Despite a century of investigation, Streptococcus pneumoniae remains a major human pathogen, causing a number of diseases, such as pneumonia, meningitis, and otitis media. Like many encapsulated pathogens, the capsular polysaccharide (CPS) of S. pneumoniae is a critical component for colonization and virulence in mammalian hosts.
KEYWORDS: Streptococcus pneumoniae, capsular polysaccharide, colonization, complement, glycoside hydrolase, invasive pneumococcal diseases, serotype 3
ABSTRACT
Despite a century of investigation, Streptococcus pneumoniae remains a major human pathogen, causing a number of diseases, such as pneumonia, meningitis, and otitis media. Like many encapsulated pathogens, the capsular polysaccharide (CPS) of S. pneumoniae is a critical component for colonization and virulence in mammalian hosts. This study aimed to evaluate the protective role of a glycoside hydrolase, Pn3Pase, targeting the CPS of type 3 S. pneumoniae, which is one of the most virulent serotypes. We have assessed the ability of Pn3Pase to degrade the capsule on a live type 3 strain. Through in vitro assays, we observed that Pn3Pase treatment increases the bacterium's susceptibility to phagocytosis by macrophages and complement-mediated killing by neutrophils. We have demonstrated that in vivo Pn3Pase treatment reduces nasopharyngeal colonization and protects mice from sepsis caused by type 3 S. pneumoniae. Due to the increasing shifts in serotype distribution, the rise in drug-resistant strains, and poor immune responses to vaccine-included serotypes, it is necessary to investigate approaches to combat pneumococcal infections. This study evaluates the interaction of pneumococcal CPS with the host at molecular, cellular, and systemic levels and offers an alternative therapeutic approach for diseases caused by S. pneumoniae through enzymatic hydrolysis of the CPS.
INTRODUCTION
Streptococcus pneumoniae, the causal agent of pneumonia, meningitis, and otitis media, remains a major threat to human health. This bacterium can stably colonize the human nasopharynx as a part of the normal commensal microflora (1–3). Colonization is the primary mode of transmission and a key step in the initiation of disease, despite asymptomatic carriage (4, 5). A critical component for survival within the host and full pathogenicity of most S. pneumoniae strains is the capsular polysaccharide (CPS) (6, 7). The CPS is a large and distinct polysaccharide structure coating the entire surface of the bacterium. The capsule helps S. pneumoniae evade the host immune system through resisting or inhibiting its phagocytosis by host macrophages while also limiting mucus-mediated clearance (8–11). S. pneumoniae has over 90 unique capsular serotypes, each differing in monosaccharide composition and linkage, as well as other modifications, such as acetylation (12). The requirement of the CPS in bacterial virulence, surface accessibility, and antigenicity has made it a target in vaccination studies for over 100 years (12–16). Great strides have been made in increasing the immunogenicity and efficacy of pneumococcal vaccines that utilize the CPS by conjugation to a protein carrier (17, 18). Current pneumococcal vaccines aim to provide serotype-specific protection for some of the most relevant clinical serotypes (12, 13). The use of the 7- and 13-valent pneumococcal conjugate vaccines PCV7 and PCV13 (Prevnar; Pfizer) has been a major success, reducing invasive pneumococcal disease (IPD) rates significantly in both vaccinated and unvaccinated populations (15, 19–22).
While the conjugate vaccines have been effective for preventing carriage and IPD caused by most included serotypes, the exception has been serotype 3. The pneumococcal type 3 polysaccharide (Pn3P) component of the current PCV13 induces variable immune responses to S. pneumoniae serotype 3 (23–25). Pn3P is a linear polymer of -3)βGlcA(1–4)βGlc(1- disaccharide repeating units with an average molecular weight of >400 kDa (26, 27). It was noted that significantly higher serum titers are required for serotype 3 in comparison with other serotypes for opsonophagocytic killing of S. pneumoniae (12, 28, 29). Numerous animal model and epidemiology studies have associated type 3 strains with increased virulence and risk of death compared to other pneumococcal serotypes (30–32). A recent case report demonstrated a fatal case of IPD caused by serotype 3, highlighting increased complications and generally poor outcomes associated with this invasive serotype (33). In addition, recent data indicate that S. pneumoniae strains are resistant to one or more antibiotics in 30% of IPD cases (34). The Centers for Disease Control and Prevention predict a rise in antibiotic resistance features of S. pneumoniae (35, 36).
The inability of vaccination to provide adequate protection against one of the most aggressive serotypes of this major human pathogen necessitates the urgent exploration of alternative approaches for type 3 pneumococcal infections. This, along with a rise in the prevalence of antibiotic-resistant strains (37, 38), led us to revisit early studies by Avery and Dubos, who discovered a soil-dwelling bacterium producing an enzyme that hydrolyzes Pn3P (39–41). Previously, we identified this bacterium as a Paenibacillus species, cloned its type 3-specific glycosyl hydrolase, Pn3Pase, and characterized its degradation products (26, 42). In light of the continued prevalence and severity of serotype 3 S. pneumoniae, we postulated the potential use of this purified protein as a therapeutic agent for hypervirulent serotype 3 infections. Here, we investigated the ability of Pn3Pase to degrade the capsule on a live virulent type 3 S. pneumoniae strain and therefore render the bacterium susceptible to host immune clearance.
RESULTS
Pn3Pase removes capsule from growing type 3 S. pneumoniae.
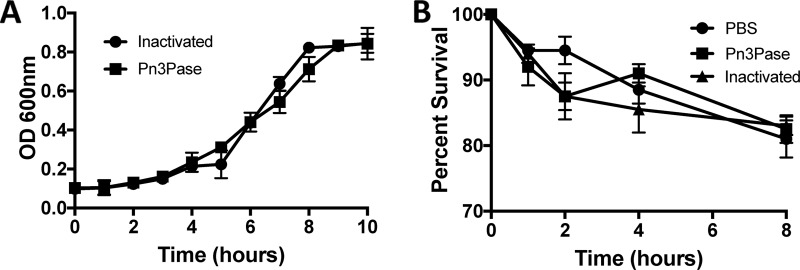
The encapsulated type 3 WU2 strain was cultured for 10 h with the recombinant enzyme added to the growth medium, and bacterial growth was monitored by measuring the optical density at 600 nm (OD600) to assess effects of Pn3Pase treatment on growing type 3 S. pneumoniae cells. Pn3Pase treatment of cells demonstrated no adverse growth or cytotoxic effects on the bacteria (Fig. 1A). In the same experiment, we obtained comparable CFU values for both enzyme-treated and nontreated groups at 2-h intervals (data not shown). To assess the direct impact of enzyme treatment on bacterial survival, a log-phase culture was isolated and suspended in nutrient-free buffer with active or heat-inactivated Pn3Pase. Enzyme-treated cells showed comparable counts to inactivated and phosphate-buffered saline (PBS) controls over the 8-h time course (Fig. 1B).
FIG 1.
Effects of Pn3Pase treatment on type 3 S. pneumoniae viability. (A) Growth curve of WU2 in THY broth in the presence of 100 μg/ml Pn3Pase following the OD600. (B) WU2 survival in PBS in the presence of 100 μg/ml active or heat-inactivated Pn3Pase.
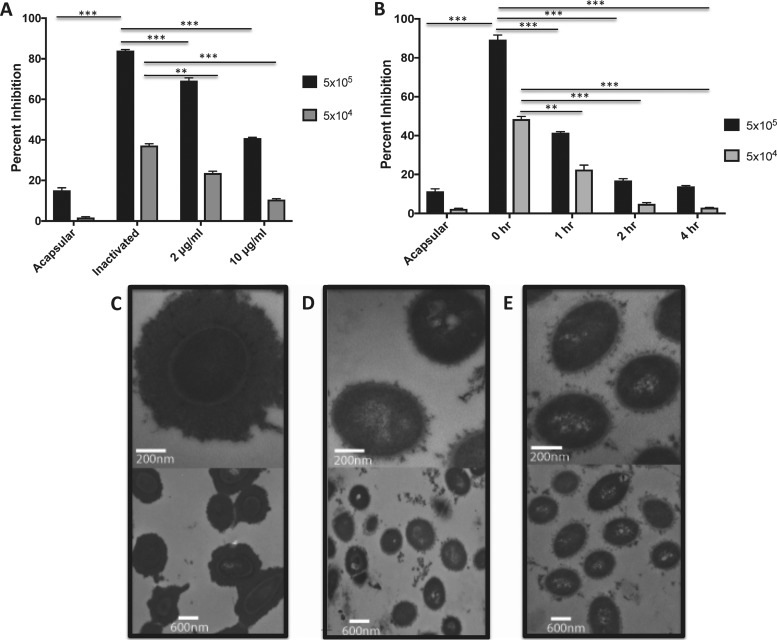
The bacterial cells grown in the presence of Pn3Pase were then examined by competition enzyme-linked immunosorbent assay (ELISA) to determine if the enzyme led to efficient capsule removal in the growing cultures. After treatment, fixed whole cells at two different concentrations were used to compete for Pn3P-specific antibody binding to the Pn3P-coated plate. The acapsular WU2 mutant strain (JD908) showed minimal to no inhibition of antibody binding due to its lack of capsule. Heat-inactivated Pn3Pase-treated cells demonstrated the highest percentage of inhibition as cell surfaces should be fully decorated with CPS. With active Pn3Pase treatment, inhibition of antibody binding begins to decrease in a Pn3Pase concentration-dependent manner (Fig. 2A), suggesting that the enzyme strips the capsule from live, growing type 3 S. pneumoniae.
FIG 2.
Depleting the capsule on live type 3 S. pneumoniae by Pn3Pase treatment. (A and B) Competition ELISA in which cells of acapsular WU2, WU2 treated with heat-inactivated Pn3Pase, or WU2 treated with Pn3Pase at two different concentrations (2 or 10 μg/ml) were used to compete for Pn3P-specific antibody binding to a Pn3P-coated ELISA plate. Data are presented as percentage of inhibition of antibody binding. Statistical significance was determined with the two-tailed Student's t test. **, P < 0.01; ***, P < 0.001. Transmission electron microscopy images of WU2 mock treated with 2 μg/ml heat-inactivated Pn3Pase (C), acapsular WU2 strain (D), and 2 μg/ml active-Pn3Pase treated WU2 (E). Bottom panels in panels C to E are imaged at 15,000× direct magnification, and top panels are imaged at 10,000× direct magnification.
A time course experiment was performed in which the bacterial cells were grown to mid-log phase, suspended in PBS, and treated with Pn3Pase for 0, 1, 2, or 4 h. The Pn3Pase dose was lowered to 1 μg/ml for this assay, given that the WU2 strain should not be actively growing and therefore should not be producing a substantial amount of new CPS under these conditions. At each time point, the treated cells were fixed and examined by competition ELISA as described above. The acapsular WU2 mutant strain (JD908) again showed minimal to no inhibition due to its lack of capsule. Untreated cells exhibited the highest percentage of inhibition, indicative of a cell surface fully decorated with CPS. With increasing Pn3Pase incubation time, inhibition of antibody binding begins to decrease significantly, appearing essentially acapsular after 2- or 4-h treatments (Fig. 2B).
The Pn3Pase-treated cells were visualized by transmission electron microscopy and compared to the cells treated with heat-inactivated Pn3Pase. The heat-inactivated enzyme-treated cells displayed a thick, complete CPS coat across their surface (Fig. 2C), undoubtedly distinct from the acapsular mutant (Fig. 2D). The capsule layer of the enzyme-treated cells exhibited little to no capsule (Fig. 2E), appearing acapsular.
Pn3Pase treatment of type 3 S. pneumoniae allows phagocytic cell uptake and killing.
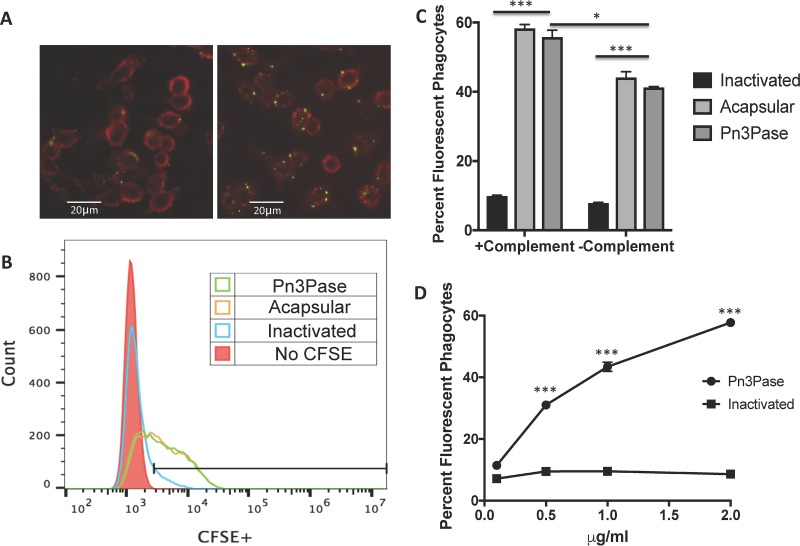
A major virulence mechanism of the CPS is to provide the bacterium the ability to resist phagocytosis by host phagocytic cells (1, 6). To investigate the effect of Pn3Pase treatment on uptake by macrophages in vitro, we stained mid-log-phase bacterial cultures with carboxyfluorescein succinimidyl ester (CFSE) and then treated them with Pn3Pase followed by incubation with RAW 264.7 macrophages. Macrophages were then washed extensively and imaged by fluorescence microscopy (Fig. 3A). The extent of bacterial uptake by macrophages was quantified by flow cytometry (Fig. 3B). Bacteria treated with the enzyme were taken up by macrophages significantly more than the encapsulated strain. We then determined the percentage of fluorescent macrophages, representing the phagocytosis of fluorescent bacteria (Fig. 3C and D). The encapsulated type 3 strain incubated with heat-inactivated Pn3Pase had minimal fluorescently labeled phagocytes, whereas Pn3Pase-treated bacteria were as efficiently taken up by the macrophages as the acapsular mutant strain. Uptake was partially dependent on complement, as evidenced by higher bacterial uptake in the presence of complement (Fig. 3C). Pn3Pase treatment rendered the bacteria more susceptible to phagocytic engulfment by RAW macrophages in a dose-dependent manner, while even high doses of the inactivated enzyme had no significant effect on bacterial uptake (Fig. 3D).
FIG 3.
Macrophage uptake of type 3 S. pneumoniae treated with Pn3Pase. (A) RAW 264.7 macrophages containing fluorescent streptococci following heat-inactivated (left) or active (right) Pn3Pase treatment. Streptococci labeled with CFSE are in green, and macrophages labeled with biotinylated wheat germ agglutinin (WGA) or streptavidin-APC are in red. (B) Histogram of the flow cytometry analysis of fluorescent phagocytes demonstrating increased fluorescence intensity for the acapsular control and Pn3Pase-treated WU2 strain. (C) Influence of Pn3Pase treatment and complement on macrophage uptake of CFSE-labeled S. pneumoniae quantified by flow cytometry. (D) Pn3Pase dose-dependent effect on the percentage of fluorescent phagocytes. Statistical significance was determined with the two-tailed Student's t test. ***, P < 0.001; *, P < 0.05.
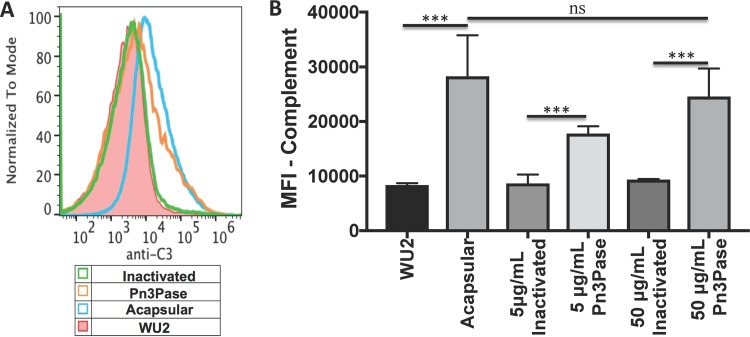
Complement deposition on the pneumococcal surface is an important mechanism aiding in efficient phagocytosis and clearance (10, 11). Since CPS provides complement evasive properties to the bacterium (10, 11), we investigated the effect of capsule removal by Pn3Pase treatment on C3b deposition on the bacterial surface. The encapsulated type 3 WU2 strain was treated with active or inactive Pn3Pase. The untreated type 3 strain and the acapsular mutant were included as additional controls. Bacteria were then incubated with normal mouse serum, washed, and stained with fluorescein isothiocyanate (FITC)-conjugated antibody to mouse complement. Fixed samples were then analyzed by flow cytometry. In a dose-dependent manner, Pn3Pase treatment increased the deposition of complement on the bacterial surface, reaching the levels of deposition on the acapsular strain. Complement had minimal binding to the bacteria either untreated or treated with inactivated Pn3Pase (Fig. 4).
FIG 4.
Effect of Pn3Pase treatment on complement deposition on S. pneumoniae surface. Shown are the results from analysis of mouse complement deposition on Pn3Pase-treated or untreated type 3 S. pneumoniae by flow cytometry. The histogram (A) and quantification by mean fluorescent intensity (MFI) (B) of FITC-A were calculated from gating of Hoechst-positive cells. Statistical significance was determined with the two-tailed Student's t test. ***, P < 0.001; ns, not significant.
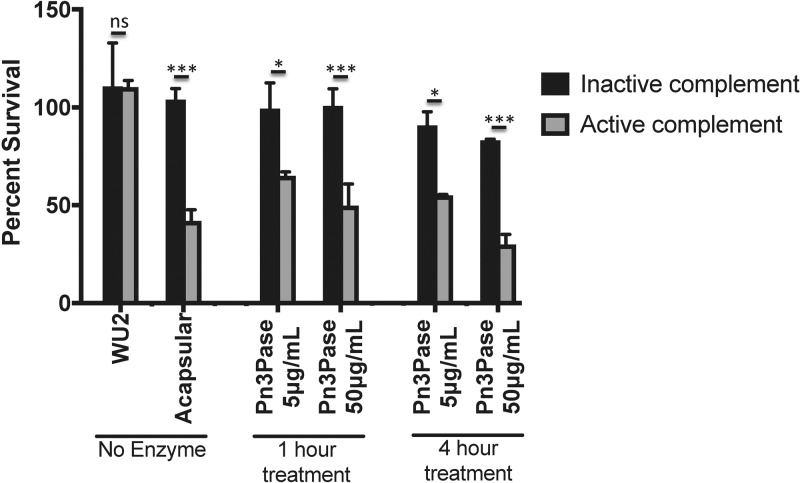
The standard in vitro assay to assess phagocytic killing of S. pneumoniae is an opsonophagocytosis assay (OPA) in which HL-60 cells are differentiated into neutrophils to engulf and clear antibody-opsonized bacteria. This method has been widely used to measure the quality of antibody responses in numerous vaccine studies (43–48). Neutrophils are one of the most important components of innate immunity against pathogenic bacteria in the lungs (49–51). While the focus of this study is not on humoral immune responses to S. pneumoniae, we have used a modified OPA to evaluate complement-mediated neutrophil killing of type 3 S. pneumoniae treated with Pn3Pase in vitro. Encapsulated type 3 S. pneumoniae was incubated with active or heat-inactivated Pn3Pase. Differentiated HL-60 cells were preincubated with active or heat-inactivated complement. The mixture of HL-60 cells and complement was added to the bacteria and incubated at 37°C for 1 h. To quantify the surviving bacteria in the experimental groups, the reaction mixtures were plated and CFU were counted the next day. Percentage of survival was calculated as each duplicate reaction normalized to mean values obtained from reactions without neutrophils, which served as control samples (100% survival). The enzyme-untreated, encapsulated type 3 strain was able to escape neutrophil killing to show maximum survival, while the acapsular mutant strain was reduced to nearly 40% viability upon incubation with active complement and neutrophils (Fig. 5). Pn3Pase treatment to remove the capsule rendered the type 3 strain susceptible to complement-dependent neutrophil killing similar to the acapsular strain (Fig. 5).
FIG 5.
Effect of Pn3Pase treatment on complement-mediated killing by neutrophils. Shown is the complement-mediated killing capacity of differentiated HL-60 cells on Pn3Pase-treated S. pneumoniae. The percentage of survival was calculated as each duplicate reaction normalized to mean values obtained for control samples (where reactions without HL60 cells represent 100% survival). Statistical significance was determined with the two-tailed Student's t test. ***, P < 0.001; *, P < 0.05; ns, not significant.
Pn3Pase limits nasopharyngeal colonization.
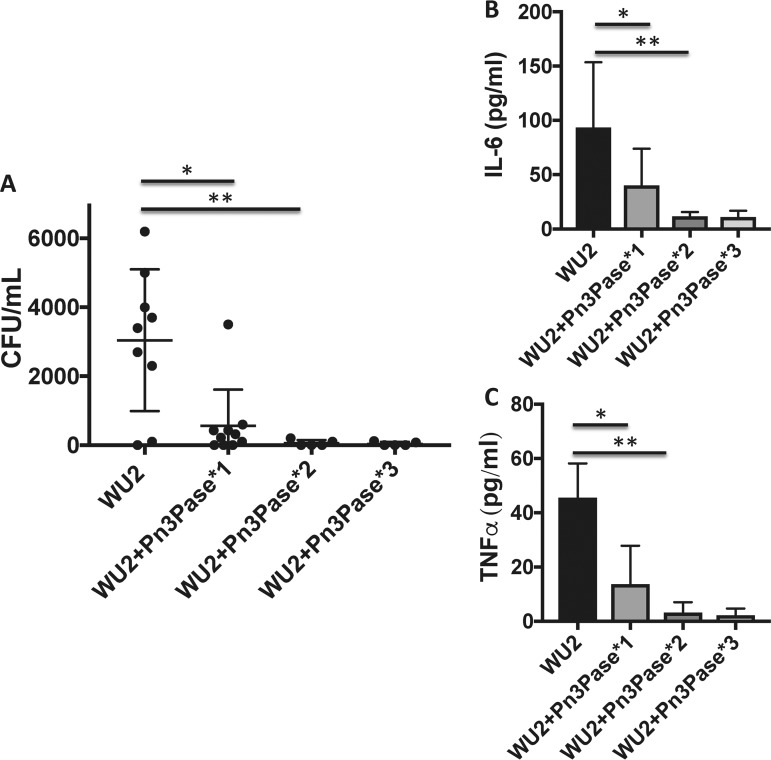
To investigate the enzyme's protective abilities in vivo, we first performed an intranasal colonization experiment with BALB/c mice. Nasal colonization by S. pneumoniae is essential for transition to invasive pneumococcal disease (4, 5). It is established that the capsule of this strain is required for intranasal colonization (6). Therefore, we used the nasal colonization model to assess the ability of Pn3Pase to reduce bacterial colonization in the nasopharynx through removal of the capsule of the colonizing type 3 strain. We first confirmed that the acapsular mutant, JD908, failed to colonize the nasopharynx (data not shown). Groups of mice were then intranasally inoculated with 106 log-phase wild-type (wt) encapsulated bacteria in 10 μl PBS. All inocula were chased with either Pn3Pase or buffer control. Groups were dosed with the enzyme at day 0, days 0 and 3, or days 0, 3, and 7 to assess the effects of multiple administrations. Mice were euthanized, and bacterial load was quantified on day 10. Nasal lavage fluid was obtained, serially diluted, and plated to enumerate the bacterial load. Vehicle control-treated mice were colonized with significantly higher bacterial loads than mice treated with only a single dose of Pn3Pase. Administration of two or three doses of Pn3Pase made the majority of the animal lavage fluid void of any viable bacterial colonies (Fig. 6A). Lung homogenates and serum samples showed no evidence of a bacterial burden (data not shown). Signature proinflammatory cytokine levels were measured in the nasal lavage fluid by ELISA (52). Vehicle-treated mice had significantly increased levels of the cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) compared to Pn3Pase-treated animals, a reflection of a continued host inflammatory response to the bacterial burden in this group (Fig. 6B and C) (52). A significant reduction in IL-6 and TNF-α was observed in most animals even after a single dose of Pn3Pase on day 0. The mouse with a higher bacterial load in the single-dose group contributed to the increased cytokine levels in this group.
FIG 6.
Intranasal colonization with type 3 S. pneumoniae. Shown is the ability of Pn3Pase treatment to reduce S. pneumoniae colonization in the nasopharynx of BALB/c mice. (A) Groups of mice were intranasally inoculated with 106 log-phase bacteria in 10 μl PBS. All inocula were chased with either 50 μg of Pn3Pase or buffer control (10 μl). Groups were dosed with the enzyme at either day 0 (WU2+Pn3Pase*1), days 0 and 3 (WU2+Pn3Pase*2), or days 0, 3, and 7 (WU2+Pn3Pase*3). Bacterial load was quantified on day 10. Serial dilutions of nasal lavage fluid were plated in duplicate to determine CFU values. (B and C) IL-6 (B) and TNF-α (C) concentrations in nasal lavage fluid were determined by ELISA. Statistical significance was determined with the two-tailed Student's t test *, P < 0.05; **, P < 0.01.
Pn3Pase protects mice from lethal challenge.
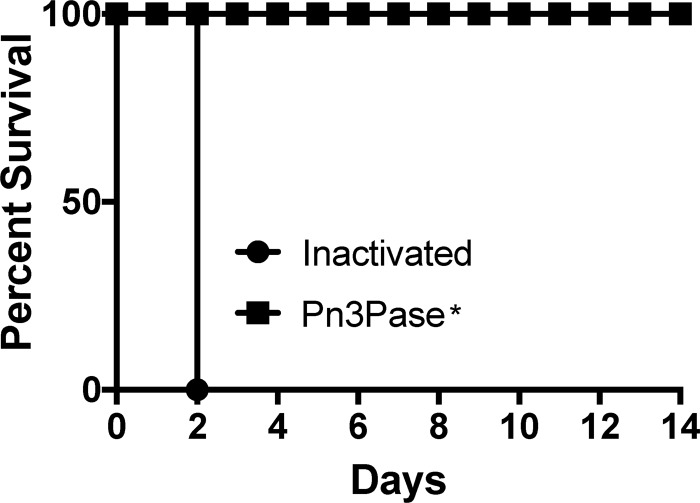
To further assess Pn3Pase for its protective abilities and to evaluate the utility of Pn3Pase as a therapeutic agent, we employed an intraperitoneal (i.p.) sepsis model (53, 54). Groups of mice were infected with 5 × 103 CFU of the log-phase WU2 type 3 strain of S. pneumoniae. We assessed the effect of a single dose of 5 μg or 0.5 μg, administered at time 0, 12, or 24 h postinfection. Control groups treated with heat-inactivated enzyme died within 48 h of infection. Regardless of the enzyme dose or the timing of the administration, all treated groups displayed no signs of illness and experienced full protection from the i.p. challenge (Fig. 7).
FIG 7.
Protective ability of Pn3Pase. Shown are the results from assessment of the ability of Pn3Pase to protect BALB/c mice from lethal challenge. Groups of mice were infected through intraperitoneal administration of 5 × 103 log-phase virulent type 3 S. pneumoniae cells. The inactivated group is heat-inactivated Pn3Pase. “Pn3Pase*” indicates the results shown represent the effect of a single dose of 5 μg or 0.5 μg administered at time 0, 12, or 24 h postinfection for n = 5 mice per group.
DISCUSSION
This study aimed to evaluate the protective role of a carbohydrate-degrading enzyme (glycoside hydrolase), Pn3Pase, targeting the CPS of the pathogenic bacterium serotype 3 S. pneumoniae. Invasive pneumococcal diseases (IPDs) caused by S. pneumoniae have been a major threat to human health, with alarming mortality rates. Despite a global vaccination program and the use of antibiotics, S. pneumoniae remains among the deadliest infectious agents worldwide. Pneumococcal vaccines are made empirically and are variably/poorly immunogenic, especially among elderly and immunocompromised individuals. Widespread use of antibiotics against IPDs has led to the spread of drug-resistant pneumococcal strains (34, 35). This study offers an alternative targeted therapeutic approach to the shortcomings of the incumbent vaccine and antibiotic solutions to IPDs.
First we demonstrated that Pn3Pase could efficiently remove the capsule from live pneumococci without having bactericidal effects on the cells. Through in vitro assays, we observed that Pn3Pase treatment increases the bacterium's susceptibility to phagocytosis by a macrophage cell line. These results were promising since the capsule is a major host immune evasion component that allows S. pneumoniae to resist engulfment by host phagocytes (9). We then concluded that enzyme treatment significantly increased complement-mediated killing by the neutrophils. A single dose of Pn3Pase reduced murine nasopharyngeal colonization by type 3 S. pneumoniae significantly, indicating that the enzyme may function as a prophylactic measure to control colonization by this serotype in at-risk populations. Finally, an intraperitoneal challenge was performed to assess the protective capacity of Pn3Pase in a sepsis model. Notably, a single low dose of 0.5 μg administered 24 h after infection was able to protect 100% of the challenged mice from the bacterial challenge, while control treated animals did not survive longer than 48 h. The robust protective capacity of Pn3Pase in this model demonstrates the enzymatic activity is sufficient within the host to effectively degrade the capsule even at low doses.
Given that Pn3Pase has therapeutic potential for pneumococcal infections, practical issues pertaining to the application of the enzyme such as immunogenicity, administration routes, and substrate specificity will need to be addressed (55). Our preliminary assessment of antibody titers generated against Pn3Pase in the challenge experiments observed no IgM or IgG response generated against the effective dose of the enzyme. Future studies will evaluate humoral and cellular immune responses to Pn3Pase and investigate alternative routes of administration, such as intravenous for a bacteremia model or aerosolized spray for the pneumonia model. A useful example of enzyme delivery to the respiratory tract by aerosol spray is the recombinant human DNase known as Pulmozyme, used to relieve airway obstruction by secreted DNA in cystic fibrosis patients (56). Another potential problem with the use of Pn3Pase as a therapeutic agent is its activity on host glycans. Relaxed substrate specificity of Pn3Pase on mammalian glycans structurally similar to Pn3P will need to be assessed.
In addition to the high potential of Pn3Pase as a therapeutic enzyme, this glycosyl hydrolase has unique properties from a structure/function point of view in that it does not fall into a currently established glycosyl hydrolase carbohydrate active enzyme (CAZY) family (57). Further examination of structural properties of this protein may lead to the discovery of structurally similar enzymes with activities toward other unique bacterial CPSs. Future investigations will explore the existence of enzymes for use against other prevalent pneumococcal serotypes and other encapsulated pathogenic bacteria. Based on earlier studies, the native species expressing Pn3Pase has the capacity to degrade two additional pneumococcal CPSs (58, 59). While this Pn3Pase-expressing Paenibacillus species was isolated from the soil (26, 42, 59), it is befitting to question why this species would evolve to possess such enzymes that are capable of degrading capsules of a human pathogen. Whether these are the natural substrates for the enzymes, indicating a coevolutionary relationship, or whether other soil-dwelling microbes or plants express similar glycan residues and linkages remains to be explored.
The results presented here indicate that enzymatic hydrolysis of the CPS may be a valid alternative or complementary therapeutic approach for diseases caused by S. pneumoniae and potentially other important encapsulated pathogens, such as Neisseria meningitidis and methicillin-resistant Staphylococcus aureus (MRSA). In summary, this study serves as the first comprehensive evaluation of the protective role of a glycoside hydrolase, Pn3Pase, debilitating an otherwise lethal bacterial pathogen through targeting its capsular polysaccharide.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Streptococcus pneumoniae type 3 (WU2 strain) and its acapsular derivative (JD908) (60, 61), generous gifts from Moon Nahm (University of Alabama at Birmingham), were cultured aerobically without shaking at 37°C on tryptic soy agar with 5% sheep blood (TSAB) or in Todd-Hewitt broth plus 0.5% yeast extract (THY) (BD Biosciences).
Mice.
Eight-week-old female BALB/c mice were obtained from Taconic Biosciences (Hudson, NY) and housed in the Central Animal Facility at the University of Georgia. Mice were kept in microisolator cages and handled under biosafety level 2 (BSL2) hoods.
Production of recombinant Pn3Pase.
Pn3Pase was produced as described previously with minor modifications (26). Briefly, BL21(DE3) cells transformed with the pET-DEST42-Pn3Pase plasmid were grown in Terrific broth supplemented with 100 μg/ml ampicillin at 37°C, and cell density was monitored by absorbance at 600 nm. Once the OD600 reached 1.0, the cells were transferred to 18°C. Protein expression was induced by the addition of IPTG (isopropyl β-d-1-thiogalactopyranoside) to a final concentration of 1 mM, and the cell culture was allowed to incubate for 18 h. Cells were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS [pH 7.2]), and lysed by pressure lysis. The lysate was clarified by centrifugation at 17,000 × g for 1 h at 4°C and passed through a 0.45-μm syringe filter. Recombinant Pn3Pase was purified by Ni2+-nitrilotriacetic acid (NTA) resin at 4°C, eluted with 300 mM imidazole, and buffer exchanged into PBS (pH 7.2). The protein concentration was determined by the bicinchoninic acid assay according to the instructions of the manufacturer. Purity was evaluated by Coomassie staining.
Pn3Pase treatment of type 3 S. pneumoniae.
Fresh Streptococcus pneumoniae type 3 (WU2) and acapsular derivative (JD908) colonies on TSAB plates were inoculated to an OD600 of 0.1 in THY broth and cultured as described above. WU2 growth in the presence of 100 μg/ml Pn3Pase was monitored over the course of 10 h by measuring OD600. WU2 was grown in the presence of 2, 10, or 10 μg/ml heat-inactivated Pn3Pase for 6 h, serially diluted, and plated to determine CFU. Cultures were harvested by centrifugation, washed in PBS, fixed in 2% paraformaldehyde for 20 min on ice, washed once more, and suspended in 1 ml PBS. For the time course experiment, WU2 and the acapsular strain were grown to the mid-log phase (OD600 of 0.6), harvested by centrifugation, washed in PBS, and then suspended in 1 ml PBS. Then 1 μg/ml Pn3Pase was added, and the mixture was incubated at 37°C for 1, 2, or 4 h. Treated cells were serially diluted and plated to determine CFU, fixed in 2% paraformaldehyde for 20 min on ice, washed once more, and suspended in 1 ml PBS.
Competition ELISA.
ELISA plates (96 well; Nunc) were coated with 5 μg/ml Pn3P in 0.1 M carbonate buffer (pH 9.0) overnight at room temperature. Plates were washed 4 times with PBS plus 0.1% Tween 20 (PBS-T) using a Biotek 405/LS microplate washer. After 1 h of blocking at room temperature with 1% BSA in PBS, microplate wells were incubated for 2 h at room temperature with fixed, treated cells that were preincubated for 30 min with Pn3P-specific antiserum in PBS-T. Plates were washed and then incubated for 2 h at room temperature with a 1:2,000 dilution of goat anti-mouse IgG-alkaline phosphatase (AP) (Southern Biotech 1030-04) in PBS-T. After washing, plates were incubated for ∼30 min at 37°C with 2 mg/ml phosphatase substrate (Sigma S0942) in 1 M Tris–0.3 mM MgCl2. Absorbance at 405 nm was measured on a Biotek synergy H1 microplate reader. The percentage of inhibition of antibody binding was calculated as [(uninhibited at OD405 − inhibited at OD405)/uninhibited at OD405] × 100.
Electron microscopy.
Electron microscopy was performed by the Georgia Electron Microscopy core facility at the University of Georgia according to a modified method by Hammerschmidt et al. (62). Treated cells were fixed in a mixture of 2% glutaraldehyde, 2% paraformaldehyde, 0.075 M lysine-acetate, and 0.075% ruthenium red in PBS buffer for 1 h on ice and then rinsed 2× with buffer containing 0.15% ruthenium red for 15 min per rinse. Cells were then fixed in 1% osmium tetroxide in buffer containing 0.15% ruthenium red for 1 h at room temperature followed by two rinses in buffer containing 0.15% ruthenium red for 15 min per rinse. The pellet was dehydrated in a graded ethanol series (30, 50, 75, 95, 100, and 100%) and two changes in 100% acetone for 15 min each step. The pellet was then infiltrated with 25% Spurr's resin and 75% acetone for 2 h followed by sequential infiltration with 50% Spurr's resin and 50% acetone, 75% Spurr's resin and 25% acetone, and 100% Spurr's resin and then polymerized in a 70°C oven for 24 h. Samples were sectioned at 60 nm with a Diatome diamond knife and picked up on slot grids. Grids were poststained on drops of uranyl acetate and lead citrate for 5 min each and rinsed with H2O for 30 s between stains. Samples were scoped using a JEOL JEM 1011 transmission electron microscope (JEOL USA, Peabody, MA) operated at 80 kV.
Phagocytosis.
Mid-log-phase WU2 and JD908 (acapsular) cultures were washed and stained with 10 μM carboxyfluorescein succinimidyl ester (CFSE) for 30 min at room temperature. The WU2 strain was concurrently treated with 2 μg/ml of active or heat-inactivated Pn3Pase. Bacterial cell pellets were washed extensively and suspended in 1 ml sterile PBS. A total of 107 bacteria were added to a confluent monolayer of murine leukemia virus-transformed macrophage line RAW 264.7 (American Type Culture Collection [ATCC] Manassas, VA) with active or heat-inactivated baby rabbit complement (Pel-Freez) in a 24-well plate and incubated for 1 h at 37°C. Wells were washed 4× with PBS to remove extracellular bacteria. Macrophages were fixed with 2% paraformaldehyde at 4°C for 15 min and removed from the plate. For microscopy, cells were incubated at room temperature for 30 min with a 1/500 dilution of biotinylated wheat germ agglutinin (Vector Laboratories) in 1% bovine serum albumin followed by a 30-min room temperature incubation with a 1/1,000 dilution of streptavidin-allophycocyanin (APC [Biolegend]). Cells were imaged with a 40× objective lens. Flow cytometry was performed on a Beckman Cytoflex S cytometer and analyzed by FlowJo. Cells were gated on the macrophage population by scatter plot. A non-CFSE-labeled control served to gate highly fluorescent cell populations. The Pn3Pase dose-dependent experiment was performed as described above with addition of active complement.
Complement deposition.
A complement deposition assay was performed as described previously (63). Type 3 WU2 and acapsular (JD908) strains of bacteria were resuspended in 3% BSA in PBS. Aliquots (in duplicate wells on a 96-well round-bottom plate) were stained with Hoechst 33342 and treated with inactivated or functional Pn3Pase at 5 or 50 μg/ml for 1 h at 37°C. Normal mouse serum (1:10 dilution) was added to the samples for 30 min at 37°C. Cells were washed and stained with FITC-conjugated goat antibody to mouse complement (MP BioMedical, Santa Ana, CA) at 4°C for 30 min. Samples were washed with 3% bovine serum albumin (BSA) in PBS and resuspended in 2% paraformaldehyde to fix. Samples were then analyzed by flow cytometry. The mean fluorescent intensity (MFI) of FITC-A was calculated from gating of Hoechst-positive cells.
Modified OPA.
An opsonophagocytic killing assay was performed as described previously with modifications (43). Briefly, the type 3 WU2 and acapsular (JD908) strains were incubated in duplicate wells in a 96-well round-bottom plate for 1 or 4 h at 37°C with or without Pn3Pase (inactivated or functional at concentrations of 5 or 50 μg/ml) in opsonization buffer B (OBB: sterile 1× PBS with Ca2+/Mg2+, 0.1% gelatin, and 5% heat-inactivated FetalClone). Cells of the human promyelocytic leukemia cell line HL-60 (ATCC, Manassas, VA) were cultured in RPMI with 10% heat-inactivated FetalClone (HyClone) and 1% l-glutamine. HL-60 cells were differentiated using 0.6% N,N-dimethylformamide (DMF [Fisher]) for 3 days before performing the OPA assay, harvested, and resuspended in OBB. Active or heat-inactivated (no complement) baby rabbit complement (Pel-Freez) was added to HL-60 cells at a 1:5 final volume. The HL-60–complement mixture was added to the serum/bacteria at 5 × 105 cells/well. (For controls, no HL-60/complement was added; equal volumes of OBB buffer was added instead.) The final reaction mixtures were incubated at 37°C for 1 h. The reactions were stopped by incubating the samples on ice for approximately 20 min. Then 10 μl of each reaction mixture was diluted to a final volume of 50 μl and plated onto blood agar plates in duplicate. Plates were incubated overnight at 30°C under anaerobic conditions and counted the next day. The percentage of survival was calculated as each duplicate reaction normalized to mean values obtained for control samples (with reactions without HL-60 cells representing 100% survival).
Murine intranasal colonization.
An intranasal colonization was performed essentially as described by Puchta et al. (64). Mid-log-phase WU2 cultures were washed with sterile PBS and suspended at a concentration of 108 or 106 CFU/10 μl. Groups of 5 to 10 unanesthetized 8-week-old female BALB/c mice (Taconic) were intranasally inoculated with 106 CFU/10 μl as previously described. Mice were either inoculated with 10 μl of PBS as vehicle on days 0, 3, and 7 or treated by administering 50 μg of Pn3Pase in 10 μl PBS on days 0, 0, and 3 or days 0, 3, and 7. Nasal lavage fluid was obtained on day 10 by flushing out the nasopharynx with PBS by insertion of a 25-gauge needle into the trachea to expel 500 μl through the nares. Serial dilutions of the nasal lavage fluid were plated on TSAB to enumerate the CFU. A sandwich ELISA (Biolegend mouse ELISA Max) was performed according to the manufacturer's instructions to determine IL-6 and TNF-α cytokine levels in the lavage fluid.
Murine sepsis challenge.
Mid-log-phase WU2 cultures were washed with sterile PBS and suspended at a concentration of 5 × 103 CFU/100 μl. Groups of 4 unanesthetized 8-week-old female BALB/c mice (Taconic) were injected intraperitoneally (i.p.) with 5 × 103 CFU. Control mice were injected i.p. with 5 μg of heat-inactivated Pn3Pase in 100 μl of PBS at time 0 or directly after infection. Treated mice were administered i.p. 0.5 or 5 μg of Pn3Pase in 100 μl PBS at time 0, 12, or 24 h postinfection. Animals were monitored every 12 h.
Ethics statement.
All mouse experiments were in compliance with the University of Georgia Institutional Animal Care and Use Committee under the approved animal use protocol 2478 A2016 11-022-Y1-A0. Our animal use protocol adheres to the principles outlined in U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training, the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the AVMA Guidelines for the Euthanasia of Animals.
ACKNOWLEDGMENTS
We thank Moon Nahm (University of Alabama Birmingham) for providing us with the type 3 WU2 strain and the acapsular WU2 mutant strain (JD908) of S. pneumoniae. We thank Christine Szymanski for critical review. Transmission electron microscopy (TEM) work was conducted in the Georgia Electron Microscope Lab at the University of Georgia, Athens.
REFERENCES
- 1.Jochems SP, Weiser JN, Malley R, Ferreira DM. 2017. The immunological mechanisms that control pneumococcal carriage. PLoS Pathog 13:e1006665. doi: 10.1371/journal.ppat.1006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austrian R. 1986. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother 18(Suppl A):35–45. [DOI] [PubMed] [Google Scholar]
- 3.Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, Pebody R, George R, Soininen A, Edmunds J, Gay N, Kayhty H, Miller E. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis 192:387–393. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 5.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O'Brien KL, Pneumococcal Carriage Group . 2012. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 6.Magee AD, Yother J. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun 69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson DA, Musher DM. 1990. Interruption of capsule production in Streptococcus pneumoniae serotype 3 by insertion of transposon Tn916. Infect Immun 58:3135–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyn GA, Zegers BJ, van Furth R. 1992. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin Infect Dis 14:251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 10.Winkelstein JA. 1984. Complement and the host's defense against the pneumococcus. Crit Rev Microbiol 11:187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]
- 11.Winkelstein JA. 1981. The role of complement in the host's defense against Streptococcus pneumoniae. Rev Infect Dis 3:289–298. doi: 10.1093/clinids/3.2.289. [DOI] [PubMed] [Google Scholar]
- 12.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. 2015. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabenstein JD, Klugman KP. 2012. A century of pneumococcal vaccination research in humans. Clin Microbiol Infect 18(Suppl 5):S15–S24. doi: 10.1111/j.1469-0691.2012.03943.x. [DOI] [PubMed] [Google Scholar]
- 14.MacLeod CM, Hodges RG, Heidelberger M, Bernhard WG. 1945. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med 82:445–465. doi: 10.1084/jem.82.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wantuch PL, Avci FY. 2018. Current status and future directions of invasive pneumococcal diseases and prophylactic approaches to control them. Hum Vaccin Immunother 14:1–19. doi: 10.1080/21645515.2018.1420843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avci F, Kasper D. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol 28:107–130. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 17.Avci F. 2013. Novel strategies for development of next-generation glycoconjugate vaccines. Curr Top Med Chem 13:2535–2540. doi: 10.2174/15680266113136660180. [DOI] [PubMed] [Google Scholar]
- 18.Avci F, Li X, Tsuji M, Kasper D. 2011. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alicino C, Paganino C, Orsi A, Astengo M, Trucchi C, Icardi G, Ansaldi F. 2017. The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on hospitalization for pneumonia in children: a systematic review and meta-analysis. Vaccine 35:5776–5785. doi: 10.1016/j.vaccine.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. 2015. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 21.Tin Tin Htar M, Stuurman AL, Ferreira G, Alicino C, Bollaerts K, Paganino C, Reinert RR, Schmitt HJ, Trucchi C, Vestraeten T, Ansaldi F. 2017. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS One 12:e0177985. doi: 10.1371/journal.pone.0177985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. 2014. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 59:1066–1073. doi: 10.1093/cid/ciu524. [DOI] [PubMed] [Google Scholar]
- 23.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. 2013. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 24.Gruber WC, Scott DA, Emini EA. 2012. Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann N Y Acad Sci 1263:15–26. doi: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- 25.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. 2013. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011. Emerg Infect Dis 19:1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton DR, Zhang X, Wantuch PL, Ozdilek A, Liu X, LoPilato R, Gangasani N, Bridger R, Wells L, Linhardt RJ, Avci FY. 2018. Identification and characterization of the Streptococcus pneumoniae type 3 capsule-specific glycoside hydrolase of Paenibacillus species 32352. Glycobiology 28:90–99. doi: 10.1093/glycob/cwx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Li L, Xue C, Middleton D, Linhardt RJ, Avci FY. 2015. Profiling pneumococcal type 3-derived oligosaccharides by high resolution liquid chromatography-tandem mass spectrometry. J Chromatogr A 1397:43–51. doi: 10.1016/j.chroma.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, Jansen KU, Devlin C, Gruber WC, Emini EA, Scott DA, 006 Study Group . 2010. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 28:4192–4203. doi: 10.1016/j.vaccine.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Jackson L, El Sahly H, George S, Winokur P, Edwards K, Brady R, Rouphael N, Keitel W, Mulligan M, Burton R, Nakamura A, Ferreria J, Nahm M. 2018. Randomized clinical trial of a single versus a double dose of 13-valent pneumococcal conjugate vaccine in adults 55 through 74 years of age previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 36:606–614. doi: 10.1016/j.vaccine.2017.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun 60:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Rückinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, O'Brien KL, Scott JA, Lipsitch M. 2010. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis 51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. 2004. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect Dis 4:21. doi: 10.1186/1471-2334-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto N, Yamagishi Y, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Mikamo H. 2017. Invasive pneumococcal disease caused by mucoid serotype 3 Streptococcus pneumoniae: a case report and literature review. BMC Res Notes 10:21. doi: 10.1186/s13104-016-2353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim L, McGee L, Tomczyk S, Beall B. 2016. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev 29:525–552. doi: 10.1128/CMR.00058-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Metcalf BJ, Chochua S, Li Z, Gertz RE, Walker H, Hawkins PA, Tran T, Whitney CG, McGee L, Beall BW. 2016. Penicillin-binding protein transpeptidase signatures for tracking and predicting β-lactam resistance levels in Streptococcus pneumoniae. mBio 7:e00756-. doi: 10.1128/mBio.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metcalf BJ, Gertz RE, Gladstone RA, Walker H, Sherwood LK, Jackson D, Li Z, Law C, Hawkins PA, Chochua S, Sheth M, Rayamajhi N, Bentley SD, Kim L, Whitney CG, McGee L, Beall B, Active Bacterial Core Surveillance Team . 2016. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect 22:60.e9–60.e29. doi: 10.1016/j.cmi.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obolski U, Lourenço J, Thompson C, Thompson R, Gori A, Gupta S. 2018. Vaccination can drive an increase in frequencies of antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae. Proc Natl Acad Sci U S A 115:3102–3107. doi: 10.1073/pnas.1718712115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao C, Li Z, Zhang F, Zhang X, Ji P, Zeng J, Hu B, Hu Z, Liao K, Sun H, Zhang R, Cao B, Zhuo C, Jia W, Mei Y, Chu Y, Xu X, Yang Q, Jin Y, Fu Q, Li H, Wang L, Ni Y, Liang H, Wang H. 2017. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates from 17 Chinese cities from 2011 to 2016. BMC Infect Dis 17:804. doi: 10.1186/s12879-017-2880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avery OT, Dubos R. 1930. The specific action of a bacterial enzyme on pneumococci of type III. Science 72:151–152. doi: 10.1126/science.72.1858.151. [DOI] [PubMed] [Google Scholar]
- 40.Dubos R, Avery OT. 1931. Decomposition of the capsular polysaccharide of pneumococcus type III by a bacterial enzyme. J Exp Med 54:51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avery OT, Dubos R. 1931. The protective action of a specific enzyme against type III pneumococcus infection in mice. J Exp Med 54:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middleton DR, Lorenz W, Avci FY. 2017. Complete genome sequence of the bacterium Bacillus circulans Jordan strain 32352. Genome Announc 5:e00289-17. doi: 10.1128/genomeA.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton RL, Nahm MH. 2012. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol 19:835–841. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels CC, Kim KH, Burton RL, Mirza S, Walker M, King J, Hale Y, Coan P, Rhee DK, Nahm MH, Briles DE. 2013. Modified opsonization, phagocytosis, and killing assays to measure potentially protective antibodies against pneumococcal surface protein A. Clin Vaccine Immunol 20:1549–1558. doi: 10.1128/CVI.00371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleck RA, Romero-Steiner S, Nahm MH. 2005. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol 12:19–27. doi: 10.1128/CDLI.12.1.19-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song JY, Moseley MA, Burton RL, Nahm MH. 2013. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 19:412–425. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middleton DR, Sun L, Paschall AV, Avci FY. 2017. T cell-mediated humoral immune responses to type 3 capsular polysaccharide of Streptococcus pneumoniae. J Immunol 199:598–603. doi: 10.4049/jimmunol.1700026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craig A, Mai J, Cai S, Jeyaseelan S. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanchette-Cain K, Hinojosa CA, Akula Suresh Babu R, Lizcano A, Gonzalez-Juarbe N, Munoz-Almagro C, Sanchez CJ, Bergman MA, Orihuela CJ. 2013. Streptococcus pneumoniae biofilm formation is strain dependent, multifactorial, and associated with reduced invasiveness and immunoreactivity during colonization. mBio 4:e00745-. doi: 10.1128/mBio.00745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med 153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiavolini D, Pozzi G, Ricci S. 2008. Animal models of Streptococcus pneumoniae disease. Clin Microbiol Rev 21:666–685. doi: 10.1128/CMR.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fenton M, Ross P, McAuliffe O, O'Mahony J, Coffey A. 2010. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. 1994. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 57.Henrissat B, Bairoch A. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw M, Sickles GM. 1950. Production of specific pneumococcus carbohydrate-splitting enzymes in media to which the specific substrate was not added. J Immunol 64:27–32. [PubMed] [Google Scholar]
- 59.Sickles GM, Shaw M. 1934. A systematic study of microörganisms which decompose the specific carbohydrates of the pneumococcus. J Bacteriol 28:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dillard JP, Vandersea MW, Yother J. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J Exp Med 181:973–983. doi: 10.1084/jem.181.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dillard JP, Yother J. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol Microbiol 12:959–972. doi: 10.1111/j.1365-2958.1994.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 62.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Müller E, Rohde M. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun 73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puchta A, Verschoor CP, Thurn T, Bowdish DM. 2014. Characterization of inflammatory responses during intranasal colonization with Streptococcus pneumoniae. J Vis Exp 2014:e50490. doi: 10.3791/50490. [DOI] [PMC free article] [PubMed] [Google Scholar]