Abstract
Scleroderma-associated pulmonary arterial hypertension (SSc-PAH) is associated with worse outcome than idiopathic pulmonary arterial hypertension (IPAH), potentially due to worse right ventricular adaptation to load as suggested by pressure–volume loop analysis. The value of non-invasive load-adaptability metrics has not been fully explored in SSc-PAH. This study sought to assess whether patients with incident SSc-PAH have worse echocardiographic load-adaptability metrics than patients with IPAH. Twenty-two patients with incident SSc-PAH were matched 1:1 with IPAH based on pulmonary vascular resistance. Echocardiographic load-adaptability indices were divided into: surrogates of ventriculo-arterial coupling (e.g. right ventricular area change/end-systolic area), indices reflecting the proportionality of load adaptation (e.g. tricuspid regurgitation velocity-time integral normalized for average right ventricular radius), and simple ratios (e.g. tricuspid annular plane systolic excursion/right ventricular systolic pressure). The prognostic value of these indices for clinical worsening (i.e. death, transplant, or hospitalization for heart failure) at one year was explored. The two groups were comprised of patients of similar age, with similar cardiac index, pulmonary resistance, capacitance and NT-proBNP levels (p > 0.10). There was no difference in baseline right ventricular dimension, function or load-adaptability indices. At one year, eight (36.4%) SSc-PAH patients had experienced clinical worsening (eight hospitalizations and two deaths) versus one hospitalization in the IPAH group. Load adaptation at one year in survivors was not worse in SSc-PAH (p > 0.33). Patients with IPAH responded better to therapy than SSc-PAH in terms of reduction of right ventricular areas at one year (p < 0.05). Right ventricular load-adaptability echocardiographic indices do not appear to capture the increased risk of negative outcomes at one year associated with SSc-PAH.
Keywords: connective tissue disease, echocardiography, outcomes, pulmonary arterial hypertension, right ventricle, scleroderma
Introduction
Systemic sclerosis (i.e. scleroderma) is a connective tissue disease characterized by excess fibrosis and vascular remodeling that can affect multiple organ systems, including the pulmonary arterial tree.1 Scleroderma-associated pulmonary arterial hypertension (SSc-PAH) occurs in 8–13% of patients and is associated with a median survival of three years after diagnosis.2–4 Despite a similar level of afterload, SSc-PAH has been associated with worse outcomes than other forms of pulmonary arterial hypertension including idiopathic (IPAH) as well as pulmonary arterial hypertension secondary to other connective tissue diseases, as shown in the Registry to Evaluate Early And Long-term PAH Disease Management (REVEAL Registry).5–9
Several factors have been alleged to contribute to this increased mortality: increased prevalence of left ventricular dysfunction and comorbidities such as renal impairment, but also intrinsically worse right ventricular (RV) adaptation to load.4,5,7,10 A mechanistic and invasive study by Tedford et al. compared the RV pressure–volume relation in seven patients with SSc-PAH and five with IPAH, suggesting altered ventriculo-arterial coupling in SSc-PAH.11 Coupling, which refers to matching between contractility and afterload, was invasively measured as the ventricular elastance (Ees) over arterial elastance (Ea) ratio (Ees/Ea).12 However, a recent larger study has failed to replicate these results when assessing coupling using an invasive and magnetic resonance imaging combined Ees/Ea ratio.9
Different non-invasive indices of right load adaptability have been proposed in the literature in patients with heart failure or pulmonary hypertension.13,14 These indices can be divided into three categories (as presented in Fig. 1): surrogates of ventriculo-arterial coupling, indices assessing the proportionality of adaptation, and simple ratio of function and load.15–17 The present study tested the hypothesis that patients with SSc-PAH have worse resting right load adaptability using echocardiography than patients with IPAH matched for load (using pulmonary vascular resistance). The value of these indices of load adaptability has not been compared between patients with incident SSc-PAH and IPAH.
Fig. 1.
Right ventricular load-adaptability indices.
CI: cardiac index; Ea: arterial elastance; Ees: ventricular elastance; MPAP: mean pulmonary arterial pressure; PH: pulmonary hypertension; PP: pulse pressure; RAP: right atrial pressure; RV: right ventricular; RVEDV: right ventricular end-diastolic volume; RVESA: right ventricular end-systolic area; RVESV: right ventricular end-systolic volume; RVSP: right ventricular systolic pressure; SPAP: systolic pulmonary arterial pressure; SV: stroke volume; TAPSE: tricuspid annular plane systolic excursion; VTI TR: velocity time integral using continuous Doppler signal of the tricuspid regurgitation
The first objective was to compare baseline right heart dimensions, function and RV load adaptability between patients with incident SSc-PAH or IPAH. The second objective was to compare therapeutic response in terms of right heart remodeling and load-adaptability indices in the two groups. The third objective was to explore potential differences in clinical outcomes in the SSc-PAH or IPAH groups and the prognostic value of load-adaptation indices.
Methods
Patients with pulmonary arterial hypertension
Between November 2002 and April 2015, 56 patients with SSc-PAH were included in the prospective observational Vera Moulton Wall Center Pulmonary Hypertension database at Stanford. Stanford University Institutional Review Board approved the study, which was conducted in agreement with the Helsinki-II-Declaration; all patients gave written informed consent. This study first included all consecutive adults with incident SSc-PAH (SSc-PAH group) and 1:1 pulmonary vascular resistance-matched patients with incident IPAH (IPAH group), who all underwent right heart catheterization during this period, as presented in Fig. 2. Inclusion criteria were: diagnosis of pulmonary arterial hypertension according to latest guidelines (mean pulmonary arterial pressure (MPAP) ≥25 mmHg and pulmonary arterial wedge pressure ≤15 mmHg)18 and baseline echocardiogram available within three months of catheterization. Patients in the SSc-PAH group met American College of Rheumatology/European League Against Rheumatism 2013 criteria for scleroderma.19 Patients were excluded from the study if they had evidence of severe interstitial lung disease (defined as having moderate or severe interstitial lung disease on chest X-ray or high resolution computed tomography and pulmonary function tests with at least moderate restriction defined as forced vital capacity and/or total lung capacity <70% of the predicted value) or if they had evidence of increase left ventricular filling pressure (pulmonary arterial wedge pressure >15 mmHg). Treatments and decisions regarding referral for lung transplantation were left to the discretion of the treating physicians according to current clinical practice.
Fig. 2.
Study design.
echo: follow-up echocardiogram available at one year; IPAH: idiopathic pulmonary arterial hypertension; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; RHC: right heart catheterization; SSc-PAH: scleroderma-associated pulmonary arterial hypertension
Healthy controls
In order to compare the incident SSc-PAH cohort with controls, we also included 1:1 age- and sex-matched healthy controls from the Stanford Healthy Aging research database, in whom pulmonary hypertension or heart failure was excluded by a 60-point health questionnaire, physical exam and echocardiography.
Right heart catheterization
Catheterization was performed through the internal jugular or right femoral vein, after local anesthesia, using mild sedation as required.20 Right atrial pressure, MPAP, systolic (SPAP) and diastolic pulmonary arterial pressures, and cardiac output were measured. Pulmonary arterial wedge pressure was measured at the end of expiration. Pulmonary vascular resistance was measured as transpulmonary gradient divided by cardiac output and indexed to body surface area using the DuBois formula adjusted to the ideal weight and gender (PVRI). Pulmonary arterial capacitance was estimated as stroke volume divided by pulse pressure (difference between SPAP and diastolic pulmonary arterial pressures), and indexed on body surface area.21 Pulmonary arterial elastance was defined as SPAP × 0.9 divided by stroke volume.22,23
Echocardiography
Resting echocardiographic studies were acquired using Philips IE 33 ultrasound systems (Philips, Andover, MA, USA). All measures were averaged over three cycles and performed according to latest guidelines.24,25 Two blinded cardiologists measured all echocardiograms. Right heart dimensions were measured on RV-focused apical four-chamber view using RV end-diastolic area, RV end-systolic area (RVESA) and right atrial maximal area; all indexed to body surface area. RV systolic function was quantified using fractional area change (RVFAC), tricuspid annular plane systolic excursion (TAPSE), and free-wall Lagrangian longitudinal strain (RVLS). Pericardial effusion was considered significant when > 0.5 cm in diastole.26 RV systolic pressure (RVSP) was estimated based on the modified Bernoulli equation applied to the peak tricuspid regurgitation velocity and estimated right atrial pressure.20 Inter-observer variability for RV measurements in our laboratory has been recently published.26
Right load adaptation
As a surrogate of ventriculo-arterial coupling, we measured the RV area change/RVESA ratio, similar to the previously reported stroke volume/end-systolic volume ratio.12 Proportionality indices included the Dandel’s index described in end-diastole (velocity-time integral of the tricuspid regurgitation signal × RV length/RV area)17 and the index derived from the allometric relation between RV function (RVFAC or RVLS) and load (PVRI) as recently published:14 RV function/loadcoefficient. The allometric modeling of the function/load relationship was first demonstrated by Stevens et al. in a magnetic resonance based study in patients with suspected pulmonary hypertension, and recently confirmed in patient with PAH.14,27 Simple ratio metrics included the TAPSE/RVSP ratio,15 the SPAP/cardiac index ratio,28 the MPAP/cardiac index ratio, the pulmonary artery pulsatility index (pulse pressure/right atrial pressure), and the MPAP/right atrial pressure ratio.29
Clinical and laboratory characteristics
New York Heart Association functional class, six-minute walking distance (m), diffusing capacity of the lung for carbon monoxide, serum creatinine and derived MDRD calculation of creatinine clearance, and serum N-terminal pro-B type natriuretic peptide (NT-proBNP; Roche Diagnostics, Mannheim, Germany) were also collected when available within one year of inclusion. The REVEAL score was calculated as previously published6 in patients with all criteria available. Low risk was defined by a score between 0 and 7, average if equal to 8, moderately high if equal to 9, high if equal to 10 or 11 and very high if ≥12. Patients were combined into three groups (REVEAL 1 = low, REVEAL 2–3 = average/moderate–high, and REVEAL 4–5 = high/very high) instead of the five original groups to obtain balanced groups in terms of number of patients. A modified REVEAL score was calculated excluding the connective tissue disease criteria, and classified as modified REVEAL I = low (0–6), REVEAL II–III = average/moderate high (7–8) and REVEAL IV–V = high/very high (≥9).
Exploratory outcomes analysis
Follow-up was concluded in September 2016. The combined end-point of clinical worsening was defined as death, lung transplantation or hospitalization for acute right heart failure (defined by more than 24 h of hospitalization). The secondary end-point consisted of death or lung transplant. One-year follow-up echocardiograms were available in 39 patients (93% of survivors). Patients were matched for therapy at one year in order to compare right heart remodeling, function and load adaptation in the two groups according to four categories: single oral agent, dual oral agent, intravenous (i.v.) prostacyclins and i.v. prostacyclins plus additional oral therapy. Inhaled prostacyclins (n = 1 SSc-PAH and n = 4 IPAH) were classified as “oral” agents.
Statistical analysis
Continuous variables are presented as median and interquartile range for values that were not normally distributed using the Wilcoxon–Mann–Whitney test and mean ± standard deviation for normally distributed variables using Student’s t-test. Normality was assessed using the Shapiro–Wilk test. Categorical data are presented as number and percentage and compared using Fischer’s exact test. Cox proportional hazards regression univariate models were used to define association between SSc-PAH etiology, REVEAL score, RV dimension and function at baseline, and all load-adaptability indices at baseline with the primary endpoint and presented as hazard ratios and their 95% confidence intervals (95% CIs). In order to assess the influence of excluding the two patients who died before one year in the one-year analysis, sensitivity analyses were performed using imputation of the RV metrics from the latest echocardiography available prior to death (one month prior to death for both). All statistical analyses were performed using SPSS version 23.0 (IBM SPSS, Inc., Armonk, NY, USA). The results were considered significant when two-sided p values were < 0.05.
Results
Baseline characteristics
Of the 56 SSc-PAH patients originally referred for catheterization, 22 SSc-PAH met inclusion criteria, matched to 22 IPAH (Figure 2). The average scleroderma disease duration from first non-Raynaud phenomenon symptom was 5.1 ± 2.4 years with a majority of patients with limited cutaneous scleroderma (90.9%); half displayed positive anti-centromere antibody. Baseline clinical characteristics and resting hemodynamics of patients with SSc-PAH and IPAH are presented in Table 1. Hemodynamics showed severe pulmonary hypertension in both groups, with significantly higher MPAP in patients with IPAH, despite being matched for pulmonary resistance and having similar moderately decreased cardiac index (p = 0.19), capacitance or arterial elastance (p > 0.78). There were no statistically significant differences in the six-minute walk distance, NT-proBNP or prevalence of renal insufficiency (≥stage 3 of chronic kidney disease). A higher percentage of patients with SSc-PAH had high-risk REVEAL scores, even after excluding the connective tissue disease criteria. No patients were receiving treatment for pulmonary hypertension at baseline; however, two SSc-PAH patients were being treated with phosphodiesterase inhibitors for severe Raynaud's phenomenon. As shown in Table 1, both groups had similarly increased right heart dimension and RV dysfunction. Most patients had moderate to severe RV dysfunction at baseline: 63.6% of SSc-PAH and 72.7% of IPAH using RVFAC (≤25%) and 68.2% in both groups using RVLS (absolute value ≤15%). Despite similar left ventricular ejection fractions, patients with SSc-PAH had a larger left atrial dimension than IPAH patients (p = 0.01).
Table 1.
Comparative baseline clinical and echocardiographic characteristics of patients with incident idiopathic or scleroderma-associated pulmonary arterial hypertension.
| Scleroderma-associated PAH n = 22 | Idiopathic PAH n = 22 | p-value | |
|---|---|---|---|
| Age, years | 61.6 (53.4; 68.0) | 52.0 (43.8; 66.1) | 0.10 |
| Female sex (%) | 21 (95.5) | 17 (77.3) | 0.08 |
| Adjusted body surface area, m2 | 1.67 (1.55; 1.74) | 1.75 (1.67; 1.83) | 0.04 |
| New York Heart Association functional class (%) | |||
| I | 1 (4.5) | 0 (0) | 0.32 |
| II | 2 (9.1) | 8 (36.4) | 0.03 |
| III | 13 (59.1) | 10 (45.5) | <0.01 |
| IV | 6 (27.3) | 4 (18.2) | 0.48 |
| 6MWT distance, m | 304.8 (182.9; 499.8) | 411.5 (185.9; 518.2) | 0.30 |
| REVEAL score | |||
| 1 (%) | 4 (22.2) | 9 (50.0) | 0.09 |
| 2–3 (%) | 2 (11.1) | 5 (27.8) | 0.21 |
| 4–5 (%) | 12 (66.7) | 4 (22.2) | <0.01 |
| Modified REVEAL scorea | |||
| I (%) | 4 (22.2) | 6 (33.3) | 0.46 |
| II–III (%) | 2 (11.1) | 7 (38.9) | 0.06 |
| IV–V (%) | 12 (66.7) | 5 (27.8) | 0.02 |
| Hemodynamics | |||
| Heart rate >92 beats/min | 7 (31.8) | 5 (22.7) | 0.50 |
| Systolic blood pressure <110 mmHg | 5 (22.7) | 1 (4.5) | 0.08 |
| Right atrial pressure, mmHg | 6.5 (3.8; 12.3) | 7.5 (4.8; 12.3) | 0.67 |
| Mean PAP, mmHg | 47.5 (39.8; 52.3) | 56.0 (45.8; 60.0) | 0.01 |
| Systolic PAP, mmHg | 79.5 (63.0; 87.0) | 94.0 (77.5; 101.3) | <0.01 |
| Diastolic PAP, mmHg | 27.5 (23.0; 35.0) | 30.0 (29.0; 40.3) | 0.11 |
| Pulmonary arterial wedge pressure, mmHg | 8.5 (6.0; 10.0) | 9.5 (7.0; 12.3) | 0.40 |
| Cardiac index, L/min per m2 | 1.9 (1.6; 2.0) | 2.0 (1.8; 2.2) | 0.19 |
| Pulmonary vascular resistance, WU | 12.1 (8.3; 15.7) | 12.5 (8.8; 14.7) | 0.99 |
| Pulmonary vascular resistance indexed, WU.m2 | 19.8 (14.6; 26.0) | 21.6 (15.3; 25.8) | 0.78 |
| Pulmonary arterial capacitance, mL/mmHg | 0.74 (0.63–1.06) | 0.81 (0.65–1.09) | 0.78 |
| Pulmonary arterial capacitance indexed, mL.m2 per mmHg | 1.22 (1.03–1.92) | 1.41 (1.05–1.95) | 0.61 |
| Pulmonary arterial elastance, mmHg/mL | 1.95 (1.40–2.29) | 1.68 (1.36–2.87) | 0.92 |
| Laboratory data | |||
| Anti-nuclear antibodies (%) | 20 (91.0) | 0 | N/A |
| Anti-centromere antibodies (%) | 11 (50.0) | 0 | N/A |
| Anti-Scl-70 antibodies (%) | 1 (4.5) | 0 | N/A |
| Log(NT-proBNP) | 7.7 (6.4; 7.9) | 6.0 (4.8; 7.7) | 0.52 |
| Serum creatinine, mg/dL | 1.1 (0.9; 1.2) | 1.1 (0.9; 1.3) | 0.97 |
| Renal insufficiency (%) | 12 (54.5) | 7 (31.8) | 0.13 |
| Baseline therapy | |||
| Phosphodiesterase inhibitorsb | 2 (9.1) | 0 (0) | 0.49 |
| Echocardiography data | |||
| RV end-diastolic area index, cm2/m2 | 15.8 (11.4; 18.8) | 18.3 (13.5; 22.1) | 0.10 |
| RV end-systolic area index, cm2/m2 | 11.6 (7.6; 14.9) | 14.8 (9.0; 18.4) | 0.14 |
| Right atrial area index, cm2/m2 | 13.0 (10.4; 16.1) | 13.0 (9.9; 14.8) | 0.74 |
| RVFAC, % | 25.0 (20.2; 27.6) | 22.3 (19.4; 29.3) | 0.47 |
| TAPSE, mm | 15.5 (11.8; 22.0) | 17.0 (14.0; 18.3) | 0.47 |
| RVLS, absolute value, % | 13.2 (9.7; 17.7) | 12.8 (9.9; 18.0) | 0.94 |
| RVSP, mmHg | 57.3 (40.6; 68.7) | 62.7 (42.4; 70.4) | 0.41 |
| Left ventricular ejection fraction (%) | 63.9 (54.7; 66.8) | 63.1 (55.8; 69.2) | 0.98 |
| Left atrial area index, cm2/m2 | 9.8 (7.5; 11.9) | 7.8 (6.5; 9.3) | 0.01 |
| Pericardial effusion | 7 (31.8) | 2 (9.1) | 0.07 |
| Ventricular-arterial coupling indices | |||
| RV area change/RVESA | 0.33 (0.25; 0.38) | 0.29 (0.24; 0.41) | 0.47 |
| Combined indices | |||
| TAPSE/RVSP | 0.27 (0.19; 0.39) | 0.27 (0.22; 0.40) | 0.81 |
| SPAP/cardiac index | 42.7 (32.3; 49.8) | 48.1 (33.2; 53.4) | 0.39 |
| MPAP/cardiac index | 26.7 (20.1; 30.7) | 26.4 (20.9; 30.4) | 0.69 |
| Pulse pressure/right atrial pressure | 6.3 (4.2; 12.1) | 7.5 (4.9; 10.9) | 0.47 |
| MPAP/right atrial pressure | 6.0 (4.3; 10.6) | 7.2 (4.3; 10.9) | 0.67 |
| Proportionality indices | |||
| Dandel’s index | 42.9 (34.9; 47.1) | 30.2 (27.2; 45.7) | 0.049 |
| RVFAC/PVRI–0.29 | 57.2 (49.4; 65.0) | 57.6 (42.8; 67.8) | 0.80 |
| RVLS/PVRI–0.34 | 37.9 (25.7; 42.1) | 36.0 (28.0; 48.1) | 0.66 |
Values are expressed as median (interquartile range) or number (percentage). Renal insufficiency was defined by stage 3 or more of chronic kidney disease. NT-proBNP levels were not available for five patients, REVEAL score in eight patients and 6MWT distance in two.
The modified REVEAL score included all criteria except for the connective tissue disease criteria.
Two patients were being treated with phosphodiesterase inhibitors for severe Raynaud phenomenon.
6MWT: six-minute walk test; MPAP: mean pulmonary arterial pressure; NT-proBNP; N-terminal pro-B type natriuretic peptide; PAH: pulmonary arterial hypertension; PAP: pulmonary arterial pressure; PVRI: pulmonary vascular resistance index; RV: right ventricular; RVESA: right ventricular end-systolic area; RVFAC: fractional area change; RVLS: RV free-wall longitudinal strain; RVSP: right ventricular systolic pressure; SPAP: systolic pulmonary arterial pressure; TAPSE: tricuspid annular plane systolic excursion
Load-adaptation in SSc-PAH versus IPAH
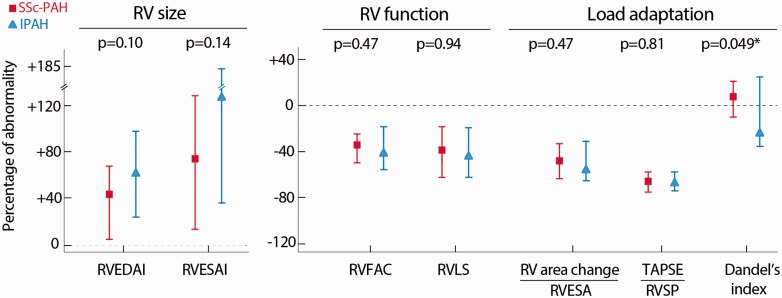
Fig. 3 illustrates the variability of RV function (using RVFAC and RVLS) and afterload (using pulmonary vascular resistance), which can be modeled by a curvilinear relation, as previously demonstrated.14,27 The allometric relation allowed to derive indices of proportionality of load adaptation as previously reported:14 RVFAC/PVRI–0.29 and RVLS/PVRI–0.34. Among the several load-adaptability indices, the Dandel’s index tended to be better in patients with SSc-PAH than IPAH (p = 0.049). None of the other indices were significantly different between the two groups (Table 1). The normalized values compared with the healthy reference group (95th or 5th percentile) are presented in Fig. 4. End-systolic dimensions presented on average a higher increase than end-diastolic dimensions. Values of the healthy cohort can be found in Supplementary Material Table S1 online.
Fig. 3.
Allometric relationship between load and right ventricular function in incident patients with SSc-PAH (dots) or IPAH (triangles).
IPAH: idiopathic pulmonary arterial hypertension; PVRI: pulmonary vascular resistance index; RV: right ventricular; RVFAC: right ventricular fractional area change; RVLS: right ventricular longitudinal strain; SSc-PAH: scleroderma-associated PAH
Fig. 4.
Percentage of abnormal right ventricular size, function and adaptation to load in patients with incident SSc-PAH or IPAH. The percentage of abnormality was calculated according to the 95th or 5th percentile of the healthy controls (n = 22), presented as median and interquartile range and compared using the Mann–Whitney test. Dandel’s index is defined in end-diastole as [velocity-time integral of the tricuspid regurgitation signal × RV length/RV area].
IPAH: idiopathic pulmonary arterial hypertension; RV: right ventricular; RVEDAI: right ventricular end-diastolic area index; RVESA: right ventricular end-systolic area; RVESAI: RVESA index; RVFAC: fractional area change; RVLS: right ventricular free-wall longitudinal strain; RVSP: right ventricular systolic pressure; SSc-PAH: scleroderma-associated pulmonary arterial hypertension; TAPSE: tricuspid annular plane systolic excursion
Exploratory outcome analysis
The mean follow-up was 3.0 ± 2.6 years. No patient was lost to follow-up at one year. The primary endpoint of death, transplant or hospitalization was reached in eight (36.4%) patients in the SSc-PAH group (eight hospitalizations and two deaths) and one (4.6%) in the IPAH group (one hospitalization) at one year (p = 0.02). Event-free survival rate for the primary endpoint (±standard error) was 79.5 ± 6.3 % at one year: 63.6 ± 10.5% for patients with SSc-PAH and 95.5 ± 4.4% patients with IPAH. The secondary end-point of death or transplant at one year was reached in two (9.1%) patients with SSc-PAH and none in patients with IPAH (p = 0.15). In univariate analysis, only SSc-PAH etiology (hazard ratio 9.5, 95% confidence interval (1.2–76.1), p = 0.03) and REVEAL score (hazard ratio 1.4 (1.1–1.9), p < 0.01) were significantly associated with the primary endpoint, while none of the baseline or changes in echocardiographic parameters including load-adaptability indices were associated with clinical worsening. None of the changes in RV metrics between baseline and follow-up echocardiography were significantly associated with the primary endpoint, even when performing imputation for the two non-survivors at one year (all p > 0.13).
One-year follow-up
At one year, in each group, five patients received a single oral agent, seven dual oral agents and two i.v. prostacyclins. There was no significant difference in terms of right heart dimensions at one year between the two groups in therapy-matched patients (n = 14 SSc-PAH vs. 14 IPAH) as presented in Table 2. Both SSc-PAH and IPAH demonstrated improvement in RV function (as assessed by all metrics) and reverse right heart remodeling at one year. Patients with IPAH had better RV reverse remodeling at one year (as shown by RV areas) than SSc-PAH despite similar functional improvement and load reduction. Non-invasive load-adaptability metrics did not differ between the two groups at one year, while the Dandel’s index improved in patients with IPAH. Similar results were found in non-therapy matched patients with available follow-up echocardiogram at one year (17 SSc-PAH versus 22 IPAH), as presented in Supplementary Table S2. The two patients with SSc-PAH who died before one year (at eight months and 10.5 months) underwent echocardiography one month prior to death. Follow-up echocardiography showed marked RV enlargement (+21% and +8% increase in RV end-systolic area index) in the context of increased RVSP (+144% and +8%) outside the range of survivors with SSc-PAH (Supplementary Table S3). Among load-adaptability indices, only the TAPSE/RVSP ratio seemed to reflect RV worsening in these patients when compared with other patients with SSc-PAH. Sensitivity analysis imputing RV metrics (including load-adaptability indices) for the two non-survivors is presented in Supplementary Table S4, showing significant difference in changes in the Dandel’s index between patients with SSc-PAH and IPAH.
Table 2.
Evolution of right heart size, function and load adaptation at one year in therapy-matched patients with scleroderma-associated or idiopathic pulmonary arterial hypertension.
| Scleroderma-associated PAH n = 14 | Idiopathic PAH n = 14 | p-value | |
|---|---|---|---|
| RV end-diastolic area index, cm2/m2 | 17.1 (10.9; 21.2) | 14.2 (11.8; 17.3) | 0.60 |
| RV end-systolic area index, cm2/m2 | 12.3 (7.0; 17.0) | 9.3 (7.5; 11.7) | 0.57 |
| Right atrial area index, cm2/m2 | 12.1 (8.6; 17.7) | 10.4 (8.8; 11.2) | 0.08 |
| RVFAC, % | 27.4 (17.7; 35.1) | 33.3 (23.6; 36.5) | 0.33 |
| TAPSE, mm | 18.0 (16.5; 20.8) | 19.5 (17.8; 22.3) | 0.29 |
| RVLS, absolute value, % | 17.4 (8.7; 21.5) | 20.9 (14.6; 22.3) | 0.18 |
| RVSP, mmHg | 51.8 (34.4; 74.7) | 52.5 (38.1; 64.5) | 0.98 |
| Left ventricular ejection fraction, % | 67.4 (64.8; 69.0) | 66.6 (63.9; 68.9) | 0.45 |
| Left atrial area index, cm2/m2 | 10.1 (7.3; 12.2) | 9.2 (7.9; 11.0) | 0.67 |
| Pericardial effusion | 2 (14.3) | 0 (0) | 0.15 |
| Load-adaptability indices at one year | |||
| RV area change/RVESA | 0.38 (0.21; 0.54) | 0.50 (0.31; 0.58) | 0.33 |
| TAPSE/RVSP | 0.31 (0.22; 0.58) | 0.38 (0.32; 0.62) | 0.40 |
| Dandel’s index | 36.2 (31.1; 43.2) | 41.4 (28.7; 55.5) | 0.64 |
| Delta change between one year and baseline | |||
| Delta RV end-diastolic area index, % | −4.4 (–13.0; 10.8) | −13.0 (–37.1; –4.0) | 0.04 |
| Delta RV end-systolic area index, % | −13.3 (–18.6; 12.7) | −30.5 (–46.9; –5.3) | 0.03 |
| Delta right atrial area index, % | −3.8 (–19.4; 21.4) | −15.5 (–28.8; –3.1) | 0.15 |
| Delta RVFAC, % | 21.9 (0.2; 33.0) | 45.9 (–0.2; 92.5) | 0.10 |
| Delta TAPSE, % | 16.7 (0; 53.5) | 15.0 (0; 49.5) | 0.95 |
| Delta RVLS, % | 15.4 (–7.6; 47.7) | 50.6 (–2.0; 125.7) | 0.18 |
| Delta RVSP, % | −8.3 (–40.5; 15.4) | −1.7 (–22.6; 6.2) | 0.98 |
| Delta left ventricular ejection fraction, % | 8.4 (0.3; 23.4) | 5.3 (–6.1; 16.5) | 0.08 |
| Delta left atrial area index, % | 3.1 (–1.6; 27.0) | 16.3 (–5.6; 61.1) | 0.45 |
| Delta RV area change/RVESA, % | 27.7 (0.3; 47.1) | 69.3 (–0.12; 142.6) | 0.15 |
| Delta TAPSE/RVSP, % | 33.3 (2.4; 111.8) | 41.8 (–5.9; 98.1) | 0.87 |
| Delta Dandel’s index, % | −3.9 (–19.2; 7.3) | 15.6 (–0.5; 31.9) | 0.03 |
| 6MWT at one year | n = 9 | n = 14 | |
| 6MWT distance, m | 322 (230; 402) | 466 (320; 549) | 0.31 |
| Absolute delta 6MWT distance, m | 48 (–25; 138) | 284 (51; 459) | 0.03 |
| Laboratory data at one year | n = 8 | n = 11 | |
| NT-proBNP level, pg/mL | 540 (138; 2241) | 58 (46; 473) | 0.08 |
| Delta NT-proBNP level, % | −10 (–60; –10) | −90 (–100; – 70) | 0.09 |
Values are expressed as median (interquartile range) or number (percentage).
6MWT: six-minute walk test; NT-proBNP: N-terminal pro-B-type natriuretic peptide; PAH: pulmonary arterial hypertension; RV: right ventricular; RVESA: right ventricular end-systolic area; RVFAC: fractional area change; RVLS: right ventricular free-wall longitudinal strain; RVSP: right ventricular systolic pressure; TAPSE: tricuspid annular plane systolic excursion.
Discussion
Our study is one of the first providing comprehensive RV adaptation phenotyping of patients with incident SSc-PAH using echocardiography. Indices of RV load-adaptability do not appear to capture the increased risk of heart failure and mortality in SSc-PAH. Consistent with previous studies, our exploratory analysis suggests worse one-year outcomes in SSc-PAH and shows less improvement in RV remodeling in response to therapy in SSc-PAH than in IPAH.7,30,31
Our main hypothesis was that worse clinical outcomes in SSc-PAH might be partially explained by poor ventricular adaptation to pulmonary vascular load in comparison with other forms of pulmonary hypertension. Recent studies using hemodynamic monitoring with pressure–volume loop analysis have shown differences in contractility between SSc-PAH patients and other forms of PAH, both at rest and with exercise.11,8,32 Tedford et al. compared the pressure–volume relationship using right heart catheterization with Valsalva in seven patients with SSc-PAH and five with IPAH, showing altered RV coupling in SSc-PAH (Ees/Ea ratio 1.0 ± 0.5) compared with IPAH (Ees/Ea 2.1 ± 1.0, p = 0.03) despite similar afterload and cardiac index.11 Hsu et al. performed a similar analysis of RV contractility during exercise to compare contractile reserve in SSc-PAH versus IPAH.32 Despite similar resting RV function and morphology, SSc-PAH subjects had depressed RV functional reserve during exercise. Finally, Kelemen et al. showed that SSc-PAH patients (n = 35) had lower RV mass than IPAH patients (n = 18) with similar pulmonary resistance, reflecting a potential difference in adaptive hypertrophy.8
There has been recent interest in focusing on non-invasive surrogate markers of RV load adaptability. The RV area change/RVESA ratio, similar to the previously reported stroke volume/end-systolic volume ratio as a surrogate of the Ees/Ea ratio, failed to distinguish SSc-PAH from IPAH in our study, showing the complexity of RV geometry. Ratio metrics (such as TAPSE/RVSP) address the question of how to best combine right heart function and load metrics into a simple index, to more accurately assess RV function and pulmonary hypertension. The TAPSE/RVSP ratio, proposed by Guazzi et al., is a simplified index of RV length–force relationship.15 A value less than 0.36 mm/mmHg was associated with an increased cardiovascular mortality (hazard ratio of 10.4 (5.4–19.8), p < 0.001) in 293 patients with heart failure with reduced or preserved ejection fraction.15 However, simple ratios may not address the question of disproportionality of adaptation, as the relationship between function and afterload follows a non-linear and often inverse fit.14,27 Dandel et al. proposed an index defined as delta pressure between the RV and the right atrium divided by the end-diastolic volume/length ratio.16,17 First demonstrated for the assessment of RV function recovery in patients with end-stage left heart failure on left ventricular assist devices,16 the prognostic value of this index was validated in 79 patients with PAH (including <8% with connective tissue disease) awaiting lung transplantation.17 A low index (reflecting excessive RV dilation and high right atrial pressure with relative low RV pressure) indicated impaired right heart adaptation to load and was associated with an increased risk of RV failure and worse transplant-free survival at one and three years.17 Although we found in our cohort a borderline significance (p = 0.049) for a higher Dandel’s index at baseline in patients with SSc-PAH than in those with IPAH (which would suggest a better adaptation), taking into account the multiple comparison analysis performed, we can only conclude that this index was not able to capture the increased risk of patients with SSc-PAH in our cohort.
Although negative, our study has several features of originality including being the first to provide complete resting ventricular function and remodeling data in incident SSc-PAH and IPAH patients. In a field in which numerous RV metrics of remodeling, function and load adaptation are available, comparing those metrics head to head is necessary in order to understand which provide the most accurate prognostication for patients. Consistent with previous studies, our study did not show a difference in terms of echocardiographic simple RV functional metrics.10 However, unlike previous studies in which RV function was usually assessed by qualitative right heart dilation from reports or by the TAPSE (which gives limited information on lateral basal contraction),10,33 our study analyzed quantitative indices of right heart remodeling and function including novel indices of ventricular function such as RV longitudinal strain.34,35 In addition, our study comprehensively explored previously reported non-invasive load-adaptability metrics. Finally, the longitudinal assessment of these metrics at one year after introduction of therapy enables us to explore the value of these metrics as markers of response to therapy, although larger randomized studies are needed.
Potential explanations for the worse intrinsic RV function in SSc-PAH need further investigation, but include the presence of myocardial fibrosis, inflammation, depressed sarcomeric function, microvascular disease or neurohumoral effectors.36,37 Cardiac magnetic resonance enabling extra-cellular matrix imaging by contrast enhancement or T1 mapping sequences could be potentially useful for detection of intrinsic RV differences in SSc-PAH and IPAH patients. Exercise or dobutamine echocardiography may also provide an alternative means to identify early RV dysfunction non-invasively, as exercise has been shown to detect those with early PAH38,39 and unmask ventriculo-arterial uncoupling in SSc-PAH.32
This study has several limitations. First, it is a retrospective study performed in a prospective cohort with missing imaging data at one year in three survivors with SSc-PAH. Second, the stringent matching by resistance and pulmonary hypertension therapy resulted in a relatively small sample size, which may have hindered our ability to detect subtle differences in RV dysfunction and load adaptation. Similarly, the small number of patients and events during follow-up limits our confidence in interpreting relationship with outcomes. Third, pulmonary vascular resistance was used to match for load acknowledging that RV afterload is a dynamic interplay between resistance, capacitance and wave reflections. However, capacitance and estimation of arterial elastance did not significantly differ between the two groups at baseline. Lastly, in this cohort, patients did not undergo cardiac magnetic resonance imaging, thus RV mass could not be accurately assessed.
In conclusion, resting non-invasive right load-adaptability indices at baseline and one-year follow-up do not appear to capture the complexity of ventricular adaptation and increased risk of heart failure in patients with SSc-PAH. Other modalities such as exercise testing or extracellular imaging using cardiac magnetic resonance imaging should be assessed in future studies.
Supplemental Material
Supplemental material for Non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension by Sarah French, Myriam Amsallem, Nadia Ouazani, Shufeng Li, Kristina Kudelko, Roham T. Zamanian, Francois Haddad and Lorinda Chung in Pulmonary Circulation
Acknowledgements
The authors would like to thank the Vera Moulton Wall Center at Stanford and Stanford Cardiovascular Institute for their support. Ethical approval: all procedures performed in studies involving human participants were approved by Stanford University Institutional Review Board in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Author contribution: SF, MA, FH and LC contributed equally to the study. SF and MA (co-first authors) have both equally contributed to the conception and design of the study, analysis and interpretation of data, drafting of the manuscript, and final approval of the version to be submitted. NO has contributed to collection of echocardiographic data and final approval of the manuscript. SL has contributed to the design of the study, statistical analysis and final approval of the manuscript. KK and RTZ have contributed to the conception and design of the study, revising the article critically and final approval of the article. FH and LC (co-senior authors) have both equally contributed to the conception and design of the study, interpretation of data, revising the article critically and final approval of the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Vera Moulton Wall Center (Young Investigator Seed Grant), the French National Research Agency as part of the second Investissements d’Avenir program (ANR-15-RHUS-0002) and the Pai Chan Lee Research Fund.
References
- 1.Sundaram SM, Chung L. An update on systemic sclerosis-associated pulmonary arterial hypertension: A review of the current literature. Curr Rheumatol Rep 2018; 20: 10. [DOI] [PubMed] [Google Scholar]
- 2.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: Application of a registry approach. Ann Rheum Dis 2003; 62: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigley FM, Lima JAC, Mayes M, et al. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum 2005; 52: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 4.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123: 344–350. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 7.Chung L, Farber HW, Benza R, et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest 2014; 146: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelemen BW, Mathai SC, Tedford RJ, et al. Right ventricular remodeling in idiopathic and scleroderma-associated pulmonary arterial hypertension: Two distinct phenotypes. Pulm Circ 2015; 5: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swift AJ, Capener D, Johns C, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2017; 196: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum 2006; 54: 3043–3050. [DOI] [PubMed] [Google Scholar]
- 11.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulate D, Mercier O, Guihaire J, et al. Pulmonary circulatory – right ventricular uncoupling: New insights into pulmonary hypertension pathophysiology. In: Maron BA, Zamanian RT and Waxman AB. (eds) Pulmonary hypertension: Basic science to clinical medicine. Basel, Switzerland: Springer International Publishing, 2016.
- 13.Amsallem M, Kuznetsova T, Hanneman K, et al. Right heart imaging in patients with heart failure: A tale of two ventricles. Curr Opin Cardiol 2016; 31: 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amsallem M, Boulate D, Aymami M, et al. Load adaptability in patients with pulmonary arterial hypertension. Am J Cardiol 2017; 120: 874–882. [DOI] [PubMed] [Google Scholar]
- 15.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013; 305: 1373–1381. [DOI] [PubMed] [Google Scholar]
- 16.Dandel M, Potapov E, Krabatsch T, et al. Load dependency of right ventricular performance is a major factor to be considered in decision making before ventricular assist device implantation. Circulation 2013; 128: 14–23. [DOI] [PubMed] [Google Scholar]
- 17.Dandel M, Knosalla C, Kemper D, et al. Assessment of right ventricular adaptability to loading conditions can improve the timing of listing to transplantation in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2015; 34: 319–328. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 19.Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amsallem M, Sternbach JM, Adigopula S, et al. Addressing the controversy of estimating pulmonary arterial pressure by echocardiography. J Am Soc Echocardiogr 2016; 29: 93–102. [DOI] [PubMed] [Google Scholar]
- 21.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006; 47: 799–803. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992; 86: 513–521. [DOI] [PubMed] [Google Scholar]
- 23.Chemla D, Antony I, Lecarpentier Y, et al. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 2003; 285: 614–620. [DOI] [PubMed] [Google Scholar]
- 24.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 26.Amsallem M, Sweatt AJ, Aymami MC, et al. Right heart end-systolic remodeling index strongly predicts outcomes in pulmonary arterial hypertension: Comparison with validated models. Circ Cardiovasc Imaging 2017; 10: pii:e005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens GR, Garcia-Alvarez A, Sahni S, et al. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging 2012; 5: 378–387. [DOI] [PubMed] [Google Scholar]
- 28.Saydain G, Awan A, Manickam P, et al. Pulmonary hypertension an independent risk factor for death in intensive care unit: Correlation of hemodynamic factors with mortality. Clin Med Insights Circ Respir Pulm Med 2015; 9: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 2016; 35: 67–73. [DOI] [PubMed] [Google Scholar]
- 30.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin V, Channick RN, Ghofrani H-A, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 32.Hsu S, Houston BA, Tampakakis E, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016; 133: 2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathai SC, Sibley CT, Forfia PR, et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J Rheumatol 2011; 38: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 34.Haeck MLA, Scherptong RWC, Marsan NA, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 2012; 5: 628–636. [DOI] [PubMed] [Google Scholar]
- 35.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013; 6: 711–721. [DOI] [PubMed] [Google Scholar]
- 36.Overbeek MJ, Mouchaers KTB, Niessen HM, et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010; 2010: 604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu S, Kokkonen-Simon KM, Kirk JA, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation 2018; 137: 2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callejas-Rubio JL, Moreno-Escobar E, de la Fuente PM, et al. Prevalence of exercise pulmonary arterial hypertension in scleroderma. J Rheumatol 2008; 35: 1812–1816. [PubMed] [Google Scholar]
- 39.Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension by Sarah French, Myriam Amsallem, Nadia Ouazani, Shufeng Li, Kristina Kudelko, Roham T. Zamanian, Francois Haddad and Lorinda Chung in Pulmonary Circulation