The gonococcal Opa proteins are an antigenically variable family of surface adhesins that bind human carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), CEACAM3, CEACAM5, and/or CEACAM6, cell surface glycoproteins that are differentially expressed on a broad spectrum of human cells and tissues. While they are presumed to be important for infection, the significance of various Opa-CEACAM-mediated cellular interactions in the context of the genital tract has remained unclear.
KEYWORDS: CEACAM1, Neisseria gonorrhoeae, carcinoembryonic antigen (CEA), carcinoembryonic antigen-related cellular adhesion molecules (CEACAM), gonorrhea, inflammation, mucosal infection, pelvic inflammatory disease (PID), sexually transmitted disease (STD)
ABSTRACT
The gonococcal Opa proteins are an antigenically variable family of surface adhesins that bind human carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), CEACAM3, CEACAM5, and/or CEACAM6, cell surface glycoproteins that are differentially expressed on a broad spectrum of human cells and tissues. While they are presumed to be important for infection, the significance of various Opa-CEACAM-mediated cellular interactions in the context of the genital tract has remained unclear. Here, we observed that CEACAM1 and CEACAM5 are differentially expressed on epithelia lining the upper and lower portions of the human female genital tract, respectively. Using transgenic mouse lines expressing human CEACAMs in a manner that reflects this differential pattern, we considered the impact of Opa-CEACAM interactions during uncomplicated lower genital tract infections versus during pelvic inflammatory disease. Our results demonstrate that Opa-CEACAM5 binding on vaginal epithelia facilitates the long-term colonization of the lower genital tract, while Opa protein binding to CEACAM1 on uterine epithelia enhances gonococcal association and penetration into these tissues. While these Opa-dependent interactions with CEACAM-expressing epithelial surfaces promote infection, Opa binding by neutrophil-expressed CEACAMs counterbalances this by facilitating more effective gonococcal clearance. Furthermore, during uterine infections, CEACAM-dependent tissue invasion aggravates disease pathology by increasing the acute inflammatory response. Together, these findings demonstrate that the outcome of infection is determined by both the cell type-specific expression of human CEACAMs and the CEACAM specificity of the Opa variants expressed, which combine to determine the level of gonococcal association with the genital mucosa versus the extent of CEACAM-dependent inflammation and gonococcal clearance by neutrophils.
INTRODUCTION
Neisseria gonorrhoeae is an exquisitely human-adapted pathogen that infects the urogenital, pharyngeal, or rectal mucosa after sexual transmission. The host restriction of N. gonorrhoeae stems, at least in part, from the specificity of key gonococcal virulence factors for human forms of their protein targets, which serve essential functions during infection. These human targets include cell surface-expressed carcinoembryonic antigen-related cell adhesion molecules (CEACAMs), complement receptor 3 (CR3), CD46, and asialoglycoprotein (ASGP) receptors for attachment (1–4); negative complement regulators factor H and C4bp for avoidance of the opsonizing and bactericidal activity of serum complement (5, 6); and transferrin and lactoferrin, utilized for nutrient acquisition (7, 8).
Typifying the human specificity of its virulence factors, gonococcal opacity-associated (Opa) proteins, found within the bacterial outer membrane, engage CEACAMs on the surface of human cells but do not bind CEACAMs from other (nonhuman) species (1, 9, 10). The gonococcal genome encodes ∼11 different opa genes, each encoding antigenically and phenotypically distinct Opa protein variants. Each Opa variant is phase variable, which means that protein expression can be reversibly turned on and off at random, with the frequency estimated to be 10−3/cell/generation (11). Despite this phase-variable expression, colonies recovered from infected male volunteers are almost exclusively opaque (Opa+), even when the men had been inoculated with N. gonorrhoeae bacteria that did not express Opa (Opa−) (12), indicating a selective pressure in favor of Opa protein expression in vivo. In females, low-passage-number clinical isolates typically express one or two Opa proteins, suggesting that Opa proteins are also required for colonization of the female genital tract (13).
Different Opa variants have distinct binding specificities for human CEACAM1, CEACAM3, CEACAM5, and/or CEACAM6, one or more of which may be expressed on the surface of various host cells encountered during the course of an infection. Structurally, the extracellular domain of all four CEACAMs comprises a variable immunoglobulin-like amino (N)-terminal domain, which contains the surface recognized by the different Opa proteins (14, 15). However, membrane anchorage and, when present, the cytoplasmic portions vary among different CEACAMs, and this confers functional specificity. CEACAM1, the evolutionary precursor to the carcinoembryonic antigen (CEA) family, is a transmembrane protein which harbors an immunoreceptor tyrosine-based inhibitory motif (ITIM) on its cytoplasmic tail and, consequently, transmits inhibitory signals inside the cell upon ligation (16). This is in contrast to the cytoplasmic tail of CEACAM3, which instead contains an immunoreceptor tyrosine-based activation motif (ITAM) that propagates activating signals into the cell (17, 18). CEACAM5 and CEACAM6 are glycosylphosphatidylinositol-anchored proteins that have no cytoplasmic domain but signal inside cells by their association with lipid rafts (19, 20).
Attachment to the mucosal epithelium represents the first step in genital colonization by N. gonorrhoeae. Depending upon the tissue from which they are derived, epithelial cells have the capacity to express up to three of these CEACAMs, CEACAM1, CEACAM5, and CEACAM6. Different Opa variants can bind a single CEACAM or combinations of these CEACAMs to mediate a tight bacterial association on various cell types (1, 14, 21, 22). On the mucosal epithelia, Opa-CEACAM binding can presumably withstand the flow of mucus, and in vitro studies suggest that this interaction promotes gonococcal engulfment and transcytosis across polarized epithelia (23). Additionally, Opa binding to CEACAM1, CEACAM5, or CEACAM6 can trigger intracellular signaling cascades that stimulate integrin-mediated focal adhesions in epithelial cells, which prevents normal shedding of infected mucosal cells (20, 24). Furthermore, CEACAM1 has also been reported to depress the proinflammatory response of infected epithelia (25) and suppress the leukocytic activities necessary for the induction of an adaptive immune response (16, 26–29), which would presumably provide a means for the infection to go undetected. Given these important functions, CEACAMs are generally considered to be important for facilitating mucosal colonization by N. gonorrhoeae.
In the ceaseless evolutionary combat between Neisseria and humans, however, the ability to engage CEACAMs does come at a price. Human neutrophils, which are rapidly recruited to sites of infection, constitutively express CEACAM1, CEACAM3, and CEACAM6, all of which enable efficient recognition and phagocytosis of Opa+ gonococci (9). Fascinatingly, CEACAM3, which is expressed only by human neutrophils, appears to be a decoy receptor that activates the neutrophil upon N. gonorrhoeae binding. Specifically, engagement of CEACAM3 leads to a highly efficient opsonin-independent phagocytosis of the bound bacteria, elicits a potent bactericidal response, and stimulates increased production of proinflammatory cytokines by neutrophils (9, 30, 31). Together, the net effect of Opa+ N. gonorrhoeae binding to CEACAMs on neutrophils appears to be efficient bacterial engulfment and killing (31), as well as an inflammatory response that recruits more neutrophils to the site of infection (30).
While in vitro studies have characterized the various effects of Opa-CEACAM interactions on isolated cell types, it is unclear how these individual interactions, collectively, influence infection and disease within human tissues. Particularly interesting in this regard is the diversity of clinical effects seen during genital infection of women, which can range from asymptomatic colonization of the cervical canal to an intense inflammatory response and tissue damage within the upper genital tract during ascending infections, which occur in approximately 20% of untreated cases (32, 33). Considering the diversity of cell types present within the female genital tract, we sought to determine whether binding to individual CEACAMs might influence colonization and/or the pathology associated with gonococcal infection. In this study, we probed human biopsy specimens to reveal that CEACAM1 and CEACAM5 are differentially expressed along the female genital lining, implying that CEACAMs are available as mucosal receptors during infection of women. Next, we use transgenic mouse lines expressing various combinations of human CEACAMs to dissect how the Opa-dependent binding to each CEACAM impacts colonization, clearance, and acute tissue inflammation.
RESULTS
Differential expression of CEACAMs in the reproductive tract of women.
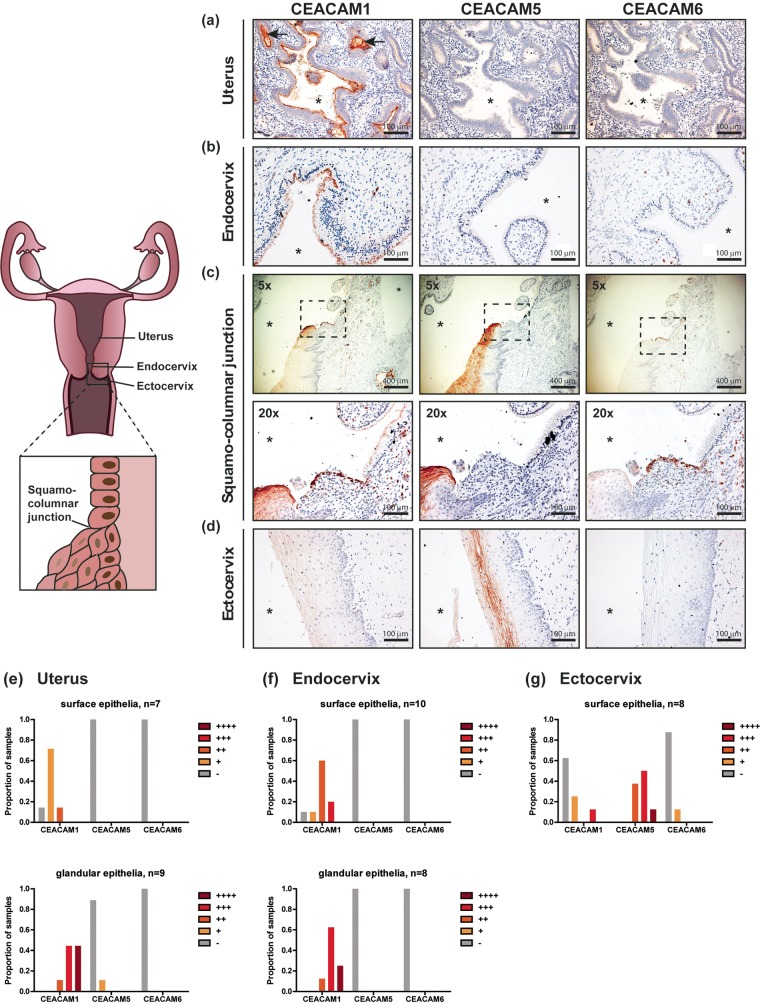
Considering that the female reproductive tract consists of distinct functional compartments (34), characterizing whether CEACAMs are expressed in these different regions would provide the first indication regarding their relevance during gonococcal infections. To map the expression of epithelial CEACAMs in the female reproductive tract, we probed hysterectomy samples from 15 noncancerous patients (median age, 48 years; age range, 33 to 76 years) using monoclonal antibodies that can differentiate between CEACAM1, CEACAM5, and CEACAM6 (representative images are depicted in Fig. 1a to d). In addition, we qualitatively scored the signal intensity of each CEACAM on surface and glandular epithelia in different parts of the genital tract (Fig. 1e to g).
FIG 1.
Expression of CEACAMs along the human female reproductive tract. (a to d) Hysterectomy samples were stained with mouse monoclonal antibodies raised against human CEACAM1, CEACAM5, or CEACAM6 (red-brown), as indicated. Nuclei were counterstained with hematoxylin (purple). Images were obtained at a ×20 magnification unless otherwise specified. Asterisk, lumen; arrows, uterine glands. (e to g) The CEACAM staining intensity (red-brown) was qualitatively scored to distinguish low (+), moderate (++), strong (+++), and very strong (++++) levels of expression in individual patient samples. The proportion of individuals with the respective levels of each CEACAM is depicted graphically. −, undetectable; n, the number of individual patient samples that were examined for CEACAM expression on surface/glandular epithelia.
Striking differences in the pattern of expression of CEACAMs along the upper (uterus) relative to the lower (endocervix and ectocervix) portion of the genital tract were observed (Fig. 1). CEACAM1 was consistently detected at low to moderate levels on the apical side of the columnar epithelial cells that line the surface of the uterus and endocervix (Fig. 1a, b, and e and f [top]). Additionally, glandular epithelia found within the uterus and endocervix also expressed CEACAM1 apically (Fig. 1a, glands indicated by arrows), and these expression levels tended to be higher than those in the respective surface epithelia (Fig. 1e and f, bottom). Aside from the epithelial cells, CEACAM1-positive cells could be detected within the stroma along the entire genital tract due CEACAM1 expression by various lymphoid and myeloid cells. CEACAM5 and CEACAM6 were not detected on columnar epithelia (Fig. 1a and b [middle and right], e, and f), with the exception of glandular cells from 1 patient, which had low levels of CEACAM5.
Close to the squamocolumnar junction, where the endocervix meets the ectocervix (and the epithelium transitions abruptly from simple columnar to multilayered squamous cells), both CEACAM1 and CEACAM5 could be detected within the superficial layers of the ectocervix (Fig. 1c, left and middle). However, further along the ectocervix, CEACAM1 was no longer expressed, and only CEACAM5 was found both apically and basally at moderate to high levels on cells that make up the intermediate and superficial layers (Fig. 1d [middle] and g).
CEACAM6 was not found on epithelial cells in any part of the female genital tract (Fig. 1a to d, right), with the exception of 1 patient, where CEACAM6 could be detected at low levels in the ectocervix. This general lack of a signal on epithelia is not due to the strength or specificity of the primary antibody, since CEACAM6 on neutrophils (which are known to express CEACAM6) was detected within the same tissue sections (Fig. 1c [right], ×20 magnification). The CEACAM expression profile in the available samples was consistent irrespective of patient age, genital pathology, menstrual status, and the use of a contraceptive.
Overall, the differential expression of CEACAM5 and CEACAM1 on the lining of the lower and upper reproductive tract, respectively, suggests that these two epithelial CEACAMs would each be available for N. gonorrhoeae colonization of these respective regions.
The pattern of epithelial CEACAM expression in the genital tract of transgenic mouse lines mirrors the pattern in humans.
The specificity of Opa proteins for CEACAM receptors of human origin and the lack of CEACAM3, CEACAM5, and CEACAM6 in nonhuman mammals make it impractical to study Opa-CEACAM interactions using wild-type mice. We therefore utilized various transgenic mouse lines in which different combinations of human CEACAMs (hCEACAMs) were introduced, with expression being driven by their native human promoter (summarized in Table 1) (35–37).
TABLE 1.
Human CEACAM expression in transgenic mouse lines
| Genotype | Human CEACAM detected |
Source or reference | ||||
|---|---|---|---|---|---|---|
| Epithelial cells |
Stromal cells |
|||||
| Uterus | Cervix | Vagina | Neutrophils | Others | ||
| Wild type | —a | — | — | — | — | This study |
| hCEACAM1 | 1 | — | — | 1 | 1b | This study, 36 |
| CEABAC | — | 5 + 6 | 5 + 6 | 3 + 6 | — | This study, 30 |
| hCEACAM5 | — | 5 | 5 | — | — | This study, 20 |
—, no signal.
Macrophages and T cells.
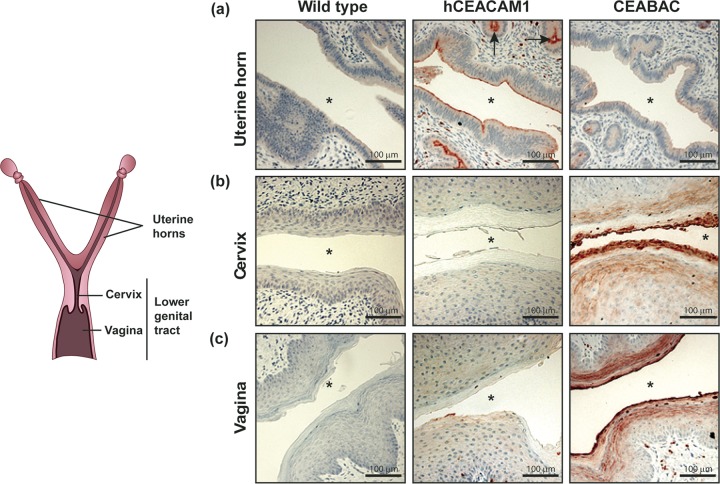
To determine whether the differential expression of epithelial CEACAMs that we observed in human tissues is faithfully recapitulated in mice, we examined the expression of human CEACAMs in the genital tract of the transgenic animals by immunohistochemistry. We utilized an antibody that recognizes all four relevant (N. gonorrhoeae-binding) human CEACAMs (hCEACAM1, -3, -5, and -6) but does not recognize any CEACAM of mouse origin (Fig. 2). The specificity of the antibody was demonstrated by the complete lack of staining in wild-type mice (Fig. 2a to c, left).
FIG 2.
Expression of human CEACAMs in the reproductive tract of transgenic mouse lines. Tissues from wild-type mice and two different transgenic mouse lines were stained using a rabbit polyclonal antibody that recognizes human CEACAM1, CEACAM3, CEACAM5, and CEACAM6 (red-brown) but not any mouse CEACAM orthologues. Tissues depicted here were collected from mice at the estrus stage. Nuclei were counterstained with hematoxylin (purple). Asterisk, lumen; arrows, uterine glands. Images were obtained at a ×20 magnification and are representative of those from at least 3 animals.
Reflecting the restricted expression profile observed in human tissues, human CEACAM1 was found on the apical surface of columnar epithelia in the uterine horns of hCEACAM1 transgenic mice (Fig. 2a, middle) but was not detected in the cervical or the vaginal lining (Fig. 2b and c, middle). Strong expression of hCEACAM1 was also detected on glandular epithelia (Fig. 2a, arrows) and leukocytes within the stroma.
Consistent with the pattern in humans, CEACAM staining could be detected on stratified squamous epithelia lining the vagina and cervix of CEABAC mice (which contain the genomic region encoding human CEACAM3, −5, −6, and −7) (Fig. 2b and c, right) and hCEACAM5 mice (which express only human CEACAM5) (see Fig. S1a in the supplemental material). Since CEABAC mice have the human genomic region encoding both hCEACAM5 and hCEACAM6, we utilized monoclonal antibodies that are able to differentiate between the two by immunohistochemistry (data not shown) and by size difference using Western blotting. In contrast to human tissues, both CEACAM5 and CEACAM6 could be detected on the stratified epithelia (Fig. S1a). CEABAC neutrophils within the stroma were also hCEACAM positive due to their expression of hCEACAM3 and hCEACAM6 (30) (Fig. 2a to c, right).
Considering that gene expression in the female genital tract may be globally modulated by the female reproductive cycle, we compared expression of human CEACAMs in the mucosal lining of transgenic mice during the estrus stage (Fig. 2) as well as the diestrus stage (data not shown) and found no differences in the pattern. Taken together, in the context of the female genital tract, epithelial expression of human CEACAMs in transgenic mice reflected the differential pattern that was observed in human samples, thus making these mice suitable models for studying the impact of Opa-CEACAM interactions during gonococcal infections.
Opa-CEACAM5 interaction on vaginal epithelia facilitates long-term colonization of the lower genital tract.
To understand the significance of various Opa-CEACAM interactions during lower genital tract (vagina and cervix) infections, we vaginally inoculated mice that had been pretreated with β-estradiol to halt the murine estrous cycle at an estrus-like stage and administered antibiotics to selectively suppress the growth of commensal microbes. These conditions have been previously shown to promote gonococcal colonization of the lower genital tract of wild-type mice (38). A well-characterized, nonpiliated N. gonorrhoeae strain, MS11, that constitutively expresses Opa57 was used for infections, unless otherwise noted (39). This particular Opa variant was chosen due to its ability to bind all four relevant CEACAMs (CEACAM1, CEACAM3, CEACAM5, CEACAM6) and was heretofore termed OpaCEA (19). Vaginal lavage samples were recovered from infected mice to quantify the gonococcal burden.
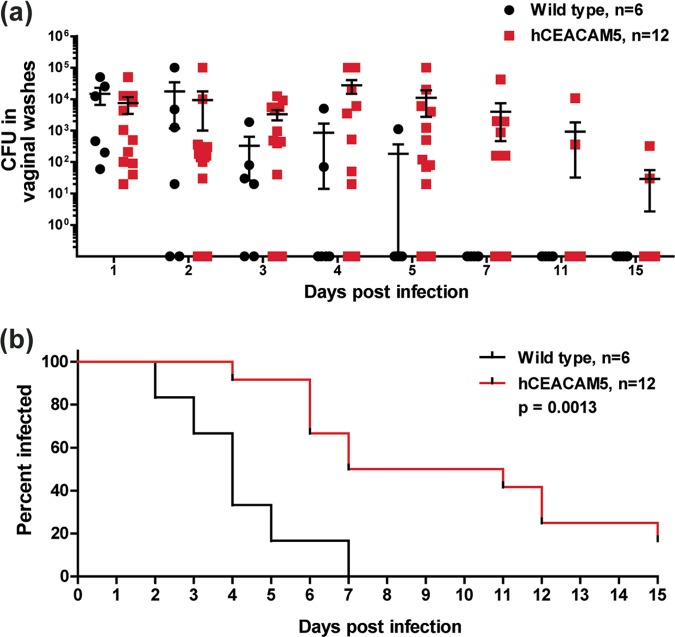
Based on the epithelial expression profile of CEACAMs in humans and transgenic mice, we were interested in determining whether the gonococcal association with hCEACAM5 would promote long-term colonization in the lower genital tract. To this end, we compared gonococcal recovery from hCEACAM5 mice to that from wild-type littermate controls. The number of colonies recovered from infected mice ranged from 10 to 105 (Fig. 3a) and tended to follow a cyclic pattern that has previously been described to be characteristic of the lower genital tract infection model (38). Notably, while wild-type mice had completely cleared the infection by 7 days, hCEACAM5 mice remained infected for a significantly longer duration (P = 0.0013), and viable gonococci could still be recovered from 2 of 12 hCEACAM5 mice past 15 days (Fig. 3b). Infection in these remaining hCEACAM5 mice had been cleared when the mice were checked at 30 days, presumably due to their reentry into the estrous cycle. Overall, the availability of CEACAM5 within the lower genital tract prolongs colonization by OpaCEA-expressing N. gonorrhoeae.
FIG 3.
Contribution of Opa-CEACAM5 interaction on vaginal epithelia during lower genital tract colonization. (a) β-Estradiol-treated wild-type and hCEACAM5 mice were vaginally infected with 107 OpaCEA-expressing N. gonorrhoeae bacteria, and viable gonococci in vaginal lavage samples were enumerated by plating at the indicated time points. The N. gonorrhoeae counts from individual animals and the mean number of N. gonorrhoeae bacteria recovered from all mice (including mice that were still colonized and those that had cleared N. gonorrhoeae) are plotted; error bars represent standard errors. Bacterial counts did not reach statistical significance using a two-way analysis of variance. (b) Graph showing the percentage of mice colonized, as determined on the last day that viable N. gonorrhoeae bacteria could be recovered. The log-rank (Mantel-Cox) test was used to compute the P value, which was statistically significant. Statistical analysis was performed using GraphPad Prism (version 5.04) software.
hCEACAM3 and hCEACAM6 expression by neutrophils impedes gonococcal persistence in the lower genital tract.
In considering that neutrophils have been reported to infiltrate into the lower genital tract within 5 days after infection (40), we further probed whether Opa protein recognition by neutrophils has the potential to alter colonization kinetics. To this end, we utilized CEABAC mice, which constitutively express CEACAM3 and CEACAM6 on neutrophils, in addition to CEACAM5 (and CEACAM6) on the vaginal epithelia.
To verify that CEABAC neutrophils are able to interact with OpaCEA-expressing N. gonorrhoeae, we infected bone marrow-derived neutrophils from wild-type and CEABAC mice ex vivo and looked at gonococcal binding by microscopy (Fig. 4ai). CEABAC neutrophils efficiently recognized OpaCEA-expressing N. gonorrhoeae, while wild-type neutrophils did not, and neither CEABAC nor wild-type neutrophils were able to detect N. gonorrhoeae bacteria that lacked Opa expression (Opa−) (Fig. 4ai and aii). This was consistent with the findings of our previous studies using CEABAC and other mouse neutrophils, where the oxidative burst, degranulation, and proinflammatory cytokine response of infected neutrophils was demonstrated to be driven by Opa-CEACAM3 binding (9, 30).
FIG 4.

Contribution of Opa-CEACAM interactions on neutrophils during gonococcal clearance from the lower genital tract. (ai) Immunofluorescence staining of bone marrow-derived neutrophils from wild-type and CEABAC mice infected for 30 min with Opa− and OpaCEA-expressing N. gonorrhoeae. N. gonorrhoeae bacteria are stained red, while nuclear DNA is stained with DAPI (blue). Images were taken at a ×63 magnification. (aii) Quantification of wild-type and CEABAC neutrophil-associated OpaCEA and Opa− gonococci for 30 to 50 cells per group using microscopy. The bar graph depicts the mean; error bars represent standard deviations. (b) β-Estradiol-treated wild-type and CEABAC mice were vaginally infected with 107 OpaCEA-expressing N. gonorrhoeae bacteria, and viable gonococci in vaginal lavage samples were enumerated by plating at the indicated time points. The graph shows the N. gonorrhoeae counts from individual animals and the mean number of N. gonorrhoeae recovered from all mice (including mice that were still colonized and those that had cleared N. gonorrhoeae); error bars represent standard errors. Bacterial counts did not reach statistical significance using a two-way analysis of variance. (c) Graph showing the percentage of mice colonized, as determined on the last day that viable N. gonorrhoeae bacteria could be recovered. The log-rank (Mantel-Cox) test was used to compute the P value to determine the difference between wild-type and CEABAC mice, which was not statistically significant. (d) Neutrophils from the indicated mice were depleted by systemically administering anti-Gr-1 antibody clone RB6-8C5 prior to infection, and the mean number of N. gonorrhoeae bacteria recovered from homogenized tissues at 2 days postinfection is plotted. Error bars represent standard errors. The log increase in N. gonorrhoeae recovery upon neutrophil depletion in CEABAC mice did not reach statistical significance, as measured by one-way analysis of variance. A one-tailed chi-square test comparing the percentage of culture-positive animals among neutrophil-depleted versus control animals produced a P value of 0.09 and 0.02 for wild-type and CEABAC mice, respectively. Statistical analysis was performed using GraphPad Prism (version 5.04) software.
To test whether the ability of CEABAC neutrophils to recognize OpaCEA-expressing N. gonorrhoeae is relevant in vivo, we quantified the viable N. gonorrhoeae bacteria in vaginal lavage samples from β-estradiol-treated wild-type and CEABAC mice that had been vaginally infected. While gonococci were able to persist for a maximum of 8 days in CEABAC mice but only 5 days in wild-type mice, this difference was not statistically significant. Hence, the substantial colonization advantage due to epithelial interactions that became apparent when comparing hCEACAM5 mice to wild-type mice was no longer observed in the CEACAM3-, CEACAM5-, and CEACAM6-expressing CEABAC mice (Fig. 4b and c).
The lack of persistence in CEABAC mice is not explained by low levels of epithelial CEACAM expression (Fig. S1a), since CEABAC mice had appreciably higher levels of CEACAM5 than hCEACAM5 mice, as well as high levels of CEACAM6 on the epithelia. The lack of persistence also cannot be attributable to the sampling method used (bacterial recovery from vaginal lavage samples) since mice that had a zero bacterial count in lavage samples also had no bacterial recovery from homogenized tissues (Fig. S1b). Together, these findings rule out the possibility of differences in epithelial CEACAM expression or deficiencies with the sample collection technique as plausible explanations for the apparent lack of persistence in CEABAC mice.
Upon infection, a parallel increase in myeloperoxidase (MPO) levels over those at the baseline was detected within vaginal lavage samples collected from wild-type and CEABAC mice within 3 days, indicative of similar neutrophil influx in the lower genital tract (data not shown). To confirm that neutrophils do play a role in controlling the N. gonorrhoeae infection and corroborate whether the CEACAM3- and CEACAM6-expressing neutrophils facilitated clearance in the CEABAC mice, we systemically depleted neutrophils by administering a Gr-1 antibody at 48 h and 6 h prior to infection. We have previously shown that this approach effectively suppresses neutrophil levels in the circulation for up to 5 days from the time that the antibody is first administered (41); therefore, day 2 postinfection was chosen to examine the effects of depletion since neutrophil levels were effectively suppressed up to this point. Neutrophil depletion led to an increase in the percentage of mice that were culture positive at 2 days postinfection compared to the percentage of the untreated controls for both wild-type mice (62% versus 29%, P = 0.09 [not significant]) and CEABAC mice (67% versus 17%, P = 0.02 [significant]), which is suggestive of a role of neutrophils in controlling infection in the lower genital tract. Additionally, there was a 10-fold increase in the maximum bacterial load when neutrophils were depleted in CEABAC mice but no similar effect on the burden in infected wild-type mice, suggesting that CEABAC neutrophils may be able to efficiently suppress bacterial numbers (Fig. 4d). However, this increase in bacterial load upon neutrophil depletion did not reach statistical significance due to high mouse-to-mouse variations.
The lack of gonococcal persistence in the lower genital tract of CEABAC mice, together with the increase in the percentage of culture-positive animals and the 10-fold (albeit nonsignificant) increase in the maximal bacterial load upon neutrophil depletion, suggests that CEACAM3- and CEACAM6-expressing neutrophils in these animals play a role in controlling the gonococcal load in the lower genital tract. This is consistent with the findings of a large body of existing cell-based studies using primary CEABAC and human neutrophils, in which Opa-CEACAM interactions resulted in efficient phagocytosis and killing of Opa-expressing gonococci ex vivo (9, 30, 31).
Opa-CEACAM1 interactions enhance gonococcal tissue association and penetration within the upper genital tract.
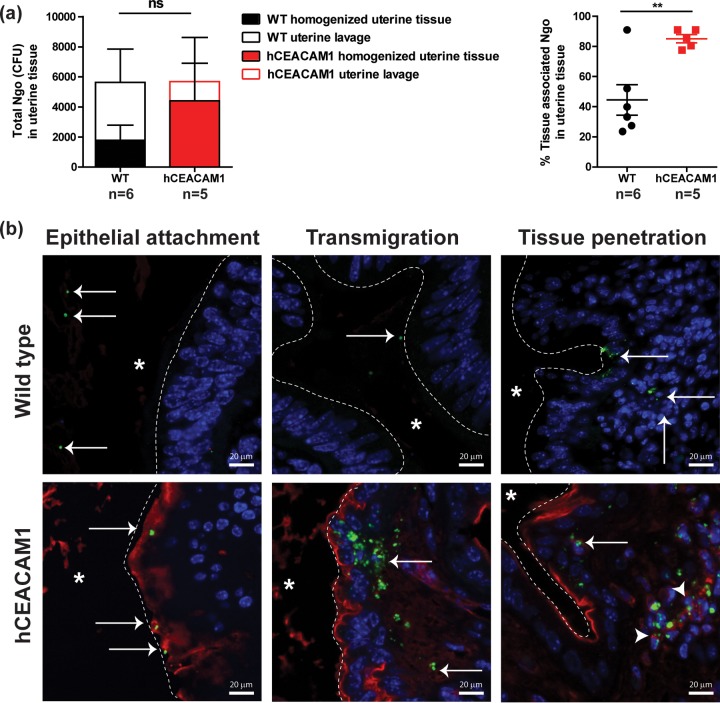
Since hCEACAM5 on the cervicovaginal lining facilitated colonization of the lower genital tract, we contemplated whether hCEACAM1-expressing columnar epithelia would similarly enable colonization of the upper genital tract and, if so, whether this would have an effect on the development of pelvic inflammatory disease (PID). We have recently established a novel model to study gonococcal PID, which involves directly instilling gonococci into the uterine horn of mice as a means of simulating an ascendant infection (42). Uterine infections in wild-type β-estradiol-treated mice are resolved within 3 days, at least partially due to the inability of N. gonorrhoeae to associate with the tissues of wild-type mice (42). To determine whether N. gonorrhoeae is able to bind hCEACAM1 on the uterine lining, we quantified gonococcal recovery from uterine lavage samples and homogenized uterine tissues from wild-type and hCEACAM1 mice at 6 h postinfection. Interestingly, while there was no difference in the total number of CFU recovered from lavage and tissue samples combined, a significantly higher proportion of recovered N. gonorrhoeae was associated with the uterine tissues of hCEACAM1 mice (Fig. 5a, right). This suggests that the bacteria were either tightly associated with the uterine tissues or had penetrated into the uterine tissues in these mice, or both.
FIG 5.
Opa-hCEACAM1-mediated gonococcal association and invasion of the uterine tissues. (a) The uterine horns of β-estradiol-treated wild-type (WT) and hCEACAM1 mice that were transcervically infected with 107 OpaCEA-expressing N. gonorrhoeae (Ngo) bacteria for 6 h were flushed with PBS++ to remove any nonadherent bacteria. (Left) Uterine lavage and homogenized uterine tissues were cultured to quantify nonadherent and tissue-associated bacteria, respectively. Bars represent means; error bars indicate standard errors. ns, not significant, as determined using two-way analysis of variance. (Right) The percentage of tissue-associated N. gonorrhoeae bacteria was calculated by dividing the number of CFU recovered from homogenized tissues by the total number of CFU in homogenized tissues and uterine lavage samples. Error bars represent standard errors. **, P < 0.05, as determined by a t test. Statistical analysis was performed using GraphPad Prism (version 5.04) software. (b) Bacterial localization in the uterine horn of β-estradiol-treated mice 8 h after transcervical infection with 107 OpaCEA-expressing N. gonorrhoeae bacteria. Sections were immunostained with anti-N. gonorrhoeae (green) and anti-human CEACAM (red) antibodies, while nuclei were counterstained with DAPI (blue). White dashed line, uterine epithelial surface; asterisk, the luminal side. Arrows point at N. gonorrhoeae. Arrowheads point at N. gonorrhoeae associated with CEACAM-positive cells in the uterine stroma. Images are representative of those from 3 animals.
To directly visualize OpaCEA-expressing N. gonorrhoeae within the uterine horns of wild-type and transgenic hCEACAM1 mice, we immunostained fixed tissue samples that have been infected for 8 h using antigonococcal and anti-hCEACAM antibodies. Consistent with the colony counts, gonococci could be found to be attached to the uterine lining and appeared to transmigrate and penetrate the tissues more effectively in hCEACAM1 mice than wild-type mice (Fig. 5b, arrows). Furthermore, bacteria that had penetrated into the tissues of hCEACAM1 mice were also able to associate with hCEACAM1-positive stromal cells (Fig. 5b, arrowheads).
To further determine whether N. gonorrhoeae bacteria are able to persist in the uteri following CEACAM1-mediated epithelial invasion, the bacterial load in homogenized tissues was quantified by quantitative PCR (qPCR) at 24 h and 48 h postinfection. qPCR was used in order to increase the sensitivity of detection due to the low/negligible recovery of N. gonorrhoeae from uterine tissues past 24 h, presumably due to lost viability due to homogenization in the presence of abundant neutrophils and/or a poor ability to recover and quantify cell-associated bacteria. Surprisingly, despite the early mucosal association and penetration observed at between 6 and 8 h postinfection in hCEACAM1 mice, the bacterial load determined by qPCR at these later time points was not significantly different from that in wild-type mice (Fig. S2a). In considering these observations, we reasoned that efficient N. gonorrhoeae detection by CEACAM1-expressing immune cells within 24 h may contribute to N. gonorrhoeae clearance in hCEACAM1 animals.
We have previously shown that in wild-type mice neutrophils are recruited into the genital tract within hours after uterine infection, as opposed to days in the case of lower genital tract infections only (40, 42). To determine whether this rapid neutrophil influx also occurs in hCEACAM1 mice, we quantified myeloperoxidase levels in homogenized uterine tissues as a readout for neutrophil levels and observed a 2- to 6-fold increase over baseline MPO levels within 24 h in hCEACAM1 mice, similar to the findings for wild-type mice (Fig. S2b). Considering this rapid recruitment of neutrophils into the uterine tissues in hCEACAM1 mice and the ability of CEACAM1-expressing neutrophils to efficiently recognize N. gonorrhoeae (Fig. S2c) (9), it is plausible that recognition by hCEACAM1-expressing neutrophils in the stroma is one mechanism by which N. gonorrhoeae may be cleared from the uterus of hCEACAM1 mice. However, since CEACAM1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), as opposed to the immunoreceptor tyrosine-based activation motif (ITAM), as in CEACAM3, the signaling cascade and net effect of CEACAM1 engagement on neutrophils will require further characterization.
On the whole, we show that Opa-CEACAM1 enables an increased tissue association with the potential to facilitate tissue penetration during uterine infection and that hCEACAM1 expression also promotes neutrophil association with the gonococci.
Distinct Opa-CEACAM interactions aggravate inflammation during upper genital tract infections.
We have previously observed that uterine infection of wild-type mice in the estrus stage (induced by β-estradiol) leads to a relatively mild proinflammatory cytokine response, apparently due to the lack of tissue penetration (42). Since Opa-CEACAM interactions noticeably affected mucosal colonization, uterine invasion, and neutrophil-mediated recognition, we reasoned that the inflammatory response during gonococcal infections may be altered in an Opa-CEACAM-dependent manner.
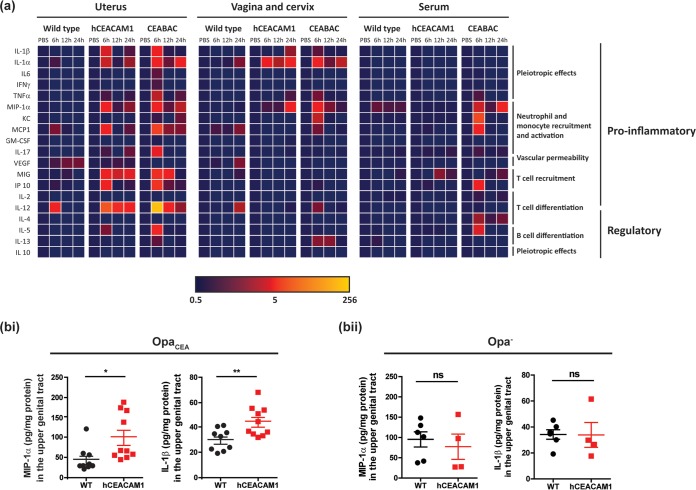
To investigate this, we quantified a broad panel of cytokines expressed in wild-type and hCEACAM1 mice that had been infected with 107 OpaCEA-expressing N. gonorrhoeae bacteria (Fig. 6). Included in this panel were cytokines, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), and a homologue of IL-8 (keratinocyte-derived chemokine [KC]), which are expressed during gonococcal infection in humans (43–45). Local and serum cytokine levels were measured at 6 h, 12 h, and 24 h postinfection, which is a relevant time frame for studying cytokine responses to symptomatic gonococcal infections on the basis of the rapid cytokine induction observed after human urethral challenge (43). Intriguingly, the levels of several proinflammatory cytokines, including IL-1β, macrophage inflammatory protein 1α (MIP-1α), IL-1α, monocyte chemotactic protein 1 (MCP-1), monokine induced by gamma interferon (MIG), gamma interferon-induced protein 10 (IP-10), IL-12, and IL-5, were substantially higher as early as 6 h postinfection in the uterine tissues of hCEACAM1 versus wild-type mice (Fig. 6a, left). To confirm that these effects were due to Opa-CEACAM interactions in the context of infection and not a differential response of the transgenic mice to infection alone, we compared IL-1β and MIP-1α levels by enzyme-linked immunosorbent assay (ELISA) in separate cohorts of wild-type and hCEACAM1 mice that had been infected with either an OpaCEA or an Opa− N. gonorrhoeae strain (Fig. 6b). When infected with an Opa-deficient strain, these cytokines were produced at similar levels in wild-type and hCEACAM1 transgenic mice, whereas higher levels of cytokines were induced in hCEACAM1 mice infected with OpaCEA-expressing bacteria. Together, these observations demonstrate that the Opa-dependent association of N. gonorrhoeae with hCEACAM1 elicits a proinflammatory response within the infected uterine tissues. Considering that these mice express hCEACAM1 on a variety of cells (myeloid and lymphoid cells) as well as uterine epithelia, the increased inflammation may have arisen from increased tissue penetration as well as specific interactions with hCEACAM1-expressing cells.
FIG 6.
Cytokine response during upper genital tract infections of wild-type and CEACAM-expressing mice. (a) Cytokine levels within homogenized tissues and serum, as determined by Luminex multiplex assays after transcervical infection of β-estradiol-treated mice with 107 OpaCEA-expressing N. gonorrhoeae bacteria for 6 h, 12 h, and 24 h. The heat map depicts the fold change normalized to the value for uninfected PBS-treated controls. Data are for 3 mice of each genotype per time point for infected groups and 2 to 3 mice per genotype for uninfected PBS-treated controls. The heat map was generated in Microsoft Excel software. GM-CSF, granulocyte-macrophage colony-stimulating factor; VEGF, vascular endothelial growth factor. The other abbreviations are defined in the text. (b) MIP-1α and IL-1β levels in the upper genital tract of β-estradiol-treated mice at 24 h postinfection with OpaCEA (bi) and Opa− (bii) N. gonorrhoeae. Horizontal bars represent means; error bars represent standard errors. P values were determined by a t test. **, P < 0.01; *, P < 0.05; ns, not significant. For panel bi, data points from two independent experiments were pooled and data are for 9 or 10 mice per genotype. For panel bii, data are for 4 or 5 mice per genotype. Statistical analysis was performed using GraphPad Prism (version 5.04) software.
Besides the hCEACAM1-dependent phenotype, we also considered the effect that other hCEACAMs may have on uterine inflammation by concomitantly measuring cytokine levels in infected CEABAC mice (Fig. 6a, left). As described above, CEABAC mice express hCEACAM5 and hCEACAM6 on the stratified epithelia and express hCEACAM3 and hCEACAM6 on neutrophils. Interestingly, the cytokine response observed in the infected CEABAC mice closely reflected that observed in the hCEACAM1 mice, with the exception that TNF-α and IL-17 levels were elevated in CEABAC (but not hCEACAM1) mice (Fig. 6a, left). Considering that there was no difference in cytokine levels when hCEACAM5 transgenic mice were compared to wild-type littermate controls (Fig. S3), it is likely that the inflammatory response in CEABAC mice is driven mainly by CEACAM3- and CEACAM6-expressing neutrophils.
Uterine inoculation with gonococci results in infection of the lower genital tract (vagina and cervix) as well, likely due to the downward movement of the bacteria within the mucus flow (42). Of note, the inflammatory cytokines detected within the lower genital tract were distinct from those apparent within the uterine tissues (Fig. 6a, middle). Both hCEACAM1 and CEABAC mice had higher levels of IL-1α than did the wild-type animals. MIP-1α was also produced at higher levels in both the transgenic mouse lines but peaked much earlier in CEABAC mice (6 h in CEABAC mice versus 24 h in hCEACAM1 mice), presumably because CEACAM3 binding drives a robust MIP-1α response in neutrophils (30). Importantly, Opa-CEACAM interactions appeared to have a less pronounced effect on local cytokine production in the lower genital tract than in the uterus, presumably reflecting inherent differences between how distinct portions of the genital tract respond to bacterial infections (42). Another plausible explanation for this differential response is the lack of tissue penetration in the lower genital tract, since gonococci typically associate only with superficial epithelial layers of the several-layer-thick epithelial lining in the lower genital tract (20).
Systemically, while there was little cytokine response detected in either wild-type or hCEACAM1 mice, elevated levels of MIP-1α, KC, MCP-1, IP-10, IL-4, and IL-5 responses were detected in the serum of CEABAC mice (Fig. 6a, right). Some of these reflect expression locally within the uterus. However, given that IL-4 was not evident in either the uterus or the lower genital tract, it is enticing to consider that at least some of this cytokine response may reflect N. gonorrhoeae and/or N. gonorrhoeae-derived products entering into the deeper tissues and/or bloodstream in CEABAC mice.
Intriguingly, upon conducting similar studies in mice that were infected at the diestrus stage of the reproductive cycle, we did not detect any differences in the cytokine response in hCEACAM-expressing transgenic mice from that in wild-type mouse lines. In fact, there was a significant increase in several cytokines even in the wild-type mice infected during diestrus (Fig. S4a), which is in marked contrast to their lack of response during estrus (compare to Fig. 6a). This is presumably due to the massive penetration of N. gonorrhoeae into uterine tissues that occurred in a CEACAM-independent manner during the diestrus stage (Fig. S4b; compare to Fig. 5). Consequently, there is a clear distinction in the contribution of hCEACAMs to N. gonorrhoeae upper genital tract infection during different phases of the reproductive cycle, with the tissue association and the resulting inflammatory response being most obviously affected during the estrus stage.
DISCUSSION
This is the first in vivo study to consider the specific contributions of individual CEACAMs to gonococcal tissue tropism and immune responses within the female genital tract. The presence of CEACAMs on the epithelial lining of healthy (noncancerous) human genital tract tissues was a critical finding in this study, since it provides direct evidence that CEACAMs are available to the gonococci during natural infection. In this regard, the differential expression of CEACAM5 on squamous epithelia within the lower genital tract and CEACAM1 on columnar epithelia of the endocervix and uterine tissues implies that Opa variants with different binding specificities would facilitate uncomplicated (cervical) versus ascending (uterine) infection.
Using conditions developed to allow N. gonorrhoeae infection of wild-type mice (38), a previous study reported that human CEACAM5 expression improves N. gonorrhoeae attachment to vaginal epithelia and reduces epithelial sloughing after 24 h of infection (20). Expanding on this notion, we have established that binding to CEACAM5 can potentiate long-term N. gonorrhoeae colonization of the lower genital tract, with some animals remaining infected for over 2 weeks. Critically, gonococcal recognition by neutrophil-expressed CEACAMs opposed this effect. This is consistent with the innate decoy receptor function of CEACAM3, which triggers gonococcal engulfment and neutrophil degranulation (9) and elicits a potent neutrophil inflammatory cascade (30) when engaged by the gonococcal Opa proteins. Considering that gonococcal isolates are able to express 11 different Opa variants, each with differential specificities for the different CEACAMs, these studies predict that Opa-expressing variants that are able to bind epithelial CEACAMs but avoid detection by neutrophil-restricted CEACAMs would be more successful at colonizing the mucosal surface. Indeed, a selection bias in favor of bacteria expressing Opa proteins that recognize CEACAM1 and/or CEACAM5 has been reported for isolates cultured from endocervical swab specimens from female patients (13); this reflects the expression of CEACAM1 in the endocervix and CEACAM5 in the adjoining ectocervical region. Furthermore, there appears to be a strong selection against the expression of Opa variants that bind neutrophil-restricted CEACAM3 in isolates from both women and men (13). Finally, turning off Opa expression in a subset of the population may be another immune evasion strategy, given that Opa− gonococci are not as effectively cleared by human neutrophils (31, 46).
In addition to enabling lower genital tract colonization, we observed that Opa-CEACAM interactions also govern the pathological outcomes during upper genital tract infections. We have previously reported that upper genital tract infections arising during the estrus stage of the cycle lead to only mild inflammation, presumably due to the inability of N. gonorrhoeae to associate with the uterus in wild-type mice (42). Herein, we observed that hCEACAM1 expression on the uterine lining enhanced gonococcal attachment and tissue penetration and exacerbated the acute inflammatory response in both the uterus and the lower genital tissues. Interestingly, the fact that increased levels of proinflammatory cytokines were induced in both hCEACAM1 and CEABAC transgenic mice, which have different CEACAMs expressed on a different spectrum of cells, indicates that distinct CEACAM-dependent mechanisms can contribute to the elevated inflammatory response.
On the basis of previous studies that have demonstrated the ability of genitally derived epithelia to respond to N. gonorrhoeae (45), one potential source of proinflammatory cytokines in the upper genital tract of hCEACAM1 mice may be uterine epithelial cells. Furthermore, CEACAM1-mediated penetration into the uterine stroma also exposes N. gonorrhoeae to resident and/or recruited phagocytic cells, including CEACAM1-expressing neutrophils and macrophages, which could also contribute to this effect. Indeed, considering that CEACAM1 facilitates N. gonorrhoeae uptake by phagocytic cells, this interaction may also facilitate innate signaling via pattern recognition receptors that detect bacterium-derived fragments. Furthermore, interactions with CEACAM1-expressing T cells may also contribute to the cytokine microenvironment. Future work must dissect the specific contribution of the various CEACAM-expressing cells during infection, immune evasion, and immunopathogenesis, with a particular focus on mechanisms contributing to gonococcal clearance.
While CEACAM1 is broadly expressed, the more restricted expression patterns of the human CEACAMs expressed by CEABAC mice make the increased inflammation apparent in these animals more easily explained. Critical in the context of inflammation, N. gonorrhoeae binding to CEACAM3, which is expressed exclusively on CEABAC neutrophils, has been shown to potently induce their proinflammatory cytokine response, which recruits even more neutrophils to the infected tissues (30). Consistent with the premise that the human CEACAM3-expressing neutrophils may be driving the inflammatory response, hCEACAM5 transgenic mice (which have wild-type neutrophils) did not have a stronger response than wild-type controls.
Intriguingly, despite the clear differences observed during estrus, the CEACAM-mediated alteration of the inflammatory response was barely noticeable during diestrus. This effect is presumably due to the overwhelming number of N. gonorrhoeae bacteria that penetrate into the tissue at this stage (42), which occurs in a CEACAM-independent manner (see Fig. S4b in the supplemental material). Therefore, it is important to recognize that Opa-CEACAM interactions may play an obvious role in determining disease pathology only during certain stages of the female reproductive cycle.
In summary, we established that differential Opa-CEACAM interactions significantly affect the outcome at various stages of infection, including mucosal association, bacterial clearance, and acute inflammation. The contribution of each CEACAM to tissue association and disease depends upon the tissue distribution and stage of the female reproductive cycle. It is crucial to appreciate that the effects of Opa-CEACAM-dependent interactions that we report in this study could not have been observed in wild-type mice, making it clear that the incorporation of human CEACAM transgenes into the mouse model represents a significant advancement toward developing more physiologically relevant infection models. Given that the focus of this study was demonstration of the range of potential CEACAM-mediated effects that can occur during genital tract infections in females, we selected a gonococcal strain which constitutively expresses an Opa variant that binds all four relevant CEACAMs. An interesting avenue for future studies would be to utilize clinical gonococcal isolates which have the ability to switch Opa variants or turn off Opa expression to further tease apart Opa-CEACAM dynamics.
While the incorporation of CEACAMs into mouse models represents a key first step in developing humanized models, it is important to consider that aside from CEACAMs, N. gonorrhoeae also binds to several other factors in a human-restricted manner, including other mucosal receptors, the iron binding proteins transferrin and lactoferrin, and complement regulatory factor H and C4bp. Since each of these has the potential to promote N. gonorrhoeae survival and immune evasion within the host (2, 3, 5–8, 47), it is enticing to consider that future efforts to integrate these human factors will amplify the effect of human CEACAM expression, further enhancing the utility of this animal model by promoting gonococcal replication and/or persistence within the genital tract.
MATERIALS AND METHODS
All animal procedures were approved by the Local Animal Care Committee of the University of Toronto (permit number 20010551), which is subject to the ethical and legal requirements under the Province of Ontario, Canada, Animals for Research Act and those of the federal Canadian Council on Animal Care (CCAC). All efforts were made to avoid and/or minimize suffering.
Reproductive tract tissues, including the endometrium and cervix, were obtained, following the provision of written informed consent, from women undergoing hysterectomies for nonmalignant gynecological purposes at McMaster University Medical Centre in Hamilton, ON, Canada. This study was approved by the Hamilton Health Sciences-McMaster University Research Ethics Board.
Mouse strains.
Transgenic hCEACAM1 (36) and CEABAC2 (35) mice were of the FVB background, while hCEACAM5 (37) mice were of the C57BL/6 background. In each case, wild-type controls were littermates of the respective animals. Mice were bred and housed in DCM CCBR at the University of Toronto. Six- to 10-week-old mice were used for the experiments.
Preparation of N. gonorrhoeae inoculum.
N. gonorrhoeae strain MS11 N313 constitutively expressing Opa57 (referred to herein as OpaCEA) and nonopaque strain MS11 N302 (referred to herein as Opa−) (39) were used in this study. Opa57 binds CEACAM1, CEACAM3, CEACAM5, and CEACAM6 (19). The parental strain used to generate N302 and N313 does not produce pilus and lacks the heparan sulfate proteoglycan-specific OpaC30. These well-characterized strains are routinely subcultured by selection of single colonies of the respective phenotype under a dissecting microscope and subject to immunoblot analysis to verify transparent (Opa−) or Opa57 expression in the absence of other phase-variable chromosomally expressed Opa variants (19, 39). These strains are resistant to the antibiotics used for suppressing vaginal commensals (below) and, therefore, suitable for in vivo studies.
To prepare the inocula used for the mice, bacteria were streaked on GC agar (Becton Dickinson, Mississauga, Canada) supplemented with IsoVitaleX (Becton Dickinson, Mississauga, Canada) and incubated overnight at 37°C in a humidified 5% CO2 atmosphere. An overnight lawn of gonococci was harvested into 1 ml of phosphate-buffered saline (PBS) supplemented to obtain 0.9 mM CaCl2 and 0.5 mM MgCl2 (PBS++; Life Technologies, Burlington, Canada), and the optical density at 550 nm was measured to calculate the number of bacteria. Appropriate dilutions were made in PBS++ to obtain the desired concentration.
Staging the estrous cycle.
To determine the stage of the reproductive cycle, the vagina was lavaged daily with 30 μl phosphate-buffered saline (Life Technologies, Burlington, Canada) using a 100-μl micropipette. Wet smears were examined under a 40× objective, and the stage of the estrous cycle was determined based on cytology (48).
Hormone administration.
Mice were subcutaneously (s.c.) injected with water-soluble β-estradiol (0.5 mg/mouse; Sigma-Aldrich, Oakville, Canada) to induce estrus or with medroxyprogesterone acetate (2 mg DepoProvera/mouse; Pfizer Canada Inc., Quebec, Canada) 5 days prior to infection to synchronize the mice at diestrus.
Vaginal infection.
The natural estrous cycle of 6- to 8-week old mice was monitored for approximately 5 days, and once diestrus was reached (day −2 relative to the day of infection), hormone and antibiotic treatments were initiated. Mice received 0.5 mg β-estradiol s.c. on day −2, day 0, and day 3. Streptomycin sulfate (2.4 mg) plus vancomycin HCl (0.6 mg) in 200 μl PBS per animal was injected intraperitoneally (i.p.) once on day −2 and days 0 to day 5 and twice on day −1. Trimethoprim was administered orally via drinking water (0.04 g/100 ml), starting at day −2, and maintained until the experimental endpoint. On day 0, just prior to infection, the vagina was washed three times with 30 μl PBS and 107 N. gonorrhoeae bacteria suspended in 5 μl PBS++ (Life Technologies, Burlington, Canada) were inoculated using a P10 pipette tip. Infections were performed on alert mice. For gonococcal recovery, the vagina was washed once with 10 μl PBS++ and serial dilutions were plated on GC agar supplemented with IsoVitaleX and vancomycin, streptomycin, nystatin, and trimethoprim (VCNT) inhibitors, which suppress the growth of commensals while allowing recovery of N. gonorrhoeae (Becton Dickinson, Mississauga, Canada).
Neutrophil depletion.
While a single dose of 250 μg of the Gr-1 epitope-specific RB6-8C5 monoclonal antibody is sufficient to deplete neutrophils in peripheral blood, we have found that two doses are required to substantially deplete neutrophils within the female genital tract. Repeated administration of RB6-8C5 extends the duration of neutrophil depletion, but it does not consistently extend beyond ∼5 days (presumably due to development of a humoral response against the rat antibody). Thus, RB6-8C5 or PBS (as a control group) was administered intraperitoneally (i.p.) at 48 h prior to infection and then again at 6 h prior to infection. All mice received β-estradiol (0.5 mg/mouse) s.c. at 48 h and 6 h prior to infection. Streptomycin sulfate (1.2 mg) and vancomycin HCl (0.6 mg) were i.p. injected at 48 h, 24 h, and 6 h prior to infection. Mice were then vaginally infected with 107 N. gonorrhoeae bacteria as previously described.
Neutrophil isolation.
Mouse bone marrow neutrophils were taken from 8- to 10-week-old mice that were humanely euthanized by CO2 inhalation. Femurs and tibias were removed, and bone marrow was isolated and separated on a discontinuous Percoll gradient (80%, 65%, 55%). Neutrophils were recovered at the 80%-65% interface. Where indicated, 5 × 105 neutrophils were infected for 30 min with N. gonorrhoeae at a multiplicity of infection (MOI) of 25 and then fixed in 3.7% paraformaldehyde as previously described (30). Coverslips were stained using rabbit antigonococcal antibody (UTR01 [18]), followed by goat anti-rabbit IgG-Alexa Fluor 594 (Life Technologies, Burlington, Canada), and mounted using Prolong Gold antifade mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Life Technologies, Burlington, Canada).
Transcervical infection.
Hormone-treated mice were anesthetized via isoflurane inhalation. We have recently described the transcervical infection protocol (42). Briefly, mice were placed in a prone position with the lower back tilted at a 45-degree angle. A blunted 25-gauge needle was inserted through the vagina so as to bypass the cervix and deposit a total of 20 μl of a suspension with the number of gonococci indicated above into the uterine horn(s). At the time points after infection indicated above, animals were humanely sacrificed by CO2 inhalation, and cardiac puncture was performed to drain the blood and obtain sera. The genital tract was dissected and cut at the point where the cervix joins the uterus to separate the uterus and the lower (cervix and vagina) sections. Tissues were flash frozen in liquid nitrogen and stored at −80°C until further processing for quantifying gonococcal DNA and/or performing ELISA.
Gonococcal quantification by qPCR.
Infected tissues were homogenized in PBS, and the total genomic DNA was extracted using a Qiagen DNeasy blood and tissue kit per the manufacturer's instructions. For gonococcal quantification, a 132-bp region of the gonococcal porA pseudogene was amplified in infected samples using the following primers: 5′-CGGTTTCCGTGCGTTACGA-3′ and 5′-CTGGTTTCATCTGATTACTTTCCA-3′ (49). The values were normalized to the levels of mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase), amplified using the primers 5′-TGAGCAAGAGAGGCCCTATC-3′ and 5′-AGGCCCCTCCTGTTATTATG-3′. The normalized porA copy numbers were then used to calculate bacterial counts using a standard curve, generated by spiking uninfected tissue samples with known numbers of gonococci.
ELISA for detection of cytokines.
Frozen tissues were homogenized in 1 ml ice-cold PBS containing 0.1% Triton, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/ml aprotinin, and 1 μg/ml pepstatin (all obtained from Sigma-Aldrich, Oakville, Canada) and sonicated for 30 s at a 40-kHz frequency using a water bath sonicator. Samples were then spun on a tabletop centrifuge at 16,060 × g for 15 min at 4°C, and the supernatant was recovered and sterile filtered through 0.22-μm-pore-size cellulose acetate SpinX columns (Corning Inc., Corning, NY, USA). Cytokines were quantitatively measured using enzyme-linked immunosorbent assay (ELISA) kits for MIP-1α, KC, and MIG (R&D Systems, Burlington, Canada) and for IL-1α and IL-1β (BD Biosciences, Mississauga, Canada), and the levels were normalized to the total protein yield, as determined using a Bradford assay (Bio-Rad Laboratories, Inc., Mississauga, Canada).
Luminex assay.
For total protein extraction, tissue samples were individually pulverized using a scalpel blade and then resuspended in 500 μl of tissue homogenization buffer (1× PBS containing 0.1% Triton, 5 mM EDTA, 1 mM PMSF, 2 μg/ml aprotinin, and 1 μg/ml pepstatin). Samples were homogenized with a 5-mm stainless steel bead (Qiagen, Valencia, CA) at 500 oscillations/min for 15 min at 4°C using a TissueLyser LT sample disruptor (Qiagen). Samples were then centrifuged for 10 min at 16,060 × g and 4°C. The supernatant was collected, and the total protein concentration was determined by a Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard. Fifty micrograms of total protein in 50 μl was analyzed to determine the levels of cytokines and chemokines using a mouse cytokine magnetic 20-plex panel (Novex Life Technologies, Carlsbad, CA) per the manufacturer's instructions. Plates were read on a Bio-Plex Magpix multiplex reader (Bio-Rad, CA) using xPONENT software (Life Technologies).
Immunohistochemistry staining.
Paraffin-embedded hysterectomy sections were stained using mouse monoclonal antibody clone 283324 (anti-hCEACAM1; R&D Systems, Minneapolis, MN, USA), COL-1 (anti-hCEACAM5, weakly reactive with hCEACAM3; Invitrogen, Burlington, Canada), and 9A6 (anti-hCEACAM6; Abcam, Cambridge, MA, USA), followed by goat anti-mouse immunoglobulin-horseradish peroxidase (HRP) (Jackson ImmunoResearch, West Grove, PA, USA). For detection of hCEACAM in transgenic mice, the pan-hCEACAM polyclonal antiserum CEA-Dako raised in rabbit was used, followed by goat anti-rabbit immunoglobulin-HRP (Jackson ImmunoResearch, West Grove, PA, USA) and visualization by incubating with 3,3′-diaminobenzidine (Sigma-Aldrich, Oakville, Canada) according to the manufacturer's recommendations. Nuclei were counterstained using Harris's hematoxylin (VWR, Mississauga, Canada) before the samples were dehydrated and mounted with SHUR/Mount mounting medium (Triangle Biomedical Sciences, Durham, NC, USA).
Immunohistochemistry scoring.
The CEACAM staining intensity on human tissues was scored manually. Multiple fields of view for each stained sample were imaged at a ×20 magnification with a Leica DMIRB/E epifluorescence microscope using the same image acquisition settings. After a preliminary examination of all the images, an intensity scale for scoring CEACAM expression on various surface and glandular epithelia was established, as follows: no signal (−), no detectable staining; low (+), weak apical staining of at least 50% of the epithelial monolayer (for uterine/endocervical surface epithelia and glandular epithelia) or weak circumferential staining of at least 50% of the cells within the superficial epithelial layers (ectocervix); intermediate (++), low- to moderate-intensity apical staining of the entire epithelial monolayer (for uterine/endocervical surface epithelia and glandular epithelia) or low- to moderate-intensity circumferential staining of cells making up the superficial epithelial layers (ectocervix); strong (+++), moderate- to high-intensity apical staining of the entire epithelial monolayer (for uterine/endocervical surface epithelia and glandular epithelia) or moderate- to high-intensity circumferential staining of cells making up the superficial epithelial layers (ectocervix); and very strong (++++), uniformly high-intensity apical staining of the entire epithelial monolayer (for uterine/endocervical surface epithelia and glandular epithelia) or uniformly high-intensity circumferential staining of cells making up the superficial epithelial layers (ectocervix). Intensity scores were recorded for tissue samples derived from each patient.
Immunofluorescence staining.
Paraffin-embedded sections of the mouse genital tract were stained with a rabbit antigonococcal antibody (UTR01 [18]), which was subsequently detected using goat anti-rabbit IgG-Alexa Fluor 594 or -Alexa Fluor 488 (Life Technologies, Burlington, Canada) as a secondary probe. In some assays, hCEACAM was costained using mouse anti-pan-hCEACAM antibody clone D14HD11 (Abcam, Cambridge, MA, USA) and detected using goat anti-mouse IgG-Alexa Fluor 594. Sections were mounted using Prolong Gold antifade mounting medium with DAPI (Life Technologies, Burlington, Canada) and imaged with a Leica DMIRB/E epifluorescence microscope (Leica, Wetzlar, Germany).
Western blotting.
Epithelial cells were scraped from dissected lower genital tract tissues of female mice using a razor and boiled in 2× SDS buffer containing 10% beta-mercaptoethanol. Whole-cell lysates were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. After blocking in 5% skim milk, membranes were incubated with a pan-hCEACAM polyclonal antiserum (CEA-Dako) raised in rabbit (Dako, Burlington, Canada), while anti-mouse tubulin served as a loading control, followed by HRP-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA). Chemiluminescent detection was performed using an ECL Plus system (Amersham Biosciences).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Department of Comparative Medicine at the University of Toronto and the Histology Facility, McMaster Immunology Research Center, for technical assistance.
This work has been funded by Canadian Institutes of Health Research (CIHR) operating grant MOP-15499 (to S.D.G.-O.) and U.S. National Institutes of Health (NIH) grant R01A1103400-01A1 (to L.M.W.).
Epshita A. Islam designed and performed experiments, analyzed data, and wrote the paper, Varun C. Anipindi and Charu Kaushic contributed data for Fig. 1 and 2, Ian Francis, Yazdan Shaik-Dasthagirisaheb, and Lee M. Wetzler contributed data for Fig. 5 and 6, Stacey Xu contributed data for Fig. S3 in the supplemental material, Nelly Leung contributed data for Fig. S1a in the supplemental material, Anna Sintsova contributed data for Fig. 4a, Mohsen Amin contributed to the establishment of standard operating procedures, and Scott D. Gray-Owen designed experiments, analyzed data, and edited the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00092-18.
REFERENCES
- 1.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J 16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson L, Rytkonen A, Wan H, Bergman P, Plant L, Agerberth B, Hokfelt T, Jonsson AB. 2005. Human-like immune responses in CD46 transgenic mice. J Immunol 175:433–440. doi: 10.4049/jimmunol.175.1.433. [DOI] [PubMed] [Google Scholar]
- 3.Jennings MP, Jen FE, Roddam LF, Apicella MA, Edwards JL. 2011. Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells. Cell Microbiol 13:885–896. doi: 10.1111/j.1462-5822.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porat N, Apicella MA, Blake MS. 1995. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect Immun 63:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O'Connell C, Boden R, Elkins C, Pangburn MK, Dahlback B, Rice PA. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med 193:281–295. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, Rice PA. 2008. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol 180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JE, Hobbs MM, Biswas GD, Sparling PF. 2003. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol Microbiol 48:1325–1337. doi: 10.1046/j.1365-2958.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- 8.Schryvers AB, Stojiljkovic I. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol 32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarantis H, Gray-Owen SD. 2012. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun 80:345–358. doi: 10.1128/IAI.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voges M, Bachmann V, Kammerer R, Gophna U, Hauck CR. 2010. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol 10:117. doi: 10.1186/1471-2180-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer LW. 1982. Rates of in vitro changes of gonococcal colony opacity phenotypes. Infect Immun 37:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson J, Barrera O, Sola J, Boslego J. 1988. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med 168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sintsova A, Wong H, MacDonald KS, Kaul R, Virji M, Gray-Owen SD. 2015. Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect Immun 83:1372–1383. doi: 10.1128/IAI.03123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virji M, Watt S, Barker K, Makepeace K, Doyonnas R. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol 22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 15.Popp A, Dehio C, Grunert F, Meyer TF, Gray-Owen SD. 1999. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol 1:169–181. doi: 10.1046/j.1462-5822.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 16.Gray-Owen SD, Blumberg RS. 2006. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 17.Sarantis H, Gray-Owen SD. 2007. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol 9:2167–2180. doi: 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 18.McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. 2003. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol 49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- 19.McCaw SE, Liao EH, Gray-Owen SD. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect Immun 72:2742–2752. doi: 10.1128/IAI.72.5.2742-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muenzner P, Bachmann V, Zimmermann W, Hentschel J, Hauck CR. 2010. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science 329:1197–1201. doi: 10.1126/science.1190892. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med 185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos MP, Grunert F, Belland RJ. 1997. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun 65:2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Gray-Owen SD, Knorre A, Meyer TF, Dehio C. 1998. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol Microbiol 30:657–671. doi: 10.1046/j.1365-2958.1998.01102.x. [DOI] [PubMed] [Google Scholar]
- 24.Muenzner P, Rohde M, Kneitz S, Hauck CR. 2005. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol 170:825–836. doi: 10.1083/jcb.200412151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, N′Guessan PD, Lucka L, Riesbeck K, Zimmermann W, Zweigner J, Temmesfeld-Wollbrueck B, Suttorp N, Singer BB. 2008. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol 9:1270–1278. doi: 10.1038/ni.1661. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Chow EM, McCaw SE, Hu N, Byrd D, Amet T, Hu S, Ostrowski MA, Gray-Owen SD. 2013. Association of Neisseria gonorrhoeae Opa(CEA) with dendritic cells suppresses their ability to elicit an HIV-1-specific T cell memory response. PLoS One 8:e56705. doi: 10.1371/journal.pone.0056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulton IC, Gray-Owen SD. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol 3:229–236. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Ostrowski MA, Gray-Owen SD. 2008. CEACAM1 dynamics during Neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J Immunol 180:6827–6835. doi: 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Zimmermann W, Parker J, Chen I, Maeda A, Bolland S. 2001. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol 70:335–340. [PubMed] [Google Scholar]
- 30.Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CH, Stanners CP, Glogauer M, Gray-Owen SD. 2014. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog 10:e1004341. doi: 10.1371/journal.ppat.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, Criss AK. 2015. Opa+ Neisseria gonorrhoeae exhibits reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. Cell Microbiol 17:648–665. doi: 10.1111/cmi.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrazzo JM, Handsfield HH, Sparling PF. 2010. Neisseria gonorrhoeae, p 2753–2770. In Mandell GL, Bennett JE, Dolin R (ed), Principles and practice of infectious diseases, 7th ed Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 33.Hook EW III, Holmes KK. 1985. Gonococcal infections. Ann Intern Med 102:229–243. doi: 10.7326/0003-4819-102-2-229. [DOI] [PubMed] [Google Scholar]
- 34.Rendi MH, Muehlenbachs A, Garcia RL, Boyd KL. 2012. Female reproductive system. In Treuting P, Dintzis S, Liggitt D, Frevert CW (ed), Comparative anatomy and histology. Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 35.Chan CH, Stanners CP. 2004. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther 9:775–785. doi: 10.1016/j.ymthe.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Gu A, Zhang Z, Zhang N, Tsark W, Shively JE. 2010. Generation of human CEACAM1 transgenic mice and binding of Neisseria Opa protein to their neutrophils. PLoS One 5:e10067. doi: 10.1371/journal.pone.0010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist S, Zimmermann W. 1994. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res 54:4169–4176. [PubMed] [Google Scholar]
- 38.Jerse AE. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun 67:5699–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. 1993. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J 12:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song W, Condron S, Mocca BT, Veit SJ, Hill D, Abbas A, Jerse AE. 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine 26:5741–5751. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johswich KO, McCaw SE, Islam E, Sintsova A, Gu A, Shively JE, Gray-Owen SD. 2013. In vivo adaptation and persistence of Neisseria meningitidis within the nasopharyngeal mucosa. PLoS Pathog 9:e1003509. doi: 10.1371/journal.ppat.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam EA, Shaik-Dasthagirisaheb Y, Kaushic C, Wetzler LM, Gray-Owen SD. 2016. The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice. Mucosal Immunol 9:1051–1064. doi: 10.1038/mi.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, Kuschner RA, Deal CD. 1995. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis 172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 44.Singer M, Ouburg S. 2016. Effect of cytokine level variations in individuals on the progression and outcome of bacterial urogenital infections—a meta-analysis. Pathog Dis 74:ftv126. doi: 10.1093/femspd/ftv126. [DOI] [PubMed] [Google Scholar]
- 45.Fichorova RN, Desai PJ, Gibson FC III, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ball LM, Criss AK. 2013. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J Bacteriol 195:2982–2990. doi: 10.1128/JB.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngampasutadol J, Ram S, Blom AM, Jarva H, Jerse AE, Lien E, Goguen J, Gulati S, Rice PA. 2005. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A 102:17142–17147. doi: 10.1073/pnas.0506471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caligioni CS. 2009. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 48:A.4I.1–A.4I.8. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiley DM, Buda PJ, Bayliss J, Cover L, Bates J, Sloots TP. 2004. A new confirmatory Neisseria gonorrhoeae real-time PCR assay targeting the porA pseudogene. Eur J Clin Microbiol Infect Dis 23:705–710. doi: 10.1007/s10096-004-1170-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.