Abstract
Arthropod-borne disease outbreaks, facilitated by the introduction of exotic mosquitoes, pose a significant public health threat. Recent chikungunya virus (CHIKV) epidemics in Europe highlight the importance of understanding the vector potential of invading mosquitoes. In this paper we explore the potential of Aedes koreicus, a mosquito new to Europe, to transmit CHIKV. Mosquitoes were challenged with CHIKV and maintained at two temperatures: 23 °C and a fluctuating temperature. Total CHIKV infection rates at 3, 10 and 14 days post-feeding were low for both temperature treatments (13.8% at 23 °C; 6.2% at fluctuating T). A low percentage (6.1%, n = 65) of mosquitoes maintained at a constant 23 °C showed dissemination of the virus to the wings and legs. Infection of mosquito saliva, with live virus, occurred in 2 mosquitoes. No dissemination was noted under the fluctuating temperature regime. Based on these results we conclude that CHIKV transmission by this species is possible.
Keywords: Aedes koreicus, invasive mosquito species, fluctuating temperature, chikungunya, arbovirus, vector competence, arthropod-borne disease, public health
Introduction
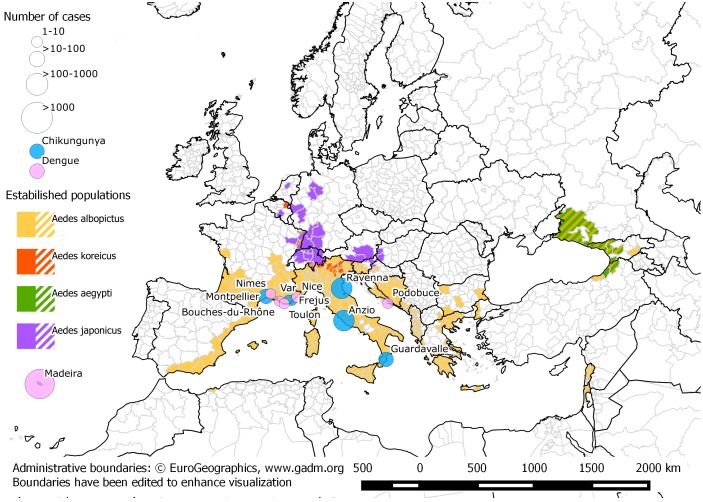
Globalization of trade and travel often results in the introduction of new species into non-native territories [1–4]. Arthropod-borne virus (arbovirus) outbreaks of public health significance have occurred as a consequence [5–12]. In 2012, an Aedes aegypti population that had established in Madeira (Portugal) in 2004 [13,14] was responsible for the largest outbreak of dengue in Europe since 1928 [15]. More than 2000 cases were recorded [16] (Figure 1). Similarly, the continuing expansion of Aedes albopictus and Ae. aegypti [17–19] might aggravate the ongoing pandemic of Zika fever through South and Central America and the Caribbean [20]. A mutation in the chikungunya virus (CHIKV) that facilitates enhanced transmission by Ae. albopictus was introduced from India to Ravenna Province, Italy in 2007. The local presence of Ae. albopictus set off an epidemic of over 200 human cases [9]. The establishment of Ae. albopictus across southern Europe has also led to autochthonous outbreaks of dengue and chikungunya in France in 2010 and 2014 (Figure 1). Further chikungunya outbreaks were reported in France and Italy during 2017 [10–12]. In recent years, a new invader: Aedes koreicus has entered Europe, with the largest populations found in Italy [21–24].
Figure 1.
Distribution of invasive mosquito species in Europe (data collated from ECDC maps https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps) and locations and severity of autochthonous dengue and chikungunya outbreaks in Europe from 2007 [9,11,12,16,53–55,83–87]. Map created by Sandro Savino (University of Padua) and Silvia Ciocchetta (QIMR Berghofer).
Invasive mosquito species in Europe: an overview
Europe has suffered a number of mosquito invasions in recent decades. The first report of Ae. albopictus in Europe came from Albania in 1979 [25]. It was then reported from Italy: first at the Genoa docks in 1990 and then, one year later, in Padua. The species is presumed to have been introduced through the import of used tyres from the United States [26,27] and has now established over almost all of the Italian peninsula [28] and in 22 other European countries (www.ecdc.europa.eu).
The commercial trade in tiers was also the most likely cause of the establishment of Aedes japonicus in Europe [29]. This species has colonized most of Switzerland, large regions in Austria and Germany and is also present in Belgium, France, Netherlands, Hungary, Slovenia, Croatia and Lichtenstein [30–32]. In 2016, it was also discovered in Italy [33].
Ae. aegypti, the most important arbovirus vector of all [34] was present from the late 1700s in many southern European countries. Reasons for its subsequent disappearance from the region during the 1900s are unclear but it has since re-invaded Madeira (Portugal), European Russia, Georgia and North East Turkey [35,36]. Figure 1 is an updated representation of extent of exotic mosquito introductions in Europe.
A new European invader: Aedes koreicus
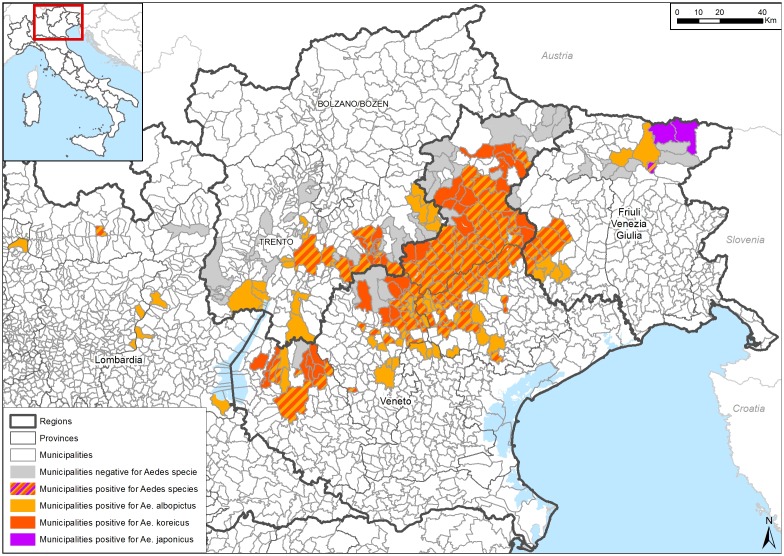
In 2011 a new aedine mosquito species, Aedes (Finlaya) koreicus (Edwards, 1917), was detected in Italy [21]. It has since established in Belgium, Russia, Switzerland, Germany, Hungary and Slovenia [37–42]. The species is well adapted to the temperate Italian climate and, in northern Italy, its distribution overlaps with Ae. albopictus. It has also colonized mountainous districts where Ae. albopictus remains absent [22,43] (Figure 2). Both mosquitoes breed in artificial containers, although Ae. koreicus is seldom found in the same containers as Ae. albopictus [44]. Ae. albopictus is a highly competitive mosquito and its displacement by Ae. koreicus seems unlikely [44]. The distribution of Ae. koreicus in Italy suggests that the area infested by the species represents only a small part of its potential European range. Dispersal models predict that parts of the Po and Adige valleys will be colonised over the next decade [24] and that it may establish in areas 400–1500 m above sea level, well above the range of Ae. albopictus (which remains below 600–800 m [45]). Despite this ongoing spread, the vector competence of Ae. koreicus for human arboviruses is under-explored. There are some limited observations on Japanese Encephalitis virus transmission [46–48] but nothing about its potential to mediate outbreaks of chikungunya.
Figure 2.
Distribution of Ae. koreicus, Ae. albopictus and Ae. japonicus in northeastern Italy (data and map provided by Geographic Information Systems office, IZSVe).
Chikungunya virus: European outbreaks caused by invasive mosquitoes
Chikungunya is an arboviral disease caused by an alphavirus of the family Togaviridae. CHIKV is transmitted by Aedes mosquitoes and characterized by acute febrile arthralgia in symptomatic human patients [49]. Phylogenetic analysis has identified three different genotypes of the virus: West African, Asian and East/Central/South African (ECSA) [50].
The first outbreak of CHIKV in Europe occurred in 2007 in Italy and was mediated by the invasive vector Ae. albopictus, and the introduction of a virus with the E1–226 V mutation belonging to the ECSA genotype (Indian Ocean lineage) by a viraemic traveler returning from India to Ravenna province. The E1–226 V mutation increases virus infectivity in Ae. albopictus [9,51,52]. The high density of Ae. albopictus in the outbreak area facilitated an epidemic involving more than 200 symptomatic human cases [9]. Three years later, autochthonous transmission of CHIKV occurred in Fréjus in southeastern France, and involved two people, Ae. albopictus and an ECSA CHIKV strain lacking the adaptive mutation for Ae. albopictus [53]. In 2014, Ae. albopictus was responsible for transmitting CHIKV (E1–226 V) which resulted in 11 chikungunya cases in Montpellier, southern France [54]. In August 2017 eight autochthonous cases of chikungunya were diagnosed in the Var department, south-eastern France, an area where Ae. albopictus is established [55]. In the same month, an outbreak of CHIKV (without the E1–226 V mutation) in the Lazio and Calabria Regions of Italy caused more than 300 cases (Figure 1). Ae. albopictus is the only potential vector in the area [10–12].
Despite the rapid spread and anthropophilic habits of Ae. koreicus [56] and the now common transmission of CHIKV in some parts of Europe, the risk of transmission of human disease agents by this species is largely unknown. This manuscript presents preliminary data on the potential of Ae koreicus to transmit CHIKV and provides the first indication of the susceptibility of Ae. koreicus to infection with CHIKV ‘La Reunion’ strain.
Materials and methods
We tested the potential of Ae. koreicus to transmit CHIKV ‘La Reunion’ (carrying the mutation E1-A226 V) under constant and fluctuating temperature regimes in the laboratory. The fluctuating temperatures mimicked those occurring during a typical summer in Belluno, Italy, an area where there are established and thriving populations of Ae. koreicus. Mosquitoes, sourced from Belluno and kept in colony for 3 years, were reared in the quarantine insectary at QIMR Berghofer Medical Research Institute (QIMR Berghofer) as per Ciocchetta et al. [57]. Infected Ae. albopictus mosquitoes, sourced from Hammond Island, Torres Strait (Australia) and kept in colony for 3 years (also reared at QIMR Berghofer: Hugo et al. [70]), were used as a validation of our infection technique. Mosquito infection and sample processing were performed in a biosafety level 3 quarantine facility at QIMR Berghofer.
After a 24 h starvation period (in which the standard 10% sucrose solution was substituted with distilled water only) 342 adult female mosquitoes, 3–5 days old, were transferred to 750 mL plastic containers (ca. 100 individuals per container) covered with gauze lids. The mosquitoes were allowed to feed for 1 h through glass membrane feeders (37 °C) covered by a porcine intestinal membrane filled with an infectious blood meal [58]. The infectious blood meal was obtained by adding 1 mL of stock virus CHIKV ‘La Reunion’ strain (LR2006-OPY1; GenBank KT449801 [59]) to 24 mL of defibrinated sheep blood (Life Technologies, Mulgrave, VIC, Australia) at a final titre of 108 TCID50/mL (TCID50 is the dilution ratio of the virus required to cause 50% mortality of cells used as a substrate for inoculation: in this experiment these were C6/36 Ae. albopictus cells). The infectious blood meal was sampled before and after feeding to determine that there was no degradation of virus titre over the feeding period. During feeding, a tube containing dry ice generated a small amount of CO2 that encouraged feeding activity (Ciocchetta, unpublished observations). After feeding, mosquitoes were anesthetized with CO2 and sorted in a cold Petri dish. Males and non-engorged females were discarded. Engorged females were transferred to a fresh container and maintained for 14 days in environmental chambers (MLR-352-PE Climate Chamber, Panasonic, Osaka, Japan) at two temperature regimes: (1) constant temperature of 23 °C, with a 12 h light: 12 h dark cycle and 75 ± 5% relative humidity; (2) fluctuating temperature based on the average temperatures registered during summer in Belluno (Italy) (Table 1) (data from Belluno Airport Meteorological Station, code 264, 46°42′00″N-12°07′48″E, Regional Agency for Environmental Prevention and Protection in Veneto, http://www.arpa.veneto.it/bollettini/storico, May to October 2011–2014), with a 12 h light: 12 h dark cycle and 75 ± 5% relative humidity. During the holding period mosquitoes were provided with 10% sucrose ad libitum.
Table 1.
The daily fluctuating temperature regime under which Ae. koreicus was maintained (75 ± 5% relative humidity, 12 h light: 12 h dark cycle).
| Phase | Degrees (°C) | Light step (Illuminance) |
|---|---|---|
| 1 (0.15 h) | 12 | 1 (1667 Lx) |
| 2 (0.15 h) | 12 | 2 (3334 Lx) |
| 3 (2.30 h) | 12 | 3 (5000 Lx) |
| 4 (3 h) | 17 | 3 (5000 Lx) |
| 5 (3 h) | 22 | 3 (5000 Lx) |
| 6 (2.30 h) | 27 | 3 (5000 Lx) |
| 7 (0.15 h) | 27 | 2 (3334 Lx) |
| 8 (0.15 h) | 27 | 1 (1667 Lx) |
| 9 (3 h) | 27 | 0 (0 Lx) |
| 10 (3 h) | 22 | 0 (0 Lx) |
| 11 (3 h) | 17 | 0 (0 Lx) |
| 12 (3 h) | 12 | 0 (0 Lx) |
At 3, 10 and 14 days post-feeding, Ae. koreicus females were anesthetized using CO2, and dissected (25 mosquitoes on each day). The Ae. albopictus controls were included to validate our infection technique and were maintained at the constant temperature regimen. Dissection occurred at day 10 only. Legs and wings were removed from each mosquito and transferred to separate 1.5 mL screw cap microfuge tubes containing 4 zirconium silica beads stored at −80 °C. Live mosquitoes deprived of wings and legs were stuck to a glass plate with double-sided sticky tape. These mosquitoes were permitted to salivate for 20 min by inserting their proboscis into a P200 tip loaded with 50 μL of collecting medium (RPMI 1640, 2% Foetal Bovine Serum (FBS), 1% Penicillin-streptomycin, 0.25 μg mL−1 Amphotericin B) (Gibco; Thermo Scientific, Waltham, MA, USA). Peristaltic movements of the abdomen and labellae were observed for each individual mosquito under a stereoscope, confirming that saliva was expectorated. After 20 min, the contents of the P200 tips were emptied into a 1.5 mL microfuge tube. Each mosquito body was placed in a separate tube. All samples were stored at −80 °C until processing.
Each mosquito body was placed in a tube with collecting medium (as above) buffered with 1:100 10 mM HEPES, homogenized and inoculated onto C6/36 cells cultured in 5% CO2 atmosphere at 27 °C [60]. Wings and legs were combined and processed in the same way as the body. Inoculations with saliva and infected blood followed the same procedure except for the initial homogenization step: 10μL of collecting medium with the mosquito saliva were directly inoculated to the plates after thawing. After a 3-day incubation period, all plates were assayed using an established ELISA technique [61] in which the conjugate solution (horseradish peroxidase labelled affinity purified goat-antimouse immunoglobulin G: DAKO Corporation, Carpinteria, CA, USA) was diluted at 1:1000. Non-neutralizing monoclonal antibodies (Hybridoma clone D7) were diluted 1:200 in blocking buffer and 50 μL were added to each well. Cells infected with CHIKV ‘La Reunion’ provided a positive control for the assay. The final chromogenic substrate added to the plates consisted of 50μL/well of TMB (3,3′,5,5′-Tetramethylbenzidine Substrate System, Sigma-Aldrich®). The plates were then incubated in the dark for 30 min. A 50μL/well stop solution (Stop Reagent for TMB Substrate, Sigma-Aldrich®) was added and the absorbency at 450 nm was measured in a microplate reader (BioTek™ Synergy™ H4 Hybrid Microplate Reader).Wells were scored as positive for virus when the optical density (OD) was greater than twice the mean OD of the uninfected control wells [62]. The virus titres of individual mosquitoes were determined by calculating 50% end points [63] expressed as the log10 TCID50 mL−1. We first tested for CHIKV ‘La Reunion’ infection in mosquito bodies. Wings, legs and saliva were processed only if body samples were positive for that particular mosquito.
Data analysis
CHIKV dissemination in Ae. koreicus under constant and fluctuating temperature regimes was compared using Fishers exact test (Prism GraphPad 6®, GraphPad Software, San Diego, CA, USA).
Results and discussion
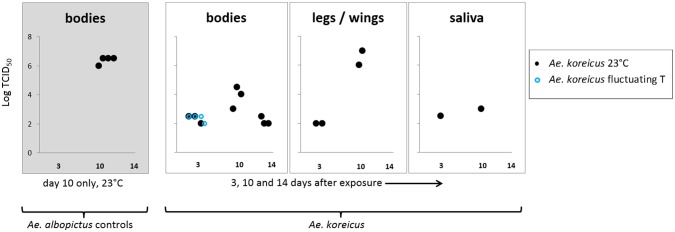
All Ae. albopictus (n = 4) were positive for CHIKV at values consistent with previous experiments (106 TCID50 mL−1, n = 1; 106.5 TCID50 mL−1, n = 3) [60] (Figure 3). This expected result validated our infection protocols. Ae. koreicus demonstrated a high survivorship after 14 days at constant and fluctuating temperatures (95.8%, n = 71; 98.1%, n = 53) and high feeding rates (65.5%, n = 342). Virus titres in the blood before and after the feeding period (1 h) were: 108 TCID50 mL−1 and 106.5 TCID50 mL−1, respectively. Despite these very favourable infection conditions, CHIKV ‘La Reunion’ was subsequently detected in a very small percentage of mosquito bodies (13.8%, n = 65, T = 23 °C; 6.2%, n = 64, T = fluctuating). Titres ranged from 102 –104.5 TCID50 mL−1 (at 23 °C) to 102 –102.5 TCID50 mL−1 (at the fluctuating temperature) (Figure 3).
Figure 3.
CHIKV ‘La Reunion’ titres in Ae. koreicus measured 3, 10 and 14 days post-infection in mosquitoes at 23 °C and at fluctuating temperature (75 ± 5% relative humidity, 12 h light: 12 h dark cycle).
The validation of the technique, using a small number of Ae. albopictus, is also shown.
Dissemination to wings and legs was observed in just four mosquitoes at days 3 and 10 post-feeding (102–107 TCID50 mL−1, Figure 3) and salivary dissemination occurred in two of those four individuals: 102.5 TCID50 mL−1 at day 3 post-feeding and 103 TCID50 mL−1 at day 10 post-feeding (Figure 3). Only when held at a constant temperature did CHIKV ‘La Reunion’ disseminate to the wings and legs and reach the saliva of Ae. koreicus (Table 2). These low infection rates are unlikely to be a consequence of insufficient incubation periods at low temperature: infection of Ae. albopictus salivary glands by CHIKV E1–226 V has been observed to occur 6 days post-exposure at 20 °C [64].
Table 2.
CHIKV ‘La Reunion’ infection and dissemination to the wings/legs and saliva in Ae. koreicus mosquitoes maintained at 23 °C and fluctuating temperature (75 ± 5% relative humidity, 12 h light: 12 h dark cycle).
| Treatment | Day post feeding | Engorged mosquitoes | Infection (tested) | Dissemination wings/legs (tested) | Dissemination saliva (tested) |
|---|---|---|---|---|---|
| 23 °C | 3 | 21 | 3 (21) | 2 (3) | 1 (3) |
| 10 | 19 | 3 (19) | 2 (3) | 1 (3) | |
| 14 | 25 | 3 (25) | 0 (3) | 0 (3) | |
| Fluctuating | 3 | 21 | 4 (21) | 0 (4) | 0 (4) |
| T °C | 10 | 21 | 0 (21) | NT* | NT* |
| 14 | 22 | 0 (22) | NT* | NT* |
NT* = Not tested as bodies were negative.
Artificially constant temperatures (23 °C) appeared to encourage greater dissemination of virus (4/65) when compared to ‘real world’ temperature fluctuations (0/64) but low infection rates meant that these differences were not significant (Fishers exact test, p = 0.12). A posteriori, assuming similar infection rates, we would need to have tested approximately double the number of mosquitoes (calculated using G*Power 3.1.9.2, n = 296, α = 0.05, 1−β = 0.95; [65]) to prove the impact of temperature regimes.
Ae. aegypti and Ae. albopictus are the main vectors of CHIKV [66–71] with Ae. albopictus being responsible for all CHIKV outbreaks in Europe [12,72–75]. The average temperature in European cities experiencing CHIKV outbreaks is 20 °C or above [73] although maximum transmission potential is realised between 26 and 29 °C [76]. A number of other Aedes species are effective vectors of CHIKV E1-A226 V under laboratory conditions and this includes Ae. japonicus [77] which is phylogenetically close to Ae. koreicus [78,79]. Salivary dissemination occurred in 38.5% of engorged Ae. japonicus after 14 days at 28 °C [77]. It is therefore unsurprising that Ae. koreicus shows vector potential under laboratory conditions, but its increasing geographic range in Italy (Figure 2), its human biting habit [56] and its capacity to expectorate live CHIKV after just three days suggests that CHIKV transmission by this species under field conditions should not be discounted.
Our work indicated that only a small proportion of Ae. koreicus from our laboratory colony could vector CHIKV under optimal rearing temperatures and that realistic temperature fluctuations might further mitigate the risks of transmission. This would be consistent with studies on dengue virus in which mosquitoes exposed to constant temperatures showed higher midgut infection levels [80] and higher dissemination [81] rates compared with mosquitoes maintained at fluctuating temperatures.
In our temperature regimens, relative humidity was a constant parameter although it may also be a variable that could affect Ae. koreicus vector competence. We recommend that further studies mimic the relative humidity fluctuations that may impact virus dynamics and mosquito survival in the field [82].
Conclusions
Our results suggest that low-level transmission of the CHIKV ‘La Reunion’ strain by Ae. koreicus is possible in regions with temperatures similar to those used in our experiments. These findings help define the relative public health risks of this new mosquito invasion in comparison with the existing threats posed by Ae. albopictus and may assist in prioritising the resources that might be directed towards its surveillance or control.
We encourage further studies on the risks related to Ae. koreicus invasion across the range of climates and geographic regions suitable for the establishment of this invader. According to the European Centre for Disease Prevention and Control database (http://atlas.ecdc.europa.eu/public/index.aspx), at least 9332 travel-associated cases of CHIKV were imported to Europe between 2008 and 2015. These viraemic importations present a clear risk of autochthonous viral transmission where competent vectors exist. Ae. koreicus may now be added to that list.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Disclosure statements
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the QIMR Berghofer Medical Research Institute (Brisbane, Australia).
Acknowledgements
The authors thank Leon E. Hugo and Elise A. Kho (QIMR Berghofer Mosquito Control Laboratory) for their assistance with mosquito infection and dissection and for providing the non-neutralizing D7 monoclonal antibodies used in this study. We thank Matteo Mazzucato (IZSVe, Geographic Information Systems office) for providing the map in Figure 2.
References
- [1].Meyerson LA, Mooney HA. Invasive alien species in an era of globalization. Front Ecol Environ. 2007;5(4):199–208. 10.1890/1540-9295(2007)5[199:IASIAE]2.0.CO;2 [DOI] [Google Scholar]
- [2].Hulme PE. Biological invasions in Europe: drivers, pressures, states, impacts and responses In: Hester R and Harrison RM, editors. Biodiversity under threat Cambridge, UK: Cambridge University Press; 2007. p. 56–80. [Google Scholar]
- [3].Early R, Bradley BA, Dukes JS, et al. . Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun. 2016;7:12485. 10.1038/ncomms12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rabitsch W, Essl F, Schindler S. Impact of biological invasions on ecosystem services. Cham: Springer; 2017. The rise of non-native vectors and reservoirs of human diseases; p. 263–275. 10.1007/978-3-319-45121-3 [DOI] [Google Scholar]
- [5].Kilpatrick AM. Globalization, land use, and the invasion of West Nile Virus. Science. 2011;334(6054):323–327. 10.1126/science.1201010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: a global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. 10.1016/j.actatropica.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kindhauser MK, Allen T, Frank V, et al. . Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94(9):675–686C. 10.2471/BLT.16.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Failloux A-B, Bouattour A, Faraj C, et al. . Surveillance of arthropod-borne viruses and their vectors in the Mediterranean and Black Sea Regions within the MediLabSecure Network. Curr Trop Med Rep. 2017;4(1):27–39. 10.1007/s40475-017-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rezza G, Nicoletti L, Angelini R, et al. . Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- [10].Venturi G, Di Luca M, Fortuna C, et al. . Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill. 2017;22 (39):17–00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cella E, Riva E, Salemi M, et al. . The new Chikungunya virus outbreak in Italy possibly originated from a single introduction from Asia. Pathog Glob Health. 2017;72:1– 8 10.1080/20477724.2017.1406565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manica M, Guzzetta G, Poletti P, et al. . Transmission dynamics of the ongoing chikungunya outbreak in Central Italy: from coastal areas to the metropolitan city of Rome, summer 2017. Euro Surveill. 2017;22 (44):17–00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Margarita Y, Grácio A, Lencastre I, et al. . First record of Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera, Culicidae) in Madeira Island-Portugal. Acta Parasitológica Portuguesa. 2006;13(1):59–61. [Google Scholar]
- [14].Almeida A, Gonçalves Y, Novo M, et al. . Vector monitoring of Aedes aegypti in the autonomous region of Madeira, Portugal. Euro Surveill. 2007;12(11):E071115. [DOI] [PubMed] [Google Scholar]
- [15].Theiler M, Casals J, Moutousses C. Etiology of the 1927–28 epidemic of dengue in Greece. Proc Soc Exp Biol Med. 1960;103(1):244–246. 10.3181/00379727-103-25474 [DOI] [PubMed] [Google Scholar]
- [16].Wilder-Smith A, Quam M, Sessions O, et al. . The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill. 2014 Feb 27;19(8):20718. [DOI] [PubMed] [Google Scholar]
- [17].Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4(5):e646. 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hawley WA, Reiter P, Copeland RS, et al. . Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. 10.1126/science.3576225 [DOI] [PubMed] [Google Scholar]
- [19].Pless E, Gloria-Soria A, Evans BR, et al. . Multiple introductions of the dengue vector, Aedes aegypti, into California. PLoS Negl Trop Dis. 2017;11(8):e0005718. 10.1371/journal.pntd.0005718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fauci AS, Morens DM. Zika virus in the Americas – yet another arbovirus threat. N Engl J Med. 2016;374(7):601–604. 10.1056/NEJMp1600297 [DOI] [PubMed] [Google Scholar]
- [21].Capelli G, Mathis A, Di Luca Simone, et al. . First report in italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasit Vectors. 2011;4(1):188–188. doi: 10.1186/1756-3305-4-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Montarsi F, Drago A, Martini S, et al. . Current distribution of the invasive mosquito species, Aedes koreicus [Hulecoeteomyia koreica] in northern Italy. Parasit Vectors. 2015;8(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baldacchino F, Montarsi F, Arnoldi D, et al. . A 2-yr mosquito survey focusing on Aedes koreicus (Diptera: Culicidae) in Northern Italy and implications for adult trapping. J Med Entomol. 2017;54(3):622–630. 10.1093/jme/tjw216 [DOI] [PubMed] [Google Scholar]
- [24].Marcantonio M, Metz M, Baldacchino F, et al. . First assessment of potential distribution and dispersal capacity of the emerging invasive mosquito Aedes koreicus in Northeast Italy. Parasit Vectors. 2016;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998;14(3):340–343. [PubMed] [Google Scholar]
- [26].Sabatini A, Raineri V, Trovato G, et al. . Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area. Parassitologia. 1990;32(3):301–304. [PubMed] [Google Scholar]
- [27].Dalla Pozza G, Majori G. First record of Aedes albopictus establishment in Italy. J Am Mosq Control Assoc. 1992;8(3):318–320. [PubMed] [Google Scholar]
- [28].Dutto M, Mosca A. Preliminary considerations about the presence of Aedes albopictus (Skuse 1897)(Diptera: Culicidae) during winter in the Northwestern Italy. Annali di igiene: medicina preventiva e di comunita. 2017;29(1):86–90. [DOI] [PubMed] [Google Scholar]
- [29].Schaffner F, Kaufmann C, Hegglin D, et al. . The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23(4):448–451. 10.1111/mve.2009.23.issue-4 [DOI] [PubMed] [Google Scholar]
- [30].Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu Rev Entomol. 2014;59:31–49. 10.1146/annurev-ento-011613-162012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krebs T, Bindler P, L’Ambert G, et al. . First establishment of Aedes japonicus japonicus (Theobald, 1901) (Diptera: Culicidae) in France in 2013 and its impact on public health. J Vector Ecol. 2014;39(2):437–440. 10.1111/jvec.2014.39.issue-2 [DOI] [PubMed] [Google Scholar]
- [32].Zielke DE, Ibáñez-Justicia A, Kalan K, et al. . Recently discovered Aedes japonicus japonicus (Diptera: Culicidae) populations in The Netherlands and northern Germany resulted from a new introduction event and from a split from an existing population. Parasit Vectors. 2015;8(1):40. 10.1186/s13071-015-0648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seidel B, Montarsi F, Huemer HP, et al. . First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: invasion from an established Austrian population. Parasit Vectors. 2016;9(1):223. 10.1186/s13071-016-1566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lindsay SW, Wilson A, Golding N, et al. . Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull World Health Organ. 2017;95(8):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dallimore T, Hunter T, Medlock JM, et al. . Discovery of a single male Aedes aegypti (L.) in Merseyside, England. Parasit Vectors. 2017;10(1):329. 10.1186/s13071-017-2251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Akiner MM, Demirci B, Babuadze G, et al. . Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4):e0004664. 10.1371/journal.pntd.0004664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bezzhonova OV, Patraman IV, Ganushkina LA, et al. . The first finding of invasive species Aedes (Finlaya) koreicus (Edwards, 1917) in European Russia. Med Parazitol (Mosk). 2014 Jan-Mar(1):16–19. rus. [PubMed] [Google Scholar]
- [38].Kurucz K, Kiss V, Zana B, et al. . Emergence of Aedes koreicus (Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitol Res. 2016;115(12):4687–4689. 10.1007/s00436-016-5229-5 [DOI] [PubMed] [Google Scholar]
- [39].Suter T, Flacio E, Fariña BF, et al. . First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasit Vectors. 2015;8(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Versteirt V, de Clercq E, Fonseca D, et al. . Bionomics of the established exotic mosquito species Aedes koreicus in Belgium. Europe. J Med Entomol. 2012;49(6):1226–1232. 10.1603/ME11170 [DOI] [PubMed] [Google Scholar]
- [41].Werner D, Zielke D, Kampen H. First record of Aedes koreicus (Diptera: Culicidae) in Germany. Parasitol Res. 2015;2015(11/28):1–4. [DOI] [PubMed] [Google Scholar]
- [42].Kalan K, Šušnjar J, Ivović V, et al. . First record of Aedes koreicus (Diptera, Culicidae) in Slovenia. Parasitol Res. 2017;116(8):2355–2358. [DOI] [PubMed] [Google Scholar]
- [43].Montarsi F, Ravagnan S, Ciocchetta Marco, et al. . Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern Italy. Parasit Vectors. 2013;6(1):292–292. 10.1186/1756-3305-6-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Baldacchino F, Arnoldi D, Lapère C, et al. . Weak larval competition between two invasive mosquitoes Aedes koreicus and Aedes albopictus (Diptera: Culicidae). J Med Entomol. 2017;54(5):1266–1272. 10.1093/jme/tjx093 [DOI] [PubMed] [Google Scholar]
- [45].Schaffner F, Hendrickx G, Scholte E-J, et al. . Development of Aedes albopictus risk maps. Stockholm: European Centre for Disease Prevention and Control; 2009. [Google Scholar]
- [46].Miles J. Some ecological aspects of the problem of arthropod-borne animal viruses in the Western Pacific and South-East Asia regions. Bull World Health Organ. 1964;30(2):197–210. [PMC free article] [PubMed] [Google Scholar]
- [47].Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera: Culicidae). J Med Entomol. 1989;26(5):454–458. 10.1093/jmedent/26.5.454 [DOI] [PubMed] [Google Scholar]
- [48].Gutsevich A, Monchadskii A, Shtakel’berg A. Fauna SSSR. Family Culicidae. 1971;3(4):73–405. [Google Scholar]
- [49].Couderc T, Lecuit M. Focus on Chikungunya pathophysiology in human and animal models. Microbes Infect. 2009;11(14):1197–1205. 10.1016/j.micinf.2009.09.002 [DOI] [PubMed] [Google Scholar]
- [50].Volk SM, Chen R, Tsetsarkin KA, et al. . Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497–6504. 10.1128/JVI.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pialoux G, Gaüzère B-A, Jauréguiberry S, et al. . Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7(5):319–327. 10.1016/S1473-3099(07)70107-X [DOI] [PubMed] [Google Scholar]
- [52].Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491–500. 10.1038/nrmicro2368 [DOI] [PubMed] [Google Scholar]
- [53].Grandadam M, Caro V, Plumet S, et al. . Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910. 10.3201/eid1705.101873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Delisle E, Rousseau C, Broche B, et al. . Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):21108. 10.2807/1560-7917.ES2015.20.17.21108 [DOI] [PubMed] [Google Scholar]
- [55].Calba C, Guerbois-Galla M, Franke F, et al. . Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22 (39):17–00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ciocchetta S, Luca T, Camuffo S, et al. . editors Indications on the host preference of Aedes koreicus [Hulecoeteomyia koreica], a new invasive mosquito to Italy. 9th CVBD symposium; 2014; Lisbon, Portugal.
- [57].Ciocchetta S, Darbro JM, Frentiu FD, et al. . Laboratory colonization of the European invasive mosquito Aedes (Finlaya) koreicus. Parasit Vectors. 2017;10(1):209. 10.1186/s13071-017-2010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rutledge L, Ward R, Gould D. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq News. 1964;24(4):407–409. [Google Scholar]
- [59].Poo YS, Rudd PA, Gardner J, et al. . Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis. 2014;8(12):e3354. 10.1371/journal.pntd.0003354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hugo LE, Prow NA, Tang B, et al. . Chikungunya virus transmission between Aedes albopictus and laboratory mice. Parasit Vectors. 2016;9(1):420. 10.1186/s13071-016-1838-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jeffery JA, Ryan PA, Lyons SA, et al. . Vector competence of Coquillettidia linealis (Skuse)(Diptera: Culicidae) for Ross River and Barmah Forest viruses. Aust J Entomol. 2002;41(4):339–344. 10.1046/j.1440-6055.2002.00316.x [DOI] [Google Scholar]
- [62].Knox T, Kay B, Hall R, et al. . Enhanced vector competence of Aedes aegypti (Diptera: Culicidae) from the Torres Strait compared with mainland Australia for dengue 2 and 4 viruses. J Med Entomol. 2003;40(6):950–956. 10.1603/0022-2585-40.6.950 [DOI] [PubMed] [Google Scholar]
- [63].Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493–497. 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- [64].Zouache K, Fontaine A, Vega-Rua A, et al. . Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proceedings of the Royal Society of London B: Biological Sciences. 2014;281(1792):20141078. 10.1098/rspb.2014.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Faul F, Erdfelder E, Buchner A, et al. . Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- [66].Delatte H, Paupy C, Dehecq J, et al. . Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite (Paris, France). 2008;15(1):3–13. 10.1051/parasite/2008151003 [DOI] [PubMed] [Google Scholar]
- [67].Paupy C, Delatte H, Bagny L, et al. . Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11(14):1177–1185. 10.1016/j.micinf.2009.05.005 [DOI] [PubMed] [Google Scholar]
- [68].Paupy C, Ollomo B, Kamgang B, et al. . Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10(3):259–266. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- [69].Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks – the globalization of vectorborne diseases. N Engl J Med. 2007;356(8):769. 10.1056/NEJMp078013 [DOI] [PubMed] [Google Scholar]
- [70].Thavara U, Tawatsin A, Pengsakul T, et al. . Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae). Southeast Asian J Trop Med Public Health. 2009;40(5):951. [PubMed] [Google Scholar]
- [71].Pagès F, Peyrefitte CN, Mve MT, et al. . Aedes albopictus Mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE. 2009;4(3):e4691. 10.1371/journal.pone.0004691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fischer D, Thomas SM, Suk JE, et al. . Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int J Health Geogr. 2013;12(1):51. 10.1186/1476-072X-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tilston N, Skelly C, Weinstein P. Pan-European Chikungunya surveillance: designing risk stratified surveillance zones. Int J Health Geogr. 2009;8(1):61. 10.1186/1476-072X-8-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sigfrid L, Reusken C, Eckerle I, et al. . Preparing clinicians for (re-) emerging arbovirus infectious diseases in Europe. Clin Microbiol Infect. 2018;24(3):229–239. 10.1016/j.cmi.2017.05.029 [DOI] [PubMed] [Google Scholar]
- [75].Erguler K, Chandra NL, Proestos Y, et al. . A large-scale stochastic spatiotemporal model for Aedes albopictus-borne chikungunya epidemiology. PLOS ONE. 2017;12(3):e0174293. 10.1371/journal.pone.0174293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mordecai EA, Cohen JM, Evans MV, et al. . Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11(4):e0005568. 10.1371/journal.pntd.0005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schaffner F, Vazeille M, Kaufmann C, et al. . Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosq Bull. 2011;29:141–142. [Google Scholar]
- [78].Cameron EC, Wilkerson RC, Mogi M, et al. . Molecular phylogenetics of Aedes japonicus, a disease vector that recently invaded Western Europe, North America, and the Hawaiian islands. J Med Entomol. 2010 Jul;47(4):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Versteirt V, Nagy Z, Roelants P, et al. . Identification of Belgian mosquito species (Diptera: Culicidae) by DNA barcoding. Mol Ecol Resour. 2015;15(2):449–457. 10.1111/1755-0998.12318 [DOI] [PubMed] [Google Scholar]
- [80].Lambrechts L, Paaijmans KP, Fansiri T, et al. . Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proceedings of the National Academy of Sciences. 2011;108(18):7460–7465. 10.1073/pnas.1101377108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yixin HY, Carrasco AM, Dong Y, et al. . The effect of temperature on Wolbachia-mediated dengue virus blocking in Aedes aegypti. Am J Trop Med Hyg. 2016;94(4):812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Thu HM, Aye KM, Thein S. The effect of temperature and humidity on dengue virus propagation in Aedes aegypti mosquitos. Southeast Asian J Trop Med Public Health. 1998 Jun;29(2):280–284. [PubMed] [Google Scholar]
- [83].Succo T, Leparc-Goffart I, Ferré J-B, et al. . Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Euro Surveill. 2016;21(21):3. 10.2807/1560-7917.ES.2016.21.21.30240 [DOI] [PubMed] [Google Scholar]
- [84].Gjenero-Margan I, Aleraj B, Krajcar D, et al. . Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16(9):19805. [PubMed] [Google Scholar]
- [85].Gould E, Gallian P, de Lamballerie X, et al. . First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality!. Clin Microbiol Infect. 2010;16(12):1702–1704. 10.1111/j.1469-0691.2010.03386.x [DOI] [PubMed] [Google Scholar]
- [86].Marchand E, Prat C, Jeannin C, et al. . Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;201:18–50. [DOI] [PubMed] [Google Scholar]
- [87].Giron S, Rizzi J, Leparc-Goffart I, et al. . New occurrence of autochthonous cases of dengue fever in Southeast France, August–September. Bull Epidemiol Hebd (Paris). 2014;2015:13–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.