SUMMARY
This document outlines a comprehensive practical approach to a laboratory quality management system (QMS) by describing how to operationalize the management and technical requirements described in the ISO 15189 international standard. It provides a crosswalk of the ISO requirements for quality and competence for medical laboratories to the 12 quality system essentials delineated by the Clinical and Laboratory Standards Institute. The quality principles are organized under three main categories: quality infrastructure, laboratory operations, and quality assurance and continual improvement. The roles and responsibilities to establish and sustain a QMS are outlined for microbiology laboratory staff, laboratory management personnel, and the institution's leadership. Examples and forms are included to assist in the real-world implementation of this system and to allow the adaptation of the system for each laboratory's unique environment. Errors and nonconforming events are acknowledged and embraced as an opportunity to improve the quality of the laboratory, a culture shift from blaming individuals. An effective QMS encourages “systems thinking” by providing a process to think globally of the effects of any type of change. Ultimately, a successful QMS is achieved when its principles are adopted as part of daily practice throughout the total testing process continuum.
KEYWORDS: quality management system, ISO standard, quality system essentials, continual improvement, quality indicators, ISO 15189, QMS
INTRODUCTION
The role of the clinical microbiology laboratory is rapidly evolving as the delivery of health care undergoes drastic changes. Laboratories are no longer revenue-generating centers. Now they are considered cost centers that must justify their existence by demonstrating added quality and safety to improve patient care. Laboratories are no longer silos of information but integrated into the quality framework to provide patient-focused services. With this evolution, laboratory staff require an introduction into how their processes and procedures should align with their organization's quality management system (QMS). The purpose of this document is to educate clinical microbiologists about the fundamental quality elements and provide practical guidance on how to meet and sustain these QMS requirements. Although there are several QMS models to follow, this document primarily addresses the standards described in ISO document 15189 (2012), Medical Laboratories—Requirements of Quality and Compliance, of the International Organization for Standardization, which is universally recognized throughout the world (1). This document complements published guidelines, translates for the reader how to operationalize each of the management and technical requirements described in the ISO 15189 standard, and crosswalks these with the 12 quality system essentials (QSEs) delineated by the Clinical and Laboratory Standards Institute (CLSI) (2). This document includes suggested roles and responsibilities of the clinical microbiology laboratory staff, laboratory management personnel, and the organization to operate under a QMS.
HOW TO USE THIS DOCUMENT
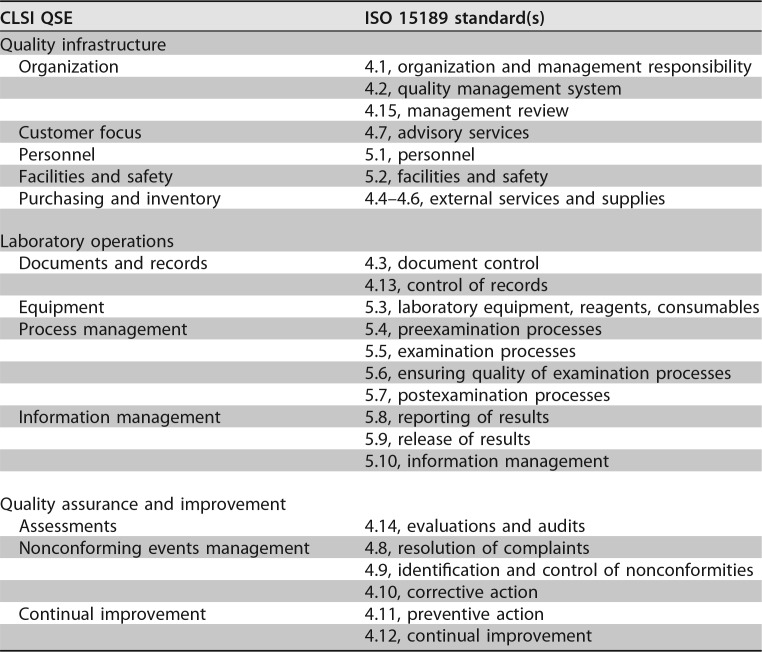
The laboratory quality standards are divided into three overarching areas: the infrastructure of the quality system, the laboratory operations, and the activities under quality assurance and continual improvement (CI). For those beginning to learn about a QMS, it may be easier to assimilate and apply the 12 quality system essentials defined by the CLSI (2) than the 10 management and 15 technical standards described in the ISO 15189 document. Table 1 is a crosswalk linking each CLSI quality system essential to an ISO 15189 requirement.
TABLE 1.
Crosswalk of CLSI quality system essentials and ISO 15189 standards
It is critical that the reader recognize the distinction between the verbs “shall” and “should” as used in this document and in concordance with ISO terminology. When something is a requirement and must be performed to achieve or remain in compliance, the verb “shall” is used. If the statement is a recommendation and a laboratory may opt out, or modify, what is prescribed, then the verb “should” is used.
Each laboratory has a unique organizational structure. Some may have laboratory directors, section directors, supervisors, and managers, while smaller laboratories may consolidate these responsibilities. In the tables listing potential roles and responsibilities for the institution, laboratory management, and staff, the reader may modify these roles and responsibilities according to how they apportion duties within their structure.
A glossary of terms guides the reader in the language of quality management (see the Appendix). Examples of policies, processes, procedures, and forms appear as figures to enable the reader to apply them to their laboratories. This document serves as a guideline only, and the sample procedures and forms are not meant to be prescriptive and may be adapted to each laboratory's practices. Publications that are referenced throughout the document provide excellent starting points to learn more about QMS standards and guidance that currently exist for clinical laboratories.
WHAT IS A QMS?
A laboratory quality management system is a systematic, integrated set of activities to establish and control the work processes from preanalytical through postanalytical processes, manage resources, conduct evaluations, and make continual improvements to ensure consistent quality results. Approaches to quality management in clinical microbiology were described previously by Bartlett et al. (3) and Schifman et al. (4). Government regulations of laboratory practices and the establishment of quality management have been debated vigorously over the decades. Laboratories improve their processes to be cost-effective and scientifically sound and effect a positive outcome for patient care. Process improvement typically occurs as laboratories adopt new technologies. Having a quality system to guide the laboratory in the implementation of new processes and procedures ensures that all elements will be considered prior to adopting a new practice or modifying a current one.
Clinical microbiology laboratories perform quality control (QC) for their analytical test methods, which is the initial level of controlling a procedure. QC requirements are mandated by regulatory agencies and specified in package inserts by the manufacturers of commercially prepared products. The next level of quality oversight is quality assurance (QA), where quality controls and quality indicators are tracked and trends are analyzed. QA includes monitoring supplies, equipment calibration and maintenance, procedures, personnel competency, proficiency testing (PT), specimen collection and transport, and accuracy and timeliness of result reporting.
Working under a true QMS broadens the scope of quality activities to be considered to obtain the highest level of quality in the product produced or service rendered. A QMS integrates all the quality elements into the work process. When a new test is initiated, the laboratory considers not only the QC and QA activities but also the purchasing and inventory processes, personnel training, test system assessment, instrument qualification, information technology support, and document control (and so forth). In a QMS, deviations from the expected performance, known as nonconforming events (NCEs), are reviewed by a higher level of management so that cross-cutting problems, such as specimen transport delays, can be resolved at the institutional level. Nonconforming events are addressed in Quality Assurance and Continual Improvement, below.
It is imperative that a laboratory review all regional and national regulatory requirements for a quality system since they may differ from the ISO standard. The most stringent requirements are the ones that must be followed to ensure compliance. For U.S. laboratories, a QMS is a requirement of the Clinical Laboratory Improvement Amendment (CLIA) (5). Laboratories have written policies and procedures to implement and monitor their quality for all phases of the testing process and general laboratory systems. A QMS meets the basic requirements defined in CLIA regulations and goes beyond the minimum requirements of the CLIA to enable continual quality improvement. In addition to meeting the requirements of regulatory authorities and accrediting bodies, a QMS focuses on exceeding the expectations of an organization's external and internal customers and encouraging the best use of resources.
At whatever level the QMS is implemented, either across the entire organization or within the laboratory system, leadership must be engaged and supportive of the QMS in order for it to succeed. The quality policy and standards shall be established at the highest organizational level, and the laboratory shall align with the organization's quality goals and objectives. How to operationalize the quality policy and standards varies according to the unique technical procedures, equipment, and personnel requirements within the laboratory. The goal is to produce a consistent, accurate, and reliable service or product for patient care. A QMS consists of tracking where inconsistencies occur, uncovering the root causes of why they happened, and correcting the system to prevent them from recurring.
QUALITY INFRASTRUCTURE
In this overarching section, we describe the CLSI QSEs that form the foundational building blocks for the quality management system. These five QSEs include (i) organization and management responsibilities, (ii) commitment to customers, (iii) personnel, (iv) provisions for adequate facilities and safety, and (v) purchasing and inventory.
Organization and Management Responsibility
Management shall visibly endorse and support the establishment of the QMS in order for the culture to change and the QMS to function successfully. Leadership shall demonstrate their commitment to the implementation of a QMS by providing the necessary budgetary resources, communications, personnel, and environment (6). Leadership shall establish the overarching quality policy and standards, which are defined in a quality manual. The quality policy should be widely communicated and understood by all laboratory personnel. Quality objectives for the laboratory should align with the organization's quality policy and standards and be specific, measurable, achievable, realistic, and time bound. Responsibilities for meeting the quality objectives shall be clearly defined within the laboratory organization. To be effective across the organization, communications shall be consistent, frequent, and delivered by multiple methods (e.g., all-hands meetings, one-on-one meetings, e-mails, and newsletters). Minutes for work group and committee meetings should be recorded and published, as appropriate, to ensure transparency and coordination of activities.
All personnel shall follow the organization's code of ethical conduct. The laboratory director shall be competent and fulfill his or her responsibilities. The laboratory director may delegate responsibilities for individual specialty areas (e.g., chemistry, microbiology, and molecular testing); however, the laboratory director is ultimately responsible for the successful completion of the activities and documentation of records.
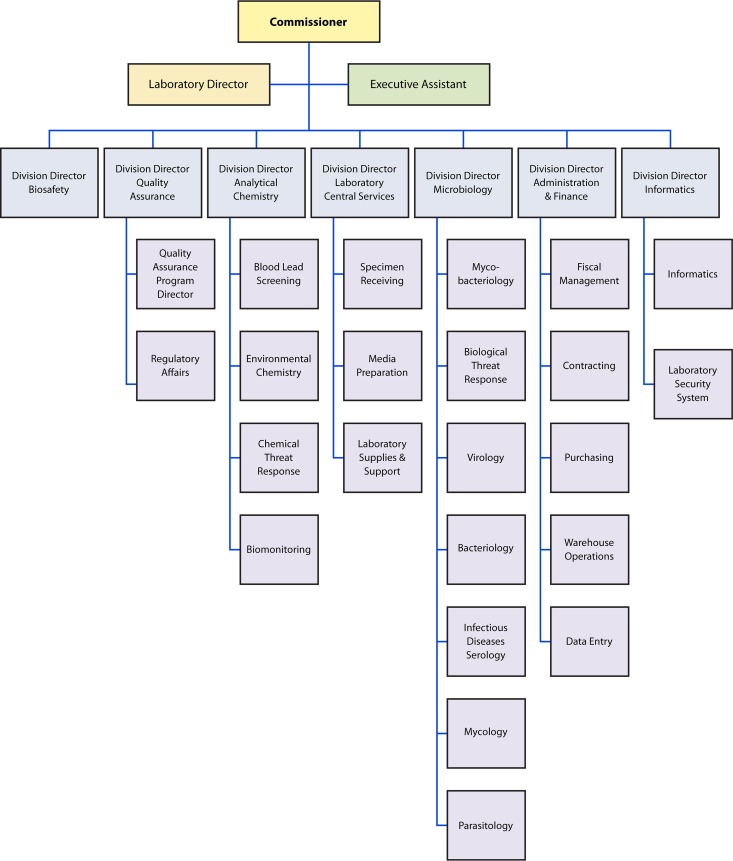
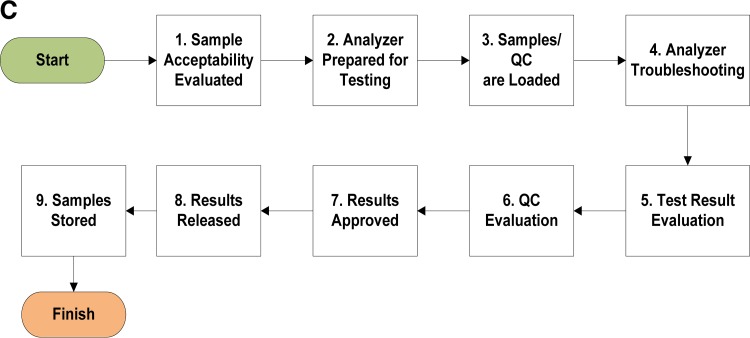
A quality manager should be appointed to oversee the processes needed and to ensure that the QMS is established, maintained, and evaluated for its effectiveness. There is a role for everyone in the QMS. It is not the sole responsibility of the quality manager. A laboratory organizational chart should be created and published so that everyone knows how they align in the organization and the supervisory chain of command. An example of an organization chart for a public health laboratory is shown in Fig. 1. Suggested roles and responsibilities for key activities are listed in Table 2.
FIG 1.
Sample organization chart for a public health laboratory system. The organizational chart demonstrates the chain of command. Organizational charts will vary based on areas of responsibility and disciplines covered.
TABLE 2.
Roles and responsibilities for the organization
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Organizational commitment to the QMS | Provide commitment and resources | Adhere to quality policy and standards | Describe the principles of the QMS |
| Ensure that QMS elements are considered in all work practices | |||
| Communication of quality policy and objectives | Establish quality policy and standards | Provide timely and transparent communications regarding quality | Read the quality manual and adhere to QMS policies |
| Responsibilities for the QMS | Define roles for all personnel | Create a quality team to accomplish quality objectives | Perform duties as assigned to ensure quality |
| Participate as a leader of a quality team | Participate as a member of a quality team | ||
| Coordinate quality activities | Participate in management review | Appoint a quality manager | Participate in quality initiatives |
| Work closely with the quality manager to achieve the laboratory's quality objectives | |||
Quality management system.
Documentation serves as evidence of what is required and if the requirements are being met. For the QMS, this includes the quality policy, quality objectives, the quality manual, all procedures and records, and the applicable regulations and standards. The documentation may be in electronic or hard-copy format and shall be communicated to laboratory staff and management. It is the responsibility of each laboratory section to contribute to the development of quality indicators and objectives. The quality manual should be created at the highest level in the organization participating in the QMS. It is the responsibility of the personnel to read and understand the contents of the quality manual since continual improvement is a process that involves all staff.
The laboratory director and management team shall determine the processes that enable their laboratory to meet institutional quality standards. The laboratory is responsible for processes and procedures for their methods, operational controls, and resources. The laboratory shall monitor these processes, such as the turnaround time for reporting positive blood cultures, and continue to look for opportunities for improvement. The roles and responsibilities for the QMS are listed in Table 3.
TABLE 3.
Roles and responsibilities for establishing a quality management system
| Document or activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Quality manual | Develop, approve, distribute, and periodically review, e.g., every 1–3 yr | Read and ensure that laboratory staff are aware of content | Read the quality manual |
| Quality policy and standards | Establish, communicate, and periodically review, e.g., every 1–3 yr | Establish or contribute to development of quality policy and standards | Adhere to policy and standards |
| Read, adhere to, and openly endorse policy and standards | |||
| Quality objectives | Evaluate for effectiveness | Establish specific, measurable, achievable, realistic, and time-bound quality objectives | Work to achieve objectives as applicable for the level of responsibility |
| Laboratory procedures, forms, and records | None | Write and approve procedures and related forms | Follow procedures as written Complete forms as required Maintain all documents and records Provide input where changes could improve the process, procedure, or form or where job aids could be beneficial |
| Educate staff on content | |||
| Provide training to perform procedures | |||
| Review documents annually and revise if needed | |||
| Retain documents and records for the prescribed period of time | |||
| Regulations | Comply with federal, state, local, and other regulatory requirements | Comply with federal, state, local, and other regulatory requirements | Adhere to all regulatory policies and procedures |
| Educate staff on all regulations that apply to the science and operation of the microbiology laboratory | |||
Management review.
Management review is an essential step for having an effective quality management system. The review should occur as a planned activity at a designated frequency throughout the year. The review should occur at least semiannually when the quality management system is newly created. Once a system is mature, an annual review may be sufficient. The data for the review may be collated by microbiology laboratory management and merged into an overall laboratory services report. Items to include in the review may be set by those higher in the organization and focus on risk management, which affects the financial and legal health of the organization (7). The review should be conducted by a management review committee comprised of higher-level laboratory leadership, such as the director of clinical pathology, the vice president of laboratory services, and directors and supervisors of the laboratory sections, to determine the continued suitability, adequacy, and effectiveness of the policies, processes, and procedures that support patient care.
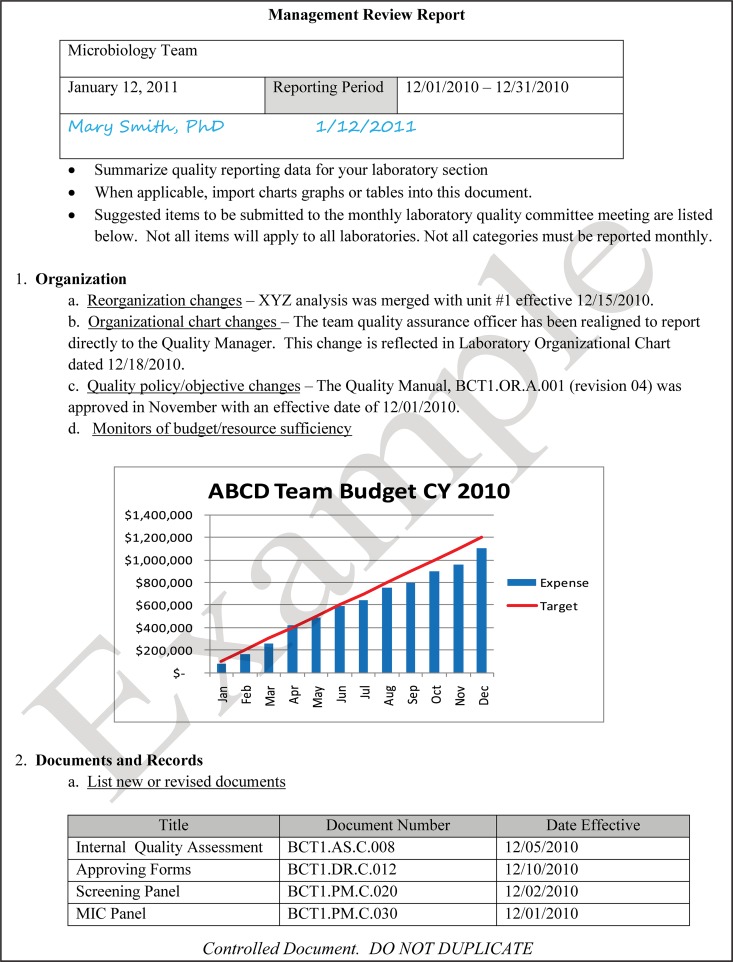
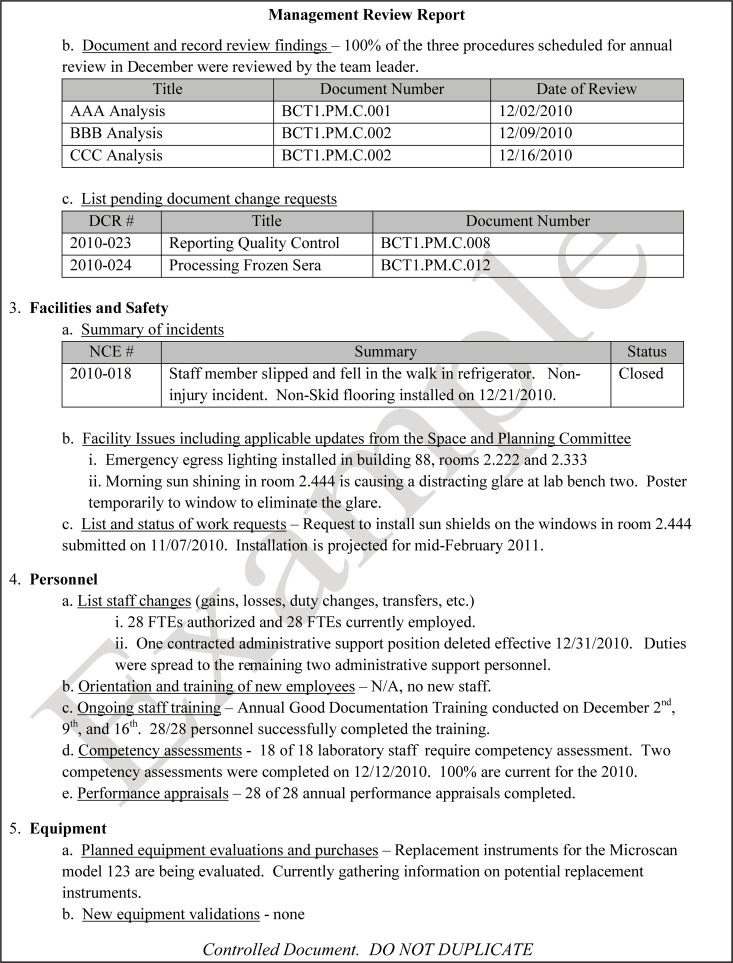
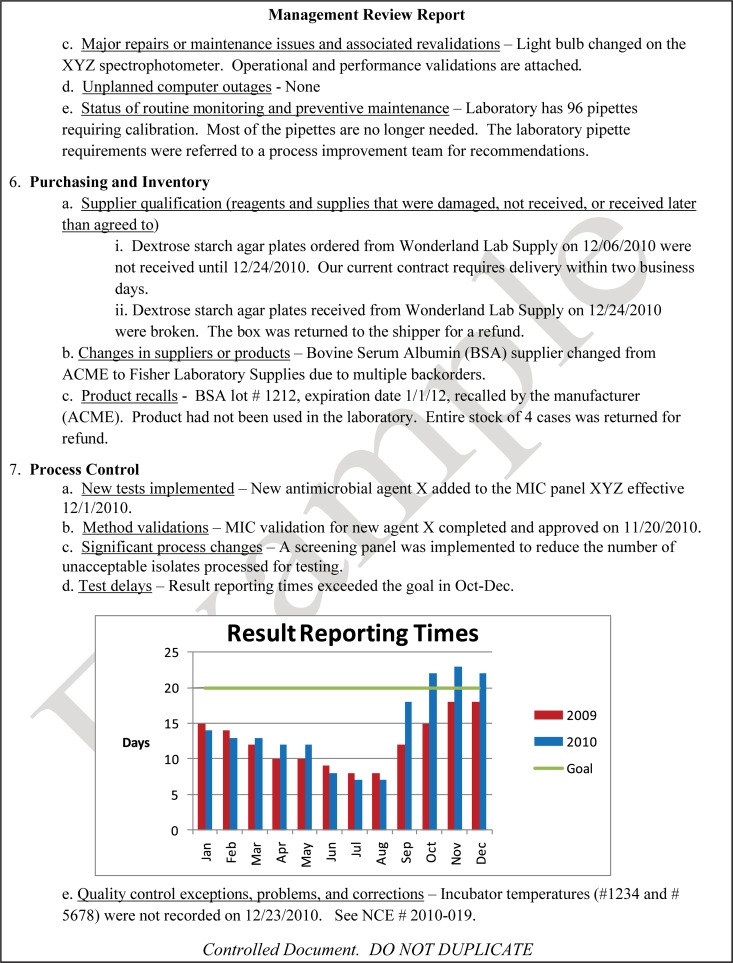
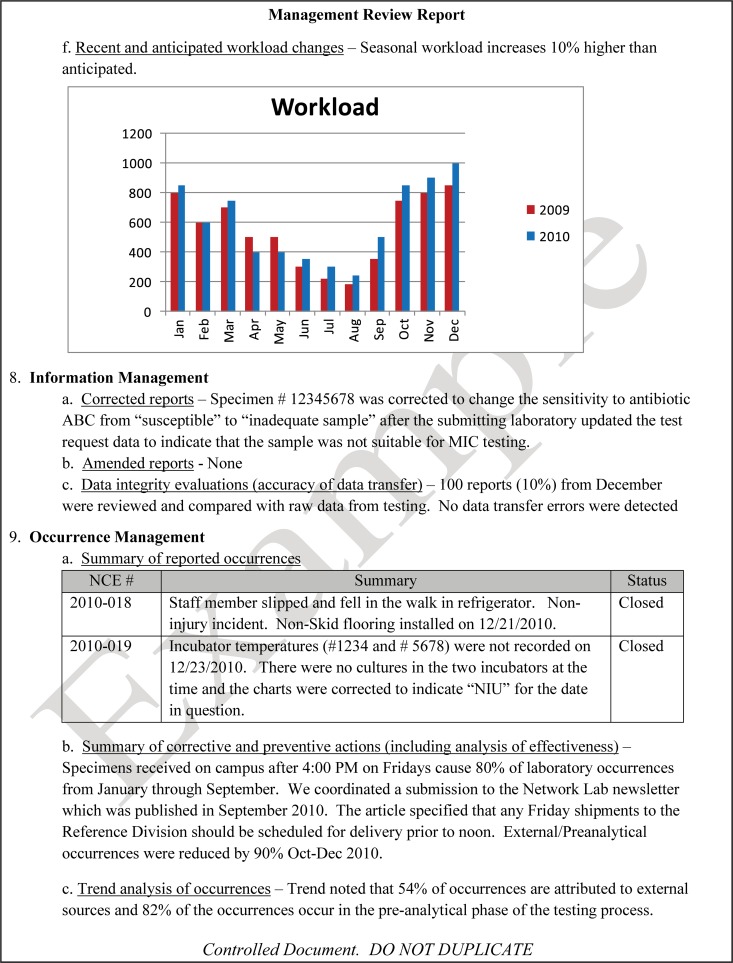
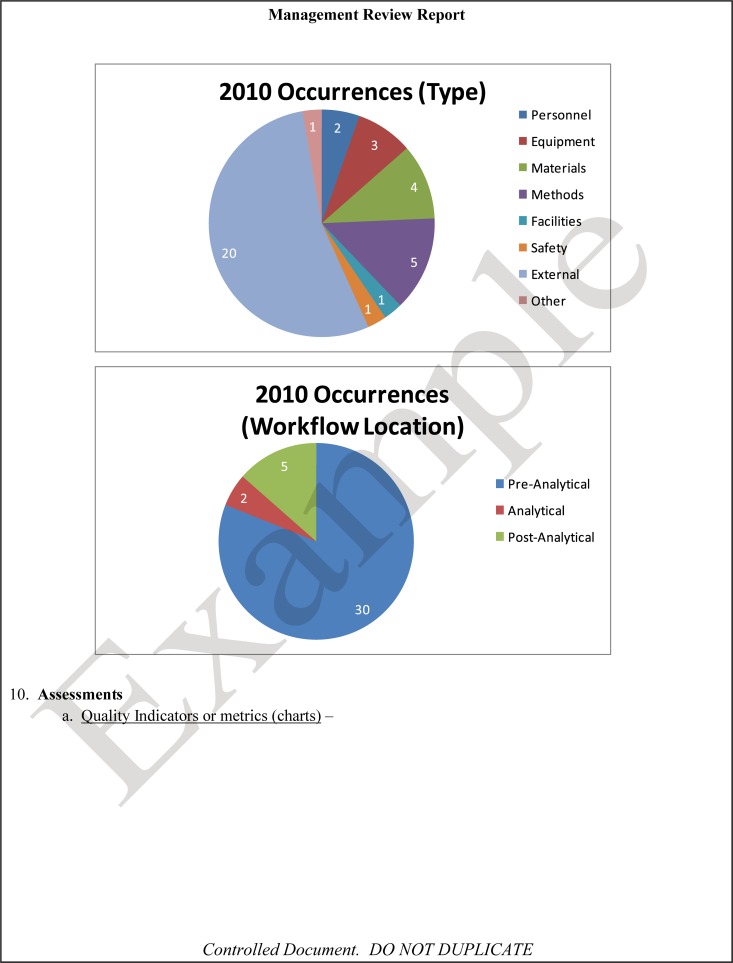
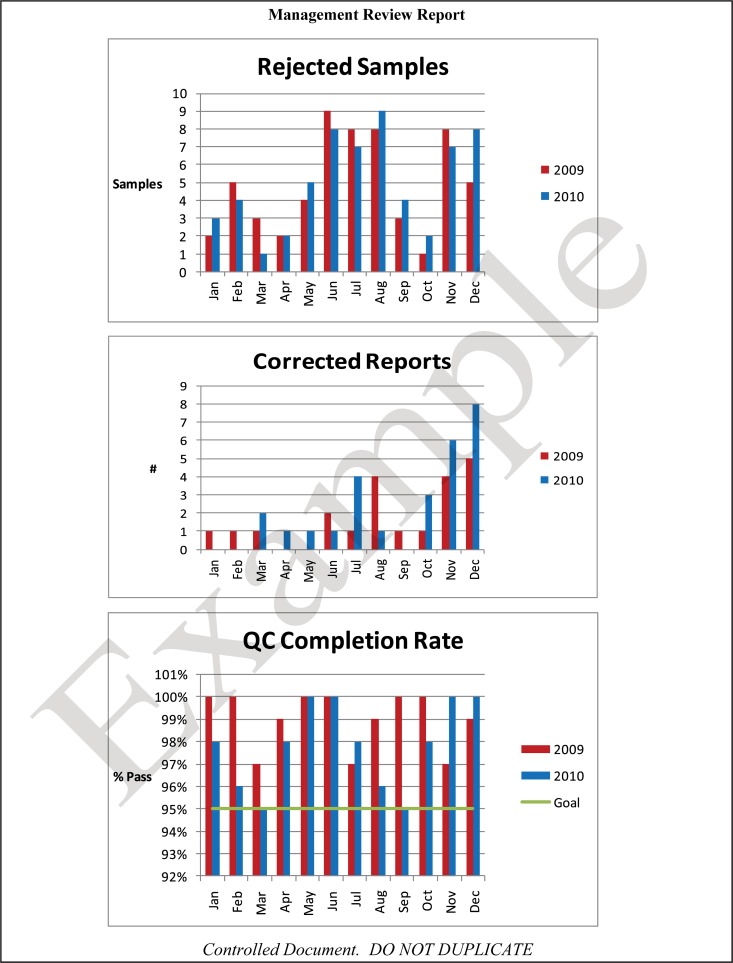
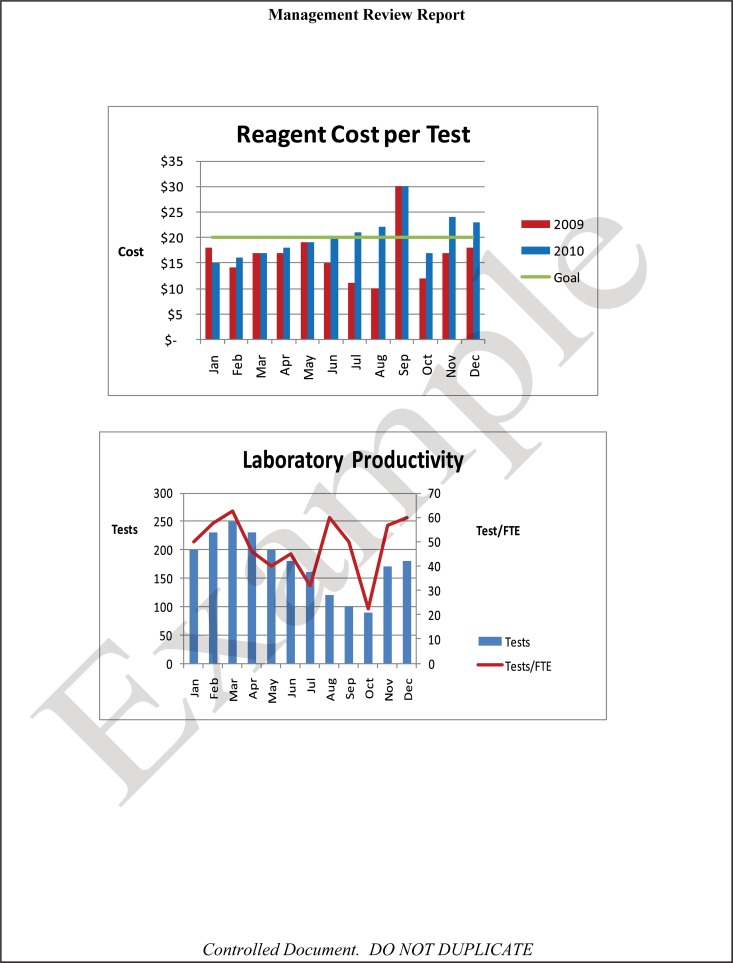
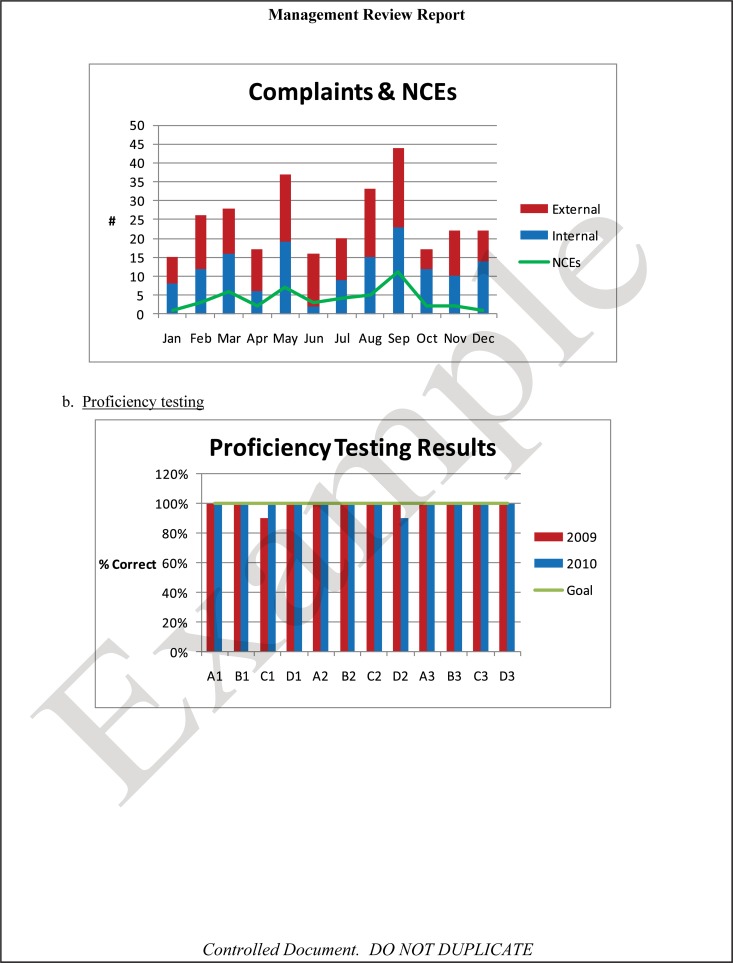
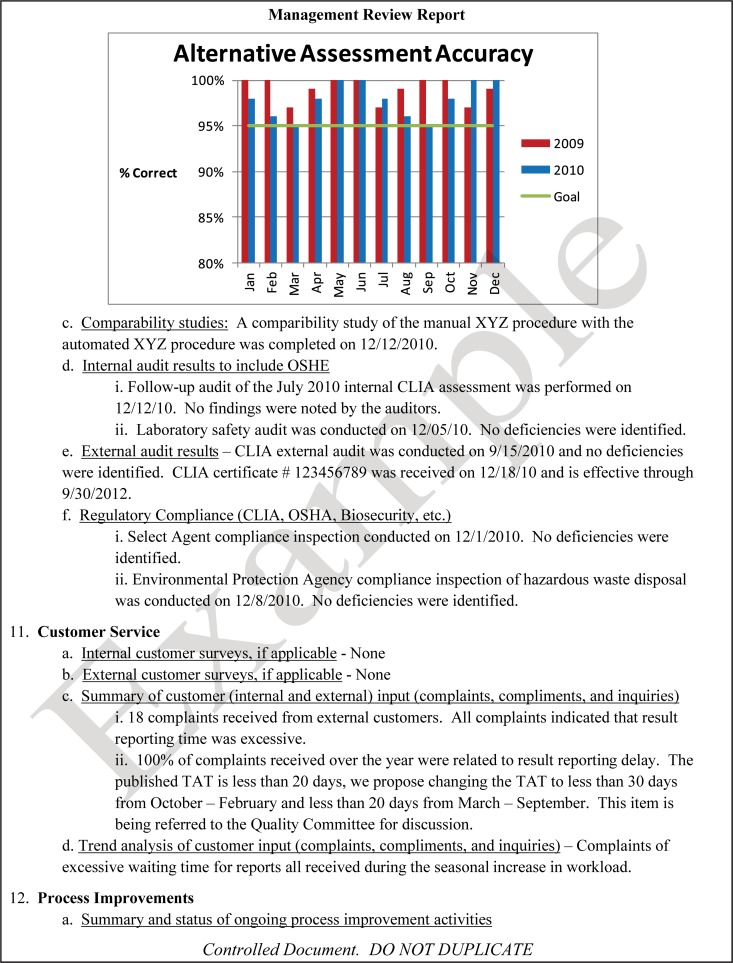
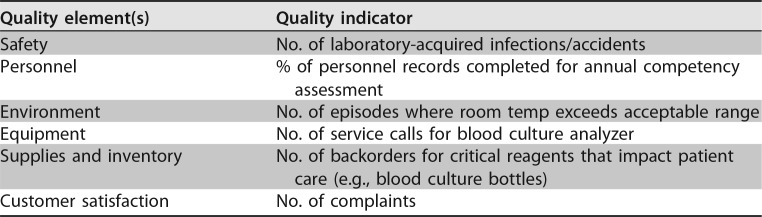
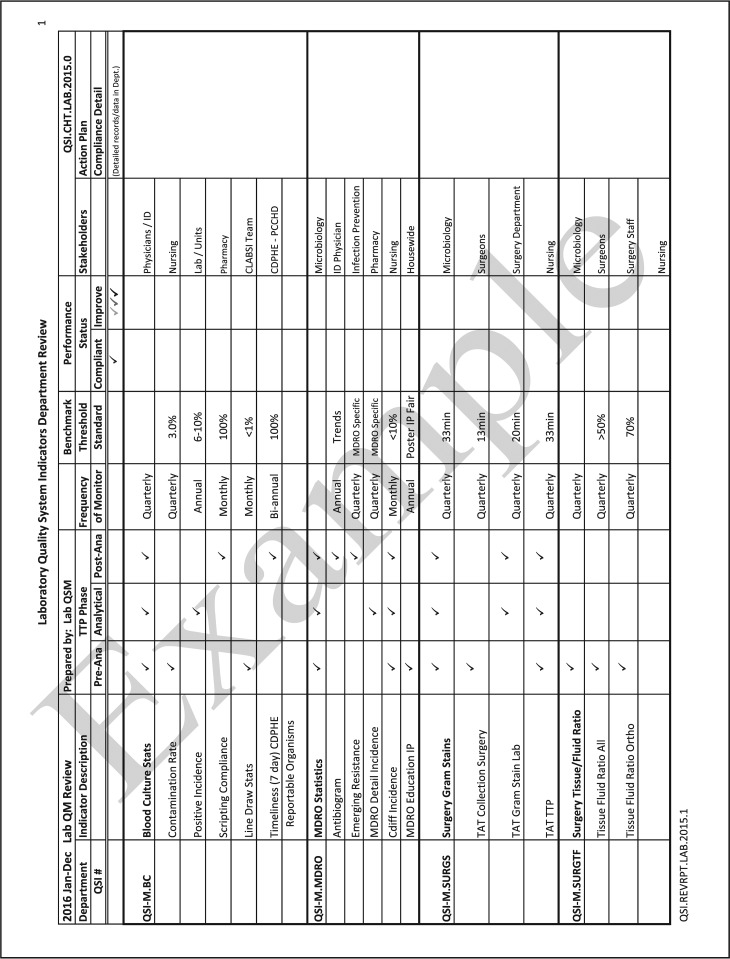
The quality and appropriateness of the laboratory's contribution to patient care should be objectively evaluated. Management review should include a review of the quality policy and quality goals and objectives annually. Individual laboratory sections, such as microbiology, serology, and molecular pathology, etc., should collect data throughout the year, which can be consolidated into an overarching report. As data are filtered upward, less detail is required for higher-level review; for example, documentation of contaminated purchased media at the technical level may contain details of the manufacturer, lot numbers, and types of contaminants, while the report to the vice president of laboratory services may have data on the impact on budget and patient services. All quality system elements should be covered in the annual review; however, data do not have to be collected from each area every month or quarter. Wherever possible, data should be presented graphically to track and analyze patterns and trends to highlight activities that require corrective or preventive action. A visual dashboard may be created to highlight specific issues for process improvement. A sample management review report, shown in Fig. 2, outlines the quality system elements to include and graphic presentations of data that allow performance comparisons over time and across laboratory sections.
FIG 2.
Sample monthly management review report for the microbiology laboratory. The report is meant to highlight trends and changes in each quality area. It is intended to be visual and not contain unnecessary explanatory text. FTEs, full-time employees; N/A, not applicable; TAT, turnaround time.
All recommendations of the management review committee and follow-up actions shall be documented in writing, which serves as a formal record of review. Evaluation of the follow-up actions shall be performed in subsequent reviews to ensure that actions have addressed the problem as intended. The recommendations and action items from the management review should be shared with the laboratory staff so that they are aware of improvement initiatives and how they may contribute to the improvement process. Examples of topics where information may be collected and shared in a report for management review are shown in Table 4. Suggested roles and responsibilities across the organization and the microbiology laboratory for management review are outlined in Table 5.
TABLE 4.
Some suggested topics for management review and evaluation
| Topic area | Item(s) for evaluation |
|---|---|
| Changes in technical or procedural process | Addition or deletion of tests |
| Changes in scope of work or workload | |
| New regulatory requirements for technical procedures | |
| Purchasing and inventory | Problems with suppliers of critical materials |
| Equipment | Equipment failures or need for preventive maintenance contracts |
| Customer feedback | Customer surveys |
| Staff suggestions | |
| Monitoring and resolving complaints | |
| Nonconforming events | Analysis of nonconformities and near misses |
| Personnel | Competency assessments completed |
| Vacancies or scheduling issues | |
| Evaluations and audit reports | Performance in proficiency testing events |
| Inspections by external organizations | |
| Reports from internal audits | |
| Risk management and safety reviews | |
| Overarching laboratory systems | Follow-up actions from previous management reviews |
| Quality indicators being monitored | |
| Results of continuous improvement efforts, including the status of corrective and preventive actions |
TABLE 5.
Roles and responsibilities for management review
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Determine quality topics and timeline for management review | Senior management decides what topics and level of detail should be presented | Suggest key areas important for management review | Provide suggestions to laboratory management |
| Responsible for scheduling planned meetings to review reports | |||
| Collect and analyze data for reports | Decide presentation format that would be most beneficial to capture data | Collate monthly data into comprehensive data that can be presented graphically over time | Assist in the completion of records that will be used to document quality issues |
| Conduct periodic management review | Hold the meeting as scheduled | Attend management review meeting | Understand what process improvements are in progress |
| Record minutes of the meeting, including recommendations made and follow-up actions Monitor the effectiveness of action plans for their efficacy and revise plans as needed |
Share outcome of management review meeting with laboratory staff Complete follow-up actions as requested Monitor the effectiveness of recommendations that pertain to the microbiology laboratory |
||
Customer Focus—Advisory Services
The microbiology laboratory provides the content expertise and leadership in infectious disease diagnosis, pathogen discovery, antibiogram, biosafety, and biosecurity areas. As such, the microbiology laboratory partners with any discipline or person in the health care system that requires their expertise to address current and changing needs. The microbiology laboratory is responsible for advising on the total testing process, including the preanalytical process (e.g., advising on the appropriate test for accurate diagnosis and providing instruction on ordering and collecting the optimal specimen), the analytical process (e.g., selecting the optimum methods and technologies used), and the postanalytical process (e.g., reporting final results and providing interpretations and data analyses). Clear and timely communication of this information is essential for successful consultation in microbiology. Miscommunication or inadequate communication is often at the heart of errors. Therefore, it is paramount for the microbiology laboratory to develop effective communication strategies to avoid misinterpretations that may compromise patient care.
The microbiology laboratory shall establish how to communicate its advice to users and have the input of its stakeholders (e.g., infection prevention, pharmacy, and infectious diseases) for the message that is being delivered. Communication may be in a written or electronic format to provide guidance, such as the names of tests performed that are clearly linked to reimbursement codes, a periodic antibiogram, specimen collection instructions, interpretive comments on patient reports, or alerts on antimicrobial resistance or notification of circulating influenza viruses. Timely communication may also be verbal, such as a phone call to the physician for a critical test result. In-person teleconferences on novel pathogens or staff in-services when a new blood culture instrument is instituted are also good forms of communication. Where appropriate, communications should be saved, archived, and readily available for future reference and can be used as evidence of advisory services for audit purposes.
In order to contact the laboratory for advice, key information should include the hours of operation of the microbiology laboratory and contact information during and after normal hours of operation. This may require creating and disseminating on-call schedules for accessing medical/scientific consultation and logistic or laboratory operations advisory services.
In addition to the advice provided directly to individuals, the microbiology laboratory should play a meaningful role in the planning and development of new services and facilities as well as strategic planning for the organization. Laboratory leadership together with the organization's leadership should collaborate to align the laboratory's vision with the needs of the patients, their health care providers, and the organization. Microbiology is currently undergoing a revolution in testing technologies and instrumentation that needs to be communicated effectively to the organization in order to secure the resources to implement these new changes. Poor communication could result in the failure to meet current standards of care if the organization's leadership is not kept abreast of new advancements in diagnostics. Similarly, to maintain high standards of care, the expertise of microbiologists is a great resource to inform policies and decisions regarding emerging infectious diseases, biosafety and biosecurity in all laboratories, infection prevention and control across the organization, antimicrobial stewardship programs, and other institutional programs. Table 6 outlines specific advisory services that shall be addressed by the microbiology laboratory and how these services may be shared or delegated.
TABLE 6.
Roles and responsibilities for advisory services and consultation
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Advising on selection of tests and services | May provide resource support for the creation and accessibility of advisory services | Create the test menu and counsel on choice and use of services | Follow and enforce the policies and procedures that govern the criteria for appropriate choice and use of microbiology services |
| Write, promote, and enforce the policies and procedures that govern the criteria for appropriate choice and use of microbiology services | |||
| Advising on clinical cases for treatment or infection control | None | Consult on individual cases | Provide initial advice on testing based on policies and procedures |
| Facilitate contacting the appropriate personnel to respond to request for advice | |||
| Advise infection preventionists | |||
| Professional judgments on the interpretation of results | None | Counsel on the interpretation of results | Provide initial interpretations based on policies and procedures |
| Promoting effective utilization of laboratory services | Support and promote the effective utilization of laboratory services across the institution | Select, create, promote, and enforce policies and procedures that govern effective utilization of laboratory services | Identify, follow, and enforce laboratory utilization policies and procedures |
| Consulting on scientific and logistic matters | None | Counsel on clinical and technical matters and operational logistic matters | Provide initial advice based on standard operating policies and procedures |
| Strategic planning | Collaborate with laboratory leadership to consider patients, health care providers, and the organization in creating new directions | Collaborate with organization leadership to consider patients, health care providers, and the microbiology laboratory in creating new directions | Follow the new directions |
Personnel
The laboratory's human resources are its most valuable asset. Therefore, the tasks of hiring, orienting, training, and managing staff within a supportive working environment are critical for maintaining high-quality work.
As part of the hiring process, the institution shall provide clear job descriptions that specify the desired qualifications and competencies. In some jurisdictions, national or regional requirements, as defined by the U.S. Centers for Medicare and Medicaid CLIA laboratory program (5) or the Canadian body, the Institute for Quality Management in Healthcare (IQMH) (8), specify qualifications and competencies for laboratory directors, supervisors, and clinical consultants that comprise microbiology laboratory management as well as the microbiology laboratory staff who are the testing personnel. Requirements may include licensure for personnel who perform laboratory testing and oversight. The job description should detail the skills, knowledge, behaviors or attitudes, and experience that are required as well as the level of authority of the position and the chain of command (9). The description should be specific and comprehensive so that there is no ambiguity in the expectations of employment. This ensures that all tasks in the laboratory are completed by the appropriate personnel (10).
Orientation and training.
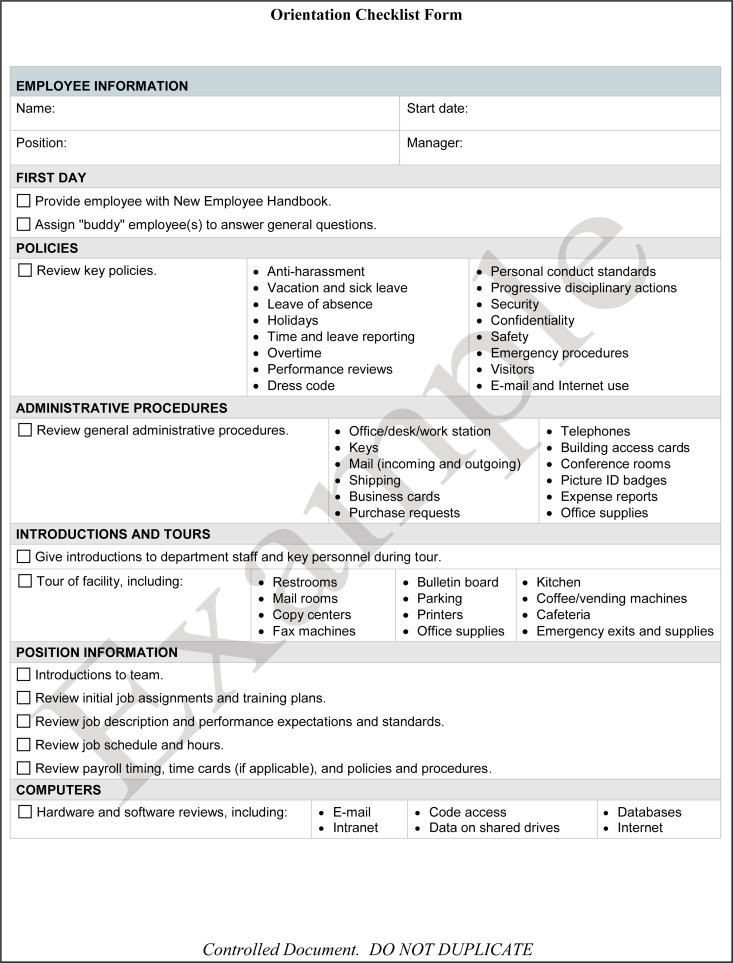
Laboratory management shall provide new staff members orientation to the organization. This includes familiarizing new staff with personnel policies (e.g., dress code, ethics, compensation, and vacation and sick days), all relevant staff facilities (e.g., personal lockers and lounges), biosafety risks, occupational health and safety requirements (e.g., workplace hazardous materials information system [WHMIS], safety data sheets [SDSs], and fire and emergency), and occupational health services. The World Health Organization (WHO) orientation checklist (11) in Fig. 3 is an example of items that should be included in the initial personnel orientation.
FIG 3.
World Health Organization new personnel orientation checklist.
An effective training program equips new staff with the knowledge, skills, and attitude required to meet the job description expectations. Currently, many microbiology laboratories are undergoing technical evolution, and new equipment, assays, and workflow processes are being introduced. Ongoing training of personnel is critical for retaining high-quality outcomes in an ever-changing laboratory environment. The training program shall be planned, documented, and evaluated periodically for its effectiveness and revised based on the feedback and needs of the staff to meet institutional and regulatory requirements. Direct observation is essential to ensure competency for high-quality independent work, especially for tasks that have a high impact on patient outcomes. Training programs should be inclusive of the specific tasks and duties reflected in the job description. Additional areas of training should include ethics, patient privacy, information technology systems, biosafety and biosecurity, occupational health and safety, as well as the QMS of the organization.
Competency assessment.
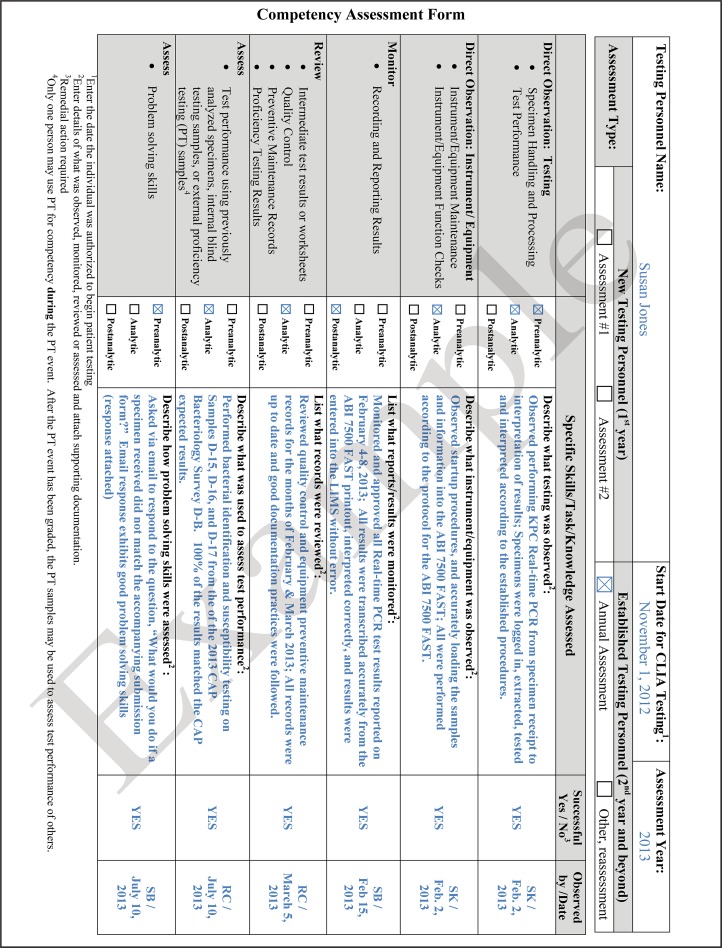
Competency testing of all technical and managerial staff shall be conducted and documented after training at the time of hiring and regularly thereafter. The frequency of competency assessments will depend on the skill or task and may be dictated by the laboratory's regulatory or accrediting body; e.g., the CLIA requires annual competency testing for personnel. The evaluations shall be objective and consistent to eliminate any assessment bias between employees. Laboratories should assess their technical staff throughout the year for the tests that they may perform. Suggested types of examinations are written or multiple-choice tests, direct observations, review of records, or testing of blind samples. The assessments should be diverse and address the knowledge, skills, or attitude that is expected of the staff member. Examples of technical skills to be assessed include the interpretation of Gram-stained smears or performance of antimicrobial susceptibility testing. Problem-solving skills could also be assessed, for example, by requesting staff to write an approach for troubleshooting a freezer or incubator whose temperature is out of range. Other types of monitoring may include a review of the records of equipment maintenance or direct observation of equipment function checks. Any observed deficiencies should be addressed, with appropriate retraining as necessary, to avoid any adverse patient care outcomes. Deficiencies may be used as the basis for continuous quality improvement (12). Competency guidelines for public health laboratory professionals have been published and may be used for those in the clinical laboratory as well (13). An example of a competency assessment form for laboratory personnel is shown in Fig. 4. The form can be duplicated to document competency assessment for multiple specialty areas where the staff is authorized to perform patient testing.
FIG 4.
Sample of a competency assessment form that may be used to meet Clinical Laboratory Improvement Amendment (CLIA) personnel requirements.
Performance reviews conducted by laboratory management or human resources shall be performed regularly, at least annually, to complement the competency assessments. They should address both the individual's and the laboratory's needs and expectations in order to maintain a healthy working environment and high-quality service. If issues arise in the employee's performance or behavior, they should be addressed in a timely manner as appropriate, and discussion and documentation, if required, should not be delayed until the annual review. Feedback from personnel should be encouraged regularly during laboratory meetings and one-on-one informal exchanges or by having an open-door policy and not just during annual reviews. Addressing staff concerns and desires as they arise can help to motivate and retain personnel and avoid high personnel turnover rates. Personnel retention is desirable to maintain continuity, efficiency, and expertise.
The employer shall provide opportunities for every staff member to enhance their supervisory and/or technical skills, and staff shall participate in professional-development or continuing-education programs. These activities can take many forms based on the needs and resources available. Examples include attending local microbiology journal clubs, webinars, or conferences and participating in interactive online courses, invited lectures, and study groups. To determine their effectiveness, these programs shall be reviewed routinely and revised accordingly. Continuing staff development, periodic assessments, and program evaluations may be achieved by (i) monitoring the percentage or number of staff participating, (ii) assessing participants' knowledge improvement with a posttest, (iii) monitoring the level of satisfaction with the professional-development or continuing-education program, and (iv) changing laboratory practices in response to continuing education, such as updating the breakpoints for antimicrobial agents. The latter two may be captured in course evaluations that specifically ask about the participant's satisfaction and how likely the course would change their practice.
Maintaining up-to-date personnel records is a shared responsibility between the staff, who provide the required documentation, and management, who record and maintain the records with the highest confidentiality and storage security. Electronic document control systems provide an efficient and secure method to maintain up-to-date records that are accessible to the required parties. An electronic system may reduce the duplication of documents that may otherwise be housed in several areas and permit the efficient storage of archived records. Personnel roles and responsibilities should include, but are not limited to, those listed in Table 7. Microbiology laboratory management should contribute to the development of these relevant documents for all microbiology personnel. Table 7 outlines possible activities that could contribute to the development and oversight of personnel records for microbiology laboratory staff. The medical and scientific staff also require similar documents and records for their jobs as well; however, this usually falls outside the scope of microbiology management and resides with human resources and/or other institutional administrators. Records of professional-development activities, competency, and licensure are fundamental requirements of the professional licensing and regulatory bodies for medical or scientific staff. These records shall be maintained up to date as proof of competency. Each institution should decide with whom the responsibility resides.
TABLE 7.
Roles and responsibilities for personnel
| Document(s) and/or activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Educational and professional qualifications | Request and maintain up-to-date records | Request and maintain up-to-date records | Provide proof of education and professional qualifications |
| Request and maintain copies of transcript or diploma | |||
| Copy of certification or license | Maintain up-to-date records for each staff member | Request and maintain copies from each staff member and acknowledge receipt | Provide copy and record of annual renewal |
| Previous work experience | Request and maintain records at the time of hiring | Request and maintain records of previous work experience at the time of hiring | Provide documentation of work experience |
| Job description | Establish job description standards | Contribute to the creation of microbiology job descriptions | Perform job responsibilities as described |
| Assign job descriptions to each staff member | |||
| Introduction of new staff to the laboratory environment | Create and maintain records of orientation to the institution | Develop the orientation program for the microbiology laboratory | Participate in the orientation of new laboratory staff |
| Orient new staff to the microbiology laboratory (e.g., checklist orientation) | |||
| Maintain records of microbiology orientation | |||
| Evaluate effectiveness of the orientation plan | |||
| Training in current job tasks | Review assessments as needed | Contribute to the development of the training program | Competently complete training in current job tasks |
| Maintain records of training | |||
| Create and update training checklist for each job task | |||
| Evaluate its effectiveness | |||
| Competency assessments | Review assessments as needed | Create competency assessments for microbiology personnel | Complete competency assessments for assigned job |
| Evaluate testing personnel for their competency for their assigned job tasks in the laboratory | Retrain where weaknesses are noted | ||
| Maintain records of competency assessments | |||
| Records of continuing education and achievements | Review records as needed Provide resources for continuing-education opportunities for employees |
Facilitate educational activities and achievements Maintain records of continuing education and achievements Establish personnel competencies where continuing education and training may be appropriate |
Identify gaps and interests in microbiology Request continuing professional and education development program activities Provide documentation of achievements Evaluate effectiveness of continuing-education programs |
| Reviews of staff performance | Maintain documentation of staff performance | Conduct staff performance reviews | Participate in peer or manager performance review as applicable |
| Maintain documentation of staff performance | |||
| Reports of accidents and exposure to occupational hazards | Maintain individual records of accident and exposure for human resources and/or occupational health | Maintain records of accidents and exposures | Report any incidents or exposures to microbiology laboratory management and occupational health |
| Complete an incident report for any laboratory accident or exposure | |||
| Track accidents or exposures and retrain employees where applicable | |||
| Immunization status, relevant to assigned duties | Maintain individual records in human resources and/or occupational health records | Ensure that laboratory staff are aware of pertinent vaccines, such as Neisseria meningitidis or hepatitis B vaccines | Maintain immunization status per institutional policies |
| Require evidence of vaccination where applicable to the task | |||
Facilities and Safety
Managing the facilities.
The laboratory's sample collection and testing sites, as well as point-of-care testing locations, shall have sufficient space to perform quality work and ensure patient, staff, and visitor safety. The laboratory shall evaluate and determine the adequacy of the space for both equipment and personnel. Special attention should be paid to the provision of a safe environment. Although specific safety standards are not included in ISO 15189, they are specified in the related ISO 15190 document (14). Safety must be an integral part of a good quality management system. A full discussion of biosafety levels, personal protective equipment, and facility and engineering controls is beyond the scope of this document. There are several excellent reference documents for consideration (15–17).
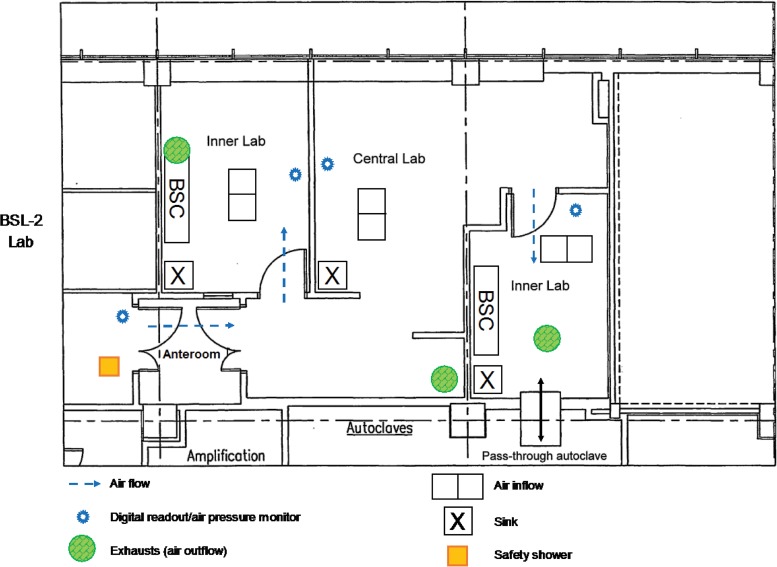
The laboratory shall have controlled access, with the level of access being based upon biological, chemical, and radiation hazards; biosecurity; and confidentiality. The levels of access can vary from space to space within the laboratory and should be restricted in areas where testing is performed at biosafety level 2 (BSL-2) or higher. In addition, signs identifying the hazards within should be posted at the entrance to and exit from the laboratory. Entrance signs for microbiology laboratories should identify the biohazard, and contact information shall be displayed in case of emergency. See Fig. 5 for an example of appropriate signage for a laboratory door. Access controls, such as key pads or double-door, locked anterooms, provide limited access to laboratories operating at BSL-3, such as mycobacteriology laboratories (16). See Fig. 6 for an example of a BSL-3 laboratory suite floor plan.
FIG 5.
Example of a door sign for a microbiology laboratory, indicating the level of biosafety, proper work practices, and type of personal protective equipment (PPE) required.
FIG 6.
Sample floor plan for a biosafety level 3 laboratory suite located within a larger BSL-2 central laboratory. The two inner BSL-3 laboratories are separate rooms with negative air flow to contain microorganisms or their molecular components. BSC, biological safety cabinet.
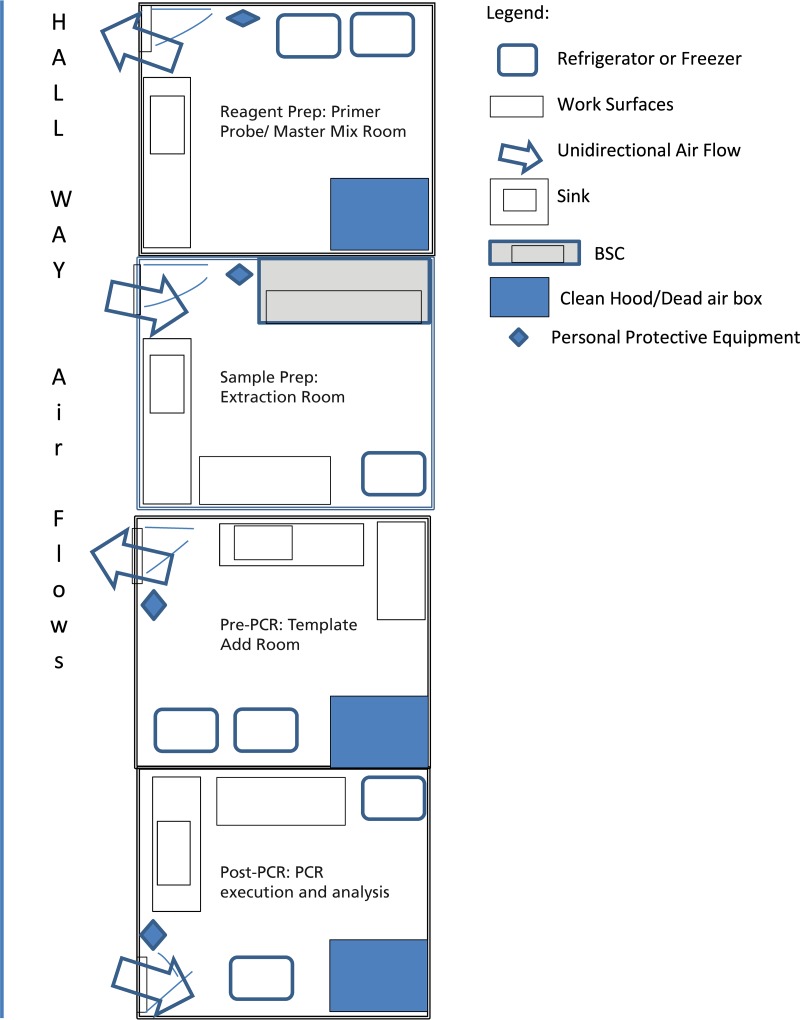
Of special note, laboratories performing molecular testing using a platform where reactions occur in an open system should limit access not only for biosafety purposes but also for decreasing the likelihood of amplicon contamination. Amplicon contamination is reduced through the use of separate work areas; engineering controls, such as dead-air boxes and unidirectional workflow; as well as stringent chemical and/or enzymatic decontamination. See Fig. 7 for a sample floor plan for molecular testing with unidirectional airflow. Although commercially available, molecular testing may be performed in a closed system; concern for contamination is still paramount throughout the testing process. Additional guidance for molecular testing in clinical laboratory environments has been reported (18).
FIG 7.
Sample floor plan for a molecular laboratory to ensure the segregation of specimens and high-amplicon material from clean reagents and PCR tubes. BSC, biological safety cabinet.
The laboratory environment shall be in compliance with federal, state, and local building codes. All areas and surfaces in the laboratory, including work benchtops, floors, ceilings, and walls, shall be clean and well maintained. Ideally, these surfaces are nonporous in order to be easily decontaminated. Laboratories shall never be carpeted in areas where testing is performed. The work environment, both the laboratory and office space, shall be ergonomically designed to prevent the occurrence of work-related musculoskeletal distress. In addition, there should be adequate separation of spaces between incompatible work environments. For example, biosafety cabinets (BSCs) should be located away from traffic flow and air ventilation systems in order to maintain proper function, and areas with potential amplicon contamination should be separate from molecular reagent and master mix preparation areas. Dedicated sinks for reagent preparation and equipment cleaning shall be available, and separate dedicated hand-washing sinks shall be hands-free and not utilized for other purposes. Additional environmental conditions that may affect the quality of the results that shall be monitored, recorded, and adjusted accordingly and include lighting, noise, vibrations, dust levels, fumes, radiation, electrical supply, temperature, and humidity.
In addition to the environment, the facility shall have adequate infrastructure for the tasks performed. Water of the type required for patient testing that meets the standards for clinical laboratory reagent water (CLRW) shall be available (19). Some laboratory procedures (e.g., molecular testing and virus culture media) require water of a purity higher than CLRW. Manufacturer specifications for the quality of water shall be followed. Electrical power with sufficient numbers of regular and emergency-backup outlets for the size and complexity of the laboratory shall be available. Multiple outlet adaptors and the use of extension cords should be limited to emergency situations only. Consideration of which critical instrumentation requires connection to emergency power shall be established; for example, automated blood culture instruments should be connected to emergency power.
Laboratory ventilation shall be monitored and adjusted to meet the air exchange, humidity, and temperature requirements of all areas. Ventilation can be affected by the configuration of the room, the location of heat-generating equipment (e.g., blood culture instruments) and ventilation equipment (e.g., biosafety cabinets and chemical fume hoods), and traffic patterns. Whenever possible, airflow should be in an inward direction from corridors into the laboratory space. Negative air pressure with adequate frequent air exchanges shall be used for mycobacteriology, mycology, and virology laboratories. In general, the acceptable frequency of air exchanges is between 3 and 15 air changes per h for BSL-2, which is generally more frequent in areas with higher concentrations of chemicals or hazards, such as mycobacteriology laboratories. Gas and vacuum lines should be available as applicable and clearly marked. Gas cylinders shall be secured in an upright fashion at all times, preferably mounted onto a solid surface, such as a wall, and placed away from flammable material. Valve safety covers shall be in place when valves are not in use.
An adequate storage facility of the appropriate size, temperature, and humidity to maintain the integrity of reagents, samples, equipment, supplies, documents, and records shall be available. Equipment and reagents may have manufacturer-specified temperature and humidity requirements that must be met. The temperature must be maintained under the required conditions 24 h per day and not altered for energy savings during off-peak hours. Sample storage space should be chosen to eliminate the potential for cross-contamination.
Safety programs.
The laboratory shall develop and implement a comprehensive safety program. The program typically includes biosafety, biosecurity, blood-borne pathogens, the transportation of dangerous goods, and the use of personnel protective equipment. A laboratory safety program shall also address fire safety; chemical hygiene; safe practices associated with electrical hazards, radiation, and hazardous waste; accessibility to first aid; and ergonomics.
The safety program shall be based upon workplace regulatory requirements and a thorough risk assessment. Safety training that should include the direct observation of safety practices shall be provided and documented as part of staff competency. Laboratory personnel shall follow guidelines for biosafety laboratory compliance (20), and management shall provide the resources to protect staff from occupationally acquired infections (21). To ensure compliance with the safety program, all facility administrative levels shall be involved, as outlined in Table 8.
TABLE 8.
Roles and responsibilities for facilities and safety
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Controlled access to laboratory | Provide engineering controls to limit access | Encourage limited access | Monitor nonlaboratory personnel |
| Adequate work environment | Provide work environment with clean, well-maintained floor, ceiling, and walls and adequate lighting, water, ventilation, power, and communication | Advise as to accommodations and environmental-condition requirements | Inform when accommodations and environmental conditions are unsatisfactory or unsafe |
| Report accidents, incidents, or near misses | |||
| Monitor environmental conditions | |||
| Safety program | Provide resources and oversight for institutional safety program | Perform a risk assessment | Comply with safety program |
| Designate biosafety and safety officer(s) | Advise of unsafe conditions | ||
| Develop a comprehensive safety program to meet all requirements | Report accidents, incidents, or near misses | ||
| Adequate safety facility and supplies | Provide adequate safety supplies in close proximity | Be aware of and advise as to safety requirements and appropriate engineering controls | Utilize appropriate personal protective equipment and engineering controls |
| Provide appropriate safe engineering controls | Inform when supplies or engineering controls are unsafe, inadequate, or unusable | ||
| Report accidents or incidents | |||
| Occupational health | Provide occupational health services adequate for level of services provided | Advise as to necessity for baseline immunity testing and provide vaccinations | Report any exposures Undergo monitoring for exposures (e.g., status of immunity, chest radiograph, or skin testing or interferon gamma release assay for tuberculosis) |
| Monitor for exposures and recommend prophylaxis or treatment as required | |||
| Receive vaccinations, prophylaxis, or treatments as recommended | |||
Safety supplies and storage shall be adequate and in close proximity to use. The safety supplies required are dependent upon the biosafety level of the laboratory as well as the chemical hazards present. Safety supplies are not limited to personal protective equipment but also include engineering controls, such as biosafety cabinets, centrifuges with safety cups and rotors with covers, pipetting aids, splash guards, and safe sharps containers. Biological and chemical spill kits shall be easily accessible in the respective areas and marked appropriately. Spill drills shall be conducted by the responsible safety official, and staff shall be knowledgeable of such practices. The performance of safety equipment (e.g., biosafety cabinets, showers, and eye washes) shall be monitored and recorded regularly. Occupational health services shall be available to staff for any hazardous workplace exposure. In particular, occupational health services shall monitor for and respond to laboratory-acquired infections and provide preventative vaccines where applicable, such as a hepatitis B vaccine to those handling human blood specimens.
Purchasing and Inventory
To ensure the use of only high-quality services and supplies, a multifaceted approach is required. Policies and procedures shall be in place in the laboratory for the selection and purchasing of services and supplies that affect the quality of the tests and/or calibrations. These quality requirements shall be detailed in standard operating procedures (SOPs), typically located under the “materials required” section of each procedure, and identify the appropriate minimum specifications when necessary. The laboratory shall maintain records on the experience with a product or vendor so that selection or rejection is based on data. Problems detected by staff shall be reported to laboratory supervisors and monitored or documented as a nonconforming event. Issues should be discussed with the vendor, and unresolved issues should be reported to the purchasing agents. The purchasing of external services and supplies is a collaborative effort between the institution's administration and the laboratory. The laboratory director should have final approval on all purchases and customarily designates laboratory staff to perform the processes. Examples of purchased external services include referral laboratory services, equipment maintenance, BSC certification, or off-site waste disposal.
Services agreements are used to document a transaction where the provider of a service performs the service for a user. Examples of professional services agreements that may be appropriate for the laboratory as a user are equipment maintenance, reference testing, pathology consulting, consultant management, and facilities management or services. The provider of the services may be internal to the user, such as facilities management, or external, such as an equipment manufacturer providing an annual service contract. Agreements to provide medical laboratory services are an essential component of a quality management system. The laboratory shall ensure that the service providers are able to perform the requested services and that they have sufficient resources to provide the services before entering into the agreement. A service-level agreement (SLA) is a contract between a service provider and the laboratory that defines the level of service expected from the service provider and the individual and shared responsibilities of each party. Examples of items covered in a SLA are security services for the laboratory, occupational health services for laboratory staff, housekeeping services, medical waste management, and preventive and scheduled maintenance services. Entering into a SLA takes a team approach to ensure that the level of service is met. Table 9 lists the functions of various members of the laboratory team to establish service agreements.
TABLE 9.
Roles and responsibilities for service agreements
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Determine the need for service agreements | Provide the method to enter into a service agreement | Review operations to determine what service agreements are needed | Provide data to drive the decision for service agreements |
| Entering into service agreements | Maintain the system that allows procurement of service agreements and good relationships with providers | Work with business analyst to facilitate development of appropriate service agreements | Maintain detailed records of services provided |
| Identify expense budget for providing the services to ensure continuation of service agreements across fiscal years and budget periods, ensuring uninterrupted service coverage | |||
| Identify requirements to be provided by the service agreement | |||
| Monitoring service agreements | Ensure that service agreements are kept current, renewed, or cancelled | Review the service agreement to make certain that terms are met | Understand and adhere to the terms of the service agreement |
| Analyze data on the performance of the service agreement | |||
| Monitor the performance of the service agreement | |||
Contracting for referral laboratory services.
Because of the wide array of tests required and available for patient care, performing all requested tests in the institutional laboratory is unfeasible. A referral laboratory or reference laboratory is normally a large laboratory that performs testing not ordinarily found in a clinical laboratory. Examples of referral laboratories include commercial reference laboratories; academic medical centers; and national, state, local, or provincial public health laboratories. It is essential for every institution to have a contractual relationship with a referral laboratory, and rigorous analysis is needed before the selection of the referral laboratory is made. The laboratory director in consultation with the medical staff should determine which tests are referred and which tests are performed at the institution, also known as in-house testing. Institutions often use more than one referral laboratory. The selection of the referral laboratory is an important process that should be led by the laboratory director in collaboration with management.
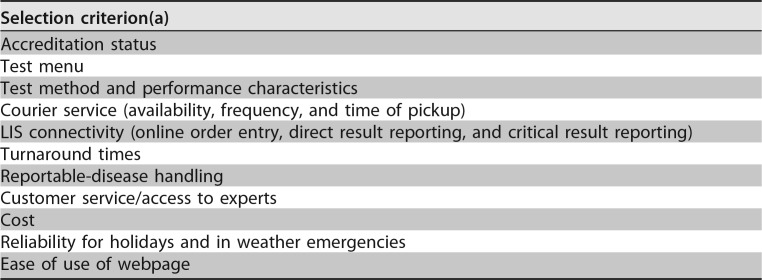
The steps in the selection process may differ for each institution, but the process should start with the development of selection criteria, as outlined in Table 10. Microbiology management is integral for the preparation of the selection criteria. The selection of a reference laboratory is based on the desired services and performance standards required. Foremost in the criteria is the requirement that the referral laboratory meet accreditation standards. Laboratory management may write a request for information (RFI) or a request for proposal (RFP), to which potential referral laboratories respond in writing to bid for the contract. After the RFI or RFP is submitted, management compares the criteria to the responses of the referral laboratory candidates, analyzes and weighs the responses, and recommends the referral laboratory. While cost is a consideration, cost cannot be the only driver of the decision. The laboratory director should have the final word on the selection of a referral laboratory since that person is knowledgeable of the regulatory testing and reporting requirements that need to be met. The laboratory director shall also ensure that the selected laboratory meets all the accreditation qualifications and the required established criteria before the institution engages in a contractual relationship with the referral laboratory.
TABLE 10.
Elements of selection criteria for assessing referral laboratories
Laboratory management has the responsibility of establishing written procedures defining how to send specimens to the selected referral laboratory, and the laboratory director shall approve these procedures. Specimens shall be appropriately packaged for shipping. Requests for testing shall have the appropriate documentation accompanying the specimens. Staff shall be adequately trained on packaging, shipping, and order entry. Management shall monitor that these procedures are performed correctly and consistently so that test results are not compromised by sending specimens to an outside laboratory.
The referring laboratory shall ensure that the results reported are exactly as intended by the laboratory performing the testing. The results shall include any comments and interpretation that the reporting laboratory intended to be part of the report. While electronic reporting through laboratory information system (LIS) connectivity is preferred, manual reporting or reentry of results into the institution's LIS is acceptable if all results, comments, and interpretations are included. It is the responsibility of the institution to ensure that procedures for reportable diseases and requirements for the submission of specific pathogens for public health purposes, according to state code, are followed by the referral laboratory. Laboratory management shall communicate with the referral laboratory regarding whose responsibility it is to notify public health authorities of reportable information. The laboratory director shall ensure that a reliable system is in place and working as expected.
After the referral laboratory contract is established, the institution shall establish the performance monitoring process. This process should be incorporated into the laboratory's quality management system. The contracting institution should request and maintain a current copy of the accreditation documentation for the referral laboratory. Reports from the referral laboratory should be reviewed for accuracy of transmission. The performance of the referral laboratory should be monitored for several performance parameters, as defined in Table 11. Problems encountered with the referral laboratory should be well documented for tracking purposes. The laboratory management and technical staff are responsible for supplying accurate data, and the laboratory director should review the performance data and review cost benefits throughout the contract period.
TABLE 11.
Referral laboratory performance parameters
Purchasing services and supplies.
Purchasing external services and supplies is a three-step process that includes the prepurchase process, the actual purchase, and the postpurchase process. In the first step, the prepurchase process, the laboratory staff determines the quality requirements. In the second step of the purchasing process, the purchasing section of the institution negotiates price, places orders in a timely manner, and ensures that bills are paid. To achieve a successful collaboration, communication between purchasing agents and laboratory staff is critical. In the third step of the process, postpurchase, the laboratory staff who use the purchased items or services verify that the items or services meet the identified quality requirements. When those requirements are not met, the staff shall report the problems to the laboratory director, and these problems are communicated to the purchasing section.
The laboratory shall ensure that purchased supplies, reagents, and consumable materials that affect the quality of tests and/or calibrations are identified and that there is a process to ensure that only services and supplies of high quality are used. The laboratory should have representation on the product selection committee to balance cost with the best product performance for an optimal quality of clinical diagnostic services. The price of the product, memberships in buying groups, and cost containment efforts should not drive product selection decisions for instruments, media, test kits, and services purchased. In addition to cost and quality, selection shall also be based on reliable delivery and service, such as the ability of the provider to ship kits and reagents with a single lot within an order and with a long shelf-life.
Purchasing documents must be reviewed and approved for technical content prior to the placement of an order. Purchasing documents shall contain specific information describing the services and supplies ordered. The information may include precise identification such as type or class of service or grade of reagent; specifications; drawings; inspection instructions; and other technical data, including approval of test results, quality required, and the quality management system standard under which they were produced. Before substitutions are made, the purchasing agent shall contact the laboratory staff for approval. Communication on back orders and substitutions to the end user is critical.
Upon receipt of the purchase order, the laboratory staff shall review packing slips and the package contents and match them with the purchase request. A certificate of analysis (COA) shall be maintained on file after the COA is examined to ensure that the received item meets minimum specifications. Chemicals and reagents should be purchased with manufacturer certificates, where possible. When uncertified chemicals are purchased from ISO/IEC 17025- or ISO 9000-registered companies, records of action taken to check compliance shall be maintained. For examples, laboratories can use QC data to demonstrate the quality of supplies, reagents, and consumables.
All reagents and supplies shall be stored under the proper conditions (e.g., temperature, humidity, and light exposure) specified by the manufacturer. Inventory shall be controlled and monitored so that critical reagents and supplies are available and fit for use. Inventory items may be managed manually or with electronic control systems. Additional information on equipment and reagent inventory control is described in Laboratory Operations, below. Suggested roles and responsibilities for purchasing and inventory are outlined in Table 12.
TABLE 12.
Roles and responsibilities for purchasing services and supplies
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Before decision to purchase products or services or renewal of an agreement | Negotiate best price, terms, and conditions | Establish criteria for the selection of quality products and services | Provide input into the level of quality required for products and services |
| Purchasing of products or services | Place orders accurately and promptly | Address deficiencies with vendors | Monitor inventory and place orders |
| Inform users of backorders or unavailability of products | |||
| After receipt of products or services | Timely payment of purchases | Document nonconformities Keep performance records to inform renewal or terminate agreements |
Inform management of incomplete orders or lack of service |
| Monitor quality of the products and services received | |||
LABORATORY OPERATIONS
This overarching section builds on the foundation and contains four QSEs that focus on routine laboratory operations. These QSEs include (i) the establishment and documentation of procedures and record retention, (ii) equipment qualification and maintenance, (iii) processes to manage the total testing pathway from preanalytical to postanalytical steps, and (iv) processes to manage information either electronically or manually.
Control of Documents and Records
Documents.
Documents may be electronic or paper based, but the principles of document control apply to all formats. All documents shall be uniquely identified with document and revision numbers to avoid the use of outdated documents. All documents shall be approved by the appropriate person who has the authority, as defined in a written procedure, before they are put into use. Documents may have different formats based on the purpose for communicating the information. These include policies, processes, SOPs, forms, and job aids.
A policy is a documented statement of the intentions or directions endorsed by management to provide efficiency and consistency. For example, the laboratory may have a written policy on attending continuing-education events locally or out of town or a policy regarding how holiday coverage is assigned.
A process is a set of interrelated activities that transforms inputs into outputs. Steps in the overall process of culturing sputum would include specimen collection, transportation to the laboratory, accession into the information system, evaluation of the quality of the sample, inoculation into appropriate media, and interpretation of the culture result. There are several formats that can be used to display a process, such as flowcharts or process maps.
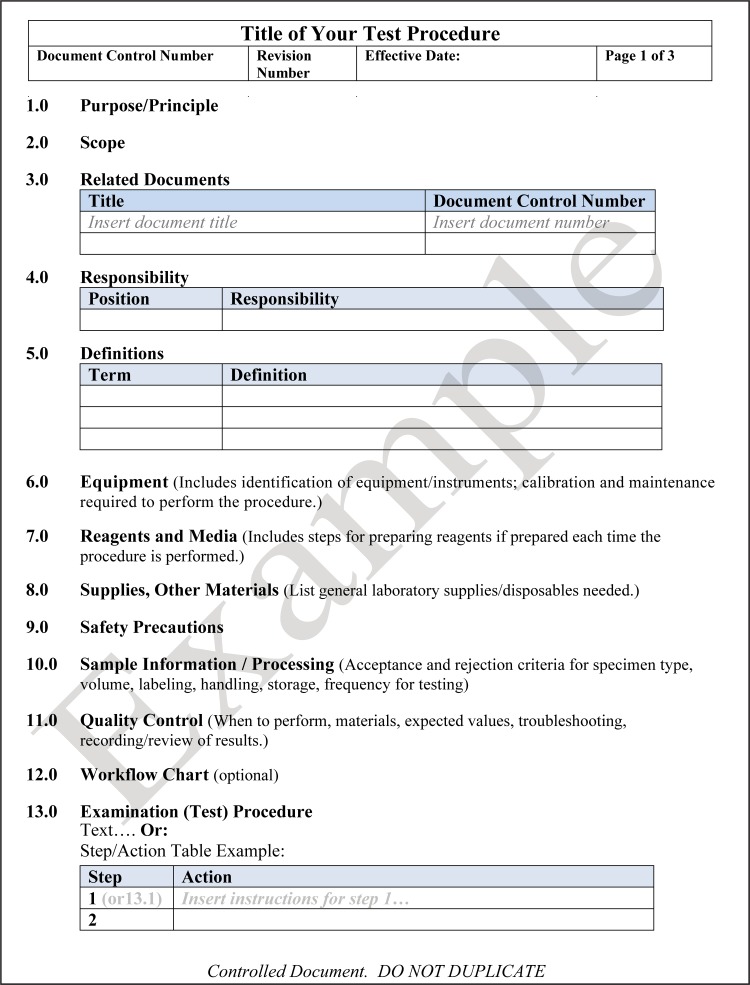
A SOP is a set of fixed instructions to carry out a routine activity or process. An example of a procedure would be a step-by-step description of how to perform a disk diffusion antimicrobial susceptibility test on a bacterial isolate. SOPs should have a consistent format and should be clear to all users so that the task being described is consistently performed. A template for writing a technical procedure is shown in Fig. 8.
FIG 8.
Template for writing a technical laboratory procedure. If a section is not applicable to a procedure, it is best not to delete the heading but to state “not applicable” in case the procedure should change and require this information in the future.
For changes to any document, there shall be a procedure that describes how to amend a document and who has authority to do so. A good practice may include the use of a “document change request” (DCR) form to initiate a change and to keep track of modifications in progress. A template for a DCR form is shown in Fig. 9.
FIG 9.
Template for writing a document change request (DCR). The DCR form allows traceability for amending or archiving technical or quality management policies and procedures.
Documentation of all changes shall be clearly recorded and should follow good documentation practices according to CLSI method QMS02-A6 (22). When making modifications, cross out original wording with a single line but allow it to remain legible. Never use correction fluid, and never delete the original information. Clearly state the revised wording. Ensure that an authorized person signs his or her initials and marks the date of the change. As soon as possible, update the revision history, assign the updated document a new revision number, and approve the document. Archive and appropriately retain outdated documents, including obsolete documents.
A form is a document to capture information, data, or test results. Examples include a communications log for inquiries and complaints, a form to capture the zone of inhibition for quality control organisms used in a disk diffusion test, or a form to document results observed for a lateral flow immunochromatographic test for Legionella antigen.
A job aid is an abbreviated set of instructions that briefly describes in words or pictures how to accomplish a task. An example of a job aid would be a diagram showing the order in which one should apply reagents for a Gram stain. It is not the complete SOP, but it indicates the necessary steps. All job aids should link to an approved SOP and should be signed and dated to ensure that the most recent SOP job aid is posted.
All documents, regardless of format, shall be reviewed periodically for their relevance. Some regulatory authorities or accrediting bodies set specific requirements for the frequency of review, which shall be observed to be in compliance.
Records.
A record is defined as documentation that provides evidence of results achieved or activity performed and can be either a hard copy or electronic. Forms become records after they are completed. The control of records applies to all technical and quality records. Technical records include observations, calculations, derived data, calibration records, instrument maintenance logbooks, spreadsheets used to calculate the accuracy and precision of instruments, sample logbooks, personnel records, and test reports. Quality management documentation includes audit reports, management review, preventive action taken, nonconforming events, and corrective action taken. A facility should develop a master index of records to provide order and structure to the record retention system.
The requirements for the control of records can originate from multiple sources, including compliance requirements of the accreditation agency, government regulations, and legal requirements. Customer requests shall be honored and may include requests from risk management to resolve legal inquires.
The “owner of the records,” sometimes known as the “records custodian,” is responsible for ensuring that records in the quality management system are handled in accordance with the stated requirements of the facility. This role can be served by the laboratory supervisor, the quality manager, or a person delegated for this function. The records custodian shall ensure that the records are properly indexed, stored on- or off-site, and maintained for the required retention period. It is the responsibility of all personnel to ensure that all records are legible and preserved for the retention process.
Records retention refers to how long the record is kept before it is either discarded or destroyed. The records custodian is required to ensure not only that the record is maintained for the required time but also that the confidentiality of any patient information is maintained. In the United States, the record retention period is based on local, state, or federal governmental regulations; the requirements of the compliance accrediting body, e.g., the College of American Pathologists (CAP) or the Joint Commission; and the procedures of the organization. There may be legal requirements depending on the type of testing performed by the laboratory. Records may be stored in the originating facility or sent off-site for long-term storage. Normally, records are retained on-site for the minimum retention time. Retention times for work area-specific records are determined by the laboratory and stated in their quality management system procedures. Suggested roles and responsibilities for controlling documents and records are listed in Table 13.
TABLE 13.
Roles and responsibilities associated with document control
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Document creation | May provide a template for official documents | Establish a document control system that meets any institutional requirements | Inform supervisor if a new or revised document is needed |
| Prepare new or revised documents with the appropriate control and revision no. | |||
| Ensure that appropriate documents capture required information, data, and results to meet regulatory requirements | |||
| Approve and review documents as required | |||
| Document access and storage | Provide a system for adequate and accessible storage of electronic and hard-copy documents | Provide a secure system for accessing documents | Use current versions of processes and procedures |
| Determine if access to documents is appropriate for work | |||
| Instruct personnel on document location and editing rules | |||
| Determine the life span of a document according to regulations and standards | |||
| Archive obsolete documents | |||
| Record creation | None | Review records for completion and follow-up of outlying entries | Enter information on forms according to good laboratory practices |
| Sign and date records in a timely manner | |||
| Record access and retention | Provide a system for adequate storage of electronic and hard-copy records | Create a system for maintaining records for easy retrieval Ensure secure access to records Determine if access to records is appropriate for the work to be performed |
Follow policy for restricting access to and sharing records Maintain all records of work |
| Set institutional policy for record retention that complies with regulatory requirements | |||
| Store, archive, or discard records according to record retention policies | |||
Equipment and Reagents
Equipment.
The laboratory shall have the necessary equipment to provide services, including general office and laboratory-specific equipment, analytical instruments, computer hardware and software, and the LIS. There are multiple steps to qualify new equipment coming into the laboratory that encompass its selection, installation, operation, and performance before it may be used for testing.
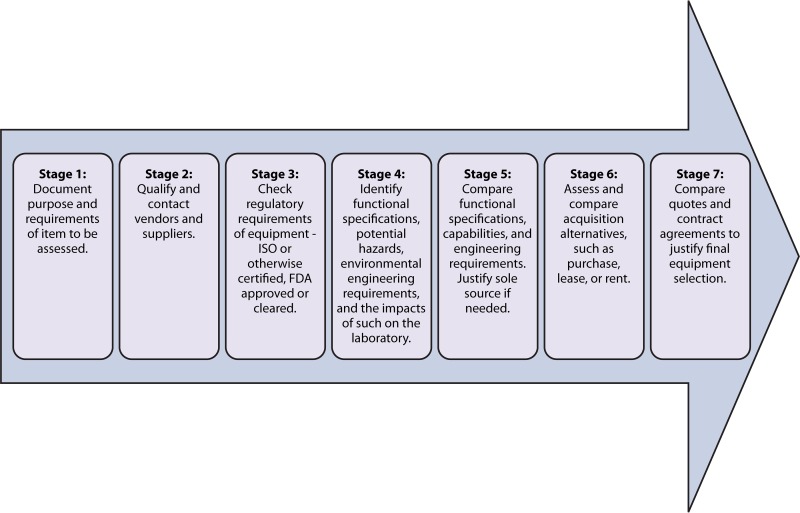
The laboratory shall have a process for identifying qualified suppliers from whom equipment purchases can be made. The laboratory shall follow approved procedures for the selection, purchase, and acquisition of equipment, including the preventive maintenance agreements for major equipment based on the manufacturer's requirements and the need for the equipment to perform reliably and safely throughout its life span of service. This initial process is known as “selection qualification” (SQ) and includes the steps outlined in Fig. 10. Note that the steps may not be followed necessarily in the particular order presented, and the ability to move to the next step is not contingent upon the completion of the prior step. Rather, Fig. 10 is a general guideline for SQ of equipment.
FIG 10.
Flow diagram for selection qualification of equipment. Each step does not necessarily need to be performed before others are completed. Some may be performed simultaneously.
The second phase of equipment qualification is designated installation qualification (IQ). The laboratory shall have a process in place to ensure that equipment has been installed according to the manufacturer's requirements. The manufacturer or the vendor may perform this task and provide documentation showing that the equipment was correctly installed and has met the specific requirements.
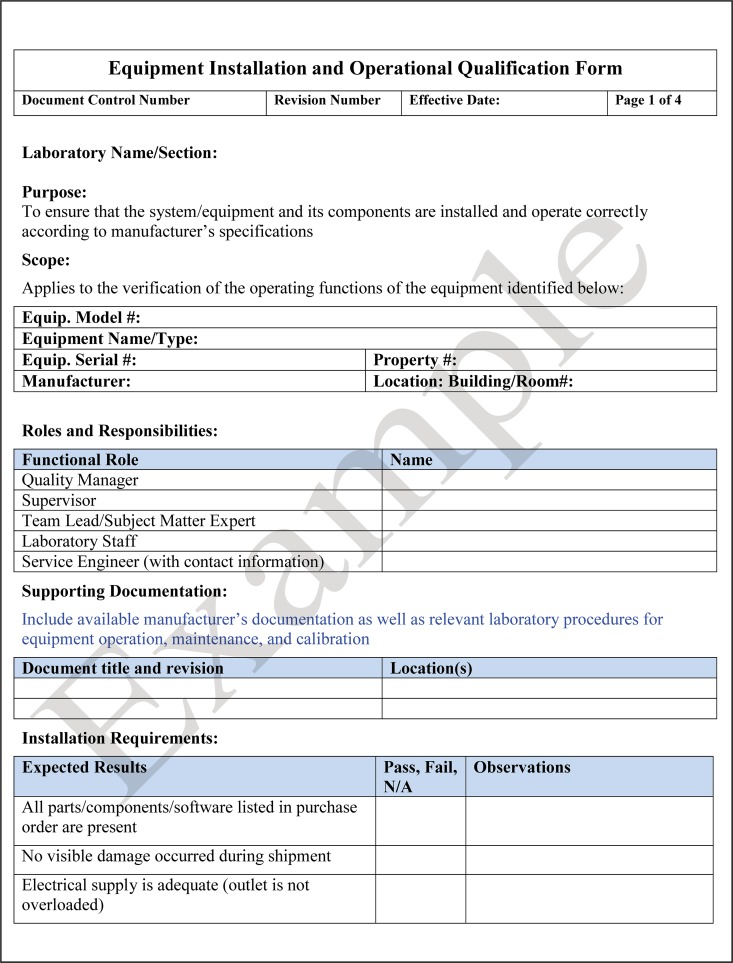
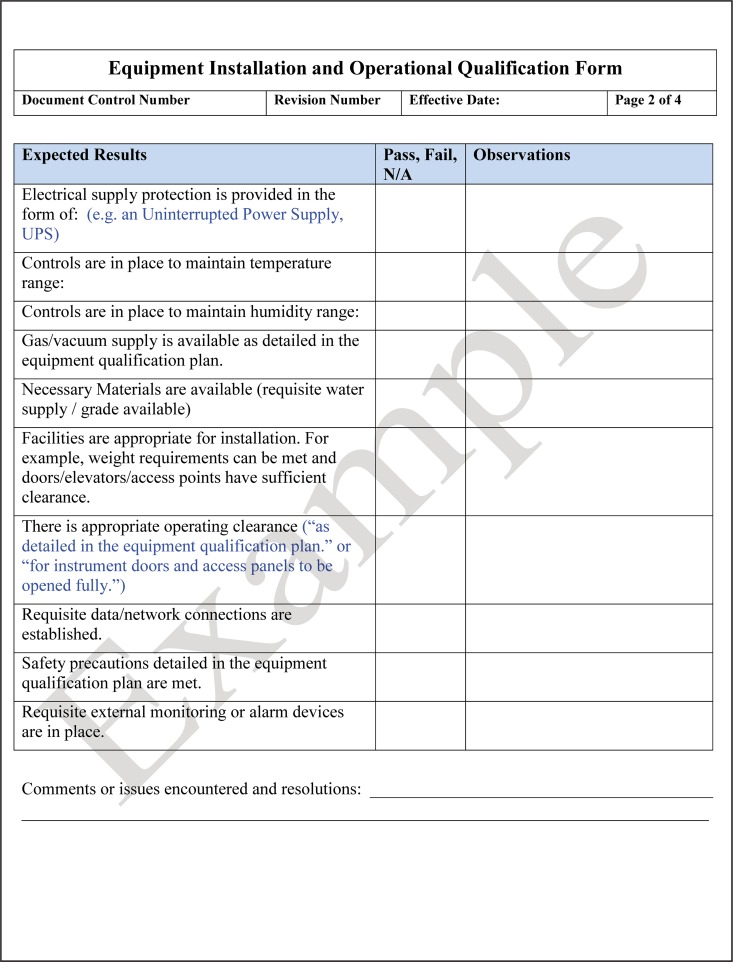
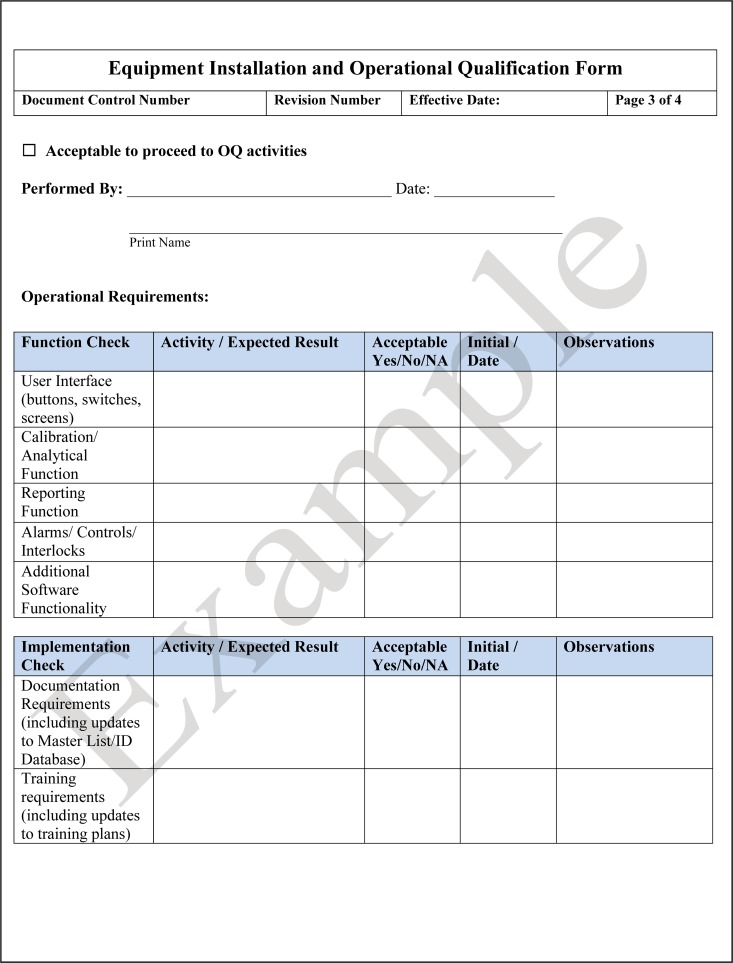
Following installation, the equipment is qualified that it operates in accordance with the manufacturer's specifications, known as operational qualification (OQ). OQ includes activities such as power-up, initial calibration, and verification of functionality. The vendor or manufacturer may perform both the IQ and OQ on the instrument and provide the required documentation, but they may not perform the performance qualification (PQ). The laboratory personnel who will perform the testing going forward are the ones responsible for performing PQ testing. An example of a checklist for installation qualification and operation qualification is shown in Fig. 11.
FIG 11.
Template for an equipment installation qualification (IQ) and operation qualification (OQ) checklist. IQ and OQ are performed before the laboratory initiates the performance qualification (PQ) for an instrument.
The laboratory shall develop a PQ plan to verify that the equipment and test system, connected together, perform effectively and reproducibly, based on the acceptance criteria specified by the laboratory. Review and approval of the PQ data confirm that the equipment produces acceptable results under normal operating conditions for the purpose intended and that the equipment meets regulatory requirements.
As a best practice, each item of equipment should be uniquely labeled for inventory, service, and maintenance. A bar code label placed at the time of receipt into the laboratory usually serves this purpose. A master list of all equipment that lists all equipment with its manufacturer, model number, serial number, bar code identification, current software version, and required frequency of maintenance and calibration should be maintained. This comprehensive master list serves as a checklist for periodic audits of equipment inventory.
An individual file should also be created for each piece of equipment, with the manufacturer, model number, serial number, identification bar code where utilized, and dates of purchase and installation, and the file should include all records of preventive maintenance and service performed or discontinuation of service. This file is beneficial as a single source of information on the history of the equipment and whom to contact for emergency repair services or for phone or online consultation.
Equipment shall be operated by trained and authorized personnel according to the manufacturer's instructions for safe handling, use, and maintenance. The laboratory shall take reasonable measures to decontaminate equipment before service, repair, or decommissioning and remove any patient-identifying information before it is discarded.
The laboratory shall identify the devices and equipment, including ancillary equipment, that are critical to the integrity of the test results. All devices and critical instruments shall be calibrated, demonstrating traceability to acceptable reference standards. For example, the laboratory calibrates thermometers to a National Institute of Standards and Technology (NIST)-certified thermometer. Any deviation from the approved protocol or manufacturer's recommendations or substitution for approved reference standards shall be documented in the equipment records. The laboratory shall have a system to document all the calibration results and shall have an indication of the current calibration status and date for the next calibration. Per laboratory equipment policy, there should be schedules for verifying the measurement of accuracy and the functioning of the equipment to prevent unauthorized adjustments or tampering with test results. A good practice is to schedule all calibration and maintenance for the upcoming year on a calendar. The equipment performance and maintenance records should be reviewed annually, maintained, and readily available for the life of the equipment, as specified in the laboratory's operating procedure. Suggested roles and responsibilities for installing, maintaining, and discarding equipment are outlined in Table 14.
TABLE 14.
Roles and responsibilities for equipment
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Equipment acceptance | Approve purchases and preventative maintenance payment | Specify acceptance criteria for purchasing equipment | Perform testing and provide tech support |
| Review documentation of purchase | |||
| Equipment usage | Provide backup power for critical instruments | Approve purpose and authorize personnel who may use the equipment | Verify and validate prior to use Use according to SOP |
| Review performance periodically | |||
| Calibration and traceability | None | Review calibration and traceability data | Perform calibration as required |
| Periodically review and analyze data for trends | Document all data to verify performance of calibration according to standards | ||
| Maintenance and repair | Provide funds and approve maintenance contracts | Approve maintenance contracts Oversee service schedules Periodically review performance, adverse effects, and accidents and maintain staff training records |
Perform maintenance as required Decontaminate equipment before vendor service is performed Maintain logs of care and calibration |
| Inventory control | May require annual audit | Conduct periodic inventory audit | Assist in conducting equipment audit and inventory control |
| Ensure that the unit is currently working in the designated laboratory location | |||
| Update status or decommission | |||
Reagents and supplies.
The laboratory shall have written procedures for the receipt, storage, inventory control, and quality control acceptance criteria for reagents and consumables. The laboratory shall ensure that purchased consumables are not used until they have been inspected or otherwise verified as complying with standard specifications or defined requirements. For the appropriate use of reagents and consumables, the laboratory shall have SOPs, which would encompass instructions and quality control parameters provided by the manufacturers.
All the consumable materials shall be stored according to the manufacturer's instructions and should be part of the QC records. In the event of unusual test results, this QC documentation is essential for event tracking and troubleshooting. An inventory control system shall be in place to track reagents and consumables and their shelf-life. A robust inventory control system should include records for each reagent and consumable, including the identity of the reagent or consumable, source (manufacturer's name and batch or lot number), contact information, date received, expiration date, condition when received (e.g., acceptable or damaged), and the manufacturer's instructions and reagent performance records confirming acceptance for use. Optional information may include the initials or name of the person who unpacked and received the product into the inventory. For in-house reagents, the records should additionally include the preparation date, expiration date, name of the person who prepared the product, product components and corresponding lot numbers and manufacturer's name, and the name of the person responsible for performing QC.
A QA program shall be in place to monitor acceptable performances of all reagents for laboratory testing. Reagents used as standards and calibrators shall be traceable, and manufacturer's certificates of analysis shall be retained and easily accessible. The laboratory shall verify the quality of the standards by comparing the new batch of standards to the previous lot. Laboratories shall follow the manufacturer's recommendations on storage and shelf-life. If external assurance of the calibrator or standards is not available or is insufficient, the laboratory should verify the performance characteristics in-house to ensure that purchased reagents and consumables comply with specified testing requirements. This should be performed in addition to other quality control testing in accordance with regulatory requirements. Suggested roles and responsibilities for reagents and supplies are shown in Table 15.
TABLE 15.
Roles and responsibilities for reagents and supplies
| Documents or activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Purchase agreements/contracts | Execute contracts and adhere to requirements | Recommend and approve suppliers/manufacturers | Perform testing and provide feedback |
| Review performance records and QC records for acceptable performance for the purpose of contract renewal | |||
| Order supplies and storage | Approve payment | Approve purchase order | Request supplies |
| Review reagent performance | Store and use according to SOP | ||
| Verify lots and traceability | None | Review performance data and trend analysis | Perform quality checks |
| Maintain certificates of analysis | Record lot performance and any variation from expected performance | ||
| Inventory control | Provide adequate storage space | Approve request for supplies in a timely manner | Perform inventory check Stock supplies Follow FIFOa usage |
| Maintain appropriate inventory for use | |||
| Monitor expiration dates of materials to avoid their use | |||
| Record keeping | None | Ensure that QA and QC programs are in place | Perform scheduled QC, and document all required information |
| Review reagent performance record | |||
FIFO, first in, first out.
Process Management
Preanalytical processes.
The preanalytical phase of the examination process encompasses all the steps that occur before the specimen reaches the microbiology laboratory and specimen accessioning and processing in the laboratory. Despite the fact that most of the preanalytical activities occur outside the walls of the microbiology laboratory, and they are the greatest source of errors, the laboratory remains responsible for the total testing process. Table 16 lists potential errors in preanalytical steps that can lead to specimen rejection or compromise the validity of the results. These errors should be monitored and serve as potential areas for quality improvement in the preanalytical phase of laboratory testing.
TABLE 16.
Examples of errors occurring in the preanalytical phase
| Preanalytical-phase step(s) | Example of error |
|---|---|
| Ordering | Urine cultures ordered for asymptomatic patients |
| Physician orders incorrect hepatitis markers | |
| Single set of blood cultures ordered to detect sepsis | |
| Patient/specimen identification | Mislabeled source of the specimen |
| Unlabeled/mislabeled specimen | |
| Missing requisition | |
| Collection | Swab collected and submitted when the preferred sample is tissue or fluid |
| Incorrect specimen container used for stool ova and parasites | |
| Formed stool submitted for Clostridium difficile testing | |
| Incorrect swab submitted for virus molecular testing | |
| Poor-quality expectorated sputa | |
| Insufficient vol of blood drawn for blood cultures | |
| Incorrect disinfection of venipuncture site before drawing of blood cultures | |
| Urine collected from the catheter reservoir bag | |
| Transportation | Specimens not sent to the laboratory within the specified time or at the appropriate temperature |
| Specimen registration and preparation | Incorrect test code registered |
| Incorrect loop size used for plating urine specimens | |
| Cultures poorly streaked; no isolated colonies | |
| Specimens improperly aliquoted | |
| Specimens inappropriately centrifuged/not centrifuged per SOP |
The main aspects of this complex preanalytical phase are outlined in the sections that follow. Each aspect should be tailored to the institution's workflow. Because a significant part of these processes occurs outside the laboratory, institutional support from various groups will be required to adhere to best practices in order to ensure that the highest-quality specimen is received in the laboratory. If the quality of the specimen is compromised, then the validity of the results is compromised, i.e., “garbage in, garbage out.”
Preanalytical processes are the cornerstones of valid results, and their magnitude should not be underestimated. These processes can be difficult to control because of the complexity and diversity of decisions that need to be made to order, collect, and transport specimens correctly. The right test for the right reason, collected in the right way, should be the goal for every patient's specimen.
The laboratory shall communicate their general laboratory services and specific specimen collection guidelines to all users and patients. The specimen collection guidelines shall be comprehensive and describe the tests that are performed in-house or by an outside entity. Test names should be linked clearly to those used in the reimbursement codes to simplify correct coding. An example of general laboratory information and the specimen collection and handling guidance that may be provided by a laboratory is shown in Table 17.
TABLE 17.
Informational items for inclusion in specimen collection and submission guidelines
| Guideline | Informational item(s) |
|---|---|
| General laboratory information | Laboratory name |
| Location of laboratory (address) | |
| Laboratory phone no. | |
| Laboratory hours of operation | |
| Laboratory director's or clinical consultant's name and contact no. | |
| Laboratory administrator's name and contact no. | |
| Contact times for director, consultant, and administrator | |
| Instructions for completing requisitions | |
| Laboratory's patient confidentiality policy | |
| Shipping address and available days and time for package receipt | |
| Specimen collection and submission | Name of test |
| Test identification or ordering code, test synonyms or aliases | |
| Method of testing | |
| Acceptable specimen types and sources | |
| Acceptable specimen containers or tubes | |
| Acceptable specimen volume and minimum volume | |
| Collection instructions for health care providers or patient self-collected specimens | |
| Specimen stability information, including transport medium and conditions | |
| Rejection criteria | |
| Repeat specimens within a specific time period | |
| Specimen retention time | |
| Specimen storage temperature | |
| Analytical time to perform test or turnaround time | |
| Day(s) and time(s) performed | |
| Notification of suspected disease or infectious agent | |
| Transport instructions relevant to specimen | |
| Regulatory information | |
| Testing location |
Detailed instructions for correct specimen collection, labeling, transportation, storage, and waste disposal shall be available to all sample collectors, e.g., laboratory staff, nurses, and patients (see reference 23 for published guidelines on specimen management). These standardized instructions are best when developed by sample collectors and the laboratory together. Any person who collects a sample is responsible for noting any changes in specimen collection for the laboratory so that results can be reported and interpreted with these noted changes. Consent may be inferred when a patient presents with a requisition for specimen collection, or it may be verbal or written; however, all patients have the right to refuse collection at any time.
Correct collection procedures begin with recognizing and adhering to the requirements to collect the requested samples, verifying the identity of the patient, and then preparing supplies and the patient for sample collection. Incorrectly collected or mislabeled specimens are significant errors that may lead to misinterpretation or, worse, reporting of results for the wrong patient. Specimens shall be labeled with specific information to unequivocally link them to the patient from whom they were collected. The label shall have at least two patient identifiers (e.g., name, date of birth, or another unique patient identification number), the date, and, where applicable, the time of specimen collection. The specimen label shall include the source of the sample (e.g., leg abscess) and, where relevant, the anatomical site sampled (e.g., inner right thigh).
All requisition forms, whether paper or electronic, shall provide space to document required and relevant information to accept and process a specimen. Customer-specific requisition forms are most effective when developed together by the customer and microbiology staff. Maintaining consistency in requisition formats can help the microbiology staff efficiently identify test requests and determine specimen acceptability. Regardless of the format, all requisitions require three types of information: patient demographics, test requested, and specimen description. Patient demographics include at least two patient identifiers, e.g., name and date of birth or a unique patient identification number, as well as the gender, age or date of birth, and location or contact information of the patient. The name and address of the authorized individual requesting the test or the contact person for the submitting laboratory, the identity of the collector where applicable, as well as the tests to be performed shall be clearly identified. Pertinent clinical information should also be provided, such as unique travel history or immunocompromised status. The requisition order shall contain the type of sample, the anatomical site sampled where relevant, and the date and time of collection when relevant. All the information on the requisition shall match that on the specimen label before the specimen is acceptable for testing. In addition, other jurisdictions or regulatory bodies may mandate additional information, or additional data may be desirable; for example, information for billing and insurance purposes may be requested. Requisitions with well-marked, mandatory completion fields help to reduce the number of incomplete requisitions.
Although verbal test requests to order additional tests are generally discouraged, they are inevitable. The laboratory shall develop and adhere to a procedure that mandates a documented confirmation of such requests within a specified time frame to adhere to the regulatory requirements. Since the majority of errors in the testing process occur in the preanalytical phase, microbiology management should be cooperative and, where applicable, resolve as many preanalytical errors as possible in order to mitigate the downstream negative impact on patient care and outcome.
Where applicable, the identity of the specimen collector shall be recorded for traceability. This information shall be accessible to the laboratory, which is particularly important in the event that the sample is compromised and follow-up and retraining are warranted. The collector's identity may be recorded in various places, including the requisition, the sample container, or the medical record. The roles and responsibilities for establishing preanalytical guidance documents are shown in Table 18.
TABLE 18.
Roles and responsibilities for preanalytical processes
| Documents | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Specimen collection, transportation, and storage guidelines | Provide support for disseminating guidelines | Write and review guidelines periodically | Assist in writing guidelines |
| Follow SOPs | |||
| Document nonconformities | |||
| Requisition forms | Collaborate to create forms | Review requisition forms to ensure adherence to regulatory requirements | Collaborate to create forms |
The integrity of the sample is dependent on time, temperature, and, in some cases, preservatives. It is the microbiology laboratory's responsibility to outline the transportation requirements for each sample type and monitor them. Examples of a laboratory's quality assurance program may include monitoring of environmental conditions, such as the temperature in courier bags, the transit time by porters or pneumatic tube systems, and the consistent use of preservatives and their expiration dates.
It is equally important to outline and follow the safety requirements for packaging biological specimens or control strains to minimize exposures of the carrier and the general public. The International Air Transport Association (IATA) is an international regulatory body that oversees the transportation of dangerous goods by air. Annual certification is required by the designated laboratory personnel who perform packing and shipping to ensure safe transportation practices.
Each laboratory shall establish and adhere to acceptance and rejection criteria for primary samples. If the integrity of the sample is compromised, but it is irreplaceable, there should be clear instructions on how to proceed and document the cause of the compromised sample. There should be a process for handling irreplaceable specimens and a record of authorization with the reason why the sample could not be recollected. The final report should denote the potential compromised state of the specimen and suggest caution when interpreting the test results.
Each primary sample and its aliquots shall be traceable within the microbiology laboratory and throughout the total testing process. This can be achieved by recording the receipt of the sample in the laboratory information system or in an accession logbook and applying laboratory identification numbers to each sample, its aliquots, the testing vial, and culture media. Recording each action performed on the sample and who performed this task can also aid in tracking the sample.
All primary samples and aliquots of primary samples shall be stored for an appropriate length of time under conditions that will minimize the loss of sample integrity. Evidence of an excellent biosecurity plan includes an inventory system for all stored clinical specimens, patient-derived strains, and reference strains. Stored samples should have a unique identifier and be easily retrievable and traceable.
Time limits may be established for ordering additional tests on the original sample that should consider the integrity of the sample, depending on its storage; e.g., DNA can be stored for longer times than specimens for Neisseria gonorrhoeae culture. Refer to the applicable accrediting body and regional, state, or federal requirements for specimen or isolate retention requirements.
To store primary specimens for research purposes, most jurisdictions may require patient consent and strict confidentiality. Refer to the local research ethics board or institutional review board (IRB) for the most up-to-date requirements. There may be additional requirements for unique tests, like molecular testing, regarding their handling, processing, and storage.
Analytical processes.
The laboratory shall be responsible for selecting appropriate test methods to meet the needs of their patient population, their programs, and their organizational operation. There are four main activities under the analytical process: test verification, test validation, writing and reviewing SOPs, and establishing the measurement of uncertainty for tests with quantitative results.
(i) Verification.
In the United States, U.S. Food and Drug Administration (FDA)-cleared or -approved tests require verification of their performance standards before being put into use. In Canada, the approving agency is Health Canada. As defined by ISO guidance (1), verification is the process by which a laboratory determines that an FDA-cleared/approved test performs according to the performance specification claimed by the manufacturer and is acceptable for use in their laboratory. The laboratory shall perform the test method according to the manufacturer's instructions using selected samples or isolates, document the process, and record the results. The performance characteristics of accuracy, precision/reproducibility, and, where applicable, the reference interval of normal values and the reportable range shall be established by the laboratory for the performance of the test procedure. Reference intervals are the expected values for 95% of the normal healthy population for whom the test is performed. The population may vary according to gender (male versus female) or age (pediatric versus adult), and the reference interval should mirror the type of patient for whom the test is being performed. Reference intervals are a decision support tool to assist in the interpretation of data from numerical laboratory reports. Most normal reference ranges are applicable to quantitative tests, such as blood glucose concentrations or red blood cell counts. Qualitative microbiology assays may have a reportable reference range, for example, “no growth” for a urine culture or “negative for group A streptococci” for a group A streptococcus assay. Performance specifications will apply according to the type of assay and may be more extensive for molecular assays (24, 25).
All verification results should be reviewed and approved by the laboratory director, or designee, and retained for inspections by regulatory and accrediting bodies. Although the performance specifications may not match those claimed by the manufacturer, they shall meet the specific requirements for the intended use of the laboratory. The performance specifications should be included in the test information in the SOP.
(ii) Validation.
Any deviation from the manufacturer's guidelines for a FDA-cleared or -approved test requires a validation of the test performance beyond the simpler comparison to the manufacturer's data in a verification study. A modified FDA-cleared/approved test, such as testing a specimen source not included in the manufacturer's claims, requires a validation process to confirm that the test performs as intended and the performance specifications expand beyond those required for the verification process. Likewise, the laboratory shall establish performance standards for laboratory-developed tests (LDTs) or when no performance standards are provided by a manufacturer, including analytical sensitivity, analytical specificity, accuracy, precision/reproducibility, reference intervals of normal values, and reportable range over which the test method is applicable to meet the requirements under CLIA 493.1253 (5). Validation shall include information on interfering substances or inhibitors and the frequency of such inhibitions. In addition, the laboratory shall establish the limit of detection, measurement of uncertainty, diagnostic sensitivity, and diagnostic specificity to validate a LDT under ISO requirements (1). Establishment of the diagnostic specificity and diagnostic sensitivity of the test method may be required by regulatory agencies such as the FDA.
The validation study should be planned and approved prior to beginning the process. The validation shall be performed by authorized laboratory staff who are trained to perform the procedure using equipment that is within the calibration period. Validation shall be performed in the same laboratory location where testing will be performed after validation. The test method validation results shall be reviewed and approved by the laboratory director, or designee, before beginning patient testing. Any change to a validated test procedure shall be documented with version controls and approved before implementation. Any such method modification would require additional validation according to the scope of the changes. The summary of the validation study and all raw data shall be retained throughout the life of the test procedure and for a period of time after the procedure has been discontinued according to institutional and regulatory requirements. Microbiologists may be familiar with the guidance in Cumitech 31A, Verification and Validation of Procedures in the Clinical Microbiology Laboratory (26). Note that the authors of Cumitech 31A use the term verification as a one-time-only process to determine the performance specifications for both FDA-cleared/approved tests and LDTs. Validation is described as an ongoing check for all tests to determine if they are still working as intended. The CAP accrediting body uses the terms “analytical verification” and “analytical validation.” Analytical verification is defined as the process by which a laboratory determines that an unmodified FDA-cleared/approved test performs according to the specifications set forth by the manufacturer when used as directed. The CAP defines analytical validation as the process used to confirm with objective evidence that a laboratory-developed or modified FDA-cleared/approved test method or instrument system delivers reliable results for the intended application (27). Both CAP definitions are similar to the ISO definitions of verification and validation.
(iii) Writing standard operating procedures.
The laboratory shall have written procedures for all tests that describe in detail the purpose of the test, sample requirements, equipment and supplies needed, and step-by-step instructions on how to perform the test. The procedure may be a hard copy or electronic and shall be available to personnel who perform the test. Required elements to be included in a technical SOP may be specified by regulatory and accrediting bodies. See Fig. 8 for a sample template of technical procedures. The manufacturer's instructions may be available electronically to be incorporated into the procedural format used by the laboratory. Package inserts may be included as an appendix to the authorized SOP. Package inserts do not fulfill the requirement for a SOP for a nonwaived CLIA test. Test procedures shall be revised when the manufacturer updates the package insert instructions. Periodic review of the package insert and the current laboratory SOP ensures that any changes have been incorporated into the working procedure and staff are apprised and retrained as necessary. Test procedures shall be approved by the laboratory director, or designee, before being used for testing patient samples. If the laboratory is CLIA certified, the CLIA laboratory director shall approve the SOP. Laboratory procedures shall be reviewed at a frequency required by regulatory and accrediting bodies and should be reviewed annually and revised as appropriate, with a revision history of significant changes being noted in the procedure.
(iv) Measurement of uncertainty.
For tests with quantitative results, the laboratory shall define the estimates of measurement uncertainty. An estimate of uncertainty provides a quantitative indication of the reliability of the patient result and is based on available information about the performance characteristics of the measurement procedure. A measured value is only an estimate of the true value of what is being measured because of imprecision, imperfect bias correction, and imperfect analytical specificity. Measurement results can be affected by preanalytical and postanalytical factors, which should be minimized by standard procedures. By running a sample multiple times and averaging the result, the laboratory would arrive more closely to the true value. Although this process may apply more to chemistry and hematology analytes, infectious disease testing has quantitative methods, such as urine colony counts, viral load assays (e.g., hepatitis, cytomegalovirus, Epstein-Barr virus, and HIV), or rubella IgG antibody titers, to which measurement of uncertainty applies. Clinicians and laboratory scientists need to understand how a PCR result of 5,000 copies/ml on one day compares to a result of 10,000 copies/ml reported the next week. A measurement of uncertainty will provide information to interpret if these results are clinically significantly different or fall within the variation of the test parameters. Similarly, a laboratory can perform multiple cultures on the same urine sample to establish the measurement of uncertainty for the method. For example, the true value for a urine culture result of 60,000 CFU/ml may lie between 45,000 and 75,000 CFU/ml.
The measurement of uncertainty can be best calculated by using a known standard of 100% trueness, but there are very few things that have full metrological traceability and trueness. An international reference standard may be available for some test analytes. The measurement of uncertainty serves as another quality indicator and does not need to be reported with each laboratory test result. However, it shall be available and provided to the health care provider if requested. A measurement of uncertainty does not apply to any test that has a qualitative result, such as positive versus negative, reactive versus nonreactive, or growth versus no growth. A more expansive discussion of measurement of uncertainty is provided by Allen and Crawford (28). The roles and responsibilities for the laboratory analytical processes are shown in Table 19.
TABLE 19.
Roles and responsibilities for analytical processes
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Test method selection | Provide budget, staff, and other resources | Select new procedures for patient population | Perform test method comparison studies |
| Suggest new testing based on customers' feedback | Review send-out tests to decide if testing can be performed in-house | ||
| Test method verification and validation | None | Develop, review, and approve verification and validation protocols and data | Perform verification or validation testing |
| Retain all records of verification and validation studies and raw data | |||
| Maintenance current version of SOPs | None | Create and approve test method procedures | Review procedures by comparing actual practice to what is written in the SOP |
| Periodically review and revise SOPs | |||
| Ensure that current version of SOP is available to staff | |||
| Archive obsolete versions of SOPs | |||
| Establishment of measurement of uncertainty for quantitative test methods | None | Review all test parameters that would contribute to variation in the test result | Perform test as specified and do not deviate from established protocol |
| Minimize variations in sample, test procedure, the environment, personnel, and reporting | |||
Ensuring quality in the process.
To ensure the quality of the test results, a comprehensive test review program is required, which begins with the monitoring of the individual test parameters with QC, the ongoing tracking of these parameters by QA, and assessing laboratory testing performance with PT. Any deviations should be easily recognized and used as a trigger to prevent the release of patient results until the nonconformance can be resolved. No test result shall be released if the QC results are out of range for that analyte. No test result or QC result shall be fabricated at any time.
A risk assessment of the total testing process for each examination should dictate the extent of QC performed (29). However, the amount and frequency of QC performance shall never be less than the minimum requirements stated by the manufacturer. Additional control materials may be added, and QC materials may be tested more frequently, as determined by the laboratory or regulatory bodies. In the United States, an individualized quality control plan (IQCP) may replace the regulatory requirements for QC specified by CLIA regulations. An IQCP is based on determining the risks for error and the frequency and severity of harm, developing a plan to mitigate these risks, and monitoring the outcomes. Wherever feasible, the QC materials used during testing shall be traceable to a known standard and simulate the matrix of the patient samples as closely as possible. The performance of the QC materials should be tracked and trends analyzed as part of the laboratory's ongoing QA program to detect changes in the controls before they fall outside the acceptable range. The laboratory QC program shall include a schedule of frequency, documented processes and procedures for QC performance, acceptable values, and corrective actions for any QC results falling outside the expected range. If QC fails, the supervisory staff assigned to investigate the problem shall also evaluate the results from patient samples before the incident occurred to ensure the accuracy of test results since the last successful QC event. QC failures should be documented as nonconforming events.
To ensure that the testing process is performing as required, the laboratory shall participate in a PT program. A commercially available PT program may meet the needs of an individual laboratory for most of its assays. However, when unique testing methods are performed or unusual microorganisms are detected or identified, a laboratory may not be able to subscribe to an externally prepared PT survey to assess test performance. In this case, an alternative assessment plan shall be created by the laboratory to meet the requirements of its certification agency or accrediting body.
An interlaboratory comparison program(s) may be established for examination and interpretations of examination results for test methods or analytes where a commercially available PT program does not exist or does not meet the laboratory's needs. All participating laboratories shall implement a documented procedure stating the responsibilities, instructions for participation, and acceptable performance criteria. Results shall be graded, and performance shall be documented. If the predetermined performance criteria are not met, then an investigation of the procedure shall occur, and corrective actions shall be developed to prevent future nonconformities.
If an interlaboratory assessment program is not feasible, an in-house QA program may fulfill the requirements for participation in a proficiency testing process. Qualified laboratory personnel who will not be part of the testing process may generate “unknowns” or blind samples using certified reference materials, previously examined clinical samples, or previous PT challenge samples with known values. All rules and regulatory requirements for participation in a commercially provided PT program also apply to one developed in-house.
The PT challenge samples shall be tested in the same manner as routine patient samples following routine clinical workflow and tested by all trained and competent laboratory staff who are qualified to perform the assay. No communication with outside laboratories regarding PT results is permitted until after the challenge set has been submitted. Failure to adhere to this policy may jeopardize the laboratory's authorization to legally perform testing. Failure to participate in a quality assessment program for each test method or multiple unsuccessful PT results may result in the revocation of authorization to perform a specific test or to test a specific specialty by the laboratory.
All testing personnel shall have adequate training and approval from the laboratory director, or designee, before participating in PT testing. Successful participation in PT challenges is a measure of personnel competency as well as the testing process. All PT results should be reviewed by the laboratory director, or designee, and the QA officer and discussed with relevant staff. When predetermined performance criteria are not fulfilled, corrective actions shall be put in place to improve performance. The longitudinal review of the laboratory's PT results should be part of their QA program. The roles and responsibilities for ensuring quality in the examination process are shown in Table 20.
TABLE 20.
Roles and responsibilities to ensure quality test results
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Performance of QC | None | Ensure that QC meets the manufacturer's or regulatory requirements | Accurately and completely document QC results |
| Perform a risk assessment for the test methods and design an appropriate QC plan covering the total testing process | Evaluate QC results before submitting patient results | ||
| Performance of quality assurance | None | Establish a robust QA program to monitor critical tests | Provide necessary information for QA monitors |
| Track, analyze trends for, and review QC values for shifts in performance | |||
| PT program evaluation | Provide resources for laboratory to enroll in necessary PT programs | Enroll in appropriate PT programs that reflect the testing performed in the laboratory | Perform testing of PT samples in the same manner as patient testing is performed |
| Create an alternative assessment plan for methods or analytes when a commercial provider does not meet the laboratory's needs | Document all actions associated with the workup of PT samples Learn from errors in order to improve knowledge and skills |
||
| Review results in a timely manner | |||
| Investigate all nonconformities | |||
Postanalytical processes.
The postanalytical phase of the examination process is comprised of three critical activities: the review of the test report, the storage of critical samples and isolates, and provision for safely discarding samples and isolates when they are no longer needed.
(i) Review of the test report.
The review of results is as important as performing testing. Two important factors affecting the postanalytical phase of the testing process are test result reporting and test result interpretation. The laboratory director, or, where authorized, supervisory staff or peers, may review the results of examinations before release, depending on the complexity and type. The reviewer should check for conformity to the laboratory protocols and acceptability of QC results and utilize any clinical information available for the patient to aid in ensuring the accuracy of the results. Previous results may also be of value in ascertaining the quality of the current test result. A process should be in place to review results on a regular basis to avoid delays in detecting nonconformities, which includes the review of results on evening or night shifts, weekends, and holidays.
A systematic, stepwise review process should verify that demographics, calculations, congruity of results, and notifications or disclaimers appear correctly on the report. The initial check is used to determine that patient demographics and specimen identification and associated results are accurately linked and transcribed to the test report. The patient test results should appear consistent with relevant patient information, such as age, sex, diagnosis, and relationship with other test findings. The identification of the organism should be compatible with the source; e.g., Neisseria gonorrhoeae cultured from a wound should be questioned. The reviewers should check if test results are accurately calculated and transcribed from raw data and if dilutions and other correction factors have been applied appropriately. Determining the congruity of results is a vital part of the review process. Culture results should be in agreement with the Gram stain results; e.g., a smear report of Gram-positive cocci should be questioned if the only organism recovered is Escherichia coli. The susceptibility results should follow the expected pattern for the organism tested, and unusual patterns of resistance should be confirmed by supplementary testing. If applicable, the susceptibility results should follow the protocol for cascade reporting. Applying the proper interpretations, alerts, and disclaimers requires knowledge of the test, actionable results, and regulatory requirements. Reference intervals and interpretive reporting should be included where appropriate for the test findings. Critical values and abnormal results are flagged and effectively communicated and documented. Results should include notifications to infection control, public health, or infectious diseases authorities where applicable. Finally, appropriate disclaimers should be present in the report if the test is not FDA cleared or approved or if the test is being performed under an emergency-use authorization (EUA).
QA rules should be applied to test results and can be applied automatically within a LIS to enhance the recognition of potential problems. Delta checks involve comparing current results with past results. This is often limited to quantitative testing in microbiology, but for some testing over time, such as for mycobacteria, it may be important to note previous findings. In microbiology, it would be important to note a change of organisms in a blood culture, i.e., Staphylococcus aureus to coagulase-negative staphylococci. Checks involving comparisons of microscopy and culture results should be performed. When Gram stain findings suggest the presence of a microorganism of a particular morphology, the reviewer should reconcile why the culture results do not include a microorganism compatible with those staining characteristics. Similarly, antimicrobial susceptibility results should be examined for inconsistencies. Antimicrobial susceptibility results for specific microorganisms can be reviewed for their expected results. When results differ from those expected, both the identification and the antimicrobial susceptibility results should be reviewed and repeated by an alternative method, if necessary. The report should be either amended, if incorrect, or accepted as an unusual value, which may need to be communicated immediately.
(ii) Sample storage.
The laboratory should develop and maintain an up-to-date storage/freezer inventory log and adequate labeling to locate samples or isolates for future testing. A periodic inventory of refrigerators and freezers should be performed to ensure that old, unwanted, and unnecessary materials are discarded, inventory logs are current and complete, and no select agents are stored without the appropriate approvals (30).
Transfer to storage and the type of storage should take into account the pathogenicity of the microorganism and potential for breakage or spillage. Access to stored materials should be limited, and the list of personnel granted access should be part of the procedure and records. Some of these storage, retention, and destruction requirements are governed by federal requirements, such as the U.S. Federal Select Agent Program (31).
Each facility shall determine the storage and retention requirements for clinical samples, including the need to retain the primary specimen container for a specified time frame so that the specimen and label information can be verified, if needed. The length of the retention time should be based on the most stringent regulatory requirement for the individual laboratory. It is advisable to retain invasive isolates from blood, cerebrospinal fluid (CSF), and sterile body sites or those with multidrug resistance for a longer period of time, within reason, for up to a year. A representative culture plate should be held from completed cultures for a few days in case additional workup must be performed. Primary specimens, depending on the type of specimen, may be held for several days or longer. Reasons for various lengths of storage range from the potential need to send a specimen to a reference laboratory to molecular typing of isolates for infection control.
Some clinical samples, culture isolates, or slides (such as malaria or Cyclospora) may be required to be submitted to the state public health laboratory. A review of local, state, and federal reporting requirements determines what must be submitted and the time frame for submission. Reasons for required submissions may include confirmation of the identification of rarely detected organisms, agents of concern for bioterrorism (see http://www.selectagents.gov/ [accessed 5 January 2018]), PulseNet for foodborne outbreak detection (see http://www.cdc.gov/pulsenet/ [accessed 5 January 2018]) or further characterization for outbreak determination, genotyping for Mycobacterium tuberculosis (see http://www.cdc.gov/tb/programs/genotyping/Chap3/3_CDCLab.htm [accessed 5 January 2018]), and surveillance, such as the National Antimicrobial Resistance Monitoring System (see http://www.cdc.gov/narms/ [accessed 5 January 2018]).
(iii) Sample disposal.
Safe disposal of clinical samples and any isolates shall be carried out in accordance with any facility, local, state, and federal requirements. If specimens or isolates are sent off-site for disposal, there may be additional requirements from a waste management company. A waste management program should be developed as part of the overall facility plan. A contingency plan for alternative disposal should be included in case of a failure of the normal waste management plan. Patient confidentiality shall be maintained throughout the disposal process.
For all the elements described here, it is critical that staff are trained and competent so that they are able to adhere to the procedures developed by the laboratory. The roles and responsibilities for postanalytical processes are shown in Table 21.
TABLE 21.
Roles and responsibilities for postanalytical processes
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Systematic review of results | None | Establish who can review results and policy for release of results | Submit all pertinent QC results for test review |
| Create procedure describing how to review content on test reports | Check results for problems | ||
| Identification and storage schemes | None | Establish a system to retain samples and isolates for short- and long-term needs | Log and save all samples or isolates per protocol |
| Retention requirements | Provide sufficient storage space and conditions to meet legal requirements | Establish retention policy to meet federal, state, local, and institutional requirements | Adhere to retention policy and follow retention protocol |
| Train staff to understand the importance of following sample retention policy | |||
| Safe disposal of waste | Provide contract with waste management to meet the needs of the laboratory and fulfill legal requirements | Establish waste disposal policies and procedures | Follow waste removal protocol |
| Ensure that high-risk waste is safe to remove | |||
Information Management
The laboratory produces and captures a large variety of data and information that shall be managed to ensure their integrity, security, and traceability. Data are composed of instrument results, worksheets, QC results, equipment logs, epidemiological data, and many other types of data. The majority of the information created in a clinical laboratory is in the form of patient reports, but information also includes analysis reports from data mining and management review reports to leadership. Whether the system for laboratory information is managed electronically or manually, the policies, processes, and procedures shall be followed for data integrity (maintaining and ensuring the accuracy and consistency of the data over their entire life cycle), data security (protection of data from accidental or malicious access, use, modification, or disclosure), and traceability (knowledge of who entered or modified the data, what was entered or modified, and when data were entered or modified). Verification shall be performed to confirm the accuracy of the manual or electronic transfer or manipulation of data. For example, calculations made electronically in the LIS shall be verified periodically and after software updates to ensure that they are working as intended.
Generation of patient reports.
Laboratory reports should be easy to read, unambiguous, and readily interpretable and should use standard terminology. The report shall contain all required demographic information to ensure that the right result goes to the right patient. If the test results are potentially compromised by the quality of the sample (Table 16), this shall be communicated as part of the test report. The laboratory director should ensure that the test result and any explanatory comments are understandable to the recipients of the report.
Interpretive comments should be a collaborative effort between the laboratory and the clinicians who oversee the clinical specialty associated with the tests, such as infectious diseases consultants, gastrointestinal service, or obstetrics and gynecology service. Comments advising isolation or other infection control activities may be very helpful to those reading the report; however, these statements should be formulated with the approval of the infection prevention and control programs and other stakeholders. Interpretive comments should be reviewed annually and updated when new guidelines are published. For example, when new CLSI performance standards for antimicrobial susceptibility testing are published, previous comments attached to susceptibility results may no longer be correct or applicable.
Information that may affect the interpretation of test results, such as test interferences (e.g., amplification inhibitors), shall be provided in the report. Value-added comments can help the clinician interpret results or better understand how to use the result appropriately (32). Current clinical and laboratory information systems generally provide great flexibility in how laboratory tests are formatted for viewing electronically and in print form. Informative comments and textual reports are particularly valuable to aid the clinician in patient care, because they augment and clarify culture results as well as positive-negative, semiquantitative, and quantitative data.
A value-added comment for a bacteriology report may be “Bacteria in the Streptococcus anginosus group are frequently associated with abscesses. When these organisms are isolated from the blood, the possible presence of an abscess should be considered.” For a virology report, interpretation of HIV results may be helpful, for example, “HIV-1/HIV-2 antigen/antibody immunoassay: reactive. HIV-1 NAAT (nucleic acid amplification test): detected. Laboratory evidence of HIV-1 infection consistent with an acute HIV-1 infection.”
Turnaround times for patient reports.
The laboratory shall determine clinically relevant and feasible turnaround times for test results. This should include conferring with the health care providers in specific locations of the health care facility to establish what results are clinically actionable. There is no value added when rapid results are provided when no one is available to change the treatment or management of the patient. The laboratory should report the expected turnaround time for test results from the time when the specimen has arrived in the laboratory. The turnaround times may be dictated by practice guidelines; e.g., acid-fast bacillus smear results need to be reported within 24 h from the time of collection. When the laboratory cannot report patient test results within its established time frames, the laboratory shall have a process for notifying the submitter when an examination is delayed and could compromise patient care. Communication with health care providers is essential when there are planned or unexpected delays.
Reports should be revised when an additional test is requested or further explanation is added to the existing report. This may be considered an amended report. The report may also be revised to change a result, and in this case, the new report would be considered a corrected report. The laboratory should have a process in place to monitor and evaluate its test results and reports for inconsistencies and inaccuracies. A corrected report shall be clearly identified as a “corrected report” and shall indicate the new results as well as maintain the original data. An explanation of what was corrected should be part of the report. Notification of corrected results shall be rapidly communicated to the health care provider, who can act on those results, and this additional communication should be documented in the report.
The laboratory shall release results only to authorized persons or the individuals responsible for utilizing the test results. In jurisdictions where results may be shared directly with the patient, it is extremely important to have results and interpretations stated simply without complicated scientific jargon or unexplained abbreviations. Processes for maintaining confidentiality shall be in place in the laboratory for all reports. Access to patient data and test results shall be established for personnel within the laboratory, and staff shall be granted appropriate authority to access information as required to perform their jobs. Adequate security training shall be provided to laboratory staff to maintain the confidentiality of all patient records.
Notification of critical results.
Laboratory management in collaboration with stakeholders shall determine the attributes of laboratory reports and the timing and the frequency of reporting of information. Procedures for defining and reporting critical test results shall be developed and periodically reviewed by the laboratory director in conjunction with the medical staff to ensure that clinicians are immediately notified about abnormal results when necessary. Critical results are defined as results that require immediate medical, infection control, or public health intervention, including those of a life-saving nature. These results shall be communicated to the appropriate health care providers according to the laboratory protocol. Examples of critical results include positive CSF Gram stain results and positive blood culture results. Additionally, first-time identification of Mycobacterium tuberculosis in a hospitalized patient requires notification to the infection preventionist to protect other patients and health care workers. Oral communications of critical results shall be followed with an electronic or hard copy, which may be faxed to a verified number in a secured location. Fax numbers and e-mail addresses should be verified for their functionality and accuracy as part of the laboratory's overall quality assurance plan. Documentation of the notification of the critical results should be included on the test report, with the specific time, date, results, and person's name to whom the results were communicated.
Failure to notify a clinician of a critical test result is a serious quality failure. However, there must be a balance between the health care provider's desire for communication of results and the laboratory's resources. The turnaround time for critical results shall be specified by the laboratory and medical staff, the standard of care, and the accrediting agency requirements. Communication with clinicians and the pharmacy should be focused on delivering patient information with actionable results, such as the initiation of appropriate antimicrobial therapy or isolation of a patient with a contagious disease. Likewise, regulations for the timely reporting of results to public health officials shall be followed as defined by the local and state jurisdiction's laws. These laws shall be considered when establishing reporting rules for the LIS. In some jurisdictions, the reference laboratory may be responsible for reporting results to the state public health authority of the submitting laboratory. The roles and responsibilities for reporting laboratory results are outlined in Table 22.
TABLE 22.
Roles and responsibilities for reporting results
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Generation of patient reports | Provide resources for a system that allows reliable and accurate results to be communicated to all authorized persons | Design report format that includes all regulatory requirements to ensure that the correct report goes to the authorized recipients | Follow established protocols |
| Establish the confidentiality process | Ensure that staff are aware of the confidentiality process | ||
| Turnaround time for reports | Key stakeholders provide input on turnaround times for tests with actionable results | Establish clinically actionable turnaround times | Follow established procedures for review and release of reports |
| Monitor turnaround times for key test results | |||
| Critical test results | Key stakeholders provide input to identify medically important results that require immediate notification | Develop definitions and procedures for critical test values with the medical staff | Follow notification protocol and document details of the notification |
| Adhere to local regulations for reporting notifiable diseases and organisms | |||
| Data mining | Stakeholders provide input on the type and frequency of report needed for cumulative data | Establish process for collecting, analyzing, and communicating data as needed | Generate reliable accurate individual patient results so that cumulative reports are accurate |
| Ensure quality and accessibility of data | |||
Managing information electronically.
The laboratory director and laboratory management shall approve the installation and validation of new computer systems as well as changes to existing validated systems. In addition to serving the needs of the laboratory, the LIS should meet requirements of internal and external customers, such as a bidirectional interface with other electronic systems. The laboratory shall determine the level of access for each job title or role in the laboratory. The laboratory management shall define who may read data, who may enter data, who may change or correct data, and who may report results to those outside the microbiology laboratory.
A procedure should be developed to maintain patient confidentiality when LIS maintenance is performed by nonlaboratory personnel. All materials and waste with patient information (e.g., requisitions, reports, and specimens) shall be disposed of in a manner to maintain patient confidentiality. For example, the use of document-shredding services for all laboratory worksheets, forms, and reports that include protected/personal health information (PHI), such as name, date of birth, or medical record number, would be appropriate. Individualized access to the LIS shall be utilized to allow only authorized personnel access to PHI and to track and audit access for any breach in confidentiality. Other suggestions to maintain patient confidentiality include covering PHI documents on desks, minimizing or exiting computer screens when unauthorized personnel are present, and logging off or locking the terminal when stepping away. Individuals shall never share their personal access code with others.
In addition to the software and hardware in the LIS, all peripheral hardware (e.g., keyboards, monitors, and printers) should be maintained to function properly. For data that are accessible outside the laboratory (e.g., nursing stations or clinics), the institution, in conjunction with the laboratory, shall create and enforce policies to prevent unauthorized access. When personnel are no longer employed at the institution, it is imperative that their access to the LIS is discontinued as soon as possible. The laboratory should have a procedure to review the accuracy of manual data entries into the LIS by both technical and clerical personnel. Personnel performing data entry shall have the necessary training, and their skills and understanding of the process shall be documented through competency assessment.
When the LIS is upgraded or the system is replaced, the laboratory shall have a process in place to ensure that previously entered data can be retrieved in its entirety and unaltered. Processes for data recovery include a system with appropriately timed backups of new data and a provision for the storage of records in an alternate location that would allow their accessibility in a timely manner. An ideally designed architecture includes redundant servers housed in physically separate locations to provide effective disaster recovery and the efficient use of server resources. Locating redundant servers several miles apart minimizes the likelihood that all information will be affected during the same disastrous event.
The laboratory shall establish a process to accept test requests and report test results when the LIS is experiencing downtime. Timely reporting should be appropriate for the clinical need of the test results. Hospitals that offer an emergency room or acute care should develop an alternative reporting system with the clinical laboratory that can be functional within a short period of time. Practicing for a downtime event allows both laboratory staff and health care personnel to understand the procedures and make improvements before an actual event occurs. In the event that the LIS is not operational and an alternative reporting process has been implemented, there is also a need for a process to reenter data created during the downtime and reestablish reporting through the LIS.
Automatic release of test results in the LIS.
As more automated instruments can be integrated into the laboratory's information system, there is an opportunity for the release of results without the review of laboratory staff. While rapid result reporting has its benefits, there are specific requirements to ensure that reporting is accurate and that fail-safes are in place to prevent patient harm. If the laboratory implements a system for automated selection and reporting of results, it shall establish a procedure to ensure that the criteria for automated selection and reporting are defined, approved, readily available, and understood by the laboratory staff and health care providers. The process shall be validated before use and after any changes that might affect function, such as a software upgrade or a change in susceptibility breakpoints. Warning messages generated by the instrument should be incorporated into automated reporting, where appropriate. If a problem occurs, the automated reporting process should allow for a timely discontinuation of the process until the problem is resolved. The roles and responsibilities for utilizing the laboratory computer system are listed in Table 23.
TABLE 23.
Roles and responsibilities for the computerized laboratory information system
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Data security | Provide redundant server capability | Determine level of access for each job title and access outside the laboratory's control | Follow security and confidentiality protocols for assigned roles |
| Provide institutional guidelines | Ensure that access is removed for staff who have left employment | ||
| Data integrity | Provide resources to acquire and update hardware and software for the LIS | Approve installation of computer hardware and software | Assist in validating and verifying the system after updates |
| Determine procedures for validation of hardware and software and criteria for acceptability | Alert supervisors to any unacceptable performance of the LIS | ||
| Determine procedures for verification of calculations and software | |||
| Backup system | Develop alternative accessioning-and-reporting system in case of failure of hospital system or LIS | Develop alternative procedures to follow in the event of LIS failure | Follow procedure during downtime and returning to functioning LIS |
| Periodically test the backup system | |||
| Automated result reporting | None | Determine which tests are appropriate for automated reporting | Notify supervisor if automated results appear to be incorrect |
| Establish a procedure for reporting results without prior personnel review | Assist in the investigation of potentially problematic release of results | ||
| Establish a procedure for recall of results when autoverification fails to meet requirements | |||
Data mining.
Regulatory requirements or accrediting bodies may dictate specific lengths of time to maintain laboratory records. Older laboratory data are archived to maintain the permanent record; however, there may be difficulty in obtaining this information to analyze data over time. Ready access to at least 3 years or more of routine microbiology data permits queries and analysis of recent laboratory testing results for quality management decisions.
Data mining within the institution may be used to recognize associations that unearth trends, forecast future events, and determine appropriate courses of action. For example, a review of the patterns of recovery of clinically relevant microorganisms may reveal undetected clusters of hospital-associated infections, increased frequencies of multidrug-resistant microorganisms, or other user-defined observations that can contribute measurably to improving patient safety. Preparation of a cumulative antibiogram that characterizes the antimicrobial resistance patterns for an institution or a specific location, such as the intensive care unit (ICU), is another valuable use of the collective data. The LIS in the clinical microbiology may be utilized in many ways to benefit the patients, the laboratory, and the stakeholders (33).
QUALITY ASSURANCE AND IMPROVEMENT
This final overarching section contains the QSEs that complete the building of the QMS. These three QSEs are used to assess work performance and to continually improve laboratory processes. These QSEs include (i) assessing work by audits, feedback, and quality indicators; (ii) addressing and analyzing nonconforming events; and (iii) reevaluating processes to improve effectiveness and efficiency.
Assessments
Evaluations and audits.
Evaluations and audits are conducted to ensure that processes put into place are effective and achieve the desired results. It is critical to demonstrate effectiveness across the total testing process. Evaluations are less-formal reviews of specific areas or documents that may detect practices requiring process improvement. Audits are formal processes conducted either internally or externally according to a defined procedure by using established standards or regulations to examine the laboratory practices. The frequency of assessments is determined by the type of process and the risks associated with the process being reviewed. External audits are usually performed at a defined interval, such as biennially for CAP and CLIA or every third year for ISO standards. The number and frequency of internal audits may be dictated by an accrediting body or determined by the organization.
Audits conducted by external certification or accreditation bodies assess laboratory practices for the unique specification required by that entity. Some of the external entities that inspect diagnostic microbiology laboratories include the Centers for Medicare and Medicaid (CMS) for CLIA certification, the CAP and the Joint Commission for CLIA accreditation, state and local public health entities, the FDA for good manufacturing practices and good laboratory practices, the Occupational Safety Health Association (OSHA), and the Federal Aviation Association (FAA) for shipping, packing, and biosafety inspection prior to issuing an import permit for an organism by the Centers for Disease Control and Prevention (CDC). In other jurisdictions throughout the world, there may be specific organizations that inspect for compliance to their quality system. Industry partners may audit a laboratory that is engaged in clinical trials of a new diagnostic test or treatment.
During an internal audit, the laboratory should assess its quality management system against a recognized set of standards that applies to the quality system prescribed, such as ISO 15189. Alternatively, if the quality system is created by the institution, then a checklist that focuses on key quality activities as described by the quality manual and quality policy may be used to audit the quality system. Internal audits to determine the effectiveness of the QMS may be coupled with technical and scientific self-assessment performed for CLIA certification or accreditation.
Audits shall be performed by personnel trained to assess the performance of the laboratory as it pertains to the QMS. Auditors should be knowledgeable of laboratory operations, interpretation of the standards, and the auditing process. Wherever resources permit, auditors should be independent of the activity to be audited. Having an unbiased review of the documents and records allows an objective assessment of compliance to the standards. Supervisors from other laboratory sections and laboratory medicine trainees, such as pathology residents, infectious diseases fellows, and postdoctoral medical microbiologists, may perform the internal audit after they complete audit training.
Audits shall be conducted in a manner to ensure objectivity and impartiality. The criteria, scope, and timing of the audit shall be communicated prior to the audit, and the checklist of standards shall be shared in advance. At the conclusion of the audit, a summation conference should be held to review the nonconformities and provide an opportunity for questions. A written report of nonconformities cited shall be communicated to the laboratory in a timely manner.
Immediately following an audit, the laboratory shall address any nonconformity with the appropriate corrective action plan that will ensure compliance with the requirements. Nonconformities exposed during the audit should be evaluated for the impact of the activities on the work process, patient outcomes, and safety considerations. Those nonconformities affecting patient or staff safety shall be immediately addressed by remedial actions followed by corrective or preventive action plans that require more time and analysis. Laboratories should enter all findings in the nonconforming event log to be tracked and resolved. Documentation of the findings of the external audit and all activities to rectify the problems should be saved. Results of external audits and the “lessons learned” should be shared with management and staff as part of the review of the quality system.
Soliciting feedback.
Requesting feedback on how your laboratory services are meeting the needs and requirements of the user is an integral part of a quality management system. A vital first step is to recognize who your laboratory's “customers” are. The laboratory's customers are everyone who utilizes the services and information provided by the microbiology laboratory. These customers include the health care providers in emergency departments (EDs), ICUs, outpatient clinics, patient care floors, remote affiliated health care facilities, laboratories for whom you provide testing services, infection preventionists, pharmacists who uses the data for antibiotic stewardship, the public health laboratory, epidemiologists, and so on. Specific activities may be targeted for feedback, such as input requested after the implementation of a new testing protocol; for example, “Has the implementation of group A streptococcus NAAT impacted patients seen in urgent care?” or “Has the reduced turnaround time associated with the implementation of gastrointestinal pathogen NAAT impacted patient care?” Feedback data shall be reviewed and used to direct improvement activities. Understanding what is valuable to the customers will allow the appropriate use of limited resources and concentration on improvements of processes that provide the greatest impact to the customers. The laboratory should not wait for customers to voice their complaints as the only opportunity for understanding their needs. Proactively soliciting formal and informal feedback on what is working and what could be improved will provide information that is invaluable to improve the laboratory system. Changes made based on survey results should be monitored and communicated to those who provided the constructive comments.
Those who perform the laboratory tests and services can provide another valuable source of feedback. Who is better to provide input on how to improve evening-shift coverage than the staff who work on the second and third shifts? The laboratory should provide a forum to obtain input from the staff to identify areas ripe for process improvements and solutions that are effective and sustainable. It also reassures the microbiology staff that what they do and how they do it are important to the overall quality of the laboratory. Bench technologists can review SOPs to provide a constructive opportunity to change the SOP and the associated forms if needed. Reserving time in a laboratory meeting to share suggestions as well as communicating comments by e-mail allow the staff to be engaged in the improvement process.
Quality indicators.
In addition to assessments and solicited feedback, formal quality indicators shall be used to monitor performance. Quality indicators shall cover critical activities for the total testing process. Problems identified from the nonconforming event log and processes delineated as risks in an individualized quality control plan can provide ideas for quality indicators. The laboratory should also include quality indicators to monitor the performance of both the technical and nontechnical aspects of its work. Quality indicators may be created and tracked annually for a single specialty area in the microbiology laboratory (e.g., virology, parasitology, or mycology), across all microbiology areas, or across all diagnostic laboratory domains (e.g., chemistry, hematology, blood bank, and microbiology). Cross-cutting problems may be uncovered, which require the concerted effort of multiple laboratory sections and higher-level management to be resolved.
Examples of some quality indicators for the total testing process are shown in Table 24, and examples of some nonanalytical quality indicators are listed in Table 25. The laboratory should decide what to monitor as well as the expected benchmark to measure the data or the threshold for when action must be taken. An example of a microbiology quality improvement activities form is shown in Fig. 12. Responsibilities for monitoring performance using assessments, feedback, and quality indicators are shown in Table 26.
TABLE 24.
Examples of quality indicators for the total testing process
TABLE 25.
Examples of nonanalytical quality indicators
FIG 12.
Example of a quality indicator activities form. MDRO, multiple-drug-resistant organism; TAT, turnaround time; TTP, total testing process; CV, critical value. (Courtesy of Lana Fairbanks, Parkview Medical Center, Pueblo, CO.)
TABLE 26.
Roles and responsibilities associated with monitoring performance
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Internal assessment | Provide needed resources to accomplish an internal assessment | Plan and conduct annual assessments of quality practices and technical processes and procedures | Assist in review of SOPs, as qualified to do so |
| Provide needed resources to address corrective and preventive action plans | Have documents and records accessible for easy review by the assessors | ||
| Create corrective action plans to address findings | |||
| External audit | Ensure that senior management is engaged in inspections by meeting with inspectors and attending summation conferences | Be knowledgeable of the scope and criteria for the external review Prepare documentation, staff, and physical facility for audit Review and sign all required records |
Assist in preparation of documents and records Be prepared to answer inspectors' questions Contribute to the corrective and preventive action plans if asked |
| Provide suitable facilities for the external audit to be held | |||
| Acquisition of feedback | Provide a forum for customer feedback to be addressed | Openly solicit feedback from customers and staff | Provide constructive suggestions for improvement |
| Indicate processes that need improvement | |||
| Quality indicators | Involve microbiology laboratory management when key quality indicators or practice guidelines that would affect the laboratory are established | Collect data for identified practices that impact patient care | Participate in data collection and generate reports as appropriate |
| Submit data in the required format for analysis | |||
| Attend laboratory management review meetings as required | |||
Management of Nonconforming Events
Nonconforming events (NCEs) include any aspect of laboratory operation that does not conform to its own policies and procedures or the requirements of its customers (34). The laboratory shall have a nonconforming event management program in place to detect, document, analyze, and correct nonconforming events. This should be aligned with the health care organization's risk management program, as it may provide data related to systemic problems that may impact patient or staff safety. NCEs can be identified through a variety of mechanisms, including complaints from health care providers, patients, customers, or laboratory staff or an institutional event-tracking system. The review of internal laboratory records serves as a resource when processes and procedures failed to go as expected. Examples include quality control or calibration results outside acceptable limits, incorrect or unacceptable performance of PT/external quality assessment (EQA) results, and corrected or amended patient reports, such as an incorrect organism identification, clerical error, or results reported for the wrong patient. Documentation of reagents and/or consumables that do not perform as expected, which may or may not be related to a manufacturer's recall; results of internal and external audits; or unusual occurrences and unexpected or adverse outcomes all contribute to the NCE data.
The NCE management program shall include events identified and corrected before any harm occurred (a “near miss”) as well as corrected events and events with potential for harm. It must be undertaken in the atmosphere of a “just culture,” where events are viewed primarily as process shortcomings and not the fault of the individual. The focus is on the modification of the process to eliminate the likelihood of reoccurrence. The analysis should be linked to process improvements. Providing education and follow-up with staff regarding process improvements will encourage communication. As staff see that NCEs are not punitive and process modifications are put in place, they are more likely to report and not hide NCEs.
The NCE management program shall include identification of the remedial action taken, determination of the extent of the nonconformity, and determination of corrective action. The program should include designated individuals responsible for tracking events and analyzing trends. These individuals should have either the authority to implement change or a clearly delineated process for reporting to individuals with that authority.
If the nonconformity involves patient testing, the process shall also include reporting of the event as part of institutional event management when there is potential for patient harm. The event shall be analyzed to determine whether it is necessary to halt testing and reported to a person who has authority to resolve the problem and identify corrective action. This analysis shall include review of previously reported testing to assess for trends. If the nonconforming event has medical significance, it may be necessary to notify health care providers. If patient testing has been halted, a process to identify when it is acceptable to resume testing shall be developed.
If the nonconformity involves a manufacturer recall or the identification of a defective product produced commercially or by the laboratory, the process is similar to that described above. However, it should also include a determination of the impact on the laboratory and patient care, the need for repeat testing of samples analyzed by using the defective product, and the identification of an alternate product or the need to halt testing. This may involve members of the health care team outside the laboratory to determine the clinical impact on patient care.
Complaints from health care providers, patients, customers, or laboratory staff are a means of identifying problems related to products and services that do not meet specified requirements. The laboratory shall have a documented procedure for maintaining records of all valid complaints, their investigation, and action taken. Valid complaints should be incorporated into the laboratory's NCE documentation and monitored over time for improvement.
The program shall include a process for analysis of NCEs. An example of an NCE management form is shown in Fig. 13. The first step is documentation of what occurred in the process that led to the event, including the identification of all involved parties and actions and the date, time, and location, if the occurrence is outside the laboratory. If the occurrence is inside the laboratory, the specific laboratory section shall be designated. Investigation should include a review of procedures involved and adherence to the procedure, the instrumentation and software utilized, and the training and competency of involved parties. Involved parties should be interviewed, along with a determination of how the problem was identified.
FIG 13.
Example of a nonconforming event form used to document personnel or equipment failures and customer complaints. QNS, quantity not sufficient; POCT, point of care test.
The second step is classification of the event. Events can be classified according to the path of workflow as preanalytical, analytical, or postanalytical. Events can also be classified according to the potential for patient harm. For example, an error could be classified as having potential for severe harm where a Staphylococcus aureus isolate is reported as being susceptible to oxacillin when it is actually resistant. An error could be classified as being minor if the provider was unlikely to take any action that could cause harm or if the error, even if acted upon, was unlikely to cause harm. This may be a case where Staphylococcus capitis is misidentified as Staphylococcus hominis. Events may also be classified according to the type of problem: systemic (failure in the process or procedure), knowledge based (lack of training or competence), or behavior based (deliberate nonadherence to policies and procedures).
Tracking and analyzing patterns of events are some of the most important steps in analyzing NCEs. Recurring problems can be easily identified. NCEs can be sorted according to classification, and the data can be presented as pie charts or Pareto charts. The data can then be analyzed by using various parameters, including time, work shift, location, type of event, service, or classification. NCEs can be grouped according to specimen type or labeling, technologist error, data entry errors, lack of adequate procedures, or failure to follow procedures. By following an intervention to correct the problem, the results can be monitored graphically to show improvement.
After analysis of the NCE, a determination of the need for root-cause analysis should be made. Root-cause analysis should be performed for events that are recurring, high risk, high cost, or high volume as well as for any event with a severe adverse patient outcome (sentinel event). A variety of quality improvement methodology tools may be used to perform a root-cause analysis, including process mapping, fishbone diagrams, Pareto charts, and failure mode and effects analysis (FMEA). A simple technique of the “five whys,” which is asking “why” five times to probe deeper to find the root cause of a problem, can be useful for investigation. Examples of tools to perform a root-cause analysis are shown in Fig. 14, and a more detailed discussion can be found in CLSI document QMS11-A (34).
FIG 14.
Three examples of root-cause analysis tools, including a fishbone diagram to identify sources of an analytical error (A), a Pareto chart to identify sources of error where improvement will have the highest impact (B), and a process map to identify variation in the steps to complete a process (C). Root-cause analysis tools are used to investigate and uncover the true reason (the “root cause”) that errors occurred.
Once root-cause analysis has been performed, corrective action shall be implemented. Corrective action is the action taken to remove the cause of a nonconformity. The immediate action to resolve the nonconformity is remedial action. Determination of the cause may or may not require implementation of the tools described as part of root-cause analysis. For example, a technologist reports Borrelia caucasica rather than Burkholderia cepacia complex from a bacterial culture. The remedial action is to issue a corrected report and notify the provider with the correction. The cause is that the mnemonics used to report the two organisms (BOCC and BUCC, respectively) are too similar. The corrective action is to remove the mnemonic BOCC, as it is unlikely that this organism would be reported from a culture.
Preventive action is proactive action taken to eliminate the cause of the potential nonconformity. A preventive action plan shall be developed for high-risk events. As with corrective action, the effectiveness of the preventive action shall be monitored. The laboratory shall have a process for identifying potential NCEs (near misses) that require preventive action. This can be accomplished by performing a risk analysis using a fishbone diagram, an external quality assessment, or FMEA. For example, a technologist located an empty specimen container but failed to locate aliquots of a sample and reported the sample as having an insufficient quantity to perform additional testing. The aliquots were later located, and testing was performed. As part of the FMEA shown in Fig. 15, preventive action was initiated to store empty sample containers and their aliquots in the same resealable bag to prevent this from happening in the future.
FIG 15.
Example of a failure mode and effects analysis (FMEA) form to identify high-risk processes and needed improvements to reduce the chance of an unintended adverse event.
It is imperative that a review of outcomes of the corrective and preventive action plans occurs as part of the NCE management program. This can be handled as part of the continual improvement process of the laboratory or less formally by elimination of the NCE recurrence.
The final step is to provide data and analysis in a summary report to laboratory management. Laboratory management shall determine the priority for problem resolution and coordinate with risk management for prioritization. Consideration should be given to the frequency of the event, the risk of patient harm, the risk to laboratory accreditation, customer satisfaction, and the cost of the occurrence of the event when determining priority. Laboratory management should determine the need for root-cause analysis and allocate appropriate resources. Recurring NCEs that are high-risk events should be identified as opportunities for improvement (OFIs) as part of the continual improvement process. The roles and responsibilities for managing NCEs are listed in Table 27.
TABLE 27.
Roles and responsibilities for managing nonconforming events
| Activity | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Nonconforming event management program | Review as part of risk management program | Establish, approve, and annually review nonconforming-event management program | None |
| Complaint resolution | Review as part of risk management program | Investigate complaints in a timely manner | Document complaints |
| Identify remedial action and provide timely response, if appropriate | |||
| Analyze and trend for commonalities | |||
| Investigation of nonconforming events | Part of institutional event management program, when appropriate | Perform investigation | Document the event |
| Determine clinical significance | |||
| Determine remedial and corrective actions | |||
| Analysis of nonconforming events | Review report of analysis | Track and analyze trends of events | Document the event |
| Review corrective actions as part of quality management program | Prepare report | Report issues if corrective action is not working or caused unintended consequences | |
| Monitor corrective action | Review as part of quality management program | Monitor effectiveness of corrective action | Report any nonconforming events |
| Revise corrective action as needed | |||
Continual Improvement
The continual improvement (CI) process is a key component of the quality management system. Through this process, OFIs are identified and acted upon to improve outcomes and customer satisfaction. Activities shall be prioritized based upon risk assessment. Quality improvement models and tools should be used to generate improvement plans. Plans shall be documented and implemented. The effectiveness of the plan shall be determined through ongoing review (35).
OFIs can be identified proactively and not just as a reaction to a problem. Not all OFIs need to be major changes; small improvements are not only easy to implement but also extremely valuable. OFIs can be identified from four quality system essentials, organization, customer, assessments, and nonconforming events, or by the laboratory staff. OFIs can be planned activities as part of the quality management system. They can be identified through customer and/or staff feedback solicited through surveys; strength, weakness, opportunity, threat (SWOT) analyses; literature review; the institutional mission or values; and risk assessments. OFIs can also be identified from failures or customer complaints or discovered through internal and external assessments, quality indicators, or analyses of nonconforming events.
There are several different models that can be used as an approach to CI. There is no strict rule that determines which model to employ. The selection of improvement models depends on institutional preference and expertise available as well as the problem. All models are iterative, with a review step to determine the effectiveness of the change to improve the process.
The most commonly used model for improvement in clinical settings is plan-do-check-act (PDCA) (36). This model is a four-step approach that allows CI. The “plan” phase identifies an OFI, determines the root cause, and determines a potential solution. The “do” phase is the implementation step. The solution most often employed is implementation on a small scale (e.g., try a new specimen collection method in one area of the hospital). The “check” phase analyzes the data to determine if the solution eliminated the issue without undue or unexpected consequences. The “act” phase allows the full-scale implementation or reassessment of the plan.
For example, a risk assessment of blood cultures identified that some Gram stains of positive blood cultures were not reported within 1 h of the indication that the bottle was positive. As part of the “plan” phase of PDCA, this was selected as an OFI. It was identified that these cultures were indicated as positive on the night shift. The solution generated was to implement night-shift reading of Gram stains of positive blood cultures. In the “do” phase, night-shift personnel were trained, and Gram stains were reread by microbiologists on the day shift to evaluate the effectiveness of the training and provide feedback. The time to reporting was monitored. In the “check” phase, it was determined that as night-shift personnel were now required to perform Gram stains, an unintended consequence, that personnel were not as readily available in the chemistry department, was observed. In the “act” phase, the plan was reassessed to allow laboratory assistants to prepare the slides and subculture the bottles, with technologists staining and reading the slides. This allowed the technologists more time to perform testing in other areas.
Some other models for CI include six sigma, lean, and lean six sigma. Six sigma attempts to eliminate error by eliminating variation in the process (37). The term originates from industry, where the sigma value is how often errors are likely to occur. The best processes have six sigma or < 3.4 defects per 1 million products. Six sigma uses the DMAIC model, an acronym for define, measure, analyze, improve, and control, a process improvement model similar to the PDCA model. The lean process focuses on the elimination of waste in an effort to deliver a quality product (38). The lean process requires the evaluation of processes to identify areas of waste or activities that do not add value to the product (e.g., repeat work, delays, unnecessary use of resources, or high inventory) that can be eliminated. Elimination of waste identified through lean processes often requires a redesign of work areas to improve efficiency and safety. The lean six sigma model combines the concept of eliminating waste to improve throughput with the concept of reducing variability to improve processes (39). A more detailed description of how to implement these process improvement models is beyond the scope of this document (for further information on these quality improvement topics, see references 40–45).
Regardless of the model employed, it should consist of a means to identify and select an OFI and to generate and implement a solution. The effectiveness of the solution should be evaluated and monitored for unintended consequences. It is imperative that the improvement be integrated into routine processes. Individuals can revert to old habits, so a means to sustain the improvement should be incorporated. Reporting of outcomes to management and staff facilitates this and encourages feedback.
CI is a process of continual change. Change involves risk, and the laboratory should have a process to identify risks and their potential impact. Management shall provide the team with adequate resources, including training and/or qualified individuals to perform the risk analysis, and management may provide additional support and approval for solutions. Management should develop a strategy for handling change that includes both tangible change and psychological transition. Management should review the outcomes of the solutions. The roles and responsibilities for CI activities are listed in Table 28.
TABLE 28.
Roles and responsibilities for managing continual improvementa
| Activity(ies) | Role(s) and/or responsibility(ies) |
||
|---|---|---|---|
| Institution | Microbiology laboratory management | Microbiology staff | |
| Continual improvement process | Provide data identifying OFIs Provide support for quality improvements Review reports as part of quality management program |
Ensure laboratory participation in the institutional plan Ensure that laboratory CI activities are in line with institutional quality objectives and address relevant areas of patient care |
Identify OFIs such as near misses Provide feedback on the improvement/change Participate in CI |
| Identify OFIs and formulate and implement solutions | |||
| Evaluate effectiveness and recognize success | Recognize success | Solicit staff feedback | Provide feedback on improvement solution |
| Evaluate the effect of change on the organization, where applicable | Evaluate effectiveness and revise if necessary | ||
| Communicate outcomes to management and staff | |||
| Recognize success | |||
CI, continual improvement; OFIs, opportunities for improvement.
Communication is a critical component of the CI process. It is important to share information regarding the goals and status of CI projects, provide opportunities for staff feedback, and celebrate the successes of the CI process (46). Written records shall be maintained, which include discussion items, CI tools, decisions, recommendations, and reviews. Some laboratories implement a daily information exchange on operations and quality. Daily accountability meetings (“huddle meetings”) may be held within laboratory sections and across supervisory sections to focus staff and management on the status of ongoing projects and problems in the laboratory. A visual display board can be used during daily huddle meetings. The items posted on a visual display board can vary, but they are generally organized according to lean principles and thus aim toward minimizing waste and standardizing processes. The board may be divided into two sections, as shown in Fig. 16. The information under the “hot button list” captures the daily staffing, workload, and safety issues and customers' feedback, while the “lean daily management system” section highlights current and future quality improvement projects and organizational strategic plans. Small quality improvement projects, called kaizens (kai for change and zen for good), may be included on the visual display board to inspire larger ones. Frequent communication encourages input from all levels of the laboratory staff and helps to provide a platform for shaping organizational culture, building trust within a team, and engaging and empowering staff.
FIG 16.
Example of a visual display board that highlights short- and long-term quality issues for the laboratory. Items on a visual display board are grounded on lean principles. The “Hot Button List” section of the display board focuses on laboratory workload statistics, safety issues, daily operational status, and customers’ feedback. The “Lean Daily Management System (LDMS)” section highlights progress on quality improvement projects and captures kaizens, which are small improvement projects that contribute to lean transformation. This low-cost visual media can be used during daily huddle meetings in clinical microbiology laboratories to recognize staff, celebrate successes on ongoing projects, and track efforts to meet institutional goals that align with strategic plans. A mobile white board facilitates sharing of relevant information at other meeting locations. The LDMS tool includes technical staff and management, reinforces accountability, and informs and engages everyone during the lean process.
CONCLUSION
This document outlines a comprehensive practical approach to a laboratory quality management system for the clinical microbiology laboratory by addressing the ISO 15189 requirements under three main categories: quality infrastructure, laboratory operations, and quality assurance and continual improvement. Practical examples and forms have been included to assist in the real-world implementation of this system and to allow the adaptation of the system to each laboratory's unique environment. Errors and nonconforming events are acknowledged and embraced as opportunities to improve the quality of the laboratory instead of penalizing individuals. An effective QMS encourages “systems thinking” by providing a process to think globally of the effects of any type of change.
To be truly successful, the QMS should not merely be used as a management tool but should also be practiced daily by each microbiology staff member, including laboratory management, and fully supported by the institution's leadership.
Biographies

Roberta B. Carey, Ph.D., D.(A.B.M.M.), C.M.Q./O.E. (A.S.Q.), held the positions of Director of the Laboratory Quality Management Program Infectious Diseases Laboratories at the Centers for Diseases Control and Prevention and CLIA Laboratory Director until she retired in 2016. She was the Branch Chief of Clinical and Environmental Microbiology at the CDC and Acting Director for the Division of Laboratory Systems. Before coming to the CDC in 2004, Dr. Carey was a clinical laboratory director at the Loyola University Medical Center, Maywood, IL; St. Francis Hospital, Evanston, IL; and Wyler Children's Hospital, University of Chicago. She is a board-certified diplomate of the American Board of Medical Microbiology and a Certified Manager of Quality/Organizational Excellence certified by the American Society for Quality. She earned her Ph.D. at the Cornell University Graduate School of Medical Sciences and did a postdoctoral fellowship in clinical and public health microbiology at Temple University in Philadelphia, PA.

Sanjib Bhattacharyya, Ph.D., C.Q.I.A. (A.S.Q.), is the Laboratory Director at the City of Milwaukee Health Department. He previously held Deputy Laboratory Director and Chief Molecular Scientist positions at the Milwaukee public health laboratory. He is an adjunct faculty member of the UW-Milwaukee Zilber School of Public Health and Clinical Associate Professor, College of Health Sciences. He has a Ph.D. in Medical Microbiology from the University of Kolkata, India, and a postdoc at the Medical College of Wisconsin and VA Medical Center. He is a member of the American Society for Microbiology and the Association of Public Health Laboratories and an ASQ-Certified Quality Improvement Associate with expertise in quality management practices. He currently serves on the APHL Environmental Laboratory Science and Global Health committees and Culture-Independent Diagnostic Testing subcommittee and is a member of the APHL-CDC public health laboratory competency implementation steering committee. He is the recipient of the 2013 APHL Emerging Leader Award.

Sue C. Kehl, Ph.D., D.(A.B.M.M.), earned her Ph.D. in pathology from the Medical College of Wisconsin, Milwaukee, WI. She completed a postdoctoral program in medical and public health microbiology at Mount Sinai Medical Center, University of Wisconsin, where she focused on the development of rapid technologies for identification and susceptibility testing. She is a diplomate of the American Board of Medical Microbiology. She was the director of virology at the International Clinical Laboratory, Nashville, TN, and later the associate director of the microbiology laboratory at Kaiser Permanente Regional Laboratory, Clackamas, OR. She is currently a Professor in pathology at the Medical College of Wisconsin, where she had served as director of clinical microbiology at Dynacare Laboratories. Dr. Kehl is currently the associate director of clinical pathology and director of clinical microbiology at Children's Hospital of Milwaukee. She serves as the director of the quality management program for the department of pathology.

Larissa M. Matukas, M.Sc., M.D., F.R.C.P.C., is an Assistant Professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto, Head of the Division of Microbiology, an infectious disease physician at St. Michael's Hospital, and a Consultant Medical Microbiologist at St. Joseph's Health Centre, Canada. She received her master of science in Immunology of Infectious Diseases from the London School of Hygiene and Tropical Medicine, United Kingdom, and completed her M.D. training and residencies in internal medicine, infectious disease, and medical microbiology at the University of Toronto, Canada. Her specific areas of interest and expertise are in clinical microbiology quality improvement, utilization, and stewardship and antimicrobial resistance and stewardship.

Michael A. Pentella, Ph.D., M.S., S.M.(A.S.C.P.), C.I.C., D.(A.B.M.M.), is a Clinical Professor at the University of Iowa College of Public Health He previously directed the Massachusetts State Public Health Laboratory and was associate director at the Iowa State Hygienic Laboratory. His experience spans over 40 years in clinical microbiology and public health laboratories. He is certified as an American Board of Medical Microbiology Diplomate, a specialist in microbiology through the American Society for Clinical Pathology, and certified in infection control through the Association of Professionals in Infection Control. He has held academic appointments at Thomas Jefferson University, the University of South Florida, and the University of Iowa. Over the years, he has made several contributions that have improved diagnostic testing assays. He has written over 40 articles and nine book chapters.

Max Salfinger, M.D., F.I.D.S.A.F.A.A.M., is the Executive Director of Advanced Diagnostic Laboratories at National Jewish Health in Denver, CO, and Director of the Mycobacteriology and Pharmacokinetics Laboratories. He is a Fellow of the Infectious Diseases Society of America and the American Academy of Microbiology. Before joining National Jewish Health, he was the Florida State Public Health Laboratory Director from 2006 to September 2012 as well as the Acting Florida State TB Controller for his last 19 months with the Florida Department of Health. Dr. Salfinger was with the New York State Department of Health Wadsworth Center from 1992 to November 2006, where he implemented the New York State Fast Track Program for Rapid Tuberculosis Testing. He was Director of the Tuberculosis Laboratory at the University of Zurich, which became the Swiss National Center for Mycobacteria, and a fellow at the University Hospital in Basel, Switzerland. Dr. Salfinger received his M.D. from Basel University.

Audrey N. Schuetz, M.D., M.P.H., D.(A.B.M.M.), is an Associate Professor of Laboratory Medicine and Pathology at the Mayo Clinic School of Medicine and Science in Rochester, MN. She received her M.D. and completed Pathology residency and a Medical Microbiology fellowship at Emory University School of Medicine. She is board certified in Clinical Pathology, Anatomic Pathology, and Medical Microbiology through the American Board of Pathology and is board certified by the American Board of Medical Microbiology. Dr. Schuetz is Director of Initial Processing and Codirector of Bacteriology in the Division of Clinical Microbiology at Mayo. She is a member of the Microbiology Resource Committee of the College of American Pathologists and a member of the Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing. Her interests include antimicrobial susceptibility testing, anaerobic bacteriology, Infectious Diseases Pathology, as well as evaluation of rapid diagnostic techniques to improve antimicrobial stewardship.
APPENDIX
GLOSSARY
- analytic
Activities executed in the testing process, e.g., the steps to conduct a procedure; referred to as examination in ISO documents.
- audit
Systematic, independent, and documented process for obtaining and evaluating evidence objectively to determine the extent to which audit criteria are fulfilled.
- blind sample
A selected sample whose composition is unknown except to the person submitting it for testing; used to validate the testing process or perform competency assessment of testing personnel.
- Clinical Laboratory Improvement Amendment (CLIA)
U.S. regulation that specifies the requirements for clinical laboratories to be certified to operate in the United States.
- College of American Pathologists (CAP)
An organization authorized by the U.S. Centers for Medicare and Medicaid to inspect and accredit clinical laboratories and to provide a proficiency testing program.
- continual improvement (CI)
Ongoing improvement of products, services, or processes through incremental steps using the plan, do, check, act (PDCA) model.
- corrective action
Solution meant to eliminate the cause of a detected nonconformity. Corrective action is taken to prevent reoccurrence, whereas preventive action is taken to prevent occurrence.
- customer
Organization or person(s) that receives the product, service, or information. A customer can be internal or external to the organization.
- define, measure, analyze, improve, control (DMAIC)
Six sigma model for continual quality improvement.
- delta checks
A comparison of consecutive values for a given test performed on a patient to detect significant changes from the previous result. An example includes variations in numerical results for molecular or quantitative assays, such as viral load assays, that are monitored as part of a computer-based quality control program.
- document
Written or electronically generated information and/or work instructions. Examples include manuals, protocols, policies, and forms.
- failure mode and effects analysis (FMEA)
Prospective risk analysis of high-risk processes to identify needed improvements that will reduce the chance of an unintended adverse event.
- Health Insurance Portability and Privacy Act (HIPPA)
U.S. law that requires confidentiality of patient records.
- individual quality control plan (IQCP)
Term used to describe an alternative quality control plan based on risks and customized for each laboratory setting; used in place of the quality control requirements specified by CLIA regulations.
- Institute for Quality Management in Healthcare (IQMH)
Canadian provider of accreditation and proficiency testing.
- International Organization for Standardization (ISO)
A voluntary, worldwide federation of national standard bodies that provides governance and guidance on international practice standards.
- just culture
term used to describe a working environment where events are viewed primarily as process shortcomings and not the fault of the individual, with the goal toward modification of the process to eliminate the likelihood of reoccurrence and not punishment of the individual.
- laboratory director
Person with responsibility and authority over a laboratory or laboratory section. National, regional, and local regulations may apply with regard to qualifications and training.
- laboratory error
Failure of a planned action to be completed as intended or use of a wrong plan to achieve an aim. Laboratory error may occur at any part of the testing process from specimen collection to result reporting.
- laboratory information system/laboratory information management system (LIS/LIMS)
Manual or electronic means of handling the laboratory test process from acquisition to results and storage.
- laboratory management
Persons responsible for the administrative, technical, and legal activities of the laboratory or laboratory section. This may include the pathologist, laboratory director, deputy/associate director, laboratory manager, laboratory supervisors, and consultants/corporate advisors.
- laboratory staff
Any testing personnel or administrative personnel who perform functions within the laboratory.
- lean
Focus on reducing cycle time and waste in a process by identifying and eliminating non-value-added steps.
- limit of detection (LOD)
The lowest concentration of a substance or an organism that can be detected in an assay.
- near miss
When something that could be a problem is averted before it occurs; may also be called a “near hit.”
- nonconformity/nonconforming event
Failure to fulfill a specified requirement. Examples include quality control or equipment failures, complaints from customers, or failure to follow procedures.
- plan, do, check, act (PDCA)
Cyclical model for continual quality improvement.
- policy
A documented statement of the intended action or direction of an organization that is approved and endorsed by the leadership. Policy answers the question “What do we do?”
- postanalytical
Activities in the testing process that follow the analytical or examination phase, e.g., report of the test results; referred to as postexamination in ISO documents.
- preanalytical
Activities in the testing process that precede the analytical or examination phase, e.g., specimen collection and accessioning; referred to as preexamination in ISO documents.
- preventive action
Steps taken to eliminate the cause of a potential nonconformity or undesirable situation. Preventive action addresses problems that have not yet occurred, whereas corrective action is taken to prevent reoccurrence.
- procedure
A step-by-step set of directions describing how to complete a method or activity. A written procedure to perform an assay is also referred to as a protocol.
- process
A set of interrelated or interacting activities that transforms the intent into action. Process answers the question “How does it get done?”
- protected or personal health information (PHI)
Used to identify a patient's health status or health care payment.
- quality assurance (QA)
The planned and systematic activities in a quality system to ensure that quality requirements are met, e.g., tracking and trending of quality control values over time.
- quality control (QC)
A procedure or set of procedures intended to ensure that a product or service adheres to a defined set of quality criteria.
- quality indicators (QIs)
observations, statistics, or data defined by the organization that typify the performance of a work process and provide evidence that the organization is meeting its intentions.
- quality management system (QMS)
Organizational structure, procedures, processes, and resources needed to ensure consistent quality.
- quality system essentials (QSEs)
The 12 building blocks of a quality management system as described by the CLSI.
- record
Evidence of results achieved or actions performed. A completed form becomes a record.
- reference interval/reference range
A range of values applied to the population serviced by the laboratory; includes most of the subjects in the normal population, e.g., a range of values used to define the normal concentration ranges for glucose, cholesterol, or liver enzymes. It may also be expressed as the absence of a microorganism for the normal population.
- remedial action
Immediate action put into place to correct or mitigate a nonconforming event.
- root cause
The underlying or most elemental reason for a nonconforming event, which, if corrected, prevents the reoccurrence of the nonconforming event.
- safety data sheets (SDSs)
Information describing any hazards associated with the use, storage, or disposal of the product; formerly called material safety data sheets (MSDSs).
- select agents
Regulated biological pathogens and toxins as defined in HHS 42 CFR part 73. These microorganisms, genetic elements, or toxins have been recognized to potentially pose a severe threat to public health, including humans, animals, and plants, as opposed to any biological agent that could be used as a biothreat.
- shall
A requirement to be met; no opting out.
- should
Implies that the action may be followed, but it is not an absolute requirement to do so.
- six sigma
A term used to indicate that a process is well controlled; a collection of techniques and tools to minimize variation in the process or a program of improvement.
- standard operating procedure (SOP)
The approved steps to complete a test or task.
- total testing process (TTP)
All actions associated with process, including preanalytical, analytical, and postanalytical phases.
- validation
As defined by the ISO, act of confirming that a service or product meets the requirements for which it was intended, e.g., establishing the performance specifications for a laboratory-developed test.
- verification
As defined by the ISO, act of confirming through the provision of objective evidence that specified requirements have been fulfilled, e.g., verifying that a new instrument meets the performance specifications claimed by the manufacturer.
Footnotes
[This article was published on 2 May 2018 with the title “Implementing a Quality Management System in the Medical Microbiology Laboratory.” The title was updated in the current version, posted on 20 July 2020.]
REFERENCES
- 1.International Organization for Standardization. 2012. Medical laboratories—requirements for quality and competence, 3rd ed ISO document 15189. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2011. A quality management system model for laboratory services; approved guideline, 4th ed CLSI document QMS01-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.Bartlett RC, Mazens-Sullivan M, Tetreault JZ, Lobel S, Nivard J. 1994. Evolving approaches to management of quality in clinical microbiology. Clin Microbiol Rev 7:55–88. doi: 10.1128/CMR.7.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schifman RB, Cembrowski GS, Wolk DM, Brisbois JI. 2014. Quality management, p 421–446. In Garcia LS, Bachner P, Baselski VS, Lewis MR, Linscott AJ, Schwab DA, Steele JCH Jr, Weissfeld AS, Wilkinson DS, Wolk DM (ed), Clinical laboratory management, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 5.US Department of Health and Human Services, Centers for Medicare and Medicaid Services. 2003. Medicare, Medicaid and CLIA programs; laboratory requirements relating to quality systems and certain personnel qualifications. Final rule. 42 CFR 493. Fed Regist 68:3640–3714. [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2012. Quality management system: leadership and management roles and responsibilities; approved guideline. CLSI document QMS14-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Njorogi SW, Nichols JH. 2014. Risk management in the clinical laboratory. Ann Lab Med 34:274–278. doi: 10.3343/alm.2014.34.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute for Quality Management in Healthcare, Centre for Accreditation. 2017. Medical laboratory accreditation requirements 2017, version 7.1. Institute for Quality Management in Healthcare, Toronto, Ontario, Canada. [Google Scholar]
- 9.Kurec AS. 2014. Employee selection, p 295–308. In Garcia LS, Bachner P, Baselski VS, Lewis MR, Linscott AJ, Schwab DA, Steele JCH Jr, Weissfeld AS, Wilkinson DS, Wolk DM (ed), Clinical laboratory management, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 10.Medvescek P. 2014. Staffing and scheduling, p 362–372. In Garcia LS, Bachner P, Baselski VS, Lewis MR, Linscott AJ, Schwab DA, Steele JCH Jr, Weissfeld AS, Wilkinson DS, Wolk DM (ed), Clinical laboratory management, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 11.WHO. 2008. Laboratory quality management handbook. World Health Organization, Geneva, Switzerland: http://www.who.int/ihr/training/laboratory_quality/quality_manual/en/ Accessed 9 January 2018. [Google Scholar]
- 12.Desjardin M, Fleming CA. 2014. Competency assessment of microbiology medical laboratory technologists in Ontario, Canada. J Clin Microbiol 52:2940–2945. doi: 10.1128/JCM.01035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ned-Sykes R, Johnson C, Ridderhof JC, Perlman EA, Pollock A, DeBoy JM. 2015. Competency guidelines for public health laboratory professionals. CDC and the Association of Public Health Laboratories. MMWR Suppl 64:1–81. [PubMed] [Google Scholar]
- 14.International Organization for Standardization. 2003. Medical laboratories—requirements for safety. ISO document 15190. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2012. Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened biosafety blue ribbon panel. MMWR Suppl 61:1–103. [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services, Centers for Disease Control and Prevention National Institutes of Health. 2007. Biosafety in microbiological and biomedical laboratories, 5th ed Government Printing Office, Washington, DC. [Google Scholar]
- 17.Public Health Agency of Canada. 2015. Canadian biosafety standard (CBS), 2nd ed Public Health Agency of Canada, Ottawa, Ontario, Canada: https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html Accessed 9 January 2018. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2011. Establishing molecular testing in clinical laboratory environments; approved guideline. CLSI document MM19-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2006. Preparation and testing of reagent water in the clinical laboratory; approved guideline, 4th ed CLSI document C03-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Centers for Disease Control and Prevention. 2011. Guidelines for biosafety laboratory competency. CDC and the Association of Public Health Laboratories. MMWR Suppl 60:1–23. [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2014. Protection of laboratory workers from occupationally acquired infections; approved guideline, 4th ed CLSI document M29-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2013. Quality management system: development and management of laboratory documents; approved guideline, 6th ed CLSI document QMS02-A6. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Miller JM. 1999. A guide to specimen management in clinical microbiology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 24.Wolk DM, Marlow EM. 2016. Molecular method verification, p 721–744. In Persing DH, Tenover FC, Hayden RT, Ieven M, Miller MB, Nolte FS, Tang Y-W, van Belkum A (ed), Molecular microbiology. Diagnostic principles and practice, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 25.Bankowski MJ. 2016. Molecular microbiology test quality assurance and monitoring, p 745–756. In Persing DH, Tenover FC, Hayden RT, Ieven M, Miller MB, Nolte FS, Tang Y-W, van Belkum A (ed), Molecular microbiology. Diagnostic principles and practice, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 26.Clark RB, Lewinski MA, Loeffelholtz MJ, Tibbetts RJ. 2009. Cumitech 31A, Verification and validation of procedures in the clinical microbiology laboratory. Coordinating ed, Sharp SE. ASM Press, Washington, DC. [Google Scholar]
- 27.College of American Pathologists. 2017. All common and laboratory general checklists. College of American Pathologists, Northbrook, IL. [Google Scholar]
- 28.Allen L, Crawford L. 2014. Guidance on measurement uncertainty for medical laboratories. Institute for Quality Management in Healthcare, Toronto, ON, Canada. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2011. Laboratory quality control based on risk management; approved guideline, 1st ed CLSI document EP-23-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Animal and Plant Health Inspection Services, Centers for Disease Control and Prevention. 2017. Select agents regulations. Federal Select Agent Program. Centers for Disease Control and Prevention, Atlanta, GA: http://www.selectagents.gov/Regulations.html Accessed 9 January 2018. [Google Scholar]
- 31.Centers for Disease Control and Prevention, US Department of Health and Human Services. 1997. Additional requirements for facilities transferring or receiving select agents. 42 CFR part 72. Centers for Disease Control and Prevention, Atlanta, GA: https://grants.nih.gov/grants/policy/select_agent/42CFR_Additional_Requirements.pdf. Accessed 17 November 2017. [Google Scholar]
- 32.Marcon MJ. 2003. “Value-added” reporting in clinical microbiology: using computer-based textual reports and comments as an aid to communicating appropriate utilization and application of test results. Clin Microbiol Newsl 25(4):25–31. doi: 10.1016/S0196-4399(03)80004-1. [DOI] [Google Scholar]
- 33.Campos JM. 2014. The laboratory information system: making the most of IT in the clinical microbiology laboratory, p 458–470. In Garcia LS, Bachner P, Baselski VS, Lewis MR, Linscott AJ, Schwab DA, Steele JCH Jr, Weissfeld AS, Wilkinson DS, Wolk DM (ed), Clinical laboratory management, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2007. Management of nonconforming events; approved guideline. CLSI document QMS-11A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2011. Quality management system: continual improvement; approved guideline, 3rd ed CLSI document QMS06-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Zarbo RJ, Copeland JR, Varney RC. 2017. Key management subsystem driver of knowledge-based continuous improvement in the Henry Ford Production System. Am J Clin Pathol 148:354–367. doi: 10.1093/ajcp/aqx084. [DOI] [PubMed] [Google Scholar]
- 37.Tennant G. 2001. Six sigma: SPC and TQM in manufacturing and services. Gower Publishing, Burlington, VT. [Google Scholar]
- 38.White BA, Baron JM, Dighe AS, Camargo CA, Brown DFM. 2015. Applying LEAN methodologies reduces ED laboratory turnaround times. Am J Emerg Med 33:1572–1576. doi: 10.1016/j.ajem.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaffie S, Shahbazi S. 2012. The McGraw Hill 36-hour course: lean six sigma. McGraw Hill, Chicago, IL. [Google Scholar]
- 40.Jones B, Raab S, Sesok-Pizzini D, Sirota R, Valenstein P. 2005. Approaches to managing quality and patient safety, p 17–48. In Valenstein P. (ed), Quality management in clinical laboratories: promoting patient safety through error reduction and continuous improvement. College of American Pathologists, Northbrook, IL. [Google Scholar]
- 41.Murman E, Weigel A, Haggerty A, McManus H. January 2008, posting date. Introduction to lean six sigma methods. Ses 1-2. MIT 16.660. YouTube video, posted by MIT Open CourseWare. https://www.youtube.com/watch?v=8RlA0D6cjDc Accessed 10 April 2018.
- 42.Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. 2009. The improvement guide: a practical approach to enhancing organizational performance, 2nd ed Institute for Healthcare Improvement, Jossey-Bass, San Francisco, CA. [Google Scholar]
- 43.Gras JM, Philippe M. 2007. Application of the six sigma concept in clinical laboratories: a review. Clin Chem Lab Med 45:789–796. doi: 10.1515/CCLM.2007.135. [DOI] [PubMed] [Google Scholar]
- 44.Elder BL. 2008. Six sigma in the microbiology laboratory. Clin Microbiol Newsl 30:143–147. doi: 10.1016/j.clinmicnews.2008.09.001. [DOI] [Google Scholar]
- 45.Westgard JO, Westgard SA. 2017. Six sigma quality management system and design of risk-based statistical quality control. Clin Lab Med 37:85–96. doi: 10.1016/j.cll.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell PS, Mandrekar JN, Yao JDC. 2014. Adoption of lean principles in a high-volume molecular diagnostic microbiology laboratory. J Clin Microbiol 52:2689–2693. doi: 10.1128/JCM.00430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]