Abstract
Malaria infection caused by Plasmodium parasites remains a major health burden worldwide especially in the tropics and subtropics. Plasmodium exhibits a complex life cycle whereby it undergoes a series of developmental stages in the Anopheles mosquito vector and the vertebrate human host. Malaria severity is mainly attributed to the genetic complexity of the parasite which is reflected in the sophisticated mechanisms of invasion and evasion that allow it to overcome the immune responses of both its invertebrate and vertebrate hosts. In this review, we aim to provide an updated, clear and concise summary of the literature focusing on the interactions of the vertebrate innate immune system with Plasmodium parasites, namely sporozoites, merozoites, and trophozoites. The roles of innate immune factors, both humoral and cellular, in anti-Plasmodium defense are described with particular emphasis on the contribution of key innate players including neutrophils, macrophages, and natural killer cells to the clearance of liver and blood stage parasites. A comprehensive understanding of the innate immune responses to malaria parasites remains an important goal that would dramatically help improve the design of original treatment strategies and vaccines, both of which are urgently needed to relieve the burden of malaria especially in endemic countries.
Keywords: Innate immunity, malaria, macrophages, natural killer cells, neutrophils, invasion, vaccines
Introduction
Malaria has been a significant threat to humans throughout history. According to the most recent World Malaria Report, 212 million clinical cases were reported in 2015 as well as 429,000 deaths [1]. Despite the fact that malaria cases have been steadily dropping during the last decade due to several World Health Organization (WHO) supported initiatives, the disease mortality and morbidity rates remain high exerting serious social and economic burdens on several populations, especially those living in poverty. Malaria transmission is ongoing in 91 countries worldwide with the heaviest burden occurring in Africa, particularly in the sub-Saharan region were poverty, inefficient access to adequate health services, poor infrastructure and presence of highly anthropophilic vectors, such as Anopheles gambiae and Anopheles funestus, aggravate the problem. WHO has come up with the Global Technology Strategy for Malaria 2016–2030 to reduce the number of malaria cases and deaths worldwide by at least 90% and to prevent the reestablishment of this parasite in malaria free countries [1].
The absence of an efficient vaccine has been a major hurdle in the elimination of malaria from endemic countries. In fact after a natural infection, the host’s immune system does not elicit a long term protective immunity to re-infection [2]. Hence, the aim from a vaccine, at best, is to provide partial but significant protection mainly to children below the age of 5 where disease morbidity is often high. There are several reasons why a sterilizing immunity against natural Plasmodium infection fail to develop, most of which are not entirely understood. Some obvious reasons include the complex biology of malaria parasites with several stage-specific genes many of which undergo antigenic variation [3–8], the complex population structure of malaria parasites [9], evasion of innate immune responses and failure to develop long term memory responses [10]. In this review, we start with a synopsis of the parasite life cycle followed by a description of the major innate immune responses elicited by the mammalian host against the invasive stages, namely the sporozoites and merozoites, highlighting in particular the contribution of neutrophils, macrophages and Natural killer (NK) cells to the frontline control of these parasite stages in the skin, liver and blood.
Plasmodium life cycle
Malaria is a mosquito borne disease caused by parasites of the genus Plasmodium. It is transmitted through the bite of the female Anopheles mosquito [11,12]. There are four traditional species that infect humans namely P. falciparum, P. vivax, Plasmodium ovale, P. malaria [13,14] and recently the upsurge of a fifth human infective species in South-East Asia known as P. knowlesi [15]. Plasmodium species exhibit several variations in their life cycle, including severity and frequency of infection, fever paroxysm [16] number of merozoites produced per erythrocytic cycle [17], presence of hypnozoite stage [18] and gametocyte feeding density in relationship to subsequent infections [19], duration and stages of gametocytogenesis, gametocyte maturity [19], and other factors.
Despite the complicated variations in the parasites’ life cycle, all species depend on two essential hosts, the Anopheles mosquito and the vertebrate host, for a successful completion and sustainability of the life cycle. During a blood meal, the female Anopheles injects sporozoites into the dermis from where they rapidly migrate to the liver initiating the hepatic schizogony. Infection of hepatocytes is initiated after the sporozoites traverse the sinusoidal wall by utilizing various microneme proteins including the sporozoite protein essential for cell-traversal (SPECT) [20], SPECT2 [21], and the cell-traversal protein for ookinetes and sporozoites [22]. Sporozoites traverse the sinusoidal cell wall by migrating through endothelial cells or Kupffer cells [23–25], however, cell traversal (CT)-independent routes have been also described [25]. CT traversal by sporozoites seems to be important for escaping clearance by Kupffer cells [25]. Following the CT phase, sporozoites switch to the invasive phase in order to infect hepatocytes. Sporozoite-specific molecules, such as the 6-cys domain proteins Pbs36p and Pbs36 have been shown to be required for hepatocyte invasion by P. berghei parasites [21]. Hepatic schizogony involves asexual parasite replication producing thousands of erythrocyte-infecting merozoites. In some species of the parasite, such as in P. vivax and P. ovale, there is a dormant stage referred to as hypnozoite [26,27] that resides in the liver cells and causes relapses by invading the bloodstream weeks or even years later [28–31].
Following their release into the blood stream, merozoites invade erythrocytes transforming initially into the ring form which eventually matures to form a trophozoite that grows substantially in size to prepare for the subsequent schizont stage characterized by multiple asexual cell divisions leading to the production of more merozoites. When the red blood cell ruptures, merozoites are released into the blood stream to infect new red blood cells [32]. Merozoites attach to the red blood cell at any point of its surface. After attachment, reorientation of the merozoites occurs in order to deploy the enzymatic content of a series of secretory organelles, such as rhoptries, micronemes, and dense granules [33–35]. Ligands interact with the erythrocyte surface receptors forming a junction. This junction is driven by an actin-myosin motor that is found in the inner membrane of the merozoites [36,37]. Further invasion occurs through the inward motion driven by the actin-myosin motor until the parasite is enclosed with a parasitophorous vacuole. After that, the junction is pinched off, the outer surface coat is shed, and the red blood cell and parasitophorous vacuole are resealed. After the completion of the process, the parasite matures to the ring form [38]. The pathogenesis of malaria is mainly linked to the erythrocytic stage, where known symptoms of the disease may occur. However, high prevalence of asymptomatic infections have been reported for certain Plasmodium spp, such as P. falciparum in Haiti pool of individuals [39]. Some parasites differentiate rather into gametocytes which are the sexual stage of the parasite. This commitment to sexual development occurs early on when merozoites are still inside the schizonts [40], and involves several epigenetic factors and transcription regulators [41]. When ingested by a mosquito, gametocytes differentiate into gametes initiating the sexual cycle of the parasite [42].
The fact that the early clearance of parasites by the innate immune system is almost always inefficient suggests that malaria parasites evolved several strategies to fend off host defenses in order to complete their development. Some of these evasion strategies include allelic diversity of parasite surface proteins such as the circumsporozoite protein [43,44], surfin [45], and the merozoite surface proteins (MSPs) [46] that form the merozoites outer coat surface [47,48]. In addition, certain parasites display variant surface antigens on infected red blood cells. For instance, P. falciparum possess three large multigene families, namely the var [49], the repetitive interspersed family (rif) [50], and the subtelomeric variant open reading frame (stevor) that encode variant antigens [51]. Interestingly, one of the products of var genes, PfEMP1, was shown to suppress IFN-γ secretion by naïve γδ-T cells and Natural Killer (NK) cells consequently further aiding in parasite immune evasion [48,52]. Other gene superfamilies have been recognized in P. vivax as variant interspersed repeats (vir) and in P. knowlesi as Schizont Infected Cell Agglutination (sicavar) that encode as well variant surface antigens related to parasite evasion. Functional analysis of P. vivax VIR proteins revealed different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor [7,53].
Despite these evasion strategies, several interactions do occur between cellular and humoral components of the innate immune system and the parasite. In the sections below, and as summarized in Table 1, we describe the significance and relative contribution of these interactions to parasite elimination.
Table 1.
Summary of the innate immunological responses against different stages of the malaria parasites as mounted in either human or rodent models.
| Cell | Organism | Malaria species | Stage of infection | Immunological responses | References |
|---|---|---|---|---|---|
| Neutrophils | Rodent | P. berghei | Sporozoites | Increase in neutrophil recruitment to skin | [58] |
| [59,60] | |||||
| Humans | P. falciparum | Merozoites | Phagocytosis of merozoites by neutrophils | [63,64] | |
| [65,66] | |||||
| Intra-erythrocytic | Blockage of parasite growth | [70] | |||
| NETs formation | [72–74] | ||||
| Reduced oxidative burst | [29] | ||||
| Positive correlation between ROS production in neutrophils and severe malaria and anemia | [2] | ||||
| CXCL10 secretion leading to CM | [76] | ||||
| Rodent | P. vinkei | Intra-erythrocytic | Decrease in neutrophils thus decrease in lung pathology | [71] | |
| Macrophages | P. berghei | Sporozoites | Sporozoites invade macrophages and immune sera trigger phagocytic uptake of Sporozoites leading to their destruction | [82] | |
| P. knowlesi | |||||
| Kupffer cells | Rodent | P. yoelii | Phagocytosis of some sporozoites with others escaping through CT | [25] | |
| Monocytes | Humans | P. falciparum | Intra-erythrocytic | Inhibiting growth by ADCI | [90] |
| Production of trappin-2 on the surface of merozoites leading to growth inhibition in vitro | [91] | ||||
| NK cells | Rodent | P. yoelii | Blood stage | Increased parasitemia as a result of reduce IFNγ production | [85,92] |
| P. chabaudi | |||||
| Humans | P. falciparum | Intra-erythrocytic | NK cells activation aided by monocytes and macrophages | [2,28,29,85] | |
| [71,82] | |||||
| Increased IFNγ directly by NK cells | [85,92] | ||||
| Destruction of infected RBC after NKcell interaction through the release of granzymes and loss of cell volume and integrity | [85] | ||||
| Splenic NK cells | Rodent | P. berghei | γ-irradiated sporozoites | Indirect activation of NK cells through immune players leading to the lysis of murine tumor cells | [110] |
| γδ T cells | Human | P.falciparum | schizonts | Activation of a subset of Vγ9+Vδ2+ T cells | [118] |
| Human and rodent | P.falciparum | schizonts | Upregulation of TIM3 expression, impairment of γδ T cells | [121] |
The role of neutrophils in defense against Plasmodium parasites
Interaction of neutrophils with Plasmodium sporozoites
Neutrophils are the first to be recruited to the site of infection combating microorganisms via an array of strategies [54]. There is strong evidence as well pointing to their important function in tumor microenvironment. Their infiltration and prognostic significance has been lately studied in esophageal squamous carcinoma [55] and in colorectal cancer [56]. Moreover, neutrophils are the first responders to the injection of Plasmodium sporozoites by the mosquito bite in the skin. Even a sterile insect bite can trigger neutrophil migration to the dermis and epidermis as has been previously shown for sand flies [57], suggesting that the damage inflicted to the skin by the probing behavior of the insect proboscis is sufficient to drive neutrophil migration. Using mice as model vertebrate host, it was shown that neutrophils increase steadily in the skin within 1–2 h after a mosquito bite [58]. Another study analyzed the influx of neutrophils for a longer duration after injecting the skin of mice with wild type sporozoites (WTS), radiation attenuated sporozoites (RAS), or salivary gland extracts (SGE) from uninfected mosquitoes. The neutrophil count decreased four hours after SGE infection reaching basal levels, but further increased in WTS and RAS injected mice indicating that sporozoites triggered a sustained neutrophil recruitment to the skin. In parallel, the levels of monocytes were relatively low in all cases during the first few hours of malarial infection; however, there was a sharp increase at 24 h in WTS and RAS injected mice [59,60]. This indicates that neutrophils are the first cells to be recruited to the site of infection. Recently, it was shown that a protein called Agaphelin is upregulated to several folds in the salivary glands of P. falciparum-infected A. gambiae mosquitoes and it exhibits inhibitory effects on neutrophil chemotaxis in vitro and in vivo in a mouse model of acute inflammation induced by carrageenan [61]. While this finding might seem contradictory to previous reports showing rapid neutrophil infiltration into the skin after sporozoite injection, it is not clear how much the carrageenan induced inflammation mimics that triggered by invading sporozoites. Also, it remains to be shown whether Agaphelin knockdown mosquitoes trigger differential neutrophil recruitment to the skin after sporozoite injection as compared to wild types. Despite the clear evidence for neutrophil influx as a result of sporozoite injection, it is not clear whether they contribute significantly to sporozoite killing [62]. Mac-Daniel et al. [59] showed that sporozoites can be phagocytosed by both neutrophils and monocytes leading to their imminent death, however, a small percentage of phagocytic cells harbored sporozoites that remained alive in parasitophorous vacuoles for at least 24 h before they die. These results suggest that sporozoites can actively invade phagocytic cells; however, it remains unclear whether a single parasite can traverse multiple phagocytes in the skin to facilitate the invasion mechanism.
Interaction of neutrophils with Plasmodium blood stages
Phagocytosis of P. falciparum merozoites have been observed in vivo [63,64] and in vitro [65,66]. Merozoite phagocytosis was examined morphologically by examining their adherence and ingestion by neutrophils. In vitro, phagocytosis of merozoites was shown to be enhanced in the presence of immune sera and furthermore if the inflammatory cytokines IFN-γ, TNF-α, IL-1β, and GM-CSF were added [67]. The fact that neutrophils from infected individuals were shown to be more effective in inhibiting parasite growth in vitro than those from naïve individuals [65] supports a potential role for inflammatory cytokines in boosting neutrophil activity. The mechanisms by which neutrophils kill blood stage parasites are not completely understood but several studies point to a role for the neutrophil respiratory burst in that process. This respiratory burst seems to be triggered by parasite specific antibodies, a process referred to as antibody-dependent respiratory burst (ADRB), and its intensity correlates with clinical protection from malaria [68]. In the ADRB, reactive oxygen species (ROS) production was shown to be produced intracellularly suggesting that the underlying driving mechanism is phagocytosis [69]. However, an earlier study revealed that neutrophils from chronic granulomatous disease (CGD) patients were still able to inhibit the growth of malaria parasites in vitro especially when stimulated with phorbol-myristate-acetate suggesting that, in addition to ROS, microbicidal components of neutrophil granules may also be involved in parasite killing [66]. TNF-α was also shown to boost the activity of neutrophils from CGD patients against P. falciparum blood stages. The inhibition of Plasmodium growth in vitro seems to depend on the ratio of polymorphonuclear leukocytes (PMNs) to parasitized-erythrocytes (PE). It was shown that at high ratios, even without stimulating neutrophils, parasite growth was almost completely blocked [70]. This observation was also supported by a study by Golenser et al. [28] whereby a PMN to PE ratio of 1/50 resulted in 82% inhibition of parasite growth after two days of culture. In this study, it was proposed that PMNs impede the growth of P. falciparum through an increase in oxidative stress, since glucose 6-phosphate dehydrogenase (G6PD)-deficient PE were more sensitive to PMNs than PE from normal individuals. This sensitivity in G6PD deficient PE is suggested to be due to the presence of an antagonistic environment for the Plasmodium thereby causing oxidative damage and destruction of the parasite [71].
In addition to phagocytosis and ROS release, neutrophil extracellular traps (NETs) contribute to host defense against malarial parasites. NETs are networks of extracellular fibers, mainly made up of web-like meshwork of DNA that were shown to be present in the peripheral blood circulation of children under the age of 6 years diagnosed with uncomplicated P. falciparum malaria. Circulating neutrophils exhibiting NETs had normal morphology, and were adherent to PE [72–74]. Interestingly, after a mosquito bite, NET formation at the level of the skin was inhibited by Agaphelin mainly by targeting elastase and reducing neutrophil infiltration [61].
Despite the presence of substantial evidence linking neutrophils to parasite killing as mentioned above, it remains unclear how significant is the contribution of neutrophils to the clearance of parasite blood stages. For instance, one study reported the presence of a dominant population of neutrophils in the blood of P. falciparum infected children with reduced oxidative burst as a result of the induced expression of the heme oxygenase-1 enzyme in neutrophil progenitors in the bone marrow [29].
On the other hand, neutrophils may also contribute to the pathophysiology of severe malaria cases. For instance, severe malaria cases were associated with significantly higher neutrophil counts compared to uncomplicated cases [75], and there was a positive correlation between ROS production in neutrophils and severe malaria and anemia in a cohort of Gabonese children [2]. A more recent study revealed that the secretion of IFN-γ- inducible protein 10, CXCL10, by inflammatory monocytes and neutrophils compromises the control of blood-stage malaria infection leading to severe diseases such as cerebral malaria (CM) [76]. CXCL10 seems to inhibit the accumulation of T-follicular helper cells in the spleen hence compromising the parasite-specific antibody response. In a mouse model of malaria-associated acute lung injury, depletion of neutrophils reduced lung pathology and increased mice survival [71]. On the same note, early depletion of neutrophils by injecting malaria infected mice with anti-GR1 IgG antibody prevented the appearance of acute lung injury/acute respiratory distress syndrome compared to untreated mice. Similarly inhibiting the CXCR4/CXCL12 signaling pathway that induces neutrophil migration to the lung tissue upon injuries protected almost 90% of the treated mice pool [77]. Hence, while neutrophils may play a protective role in the early phase of the disease, at later stages they might contribute to the pathophysiology that develops in severe cases.
The role of macrophages and monocytes in anti-Plasmodium immunity
Macrophages are known to be highly phagocytic cells with an array of surface receptors that allow them to interact with diverse classes of microorganisms. They possess different phenotypes and functions mainly based on the cytokine profile in their microenvironment [78]. They can either favor a pro-inflammatory or an anti-inflammatory immune response thereby playing a role in inflammation and tissue repair. Their function extends to several diseases and disorders, ranging from obesity, asthma, metabolic disorders, autoimmunity, anti-tumor activity [79,80] and fighting parasitic infections, such as Leishmaniasis [81]. In this section, we review the role of macrophages to defeat malaria infections, particularly against Plasmodium sporozoites and its blood stages.
Interaction of macrophages with Plasmodium sporozoites
Early studies utilizing peritoneal macrophages incubated with sporozoites of P. berghei and P. knowlesi in the presence of serum revealed that the fate of sporozoites and macrophages depends on the presence of Plasmodium antibodies in the serum. In normal serum, sporozoites invade macrophages actively leading to their destruction, while immune serum triggers the phagocytic uptake of sporozoites leading to their destruction [82]. Activated macrophages were also shown to phagocytose and kill Plasmodium-infected erythrocytes using oxidative burst and this response was enhanced in the presence of immune serum [83,84]. The protective role of parasite neutralizing antibodies has been documented in several studies and it is clear now that several mechanisms are involved [85]. However, the outcome of the interaction of sporozoites with macrophages seems to be context-dependent as other studies highlighted a certain level of immune dysfunction in Kupffer cells (KCs) upon encounter with sporozoites. For instance, upon contact with Plasmodium yoelii sporozoites, murine KCs in the liver directly undergo apoptosis as depicted by the blebbing of the cellular membrane, nuclear condensation and fragmentation. This process results from the down-regulation of the pro-inflammatory cytokine, TNF-α, and up-regulation of the anti-inflammatory cytokine, IL-10 [86]. Moreover, sporozoites and recombinant circumsporozoite protein suppressed the respiratory burst of KCs by increasing the levels of cAMP in these cells [87]. Additionally, Steers et al. [88] revealed that the uptake of sporozoites by KCs of naïve mice, not infected previously with malaria, lead to the impairment of antigen presentation by down-regulating the expression levels of MHC class I and co-stimulatory molecules. Sporozoites also exhibit a cell traversal (CT) activity that aids in KC evasion and liver invasion. Using intravital laser spinning disc microscopy, it was shown that CT activity prevents the clearance of sporozoites by KCs, as the majority of SPECT2- sporozoites established lasting interactions with KCs resulting in their clearance while a small percentage of wildtype sporozoites established such interactions [25]. In the same study, authors also examined the in vitro internalization of fluorescently labeled Plasmodium sporozoites by purified primary KCs and their association with the lysosomal marker, LAMP1. Interestingly, only half of the sporozoites were phagocytosed as marked by an overlapping stain with LAMP1. The remaining half was suggested to be packaged in parasitophorous vacuoles hence escaping phagocytosis through CT activity [25]. In addition to their role in parasite clearance, inflammatory macrophages may also contribute to the severity of malaria cases by priming type I IFN production by plasmacytoid dendritic cells [85]. On the other hand, M2 anti-inflammatory macrophages polarized by IL-33 administration contributed to protection from cerebral malaria in a P. berghei model of infection [89].
Interaction of macrophages with Plasmodium blood stages
Monocytes have been also implicated in inhibiting the growth of blood stages of the parasite by antibody-dependent cellular inhibition (ADCI) in the presence of protective antibodies [90]. Recently, it was shown that human monocytes incubated with P. falciparum-infected erythrocytes in the presence of IgGs from malaria immune African human sera produced the antibacterial molecule trappin-2 that localized to the surface of merozoites and inhibited P. falciparum growth in vitro [91]. The systemic and lung overexpression of trappin-2 in mice reduced parasite sequestration in the spleen, liver, lung and brain leading to increased mouse survival after infection with a P. berghei strain that causes cerebral malaria. These results suggest that monocyte secretion of trappin-2 may contribute to the process of ADCI. On the other hand, there is evidence that ingestion of the parasite pigment hemozoin, the breakdown product of hemoglobin, or malaria-infected erythrocytes impairs the function of both monocytes and macrophages repressing their ability to produce inflammatory cytokines [92,93]. More recently, this functional impairment in response to the uptake of blood stage parasites was attributed to the rapid phagosomal acidification in monocytes and macrophages which impairs toll-like receptor (TLR) interactions with their parasite ligands, hence, preventing downstream cytokine responses [28]. This is particularly significant in malaria infections whereby Plasmodium parasites seem to be recognized exclusively by endosomal TLRs [2,29,94]. Moreover, CD47, a marker of self, expressed on the cell membranes of human cells including young red blood cells acts as a shield for blood stages parasites inhibiting their clearance by phagocytic cells [71]. CD47 exerts its effects by interacting with macrophage signal-regulatory protein alpha (SIRPα), which activates intracellular signaling pathways leading to reduced phagocytic uptake and decreased production of inflammatory cytokines [95].
Direct and indirect roles of natural killer cells in anti-Plasmodium immunity
Natural killer (NK) cells are key players in innate immunity [96]. They have been traditionally linked to fighting cancers and viral infections however recent studies point to a broader role in microbial infections. They form a heterogenous pool that is distinguished from other lymphocytes based on the expression of certain surface markers, such as CD56 and CD16 [97,98], along with the absence of surface marker CD3, which is specific to T cells [99,100]. NK cells normally express a wide variety of receptors, such as lectin-like and TLRs [101,102]; and inhibitory receptors, such as LT-2, CD94/NKG2A, CD161, and killer cell immunoglobulin-like receptors (KIRs) [103]. Their role in immunomodulating parasitic [104] and fungal infections [105] and in immunotherapy is of great concern [106,107]. Hereby, we provide the behavior of NK cells against malaria infections. A quick and robust pro-inflammatory response is needed to control malaria parasites in the early phases of infection and NK cells can contribute to that by the secretion of IFN-γ. Studies in mice revealed a crucial role for IFN-γ in controlling the levels of parasitemia and parasite clearance [85,92]. The depletion of NK cells in mice infected with P. yoelii and P. chabaudi triggered an early increase in parasitemia as a result of reduced IFN-γ production [85,108]. The protective role of IFN-γ is thought to occur through the activation of macrophages to phagocytose merozoites and parasitized erythrocytes in opsonization-dependent and independent manners [93].
In humans, the early production of IFN-γ also provides protection against malaria infection. IFN-γ production was shown to correlate with mild rather than severe diseases in children and to protect from reinfection within one year of initial infection [109]. Interestingly, IFN-γ production was not detected upon placing purified NK cells, instead of NK from whole blood donors, with infected RBCs. It was therefore deduced that peripheral blood mononuclear cells (PBMC) aid in NK cells activation, particularly by monocytes through the secretion of IL-12 and IL-18 [2,28,29,85] or through direct contact with macrophages [71,82]. In fact, the dependence of NK cells on other immune cells for activation during malaria infections have been observed long time ago. In a study by Ojo-Amaize et al. [110] P. berghei sporozoites were γ-irradiated to attenuate their virulence without affecting the erythrocyte stability prior to their culture with whole blood after their injection into mice. Such a modification still induced IFN-γ release in vitro and activation of splenic NK cells to lyse murine tumor cells. This piece of finding reflects the indirect activation of NK cells through immune players rather than by the parasite itself. Later studies supported this observation. For instance, NK cells from PBMC depleted of monocytes resulted in a relative decrease in IFN-γ production after incubation with P. falciparum-infected erythrocytes; however, when placed with purified macrophages only, NK cells re-established their IFN-γ production [111]. Hence, there seems to be a close cooperation between macrophages and NK cells that enhances IFN-γ by the latter in an IL-18 and MyD88 dependent manner. In fact NK cell activation in response to malaria infected RBCs (iRBCs) seems to depend on multiple factors, including the priming cytokine IL-2, the activating cytokines IL-12 and IL-18 [112,113], direct contact with macrophages through adhesion receptors [82,114] as well as direct contact with iRBCs [113]. The significance of such interaction and the receptors involved remain unclear. Supernatant from PBMC or NK from blood donors cultured with Pf-infected red blood cells, showed high levels of IFN-γ production as assayed by ELISA [115]. It is worth noting that opposing viewpoints exist in the literature regarding the fast release of IFN- γ directly by NK cells [85,92]. Overall, the secretion of IFN- γ in humans whether via direct or indirect activation of NK cells showed a positive correlation with the protection against malaria infection.
In addition to the indirect role of NK cell in malaria defense through the release of cytokines, NK cells are also capable of directly killing parasitized cells, whether red blood cells or hepatocytes by cytotoxicity. When NK cells are activated, a contact-dependent elimination of the infected RBC occurs. Chen et al. were able to observe the process of conjugates formation between NK and infected RBC in real time where the infected red blood cells were flattened after NK cells interaction. Throughout this process, there was no sign of phagocytosis, therefore it was deduced that the killing of the infected cell was a result of the leakage of granzymes and loss of the cell volume and integrity [85]. Additionally, some NK cells showed actin relocalization at the site of contact with infected RBC, probably an indication of an immunological synapse where cytotoxic granules are released. The identity of the ligands or receptors mediating such synapse remains unclear [116].
An important role of NK cells during innate immunity is its natural killing capacity by surveilling MHC class I expression levels on nucleated cells and the binding affinity of MHC- I to certain KIRs. Such defense mechanism is most likely to take place in the pre-erythrocytic liver stage of infection since RBCs do not harbor MHC-I molecules on their cell surface. Moreover, it has been shown that individuals with certain haplotypes of KIR and MHC- I may be less prone to malarial infections [108]. Thus, the function of NK cells against malarial infections is quite heterogeneous based on the mode of NK activation, levels of pro-inflammatory cytokines, and its means of killing that culminate in variable protective and pathogenesis patterns among individuals.
Role of γδ-T cells in the pathogenesis of malaria
γδ T cells possess an immunoregulatory role by expressing pro-inflammatory molecules such as IFN-γ, TNF-α, TNF-β and releasing cytotoxic granules [117]. They occupy a small subset of T lymphocytes, and unlike normal T cells, they have restricted T cell repertoire and express abundant TLRs. γδ T cells do not recognize antigens presented by MHC molecules on antigen presenting cells and thus, are considered innate-like lymphocytes.
Analyzing the surface phenotype of T cells after a 6 day in vitro stimulation of P.falciparum schizonts on extracts from unprimed individuals showed a dominant proliferation of γδ T cells subset with T-cell receptors of Vγ9 + family type [118,119]. Such observations reflect the role of γδ-T cells in the pathogenesis of malaria as discussed in Langhorne’s review, 1992 [120]. Recent studies demonstrate the release of phosphoantigens to the extracellular milieu during the egress of parasites at the end of the intraerythrocytic phase promoting the activation of γδ T cells [118] particularly a subset Vγ9 + Vδ2+ T cells. However, the exact mechanism of action that Vγ9 + Vδ2+ T possess in immunoregulating T cells is still not fully understood. Schofield et al. reported an upregulated expression of a potential inhibitory receptor, TIM3, in acutely infected mice or children with malaria. TIM3 may result in the impairment of γδ-T cells and consequently may have implications in the management of malarial diseases [121].
Other innate immune factors involved in defense against malaria
Certain studies have elucidated the role of the complement system against malarial infections [122]. Despite the well-established activation mechanism of the classical pathway through the increased binding of C1q to CRP and the alternative pathway through the activation of C3 by fragments from lysed infected erythrocytes, to date there is still no definitive mechanism on the mode of activation of the lectin pathway [14]. In addition to complement proteins, TLRs, such as TLR-2, TLR-4, and TLR-9, are considered key elements in initiating inflammatory response against malarial infections [123]. TLR-9 resides intracellularly, namely in the endoplasmic reticulum of resting non-specific immune cells, such as monocytes, NK cells, B cells and plasmacytoid dendritic cells [124]. Upon activation, TLR-9 are transported to a LAMP-1 positive compartment by trafficking through the Golgi complex [125] and interact with hemozoin aggregates found in engulfed parasitized erythrocytes [123]. In a study by Coban et al., TLR-9 deficient mice injected with P. falciparum hemozoin showed a drastic reduction in the serum levels of MCP-1 and IL-6 [126]. On the other hand, TLR-2 and TLR-4 reside on the surface of innate immune cells and are upregulated upon parasite infection especially in CD11c+/CD14+ monocytes [127]. These TLRs induce endosome/phagosome formation upon ligand binding and elicit an increase in TNF-α, IL-6, and IL-10 levels [123]. Furthermore, Toll/IL-1 receptor family induces NO synthase expression upon its binding to cytosolic factors released by ruptured host cells and impede the progress of parasite infection in the liver [128].
Another anti-malarial innate response is mediated through the secretion of cytokines and interferons, including both type I interferon (IFN-α/β) and IFN-γ (Figure 1). Type I interferon is released by hepatocytes [129] and by plasmacytoid cells (pDC) upon the recognition of sporozoites RNA by cytosolic sensors. Whereas, IFN-γ is released by several cellular sources including NK cells and CD8+ T cells upon activation by dendritic cells [130]. High serum levels of interferons have been shown to reduce the survival of the exo-erythrocytic forms of the parasite thus reducing the blood-stage population and impairing liver infection. It is worth noting that type I IFN activates the recruitment of neutrophils to the liver during P.vivax infection in both human and rodent malaria. Such an activation induces the expression of type I IFN stimulated genes (ISGs) by neutrophils. A positive correlation was detected between ISGs and increased serum levels of alanine and aspartate aminotransferases as an indicator of liver tissue damage. Thus, type I IFN modulates liver immunopathology by inducing the expression of certain chemokines and adhesion molecules involved in neutrophils recruitment [129,131].
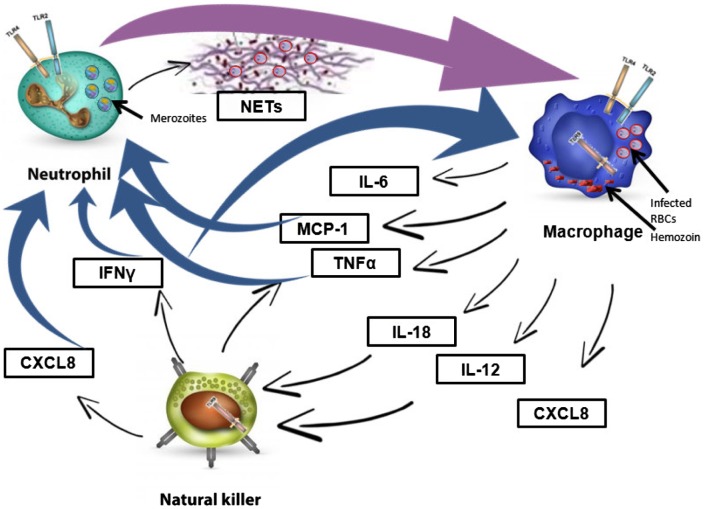
Figure 1.
Major cytokines controlling the functions of key innate immune cells against malaria parasites.
During a malaria infection, a fine-tuned communication through different cytokines elicits the activation and recruitment of the three major players of the innate immune response, namely neutrophils, macrophages, and natural killer cells. Activated macrophages stimulate neutrophils via TNF-α and MCP1 and stimulate natural killer cells via IL-12 and IL-18. Natural killer cells also exhibit a feedback loop further activating macrophages and neutrophils via IFN-γ and CXCL8. Despite the feed-forward and feedback loops mediated through different cytokines to amplify the immune response, malaria parasites can still evade the immune players.
Abbreviations: TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; RBC, red blood cells; NETs, neutrophil extracellular traps.
Conclusion
Significant progress has been done in understanding the early innate immune responses triggered against malaria parasites and their relative contribution to parasite clearance and immune pathology. However, several questions remain to be addressed in order to gain better insight into these early events which are crucial in defining the profile through which the disease will evolve. An interesting question is to understand why several immune factors both humoral and cellular act in concert to cause protection while in other instances may contribute to severe malaria. What controls the levels of cytokines in the malaria infections and how and on what basis does this change from one individual to another? These questions are difficult to infer from most studies on malaria immunity due to the reductionist approach utilized. Understanding these early events in malaria immunity is important as it may inform the design of novel more efficient vaccines and possibly help designing novel original treatment strategies, both of which are urgently needed to relieve the burden of malaria especially in endemic countries.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgment
We are grateful to Dr Mike Osta for his insightful comments and critical revision of the manuscript.
References
- [1].WHO World malaria report. Geneva: World Health Organization; 2016. [Google Scholar]
- [2].Offeddu V, Thathy V, Marsh K, et al. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol. 2012;42(6):535–548. 10.1016/j.ijpara.2012.03.011 [DOI] [PubMed] [Google Scholar]
- [3].Rich SM, Ayala FJ. Population structure and recent evolution of Plasmodium falciparum. Proc Nat Acad Sci. 2000;97(13):6994–7001. 10.1073/pnas.97.13.6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82(1):89–100. 10.1016/0092-8674(95)90055-1 [DOI] [PubMed] [Google Scholar]
- [5].Abdi AI, Fegan G, Muthui M, et al. Plasmodium falciparum antigenic variation: relationships between widespread endothelial activation, parasite PfEMP1 expression and severe malaria. BMC Infect Dis. 2014;14:170. 10.1186/1471-2334-14-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Deitsch KW, Dzikowski R. Variant gene expression and antigenic variation by malaria parasites. Annu Rev Microbiol. 2017;71:625–641. 10.1146/annurev-micro-090816-093841 [DOI] [PubMed] [Google Scholar]
- [7].Galinski MR, Lapp SA, Peterson MS, et al. Plasmodium knowlesi: a superb in vivo nonhuman primate model of antigenic variation in malaria. Parasitology. 2017;1–16. DOI: 10.1017/S0031182017001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kirkman LA, Deitsch KW. Antigenic variation and the generation of diversity in malaria parasites. Curr Opin Microbiol. 2012;15(4):456–462. 10.1016/j.mib.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buckee CO, Gupta S. Modelling malaria population structure and its implications for control. Adv Exp Med Biol. 2010;673:112–126. 10.1007/978-1-4419-6064-1 [DOI] [PubMed] [Google Scholar]
- [10].Stanisic DI, Barry AE, Good MF. Escaping the immune system: How the malaria parasite makes vaccine development a challenge. Trends Parasitol. 2013;29(12):612–622. 10.1016/j.pt.2013.10.001 [DOI] [PubMed] [Google Scholar]
- [11].Grassi B, Bignami A, Bastianelli G. Medical zoology: further researches upon the cycle of human malaria in the body of the mosquito. Indian Med Gazette. 1899;34(3):104–107. [PMC free article] [PubMed] [Google Scholar]
- [12].Ross R Dr. Manson’s mosquito-malaria theory. Indian Med gazette. 1896;31(7):264. [PMC free article] [PubMed] [Google Scholar]
- [13].Antinori S, Galimberti L, Milazzo L, et al. Biology of human malaria plasmodia including Plasmodium knowlesi. Mediterr J Hematol Infect Dis. 2012;4(1):e2012013. 10.4084/mjhid.2012.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Silver KL, Higgins SJ, McDonald CR, et al. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol. 2010;12(8):1036–1045. 10.1111/j.1462-5822.2010.01492.x [DOI] [PubMed] [Google Scholar]
- [15].Jongwutiwes S, Buppan P, Kosuvin R, et al. Plasmodium knowlesi malaria in humans and macaques, Thailand. Emerg Infect Dis. 2011;17(10):1799–1806. 10.3201/eid1710.110349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bruce MC, Macheso A, Galinski MR, et al. Characterization and application of multiple genetic markers for Plasmodium malariae. Parasitology. 2007;134(Pt 5):637–650. 10.1017/S0031182006001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev. 2007;20(4):579–592. 10.1128/CMR.00027-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Richter J, Franken G, Mehlhorn H, et al. What is the evidence for the existence of Plasmodium ovale hypnozoites? Parasitol Res. 2010;107(6):1285–1290. 10.1007/s00436-010-2071-z [DOI] [PubMed] [Google Scholar]
- [19].Da DF, Churcher TS, Yerbanga RS, et al. Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Exp Parasitol. 2015;149:74–83. 10.1016/j.exppara.2014.12.010 [DOI] [PubMed] [Google Scholar]
- [20].Ishino T, Yano K, Chinzei Y, et al. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2(1):E4. 10.1371/journal.pbio.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7(2):199–208. [DOI] [PubMed] [Google Scholar]
- [22].Kariu T, Ishino T, Yano K, et al. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59(5):1369–1379. 10.1111/j.1365-2958.2005.05024.x [DOI] [PubMed] [Google Scholar]
- [23].Frevert U, Nardin E. Arrest in the liver–a genetically defined malaria vaccine? N Engl J Med. 2005;352(15):1600–1602. 10.1056/NEJMcibr050521 [DOI] [PubMed] [Google Scholar]
- [24].Frevert U, Usynin I, Baer K, et al. Nomadic or sessile: can Kupffer cells function as portals for malaria sporozoites to the liver? Cell Microbiol. 2006;8(10):1537–1546. 10.1111/cmi.2006.8.issue-10 [DOI] [PubMed] [Google Scholar]
- [25].Tavares J, Formaglio P, Thiberge S, et al. Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med. 2013;210(5):905–915. 10.1084/jem.20121130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hollingdale MR, Collins Alan LWE, Campbell CC, et al. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am J Trop Med Hyg. 1985;34(2):216–222. 10.4269/ajtmh.1985.34.216 [DOI] [PubMed] [Google Scholar]
- [27].Mazier D, Collins WE, Mellouk S, et al. Plasmodium ovale: in vitro development of hepatic stages. Exp Parasitol. 1987;64(3):393–400. 10.1016/0014-4894(87)90052-X [DOI] [PubMed] [Google Scholar]
- [28].Markus MB. Do hypnozoites cause relapse in malaria? Trends Parasitol. 2015;31(6):239–245. 10.1016/j.pt.2015.02.003 [DOI] [PubMed] [Google Scholar]
- [29].Hulden L, Hulden L. Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malaria J. 2011;10:90. 10.1186/1475-2875-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garnham PC. Relapses in malaria: review of current studies. Ann Soc Belg Med Trop. 1985;65(3):233–242. [PubMed] [Google Scholar]
- [31].Hulden L, Hulden L, Heliovaara K. Natural relapses in vivax malaria induced by Anopheles mosquitoes. Malaria J. 2008;7:64. 10.1186/1475-2875-7-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bannister L, Mitchell G. The ins, outs and roundabouts of malaria. Trends Parasitol. 2003;19(5):209–213. 10.1016/S1471-4922(03)00086-2 [DOI] [PubMed] [Google Scholar]
- [33].Hanssen E, Dekiwadia C, Riglar DT, et al. Electron tomography of Plasmodium falciparum merozoites reveals core cellular events that underpin erythrocyte invasion. Cell Microbiol. 2013;15(9):1457–1472. 10.1111/cmi.12132 [DOI] [PubMed] [Google Scholar]
- [34].Riglar DT, Richard D, Wilson DW, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microb. 2011;9(1):9–20. 10.1016/j.chom.2010.12.003 [DOI] [PubMed] [Google Scholar]
- [35].Singh S, Alam MM, Pal-Bhowmick I, et al. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6(2):e1000746. 10.1371/journal.ppat.1000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baum J, Richard D, Healer J, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281(8):5197–5208. 10.1074/jbc.M509807200 [DOI] [PubMed] [Google Scholar]
- [37].Jones ML, Kitson EL, Rayner JC. Plasmodium falciparum erythrocyte invasion: a conserved myosin associated complex. Mol Biochem Parasitol. 2006;147(1):74–84. 10.1016/j.molbiopara.2006.01.009 [DOI] [PubMed] [Google Scholar]
- [38].Glushakova S, Yin D, Li T, et al. Membrane transformation during malaria parasite release from human red blood cells. Curr Biol. 2005;15(18):1645–1650. 10.1016/j.cub.2005.07.067 [DOI] [PubMed] [Google Scholar]
- [39].Elbadry MA, Al-Khedery B, Tagliamonte MS, et al. High prevalence of asymptomatic malaria infections: a cross-sectional study in rural areas in six departments in Haiti. Malaria J. 2015;14:510. 10.1186/s12936-015-1051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bruce MC, Alano P, Duthie S, et al. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. 10.1017/S0031182000061199 [DOI] [PubMed] [Google Scholar]
- [41].Josling GA, Llinás M. Sexual development in Plasmodium parasites: knowing when it’s time to commit. Nat Rev Microbiol. 2015;13(9):573–587. 10.1038/nrmicro3519 [DOI] [PubMed] [Google Scholar]
- [42].Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Ann Rev Microbiol. 2009;63:195–221. 10.1146/annurev.micro.091208.073403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zevering Y, Khamboonruang C, Good MF. Effect of polymorphism of sporozoite antigens on T-cell activation. Res Immunol. 1994;145(6):469–476. 10.1016/S0923-2494(94)80178-9 [DOI] [PubMed] [Google Scholar]
- [44].Zevering Y, Khamboonruang C, Good MF. Human and murine T-cell responses to allelic forms of a malaria circumsporozoite protein epitope support a polyvalent vaccine strategy. Immunology. 1998;94(3):445–454. 10.1046/j.1365-2567.1998.00514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Winter G, Kawai S, Haeggström M, et al. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med. 2005;201(11):1853–1863. 10.1084/jem.20041392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kiwanuka GN. Genetic diversity in Plasmodium falciparum merozoite surface protein 1 and 2 coding genes and its implications in malaria epidemiology: a review of published studies from 1997–2007. J Vector Borne Dis. 2009;46(1):1–12. [PubMed] [Google Scholar]
- [47].Ramasamy R. Molecular basis for evasion of host immunity and pathogenesis in malaria. Biochimica et biophysica acta 1998;1406::10–27. 10.1016/S0925-4439(97)00078-1 [DOI] [PubMed] [Google Scholar]
- [48].Wright GJ, Rayner JC. Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog. 2014;10(3):e1003943. 10.1371/journal.ppat.1003943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Singh V, Gupta P, Pande V. Revisiting the multigene families: Plasmodium var and vir genes. J Vector Borne Dis. 2014;51(2):75–81. [PubMed] [Google Scholar]
- [50].Fernandez V, Hommel M, Chen Q, et al. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190(10):1393–1404. 10.1084/jem.190.10.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol. 2017;15(8):479–491. 10.1038/nrmicro.2017.47 [DOI] [PubMed] [Google Scholar]
- [52].D’Ombrain MC, Voss TS, Maier AG, et al. Plasmodium falciparum erythrocyte membrane protein-1 specifically suppresses early production of host interferon-gamma. Cell Host Microb. 2007;2(2):130–138. 10.1016/j.chom.2007.06.012 [DOI] [PubMed] [Google Scholar]
- [53].Son UH, Dinzouna-Boutamba SD, et al. Diversity of vir genes in Plasmodium vivax from endemic regions in the Republic of Korea: an initial evaluation. Korean J Parasitol. 2017;55(2):149–158. 10.3347/kjp.2017.55.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Segal AW. How neutrophils kill microbes. Ann Rev Immunol. 2005;23:197–223. 10.1146/annurev.immunol.23.021704.115653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen CL, Wang Y, et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology. 2017;7(1):e1373234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Amicarella F, Muraro MG, Hirt C, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66(4):692–704. 10.1136/gutjnl-2015-310016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. 10.1126/science.1159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yamauchi LM, Coppi A, Snounou G, et al. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9(5):1215–1222. 10.1111/cmi.2007.9.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mac-Daniel L, Buckwalter MR, Berthet M, et al. Local immune response to injection of Plasmodium sporozoites into the skin. J Immunol. 2014;193(3):1246–1257. 10.4049/jimmunol.1302669 [DOI] [PubMed] [Google Scholar]
- [60].Hopp CS, Sinnis P. The innate and adaptive response to mosquito saliva and Plasmodium sporozoitess in the skin. Ann N Y Acad Sci. 2015;1342:37–43. 10.1111/nyas.2015.1342.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Waisberg M, Molina-Cruz A, Mizurini DM, et al. Plasmodium falciparum infection induces expression of a mosquito salivary protein (Agaphelin) that targets neutrophil function and inhibits thrombosis without impairing hemostasis. PLoS Pathog. 2014;10(9):e1004338. 10.1371/journal.ppat.1004338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Demeure CE, Brahimi K, Hacini F, et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol. 2005;174(7):3932–3940. 10.4049/jimmunol.174.7.3932 [DOI] [PubMed] [Google Scholar]
- [63].Metzger WG, Mordmüller BG, Kremsner PG. Malaria pigment in leucocytes. Trans R Soc Trop Med Hyg. 1995;89(6):637–638. 10.1016/0035-9203(95)90423-9 [DOI] [PubMed] [Google Scholar]
- [64].Sun T, Chakrabarti C. Schizonts, merozoites, and phagocytosis in falciparum malaria. Ann Clin Lab Sci. 1985;15(6):465–469. [PubMed] [Google Scholar]
- [65].Brown J, Smalley ME. Inhibition of the in vitro growth of Plasmodium falciparum by human polymorphonuclear neutrophil leucocytes. Clin Exp Immunol. 1981;46(1):106–109. [PMC free article] [PubMed] [Google Scholar]
- [66].Kharazmi A, Jepsen S, Valerius NH. Polymorphonuclear leucocytes defective in oxidative metabolism inhibit in vitro growth of Plasmodium falciparum. Evidence against an oxygen-dependent mechanism. Scand J Immunol. 1984;20(1):93–96. 10.1111/sji.1984.20.issue-1 [DOI] [PubMed] [Google Scholar]
- [67].Kumaratilake LM, Ferrante A. Opsonization and phagocytosis of Plasmodium falciparum merozoites measured by flow cytometry. Clin Diagn Lab Immunol. 2000;7(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Joos C, Marrama L, Polson HE, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE. 2010;5(3):e9871. 10.1371/journal.pone.0009871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kapelski S, Klockenbring T, Fischer R, et al. Assessment of the neutrophilic antibody-dependent respiratory burst (ADRB) response to Plasmodium falciparum. J Leukoc Biol. 2014;96(6):1131–1142. 10.1189/jlb.4A0614-283RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kharazmi A, Jepsen S. Enhanced inhibition of in vitro multiplication of Plasmodium falciparum by stimulated human polymorphonuclear leucocytes. Clin Exp Immunol. 1984;57(2):287–292. [PMC free article] [PubMed] [Google Scholar]
- [71].Clark IA, Hunt NH. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983;39(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- [74].Baker VS, Imade GE, Molta NB, et al. Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malaria J. 2008;7:41. 10.1186/1475-2875-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mahanta A, Kar SK, Kakati S, et al. Heightened inflammation in severe malaria is associated with decreased IL-10 expression levels and neutrophils. Innate Immunity. 2015;21(5):546–552. 10.1177/1753425914561277 [DOI] [PubMed] [Google Scholar]
- [76].Ioannidis LJ, Nie CQ, Ly A, et al. Monocyte- and Neutrophil-Derived CXCL10 Impairs Efficient Control of Blood-Stage Malaria Infection and Promotes Severe Disease. J Immunol. 2016;196(3):1227–1238. 10.4049/jimmunol.1501562 [DOI] [PubMed] [Google Scholar]
- [77].Sercundes MK, Ortolan LS, Debone D, et al. Correction: targeting neutrophils to prevent malaria-associated acute lung injury/acute respiratory distress syndrome in mice. PLoS Pathog. 2017;13(11):e1006730. 10.1371/journal.ppat.1006730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moghaddam AS, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization and function in health and disease. J Cell Physiol. 2018. DOI: 10.1002/jcp.26429 [DOI] [PubMed] [Google Scholar]
- [79].Li C, Xu MM, Wang K, et al. Macrophage polarization and meta-inflammation. Translational research: the journal of laboratory and clinical medicine. 2018;191:29–44. 10.1016/j.trsl.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9(3):289–302. 10.2217/imt-2016-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Soulat D, Bogdan C. Function of Macrophage and Parasite Phosphatases in Leishmaniasis. Front Immunol. 2017;8:1838. 10.3389/fimmu.2017.01838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Newman KC, Korbel DS, Hafalla JC, et al. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2006;2(12):e118. 10.1371/journal.ppat.0020118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ockenhouse CF, Tandon NN, Magowan C, et al. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989;243(4897):1469–71. 10.1126/science.2467377 [DOI] [PubMed] [Google Scholar]
- [84].Ockenhouse CF, Tegoshi T, Maeno Y, et al. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176(4):1183–1189. 10.1084/jem.176.4.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sinka ME, Bangs MJ, Manguin S, et al. A global map of dominant malaria vectors. Parasites Vectors. 2012;5:69. 10.1186/1756-3305-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Klotz C, Frevert U. Plasmodium yoelii sporozoites modulate cytokine profile and induce apoptosis in murine Kupffer cells. Int J Parasitol. 2008;38(14):1639–1650. 10.1016/j.ijpara.2008.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007;9(11):2610–2628. 10.1111/cmi.2007.9.issue-11 [DOI] [PubMed] [Google Scholar]
- [88].Steers N, Schwenk R, Bacon DJ, et al. The immune status of Kupffer cells profoundly influences their responses to infectious Plasmodium berghei sporozoites. Eur J Immunol. 2005;35(8):2335–2346. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- [89].Besnard AG, Guabiraba R, Niedbala W, et al. IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PLoS Pathog. 2015;11(2):e1004607. 10.1371/journal.ppat.1004607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bouharoun-Tayoun H, Attanath P, Sabchareon A, et al. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172(6):1633–1641. 10.1084/jem.172.6.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Roussilhon C, Bang G, Bastaert F, et al. The antimicrobial molecule trappin-2/elafin has anti-parasitic properties and is protective in vivo in a murine model of cerebral malaria. Sci Rep. 2017;7:42243. 10.1038/srep42243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stegmann KA, De Souza JB, Riley EM. IL-18-induced expression of high-affinity IL-2R on murine NK cells is essential for NK-cell IFN-gamma production during murine Plasmodium yoelii infection. Eur J Immunol. 2015;45(12):3431–3440. 10.1002/eji.201546018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mota MM, Brown KN, Holder AA, et al. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surfaces of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infect Immun. 1998;66(9):4080–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Nat Acad Sci. 2007;104(6):1919–1924. 10.1073/pnas.0608745104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ayi K, Lu Z, Serghides L, et al. CD47-SIRPalpha interactions regulate macrophage uptake of Plasmodium falciparum-infected erythrocytes and clearance of malaria in vivo. Infect Immun. 2016;84(7):2002–2011. 10.1128/IAI.01426-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Park DW, Lee HJ, Park CW, et al. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010;63(2):173–180. 10.1111/j.1600-0897.2009.00777.x [DOI] [PubMed] [Google Scholar]
- [97].Lash GE, Robson SC, Bulmer JN. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–S92. 10.1016/j.placenta.2009.12.022 [DOI] [PubMed] [Google Scholar]
- [98].Poli A, Michel T, Thérésine M, et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. 10.1111/imm.2009.126.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lanier LL, Le AM, Civin CI, et al. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136(12):4480–4486. [PubMed] [Google Scholar]
- [100].Ritz J, Campen TJ, Schmidt RE, et al. Analysis of T-cell receptor gene rearrangement and expression in human natural killer clones. Science. 1985;228(4707):1540–1543. 10.1126/science.2409597 [DOI] [PubMed] [Google Scholar]
- [101].Bellora F, Castriconi R, Dondero A, et al. Human NK cells and NK receptors. Immunol Lett. 2014;161(2):168–173. 10.1016/j.imlet.2013.12.009 [DOI] [PubMed] [Google Scholar]
- [102].Bao SH, Shuai W, Tong J, et al. Increased expression of Toll-like receptor 3 in decidual natural killer cells of patients with unexplained recurrent spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):326–330. 10.1016/j.ejogrb.2012.08.005 [DOI] [PubMed] [Google Scholar]
- [103].Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta 2008;29 Suppl A:60–66. 10.1016/j.placenta.2007.10.006 [DOI] [PubMed] [Google Scholar]
- [104].Roetynck S, Baratin M, Vivier ÉricE, et al. NK cells and innate immunity to malaria. Med Sci. 2006;22(8–9):739–744. 10.1051/medsci/20062289739 [DOI] [PubMed] [Google Scholar]
- [105].Schmidt S, Tramsen L, Lehrnbecher T. Natural ailler cells in antifungal immunity. Front Immunol. 2017;8:e711. 10.3389/fimmu.2017.01623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ding H, Yang X, Wei Y. Fusion proteins of NKG2D/NKG2DL in cancer immunotherapy. Int J Mol Sci. 2018;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Trans Med. 2009; 1 (6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yindom LM, Forbes R, Aka P, et al. Killer-cell immunoglobulin-like receptors and malaria caused by Plasmodium falciparum in The Gambia. Tissue Antigens. 2012;79(2):104–113. 10.1111/tan.2012.79.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179(4):980–988. 10.1086/jid.1999.179.issue-4 [DOI] [PubMed] [Google Scholar]
- [110].Ojo-Amaize EA, Vilcek J, Cochrane AH, et al. Plasmodium berghei sporozoites are mitogenic for murine T cells, induce interferon, and activate natural killer cells. J Immunol. 1984;133(2):1005–1009. [PubMed] [Google Scholar]
- [111].Baratin M, Roetynck S, Lepolard C, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Nat Acad Sci. 2005;102(41):14747–14752. 10.1073/pnas.0507355102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Horowitz A, Behrens RH, Okell L, et al. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185(5):2808–2818. 10.4049/jimmunol.1000844 [DOI] [PubMed] [Google Scholar]
- [113].Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol. 2003;171(10):5396–5405. 10.4049/jimmunol.171.10.5396 [DOI] [PubMed] [Google Scholar]
- [114].Baratin M, Roetynck S, Pouvelle B, et al. Dissection of the role of PfEMP1 and ICAM-1 in the sensing of Plasmodium falciparum-infected erythrocytes by natural killer cells. PLoS ONE. 2007;2(2):e228. 10.1371/journal.pone.0000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169(6):2956–2963. 10.4049/jimmunol.169.6.2956 [DOI] [PubMed] [Google Scholar]
- [116].Chen Q, Amaladoss A, Ye W, et al. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc Nat Acad Sci. 2014;111(4):1479–1484. 10.1073/pnas.1323318111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Patel SS, Wacholtz MC, Duby AD, et al. Analysis of the functional capabilities of CD3+CD4-CD8- and CD3+CD4+CD8+ human T cell clones. J Immunol. 1989;143(4):1108–1117. [PubMed] [Google Scholar]
- [118].Goodier M, Fey P, Eichmann K, et al. Human peripheral blood gamma delta T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992;4(1):33–41. 10.1093/intimm/4.1.33 [DOI] [PubMed] [Google Scholar]
- [119].Ho M, Webster HK, Tongtawe P, et al. Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25(1–3):139–141. 10.1016/0165-2478(90)90105-Y [DOI] [PubMed] [Google Scholar]
- [120].Langhorne J, Goodier M, Behr C, et al. Is there a role for gamma delta T cells in malaria? Immunol Today. 1992;13(8):298–300. 10.1016/0167-5699(92)90041-5 [DOI] [PubMed] [Google Scholar]
- [121].Schofield L, Ioannidis LJ, Karl S, et al. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of gammadelta T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Med. 2017;15(1):114. 10.1186/s12916-017-0883-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Salmon D, Vilde JL, Andrieu B, et al. Role of immune serum and complement in stimulation of the metabolic burst of human neutrophils by Plasmodium falciparum. Infect Immun. 1986;51(3):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].McCall MB, Netea MG, Hermsen CC, et al. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol. 2007;179(1):162–171. 10.4049/jimmunol.179.1.162 [DOI] [PubMed] [Google Scholar]
- [124].Leifer CA, Kennedy MN, Mazzoni A, et al. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173(2):1179–1183. 10.4049/jimmunol.173.2.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chockalingam A, Brooks JC, Cameron JL, et al. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol Cell Biol. 2009;87(3):209–217. 10.1038/icb.2008.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Coban C, Ishii kJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201(1):19–25. 10.1084/jem.20041836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Franklin BS, Parroche P, Ataide MA, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Nat Acad Sci. 2009;106(14):5789–5794. 10.1073/pnas.0809742106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Torgler R, Bongfen SE, Romero JC, et al. Sporozoite-mediated hepatocyte wounding limits Plasmodium parasite development via MyD88-mediated NF-kappa B activation and inducible NO synthase expression. J Immunol. 2008;180(6):3990–3999. 10.4049/jimmunol.180.6.3990 [DOI] [PubMed] [Google Scholar]
- [129].Liehl P, Zuzarte-Luís V, Chan J, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014;20(1):47–53. 10.1038/nm.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ocaña-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197(2):143–151. 10.1084/jem.20021072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Rocha BC, Marques PE, Leoratti FMS, et al. Type I interferon transcriptional signature in neutrophils and low-density granulocytes are associated with tissue damage in malaria. Cell Rep. 2015;13(12):2829–2841. 10.1016/j.celrep.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]