Abstract
Major depressive disorder (MDD) is one of the most common neuropsychiatric disorders affecting over one-fifth of the population worldwide. Owing to our limited understanding of the pathophysiology of MDD, the quest for finding novel antidepressant drug targets is severely impeded. Monoamine hypothesis of MDD provides a robust theoretical framework, forming the core of a large jigsaw puzzle, around which we must look for the vital missing pieces. Growing evidence suggests that the glial loss observed in key regions of the limbic system in depressed patients, at least partly, accounts for the structural and cognitive manifestations of MDD. Studies in animal models have subsequently hinted at the possibility that the glial atrophy may play a causative role in the precipitation of depressive symptoms. Antidepressants as well as monoamine neurotransmitters exert profound effects on the gene expression and metabolism in astrocytes. This raises an intriguing possibility that the astrocytes may play a central role alongside neurons in the behavioral effects of antidepressant drugs. In this article, we discuss the gene expression and metabolic changes brought about by antidepressants in astrocytes, which could be of relevance to synaptic plasticity and behavioral effects of antidepressant treatments.
Keywords: Astrocyte, monoamine hypothesis, antidepressants, noradrenaline, norepinephrine, BDNF
Introduction
Major depressive disorder (MDD) is a complex neuropsychiatric illness characterized by diverse neuropathological and physiological symptoms, involving a broad array of neuronal circuits.1 Prolonged exposure to stress can precipitate into depression in the individuals who are predisposed to stress-related mental illnesses. However, the predisposing factors as well as the mechanisms underlying the pathophysiology of these disorders are yet to be well-understood. Brain imaging studies have revealed marked structural changes in several brain regions in patients having stress-related neuropsychiatric illnesses. Most notable of these changes are severe volume reductions observed in the hippocampus and medial prefrontal cortex (mPFC), particularly in MDD.2–4 Although neuronal atrophy has been thought to underlie the structural and cognitive manifestations of MDD,4 growing evidence supports a major role for loss of nonneuronal (glial) cells in the cognitive dysfunction observed in stress-related disorders.5–11 Studies in postmortem brain samples from depressed patients have shown lowered density and number of glial cells in cortical regions, most prominently in prefrontal and cingulate areas.6,7,12 The density of astrocytes, the major glial cell type in the human brain, as measured by the glial fibrillary acidic protein immunopositivity, was found to be significantly lower selectively in the PFC of young patients with MDD.7 In addition, expression of glial high-affinity glutamate transporters and glutamine synthetase is decreased in depressed patients.13,14 In agreement with a major role for the astrocyte dysfunction in MDD, pharmacologic ablation of astrocytes in the mPFC is sufficient to induce depressive-like symptoms in experimental rats.9 Moreover, chronic administration of fluoxetine, a selective serotonin (5-HT) reuptake inhibitor (SSRI) reverses stress-induced decline in glial cell number in the hippocampus of tree shrews.10 These results suggest that antidepressant-mediated changes in astrocytes may lie at the heart of their behavioral effects. However, the mechanism underlying the modulation of behavior by astrocytes is yet to be uncovered.
Despite being the most abundant cell type in the brain,15 glial cells were assumed to be just the glue that held more important brain cells (neurons) together.16 Over time, it has become clear that glia, and particularly astrocytes, are not the merely silent spectators of neuronal activity as once thought, but play a more active role in brain function.16 Molecules secreted by astrocytes have been shown to be essential for neuronal survival, neurite outgrowth, and synaptogenesis during development.17 Astrocytes also provide trophic and metabolic support to neurons, which is essential for their normal functioning.17 Moreover, astrocytes are closely associated with synaptic compartments in the adult brain and their proximity to synapses and calcium signaling in perisynaptic astrocytic processes (PAPs) seems to be a function of neuronal activity and plasticity.18 Astrocytes can sense the neurotransmitter release in the synaptic cleft through the neurotransmitter receptors and transporters expressed at the PAPs and can secrete gliotransmitters in response, to modulate postsynaptic responses.19 Astrocytes seem to be highly responsive to changes in extracellular monoamine concentrations,20 and hence they can directly link the changes in synaptic monoamine release to synaptic plasticity. In this mini review, we aim to reconcile the data showing the effects of monoamine-mediated gene expression and metabolic changes, which may be relevant to the pathophysiology of MDD and to the behavioral effects of antidepressant drugs which act via monoamine system. With this article, we hope to integrate astrocytes in the classical monoamine hypothesis model, which will provide a theoretical framework to look for alternate drug targets influencing astrocyte-neuron interactions.
Classical Monoamine Hypothesis of Depression
Monoamine hypothesis of major depression was formulated almost half a century ago,21 which stated that deficiency of monoamine neurotransmitters, namely, norepinephrine and/or serotonin, underlies clinical depression.22 Although this hypothesis mainly originated from the mechanism of action of serendipitously discovered antidepressant drugs, it has resulted in the design of SSRI class of antidepressants that have proved quite useful in treating clinical depression.23 However, classical antidepressant drugs of the classes SSRI (eg, fluoxetine), tricyclics (eg, imipramine, desipramine, and amitriptyline), monoamine oxidase inhibitors (eg, iproniazid) etc, that act via increasing central monoamine levels, are marred by various drawbacks such as delayed effects on behavior, low efficacy, and frequent relapse.1 Although there seems to be a consensus on the notion that increased monoamine levels can alleviate depressive symptoms,22 a fuller understanding of monoamine hypothesis and novel drug targets targeting the central monoamine system are under investigation.
Mechanisms Underlying Behavioral Effects of Antidepressant Drugs
It has become evident that there is no single isolated mechanism of action for antidepressant treatments and that the therapies must exert multipronged effects on various cellular and molecular systems to effectively alleviate the depressive symptoms.1 The success of NMDA receptor (NMDAR) antagonists as effective and rapid-action antidepressant drugs has shown that excitatory synapses may be the prime targets for effective antidepressant action.24 Indeed, antidepressant-like effects of ketamine rely on protein synthesis–dependent increase in new excitatory synapses and increased excitatory neurotransmission.25 Furthermore, it was shown that acute treatment with an antidepressant imipramine enhances long-term potentiation (LTP).26 Interestingly, induction of LTP within the dentate gyrus (DG) of the hippocampus by stimulating perforant pathway is sufficient to induce antidepressant-like behavioral effects in rats.27 These results indicate that antidepressant drugs may have to aid the formation, stabilization, and potentiation of excitatory synapses to produce antidepressant-like effects.
Various classes of antidepressant drugs are known to enhance adult hippocampal neurogenesis.28,29 Adult hippocampal neurogenesis is a remarkable form of structural plasticity exhibited by adult mammalian brain, which involves birth, maturation, and integration of new neurons throughout adulthood.30 Subgranular zone of the hippocampal DG harbors a neurogenic niche containing neural progenitor cells which keep giving rise to new neurons throughout life.30 These neural progenitor cells and resulting newborn neurons seem to be highly responsive to monoamines and antidepressant drugs.29 Intriguingly, enhancement of adult neurogenesis is necessary for behavioral effects of antidepressant drugs.31 Adult hippocampal neurogenesis is a fairly protracted process encompassing progenitor proliferation, differentiation, maturation, and eventual integration of newly born neuron into the functional circuitry.30 Hence, the delay involved in the onset of behavioral effects of chronic antidepressant treatment may be, at least partially, attributed to the time it takes for the newly born neurons to get integrated into the functional circuitry. In conclusion, the enhancement of adult hippocampal neurogenesis appears to be an integral aspect of antidepressant effects on behavior.
Finally, antidepressant drugs have been shown to increase the expression of various neurotrophic factors such as BDNF (brain-derived neurotrophic factor),32 VEGF (vascular endothelial growth factor),33 and VGF (nonacronymic).34 These neurotrophic factors provide vital trophic support necessary for synapse stability and function. Furthermore, overexpression of each of these trophic factors produces antidepressant-like neurogenic and behavioral effects.33-35
In this article, we will discuss the potential role astrocytes could play in aiding these different mechanisms of action of antidepressant drugs.
Monoamine Receptors and Transporters on Astrocytes
It is important to note that astrocytes express transporters for both norepinephrine (NET)36 and serotonin (SERT),37 which are the targets of several classical antidepressant drugs. This raises a possibility that antidepressants can have direct effects on astrocytes by blocking the reuptake of monoamines by astrocytes. Moreover, astrocytes abundantly express α2A and β1 adrenoreceptors, with α1A expressed at much lower levels.38 Astrocytes also express 5-HT1A, 5-HT2A, and 5-HT2B receptors of serotonin, in addition to 5-HT5A, which is a predominantly astrocyte-specific receptor.39 Several studies have revealed that these receptors respond to physiologically relevant stimuli such as calcium influx and cyclic adenosine monophosphate (cAMP) concentrations, suggesting that these receptors could play an important role in antidepressant-mediated changes in monoamine concentrations.
Neurotrophic Factors
It has long been accepted that astrocytes provide trophic support to neurons by secreting various trophic factors. Particularly, they have been shown to secrete BDNF, nerve growth factor (NGF), neurotrophin 3 (NT3), ciliary neurotrophic factor (CNTF), fibroblast growth factor 2 (FGF2), glial cell line–derived neurotrophic factor (GDNF), insulin-like growth factor 1 (IGF-1), VGF, and VEGF.40–44 Out of these, secretion of BDNF, VEGF, and VGF is regulated by monoamines and/or by antidepressant drugs that act via monoamine system.45 Moreover, these are involved in regulating various aspects of depressive pathophysiology or antidepressant action.33–35 In this section, we summarize the results showing the regulation of trophic factor expression by monoamines and their effects on synaptic plasticity.
Brain-Derived Neurotrophic Factor
Antidepressants are known to induce the expression of BDNF, which is essential for their behavioral effects.32 Pyramidal neurons, but not interneurons, in rodent cerebral cortex and hippocampus express BDNF.46,47 Interestingly, fast-acting antidepressant treatments such as electroconvulsive therapy (ECT)32 and a combination treatment of yohimbine, an alpha-2 noradrenergic receptor antagonist, and imipramine, a norepinephrine reuptake inhibitor (Y+I),48 seem to increase the expression of BDNF transcripts in the inner molecular layer (ML) of DG. Although the subcellular localization of these transcripts is not very clear, it is interesting to note that the ML is enriched in astrocytes, and thus, at least a portion of those transcripts may be contributed by astrocytes. The ML of the DG harbors the perforant path terminals that form the inputs from entorhinal cortex to the DG, and the astrocytes present in ML are in a perfect position to modulate the entorhinal inputs to the hippocampus. Indeed, cultured astrocytes have also been shown to secrete BDNF in vitro.45 Interestingly, a recent report suggests that chronic unpredictable mild stress decreases the expression of BDNF in hippocampal astrocytes and this is reversed by the administration of 3,5,6,7,8,3ʹ,4ʹ-heptamethoxyflavone, a compound present in citrus fruits that exerts antidepressant-like behavioral effects.49 It would be interesting to study whether the BDNF expression induced by fast-acting antidepressant treatments such as ECT and Y+I is, at least in part, contributed by astrocytes. The SSRI antidepressants, namely, fluoxetine and paroxetine,45 as well as TCAs, namely, imipramine and amitriptyline,50,51 induce BDNF overexpression in cultured primary astrocytes. Interestingly, overexpression of BDNF specifically in mouse hippocampal astrocytes is sufficient to induce neurogenesis and to produce anxiolytic behavior.52 Hence, it is tempting to speculate that homeostatic secretion of BDNF by astrocytes may be necessary for mounting effective stress response and any dysregulation in astrocytic BDNF may precipitate into mood disorders.
It was shown that the BDNF induction by fluoxetine in vitro is not mimicked by application of serotonin,45 suggesting that this phenomenon could be independent of the blockade of serotonin transporters. It is important to note that fluoxetine concentration builds up in the brain to about 1 to 25 µM during MDD treatment as measured by fluorine magnetic resonance spectroscopy.53,54 These concentrations are much higher than those required for serotonin reuptake inhibition at nerve terminals, where Ki is only about 0.07 µM.55 This suggests that the BDNF induction by fluoxetine may employ additional targets, possibly the inhibition of astrocytic inward rectifying potassium channels Kir4.1.56 Astrocytic Kir4.1 channels regulate neuronal firing by spatial K+ buffering.57 Astrocytic Kir4.1 channels are blocked by antidepressants and it was recently shown that small interfering RNA–mediated knockdown of Kir4.1 channels in cultured astrocytes is sufficient to increase BDNF expression.58 However, direct effects of monoamines through their receptors on BDNF expression in astrocytes may not be ruled out just yet. It is shown that dopamine as well as norepinephrine induces BDNF expression in cultured astrocytes.59 The effects of dopamine are brought about by its cross-reactivity with norepinephrine receptors.59 Hence, astrocytic norepinephrine receptors may cell autonomously induce BDNF secretion in response to norepinephrine-enhancing antidepressant drugs. Such mechanisms need more thorough investigation as they may lie at the heart of mechanism of action of antidepressant drugs.
One study found that the BDNF induction by norepinephrine or dopamine is brought about by β-noradrenergic receptors, whereas the α1 receptors contribute to a much lesser extent59; another study found that β as well as α1-noradrenergic receptors contributes to norepinephrine-mediated BDNF induction.60 Moreover, activation of adenylate cyclase, protein kinase A (PKA) or protein kinase C (PKC) could mimic BDNF increase.60 Hence, increased cAMP levels following β-adrenergic receptor stimulation could increase CRE-binding protein (CREB)-dependent BDNF transcription via activation of PKA. However, this hypothesis warrants a direct in vivo validation.
The BDNF secreted from astrocytes in response to chronic antidepressant treatments may help boost synaptic plasticity at the presynaptic terminals by increasing quantal neurotransmitter release, aiding vesicle docking and by increasing the expression of synaptic vesicle proteins.61 Postsynaptically, BDNF may regulate actin polymerization at dendritic spines,62 increase the expression and phosphorylation of NR2B subunits,63,64 and upregulate NR2A and NR1 protein levels.64 In addition, BDNF secreted by astrocytes can boost adult hippocampal neurogenesis.52 Such synaptic and structural plasticity events are necessary to induce long-lasting behavioral effects of antidepressant drugs, and astrocytic BDNF may play a vital role in these processes.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor is an important regulator of the adult hippocampal neurogenesis.33 It has been shown to enhance progenitor proliferation33 and promote neurite outgrowth.65 Moreover, VEGF also enhances synaptic plasticity by increasing LTP in the DG, whereas blockade of VEGF completely abolishes LTP,66 suggesting that it is necessary for LTP induction under physiological conditions. Interestingly, VEGF has been shown to be necessary for neurogenic and behavioral effects of chronic antidepressant treatments.33 Furthermore, chronic intracerebroventricular infusion of VEGF is sufficient to produce neurogenic and antidepressant-like behavioral effects showing that it is both necessary and sufficient to produce antidepressant action.33
Cultured astrocytes upregulate the expression of VEGF in response to antidepressants such as fluoxetine, paroxetine, and amitriptyline.45,67 Intriguingly, lithium, a mood stabilizer used in the treatment of bipolar disorders, induces VEGF expression in the cortical astrocytes as well.68 Together, these results indicate that astrocyte-derived VEGF may be an important contributor to the enhancement of synaptic plasticity, adult hippocampal neurogenesis, and behavioral effects of chronic antidepressant treatments.
VGF
VGF, a secreted neuropeptide, is a key modulator of depressive-like behavior. VGF levels are downregulated in animal models of depression and are upregulated by various antidepressant treatments in rat hippocampus.34 Interestingly, hippocampal infusions of VGF produce antidepressant-like behavioral phenotype in experimental animals.34 Moreover, VGF +/− heterozygous mice that have reduced levels of VGF expression show depressive-like behavior.69 VGF has been shown to enhance proliferation of adult hippocampal progenitors,34 suggesting that neurogenesis may contribute to its antidepressant-like effects. VGF also increases dendritic growth,70 suggesting that VGF may even reverse the volumetric loss seen in MDD. It has been shown that fluoxetine and paroxetine increase VGF expression in cultured mouse astrocytes.45 These results must be verified in vivo; nevertheless, they do indicate that astrocytic VGF may contribute to the neurogenic and behavioral effects of chronic antidepressant treatments.
It is interesting to note that serotonin on its own does not mimic the effects of fluoxetine on astrocytic VEGF and VGF levels, suggesting that these may also be brought about by additional targets such as Kir4.1. However, any such possible links need further investigation. Alternatively, BDNF by itself is shown to upregulate the expression of VEGF71 and VGF.72 Thus, antidepressants and monoamines may directly affect BDNF levels, which can in turn induce VEGF and VGF expression, thus acting as a master regulator of trophic support provided by astrocyte.
FGF2
FGF2 regulates neurogenesis and is known to be involved in pathophysiology of depression as well as in antidepressant action. Postmortem analysis in depressed patients has revealed decreased expression of FGF2 in the hippocampus and prefrontal areas.73 Interestingly, FGF2 overexpression has been shown to be sufficient to elicit antidepressant-like effects in various animal and cellular models.74,75 Desipramine and fluoxetine upregulated FGF2 expression both in neurons and in astrocytes76 suggesting that astrocytic FGF2 can contribute to their neurogenic and behavioral effects. Whether antidepressants can directly act on astrocytes to induce FGF2 expression is debatable as FGF2 upregulation has been observed in cultured astrocytes with amitriptyline, clomipramine, fluvoxamine, and duloxetine,67 whereas no such upregulation was observed with fluoxetine, paroxetine, imipramine, and desipramine.45 Hence, it is plausible that this regulation is brought about through off-target effects independent of monoamine system. Indeed, FGF2 induction by amitriptyline is not mediated via α1 or β-noradrenergic receptors.67 Hence, more work is needed to assess whether FGF2 can contribute to astrocyte mediated behavioral effects of antidepressants.
Effects of Monoamines and Antidepressants on Metabolism in Astrocytes
Astrocytes provide metabolic support to neurons. In the events of elevated neuronal activity, astrocytes increase their glucose uptake and convert it to lactate which is used by neurons to derive energy.77,78 In addition, astrocytes also contain glycogen reserves, which can be mobilized in a process known as glycogenolysis, to obtain additional glucose to be metabolized.79 Interestingly, it has been recently shown that astrocyte-derived lactate is not just an energy metabolite but also plays an important role in synaptic plasticity.77,80 In addition, peripheral administration of lactate also exerts antidepressant-like behavioral effects.81 These behavioral effects of lactate may be brought about by its ability to increase plasticity at the excitatory synapses. Indeed, lactate is known to potentiate NMDAR currents, promote LTP, and induce expression of plasticity genes such c-Fos and BDNF.80,82 It has been shown that noradrenaline, serotonin, as well as antidepressant fluoxetine and paroxetine decrease glycogen levels in astrocytes indicating that they induce glycogenolysis.45,83 The glycogenolytic effects of norepinephrine are brought about mainly by β-adrenoreceptors, although α2-adrenoreceptors also contribute to some extent.84 A complete inhibition of norepinephrine-induced glycogenolysis can only be achieved by simultaneous inhibition of β- and α2-adrenoreceptors.84 Glycogenolysis results in increased glycolytic drive in astrocytes, which in turn could provide antidepressant-like effects by stimulating extracellular secretion of lactate. These results indicate that glycogenolysis and subsequent lactate release stimulated by certain antidepressant drugs may play an important role in their behavioral effects.
Concluding Remarks
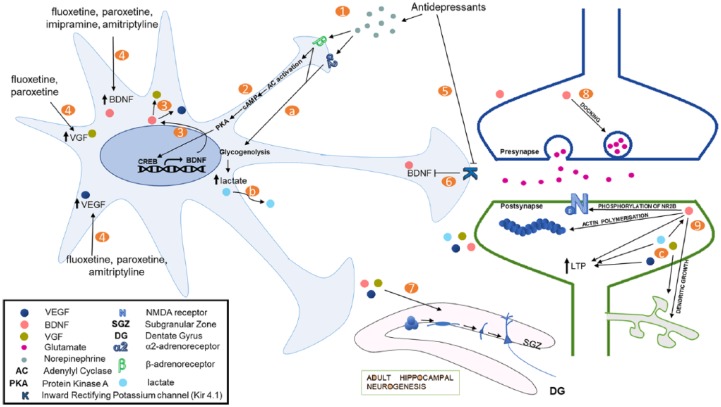
There is plenty of evidence suggesting a direct role for astrocytes in pathophysiology of depression and in antidepressant effects. Here, we formulate a theoretical model based on published literature, which incorporates astrocytes into the classical monoamine hypothesis for antidepressant action (Figure 1). Stimulation of β-adrenoreceptors by norepinephrine activates the cAMP/PKA pathway to activate CREB signaling. This is known to result in increased BDNF transcription. Increased BDNF may in turn increase the expression of VEGF and VGF. Various antidepressants through yet unknown mechanisms also mediate increased expression of trophic factors such as BDNF, VEGF and VGF in astrocytes. These trophic factors are known to produce antidepressant-like effects, at least in part, by enhancing adult hippocampal neurogenesis. Brain-derived neurotrophic factor is known to promote excitatory synaptic transmission by various mechanisms including dendritic growth and synaptogenesis, aiding in neurotransmitter vesicle docking, phosphorylation of NR2B subunit of the NMDARs, actin polymerization at the postsynapses and promoting LTP. Increased excitatory synaptic transmission is crucial for antidepressant’s behavioral effects and has been proposed as an important mediator of rapid antidepressant-like effects of NMDAR antagonists. Taken together, we postulate that astrocytes play an integral part in mediating the behavioral effects of antidepressant drugs, mainly by releasing plasticity-enhancing growth factors. The role of astrocytes in modulating depressive-like behavior needs more thorough investigation to improve our understanding of the monoamine system in pathophysiology of depression.
Figure 1.
A schematic representation showing effects of monoamines and antidepressants on astrocytes, which are relevant to their antidepressant action. Classical antidepressants lead to increase in synaptic concentrations of monoamines, depicted here is norepinephrine (1). Activation of β-adrenoreceptors by norepinephrine can lead to upregulation of CREB-mediated transcription through adenylyl cyclase/PKA activation (2). This increases the expression of BDNF (3), which can, in turn, increase the expression of other trophic factors, namely, VGF and VEGF. Antidepressant may also increase trophic factor expression in astrocytes through yet unknown mechanisms (4). For instance, antidepressants are known to decrease the activity of an inward rectifying potassium channel (Kir4.1) (5), and a decrease in Kir4.1 activity or expression decreases BDNF expression (6). These trophic factors can then enhance adult hippocampal neurogenesis, thus aiding the behavioral effects of chronic antidepressant treatments (7). BDNF also enhances excitatory transmission by increasing vesicle docking and enhancing quantal release from glutamatergic presynaptic terminals (8). Trophic factors also enhance excitatory postsynaptic responses through various mechanisms (9). BDNF increases the expression of various NMDA receptor subunits and mediates the phosphorylation of NR2B. It is also known to enhance actin polymerization. In addition, both BDNF and VEGF are known to promote LTP. Moreover, BDNF and VGF mediate dendritic outgrowth which can rescue volumetric loss observed in depressive disorders. Norepinephrine and several antidepressants are also shown to induce breakdown of glycogen through α2- and β-adrenoreceptors in astrocytes (a). This results in increase in glycolytic activity and production and secretion of lactate by astrocytes (b). Lactate, apart from acting as an energy substrate in neurons, is also known to increase NMDA currents, LTP, and plasticity-related gene expression, including expression of BDNF (c). BDNF indicates brain-derived neurotrophic factor; CREB, CRE-binding protein; LTP, long-term potentiation; PKA, protein kinase A; VEGF, vascular endothelial growth factor.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by INSPIRE faculty grant to S.V.M. Further support came from the Swiss National Foundation Project n. 31003A_163470 granted to L.A., the Indo-Swiss Excellence Doctoral Fellowship to P.B., and CSIR-NET Junior Research fellowship to G.V.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SVM conceptualized and wrote the article, PLD and GV prepared the figure, all authors discussed and edited the manuscript. PLD and GV contributed equally to this article.
ORCID iD: Swananda V Marathe  https://orcid.org/0000-0002-2539-366X
https://orcid.org/0000-0002-2539-366X
References
- 1. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. [DOI] [PubMed] [Google Scholar]
- 2. Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. [DOI] [PubMed] [Google Scholar]
- 3. Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. [DOI] [PubMed] [Google Scholar]
- 4. Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. [DOI] [PubMed] [Google Scholar]
- 5. Etiévant A, Lambás-Señas L, Scarna H, Lucas G, Haddjeri N. Astrocytes and gliotransmitters: new players in the treatment of major depression? Curr Drug Targets. 2013;14:1295–1307. [DOI] [PubMed] [Google Scholar]
- 6. Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. [DOI] [PubMed] [Google Scholar]
- 7. Miguel-Hidalgo JJ, Baucom C, Dilley G, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. [DOI] [PubMed] [Google Scholar]
- 8. Banasr M, Chowdhury G, Terwilliger R, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2005;31:1616–1626. [DOI] [PubMed] [Google Scholar]
- 11. Czéh B, Müller-Keuker JIH, Rygula R, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. [DOI] [PubMed] [Google Scholar]
- 12. Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. [DOI] [PubMed] [Google Scholar]
- 13. Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. [DOI] [PubMed] [Google Scholar]
- 16. Elsayed M, Magistretti PJ. A new outlook on mental illnesses: glial involvement beyond the glue. Front Cell Neurosci. 2015;9:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernardinelli Y, Randall J, Janett E, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24:1679–1688. [DOI] [PubMed] [Google Scholar]
- 19. Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4:e00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magistretti PJ. Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabet Metab. 1988;14:237–246. [PubMed] [Google Scholar]
- 21. Schildkraut JJ. The catecholamine hypothesis of affective disorders. A review of supporting evidence. Int J Psychiatry. 1967;4:203–217. [PubMed] [Google Scholar]
- 22. Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61:4–6. [PubMed] [Google Scholar]
- 23. Lemberger L, Fuller RW, Zerbe RL. Use of specific serotonin uptake inhibitors as antidepressants. Clin Neuropharmacol. 1985;8:299–317. [DOI] [PubMed] [Google Scholar]
- 24. Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112–117. [DOI] [PubMed] [Google Scholar]
- 25. Li N, Lee B, Liu R-J, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birnstiel S, Haas HL. Acute effects of antidepressant drugs on long-term potentiation (LTP) in rat hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:79–83. [DOI] [PubMed] [Google Scholar]
- 27. Kanzari A, Bourcier-Lucas C, Freyssin A, Abrous DN, Haddjeri N, Lucas G. Inducing a long-term potentiation in the dentate gyrus is sufficient to produce rapid antidepressant-like effects. Molec Psychiatry. 2018;23:587–596. [DOI] [PubMed] [Google Scholar]
- 28. Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. [DOI] [PubMed] [Google Scholar]
- 29. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ming G, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. [DOI] [PubMed] [Google Scholar]
- 31. Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. [DOI] [PubMed] [Google Scholar]
- 32. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF trkB mRNA in rat brain by chronic electroconvulsive seizure antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104:4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakker-Varia S, Krol JJ, Nettleton J, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27:12156–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem. 2003;84:136–144. [DOI] [PubMed] [Google Scholar]
- 37. Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem Int. 1998;33:11–22. [DOI] [PubMed] [Google Scholar]
- 38. Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. 2010;57:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berumen LC, Rodríguez A, Miledi R, García-Alcocer G. Serotonin receptors in hippocampus. Scientificworldjournal. 2012;2012:823493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckenstein FP. Fibroblast growth factors in the nervous system. J Neurobiol. 1994;25:1467–1480. [DOI] [PubMed] [Google Scholar]
- 41. Rudge JS, Alderson RF, Pasnikowski E, McClain J, Ip NY, Lindsay RM. Expression of ciliary neurotrophic factor and the neurotrophins-nerve growth factor, brain-derived neurotrophic factor and neurotrophin 3-in cultured rat hippocampal astrocytes. Eur J Neurosci. 1992;4:459–471. [DOI] [PubMed] [Google Scholar]
- 42. Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. [DOI] [PubMed] [Google Scholar]
- 43. Ballotti R, Nielsen FC, Pringle N, et al. Insulin-like growth factor I in cultured rat astrocytes: expression of the gene, and receptor tyrosine kinase. EMBO J. 1987;6:3633–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ijichi A, Sakuma S, Tofilon PJ. Hypoxia-induced vascular endothelial growth factor expression in normal rat astrocyte cultures. Glia. 1995;14:87–93. [DOI] [PubMed] [Google Scholar]
- 45. Allaman I, Fiumelli H, Magistretti PJ, Martin J-L. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl). 2011;216:75–84. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt-Kastner R, Wetmore C, Olson L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience. 1996;74:161–183. [DOI] [PubMed] [Google Scholar]
- 47. Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. [DOI] [PubMed] [Google Scholar]
- 48. Yanpallewar SU, Fernandes K, Marathe SV, et al. α2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sawamoto A, Okuyama S, Amakura Y, et al. 3,5,6,7,8,3′,4′-heptamethoxyflavone ameliorates depressive-like behavior and hippocampal neurochemical changes in chronic unpredictable mild stressed mice by regulating the brain-derived neurotrophic factor: requirement for ERK activation. Int J Mol Sci. 2017;18:E2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kittel-Schneider S, Kenis G, Schek J, et al. Expression of monoamine transporters, nitric oxide synthase 3, and neurotrophin genes in antidepressant-stimulated astrocytes. Front Psychiatry. 2012;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takano K, Yamasaki H, Kawabe K, Moriyama M, Nakamura Y. Imipramine induces brain-derived neurotrophic factor mRNA expression in cultured astrocytes. J Pharmacol Sci. 2012;120:176–186. [DOI] [PubMed] [Google Scholar]
- 52. Quesseveur G, David DJ, Gaillard MC, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bolo NR, Hodé Y, Nédélec JF, Lainé E, Wagner G, Macher JP. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology. 2000;23:428–438. [DOI] [PubMed] [Google Scholar]
- 54. Henry ME, Schmidt ME, Hennen J, et al. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: a 19-F MRS study. Neuropsychopharmacology. 2005;30:1576–1583. [DOI] [PubMed] [Google Scholar]
- 55. Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4:764–774. [DOI] [PubMed] [Google Scholar]
- 56. Furutani K, Ohno Y, Inanobe A, Hibino H, Kurachi Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol Pharmacol. 2009;75:1287–1295. [DOI] [PubMed] [Google Scholar]
- 57. Larsen BR, MacAulay N.Kir4.1-mediated spatial buffering of K+: experimental challenges in determination of its temporal and quantitative contribution to K+ clearance in the brain. Channels (Austin). 2014;8:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kinboshi M, Mukai T, Nagao Y, et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front Mol Neurosci. 2017;10:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koppel I, Jaanson K, Klasche A, et al. Dopamine cross-reacts with adrenoreceptors in cortical astrocytes to induce BDNF expression, CREB signaling and morphological transformation. Glia. 2018;66:206–216. [DOI] [PubMed] [Google Scholar]
- 60. Juric DM, Loncar D, Carman-Krzan M. Noradrenergic stimulation of BDNF synthesis in astrocytes: mediation via alpha1- and beta1/beta2-adrenergic receptors. Neurochem Int. 2008;52:297-306. [DOI] [PubMed] [Google Scholar]
- 61. Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons. Development. 2005;132:4285-4298. [DOI] [PubMed] [Google Scholar]
- 63. Carreño FR, Walch JD, Dutta M, Nedungadi TP, Cunningham JT. BDNF-TrkB pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol. 2011;23:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208-219. [DOI] [PubMed] [Google Scholar]
- 65. Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236-242. [DOI] [PubMed] [Google Scholar]
- 66. Licht T, Goshen I, Avital A, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kajitani N, Hisaoka-Nakashima K, Morioka N, et al. Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors: differential regulation of FGF-2 by noradrenaline. PLoS ONE. 2012;7:e51197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo S, Arai K, Stins MF, Chuang D-M, Lo EH. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke. 2009;40:652-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hunsberger JG, Newton SS, Bennett AH, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476-1482. [DOI] [PubMed] [Google Scholar]
- 70. Sato H, Fukutani Y, Yamamoto Y, et al. Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons. J Neurosci. 2012;32:15388-15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lin C-Y, Hung S-Y, Chen H-T, et al. Brain-derived neurotrophic factor increases vascular endothelial growth factor expression and enhances angiogenesis in human chondrosarcoma cells. Biochem Pharmacol. 2014;91:522-533. [DOI] [PubMed] [Google Scholar]
- 72. Alder J, Thakker-Varia S, Bangasser DA, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800-10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Evans SJ, Choudary PV, Neal CR, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101:15506-15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry. 2012;72:258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304-2311. [DOI] [PubMed] [Google Scholar]
- 78. Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451-461. [DOI] [PubMed] [Google Scholar]
- 80. Yang J, Ruchti E, Petit J-M, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. PNAS. 2014;111:12228-12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carrard A, Elsayed M, Margineanu M, et al. Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry. 2016;23:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Magistretti PJ, Manthorpe M, Bloom FE, Varon S. Functional receptors for vasoactive intestinal polypeptide in cultured astroglia from neonatal rat brain. Regul Pept. 1983;6:71-80. [DOI] [PubMed] [Google Scholar]
- 84. Subbarao KV, Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990;536:220-226. [DOI] [PubMed] [Google Scholar]