Abstract
Proinflammatory dietary patterns have been associated with increased cancer risk and mortality. We present a systematic review and meta-analysis of the current published literature on a dietary inflammatory index (DII) score and its association with cancer risk and mortality outcomes. Published articles from online databases (PubMed, Scopus, and Embase) examining the association between DII and any cancer risk, incidence, or mortality between 1980 and November 2016 were selected for review. Results of studies meeting inclusion criteria were summarized and meta-analyzed using STATA to generate summary measures of association across studies. Sixty-three published articles were identified from the search, and following title, abstract and full-text review, twenty-four studies met inclusion criteria. All articles calculated DII scores based on study-specific food-frequency questionnaires using methodology from the same article. Of the 24 included studies, 13 were case–control, 6 were prospective cohort, 1 was a retrospective cohort, 3 were RCTs, and 1 did not specify study design. The most common cancers examined were colorectal, breast, lung, and prostate. Individuals in the highest versus lowest DII categories had 25% increased risk of overall cancer incidence (RR: 1.25, 95% CI: 1.16–1.35), 75% higher odds of cancer (OR: 1.75, 95% CI: 1.43–2.16) and 67% increased risk of cancer mortality (RR: 1.67, 95% CI: 1.13–2.48). Upon stratification for cancer type, positive associations remained (RRbreast: RR: 1.12, 95% CI: 1.03–1.22) (RRcolorectal: 1.33, 95% CI: 1.22–1.46) (RRlung: 1.30, 95% CI: 1.13–1.50). There were consistent and significant positive associations between higher DII and cancer incidence and mortality across cancer types, study populations, and study design.
Keywords: diet, dietary inflammatory index, pro-inflammatory diet, cancer incidence, cancer mortality
Cancer is a leading cause of death worldwide, and while many factors may contribute to the development of cancer, chronic inflammation has been examined as a major contributor to its pathogenesis.1 Chronic inflammation is associated with oxidative DNA damage which can lead to mutations in key tumor suppressor genes and oncogenes leading to the development of cancer.2 The role of diet in chronic inflammation has also been extensively examined,3–5 and foods with high glycemic load or glycemic index have been shown to contribute to increased inflammation.3 One of the most extensively studied dietary patterns is the Mediterranean diet, consisting of high amounts of monounsaturated fatty acids, omega-3 and omega-6 fatty acids, fruits, vegetables, and whole grains, and is linked to anti-inflammatory properties.3 The Mediterranean diet has been associated with lower systemic chronic inflammation and mutation-causing DNA damage, and has been identified as a key factor in preventing tumorigenesis via inflammatory pathways.6 There is considerable interest in examining the inflammatory potential of specific food items and dietary patterns other than the Mediterranean diet, and in evaluating the extent to which higher dietary inflammation is associated with risk of cancer.
In 2014, Shivappa et al.7 developed a novel dietary inflammatory index (DII) as an improved measure to the version created in 2009 by Cavicchia et al.8 The DII was designed to assess the inflammatory potential of individual food items using food frequency questionnaires (FFQ), a method that has been widely used across cancer types and study populations.7 FFQs are widely used in epidemiologic studies to assess dietary patterns and consumption of micronutrients,3 and provide a valuable tool to estimate consumption pattern of common dietary items and micronutrients. The DII utilizes FFQ data to calculate a DII “score” that may be used to examine the association between diet related inflammation and risk of multiple chronic diseases, including cancer incidence and mortality.8 Higher DII scores indicate a more pro-inflammatory diet, while lower DII scores indicate a more anti-inflammatory diet with properties similar to the Mediterranean diet.8 While the DII has been used extensively in relation to the risk and outcomes for several cancer types, to our knowledge there is currently no systematic review or meta-analysis to summarize the evidence on the association between higher DII scores and cancer outcomes.
The purpose of this study is to: (i) provide a systematic review of the current published literature on the association between DII score and cancer incidence and mortality, and (ii) conduct a meta-analysis of study results to generate a summary estimate of the association between DII and cancer outcomes, where indicated. If results suggest that the DII is a consistent and significant predictor of cancer risk and mortality across study populations, then future studies may utilize the DII as a risk or prognostic factor for cancer as part of comprehensive cancer prevention strategies focused on reducing diet-related chronic inflammation.
Material and Methods
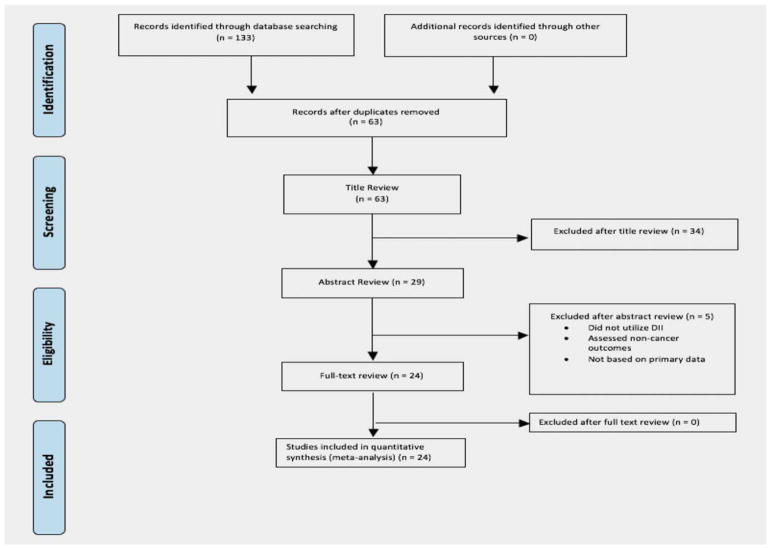
The PRISMA guidelines for systematic reviews of health were utilized for this study (Fig. 1). Articles published between 1980 and November 2016 were identified through searches in PubMed, Scopus, and Embase using the following keywords: DII[title/abstract] and diet[title/abstract] or diets[title/abstract] or dietary[title/abstract] or “diet”[mesh] or “dietary inflammatory index”[title/abstract])) and “neoplasms”[mesh] or cancer* [title/abstract] or malignan*[title/abstract] OR neoplas*[title/ abstract] or carcinoma*[title/abstract] or “mortality”[mesh] or “mortality” [subheading] or mortality[title/abstract] or death* [Title/Abstract]. The search was restricted to articles published in the English language only with no restrictions on country of origin. The year 1980 was chosen as a lower time limit to ensure that manuscripts evaluating any aspect of dietary inflammation in relation to cancer using data from existing cohort studies with FFQ data were captured.
Figure 1.
PRISMA flowchart of article selection for systematic review and meta-analysis. [Color figure can be viewed at wileyonlinelibrary.com]
Eligibility
Articles were considered eligible if (i) DII was calculated at baseline, and (ii) cancer outcomes i.e., odds, incidence and/or mortality were assessed among study participants using primary data from research studies. Studies were excluded if published in languages other than English, full text was not available, cancer outcomes were not assessed, or DII measure was missing.
Selection
Two authors (T.A. and M.F.) reviewed titles, abstracts, and full text of all studies retrieved from electronic databases, and resolved any discrepancies in selection by consensus. There were 63 articles identified after duplicates were removed; 34 articles were excluded after title review and 5 articles were excluded after abstract review, leaving 24 articles for full-text review. Articles were eliminated based on title if it was clear in the title that cancer outcomes were not assessed or irrelevant exposures were assessed. If there was any doubt, these articles were moved to abstract review and assessed more fully based on abstract. Most of the articles excluded during abstract review either did not report a DII measure (n =1), cancer outcomes were not assessed (n =3), or did not utilize primary data for analysis (n =1). All 24 articles eligible for full-text review met the inclusion criteria and were included in the systematic review and meta-analysis.
Data extraction
One author (M.F.) abstracted data from the included articles and summarized study information and results into the study database. Another author (T.A.) independently reviewed and verified the accuracy of data collected via cross-reference with the original articles. Any discrepancies were discussed and resolved by consensus. The study database included data on study characteristics such as country of origin, study design, year, author information, DII data source and calculation, sample size, cancer type studied, and race and age specifications if reported. Additionally, detailed information on the format of the DII measure (continuous or categorical), categorical cut-points, as well as lists of covariates included in the analysis were recorded. Furthermore, measures of association (odds ratio [OR] or hazard ratio [HR]) and 95% confidence intervals were recorded. Not all studies reported continuous measures for DII, and some reported continuous measures only for overall measures and not for stratified measures. Two articles only reported continuous measures and did not categorize DII measures into highest versus lowest group.
Statistical analysis
Rate ratios were reported as presented in the text of the articles. Most articles calculated rate ratios comparing highest to the lowest categories, while two articles utilized a continuous measure of DII for their overall cancer outcome rate ratios. Articles were organized based on the type of cancer outcome assessed (e.g. incidence, mortality, or case–control), and overall un-stratified results were presented when available, as well as age-, race-, or gender-stratified results. Meta-analysis was conducted separately for each cancer outcome type, and separately by cancer type when at least three articles assessed the same cancer type. Summary rate ratios were estimated by comparing the two extreme categories of the DII measure in relation to cancer outcome using random effects models, or by using the continuous DII measure when available. The Q-statistic was used to evaluate the presence of between-studies heterogeneity, while the I2 statistic was used to calculate the proportion of variation between studies due to heterogeneity. All statistical analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX).
Results
Twenty-four articles were reviewed for full text.9–32 The summary statistics for each of these are presented in Table 1. Of those, 9 examined cancer incidence,9–17 2 examined cancer mortality,31,32 and 13 examined odds of cancer in case–control studies.18–30 Additionally, 3 articles were published in 2014,12,20,29 9 articles were published in 2015,10,13,14,16,22,24,25,28,30 and 12 were published in 2016.9,11,15,17–19,21,23,26,27,31,32 There were 13 studies with case–control designs,18–30 6 were prospective cohorts,10–12,14,15,17 1 was a retrospective cohort,33 and 2 were randomized controlled trials.13,31 Others utilized data from a randomized controlled trial,16 or did not specify study design.17 Several countries were represented in the articles. One article was from Australia,15 2 were from France,9,31 1 was from Iran,24 10 were from Italy,17–19,22,25–29,32 1 each was from Jamaica,30 Korea,21 Spain,20 and Sweden,10 and 6 were from the United States.11–14,16,23 Several races were represented as well; 16 of the 24 included articles included White participants,9,10,13–15,17–19,22,25–29,31,32 three included Black participants,14,24,31 three included Asian participants,13,21,24 and others were mixed or not-specified.14,15 Multiple cancer types were represented in the included studies; two articles examined all cancers,9,31 three examined breast cancer,10,11,19 five examined colorectal cancer,12–14,20,21 three examined prostate cancer,29,30,32 three examined lung cancer,15–17 and others examined single cancer types18,22–28 (Table 1). More detailed summaries of each article are included in Table 2, which provides information regarding study country, study design, DII data collection strategy, and results for each study. DII data was not obtained from a single database for each of the original studies, rather it was collected as a part of country-specific dietary databases.
Table 1.
Summary statistics for studies included after full text review (n =24)
| Total no. studies | Incidence | Mortality | Case/control | |
|---|---|---|---|---|
| Publication years | ||||
| 2014 | 3 | 1 | 2 | |
| 2015 | 9 | 4 | 5 | |
| 2016 | 12 | 4 | 2 | 6 |
| Study design | ||||
| Case–control | 13 | 13 | ||
| Prospective cohort | 6 | 6 | ||
| Retrospective cohort | 1 | 1 | ||
| RCT | 2 | 1 | 1 | |
| RCT and cohort | 1 | 1 | ||
| Missing | 1 | 1 | ||
| Country | ||||
| Australia | 1 | 1 | ||
| France | 2 | 1 | 1 | |
| Iran | 1 | 1 | ||
| Italy | 10 | 1 | 1 | 8 |
| Jamaica | 1 | 1 | ||
| Korea | 1 | 1 | ||
| Spain | 1 | 1 | ||
| Sweden | 1 | 1 | ||
| U.S. | 6 | 5 | 1 | |
| Race1 | ||||
| White | 16 | 6 | 2 | 8 |
| Black | 3 | 1 | 2 | |
| Asian | 3 | 1 | 2 | |
| Spanish | 1 | 1 | ||
| American Indian/Alaskan Native | 1 | 1 | ||
| Hispanic/Latino | 1 | 1 | ||
| Not Specified | 5 | 5 | ||
| Cancer type | ||||
| All | 2 | 1 | 1 | |
| Bladder | 1 | 1 | ||
| Breast | 3 | 2 | 1 | |
| Colorectal | 5 | 3 | 2 | |
| Endometrial | 1 | 1 | ||
| Epithelial ovarian | 1 | 1 | ||
| Esophageal squamous cell | 2 | 2 | ||
| Gastric | 1 | 1 | ||
| Lung | 3 | 3 | ||
| Ovarian | 1 | 1 | ||
| Pancreatic | 1 | 1 | ||
| Prostate | 3 | 1 | 2 | |
| Total | 24 | 9 | 2 | 13 |
Some studies assessed several racial groups.
Abbreviation: RCT, randomized control trial.
Table 2.
Characteristics of studies included after full text review
| Author, year, country | Study design, characteristics | DII measure | Study outcome | Rate ratio (highest vs. lowest, 95% CI); estimate (95% CI)1 |
|---|---|---|---|---|
| Incidence | ||||
| Shivappa et al., 2014, United States (U.S.)12 | Prospective cohort, n = 34,703. 1,636 Incident cases, F, 55–69 yr, race not specified | 121-Item FFQ adapted from 126 item developed by Willett et al.34 | Colorectal cancer incidence |
|
| Hodge et al., 2016, Australia15 | Prospective cohort, n = 35,303, 403 incident cases, M/F, 27–75 yr, 100% White | 121-Item FFQ. used other countries’ data for nutrients where Australian data was lacking | Lung cancer incidence |
|
| Shivappa et al., 2015, Sweden10 | Prospective cohort, n = 45,257. 1,895 Incident cases, F, 100% White | 80-Item FFQ at baseline, nutrients determined from Swedish National Food Administration database | Breast cancer incidence |
|
| Maissonueve et al., 2016, Italy17 | Prospective cohort, n = 4,309. 200 incident cases, M/F, ≥ 50 yr, 100% White | 188-Item FFQ representative of Italian diet | Lung cancer incidence |
|
| Wirth et al., 2015, U.S.14 | Prospective cohort, n = 489, 442. 6,944 incident cases, M/F, 50–74 yr, European American and other races | 124-Item FFQ Data from the FFQ were linked to the United States Department of Agriculture’s 1994– 1996 Continuous survey of food intake by individuals in order to estimate nutrient, foods, and food group intake. | Colorectal cancer incidence |
|
| Tabung et al., 2015, U.S.13 | RCT and cohort, n = 152,589. 1,920 Incident cases, F, 50–70 yr, American Indian/Alaskan Native, Asian/Pacific Islander, African-American, Hispanic/Latino, European American, other | 122-Item FFQ from previous 3-mo period. FFQs were evaluated when all adjustment questions, all summary questions, 90% of foods questions, and at least 50% of every food group section was complete. University of Minnesota Nutrition Data System for Research linked to U.S. Department of Agriculture Standard Reference Releases and Manufacturer information for foods. 32 of 45 food parameters used to calculate DII score | Colorectal cancer incidence |
|
| Shivappa et al., 2016, U.S.11 | Prospective cohort, n = 33,817. 2,934 Incident cases or deaths, F, 55–69 yr, race not specified | 121-FFQ adapted from 126-item by Willett et al.34 at baseline. Twenty-nine of 45 food parameters used to calculate DII | Breast cancer incidence |
|
| Shoaibi et al., 2015, U.S.16 | Unknown, n = 110,317. 1,850 Incident cases, M/F, race not specified | Not specified | Lung cancer incidence |
|
| Graffouillere et al., 2016, France9 | RCT, n = 6,542, 559 Cancer deaths? M/F, 100% White | FFQ dietary record for 24 h every 2 mo, records from first 2 yr of f/ uΨused. Selected food consumed each day from list of ~1,000 items. Portion sizes determined via validated picture booklet distributed at enrollment. Energy, alcohol (g/d) and nutrient intake estimated using published French food composition table | Overall, breast, prostate, other (skin, colorectal, thyroid, lung, other) cancer incidence |
|
| Cancer status | ||||
| Shivappa et al., 2016, Italy26 | Case–control, n = 777, M/F 22–80 yr, 100% White | 78-Item FFQ, including most common Italian recipes and five items on alcoholic beverages. Indicated weekly frequency of consumption of each item, intake lower than once/wk but at least once/mo coded as 0.5/wk | Gastric cancer |
|
| Shivappa et al., 2014, Italy29 | Case–control, n = 2,745, M, 46–74 yr, 100% White | 78-Item FFQ with most common Italian recipes, based on diet 2 yr prior to cancer diagnosis. 0.5 for less than once/wk but at least once/mo | Prostate cancer |
|
| Shivappa et al., 2016, Italy19 | Case–control, n = 5,157, F, 23–74 yr, 100% White | 78-Item FFQ for previous 2 yr, 0.5 for less than once/wk but at least once/mo | Breast cancer |
|
| Shivappa et al., 2015, Italy22 | Case–control, n = 1,362, F, 18–79 yr, 100% White | 78-Item FFQ, including most common Italian recipes and five items on alcoholic beverages, indicated weekly frequency of consumption of each item, intake lower than once/wk but at least once/mo were coded as 0.5 per week | Endometrial cancer |
|
| Zamora-Ros et al., 2014, Spain20 | Case–control, n = 825, M/F, 100% Spanish | Habitual diet in previous year to diagnosis recorded in a personal interview using a validated Spanish dietary history questionnaire. Energy, nutrient, and flavonoid intakes were estimated from Spanish food composition tables used for the European Prospective Investigation into Cancer and Nutrition Study | Colorectal cancer |
|
| Shivappa et al., 2016, Italy27 | Case–control, n = 3,442, F, median 56 yr, 100% White | 78-Item FFQ on diet 2 yr prior to cancer diagnosis or hospital admission. Less than once/wk but at least once/mo was 0.5/wk | Ovarian cancer |
|
| Shivappa et al., 2016, Italy18 | Case–control, n = 1,355, M/F, 25–80 yr, 100% White | 80-Item Food, 15-Item beverage FFQ. Less than once/wk but at least once/mo was 0.5/wk. Nutrient and total energy intake determined using Italian food composition database | Bladder cancer |
|
| Cho et al., 2016, Korea21 | Case–control, n = 2,769, M, F, 100% Asian | 106-Item SQFFQ assessing average frequency and typical portion sizes in the previous year, which were converted to obtain daily nutrient intake | Colorectal cancer |
|
| Peres et al., 2016, U.S.23 | Case–control, n = 1,155, African-American F, 20–79 yr, 100% Black | 78-Item FFQ. 11 dietary databases around the world used to estimate global mean intake of each food parameter | Epithelial ovarian cancer |
|
| Shivappa et al., 2015, Iran24 | Case–control, n = 143, M/F, 40–75 yr, 100% Asian | 125-Item FFQ, no alcohol included | Esophageal squamous cell cancer |
|
| Shivappa et al., 2015, Italy25 | Case–control, n = 1,047, M/F, 39–77 yr, 100% White | 78-Item FFQ from 2 yr prior to diagnosis or hospital admission. Average weekly consumption of food and five alcoholic beverages. 0.5/wk for items less than once/ wk but at least once/mo | Esophageal squamous cell cancer |
|
| Shivappa et al., 2015, Italy28 | Case–control, n = 978, M/F, median 63 yr, 100% White | 78-Item FFQ from 2 yr prior to diagnosis or hospital admission. Range of most common Italian recipes. Weekly frequency assessed. 0.5/wk for items less than once/wk but at least once/ mo. Nutrient and total energy intakes determined using Italian Food Composition database | Pancreatic cancer |
|
| Shivappa et al., 2015, Jamaica30 | Case–control, n = 479, M, 40–80 yr, 100% Black | 78-Item FFQ using regionally representative world database | Prostate cancer |
|
| Mortality | ||||
| Zucchetto et al., 2016, Italy32 | Retrospective Cohort, n = 726. 76 Cancer deaths, M, 46–74 yr, 100% White | 78-Item FFQ and common Italian recipes. Less than once/wk but at least once/mo is 0.5/week | Prostate cancer mortality |
|
| Graffouillere et al., 2016, France31 | RCT, n = 8,089. 123 Cancer deaths, M/F, 35–60 yr, 100% White | First 2 yr of f/uΨdietary records, random 24 h dietary record every 2 mo, foods consumed were selected from list, portion size estimates using picture booklet, included seasoning, amounts from recipes estimated from nutritionists, energy, alcohol, nutrient intakes estimated using published French food comp table, and phenol-explorer used for total polyphenols and intakes of main groups and subgroups | All cancer mortality |
|
Highest versus lowest for categorical DII measures; estimate for risk/incidence/odds of cancer associated with one unit increase in continuous measures of DII.
Abbreviations: 95% CI, 95% confidence interval; M, male; F, female.
DII measure
All the studies utilized the same DII measure, which was calculated based on the same methodology.7 Briefly, data for the DII was obtained from FFQs and linked to a country-specific/regional dietary database to obtain nutrient composition of each item. The DII methodology as described by Shivappa et al.7 included identification of articles with food parameters of inflammatory biomarkers, assignment of scores as +1 for proinflammatory, −1 for anti-inflammatory, and 0 for no change in inflammatory biomarker. The articles were then weighted based on study characteristics and the weighted values were used to obtain food parameter-specific proinflammatory and anti-inflammatory scores. The overall inflammatory score for each food parameter was calculated by subtracting anti-inflammatory scores from proinflammatory scores for each food parameter, multiplying by the number of articles and adjusting for the total number of articles assessing the individual food parameter. A world database for the food parameters was created using data from several countries to calculate a world mean and standard deviation for each parameter. Next, individual study subject’s dietary consumption was used to calculate z scores and centered percentile for each parameter. The centered percentiles were then multiplied by the overall inflammatory score to find the DII score specific to a certain food parameter in one subject and all food parameter-specific scores were added to find overall DII score for a specific study subject. More specific details on creation, validation, and calculation of DII score have been published elsewhere.7
DII and cancer incidence
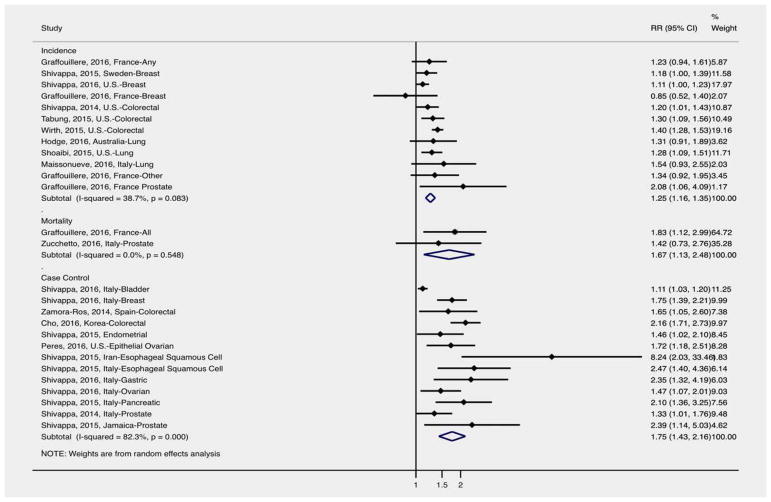
A total of nine studies examined DII and cancer incidence9–17 (Fig. 2). Higher DII was associated with increased incidence of cancer overall (RR: 1.25 (1.16–1.35)), with all studies except one9 reporting a positive association between DII and cancer incidence. Shivappa et al.9 observed a non-significant 15% reduction in breast cancer incidence among women in France, while other studies observed an 11% to more than twofold increased incidence of cancer in relation to DII.10–17 There was no evidence of statistically significant heterogeneity between the studies (I2 =39%, p =0.083).
Figure 2.
Overall meta-analysis of DII and cancer outcomes (relative risks [RR] and 95% confidence intervals). [Color figure can be viewed at wileyonlinelibrary.com]
DII and cancer case–control
Thirteen studies assessed DII and cancer case–control18–30 (Fig. 2). There was no common cancer type examined by at least three articles, therefore only the overall association was obtained via meta-analysis. Higher DII was associated with increased odds of cancer overall (OR: 1.75, 95% CI: 1.43–2.16), with all studies reporting a positive association between DII and odds of cancer. Shivappa et al.18 observed a significant 11% increased odds of bladder cancer in relation to DII, with other studies observing a 33% increase to a more than eightfold increased odds of cancer in relation to DII.18–30 There was moderate evidence of heterogeneity between the studies (I2 =48.9%, p =0.048).
DII and cancer mortality
Only two studies assessed DII and cancer mortality31,32 (Fig. 2). Neither of these studies examined the same cancer type therefore only overall results were obtained via meta-analysis. Higher DII was associated with increased cancer mortality overall (RR: 1.67, 95% CI: 1.13–2.48), with all studies reporting a positive association between DII and cancer mortality. Zucchetto et al.32 observed a non-significant 42% increase in prostate cancer mortality, while Graffouillere et al.31 observed a significant 83% increase in all cancer mortality. Overall, there was no evidence of statistically significant heterogeneity between the studies (I2 =0.0%, p =0.548).
DII and cancer specific incidence
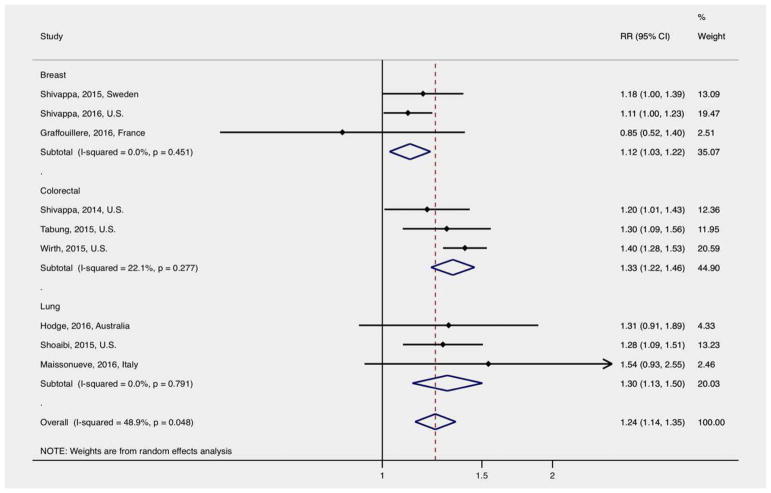
Three studies each reported results on breast,9–11 colorectal,12–14 and lung cancer incidence15–17 (Fig. 3). Higher DII was associated with 12% higher incidence of breast cancer (RR: 1.12, 95% CI: 1.03–1.22), 33% higher incidence of colorectal cancer (RR: 1.33, 95% CI: 1.22–1.46), and 30% higher incidence of lung cancer (RR: 1.30, 95% CI: 1.13–1.50). There was no evidence of statistically significant heterogeneity between the studies evaluating each cancer type (breast I2 =0.0%, p =0.451; colorectal I2 =22.1%, p =0.277; lung I2 =0.0%, p =0.791).
Figure 3.
Relative risk and 95% confidence interval (CI) of DII and cancer incidence by cancer site. [Color figure can be viewed at wileyonli-nelibrary.com]
Discussion
This review and meta-analysis summarizes the current published literature examining the association between DII and cancer risk, odds and mortality. Since the initial development and publication of the DII score,7 multiple research articles have been published assessing its ability to predict risk of cancer and cancer mortality. There were 24 articles that met our inclusion criteria, directly examining the association between DII and cancer across a wide range of geographic regions and cancer types.9–32 Overall, higher DII score was associated with higher cancer risk, odds, and mortality across cancer types, country of study, and racial groups. The results showed that higher DII score was associated with a significant 25% increase in overall cancer incidence, and a significant 75% increase in cancer odds regardless of cancer type, study design, country of study, or racial stratification. Additionally, the results showed that higher DII score was associated with a significant 67% increase in cancer mortality regardless of study design, country of study, or racial stratifications. There was limited evidence of heterogeneity observed between the studies included in the meta-analysis.
The positive association between high DII and cancer outcomes was strong and consistent throughout the review across each cancer outcome. Although there were variations in the type of FFQs used in evaluating dietary items between articles, the DII measure itself was calculated using the same methodology for each study, making these studies highly comparable. Although there were no geographic limitations, most studies in this review were conducted in Italy, and therefore may be more generalizable to the Italian population and diet. However, positive associations between cancer incidence, risk, and mortality were observed across all other observed countries, including the United States and France. The increased cancer incidence due to higher DII ranged from a 15% reduction in only one study from France9 to most studies showing a 11% to two-fold greater risk in studies from the United States and France, respectively.10–17 No study showed decreased odds of cancer due to higher DII, and results ranged from 11% increased odds to a more than eight-fold increased odds in studies from Italy and Iran, respectively.18–30 Only two studies evaluated cancer mortality, with results showing a 42% to 83% increased risk of cancer mortality in Italy and France, respectively.31,32
Diet has long been studied as a contributor to chronic inflammation status.3–5 Diets high in fruits and vegetables may contribute to reduced risk of cancer via improved vascular, inflammatory and immune function.5,6 Diet also plays a critical role in cancer risk through pathways involving overweight and obesity.6 For instance, high BMI is a strong risk factor for multiple cancers such as postmenopausal breast and colorectal cancers.6 Higher BMI is also associated with dysregulation of multiple metabolic risk factors such as insulin resistance, high blood pressure, high cholesterol, etc. which are also associated with increased cancer risk and poor prognosis.33 Giugliano et al.5 reported that diets high in carbohydrates and saturated fats and low in fiber may cause an increase in innate immune response via increased proinflammatory cytokines, and decreased anti-inflammatory cytokines leading to a proinflammatory cellular environment. Inflammation is also linked to cancer development via oxidative damage to DNA and mutation in tumor suppressor genes and oncogenes.1,2 Recent studies provide evidence that the link between inflammation and cancer may also occur via altered microbiome composition in humans, leading to immune activation and other cellular responses critical for cell proliferation and cancer.35 Furthermore, diet may directly or indirectly lead to epigenetic changes that may enhance tumorigenesis.36 Recent studies have provided compelling evidence linking specific dietary items with key epigenetic mechanisms relating to DNA repair and cell cycle regulation via pathways including DNA methylation, histone modification, and chromatin remodeling.37 Li et al.38 recently observed that certain compounds found in green tea polyphenols and broccoli sprouts interfere with epigenetic mechanisms in early breast cancer cells, for instance via reversal of normal DNA methylation and histone acetylation in genes that alter cancer cell gene regulation. While there are likely multiple, overlapping biological pathways linking specific dietary items or dietary patterns to tumorigenesis, diet remains a highly modifiable risk factor for multiple chronic diseases that may be targeted via public health interventions. The DII score provides a useful summary measure of the total inflammatory potential of multiple food items, and can be used in epidemiologic studies across sub-populations to estimate the potential burden of cancer linked to diet, and inform cancer prevention efforts. While the FFQ may be a less effective method for collecting dietary data,39 this review and DII measure serves as a starting point for the development of more effective methods for quantifying dietary inflammatory potential to inform cancer etiology studies.
There are several limitations to this review. The English language restriction may have led to an exclusion of articles from non-English-speaking countries, nevertheless a wide range of countries were represented in the reviewed articles. Furthermore, there were few articles focusing on the same specific cancer type so further stratification by cancer type was limited. Many studies also employed different categorical cut-points for DII analysis (e.g., tertiles, quartiles) and two articles only reported estimates in relation to continuous DII.18,22 However, by using the two extreme categories of high versus low, we were able to compare both ends of the spectrum, as is common practice in meta-analyses. Another potential limitation is that all of the articles utilized methodology by one author, who was also first author or co-author on all of the reviewed manuscripts. Although the results were consistent across countries and cancer types, we were unable to compare results with studies utilizing other measures of DII. Further, the ease of use of FFQ data, the basis of DII scores, as part of individual surveys is counteracted by well-documented limitations, including the potential for recall bias, limitations in assessing culture-specific food items and lack of validation in different study settings.39 Despite the limitations, this review also features several strengths. First, the review was not restricted to a specific cancer type and provides a summary and meta-analysis of the role of dietary inflammation in cancer outcomes across all cancer types. Additionally, since the DII used in each study was calculated in the same manner, comparability is increased. Also, by combining results from multiple studies, the meta-analysis provides an overall summary of studies resulting in a larger sample size to detect significant differences.
Conclusions
In conclusion, this is the first systematic review and meta-analysis of articles examining DII and cancer outcomes. Strong positive and significant associations were observed between higher DII and cancer incidence, risk, and mortality, with consistent results across cancer type and country.
What’s new?
In this meta-analysis, the authors use a dietary inflammatory index (DII) to analyze the relation between the inflammatory potential of individual food items and cancer development. They find that a higher DII (indicative of a more proinflammatory diet) was associated with substantial increases in cancer incidence, odds of cancer, and cancer mortality. These findings may be useful to establish the DII as a useful cancer risk or prognostic factor, emphasizing the need for comprehensive cancer prevention strategies reducing diet-related chronic inflammation through targeted dietary modifications.
Acknowledgments
The authors thank Catherine Smith Hogan at the UAB Lister Hill library for her assistance in compiling articles for review.
References
- 1.Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case–control study. Am J Epidemiol. 2013;177:3–13. doi: 10.1093/aje/kws359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murata M, Thanan R, Ma N, et al. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. Biomed Res Int. 2012;2012 doi: 10.1155/2012/623019. Figure 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25:634–40. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 4.Emerson SR, Kurti SP, Harms CA, et al. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr Res. 2017;8:213–25. doi: 10.3945/an.116.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Ostan R, Lanzarini C, Pini E, et al. Inflammaging and cancer: a challenge for the Mediterranean diet. Nutrients. 2015;7:2589–621. doi: 10.3390/nu7042589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavicchia PP, Steck SE, Hurley TG, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–72. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graffouillere L, Deschasaux M, Mariotti F, et al. The dietary inflammatory index is associated with prostate cancer risk in French middle-aged adults in a prospective study. J Nutr. 2016;146:785–91. doi: 10.3945/jn.115.225623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Sandin S, Lof M, et al. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer. 2015;113:1099–103. doi: 10.1038/bjc.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivappa N, Blair CK, Prizment AE, et al. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. 2016;61:7. doi: 10.1002/mnfr.201600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Prizment AE, Blair CK, et al. Dietary inflammatory index (DII) and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2014;23:2383–92. doi: 10.1158/1055-9965.EPI-14-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabung FK, Steck SE, Ma Y, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes Control. 2015;26:399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirth MD, Shivappa N, Steck SE, et al. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Br J Nutr. 2015;113:1819–27. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodge AM, Bassett JK, Shivappa N, et al. Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control. 2016;27:907–17. doi: 10.1007/s10552-016-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoaibi A, Shivappa N, Wirth M, et al. Interactions between smoking and the dietary inflammatory index in relation to lung cancer in the Prostate Lung Colorectal and Ovarian Trial. J Thorac Oncol. 2015;10:S219. doi: 10.1016/S1556-0864(16)30010-7. [DOI] [Google Scholar]
- 17.Maisonneuve P, Shivappa N, Hébert JR, et al. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr. 2016;55:1069–79. doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N, Hébert JR, Rosato V, et al. Dietary inflammatory index and risk of bladder cancer in a large Italian case–control study. Urology. 2016;100:84–9. doi: 10.1016/j.urology.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivappa N, Hébert JR, Rosato V, et al. Association between the dietary inflammatory index and breast cancer in a large Italian case–control study. Mol Nutr Food Res. 2016;61:897–906. doi: 10.1002/mnfr.201600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamora-Ros R, Shivappa N, Steck SE, et al. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case–control study. Genes Nutr. 2014;10:1–9. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YA, Lee J, Oh JH, et al. Dietary inflammatory index and risk of colorectal cancer: a case–control study in Korea. Nutrients. 2016;8:1–11. doi: 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivappa N, Hébert JR, Zucchetto A, et al. Dietary inflammatory index and endometrial cancer risk in an Italian case–control study. Br J Nutr. 2015;115:138–46. doi: 10.1017/S0007114515004171. [DOI] [PubMed] [Google Scholar]
- 23.Peres LC, Bandera EV, Qin B, et al. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. Int J Cancer. 2016;140:535–43. doi: 10.1002/ijc.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivappa N, Hebert JR, Rashidkhani B. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Iran. Nutr Cancer. 2015;67:1253–9. doi: 10.1002/aur.1474.Replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivappa N, Hébert JR, Zucchetto A, et al. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Italy. Cancer Causes Control. 2015;26:1439–47. doi: 10.1017/S0007114515004171. [DOI] [PubMed] [Google Scholar]
- 26.Shivappa N, Hébert JR, Ferraroni M, et al. Association between dietary inflammatory index and gastric cancer risk in an Italian case–control study. Nutr Cancer. 2016;68:1262–8. doi: 10.1080/01635581.2016.1224367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivappa N, Hébert JR, Rosato V, et al. Dietary inflammatory index and ovarian cancer risk in a large Italian case–control study. Cancer Causes Control. 2016;27:897–906. doi: 10.1007/s10552-016-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Bosetti C, Zucchetto A, et al. Dietary inflammatory index and risk of pancreatic cancer in an Italian case–control study. Br J Nutr. 2015;113:292–8. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivappa N, Bosetti C, Zucchetto A, et al. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. 2014;14:1–6. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivappa N, Jackson MD, Bennett F, et al. Increased dietary inflammatory index (DII) is associated with increased risk of prostate cancer in Jamaican men. Nutr Cancer. 2015;67:941. doi: 10.1080/01635581.2015.1062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graffouillere L, Deschasaux M, Mariotti F, et al. Prospective association between the dietary inflammatory index and mortality: modulation by antioxidant supplementation in the SU.VI. MAX randomized controlled trial. Am J Clin Nutr. 2016;103:878–85. doi: 10.3945/ajcn.115.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucchetto A, Gini A, Shivappa N, et al. Dietary inflammatory index and prostate cancer survival. Int J Cancer. 2016;139:2398–404. doi: 10.1002/ijc.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. J Clin Oncol. 2016;34:3664–71. doi: 10.1200/JCO.2016.67.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 35.Francescone R, Hou V, Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J. 2014;20:181–9. doi: 10.1097/PPO.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul B, Barnes S, Demark-Wahnefried W, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenet. 2015;7:112. doi: 10.1186/s13148-015-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross SA. Diet and epigenetics. Bioact Compd Cancer. 2010:101–23. doi: 10.1007/978-1-60761-627-6_5. [DOI] [Google Scholar]
- 38.Li Y, Buckhaults P, Cui X, et al. Combinatorial epigenetic mechanisms and efficacy of early breast cancer inhibition by nutritive botanicals. Epigenomics. 2016;8:1019–37. doi: 10.2217/epi-2016-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev. 2005;14:2826–8. doi: 10.1158/1055-9965.EPI-editorial. [DOI] [PubMed] [Google Scholar]