28S (mtSSU) subunit. Compared with the bacterial ribosome, the mammalian mitoribosome has reduced rRNA components. To compensate for this, 36 mitochondria-specific proteins have been recruited to the ribosome, primarily found at the periphery of the complex surrounding a highly conserved catalytic core [85–89]. In the bacterial ribosome, a 5S rRNA acts as a scaffold interconnecting the LSU, SSU and the tRNAs in the intersubunit space. However, recent structures of the mitoribosome instead identified the recruitment of a mitochondrially encoded tRNA to this site (tRNAVal in humans and rat, tRNAPhe in porcine and bovine mitochondria) [88,90,91].
The maturation of the mitoribosome requires the post-transcriptional processing of the catalytic rRNA in addition to the import and assembly of about 80 nuclear-encoded proteins (MRPL and MRPS proteins). As for other ribosomes, both the small subunit and the large subunit rRNA undergo chemical nucleotide modifications. These modifications include base methylations, 2′-O-ribose methylations and pseudouridylation, with several enzymes responsible for these modifications having been identified (TRMT61B [92]; TFB1M [93]; NSUN4 [94]; MRMs [95]; RPUSD4 [76]; reviewed in [96]). For example, the A-loop region of the 16S rRNA is methylated at position U1369 and G1370 (human mtDNA numbering). This site directly interacts with the aminoacyl-tRNA [88,97]. U1369 is methylated by MRM2, which has been shown to interact with the mtLSU. Depletion of the protein leads to defective biogenesis of the mtLSU and consequently, a deficiency in translation [97]. Also, several protein factors not directly involved in rRNA modification have been identified to coordinate the assembly of the mitoribosome reviewed in [96]. For example, ERAL1, a homolog of the bacterial Era protein that belongs to the conserved family of GTP-binding proteins, has been proposed to act as an RNA chaperone that stabilizes 12S mt-rRNA during mtSSU assembly [98].
Many of the proteins involved in mitoribosome assembly and the post-transcriptional processing of the nascent transcript, including FASTK proteins, ELAC2 or RNase P (see above) are found in distinct foci called mitochondrial RNA granules (MRG). This compartmentalization has been proposed to facilitate more efficient and accurate gene expression [40,99,100]. It has been suggested that an integral inner membrane protein, RMND1, stabilizes and anchors the mitochondrial ribosome at the inner membrane, adjacent to MRGs where the mRNAs are produced and processed [101]. However, the exact function and mechanism of this protein is still unclear.
Translation
Mitochondrial translation is fully dependent on various nuclear-encoded regulatory proteins. In the mammalian mitochondria, the mitochondrial initiation factors, mtIF2 and mtIF3, control the initiation of translation [102]. During initiation, mtIF3 positions the AUG or AUA initiation codons of the mRNA at the peptidyl (P) site in the mtSSU and prevents the premature association of the mtLSU and mtSSU [103–105]. As in all protein synthesis systems, translation in mitochondria is initiated with a methionine residue. However, mitochondria differ in that only a single tRNAMet is used for both initiation and elongation. Discrimination, instead, is achieved through a post-transcriptional modification, with the aminoacylated initiator mt-tRNAMet being subjected to formylation of methionine (fMet), thereby increasing its affinity for mtIF2 [106]. mtIF2 directs the association of the fMet-tRNAMet with the mRNA, and guides the assembly of the mitochondrial monosome and the initiation of translation [107,108].
Translation in mammalian mitochondria differs from that of the cytoplasm or that of the yeast mitochondria in part due to the general absence of 5′-untranslated regions (UTRs) on mRNAs, gene-specific RNA cis-acting regulatory elements and introns. In yeast, a 5′-UTRs allow mRNA-specific, translational activators to bind and direct the mRNA into the mitoribosome for translation. However, in mammalian mitochondria, such mRNA regulatory elements have not been identified. Hence, alternative mechanisms are in place for the regulation of translation. Unlike UTR-based regulation, these protein factors have to bind directly to the mitochondrial transcript and affect gene expression. For example, various protein factors such as TACO1, MITRAC or C12orf62 have been recruited to modulate the translation of complex IV subunit CO1. Absence of any of these protein leads to a complex IV deficiency [109–111].
Elongation of translation is mediated by mitochondrial elongation factors, EFTu (TUFM), EFTs (TSFM) and EFGM (GFM1) [112,113]. In elongation, EFTu forms a complex with GTP and aminoacyl tRNA. It directs the tRNA to the acceptor (A) site where the tRNA base pairs with the mRNA at the codon–anticodon site. The hydrolysis of GTP catalyses peptide bond formation. EFTu is released and the GTP:EFTu complex is re-established by EFTs [114]. EFG1-mt causes the release of the deacetylated tRNA from the P-site, translocates the peptidyl-tRNAs from the A and P site to the P and exit (E) site, also causing the mRNA to move along by one codon.
Termination of mitochondrial translation is finally triggered by the presence of a stop codon at the A-site. Four mitochondrial proteins with homology to ribosome release factors have been identified in humans, including mtRF1, mtRF1a, C12orf65 and ICT1. These factors are characterized by the presence of a tripeptide GGQ motif that confers peptidyl-tRNA hydrolase activity [115,116]. Structural analysis of mtRF1 suggested that it is capable of recognizing the UAA and UAG stop codons, targeting ribosomes with a vacant A-site [117,118]. However, it does not exhibit release activity in vitro [119]. mtRF1a catalyses the hydrolysis of peptidyl tRNA at the UAA and UAG stop codons [120]. mtRF1a has been proposed to be sufficient for the termination of translation of all 13 mtDNA-encoded polypeptides, despite the mRNAs for MT-CO1 and MT-ND6 lacking the UAA and UAG stop codons at the end of the open reading frame (ORF). Instead, these ORFs contain in-frame AGA and AGG as the last codons respectively. AGA and AGG are used to encode Arg according to the universal genetic code; however, they are not used for this purpose in any of the mitochondria ORFs. Fine mapping of the termination codons of the mRNAs showed that these two mRNAs terminate at the UAG stop codon possibly created as a result of a −1 frameshift of the mitoribosome [121,122]. Initially, ICT1 was suggested as the protein that performed the termination function at the AGA and AGG codons. However, neither ICT1 nor C12orf65 release factor homologues containing the specific domains required for UAA and UAG stop codon recognition [116]. Recent evidence also suggests that ICT1 is capable of inducing hydrolysis of the peptidyl tRNAs in stalled mitoribosomes [119,123]. Since ICT1 is incapable of performing the peptidyl hydrolase activity, where the RNA template extends 14 nucleotides beyond the A-site, as is the case in MT-CO1 and MT-ND6, ICT1 may not be directly involved in the termination of translation of these two mRNAs [124,125].
Finally, after the release of the polypeptide, mitochondrial ribosomal recycling factors, mtRRF and EFG2 (also known as RRF2M, a homologue of EFGM) catalyse the release of the mRNAs, deacetylated tRNAs and the ribosomal subunits [126,127].
Concluding remarks
Diseases affecting mitochondrial transcription and translation, as described in the article by Boczonadi et al. [1], can have multi-systemic and severe manifestation. The development of novel, treatments of these mitochondrial diseases can be made more effective through a deeper understanding of the underlying mechanisms that cause them.
Summary
Progression of mitochondrial transcription and translation requires the sequential recruitment of different, nuclear-encoded initiation, elongation and termination factors.
Almost the entire mitochondrial genome is transcribed as long polycistronic transcripts.
Maturation of the transcripts requires endonucleolytic cleavage, but not all mRNAs are produced through RNase P and RNase Z function.
Mitochondrial mRNA steady-state levels are mainly controlled post-transcriptionally.
The role mitochondrial mRNA polyadenylation is not fully understood.
Mitochondrial tRNAs undergo extensive chemical modifications, including the addition and removal of nucleotides during their maturation.
Aminoacyl tRNA-synthetases charge tRNAs with their cognate amino acid, many of which are unique to the mitochondria.
Mammalian mitoribosomes differ considerably from other ribosomes as far as architecture and composition are concerned, with the key differences being the reversed protein:RNA mass ratio, incorporation of mtDNA-encoded structural tRNA and many novel, mitochondria-specific protein components.
The assembly of the mitoribosome assembly pathway is likely to be considerably different from its bacterial counterpart, implying the presence of mitochondria-specific regulatory factors.
Acknowledgments
We thank Dr Christopher Powell and other members of the Mitochondrial Genetics group at the MRC MBU, University of Cambridge for stimulating discussion during the course of this work.
Abbreviations
- ARS2
aminoacyl-tRNA synthetase
- CSB2
conserved sequence block 2
- FASTK
Fas-activated serine/threonine kinase
- HSP
H-strand promoter
- hSuv3
human Suv3 protein
- LRPPRC
leucine-rich penticopeptide rich domain containing protein
- LSP
L-strand promoter
- MTERF1
mitochondrial termination factor 1
- PNPase
polynucleotide phosphorylase
- SLIRP
stem–loop interacting RNA-binding protein
- TRNT1
tRNA-nucleotidyltransferase 1
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Medical Research Council, U.K. [MC_U105697135 and MC_UU_00015/4].
Author Contribution
A.R.D. contributed to drafting this review. A.R.D. and M.M. contributed to revising it and approved the final version. A.R.D. prepared the figure.
References
- 1.Boczonadi V., Ricci G., Horvath R. (2018) Mitochondrial DNA transcription and translation: clinical syndromes. Essays Biochem., 62, 321–340 10.1042/EBC20170103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang D.D. and Clayton D.A. (1984) Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell 36, 635–643 10.1016/0092-8674(84)90343-X [DOI] [PubMed] [Google Scholar]
- 3.Montoya J., Christianson T., Levens D., Rabinowitz M. and Attardi G. (1982) Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc. Natl. Acad. Sci. U.S.A. 79, 7195–7199 10.1073/pnas.79.23.7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montoya J., Gaines G.L. and Attardi G. (1983) The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell 34, 151–159 10.1016/0092-8674(83)90145-9 [DOI] [PubMed] [Google Scholar]
- 5.Terzioglu M., Ruzzenente B., Harmel J., Mourier A., Jemt E., Lopez M.D. et al. (2013) MTERF1 binds mtDNA to prevent transcriptional interference at the light-strand promoter but is dispensable for rRNA gene transcription regulation. Cell Metab. 17, 618–626 10.1016/j.cmet.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Litonin D., Sologub M., Shi Y., Savkina M., Anikin M., Falkenberg M. et al. (2010) Human mitochondrial transcription revisited: only TFAM and TFB2M are required for transcription of the mitochondrial genes in vitro. J. Biol. Chem. 285, 18129–18133 10.1074/jbc.C110.128918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringel R., Sologub M., Morozov Y.I., Litonin D., Cramer P. and Temiakov D. (2011) Structure of human mitochondrial RNA polymerase. Nature 478, 269–273 10.1038/nature10435 [DOI] [PubMed] [Google Scholar]
- 8.Tiranti V., Savoia A., Forti F., D’Apolito M.F., Centra M., Rocchi M. et al. (1997) Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the expressed sequence tags database. Hum. Mol. Genet. 6, 615–625 10.1093/hmg/6.4.615 [DOI] [PubMed] [Google Scholar]
- 9.Jeruzalmi D. and Steitz T.A. (1998) Structure of T7 RNA polymerase complexed to the transcriptional inhibitor T7 lysozyme. EMBO J. 17, 4101–4113 10.1093/emboj/17.14.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkan A., Rojas M., Fujii S., Yap A., Chong Y.S., Bond C.S. et al. (2012) A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 8, e1002910 10.1371/journal.pgen.1002910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanki T., Nakayama H., Sasaki N., Takio K., Alam T.I., Hamasaki N. et al. (2004) Mitochondrial nucleoid and transcription factor A. Ann. N. Y. Acad. Sci. 1011, 61–68 10.1196/annals.1293.007 [DOI] [PubMed] [Google Scholar]
- 12.Falkenberg M., Gaspari M., Rantanen A., Trifunovic A., Larsson N.G. and Gustafsson C.M. (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 31, 289–294 10.1038/ng909 [DOI] [PubMed] [Google Scholar]
- 13.McCulloch V., Seidel-Rogol B.L. and Shadel G.S. (2002) A human mitochondrial transcription factor is related to RNA adenine methyltransferases and binds S-adenosylmethionine. Mol. Cell. Biol. 22, 1116–1125 10.1128/MCB.22.4.1116-1125.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posse V. and Gustafsson C.M. (2017) Human mitochondrial transcription factor B2 is required for promoter melting during initiation of transcription. J. Biol. Chem. 292, 2637–2645 10.1074/jbc.M116.751008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidel-Rogol B.L., McCulloch V. and Shadel G.S. (2003) Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat. Genet. 33, 23–24 10.1038/ng1064 [DOI] [PubMed] [Google Scholar]
- 16.Sologub M., Litonin D., Anikin M., Mustaev A. and Temiakov D. (2009) TFB2 is a transient component of the catalytic site of the human mitochondrial RNA polymerase. Cell 139, 934–944 10.1016/j.cell.2009.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillen H.S., Morozov Y.I., Sarfallah A., Temiakov D. and Cramer P. (2017) Structural Basis of Mitochondrial Transcription Initiation. Cell 171, 1072e10–1081e10 10.1016/j.cell.2017.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran A., Basu U., Sultana S., Nandakumar D. and Patel S.S. (2017) Human mitochondrial transcription factors TFAM and TFB2M work synergistically in promoter melting during transcription initiation. Nucleic Acids Res. 45, 861–874 10.1093/nar/gkw1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minczuk M., He J., Duch A.M., Ettema T.J., Chlebowski A., Dzionek K. et al. (2011) TEFM (c17orf42) is necessary for transcription of human mtDNA. Nucleic Acids Res. 39, 4284–4299 10.1093/nar/gkq1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posse V., Shahzad S., Falkenberg M., Hallberg B.M. and Gustafsson C.M. (2015) TEFM is a potent stimulator of mitochondrial transcription elongation in vitro. Nucleic Acids Res. 43, 2615–2624 10.1093/nar/gkv105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham X.H., Farge G., Shi Y., Gaspari M., Gustafsson C.M. and Falkenberg M. (2006) Conserved sequence box II directs transcription termination and primer formation in mitochondria. J. Biol. Chem. 281, 24647–24652 10.1074/jbc.M602429200 [DOI] [PubMed] [Google Scholar]
- 22.Wanrooij P.H., Uhler J.P., Simonsson T., Falkenberg M. and Gustafsson C.M. (2010) G-quadruplex structures in RNA stimulate mitochondrial transcription termination and primer formation. Proc. Natl. Acad. Sci. U.S.A. 107, 16072–16077 10.1073/pnas.1006026107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falkenberg M. (2018) Mitochondrial DNA replication in mammalian cells: overview of the pathway. Essays Biochem., 62, 287–297 10.1042/EBC20170100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agaronyan K., Morozov Y.I., Anikin M. and Temiakov D. (2015) Mitochondrial biology. Replication-transcription switch in human mitochondria. Science 347, 548–551 10.1126/science.aaa0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillen H.S., Parshin A.V., Agaronyan K., Morozov Y.I., Graber J.J., Chernev A. et al. (2017) Mechanism of transcription anti-termination in human mitochondria. Cell 171, 1082e13–1093e13 10.1016/j.cell.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez-Menendez N., Fernandez-Millan P., Rubio-Cosials A., Arnan C., Montoya J., Jacobs H.T. et al. (2010) Human mitochondrial mTERF wraps around DNA through a left-handed superhelical tandem repeat. Nat. Struct. Mol. Biol. 17, 891–893 10.1038/nsmb.1859 [DOI] [PubMed] [Google Scholar]
- 27.Martin M., Cho J., Cesare A.J., Griffith J.D. and Attardi G. (2005) Termination factor-mediated DNA loop between termination and initiation sites drives mitochondrial rRNA synthesis. Cell 123, 1227–1240 10.1016/j.cell.2005.09.040 [DOI] [PubMed] [Google Scholar]
- 28.Yakubovskaya E., Mejia E., Byrnes J., Hambardjieva E. and Garcia-Diaz M. (2010) Helix unwinding and base flipping enable human MTERF1 to terminate mitochondrial transcription. Cell 141, 982–993 10.1016/j.cell.2010.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y., Posse V., Zhu X., Hyvarinen A.K., Jacobs H.T., Falkenberg M. et al. (2016) Mitochondrial transcription termination factor 1 directs polar replication fork pausing. Nucleic Acids Res. 44, 5732–5742 10.1093/nar/gkw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyvarinen A.K., Kumanto M.K., Marjavaara S.K. and Jacobs H.T. (2010) Effects on mitochondrial transcription of manipulating mTERF protein levels in cultured human HEK293 cells. BMC Mol. Biol. 11, 72 10.1186/1471-2199-11-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojala D., Montoya J. and Attardi G. (1981) tRNA punctuation model of RNA processing in human mitochondria. Nature 290, 470–474 10.1038/290470a0 [DOI] [PubMed] [Google Scholar]
- 32.Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J. et al. (1981) Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 10.1038/290457a0 [DOI] [PubMed] [Google Scholar]
- 33.Holzmann J., Frank P., Loffler E., Bennett K.L., Gerner C. and Rossmanith W. (2008) RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 135, 462–474 10.1016/j.cell.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 34.Brzezniak L.K., Bijata M., Szczesny R.J. and Stepien P.P. (2011) Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA Biol. 8, 616–626 10.4161/rna.8.4.15393 [DOI] [PubMed] [Google Scholar]
- 35.Rossmanith W. (2011) Localization of human RNase Z isoforms: dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS One 6, e19152 10.1371/journal.pone.0019152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez M.I., Mercer T.R., Davies S.M., Shearwood A.M., Nygard K.K., Richman T.R. et al. (2011) RNA processing in human mitochondria. Cell Cycle 10, 2904–2916 10.4161/cc.10.17.17060 [DOI] [PubMed] [Google Scholar]
- 37.Boehm E., Zaganelli S., Maundrell K., Jourdain A.A., Thore S. and Martinou J.C. (2017) FASTKD1 and FASTKD4 have opposite effects on expression of specific mitochondrial RNAs, depending upon their endonuclease-like RAP domain. Nucleic Acids Res. 45, 6135–6146 10.1093/nar/gkx164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popow J., Alleaume A.M., Curk T., Schwarzl T., Sauer S. and Hentze M.W. (2015) FASTKD2 is an RNA-binding protein required for mitochondrial RNA processing and translation. RNA 21, 1873–1884 10.1261/rna.052365.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonicka H. and Shoubridge E.A. (2015) Mitochondrial RNA granules are centers for posttranscriptional RNA processing and ribosome biogenesis. Cell Rep. 10.1016/j.celrep.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 40.Jourdain A.A., Koppen M., Rodley C.D., Maundrell K., Gueguen N., Reynier P et al. (2015) A mitochondria-specific isoform of FASTK is present in mitochondrial RNA granules and regulates gene expression and function. Cell Rep. 10, 1110–1121 10.1016/j.celrep.2015.01.063 [DOI] [PubMed] [Google Scholar]
- 41.Tomecki R., Dmochowska A., Gewartowski K., Dziembowski A. and Stepien P.P. (2004) Identification of a novel human nuclear-encoded mitochondrial poly(A) polymerase. Nucleic Acids Res. 32, 6001–6014 10.1093/nar/gkh923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai Y., Srivastava S.K., Chang J.H., Manley J.L. and Tong L. (2011) Structural basis for dimerization and activity of human PAPD1, a noncanonical poly(A) polymerase. Mol. Cell 41, 311–320 10.1016/j.molcel.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapkouski M. and Hallberg B.M. (2015) Structure of mitochondrial poly(A) RNA polymerase reveals the structural basis for dimerization, ATP selectivity and the SPAX4 disease phenotype. Nucleic Acids Res. 43, 9065–9075 10.1093/nar/gkv861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagaike T., Suzuki T., Katoh T. and Ueda T. (2005) Human mitochondrial mRNAs are stabilized with polyadenylation regulated by mitochondria-specific poly(A) polymerase and polynucleotide phosphorylase. J. Biol. Chem. 280, 19721–19727 10.1074/jbc.M500804200 [DOI] [PubMed] [Google Scholar]
- 45.Rorbach J., Nicholls T.J. and Minczuk M. (2011) PDE12 removes mitochondrial RNA poly(A) tails and controls translation in human mitochondria. Nucleic Acids Res. 39, 7750–7763 10.1093/nar/gkr470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wydro M., Bobrowicz A., Temperley R.J., Lightowlers R.N. and Chrzanowska-Lightowlers Z.M. (2010) Targeting of the cytosolic poly(A) binding protein PABPC1 to mitochondria causes mitochondrial translation inhibition. Nucleic Acids Res. 38, 3732–3742 10.1093/nar/gkq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterky F.H., Ruzzenente B., Gustafsson C.M., Samuelsson T. and Larsson N.-G. (2010) LRPPRC is a mitochondrial matrix protein that is conserved in metazoans. Biochem. Biophys. Res. Commun. 398, 759–764 10.1016/j.bbrc.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 48.Sasarman F., Brunel-Guitton C., Antonicka H., Wai T., Shoubridge E.A. and Consortium L. (2010) LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol. Biol. Cell 21, 1315–1323 10.1091/mbc.e10-01-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gohil V.M., Nilsson R., Belcher-Timme C.a., Luo B., Root D.E. and Mootha V.K. (2010) Mitochondrial and nuclear genomic responses to loss of LRPPRC expression. J. Biol. Chem. 285, 13742–13747 10.1074/jbc.M109.098400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruzzenente B., Metodiev M.D., Wredenberg A., Bratic A., Park C.B., Cámara Y. et al. (2012) LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 31, 443–456 10.1038/emboj.2011.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mourier A., Ruzzenente B., Brandt T., Kühlbrandt W. and Larsson N.-G. (2014) Loss of LRPPRC causes ATP synthase deficiency. Hum. Mol. Genet. 23, 2580–2592 10.1093/hmg/ddt652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson W.C., Hornig-Do H.T., Bruni F., Chang J.H., Jourdain A.A., Martinou J.C. et al. (2014) A human mitochondrial poly(A) polymerase mutation reveals the complexities of post-transcriptional mitochondrial gene expression. Hum. Mol. Genet. 23, 6345–6355 10.1093/hmg/ddu352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chujo T., Ohira T., Sakaguchi Y., Goshima N., Nomura N., Nagao A. et al. (2012) LRPPRC/SLIRP suppresses PNPase-mediated mRNA decay and promotes polyadenylation in human mitochondria. Nucleic Acids Res. 40, 8033–8047 10.1093/nar/gks506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siira S.J., Spahr H., Shearwood A.J., Ruzzenente B., Larsson N.G., Rackham O. et al. (2017) LRPPRC-mediated folding of the mitochondrial transcriptome. Nat. Commun. 8, 1532 10.1038/s41467-017-01221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baughman J.M., Nilsson R., Gohil V.M., Arlow D.H., Gauhar Z. and Mootha V.K. (2009) A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet. 5, e1000590 10.1371/journal.pgen.1000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lagouge M., Mourier A., Lee H.J., Spahr H., Wai T., Kukat C. et al. (2015) SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation. PLoS Genet. 11, e1005423 10.1371/journal.pgen.1005423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chujo T. and Suzuki T. (2012) Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA 18, 2269–2276 10.1261/rna.035600.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borowski L.S., Dziembowski A., Hejnowicz M.S., Stepien P.P. and Szczesny R.J. (2013) Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci. Nucleic Acids Res. 41, 1223–1240 10.1093/nar/gks1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen H.W., Rainey R.N., Balatoni C.E., Dawson D.W., Troke J.J., Wasiak S. et al. (2006) Mammalian polynucleotide phosphorylase is an intermembrane space RNase that maintains mitochondrial homeostasis. Mol. Cell. Biol. 26, 8475–8487 10.1128/MCB.01002-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szczesny R.J., Borowski L.S., Brzezniak L.K., Dmochowska A., Gewartowski K., Bartnik E. et al. (2010) Human mitochondrial RNA turnover caught in flagranti: involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 38, 279–298 10.1093/nar/gkp903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toompuu M., Tuomela T., Laine P., Paulin L., Dufour E. and Jacobs H.T. (2018) Polyadenylation and degradation of structurally abnormal mitochondrial tRNAs in human cells. Nucleic Acids Res., 10.1093/nar/gky159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gammage P.A., Moraes C.T. and Minczuk M. (2017) Mitochondrial genome engineering: the revolution may not be CRISPR-Ized. Trends Genet., 34, 101–110 34, 101-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haag S., Sloan K.E., Ranjan N., Warda A.S., Kretschmer J., Blessing C. et al. (2016) NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 35, 2104–2119 10.15252/embj.201694885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakano S., Suzuki T., Kawarada L., Iwata H., Asano K. and Suzuki T. (2016) NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met). Nat. Chem. Biol. 12, 546–551 10.1038/nchembio.2099 [DOI] [PubMed] [Google Scholar]
- 65.Van Haute L., Dietmann S., Kremer L., Hussain S., Pearce S.F., Powell C.A. et al. (2016) Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat. Commun. 7, 12039 10.1038/ncomms12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Haute L., Powell C.A. and Minczuk M. (2017) Dealing with an unconventional genetic code in mitochondria: the biogenesis and pathogenic defects of the 5-formylcytosine modification in mitochondrial tRNAMet. Biomolecules 7, e24, e24 28257121 [Google Scholar]
- 67.Asano K., Suzuki T., Saito A., Wei F.Y., Ikeuchi Y., Numata T. et al. (2018) Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic Acids Res. 46, 1565–1583 10.1093/nar/gky068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villarroya M., Prado S., Esteve J.M., Soriano M.A., Aguado C., Perez-Martinez D. et al. (2008) Characterization of human GTPBP3, a GTP-binding protein involved in mitochondrial tRNA modification. Mol. Cell. Biol. 28, 7514–7531 10.1128/MCB.00946-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasarman F., Antonicka H., Horvath R. and Shoubridge E.A. (2011) The 2-thiouridylase function of the human MTU1 (TRMU) enzyme is dispensable for mitochondrial translation. Hum. Mol. Genet. 20, 4634–4643 10.1093/hmg/ddr397 [DOI] [PubMed] [Google Scholar]
- 70.Wu Y., Wei F.Y., Kawarada L., Suzuki T., Araki K., Komohara Y. et al. (2016) Mtu1-mediated thiouridine formation of mitochondrial tRNAs is required for mitochondrial translation and is involved in reversible infantile liver injury. PLoS Genet. 12, e1006355 10.1371/journal.pgen.1006355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yarham J.W., Lamichhane T.N., Pyle A., Mattijssen S., Baruffini E., Bruni F. et al. (2014) Defective i6A37 modification of mitochondrial and cytosolic tRNAs results from pathogenic mutations in TRIT1 and its substrate tRNA. PLoS Genet. 10, e1004424 10.1371/journal.pgen.1004424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Powell C.A., Kopajtich R., D’Souza A.R., Rorbach J., Kremer L.S., Husain R.A. et al. (2015) TRMT5 mutations cause a defect in post-transcriptional modification of mitochondrial tRNA associated with multiple respiratory-chain deficiencies. Am. J. Hum. Genet. 97, 319–328 10.1016/j.ajhg.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao X., Patton J.R., Davis S.L., Florence B., Ames S.J. and Spanjaard R.A. (2004) Regulation of nuclear receptor activity by a pseudouridine synthase through posttranscriptional modification of steroid receptor RNA activator. Mol. Cell 15, 549–558 10.1016/j.molcel.2004.06.044 [DOI] [PubMed] [Google Scholar]
- 74.Patton J.R., Bykhovskaya Y., Mengesha E., Bertolotto C. and Fischel-Ghodsian N. (2005) Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J. Biol. Chem. 280, 19823–19828 10.1074/jbc.M500216200 [DOI] [PubMed] [Google Scholar]
- 75.Zaganelli S., Rebelo-Guiomar P., Maundrell K., Rozanska A., Pierredon S., Powell C.A. et al. (2017) The pseudouridine synthase RPUSD4 is an essential component of mitochondrial RNA granules. J. Biol. Chem. 292, 4519–4532 10.1074/jbc.M116.771105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antonicka H., Choquet K., Lin Z.Y., Gingras A.C., Kleinman C.L. and Shoubridge E.A. (2017) A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 18, 28–38 10.15252/embr.201643391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arroyo J.D., Jourdain A.A., Calvo S.E., Ballarano C.A., Doench J.G., Root D.E. et al. (2016) A genome-wide CRISPR death screen identifies genes essential for oxidative phosphorylation. Cell Metab., 10.1016/j.cmet.2016.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagaike T., Suzuki T., Tomari Y., Takemoto-Hori C., Negayama F., Watanabe K. et al. (2001) Identification and characterization of mammalian mitochondrial tRNA nucleotidyltransferases. J. Biol. Chem. 276, 40041–40049 10.1074/jbc.M106202200 [DOI] [PubMed] [Google Scholar]
- 79.Nakamura A., Nemoto T., Heinemann I.U., Yamashita K., Sonoda T., Komoda K. et al. (2013) Structural basis of reverse nucleotide polymerization. Proc. Natl. Acad. Sci. U.S.A. 110, 20970–20975 10.1073/pnas.1321312111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearce S.F., Rorbach J., Van Haute L., D’Souza A.R., Rebelo-Guiomar P., Powell C.A. et al. (2017) Maturation of selected human mitochondrial tRNAs requires deadenylation. Elife 6, e27596 10.7554/eLife.27596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chihara T., Luginbuhl D. and Luo L. (2007) Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat. Neurosci. 10, 828–837 10.1038/nn1910 [DOI] [PubMed] [Google Scholar]
- 82.Tolkunova E., Park H., Xia J., King M.P. and Davidson E. (2000) The human lysyl-tRNA synthetase gene encodes both the cytoplasmic and mitochondrial enzymes by means of an unusual alternative splicing of the primary transcript. J. Biol. Chem. 275, 35063–35069 10.1074/jbc.M006265200 [DOI] [PubMed] [Google Scholar]
- 83.Nagao A., Suzuki T., Katoh T., Sakaguchi Y. and Suzuki T. (2009) Biogenesis of glutaminyl-mt tRNAGln in human mitochondria. Proc. Natl. Acad. Sci. U.S.A. 106, 16209–16214 10.1073/pnas.0907602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Echevarria L., Clemente P., Hernandez-Sierra R., Gallardo M.E., Fernandez-Moreno M.A. and Garesse R. (2014) Glutamyl-tRNAGln amidotransferase is essential for mammalian mitochondrial translation in vivo. Biochem. J. 460, 91–101 10.1042/BJ20131107 [DOI] [PubMed] [Google Scholar]
- 85.Sharma M.R., Koc E.C., Datta P.P., Booth T.M., Spremulli L.L. and Agrawal R.K. (2003) Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 115, 97–108 10.1016/S0092-8674(03)00762-1 [DOI] [PubMed] [Google Scholar]
- 86.Kaushal P.S., Sharma M.R., Booth T.M., Haque E.M., Tung C.S., Sanbonmatsu K.Y. et al. (2014) Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc. Natl. Acad. Sci. U.S.A. 111, 7284–7289 10.1073/pnas.1401657111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greber B.J., Boehringer D., Leibundgut M., Bieri P., Leitner A., Schmitz N. et al. (2014) The complete structure of the large subunit of the mammalian mitochondrial ribosome. Nature 515, 283–286 10.1038/nature13895 [DOI] [PubMed] [Google Scholar]
- 88.Greber B.J., Bieri P., Leibundgut M., Leitner A., Aebersold R., Boehringer D. et al. (2015) Ribosome. The complete structure of the 55S mammalian mitochondrial ribosome. Science 348, 303–308 10.1126/science.aaa3872 [DOI] [PubMed] [Google Scholar]
- 89.Brown A., Amunts A., Bai X.C., Sugimoto Y., Edwards P.C., Murshudov G. et al. (2014) Structure of the large ribosomal subunit from human mitochondria. Science 346, 718–722 10.1126/science.1258026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amunts A., Brown A., Toots J., Scheres S.H. and Ramakrishnan V. (2015) Ribosome. The structure of the human mitochondrial ribosome. Science 348, 95–98 10.1126/science.aaa1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rorbach J., Gao F., Powell C.A., D’Souza A., Lightowlers R.N., Minczuk M. et al. (2016) Human mitochondrial ribosomes can switch their structural RNA composition. Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.1609338113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bar-Yaacov D., Frumkin I., Yashiro Y., Chujo T., Ishigami Y., Chemla Y. et al. (2016) Mitochondrial 16S rRNA Is Methylated by tRNA Methyltransferase TRMT61B in All Vertebrates. PLoS Biol. 14, e1002557 10.1371/journal.pbio.1002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Metodiev M.D., Lesko N., Park C.B., Camara Y., Shi Y., Wibom R. et al. (2009) Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 9, 386–397 10.1016/j.cmet.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 94.Metodiev M.D., Spahr H., Loguercio Polosa P., Meharg C., Becker C., Altmueller J. et al. (2014) NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 10, e1004110 10.1371/journal.pgen.1004110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee K.W. and Bogenhagen D.F. (2014) Assignment of 2′-O-methyltransferases to modification sites on the mammalian mitochondrial large subunit 16 S ribosomal RNA (rRNA). J. Biol. Chem. 289, 24936–24942 10.1074/jbc.C114.581868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearce S.F., Rebelo-Guiomar P., D’Souza A.R., Powell C.A., Van Haute L. and Minczuk M. (2017) Regulation of mammalian mitochondrial gene expression: recent advances. Trends Biochem. Sci. 42, 625–639 10.1016/j.tibs.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rorbach J., Boesch P., Gammage P.A., Nicholls T.J., Pearce S.F., Patel D. et al. (2014) MRM2 and MRM3 are involved in biogenesis of the large subunit of the mitochondrial ribosome. Mol. Biol. Cell 25, 2542–2555 10.1091/mbc.e14-01-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dennerlein S., Rozanska A., Wydro M., Chrzanowska-Lightowlers Z.M. and Lightowlers R.N. (2010) Human ERAL1 is a mitochondrial RNA chaperone involved in the assembly of the 28S small mitochondrial ribosomal subunit. Biochem. J. 430, 551–558 10.1042/BJ20100757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antonicka H., Sasarman F., Nishimura T., Paupe V. and Shoubridge E.A. (2013) The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 17, 386–398 10.1016/j.cmet.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 100.Jourdain A.A., Koppen M., Wydro M., Rodley C.D., Lightowlers R.N., Chrzanowska-Lightowlers Z.M. et al. (2013) GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 17, 399–410 10.1016/j.cmet.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Janer A., van Karnebeek C.D., Sasarman F., Antonicka H., Al Ghamdi M., Shyr C. et al. (2015) RMND1 deficiency associated with neonatal lactic acidosis, infantile onset renal failure, deafness, and multiorgan involvement. Eur. J. Hum. Genet. 23, 1301–1307 10.1038/ejhg.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaur R., Grasso D., Datta P.P., Krishna P.D., Das G., Spencer A. et al. (2008) A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. Mol. Cell 29, 180–190 10.1016/j.molcel.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao H.X. and Spremulli L.L. (1989) Interaction of bovine mitochondrial ribosomes with messenger RNA. J. Biol. Chem. 264, 7518–7522 [PubMed] [Google Scholar]
- 104.Haque M.E. and Spremulli L.L. (2008) Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis. J. Mol. Biol. 384, 929–940 10.1016/j.jmb.2008.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhargava K. and Spremulli L.L. (2005) Role of the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3. Nucleic Acids Res. 33, 7011–7018 10.1093/nar/gki1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spencer A.C. and Spremulli L.L. (2004) Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 32, 5464–5470 10.1093/nar/gkh886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma J. and Spremulli L.L. (1996) Expression, purification, and mechanistic studies of bovine mitochondrial translational initiation factor 2. J. Biol. Chem. 271, 5805–5811 10.1074/jbc.271.10.5805 [DOI] [PubMed] [Google Scholar]
- 108.Liao H.X. and Spremulli L.L. (1990) Identification and initial characterization of translational initiation factor 2 from bovine mitochondria. J. Biol. Chem. 265, 13618–13622 [PubMed] [Google Scholar]
- 109.Richter-Dennerlein R., Oeljeklaus S., Lorenzi I., Ronsor C., Bareth B., Schendzielorz A.B. et al. (2016) Mitochondrial protein synthesis adapts to influx of nuclear-encoded protein. Cell 167, 471e10–483e10 10.1016/j.cell.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szklarczyk R., Wanschers B.F., Cuypers T.D., Esseling J.J., Riemersma M., van den Brand M.A. et al. (2012) Iterative orthology prediction uncovers new mitochondrial proteins and identifies C12orf62 as the human ortholog of COX14, a protein involved in the assembly of cytochrome c oxidase. Genome Biol. 13, R12 10.1186/gb-2012-13-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weraarpachai W., Sasarman F., Nishimura T., Antonicka H., Aure K., Rotig A. et al. (2012) Mutations in C12orf62, a factor that couples COX I synthesis with cytochrome c oxidase assembly, cause fatal neonatal lactic acidosis. Am. J. Hum. Genet. 90, 142–151 10.1016/j.ajhg.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling M., Merante F., Chen H.S., Duff C., Duncan A.M. and Robinson B.H. (1997) The human mitochondrial elongation factor tu (EF-Tu) gene: cDNA sequence, genomic localization, genomic structure, and identification of a pseudogene. Gene 197, 325–336 10.1016/S0378-1119(97)00279-5 [DOI] [PubMed] [Google Scholar]
- 113.Hammarsund M., Wilson W., Corcoran M., Merup M., Einhorn S., Grander D. et al. (2001) Identification and characterization of two novel human mitochondrial elongation factor genes, hEFG2 and hEFG1, phylogenetically conserved through evolution. Hum. Genet. 109, 542–550 10.1007/s00439-001-0610-5 [DOI] [PubMed] [Google Scholar]
- 114.Cai Y.C., Bullard J.M., Thompson N.L. and Spremulli L.L. (2000) Interaction of mitochondrial elongation factor Tu with aminoacyl-tRNA and elongation factor Ts. J. Biol. Chem. 275, 20308–20314 10.1074/jbc.M001899200 [DOI] [PubMed] [Google Scholar]
- 115.Frolova L.Y., Tsivkovskii R.Y., Sivolobova G.F., Oparina N.Y., Serpinsky O.I., Blinov V.M. et al. (1999) Mutations in the highly conserved GGQ motif of class 1 polypeptide release factors abolish ability of human eRF1 to trigger peptidyl-tRNA hydrolysis. RNA 5, 1014–1020 10.1017/S135583829999043X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richter R., Rorbach J., Pajak A., Smith P.M., Wessels H.J., Huynen M.A. et al. (2010) A functional peptidyl-tRNA hydrolase, ICT1, has been recruited into the human mitochondrial ribosome. EMBO J. 29, 1116–1125 10.1038/emboj.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huynen M.A., Duarte I., Chrzanowska-Lightowlers Z.M. and Nabuurs S.B. (2012) Structure based hypothesis of a mitochondrial ribosome rescue mechanism. Biol Direct 7, 14 10.1186/1745-6150-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lind C., Sund J. and Aqvist J. (2013) Codon-reading specificities of mitochondrial release factors and translation termination at non-standard stop codons. Nat. Commun. 4, 2940 10.1038/ncomms3940 [DOI] [PubMed] [Google Scholar]
- 119.Akabane S., Ueda T., Nierhaus K.H. and Takeuchi N. (2014) Ribosome rescue and translation termination at non-standard stop codons by ICT1 in mammalian mitochondria. PLoS Genet. 10, e1004616 10.1371/journal.pgen.1004616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soleimanpour-Lichaei H.R., Kuhl I., Gaisne M., Passos J.F., Wydro M., Rorbach J. et al. (2007) mtRF1a is a human mitochondrial translation release factor decoding the major termination codons UAA and UAG. Mol. Cell 27, 745–757 10.1016/j.molcel.2007.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Temperley R., Richter R., Dennerlein S., Lightowlers R.N. and Chrzanowska-Lightowlers Z.M. (2010) Hungry codons promote frameshifting in human mitochondrial ribosomes. Science 327, 301 10.1126/science.1180674 [DOI] [PubMed] [Google Scholar]
- 122.Temperley R.J., Wydro M., Lightowlers R.N. and Chrzanowska-Lightowlers Z.M. (2010) Human mitochondrial mRNAs–like members of all families, similar but different. Biochim. Biophys. Acta 1797, 1081–1085 10.1016/j.bbabio.2010.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feaga H.A., Quickel M.D., Hankey-Giblin P.A. and Keiler K.C. (2016) Human cells require non-stop ribosome rescue activity in mitochondria. PLoS Genet. 12, e1005964 10.1371/journal.pgen.1005964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chrzanowska-Lightowlers Z.M. and Lightowlers R.N. (2015) Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”. PLoS Genet. 11, e1005227 10.1371/journal.pgen.1005227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chrzanowska-Lightowlers Z.M., Pajak A. and Lightowlers R.N. (2011) Termination of protein synthesis in mammalian mitochondria. J. Biol. Chem. 286, 34479–34485 10.1074/jbc.R111.290585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rorbach J., Richter R., Wessels H.J., Wydro M., Pekalski M., Farhoud M. et al. (2008) The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic. Acids. Res 36, 5787–5799 10.1093/nar/gkn576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsuboi M., Morita H., Nozaki Y., Akama K., Ueda T., Ito K. et al. (2009) EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Mol. Cell 35, 502–510 10.1016/j.molcel.2009.06.028 [DOI] [PubMed] [Google Scholar]