Abstract
Mosquito control using chemical insecticides is facing a major challenge due to development of insecticide resistance. Improving the efficiency of existing insecticides using synergistic secondary metabolites of biological origin is increasingly being researched. Herein, we evaluated the toxicity of Fusarium oxysporum extract alone and in binary combinations with temephos, on larvae and pupae of Anopheles stephensi, Aedes aegypti and Culex quinquefaciatus. F. oxysporum extract was characterized using TLC, FT-IR and GC-MS. After 24 h of exposure, the binary combination of temephos + F. oxysporum extract (1:1 ratio) was highly toxic to larvae of An. stephensi (LC50: 35.927 μg/ml), Ae. aegypti (LC50: 20.763 μg/ml) and Cx. quinquefasciatus, (LC50: 51.199 μg/ml). For pupae LC50 values were 38.668, 26.394, and 72.086 μg/ml, respectively. Histology studies of mosquitoes exposed to F. oxysporum extract showed vacuolation in epithelium, as well as in adipose, and muscle tissues of larval midgut. Overall, our results show that the synergistic combination of temephos and F. oxysporum extract is highly effective to control mosquito young instars.
Keywords: Fusarium oxysporum, temephos, Zika virus, FT-IR, GC-MS, histopathology
Introduction
Mosquitoes (Diptera: Culicidae) spread several diseases, which include malaria, dengue, yellow fever, filariasis, Japanese encephalitis and Zika virus. Anopheles stephensi is a major one of the leading vectors of malaria in India and other tropical and subtropical areas of the world [1]. Aedes aegypti is a day-biting mosquito responsible for the transmission of chikungunya, dengue fever, Rift Valley fever, Yellow fever and Zika virus, among others [2,3]. The Southern house mosquito, Culex quinquefasciatus a night biting mosquito responsible for the transmission of lymphatic filariasis, Japanese encephalitis and West Nile virus [4].
Vector control programs currently employ several synthetic insecticides, which include organophosphates, carbamates and pyrethroids, for the effective control of mosquito vectors [5,6]. However, the continuous overuse of a limited number of chemical pesticides result in fast development of multiple resistance in mosquitoes [7,8]. Currently, insecticide resistance is a major problem in Integrated Vector Management of mosquitoes. Besides, synthetic chemical insecticides also cause side effect to humans, non-target species and environmental pollution [5]. Several methods for the management of insecticide resistance in mosquitoes have been explored. Among these methods using synergistic combinations of bioactive metabolites from plants and fungi along with chemical insecticides is gaining importance [9], this can be attributed to the multiple modes of action of bioactive “green” metabolites. Research on bioactive metabolites from plants and fungal species showed relevant insecticidal properties [10–15].
Entomopathogenic fungi are among the most important species considered as potential biological control agents [16]. As significant number of them are preferred as they exhibit selective toxicity, do not persist, and do not need to be ingested [17]. There are several entomopathogenic fungi which have been tested for mosquito larval control. Metarhizium anisopliae has excellent toxic activity against Ae. aegypti [18], its secondary metabolites shows larvicidal and adulticidal activity against An. stephensi [19]. Fusarium oxysporum is a pathogenic soil-borne fungus distributed worldwide. Agricultural pests are naturally infected by F. oxysporum [20]. F. oxysporum strains can infect and kill a large range of hosts with agricultural and medically importance [21]. Synergism is the joint action where one component of the mixture has the effect of increasing the potency of the other component of the mixture, such that their combined effect is greater than the sum of their individual effects, as recently elucidated for binary mixtures of green biopesticides [22,23]. Besides, some insecticides have the capacity to increase stress and affect insect behavior, which may lead to improved performances of entomopathogens [24].
Temephos is an organophosphate insecticide which is recommended by WHO for the control of mosquito larvae [25]. The use of larvicides is highly regulated by WHO as there is an immediate danger of biomagnification of pesticides in food chain. There have been several reports showing that An. stephensi, Ae. aegypti and Cx. quinquefasciatus have developed resistance to temephos. Hence in order to increase the effectiveness of temephos, a combination with fungal secondary metabolite might be ideal. Therefore, in the present study, we evaluated the synergistic mosquitocidal activity of F. oxysporum with temephos on larvae and pupae of three mosquito species, namely An. stephensi, Ae. aegypti and Cx. quinquefasciatus . Chemical characterization F. oxysporum extract using TLC, FT-IR, and GC MS was carried out. In addition, midgut histology of the three mosquito species exposed to the fungal extract was investigated.
Materials and methods
Isolation and identification of F. oxysporum
Fusarium oxysporum MG574894.1 (Supplementary Data Figure S1) fungal strain was isolated from a dead Spodoptera litura in cotton field, Dharmapuri, Tamilnadu, India. F. oxysporum was characterized and sequenced. The sequenced data was submitted in National Center of Biotechnology Information (NCBI), the data base accession number is MG574894.1. Pure fungal strains were cultured in Potato Dextrose Agar (PDA) (Hi-media, India) for 7 days in the dark (28 ± 2 °C) inside an incubator at the Molecular Entomology Laboratory, Department of Biotechnology, Periyar University (Salem, India). F. oxysporum was primarily identified based on morphological characteristics colony, i.e. growth, presence or absence of aerial mycelium, colony colour, and colony pigment [26]. Mainly lacto phenol cotton blue was use for primary stain and the species was photographed under light microscope (Olympus CH-20i/India) at 40X magnification, (Supplementary Data Figure S2).
Preparation of the fungal broth
F. oxysporum spores were harvested by the flooding sterile distilled water with 0.05% Tween 80 (Sigma, USA). This fungal suspension was filtered by using sterile cloth for removal of fungal hyphae, conidia clumps and media debris. The filtered fungal suspension was estimated by using heamocytometer to count the spore concentration, then adjusted to 1 × 107 conidia/ml. The fungal broth was prepared with 250 ml of potato dextrose broth (PDB) in 500 ml Erlenmeyer flasks. 1 × 107 conidia/ml was inoculated in 250 ml of broth culture, then the broth was incubated (28 ± 2 °C) and placed in an orbital shaker (Rivotech-22038A2) at 130 rpm for 7 days, (Supplementary Data Figure S1b).
Extraction and concentration of F. oxysporum metabolites
After 7 days, the fungal biomass was filtered through Whatman no. 1 filter paper. Then, the biomass was washed with sterile distilled water for 3 times for the removal of media components. Then, 25 g of fungal biomass was transferred to a 500 ml Erlenmeyer flask containing 250 ml of ethyl acetate and extracted for 7–9 days using an orbital shaker (Rivotech-22038A2) with 130 rpm at 28 ± 2 °C. After incubation the fungal filtrate was filtered through Whatman No.1 filter paper.
The fungal crude filtrate was concentrated to eliminate ethyl acetate by using a rotary evaporator at 40 ± 5 °C (Superfit, India, Model R/150/01) under reduced pressure 23–27 mm hg at 40 ± 5 °C and the residue obtained was stored at room temperature.
Larval and pupal toxicity
An stephensi, Ae. aegypti and Cx. quinquefasciatus eggs was provided from the Institute of Vector Control Zoonoses (IVCZ) Banahalli, Tamil Nadu, India. The eggs hatched in distilled water and were maintained at 28 ± 2 °C and 60 ± 10 R.H. and 12:12 (L: D) photoperiod. We provided dog biscuits and yeast (3:1 ratio) as food source for the larvae.
The F. oxysporum extract, temephos and binary mixtures of F. oxysporum extract + temephos were tested against An. stephensi, Ae. aegypti and Cx. quinquefasciatus 4th instar larvae and pupae, following the method by WHO [27]. For each replicate, 25 mosquito larvae or pupae were stored in 300 ml plastic cups containing 250 ml of distilled water plus the desired concentration (i.e. 100, 200, 300, 400 and 500 μg/ml) of the selected treatment, each concentration was replicated three times. The control was 25 individuals exposed to the same dose of ethyl acetate. After 24 h, the mortality (%) was calculated and corrected with control mortality using the formula by Abbott [28].
Midgut histology
Treated and control Ae. aegypti, An. stephensi and Cx. quinquefasciatus larvae were separately embedded in 3% formaldehyde solution for 2 h at 4 °C. The blocks were cooled at 27 °C for 3 h and cut into 8 μm thickness, with 1.3 mm ribbons, using a microtome (Leica, Germany). Cross-sectioned larval midgut sections were stained with Ehrlich’s haematoxylin and eosin, after air drying the collected sections were viewed under a light microscope (Olympus-CH20i/India), with magnification at 100X and 400X.
Thin layer chromatography (TLC)
F. oxysporum crude metabolites were separated by using thin layer chromatography (TLC) with silica gel 60 size mesh coating on 20 mm × 20 mm glass slide. The chloroform : methanol solvent system was used as mobile phase, with different mobile phase solvent ratios (i.e. 10:0; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9; 0:10), at 26 ± 2 °C and 40% R.H.).
Fourier transformed infrared (FT-IR) spectroscopy
F. oxysporum extract was dried, and powder was subjected to FT-IR spectroscopy. Characterization involved FT-IR analysis of the dried powder of ethyl acetate extract by scanning it in the range 500– 4000 cm−1 at a resolution of 4 cm−1. These measurements were carried out on a Bruker Optics (Germany) Tensor 27 model in the diffuse reflectance mode operating at a resolution value of 0.4 cm−1 in KBr pellets. The pellets were later subjected to FT-IR spectroscopy measurements.
GC-MS analysis
Clarus 680 was used in the analysis employing a fused silica column, packed with Elite-5MS (5% biphenyl 95% dimethyl polysiloxane, 30 m × 0.25 mm ID × 250 μm df and the components were separated using helium as carrier gas, at a constant flow of 1 ml/min. The injector temperature was set at 260 °C during the chromatographic run. 1 μl of extract sample was injected into the instrument, the oven temperature was as follows: 60 °C (2 min), followed by 300 °C at the rate of 10 °C min−1 and then 300 °C, where it was held for 6 min. The mass detector conditions were: transfer line temperature 240 °C, ion source temperature 240 °C, and ionization mode electron impact at 70 eV, a scan time 0.2 s and scan interval of 0.1 s. Considering fragments from 40 to 600 Da, the spectrums of the components were compared with the database of spectrum of known components stored in the GC-MS NIST (2008) library.
Statistical analysis
Ae. aegypti, An. stephensi and Cx. quinquefasciatus larval and pupal mortality data were subjected to analysis of variance (ANOVA) of arcsine square root transformed mortality percentages). The lethal concentrations required to kill 50, 90 and 99% (LC50, LC90, and LC90) of larvae and pupae 24 h post-treatment were calculated by probit analysis with a reliability interval of 95% using the SPSS 16 software.
Results
Larval and pupal toxicity
Larvicidal activity of F. oxysporum extract was investigated on An. stephensi, Ae. aegypti and Cx. quinquefaciatus. 24 h post-treatment, LC50 values achieved by the F. oxysporum extract were 70.789 μg/ml for Ae. aegypti larvae, while An. stephensi and Cx. quinquefasciatus larvae was relatively more tolerant to the fungal extract (LC50 109.248 and 320.307 μg/ml, respectively), LC90 and LC99 are detailed in Table 1. LC50 values against pupae were 247.552, 197.989 and 330.377 μg/ml, respectively. LC90 and LC99 are shown in Table 2.
Table 1.
Larvicidal activity of Fusarium oxysporum fungal extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 109.248 (26.596–160.622) | 471.871 (412.356–572.163) | 767.502 (646.431–988.095) | 0.152 |
| Aedes aegypti | 70.789 (8.635–119.862) | 358.435 (316.461–422.297) | 592.940 (507.507–742.851) | 4.408 |
| Culex quinquefasciatus | 320.307 (276.749–367.614) | 738.217 (628.484–940.646) | 1078.922 (891.394–1431.661) | 0.048 |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
Table 2.
Pupicidal activity of Fusarium oxysporum fungal extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 247.552 (168.241–306.155) | 826.426 (662.296–1216.275) | 1298.358 (998.426–2024.911) | 0.107 n.s. |
| Aedes aegypti | 197.989 (123.192–247.973) | 663.423 (558.218–871.396) | 1042.873 (844.107–1448.418) | 0.454 n.s. |
| Culex quinquefasciatus | 330.377 (276.730–393.263) | 851.210 (693.877.1192.077) | 1275.661 (1004.632–1872.644) | 0.072 n.s. |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
Temephos was investigated alone against larvae of An. stephensi, Ae. aegypti and Cx. quinquefasciatus were 50.703, 48.340 and 146.133 μg/ml, respectively (Table 3). LC50 values against pupae were 57.454, 36.439 and 117.271 μg/ml, respectively (Table 4).
Table 3.
Larvicidal activity of temephos against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 50.703 (44.643–106.710) | 360.454 (316.010–429.602) | 616.981 (519.594–783.284) | 4.561 n.s. |
| Aedes aegypti | 48.340 (26.114–92.328) | 259.645 (227.412–305.259) | 431.913 (370.118–542.836) | 3.136 n.s. |
| Culex quinquefasciatus | 146.133 (56.232–200.429) | 590.233 (501.650–760.274) | 952.290 (776.815–1304.658) | 0.719 n.s. |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
Table 4.
Pupicidal activity of temephos against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 57.454 (51.961–123.004) | 455.939 (393.263–567.673) | 780.808 (644.885–1048.881) | 0.506 n.s. |
| Aedes aegypti | 36.439 (25.801–92.338) | 429.398 (370.336–533.228) | 749.762 (618.963–1010.067) | 0.371 n.s. |
| Culex quinquefasciatus | 117.271 (47.564–162.710) | 440.433 (388.772–523.229) | 703.893 (601.028–883.063) | 4.569 n.s. |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
24 h after exposure to binary combination of temephos and F. oxysporum extract (1:1 ratio) resulted in lower LC50 values on An. stephensi (35.927 μg/ml), Ae. aegypti (20.763 μg/ml) and Cx. quinquefasciatus (51.199 μg/ml) (Table 5), if compared to the products tested alone. Furthermore, LC50 values on pupae were 38.668, 26.394 and 72.086 μg/ml, respectively (Table 6).
Table 5.
Larvicidal activity of a binary combination of Fusarium oxysporum fungal extract + temephos against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 35.927 (25.592–52.953) | 187.092 (112.472–192.010) | 287.243 (230.356–583.259) | 0.812 n.s. |
| Aedes aegypti | 20.763 (12.902–42.894) | 130.353 (85.576–168.794) | 253.552 (202.768–419.726) | 0.506 n.s. |
| Culex quinquefasciatus | 51.199 (29.819–95.321) | 225.580 (181.947–277.315) | 451.226 (372.079–622.215) | 4.830 n.s. |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
Table 6.
Pupicidal activity of a binary combination of Fusarium oxysporum fungal extract + temephos against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus 24 h post treatment.
| Mosquito species (na = 375) | LC50 (95% LCL-UCL) (μg/ml) | LC90 (95% LCL-UCL) (μg/ml) | LC99 (95% LCL-UCL) (μg/ml) | χ2 (df = 4) |
|---|---|---|---|---|
| Anopheles stephensi | 38.668 (34.401–52.921) | 195.048 (153.728–241.172) | 385.588 (317.664–536.697) | 1.668 n.s. |
| Aedes aegypti | 26.394 (22.173–42.153) | 92.331 (76.932–115.284) | 246.274 (221.394–283.249) | 0.771 n.s. |
| Culex quinquefasciatus | 72.086 (60.932–98.302) | 227.304 (190.058–275.098) | 422.610 (353.125–563.736) | 3.011 n.s. |
na = total number of mosquitoes used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = lethal concentration killing 50% of exposed organisms, LC90 = lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (not significant, p > 0.05); df = degrees of freedom.
Histology studies on mosquito larvae
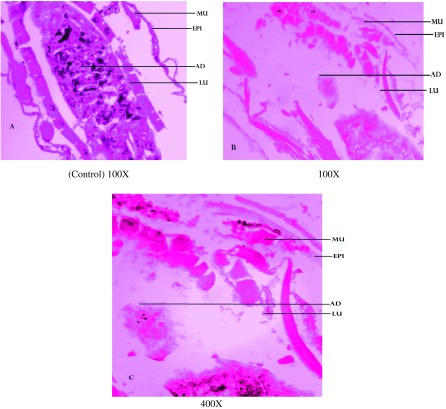
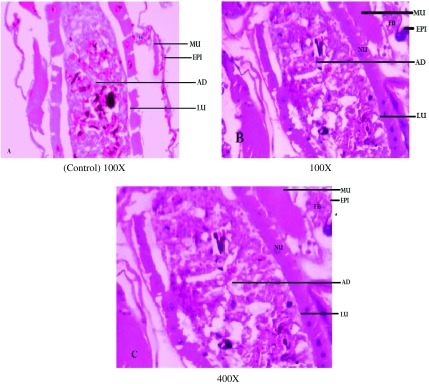
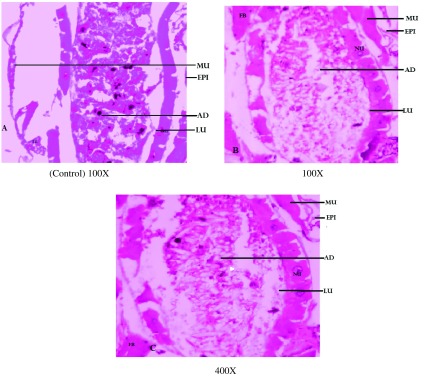
Ae. aegypti larvae were highly affected by the exposure to the fungal extract alone. Cross sections of fourth instar larvae of Ae. aegypti, An. stephensi and Cx. quinquefasciatus treated with F. oxysporum extract highlighted the damage done by the extract in midgut tissues. Ae. aegypti larval midgut showed the highest damage with distinct vacuolation in midgut epithelial cells, adipose tissue and muscles; similar damage to a lesser degree was observed in Cx. quinquefasciatus and An. stephensi larvae. These changes were not detected in control larvae at 100X magnification (Figure 1–3).
Figure 1. .
Cross sections of 4th instar larvae of Aedes aegypti treated or untreated with the Fusarium oxysporum extract. (A) Control was compared with (B) 100X and (C) 400X treated larval tissues, showing vacuolated gut epithelium (epi), gut lumen (lu), adipose tissue (ad) muscles (mu) nucleus (nu) and fat body (fb).
Notes: Larval mid-gut section was stained with Ehrlich’s haematoxylin, stained mid-gut tissues were viewed and photographed under light microscope at 100X and 400X magnification.
Figure 2. .
Cross sections of 4th instar larvae of Anopheles stephensi treated or untreated with Fusarium oxysporum extract. (A) Control was compared with (B) 100X and (C) 400X treated larval tissues, showing vacuolated gut epithelium (epi), gut lumen (lu), adipose tissue (ad) muscles (mu) nucleus (nu) and fat body (fb).
Notes: Larval mid-gut section was stained with Ehrlich’s haematoxylin, stained mid-gut tissues were viewed and photographed under light microscope at 100X and 400X magnification.
Figure 3. .
Cross sections of 4th instar larvae of Culex quinquefasciatus treated and untreated Fusarium oxysporum extract. (A) Control was compared with (B) 100X and (C) 400X treated larval tissues, showing vacuolated gut epithelium (epi), gut lumen (lu), adipose tissue (ad) muscles (mu) nucleus (nu) and fat body (fb).
Notes: Larval mid-gut section was stained with Ehrlich’s haematoxylin, stained mid-gut tissues were viewed and photographed under light microscope at 100X and 400X magnification.
Chemical characterization of the F. oxysporum extract
In TLC assays, the crude ethyl acetate extract of F. oxysporum showed a spot with Rf value of 0.5555 (Figure 4); the distance travelled by solute was 2.5 cm, while the distance travelled by the solvent was 4.5 cm.
Figure 4.
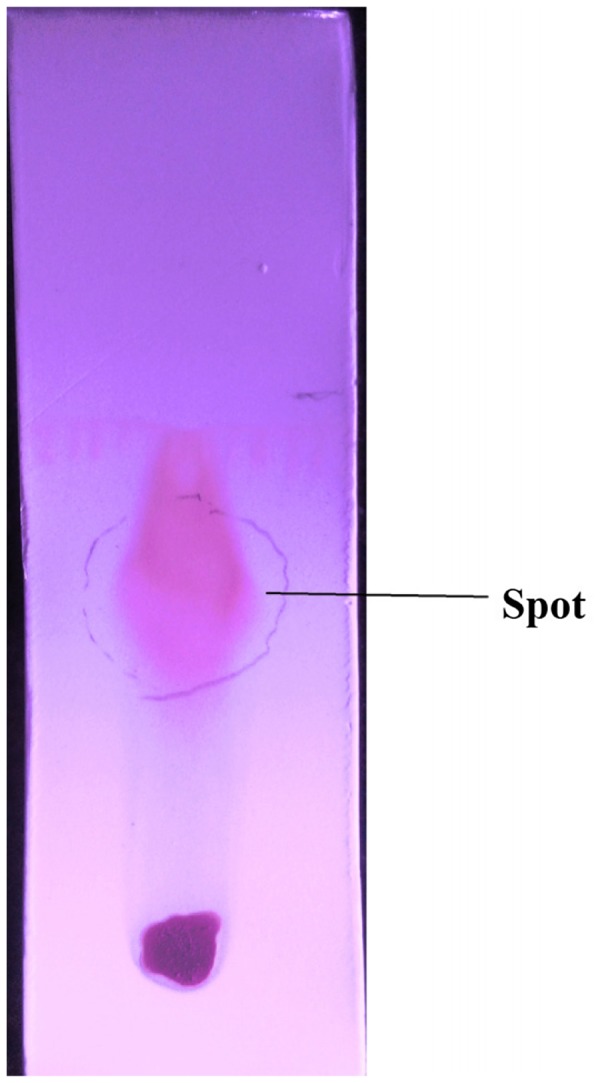
Thin layer chromatography (TLC) of the Fusarium oxysporum extract showing a major central spot.
Notes: The chloroform : methanol solvent system was used as mobile phase, with different mobile phase solvent ratios (i.e. 10:0; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9; 0:10), at 26 ± 2 oC and 40% R.H.).
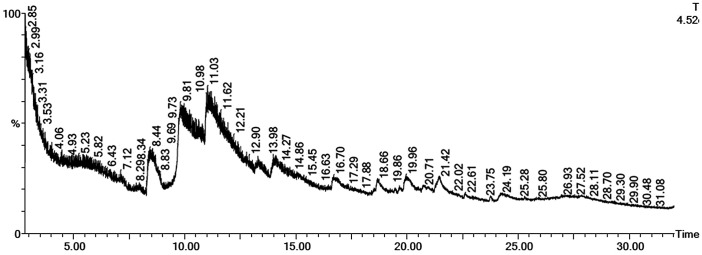
GC-MS of F. oxysporum extract showed the presence of various compounds (Figure 5), which include carbonic acid, bis (1-methylethyl) ester (9.806%), 2-propanol, 1-(1-methylethoxy)- (10.977%), 4-heptanol, 2-methyl- (19.955%), and 4 h-1-benzopyran-4-one, 5,6,7,8-tetrahydro-3-hydroxy-2-methyl- (21.451%) (Table 7).
Figure 5.
GC-MS analysis of the Fusarium oxysporum extract.
Notes: Oven initial temp 60 °C for 2 min, ramp 10 °C min−1 to 300 °C, hold 6 min, inject auto = 250 °C, volume = 1 μl, split = 10:1, carrier gas = He, solvent delay = 2.00 min, transfer temp = 240 °C, source temp = 240 °C, scan 50 to 600 Da, column 300 m~250 μm.
Table 7.
Major compounds identified in the ethyl acetate extract of Fusarium oxysporum.
| No. | R.T. | Compound name | Molecular Weight | Formula | Area (%) | Bioactivity | References |
|---|---|---|---|---|---|---|---|
| 1 | 9.806 | Carbonic acid, bis (1- methylethyl) ester | 146 | C7H14O3 | 3.375 | Pesticide | [46] |
| 2 | 10.977 | 2-Propanol, 1-(1-Methylethoxy)- | 118 | C6H14O2 | 1.893 | Unknown | – |
| 3 | 11.027 | 2-(2-Hydroxyethoxy)Ethyl Acetate | 148 | C6H12O4 | 1.699 | Unknown | – |
| 4 | 11.202 | Diisopropyl Ether | 148 | C6H12O4 | 2.069 | Unknown | – |
| 5 | 18.740 | Benzenemethanol, Alpha.-(Trichloromethyl)-, Acetate | 266 | C10H9O2Cl3 | 1.748 | Unknown | – |
| 6 | 19.955 | 4-Heptanol, 2-Methyl | 130 | C8H18O | 3.745 | Unknown | – |
| 7 | 21.451 | 4-1-Benzopyran-4-One, 5,6,7,8-Tetrahydro-3-Hydroxy-2-Methyl | 180 | C10H12O3 | 4.004 | Antimicrobial and larvicidal activity | [47] |
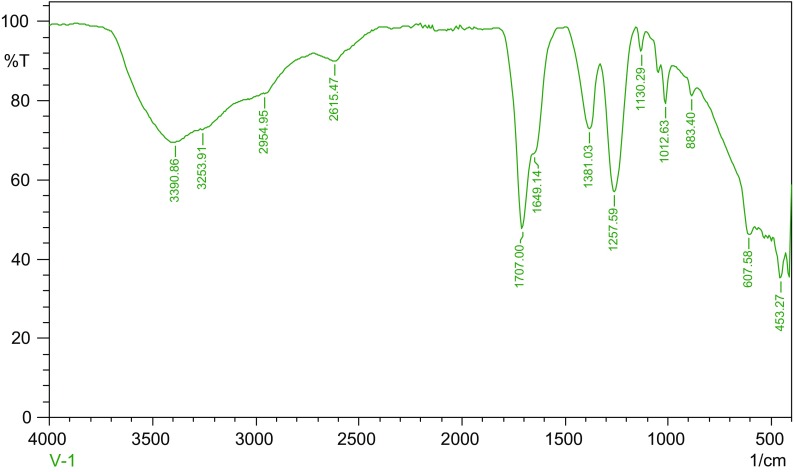
The FT-IR spectrum showed main peaks at 3390.86, 3253.91, 2954.95, 1707.00 and 1257.57 cm−1 (Figure 6); the broad peak at 3390.86 cm−1 was assigned to wagging vibration of N–H stretching, while the broad peak at 3253.91 cm−1 was assigned to N–H stretching, and the sharp peak 1707.00 cm−1 outlined C=C bending (Table 8).
Figure 6.
FT-IR spectrum of the Fusarium oxysporum extract.
Table 8.
FT-IR spectroscopy of Fusarium oxysporum mycelial extract.
| Wave number (cm−1) | Peak assignment | Visible intensity | Functional group |
|---|---|---|---|
| 3390.86 | N–H stretching | Broad peak | Amine group |
| 3253.91 | N–H stretching | Broad peak | Aliphatic group |
| 2954.95 | C–H bending | Broad peak | Alkane group |
| 2615.47 | C–H stretching | Broad peak | Ether group |
| 1707.00 | C=C bending | Medium | Alkane group |
| 1649.14 | C=O Stretching | Medium | Ether group |
| 1381.03 | C–H bending | Sharp | Alkane group |
| 1257.59 | C–O Stretching | Sharp | Alkane, Ether group |
| 1130.29 | S=O Stretching | Sharp | Sulfone group |
| 1012.63 | C–O Stretching | Sharp | Alkane |
| 883.40 | C=C bending | Medium | Alkane group |
| 607.58 | C–Br stretching | Medium | Ether group |
Discussion
The effective control of mosquito vectors with synthetic insecticides is a major concern for human health and the environment [7]. This study shows the efficacy of F. oxysporum extract conjugated with temephos as a potential mosquitocidal combination against three major mosquito vectors. Fungal metabolites known to possess insecticidal activity [29–32]. Streptomyces and Actinobacteria secondary metabolites show remarkable insecticidal activity against An. stephensi [10,33], while the ethyl acetate extracts of marine Actinobacteria, and Streptomyces are highly toxic to larvae of An. stephensi and Cx. tritaeniorhynchus 24 h post-treatment [34].
Combinations of plant extracts with chemical insecticides have been shown to increase insecticidal activity in several instances [35,25]. Similar synergistic effects have been detected also testing binary mixtures of mosquitocidal essential oils [22,23] and selected compounds [36]. In our study, the extract of F. oxysporum in binary combination with temephos showed a synergistic action against larvae and pupae of all the tested mosquito species, when compared with either temephos alone or with the fungal extract alone.
Our findings on the use of a binary combination of chemical insecticide and fungal secondary metabolites support earlier studies. Indeed, the combined use of Beauveria bassiana and Metarhizium anisopliae metabolites with permethrin has been found to be very effective to kill mosquito larvae [37]. Increased toxicity of M. anisopliae metabolites in combination with neem oil against Anopheles gambiae and C. quinquefasciatus adults has been also reported [38]. Concering tests on other insect species, B. bassiana secondary metabolites combined with synthetic insecticides abamectin, triflumuron and carbaryl showed boosted insecticidal activity against the Colorado potato beetle [39]. Furthermore, the combination of chemical insecticide imidacloprid with B. bassiana and M. anisopliae enhanced the toxic effect and reduce survival Diaprepes abbreviatus larvae [40]. Combinations of imidacloprid with secondary metabolites from M. anisopliae show remarkable toxicity against German cockroaches [41]. Lecanicillium muscarium metabolites in combination with either imidacloprid, buprofezin, diflubenzuron, and nicotine, were found to be effective for controlling adults of Bemisia tabaci [42].
Similiarly, B. bassiana and M. anisopliae conidia and their secondary metabolites in combination with sub-lethal doses of imidacloprid were effective against adults of Attasexdens rubropilosa [24]. Combination of M. anisopliae secondary metabolites with synthetic pesticides organophosphate and diflubenzuron achieved a joint toxic action against larvae of Anomala cuprea [43].
A major advantage of using binary mixtures with green metabolites as insecticides is the reduction of the amount of chemical insecticide used, reducing environmental pollution and the negative impact on non-target organisms. Second, and most importantly, natural product metabolites exert their toxicity through multiple mechanisms of action, thus reducing the possibility of resistance development in target mosquitoes [44]. In this binary combination of chemical insecticide and fungal extract, the two products exert toxicity in mosquitoes through different mechanisms of actions; the fungal extract induces mechanical stress on mosquito young instars and also contains toxic molecules detailed above, while the chemical insecticide affects the central nervous system, through inhibition of cholinesterase [21]. GC-MS data shows the presence of carbonic acid, bis (1-methylethyl) ester, 2-propanol, 1-(1-methylethoxy)-, 4-heptanol, 2-methyl-, and 4 h-1-benzopyran-4-one, 5,6,7,8-tetrahydro-3-hydroxy-2-methyl- as main compounds. Notably, it has been earlier elucidated that carbonic acid, bis (1-methylethyl) ester, 4-heptanol and 1–4-benzopyram-4-one-5, 6, 7, 8-tetrahydro-3-hydroxy-2-methyl possess insecticidal activity against mosquitoes [45], allowing us to argue that these three constituents can be actively involved in the observed synergistic effect highlighted in the present work.
Overall, our result shed light on the use of a cheap fungal extract from fungus easy to culture in combination with temephos, to achieve synergistic effects in larvicidal and pupicidal treatments for the control of An. stephensi, Ae. aegypti and Cx. quinquefasciatus. Therefore, we suggest that the selection of appropriate combinations of currently marketed insecticides and pathogenic fungal extracts offers the prospect for effective mosquito control, reducing costs and resistance problems derived from the continuous overuse of conventional insecticides.
Funding
The authors are grateful to Periyar University for providing financial support under University Research Fellowship Scheme (Ref No. PU/AD3/URF/16169/2016). We also thank the Institute of Vector Control and Zoonoses (IVCZ) Hosur for supplying eggs as well as the Vellore Institute of Technology (VIT) for FT-IR and GC-MS analysis.
Supplemental data
The supplemental data for this article can be accessed at https://doi.org/10.1080/20477724.2018.1438228
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Acknowledgements
The authors are grateful to Periyar University for providing financial support under University Research Fellowship Scheme (Ref No. PU/AD3/URF/16169/2016). We also thank the Institute of Vector Control and Zoonoses (IVCZ) Hosur for supplying eggs as well as the Vellore Institute of Technology (VIT) for FT-IR and GC-MS analysis.
References
- [1].Benelli G, Beier J. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. 10.1016/j.actatropica.2017.06.028 [DOI] [PubMed] [Google Scholar]
- [2].WHO. World Malaria Report 2015 [Internet]. WHO; [cited 2017 Jun 13]. Available from: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en/.
- [3].Benelli G, Romano D. Mosquito vectors of Zika virus. Entomol Gen. 2017: 309–318. DOI: 10.1127/entomologia/2017/0496. [DOI] [Google Scholar]
- [4].World Health Organization Global programme to eliminate lymphatic filariasis-progress report on mass drug administration in 2016. Weekly Epidemiol Record. 2016;85:365–372. [Google Scholar]
- [5].Ramkumar G, Shivakumar MS. Laboratory development of permethrin resistance and cross-resistance pattern of Culex quinquefasciatus to other insecticides. Parasitol Res. 2015;114:2553–2560. [DOI] [PubMed] [Google Scholar]
- [6].Benelli G. Research in mosquito control: current challenges for a brighter future. Parasitol Res. 2015a;114:2801–2805. 10.1007/s00436-015-4586-9 [DOI] [PubMed] [Google Scholar]
- [7].Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. 10.1146/annurev.ento.45.1.371 [DOI] [PubMed] [Google Scholar]
- [8].Muthusamy R, Shivakumar MS. Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J Vector Borne Dis. 2015;52:159–165. [PubMed] [Google Scholar]
- [9].Srivastava CN, Mohan L, Sharma P, et al. . A review on prospective of synergistic approach in insect pest management. J Ent Res. 2011;35:255–266. [Google Scholar]
- [10].Dhanasekaran D, Sakthi V, Thajuddin N, et al. . Preliminary evaluation of Anopheles mosquito larvicidal efficacy of mangrove actinobacteria. Int J App Biol Pharm Technol. 2010;1:374–381. [Google Scholar]
- [11].Benelli G. Plant-borne ovicides in the fight against mosquito vec- tors of medical and veterinary importance: a systematic review. Parasitol Res. 2015b;114:3201–3212. 10.1007/s00436-015-4656-z [DOI] [PubMed] [Google Scholar]
- [12].Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 2016a;115:23–34. 10.1007/s00436-015-4800-9 [DOI] [PubMed] [Google Scholar]
- [13].Benelli G, Lo Iacono A, Canale A, et al. . Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol Res. 2016b;115:2131–2137. 10.1007/s00436-016-5037-y [DOI] [PubMed] [Google Scholar]
- [14].Amerasan D, Nataraj T, Murugan K, et al. . Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J Pest Sci. 2016;89:249–256. 10.1007/s10340-015-0675-x [DOI] [Google Scholar]
- [15].Vivekanandhan P, Senthil-Nathan S, Shivakumar MS, et al. . Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol Mol Plant Pathol. 2017;. DOI: 10.1016/j.pmpp.2017.05.005. [DOI] [Google Scholar]
- [16].Keller S. Arthropod-pathogenic Entomophthorales of Switzerland. II. Erynia, Eryniopsis, Neozygites, Zoophthora and Tarichium. Sydowia. 1991;43:39–122. [Google Scholar]
- [17].Maurya P, Mohan L, Sharma P, et al. . Evaluation of larvicidal potential of certain insect pathogenic fungi extracts against Anopheles stephensi and Culex quinquefasciatus. Entomol Res. 2011;41:211–215. 10.1111/enr.2011.41.issue-6 [DOI] [Google Scholar]
- [18].Silva RO, Silva HHG, Luz C, et al. . Effect of Metarhizium anisopliae isolated from soil samples of the central Brazilian cerrado against Aedes aegypti larvae under laboratory conditions. Rev Patol Trop. 2008;33:207–216. [Google Scholar]
- [19].Vyas N, Dua KK, Prakash S, et al. . Efficacy of Lagenidium giganteum metabolites on mosquito larvae with reference to non-target organisms. Parasitol. Res. 2007;101:385–390. 10.1007/s00436-007-0496-9 [DOI] [PubMed] [Google Scholar]
- [20].Soni N, Prakash S. Effect of Chrysosporium keratinophilum metabolites against Culex quinquefasciatus after chromatographic purification. Parasitol Res. 2015;107:1329–1336. [DOI] [PubMed] [Google Scholar]
- [21].Hiramori H, Nishigaki J. Factors analysis of synergistic effect between the entomopathogenic fungus Metarhizium anisopliae and synthetic insecticides. Appl Entomol Zool. 2001;36:231–236. 10.1303/aez.2001.231 [DOI] [Google Scholar]
- [22].Benelli G, Pavela R, Canale A, et al. . Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: synergistic and antagonistic effects. Parasitol Int. 2017a;66:166–171. 10.1016/j.parint.2017.01.012 [DOI] [PubMed] [Google Scholar]
- [23].Benelli G, Pavela R, Iannarelli R, et al. . Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind Crops Prod. 2017b;96:186–195. 10.1016/j.indcrop.2016.11.059 [DOI] [Google Scholar]
- [24].Santos AV, Lorenz Bruno, de Oliveira Richard, et al. . Selection of entomopathogenic fungi for use in combination with sublethal doses of imidacloprid: perspectives for the control of the leaf cutting ant Attasexdens rubropilosa Forel (Hymenoptera: Formicidae). Mycopathologia. 2007;163:233–240. 10.1007/s11046-007-9009-8 [DOI] [PubMed] [Google Scholar]
- [25].Bhan S, Mohan L, Srivastava CN, et al. . Efficacy of Cuscuta reflexa extract and its combinatorial activity with Temephos against mosquito larvae, Anopheles stephensi (Liston) and Culex quinquefasciatus (Say). Int J Mosquito Res. 2015;2:34–41. [Google Scholar]
- [26].Samson RA, Evans HC, Latg′e JP, et al. . Atlas of entomopathogenic fungi. Berlin: Springer-Verlag; 1988 10.1007/978-3-662-05890-9 [DOI] [Google Scholar]
- [27].World Health Organisation , 2005. Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13. [Google Scholar]
- [28].Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–266. 10.1093/jee/18.2.265a [DOI] [Google Scholar]
- [29].Weiser J, Matha V, Zizka Z, et al. . Ultrastructural changes in larvae treated with tolypin, the insecticidal metabolite of Tolypocladium inflatum Gams (Deuteromycetes). Cytobios. 1992;69:179–186. [PubMed] [Google Scholar]
- [30].Priyanka, Srivastava JN, Prakash S, et al. . Chrysosporium tropicum efficacy against Anopheles stephensi larvae in the laboratory. J Am Mosq Con Ass. 2001;17:127–130. [PubMed] [Google Scholar]
- [31].Singh G, Prakash S. Evaluation of culture filtrates of Culicinomyces clavisporus: myco adulticides for Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi. Parasitol Res. 2012;110:267–272. 10.1007/s00436-011-2482-5 [DOI] [PubMed] [Google Scholar]
- [32].Senthilkumar N, Murugesan S, Suresh Babu D, et al. . Metabolite profiling of the extracts of endophytic fungi of entomopathogenic significance, Aspergillus flavus and Nigrospora sphaerica isolated from tropical tree species of India, Tectona grandis L. J Agric Life Sci. 2014;1:108–114. [Google Scholar]
- [33].Thimiri LD, Krishnan K, Venkatesan GK, et al. . Isolation and characterisation of acaricidal and larvicidal novel compound (2S,5R,6R)-2-hydroxy-3,5,6-trimethyloctan-4-one from Streptomyces sp. against blood-sucking parasites. Parasitol Res. 2012;111:1151–1163. [DOI] [PubMed] [Google Scholar]
- [34].Saurav K, Rajakumar G, Kannabiran K, et al. . Larvicidal activity of isolated compound 5-(2, 4-dimethylbenzyl) pyrrolidin-2-one from marine Streptomyces VITSVK5 sp. against R. microplus, An. stephensi, and Cx. tritaeniorhynchus. Parasitol Res. 2013;. 112(1):215-226. [DOI] [PubMed] [Google Scholar]
- [35].Abdel-Baky NF, Abdel-Salam AH. Natural incidence of Cladosporium spp. as a biocontrol agent against whiteflies and aphids in Egypt. J Appl Entomol. 2003;127:228–235. 10.1046/j.1439-0418.2003.00662.x [DOI] [Google Scholar]
- [36].Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some EOs against Culex quinquefasciatus Say larvae. Parasitol Res. 2015;114:3835–3853. 10.1007/s00436-015-4614-9 [DOI] [PubMed] [Google Scholar]
- [37].Farenhorst M, Bart GJ, Knols Matthew B, et al. . Synergy in efficacy of fungal entomopathogens and permethrin against West African insecticide-resistant Anopheles gambiae mosquitoes. Plos One 2010;5(8):e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Seye F, Ndiaye M, Faye O, et al. . Evaluation of entomopathogenic fungus Metarhizium anisopliae formulated with neem (neem oil) against Anopheles gambiae and Culex quinquefasciatus adults. Malaria Chemo Con Elim. 2012. Vol. 1 2012; Article ID 235494, DOI: 10.4303/mcce/235494. [DOI] [Google Scholar]
- [39].Anderson TE, Hajek AE, Roberts DW, et al. . Colorado potato beetle (Coleoptera: Chrysomelidae) effects of combinations of Beauveria bassiana with insecticides. J Econ Entomol. 1989;82:83–89. 10.1093/jee/82.1.83 [DOI] [Google Scholar]
- [40].Quintela ED, McCoy CW. Synergistic effect of imidacloprid and two entomopathogenic fungi on behavior and survival of larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in soil. J Econ Entomol. 1998;91:110–122. 10.1093/jee/91.1.110 [DOI] [Google Scholar]
- [41].Kaakeh WBL, Reid TJ, Bohnert JGW, et al. . Toxicity of imidacloprid in the German cockroach (Dictyoptera: Blattellidae), and the synergism between imidacloprid and Metarhizium anisopliae (imperfect fungi: Hyphomycetes). J Econ Entomol. 1997;90:473–482. 10.1093/jee/90.2.473 [DOI] [Google Scholar]
- [42].Cuthbertson and Walters Compatibility of the entomopathogenic fungus Lecanicillium muscarium and insecticides for eradication of sweet potato whitefly. Bemisia tabaci. Mycopathologia. 2005;160:35–41. [DOI] [PubMed] [Google Scholar]
- [43].Hiramori H, Nishigaki J. Joint action of an entomopathogenic fungus Metarhizium anisopliae and synthetic insecticides against the scarab beetle, Anomala cuprea (Coleoptera: Scarabaeidae) larvae. Appl Entomol Zool. 1998;33:77–84. 10.1303/aez.33.77 [DOI] [Google Scholar]
- [44].Pavela R, Benelli G. EOs as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. 10.1016/j.tplants.2016.10.005 [DOI] [PubMed] [Google Scholar]
- [45].Senthilkumar G, Madhanraj P, Panneerselvam S, et al. . A study on the compounds and its anti-fungal potentiality of fungi isolated from paddy field soils of Jenbagapuram Village, Thanjavur District, and South India. Asian J Pharm Res. 2011;1:19–21. [Google Scholar]
- [46].Melnikov , Derivatives of carbonic acid. Chem Pest . 1971:177–182. [Google Scholar]
- [47].Chirchir DK, Ouma RBO, Cheplogoi PK, et al. . Larvicidal activity of extracellular secondary metabolites from a Stereum species Hill ex Pers. (JO5289) against the dengue fever mosquito, Aedes aegypti (Linn) (Diptera: Culicidae). African J Biotechnol. 2013;12:6302–6309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.