Salmonella enterica infection affects a wide range of animals and humans, and a small number of serovars cause typhoid-like infections, one characteristic of which is persistent infection in convalescents. Avian-specific S. enterica serovar Pullorum produces systemic disease in young chickens, which is followed by a carrier state in convalescent birds, leading to infection of the ovary at sexual maturity and vertical transmission.
KEYWORDS: Salmonella enterica serovar Pullorum, Salmonella enterica serovar Enteritidis, macrophage, CD4+ T cells, Th1, Th2, Th17, salmonella, T cell immunity, adaptive immunity, chickens, macrophages, persistent infection
ABSTRACT
Salmonella enterica infection affects a wide range of animals and humans, and a small number of serovars cause typhoid-like infections, one characteristic of which is persistent infection in convalescents. Avian-specific S. enterica serovar Pullorum produces systemic disease in young chickens, which is followed by a carrier state in convalescent birds, leading to infection of the ovary at sexual maturity and vertical transmission. However, the immunological basis of persistent infection remains unclear. S. enterica serovar Enteritidis is taxonomically closely related but does not show this characteristic. Differences in the immune responses between S. Pullorum and S. Enteritidis were compared by using Salmonella-infected chicken monocyte-derived macrophages (chMDMs) and CD4+ T lymphocytes that had been cocultured with infected chMDMs or chicken splenocytes in vitro and also in 2-day-old chickens in vivo. In comparison with S. Enteritidis, S. Pullorum-infected chMDMs showed reduced mRNA expression levels of interleukin-12α (IL-12α) and IL-18 and stimulated the proliferation of Th2 lymphocytes, with reduced expression of gamma interferon (IFN-γ) and IL-17 and increased expression levels of IL-4 and IL-13. There was little evidence of clonal anergy or immune suppression induced by S. Pullorum in vitro. S. Pullorum also increased the levels of expression of IL-4 and decreased the levels of IFN-γ in the spleen and cecal tonsil of infected birds. This suggests that S. Pullorum is able to modulate host immunity from a dominant IFN-γ-producing Th17 response toward a Th2 response, which may promote persistent infection in chickens. S. Pullorum in chickens is presented as a good model of the typhoid group to study persistent infection.
INTRODUCTION
The majority of Salmonella enterica serovars that affect human or animal health generally cause gastrointestinal disease of various severities in a wide range of hosts (1). A small number of serovars, including Salmonella enterica serovar Typhi, S. Gallinarum, S. Pullorum, S. Dublin, S. Choleraesuis, and S. Abortusovis (S. Abortusequi), are adapted to a narrow range of host species and generally produce severe, typhoid-like disease, sometimes with high mortality rates (2). S. enterica serovar Typhimurium and S. Enteritidis are the serovars most frequently associated with food poisoning, with infection restricted to the lower gastrointestinal tract or transient systemic infection (3), and only produce characteristic typhoid experimentally in mice (4). One of the features of infection produced by the typhoid serovars is asymptomatic persistent infections in a proportion of convalescents in experimental infection involving macrophages in lymphoid tissues (5). This results in localization in the gallbladder, liver, and spleen, leading to fecal shedding by carriers for long periods and, in some cases, many years (S. Typhi in humans and S. Dublin in cattle) (6–8), or localization in the reproductive tract, leading to either abortion (S. Dublin and S. Abortusovis in sheep) or vertical transmission through hatching eggs to progeny (S. Pullorum and S. Gallinarum) (9). S. Pullorum is a good and natural model of persistent infection shown by these serovars (10).
Studies on murine typhoid with S. Typhimurium have indicated the critical role of CD4+ Th1 lymphocytes in controlling salmonellosis (11). Clearance of infection by S. Enteritidis from the intestinal tract of infected chickens was also shown to be due to a Th1-dominated response involving increased expression levels of gamma interferon (IFN-γ) mRNA in the gut and deeper tissues (12–16). S. Pullorum colonizes the gut poorly, with bacteria migrating from the intestine to deeper tissues soon after infection (17), accompanied by relatively little inflammation (18), as does the taxonomically closely related S. Gallinarum (19). This is attributed to the reduced production of the proinflammatory chemokines interleukin-1 (IL-1) and IL-6 demonstrated in vitro following S. Gallinarum infection of avian epithelial cells (20). In the case of S. Pullorum, a small number of viable bacteria have been shown to persist in macrophages in convalescent birds. These bacteria are most easily detectable in the spleen, in a proportion of animals, despite the presence of a high-titer antibody response (5, 9, 10). Recrudescence of systemic infection and spread of S. Pullorum to the reproductive tissue occur in females at sexual maturity, associated with the reduced T cell responsiveness that occurs at this time (5, 9, 10). In males, infection persists, but bacterial numbers in the spleen and liver gradually decline with time, resulting in very slow tissue clearance by ca. 18 weeks after infection (9). However, the mechanisms by which S. Pullorum and other typhoid serovars produce persistent infection in the host and the reasons for the absence of complete clearance are not known. In an initial comparative study using S. Pullorum and S. Enteritidis, S. Pullorum-infected birds expressed significantly lower levels of splenic IL-18 and IFN-γ, whereas the expression of IL-4 was increased 14 days after infection (18). This suggested that S. Pullorum might induce an immune response that more closely resembled the Th2 response in mammals and that could allow S. Pullorum to establish intracellular carriage, evading Th1-mediated clearance.
The nature of the immune response to the other serovars that typically produce typhoid-like diseases is poorly understood. In response to S. Typhi in humans, IL-17 production was first found in CD8+ T cells, which also produced IFN-γ (21). Significant increases in IL-17+ CD4+ T cells and in vitro IFN-γ production were also observed during convalescence from S. Typhi (22). Those studies suggested that in the majority of individuals, S. Typhi infection induced a predominant IFN-γ response derived from lymphocyte subsets other than Th1. Persistent infections occur in <3% of typhoid patients (23).
However, alternative potential reasons for the absence of a strong Th1 response exist, including immunosuppression, clonal anergy, or reduced lymphocyte proliferation. The aim of the study reported here was to clarify in greater detail the immunological basis for the persistent carrier state observed in S. Pullorum infection using an in vitro macrophage-T cell coculture system and in vivo infections. The results indicated that S. Pullorum is able to drive host immunity toward a Th2-like response.
RESULTS
Persistence of S. Pullorum infection is not the result of increased survival in macrophages.
The persistence of S. Pullorum in comparison with S. Enteritidis is likely to be the result of increased microbial survival within the internal macrophage environment. We therefore assessed this using chicken monocyte-derived macrophages (chMDMs) (see Fig. S1 in the supplemental material). We quantified the invasiveness and survival of the S. Pullorum and S. Enteritidis strains, macrophage viability, and nitrite ion (NO2−) activity.
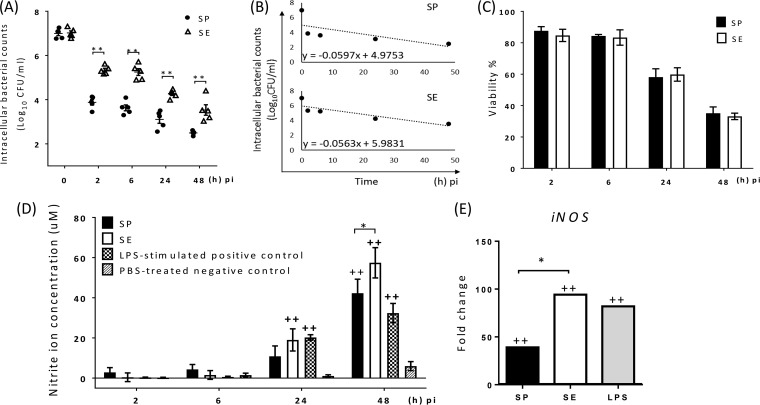
S. Pullorum invaded and/or was taken up by chMDMs in lower numbers than S. Enteritidis at 2 h postinfection (p.i.) (P < 0.01) (Fig. 1A). At later times, there was a significant difference between the viable counts of the two serovars recovered (P < 0.01), with S. Enteritidis showing a significantly higher rate of decline in vitro than that of S. Pullorum over 48 h p.i. (Fig. 1B). Approximately 85% of infected chMDMs remained alive until 6 h p.i., but this figure was significantly reduced by 24 h and 48 h p.i. (P < 0.01). However, the difference between S. Pullorum- and S. Enteritidis-infected cells was not significant (P > 0.99) (Fig. 1C).
FIG 1.
Differences between S. Pullorum (SP) and S. Enteritidis (SE) in their intracellular survival dynamics (A and B), NO production (D), and iNOS expression (E) in infected chMDMs do not correlate with their effect on the viability of infected chMDMs (C). (A and B) Infected chMDMs were lysed to quantify intracellular viable bacteria (A) and the decline rate (B). (D) The supernatant was collected to determine the nitrite ion concentration using a Griess assay. (E) Relative mRNA expression levels of iNOS shown as fold changes in comparison to those from uninfected chMDMs (shown as 1) at 6 h p.i. (C) Percentages of viable chMDMs infected with S. Pullorum and S. Enteritidis were determined by using propidium iodide. Data in panels A, C, and D are presented as means ± standard errors of the means (SEM) (n = 3) and are representative of results from at least two independent experiments. For panel B, the decline rate was determined by using averaged counts of intracellular viable bacteria at each time point. For panel E, iNOS levels were determined from chMDMs prepared from three birds. + indicates a statistically significant difference from the negative control (+, P < 0.05; ++, P < 0.01). * indicates statistical differences between different treatment (*, P < 0.05; **, P < 0.01).
NO is a major antibacterial effector during chronic infection (24), so, as expected, NO2− production was not clearly evident until 24 h after infection. S. Enteritidis produced more NO2− than did S. Pullorum, with this difference being significant (P < 0.05) at 48 h p.i. (Fig. 1D). This was mirrored by the difference in the mRNA level of inducible nitric oxide synthase (iNOS) measured at 6 h p.i., with the level induced by S. Enteritidis being significantly higher (P < 0.5) than that induced by S. Pullorum (Fig. 1E).
S. Pullorum is less effective than S. Enteritidis in inducing strong inflammatory responses by infected chMDMs.
Initiation of macrophage killing of invading bacteria also requires the activity of different chemokines and cytokines. Because the related avian serovar, S. Gallinarum, induces lower levels of proinflammatory cytokines following infection of cultured epithelial cells (20), we compared the effects of S. Pullorum and S. Enteritidis infection on the induction of the mRNA expression of IL-1β, IL-6, CXCLi1 (K60), and CXCLi2 (IL-8) by chMDMs. With the exception of IL-1β and CXCLi1, S. Pullorum induced significantly lower levels of IL-6 than did S. Enteritidis (P < 0.05), with CXCLi2 levels induced by S. Pullorum showing a marginally significant reduction compared with those induced by S. Enteritidis (P = 0.0515) (Fig. 2A). Lipopolysaccharide (LPS) stimulation enhanced IL-6 expression levels in chMDMs, which were significantly higher (P < 0.05) than those in response to S. Pullorum infection (Fig. 2A). S. Pullorum did not completely suppress the expression of proinflammatory cytokines as S. Gallinarum was observed to do in epithelial cells (20). However, they were detected at lower levels than with S. Enteritidis, suggesting that S. Pullorum may invade splenic macrophages without an extensive infiltration of neutrophils during the early stage of infection.
FIG 2.
S. Pullorum infection induces inflammatory responses that are not as strong as those induced by S. Enteritidis in chMDMs. At 6 h p.i., mRNA expression of proinflammatory cytokines (IL-1β and IL-6) and chemokines (CXCLi1 and CXCLi2) (A), IL-12α and IL-18 (driving the Th1 response) (B), and IL-4 and IL-13 (driving the Th2 response) (C) was detected in chMDMs from 3 chickens. The data are shown as fold changes in the mRNA levels of cytokines in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. + indicates differences between levels of cytokines induced by each serovar compared to PBS-treated uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
Macrophages function as antigen-presenting cells (APCs) and can also shift the direction of differentiation of naive T cells. Therefore, we investigated the expression levels of cytokines that drive the differentiation of Th1 (IL-12α and IL-18) and Th2 (IL-4 and IL-13) subsets. S. Enteritidis infection and LPS stimulation (as a positive control) induced strong expression of IL-12α and IL-18. S. Enteritidis stimulated higher levels of IL-12α than did S. Pullorum (P < 0.05), although for IL-18, this difference was of marginal statistical significance (P = 0.0509) (Fig. 2B). In contrast, S. Enteritidis induced lower levels of IL-13 than did S. Pullorum (P < 0.05) (Fig. 2C). This experiment was also repeated by using cultured macrophage-like HD11 cells, with similar results (data not shown).
A wider selection of S. Pullorum and S. Enteritidis strains also displays a similar pattern of cytokine/chemokine expression.
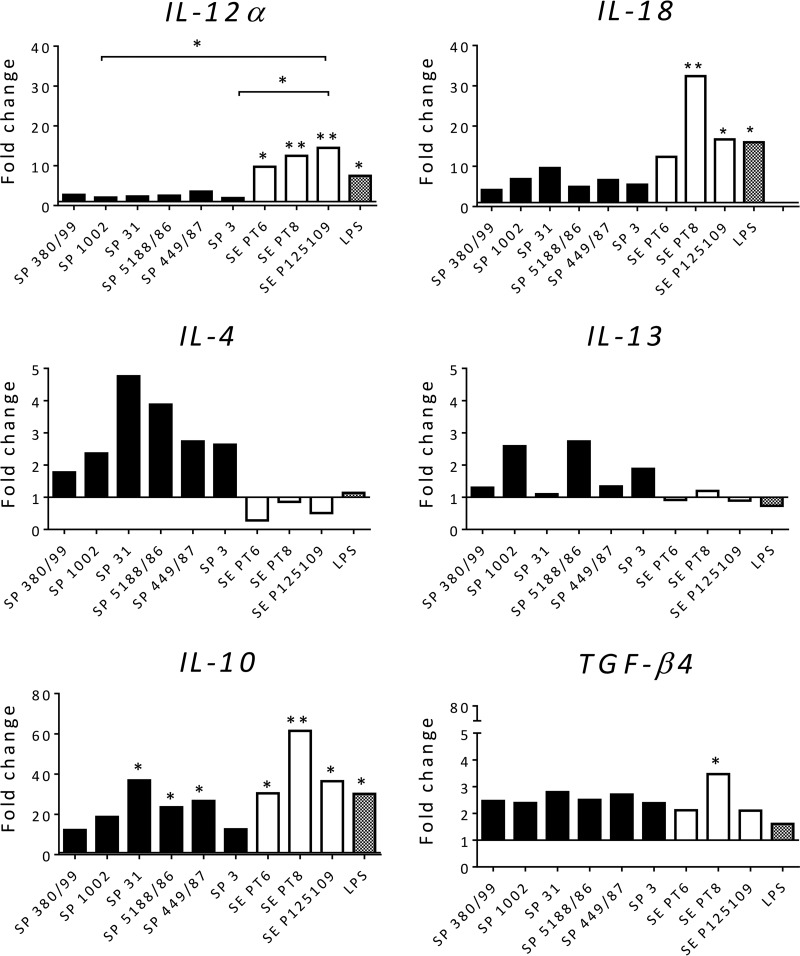
Although the strains used have been shown experimentally to produce infection with characteristics typical of S. enterica serovars Pullorum (5, 10) and Enteritidis (25), we could not be sure that other strains would behave similarly. We therefore repeated the experiments infecting chMDMs with an additional 5 strains of S. Pullorum isolated from cases of pullorum disease and 2 of different phage types (PTs) of S. Enteritidis isolated from cases of human food poisoning that were attributed to poultry consumption. The gene expression profiles of the IL-12α, IL-18, IL-4, IL-13, IL-10, and transforming growth factor β4 (TGF-β4) genes are shown in Fig. 3. Here the patterns of expression for S. Pullorum 449/87 and S. Enteritidis 125109 were very similar to those observed in the above-described experiment (Fig. 2), with the other strains of each serovar behaving in a similar manner, with little variation. The patterns of production of the proinflammatory chemokines IL-1β, IL-6, CXCLi1, and CXCLi2 and also iNOS were also similar to those produced by the strains presented in Fig. 1 and 2 (data not shown).
FIG 3.
Gene expression profiles of immune mediators in chMDMs in response to infection with a wider selection of S. Pullorum and S. Enteritidis strains maintain the patterns of the representative strains used. At 6 h p.i., mRNA expression of IL-12α, IL-18, IL-4, IL-13, IL-10, and TGF-β4 was detected in chMDMs from 3 chickens. The data are shown as fold changes in the mRNA levels of cytokines in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. + indicates differences between levels of cytokines induced by each serovar compared to PBS-treated uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
S. Pullorum suppresses IL-18 and IL-17F expression in ex vivo-infected splenocytes.
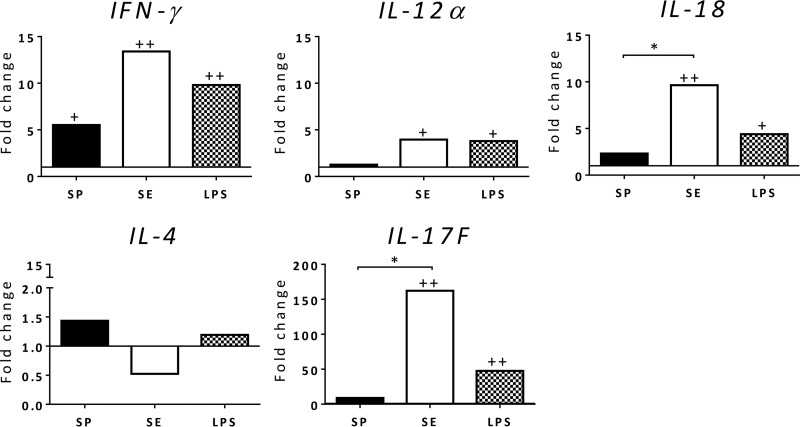
chMDMs may not reflect accurately the infection biology in the spleen, where the bacteria are mainly localized during S. Pullorum infection (5, 10), as the spleen consists of a variety of cell types. These types include dendritic cells (DCs) and lymphocytes, having different immune functions associated with combating infection and the initiation of the immune response. We therefore isolated splenocytes, which were infected with the standard strains S. Pullorum 449/87 and S. Enteritidis 125109. In this case, the expression of IL-18 and IL-4 (Fig. 4) was in accordance with that observed with chMDMs in Fig. 2B, with S. Pullorum suppressing Th1-related cytokines and increasing the expression of Th2-related cytokines. However, both S. Pullorum and S. Enteritidis induced lower expression levels of IL-12α in splenocytes than in chMDMs, which may be regulated by other cell populations other than macrophages in the spleen. We also measured IL-17F, which showed very high levels of expression by S. Enteritidis compared with those with S. Pullorum (P < 0.05) or uninfected controls (P < 0.01).
FIG 4.
S. Pullorum infection induces inflammatory responses that are not as strong as those induced by S. Enteritidis in chicken splenocytes in vitro at 6 h p.i. Expression of IFN-γ, IL-12α, IL-18, IL-4, and IL-17F mRNAs was detected in chMDMs from 3 chickens. The data are shown as fold changes in the mRNA levels of cytokines in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. + indicates differences between levels of cytokines induced by each serovar compared to PBS-treated uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
S. Pullorum suppresses the expression of Th1/Th17 cytokines by CD4+ T cells cocultured with chMDMs.
The pattern of cytokine production by S. Pullorum compared to S. Enteritidis in chMDMs and splenocytes suggested a response that was anti-inflammatory and that may induce the differentiation of Th2 cells. To test this further, we isolated CD4+ T cells, taken from the blood of different individual birds but from the same genetic line, and cocultured these cells with infected chMDMs. Initial experiments on the viability of macrophages and T cells indicated that over 60% of cells were viable after 5 days of in vitro culture (see Fig. S2 in the supplemental material).
After 5 days of coculture, the CD4+ T cells were removed to examine their cytokine profile, which would identify the Th subsets that had proliferated. Compared to those of the control for any allogeneic response, S. Enteritidis-infected chMDMs induced the proliferation of CD4+ T cells that expressed high levels of IFN-γ (P < 0.01) and IL-17F (P < 0.05), whereas S. Pullorum-infected chMDMs induced the proliferation of CD4+ T cells, which did not express IFN-γ (P > 0.05) or suppressed the expression of IL-17F (P < 0.05) (Fig. 5). The differences between S. Pullorum and S. Enteritidis were statistically significant at a P value of <0.01. Neither S. Pullorum- nor S. Enteritidis-infected chMDMs induced the expression of IL-17A in cocultured CD4+ T cells compared to the allogeneic control, although there was a significant difference between S. Pullorum and S. Enteritidis (P < 0.05). In contrast, S. Pullorum induced higher levels of expression of IL-4 than did S. Enteritidis, although this difference was not statistically significant (Fig. 5). This suggested that S. Pullorum was able to switch cytokine production of CD4+ T cells from dominant IFN-γ and IL-17F expression toward IL-4 expression in vitro.
FIG 5.
S. Pullorum infection suppresses IFN-γ-producing Th17 responses in CD4+ T cells cocultured with infected chMDMs after 5 days of coculture. Expression of IFN-γ, IL-4, IL-17A, and IL-17F mRNAs was detected in chMDMs from 3 chickens. The data are shown as fold changes in the mRNA levels of cytokines in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. + indicates differences between levels of cytokines induced by each serovar compared to PBS-treated uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
Immune evasion strategies, other than a switch from a resolving Th17/CD4+ profile to a nonresolving Th2/CD4+ profile, may explain the mechanism of carriage of S. Pullorum in convalescent birds. These include (i) decreased expansion of cognate CD4+ T cell clones, (ii) proliferation of IL-10- and/or TGF-β-producing suppressor T cells, or (iii) a failure of APCs to express costimulatory signals following the engagement of cognate CD4+ T cells, thus inducing clonal anergy.
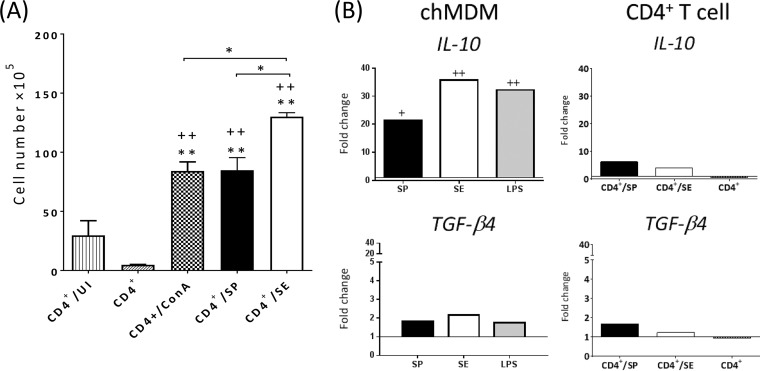
The results obtained in this study show that after 5 days of coculture, S. Enteritidis-infected chMDMs stimulated significantly increased CD4+ T cell proliferation compared with that in S. Pullorum-infected chMDMs (P < 0.05) (Fig. 6A), indicating that S. Pullorum in fact exerted a suppressive effect on proliferation.
FIG 6.
S. Pullorum infection neither suppress lymphocyte proliferation nor induces immunosuppression after 5 days of coculture in vitro. (A) Numbers of viable proliferating CD4+ T cells are presented as means ± SEM (n = 3; chMDMs and CD4+ T cells from 3 chickens each) and are representative of data from two independent experiments. (B) The gene expression levels of IL-10 and TGF-β4 (IL-10 and TGF-β4 mRNAs in chMDMs are detected at 6 h p.i. without coculture) are shown as fold changes in the mRNA levels of cytokines in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. CD4+/UI, CD4+ T cells cocultured with uninfected chMDMs (control for allogeneic response); CD4+, CD4+ T cells cultured alone; CD4+/ConA, CD4+ T cells stimulated with ConA (positive control for CD4+ T cell proliferation); CD4+/SP, CD4+ T cells cocultured with S. Pullorum-infected chMDMs; CD4+/SE, CD4+ T cells cocultured with S. Enteritidis-infected chMDMs. * indicates a statistical difference from the control for the allogeneic response (CD4+/UI) or between different serovar groups (*, P < 0.05; **, P < 0.01); + indicates a statistical difference from the unstimulated control (CD4+) (+, P < 0.05; ++, P < 0.01).
S. Pullorum, S. Enteritidis, and LPS induced significantly increased (P < 0.05) expression levels of IL-10 by chMDMs, but TGF-β4 was not significantly expressed compared to control expression by uninfected chMDMs (Fig. 6B). However, these IL-10-expressing chMDMs did not induce the proliferation of IL-10/TGF-β-producing (tolerogenic) CD4+ T cells, and in this regard, the effect of infected chMDMs on T cells was comparable to the effect of allogeneic controls (Fig. 6B). We measured levels of expression of major histocompatibility complex class II (MHCII) and also the costimulatory molecules CD40, CD80, and CD86 in infected chMDMs. A significant reduction in the number of MHCII-positive chMDMs in response to S. Pullorum infection was measured at 1 day p.i. (P < 0.05) (Fig. 7A), compared to uninfected chMDMs. However, the percentage of CD40-, CD80-, or CD86-positive cells was not lower in S. Pullorum-infected chMDMs than in uninfected cells. Compared with S. Enteritidis infection, the number of CD40-expressing S. Pullorum-infected chMDMs was low only at 24 h p.i. (Fig. 7A).
FIG 7.
S. Pullorum infection does not induce clonal anergy by reducing the number of chMDMs bearing costimulatory molecules. (A) chMDMs (P1) were gated based on side-scatter (SSC)/forward-scatter (FSC) parameters. Shown are representative histograms (top) and average numbers (bottom) of MHCII+, CD40+, CD80+, and CD86+ chMDMs in response to Salmonella infection. Black lines, secondary binding or isotype control MAbs; gray shadow, anti-chicken cell surface marker MAbs. (B and C) Numbers of CD28 + cells (B) and gene expression of CD28 and CTLA-4 (C) in CD4+ T cells from the coculture. For panels A and B, the percentages of MHCII+, CD40+, CD80+, and CD86+ cells from infected chMDMs and CD4+ CD28+ cells out of cocultured CD4+ T cells are shown as means ± SEM (n = 3; chMDMs or CD4+ T cells from 3 chickens). For panel C, the mRNA levels of CD28 and CTLA-4 of CD4+ T cells from 3 chickens are shown as fold changes in comparison to those from uninfected controls (shown as 1) and are representative of results from three independent experiments. + indicates statistically significant differences from the uninfected controls (+, P < 0.05; ++, P < 0.01). * indicates statistical differences between different serovars (*, P < 0.05; **, P < 0.01).
If cytotoxic-T lymphocyte-associated antigen 4 (CTLA-4) is overexpressed on CD4+ T cells, CD80 and CD86 will preferentially bind to this receptor rather than CD28 (which is expressed by activated T lymphocytes). Levels of CD28 protein expression by CD4+ T cells over the 5-day-p.i. period were comparable following coculture with S. Pullorum- and S. Enteritidis-infected chMDMs (Fig. 7B). However, measurement of CD28 and CTLA-4 gene expression showed that there was a shift from CD28 (day 1) to CTLA-4 (day 5) (Fig. 7C). This would normally be expected as T cells move from an activated state back toward steady-state conditions. We hypothesize that increased CTLA-4 protein levels (shifting the CD28/CTLA-4 ratio toward CTLA-4) probably also occurred over the 5-day period p.i., but due to the lack of a commercially available CTLA-4 antibody, we were unable to measure this. Thus, the lower level of CD4+ proliferation induced by S. Pullorum in vitro was not a result of the absence of a costimulatory signal and therefore was not clonal anergy.
S. Pullorum shows a greater capacity than S. Enteritidis for systemic infection in vivo.
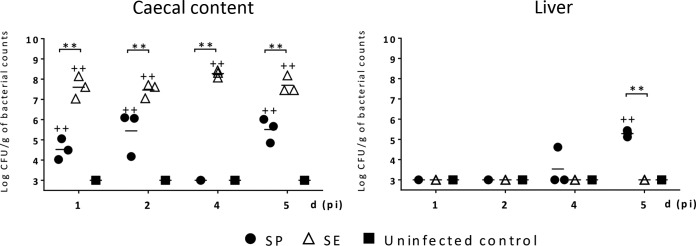
Although the evidence so far suggests that S. Pullorum is able to modulate the immune response of the chicken away from an IFN-γ-producing Th17-type response toward a Th2-type response, this is based on the use of chMDMs as representative antigen-presenting cells interacting with CD4+ T cells. However, DCs and CD8+ T cells are also involved in the early response to S. enterica infection in vivo (26). Thus, it was essential to determine whether the evidence accumulated thus far was mirrored during experimental infections in vivo. To examine this effect in vivo, 2-day-old chickens were infected orally with S. Pullorum or S. Enteritidis, and these chickens were compared with uninfected birds. Infection with approximately 108 CFU of S. Pullorum or S. Enteritidis did not induce any clinical signs of disease over the 5-day period p.i. Viable S. Pullorum and S. Enteritidis bacteria were detected in the cecal contents of infected chickens in each group after 1 day p.i. S. Enteritidis had much higher bacterial counts at all time points examined than did S. Pullorum (P < 0.01), where the counts were also more variable (Fig. 8). Neither serovar was found in the liver of infected chickens at 1 or 2 days p.i. At 5 days p.i., the mean counts of S. Pullorum recovered from the liver of infected chickens increased to 5.29 log10 CFU/g, which were significantly higher than those of S. Enteritidis (P < 0.01).
FIG 8.
S. Pullorum is a poor colonizer of the ceca but an effective invader of the liver. The numbers of viable S. Pullorum and S. Enteritidis bacteria in the cecal content and the liver of 2-day-old chickens were determined at various times (days) after oral infection. Each symbol represents data for an individual chicken (3 chickens/group) in one independent experiment. When no viable colonies were found at a 10−1 dilution after selective enrichment, this suggested a viable count of <3 of log CFU/g, and so log CFU/g=3 was used to represent the bacterial loads in negative animals for statistical analysis. + indicates a statistically significant difference from uninfected controls (+, P < 0.05; ++, P < 0.01). * indicates statistical differences between different serovars (*, P < 0.05; **, P < 0.01).
S. Pullorum infection induces a weaker proinflammatory response in vivo.
The pattern of induction of proinflammatory cytokines in the cecal tonsil was similar to that observed in chMDMs, with higher levels of all cytokines being induced by S. Enteritidis than by S. Pullorum but with the greatest statistically significant differences being found in the cecal tonsils compared to chMDMs. The differences between S. Pullorum and S. Enteritidis (P < 0.05) were more apparent earlier (1 day p.i.) for CXCLi1 and IL-1β but did not appear until 2 days p.i. for CXCLi2, IL-6, and iNOS (Fig. 9). The differences were not as marked in the spleen. Statistically significant differences between S. Pullorum and S. Enteritidis infection for CXCLi2 (P < 0.05), IL-1β (P < 0.01), IL-6 (P < 0.05), and iNOS (P < 0.01) did not appear until 4 days p.i.
FIG 9.
S. Pullorum infection suppresses inflammatory responses in the cecal tonsils and spleens of infected chickens at various times (days) after oral infection. The mRNAs of proinflammatory chemokines (CXCLi1 and CXCLi2) and cytokines (IL-1β, IL-6, and iNOS) were detected in 3 chickens from one independent experiment, and the data are shown as fold changes in comparison to values for uninfected controls (shown as 1). + indicates differences in levels of cytokines induced by each serovar compared to uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
In vivo S. Pullorum infection suppresses the expression of Th1/Th17-related cytokines but upregulates Th2-related cytokines.
The patterns of production of immune-modulating cytokines measured in the cecal tonsil and spleen were largely similar to each other, with much higher expression levels of Th1 cytokines and lower expression levels of Th2 cytokines being induced by S. Enteritidis infection than by S. Pullorum, but with some key differences (Fig. 10). In the cecal tonsils, gene expression levels of IFN-γ (P < 0.01 at 1 day p.i. and P < 0.05 at 2, 4, and 5 days p.i.), IL-12α (P < 0.05 at 1 day p.i. and P < 0.01 at 2 days p.i.), and IL-18 (P < 0.01 at 2 days p.i.) were significantly higher in response to S. Enteritidis infection than in response to S. Pullorum. In the spleen, significant levels of IL-12α and IL-18 (P < 0.05) were produced by S. Enteritidis at 1 day p.i., although no bacteria were isolated from the liver at this time (the lower limit of bacterial enumeration was 3 log10 CFU/g tissue). In the case of IL-4 (P < 0.01 at 2 and 5 days p.i. and P < 0.05 at 4 days p.i. in the cecal tonsils and P < 0.01 at 4 and 5 days p.i. in the spleens) and IL-13 (P < 0.05 at 1 day p.i. and P < 0.01 at 5 days p.i. in the cecal tonsils and P < 0.05 at 4 and 5 days p.i. in the spleens), this was reversed, with higher levels being produced by S. Pullorum infection than by S. Enteritidis. The expression of IL-17F was slightly different from that observed with CD4+ T cells in vitro. In the cecal tonsils, S. Pullorum suppressed the production of IL-17F mRNA at 1 day p.i., with statistically significant differences compared to the uninfected controls (P < 0.05) and S. Enteritidis infection (P < 0.01). S. Enteritidis infection produced higher levels of IL-17F than did S. Pullorum infection, with the difference remaining significant at 4 days p.i. (P < 0.01) and 5 days p.i. (P < 0.05), respectively. Infection with S. Enteritidis upregulated the production of splenic IL-17F mRNA, the level of which was significantly higher than that of S. Pullorum at 2 and 5 days p.i. (P < 0.05). In both organs, as with chMDMs and cocultured CD4+ T cells, the changes in TGF-β4 after infection were generally small. Increased IL-10 expression induced by S. Pullorum over those produced by S. Enteritidis (P < 0.01) and uninfected controls (P < 0.05) was observed at 5 days p.i.
FIG 10.
S. Pullorum infection modulates an IFN-γ-producing Th17 response toward an anti-inflammatory response in the cecal tonsils and spleens of infected chickens at various times (days) after oral infection. The mRNAs of Th1 (IFN-γ, IL-12α, and IL-18), Th2 (IL-4 and IL-13), Th17 (IL-17F), and regulatory (IL-10 and TGF-β4) cytokines were detected in 3 chickens from one independent experiment, and the data are shown as fold changes in comparison to values for uninfected controls (shown as 1). + indicates differences between levels of cytokines induced by each serovar compared to uninfected controls (+, P < 0.05; ++, P < 0.01); * indicates differences between levels of cytokines induced by different serovars (*, P < 0.05; **, P < 0.01).
DISCUSSION
In contrast to S. Enteritidis, S. Pullorum infection did not enhance proinflammatory cytokine expression in avian macrophages or Th1- and/or Th17-related cytokine expression in CD4+ T cells cocultured with infected chMDMs. This was also the case in the cecal tonsil and spleen of infected chickens. Although modulation of adaptive immunity by S. Pullorum toward a nonprotective Th2-like response was most evident in vivo, the results of suppressed Th1/Th17 responses derived from the in vitro coculture experiments are largely consistent with the observations of infection of 2-day-old chickens. These results support our hypothesis that the mechanisms that underlie persistent infection with S. Pullorum involve a manipulation of adaptive immune responses away from a protective IFN-γ-producing Th17-type response. This may enable S. Pullorum to evade immune clearance, resulting in persistent carriage.
S. Pullorum may inhibit the proliferation of Th1 lymphocytes by inhibiting IL-12 and IL-18. IL-12-stimulated Th1 differentiation is critical for controlling the early exponential growth of S. Typhimurium in the spleen and liver of infected mice by potentiating innate cell-killing pathways (11), while the later control of persistent infection also requires IFN-γ production by Th1 cells (4). The NO pathway is also known to be important for killing of S. Typhimurium in murine macrophages. In this case, a biphasic response occurs, such that NO pathways are activated in the later (chronic) phase of infection, whereas reactive oxygen species (ROS) are more important in the earlier stages (27) and are also IFN-γ dependent. A previous study with HD11 cells showed an increase in the oxidative burst after Salmonella infection but with no significant difference between S. Enteritidis and S. Pullorum (28). In the present study, in comparison with S. Enteritidis, the failure of S. Pullorum to increase IL-12α expression in the spleen at 1 day p.i. was followed by significantly lower levels of IFN-γ mRNA observed at 5 days p.i., which may possibly give rise to persistent infection in the spleen of infected chickens.
Metabolism of arginine utilized by macrophages involves the enzyme iNOS (M1 macrophages) or arginase (M2 macrophages) (29, 30). In a murine model of persistent infection, S. Typhimurium infection was preferentially associated with M2 macrophages activated by Th2 cytokines (31). It is not yet clear whether M1/M2 macrophage polarization occurs in avian species. We showed that S. Pullorum is a less robust stimulus for iNOS mRNA expression in chMDMs than S. Enteritidis, which is what might be expected from a more chronic, persistent infection. The expression of nitric oxide synthase by M1 macrophages metabolizes arginine to NO, whereas arginine is metabolized by M2 macrophages to urea and ornithine, and this limits the production of NO (32). It is possible, therefore, that such differences in arginine metabolism occur in S. Pullorum- or S. Enteritidis-infected chMDMs, although we have not specifically measured this. However, we also show that S. Pullorum-infected chMDMs produce low levels of IL-12α/IL-18 but much higher levels of IL-4/IL-13, which suggest that S. Pullorum infection alone may induce an M2 phenotype (33, 34).
IFN-γ production by a Th1-dominant cellular immune response and initiated by IL-12 and IL-18 is essential for host resolution of S. Typhimurium infection in chickens (12–16) and mice (35, 36). Recombinant chicken IFN-γ (chIFN-γ) enhanced NO production in peripheral blood mononuclear cell (PBMC)-derived macrophages and reduced the intracellular replication of S. enterica serovar Typhimurium or Enteritidis (37). However, S. Pullorum infection neither induced IL-12α expression in chMDMs nor promoted IFN-γ expression in CD4+ T cells in coculture, indicating that S. Pullorum does not initiate an effective IFN-γ-dependent inflammatory response to clear infection.
Virulent S. Typhimurium can show persistent infection in resistant mice despite the presence of high levels of circulating anti-S. Typhimurium antibody (4). Neutralization of IFN-γ can reactivate acute infections, probably by interfering with macrophage activation (4), suggesting that functional IFN-γ is probably required to suppress bacterial growth during persistent infection by virulent strains in resistant hosts. This implies an increase in both Th1 and Th2 cytokines in response to Salmonella infection. It is rational to consider that the ratio of these cytokine levels will govern the overall direction of the immune response to be mainly of the Th1 or Th2 type. It would be interesting to study the kinetics of Th1 and Th2 cytokines during persistent infection because S. Pullorum persists in female chickens, with gradually reducing bacterial numbers in the spleens, interrupted by the onset of sexual maturity with spread to the reproductive tract, whereas in males, elimination eventually occurs at between 10 and 18 weeks of age (9). Thus, although S. Pullorum appears to suppress the production of IFN-γ in chickens, the role of IFN-γ in Nramp1+/+mice may be very different, since IFN-γ is required to continue to suppress S. Typhimurium in an innately resistant mouse line (4).
In mice, the ablation of Treg early after infection increased the effectiveness of Th1 responses and controlled the tempo of persistent S. Typhimurium infection (38). It is unclear whether similar alterations in Treg activities can affect the Th1 responses in susceptible mice or in chickens. CTLA-4/CD80/86 ligation inhibits T cell proliferation and induces T cell apoptosis (tolerance) (39). In comparison with S. Enteritidis, S. Pullorum-infected chMDMs did not induce high levels of CTLA-4 mRNA expression in cocultured CD4+ T cells. The suppressive properties of avian Treg cells (CD4+ CD25+) were suggested to be IL-10 dependent (40). In our study, S. Pullorum infection led to the invasion of liver and increased IL-10 expression in the spleen. This suggests a possible regulatory effect of IL-10 on inhibiting cytokine production during systemic dissemination and, possibly, persistent infection. Avian CD4+ CD25+ suppressor T cells have been shown to produce high concentrations of IL-10, TGF-β4, and CTLA-4 and suppress T cell proliferation in vitro (41). IL-10 inhibits the further development of the avian Th1 response and downregulates the effects of IFN-γ to limit the inflammatory response (42). Increased TGF-β4 expression in S. Typhimurium-infected chickens was also shown to correspond to the decreased production of proinflammatory mediators (15). The measurement of IL-10 expression by gene expression rather than the presence of protein opens the possibility that the IL-10 protein may conceivably not have been produced. If this is the case, it may be the reason why IL-10/TGF-β-producing CD4+ T cells did not proliferate. This may be measured in supernatants when a reliable reagent becomes available.
In chickens infected with S. Enteritidis, early expression of IL-17 and prolonged high-level expression of IFN-γ were detected in the ceca (43, 44), which suggested a function of Th17 cells as inflammatory mediators in avian immunity. However, the functional role of Th17 cells and IL-17 in the mucosal inflammatory response to avian salmonellosis is not yet fully defined. In 17A−/− mice infected with S. Enteritidis, the recruitment of neutrophils was significantly compromised, with reduced clearance of S. Enteritidis from the spleen and liver (45), indicating the potential of Th17 cytokines to be involved in intestinal defense against S. enterica infection. Our CXCLi1/CXCLi2 data may suggest a difference between S. Pullorum and S. Enteritidis in heterophil recruitment, and avian IL-17 may potentially also function to recruit heterophils to promote inflammatory responses. IL-17 was elicited rapidly in response to S. Typhimurium infection of bovine ligated ileal loops, probably through a nonspecific activation of intestinal Th17 cells in response to inflammatory cytokines or the recognition of flagellin via the Toll-like receptor 5 (TLR-5) pathway to drive Salmonella-specific Th17 cell development (46). S. Pullorum was able to induce the expression of various proinflammatory cytokines in chMDMs. The reduced expression of IL-17F in CD4+ T cells in vitro and in spleen and cecal tonsils in vivo may thus have resulted from the absence of TLR-5 stimulation by nonflagellated S. Pullorum. This may also be the case for another nonflagellated serovar, S. Gallinarum, which is able to show persistent systemic infection in a SAL1-resistant chicken phenotype (47). Murine Th17 cells were reported to produce IFN-γ in vitro (48) and in vivo (49). Although the roles and functions of IL17 or Th17 cells in regulating immune responses have not been studied in chickens, S. Pullorum-infected chMDMs were unable to induce the gene expression of IFN-γ and IL-17F from cocultured CD4+ T cells, indicating a host immunological bias away from IFN-γ-producing Th17 immunity in response to S. Pullorum infection, which might be associated with the establishment of carriage.
In this study, S. Pullorum was shown to be less effective than S. Enteritidis in stimulating the proliferation of CD4+ T cells using commercial blood obtained from spent laying hens, which had been vaccinated more than 1 year previously. Although there are no authenticated reports of immunity against Salmonella infection lasting more than 6 to 9 months, we collected blood from unvaccinated layer breeders to repeat the proliferation assay. This produced a pattern of T cell proliferation (see Fig. S3 in the supplemental material) similar to that shown in Fig. 6A, indicating that the vaccination of the birds more than 1 year previously had no effect.
S. Typhimurium was shown to reduce T cell proliferation and cytokine production in the absence of DCs (50). Although Salmonella resides largely as an intracellular pathogen, the spread of S. Dublin from ligated intestinal loops in calves involves free bacteria that are not present within macrophages (51), although the extent to which this occurs with other host species and serovars is unknown. In chickens at the onset of lay, when S. Pullorum bacteria multiply within splenic macrophages and spread to the reproductive tract, S. Pullorum may conceivably utilize a similar strategy to directly inhibit the proliferation of T cells.
The bacterial determinants of persistent infection, as opposed to multiplication during acute disease, remain obscure. Type 3 secretion system 2 (TTSS-2) enables the replication and survival of Salmonella within macrophages and is essential for inducing systemic infection caused by S. enterica serovar Pullorum or Typhimurium in chickens (52, 53). However, Salmonella pathogenicity island 2 (SPI-2) contributes to, but is not absolutely required for, persistent S. Typhimurium infection in mice (54). Further work to identify the bacterial determinants of persistent infection in S. Pullorum will likely require investigation of all the genes associated with intracellular survival and growth, including SPI-2 genes, plus a number of genes with metabolic functions. It may be significant that one feature of the serovars that typically produce typhoid-like disease, which is associated with systemic and persistent infection, is auxotrophy (55). The fact that both S. enterica serovars Pullorum and Gallinarum are nonflagellate is unlikely to be significant, as S. enterica serovars Dublin, Typhi, and Abortusovis/Equi are all flagellate. Moreover, the importance of the host genetic background in determining persistent infection has been observed for S. Gallinarum infection. In a SAL1-resistant inbred chicken line, the organism persisted for more than 14 weeks, with infection restricted to persistence and without extensive multiplication in the liver and spleen (10). Similarly, persistent spleen infection involving fully virulent S. Typhimurium in mice has also been shown with the Nramp1+/+ haplotype (4), whereas certain auxotrophic mutants of S. Typhimurium are able to persist in the spleens of Nramp1−/− mice (56). Persistent infection thus appears to be possible in resistant host phenotypes with fully virulent wild strains or in more susceptible host strains with more attenuated bacterial mutants or with wild-type serovars. How this is related to the persistence of S. Typhi or S. Dublin in humans or cattle, respectively, also remains to be determined. That the host genetic background is also involved is suggested by the fact that the response to S. Enteritidis during intestinal colonization, in a line of chickens showing long-term fecal shedding, also shows a Th2 bias (57).
It thus seems clear that the true picture of persistence of both S. Pullorum in chickens and S. Typhimurium in mice is more complex than first appearances suggest. It is unclear how this compares with the other serovars that typically produce typhoid-like disease and show persistence after convalescence. Chronic infection with S. Typhi is associated with shedding via the gallbladder, although the spleen and the liver are known to be infected (58–60). In S. Dublin infection in cattle, persistent shedding can occur from the gut and udder, but the spleen is also affected, and the gallbladder is also sometimes involved (6, 61). Persistent infection within splenic macrophages may thus also be the key infection site for other serovars producing typhoid-like disease and chronic infections, and S. Pullorum infection in chickens may thus represent a good working model with which to study immune manipulation in greater detail and explore approaches to modifying the host response to adversely affect bacterial persistence.
MATERIALS AND METHODS
Bacterial strains.
The in vivo behavior of S. Pullorum 449/87 (5, 62) and S. Enteritidis P125109 (63, 64) in chickens has been well characterized. S. Pullorum and S. Enteritidis were cultured in nutrient broth (Oxoid, UK) at 37°C with shaking at 150 rpm prior to use in experimental infections in vitro and in vivo.
Isolation and culture of chMDMs, CD4+ T cells, and splenocytes.
Chicken peripheral whole blood, obtained from spent Lohmann Lite laying hens, was purchased from Harlan Laboratories UK Ltd. (Leicestershire, UK). The methods of isolation of chicken peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation using Histopaque 1077 and conversion into macrophages were described previously (47). chMDM enrichment was confirmed by flow cytometry analysis using chicken monocyte/macrophage marker antibody (clone KUL01; Santa Cruz Biotechnology, USA). Approximately half of the monocytes initially separated from chicken whole blood were KUL01+ MHCII+. After 2 days of conversion into macrophages and removal of nonadherent cells, this figure increased to more than 95% of adherent cells (see Fig. S1 in the supplemental material). Mouse anti-chicken CD4 monoclonal antibody (MAb) (clone CT-4; Southern Biotech, USA) and anti-mouse IgG1 microbeads (Miltenyi Biotec, UK) were used to positively select chicken CD4+ T cells according to the manufacturers' instructions. Cell viability was assessed by propidium iodide (20 μg/ml; Life Technologies, UK) uptake detected by flow cytometry analysis. CD4+ T cells and chMDMs were isolated from different individual birds. Spleens from newly hatched Lohmann Lite layer chickens were removed aseptically and homogenized gently by using a 70-μm strainer (BD Biosciences, UK) to prepare a suspension of single splenocytes.
In vitro infection of chMDMs and splenocytes with S. enterica.
chMDMs and splenocytes were produced at a final concentration of 5 × 105 cells/ml in RPMI 1640 (Gibco, Life Technologies, UK) supplemented with fetal bovine serum (FBS) (10%, vol/vol) (Gibco, Life Technologies, UK), HEPES (20 mM) (Sigma-Aldrich, UK), gentamicin sulfate (50 μg/ml) (Sigma-Aldrich, UK), streptomycin-penicillin (10 U/ml) (Gibco, Life Technologies, UK), amphotericin B (Fungizone) (1.25 μg/ml) (Gibco, Life Technologies, UK), and l-glutamine (2 mM) (Gibco, Life Technologies, UK). In vitro invasion was performed by using a multiplicity of infection (MOI) of 10 (20, 28). S. Enteritidis LPS (50 μg/ml) (Sigma-Aldrich, UK) was used as a positive control for nitrite ion (NO2−) and cytokine production, and phosphate-buffered saline (PBS) only was used as a negative control. After 1 h of incubation, the medium was replaced with fresh culture medium supplemented with 100 μg/ml of gentamicin sulfate, and the mixture was incubated for another hour to kill extracellular S. enterica bacteria. Cell preparations were then washed three times with sterile PBS and kept in fresh culture medium containing 20 μg/ml of gentamicin sulfate prior to use in subsequent studies. Salmonella-infected cells were lysed at 2, 6, 24, and 48 h postinfection (p.i.) by using Triton X-100 (1%, vol/vol) (Thermo Fisher Scientific, UK) to release and determine the intracellular survival of bacteria (log10 CFU per milliliter). The concentration of NO2− produced by infected and uninfected chMDMs was assessed by a Griess assay kit (Promega, USA) at the same time points. At 6 h p.i., Salmonella-infected cells were collected for cytokine mRNA expression analysis by quantitative real-time PCR (qRT-PCR).
Avian chMDM/CD4+ T cell model in vitro.
chMDMs infected with S. Pullorum or S. Enteritidis were cocultured with CD4+ T cells for 5 days in vitro. The ratio of cocultured cells was maintained at 1:10 (chMDMs to CD4+ T cells) throughout the study. In addition, three control groups were set up, as follows: (i) coculture of uninfected (PBS-treated) chMDMs with CD4+ T cells was used to assess the allogeneic immune response due to culture of chMDMs and CD4+ T cells isolated from different individual birds, (ii) CD4+ T cells were cultured with concanavalin A (ConA) (10 μg/ml) (Sigma-Aldrich, UK) as a positive control for the proliferation of CD4+ T cells, and (iii) CD4+ T cells cultured alone were assessed for viability and nonspecific proliferation over the 5-day culture period in vitro. All cultures were repeated in triplicate on three separate occasions. After 5 days of coculture, CD4+ T cells from each group were collected to measure the proliferation of CD4+ T cells using the CellTiter96AQueous One Solution cell proliferation assay (Promega, USA). Supernatants from infected and uninfected chMDMs cultured alone under the same conditions were also tested for cell proliferation to ensure that chMDMs did not affect the difference between S. Pullorum and S. Enteritidis induction of CD4+ T cells for proliferation. CD4+ T cells were also harvested from each group after 5 days of coculture to measure cytokine mRNA expression by qRT-PCR.
Phenotypic analysis of infected chMDMs and CD4+ T cells following coculture with chMDMs.
Cells to be analyzed for MHCII, CD40, CD80, CD86, or CD28 expression were collected and fixed with PBS–4% (vol/vol) formaldehyde. In each group, 106 cells were incubated with the antibodies indicated and their isotype controls coupled to phycoerythrin (PE), fluorescein isothiocyanate (FITC), or allophycocyanin. MAbs used are all listed in Table 1. Fluorescence analysis was performed by using a FACSCanto II fluorescence-activated cell sorter (FACS) equipped with FACSDiva software (BD Biosciences, UK).
TABLE 1.
MAbs used in this study
| Antibody | Clone | Isotype | Concn (μg/ml) |
|---|---|---|---|
| Monocyte/macrophage marker (KUL01)-PEc | KUL01 | IgG1κ | 1 |
| Mouse anti-chicken CD4d | CT-4 | IgG1κ | 1 |
| Mouse anti-chicken CD4-FITCa | 2-35 | IgG2b | 5 |
| Mouse anti-chicken CD3a | CT-3 | IgG1 | 2.5 |
| Mouse anti-chicken MHCII-FITCd | 2G11 | IgG1 | 1 |
| Mouse anti-chicken CD40a | AV79 | IgG2a | 2.5 |
| Mouse anti-chicken CD80a | IAH:F864:DC7 | IgG2a | 2.5 |
| Mouse anti-chicken CD86a | IAH:F853:AG2 | IgG1 | 2.5 |
| Mouse anti-chicken CD28a | 2-4 | IgG2a | 5 |
| Anti-mouse IgG2α-allphycocyaninb | m2a-15F8 | 2.5 | |
| Anti-mouse IgG1-FITCb | M1-14D12 | 2.5 | |
| Mouse IgG1-PEa | 1 | ||
| Mouse IgG1-FITCa | 1 | ||
| Mouse IgG2α-FITCb | 5 | ||
| Mouse IgG2b-FITCb | 5 |
AbDSerotec, UK.
eBioscience, UK.
Santa Cruz Biotechnology, USA.
Southern Biotech, USA.
In vivo Salmonella chicken infections.
A total of 36 2-day-old Lohmann Lite chickens obtained from the Millennium Hatchery (Birmingham, UK) were divided into three groups with 12 birds each in separate pens and given access to antibiotic-free feed and water ad libitum throughout the experiment. Two groups were inoculated orally with 108 CFU of S. Pullorum or S. Enteritidis in 0.1 ml of nutrient broth. All animal care and experimentation were carried out under Home Office project license PPL 40/3412 and had local ethical approval. At 1, 2, 4, and 5 days p.i., three birds from each group were euthanized. Cecal content and liver were collected aseptically, weighed, and homogenized in PBS using Griffiths tubes. Decimal dilutions of the homogenates were then plated onto Brilliant Green agar plates containing sodium nalidixate (20 μg/ml; Sigma-Aldrich, UK) and novobiocin (1 μg/ml; Sigma-Aldrich, UK) to determine bacterial counts. Spleen and cecal tonsil were collected for cytokine mRNA expression analysis.
mRNA expression analysis by qRT-PCR.
RNA extraction was performed by using the RNeasy Plus minikit (Qiagen, UK). One microgram of total cellular RNA was reverse transcribed to cDNA by using a Transcriptor first-strand cDNA synthesis kit (Roche, UK) according to the manufacturer's guidelines. The LightCycler 480 system (Roche, UK) was used to measure the gene expression levels of selected cytokines and chemokines by qRT-PCR. The sequences of primers and probes are shown in Table 2. Gene expression of CD28 and CTLA-4 was detected by SYBR green-based qRT-PCR. According to methods described previously (20, 65), relative gene expression was normalized against 28S mRNA expression and expressed as a fold difference from levels in uninfected controls.
TABLE 2.
Sequences of probes and primers used in this study
| Target RNA | Probe or primer sequence (5′–3′)a | GenBank accession no. |
|---|---|---|
| 28S | P, (FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA) | X59733 |
| F, GGCGAAGCCAGAGGAAACT | ||
| R, GACGACCGATTTGCACGTC | ||
| iNOS | P, (FAM)-TCCACAGACATACAGATGCCCTTCCTCTTT-(TAMRA) | U46504 |
| F, TTGGAAACCAAAGTGTGTAATATCTTG | ||
| R, CCCTGGCCATGCGTACAT | ||
| IL-1β | P, (FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA) | AJ245728 |
| F, GCTCTACATGTCGTGTGTGATGAG | ||
| R, TGTCGATGTCCCGCATGA | ||
| IL-6 | P, (FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA) | AJ250838 |
| F, GCTCGCCGGCTTCGA | ||
| R, GGTAGGTCTGAAAGGCGAACAG | ||
| CXCLi1 | P, (FAM)-CCACATTCTTGCAGTGAGGTCCGCT-(TAMRA) | AF277660 |
| F, CCAGTGCATAGAGACTCATTCCAAA | ||
| R, TGCCATCTTTCAGAGTAGCTATGACT | ||
| CXCLi2 | P, (FAM)-TCTTTACCAGCGTCCTACCTTGCGACA-(TAMRA) | AJ009800 |
| F, GCCCTCCTCCTGGTTTCAG | ||
| R, TGGCACCGCAGCTCATT | ||
| IFN-γ | P, (FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA) | Y07922 |
| F, GTGAAGAAGGTGAAAGATATCATGGA | ||
| R, GCTTTGCGCTGGATTCTCA | ||
| IL-12α | P, (FAM)-CCAGCGTCCTCTGCTTCTGCACCTT-(TAMRA) | AY262751 |
| F, TGGCCGCTGCAAACG | ||
| R, ACCTCTTCAAGGGTGCACTCA | ||
| IL-18 | P, (FAM)-GGAAGGAG-(TAMRA) | AJ276026 |
| F, AGAGCATGGGAAAATGGTTG | ||
| R, CCAGGAATGTCTTTGGGAAC | ||
| IL-4 | P, (FAM)-AGCAGCACCTCCCTCAAGGCACC-(TAMRA) | AJ621735 |
| F, AACATGCGTCAGCTCCTGAAT | ||
| R, TCTGCTAGGAACTTCTCCATTGAA | ||
| IL-13 | P, (FAM)-CATTGCAAGGGACCTGCACTCCTCTG-(TAMRA) | AJ621735 |
| F, CACCCAGGGCATCCAGAA | ||
| R, TCCGATCCTTGAAAGCCACTT | ||
| TGF-β4 | P, (FAM)-ACCCAAAGGTTATATGGCCAACTTCTGCAT-(TAMRA) | M31160 |
| F, AGGATCTGCAGTGGAAGTGGAT | ||
| R, CCCCGGGTTGTGTGTTGGT | ||
| IL-10 | P, (FAM)-CGACGATGCGGCGCTGTCA-(TAMRA) | AJ621614 |
| F, CATGCTGCTGGGCCTGAA | ||
| R, CGTCTCCTTGATCTGCTTGATG | ||
| IL-17A | P, (FAM)-ATCGATGAGGACCACAACCGCTTC-(TAMRA) | NM_204460.1 |
| F, TATCAGCAAACGCTCACTGG | ||
| R, AGTTCACGCACCTGGAATG | ||
| IL-17F | P, (FAM)-GTTGACATTCGCATTGGCAGCTCT-(TAMRA) | JQ776598.1 |
| F, TGAAGACTGCCTGAACCA | ||
| R, AGAGACCGATTCCTGATGT | ||
| CTLA-4 | F, CAAGGGAAATGGGACGCAAC | AM236874.1 |
| R, GTCTTCTCTGAATCGCTTTGCC | ||
| CD28 | F, GCCAGCCAAACTGACATCTAC | NM_205311.1 |
| R, CTGTAGAAACCAAGAAGTCCCG |
P, probe; F, forward primer; R, reverse primer; FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Statistical analysis.
Data were plotted and analyzed by using GraphPad Prism 6.0. Comparisons between different groups and between different groups at different time points p.i. were performed by using two-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison post hoc test. Statistical significance was determined at the 5% and 1% confidence limits as P values of <0.05 and <0.01.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the China Scholarship Council (CSC) and the University of Nottingham.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00307-18.
REFERENCES
- 1.Coburn B, Grassl GA, Finlay BB. 2007. Salmonella, the host and disease: a brief review. Immunol Cell Biol 85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 2.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. 1970. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 3.Rabsch W, Tschape H, Baumler AJ. 2001. Non-typhoidal salmonellosis: emerging problems. Microbes Infect 3:237–247. doi: 10.1016/S1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- 4.Monack DM, Bouley DM, Falkow S. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J Exp Med 199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wigley P, Berchieri A Jr, Page KL, Smith AL, Barrow PA. 2001. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect Immun 69:7873–7879. doi: 10.1128/IAI.69.12.7873-7879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sojka WJ, Thomson PD, Hudson EB. 1974. Excretion of Salmonella dublin by adult bovine carriers. Br Vet J 130:482–488. doi: 10.1016/S0007-1935(17)35791-3. [DOI] [PubMed] [Google Scholar]
- 7.Wray C, Sojka WJ. 1977. Reviews of the progress of dairy science: bovine salmonellosis. J Dairy Res 44:383–425. doi: 10.1017/S0022029900020355. [DOI] [PubMed] [Google Scholar]
- 8.House D, Bishop A, Parry C, Dougan G, Wain J. 2001. Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis 14:573–578. doi: 10.1097/00001432-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Wigley P, Hulme SD, Powers C, Beal RK, Berchieri A Jr, Smith A, Barrow P. 2005. Infection of the reproductive tract and eggs with Salmonella enterica serovar Pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infect Immun 73:2986–2990. doi: 10.1128/IAI.73.5.2986-2990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berchieri A Jr, Murphy CK, Marston K, Barrow PA. 2001. Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathol 30:221–231. doi: 10.1080/03079450120054631. [DOI] [PubMed] [Google Scholar]
- 11.Mastroeni P, Harrison JA, Robinson JH, Clare S, Khan S, Maskell DJ, Dougan G, Hormaeche CE. 1998. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun 66:4767–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol 100:151–164. doi: 10.1016/j.vetimm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Beal RK, Powers C, Wigley P, Barrow PA, Smith AL. 2004. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol 33:25–33. doi: 10.1080/03079450310001636282. [DOI] [PubMed] [Google Scholar]
- 14.Wigley P, Hulme S, Powers C, Beal R, Smith A, Barrow P. 2005. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet Res 1:2. doi: 10.1186/1746-6148-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Withanage GS, Wigley P, Kaiser P, Mastroeni P, Brooks H, Powers C, Beal R, Barrow P, Maskell D, McConnell I. 2005. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect Immun 73:5173–5182. doi: 10.1128/IAI.73.8.5173-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berndt A, Wilhelm A, Jugert C, Pieper J, Sachse K, Methner U. 2007. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun 75:5993–6007. doi: 10.1128/IAI.00695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson SC, Bounous DI, Lee MD. 1999. Early events in the pathogenesis of avian salmonellosis. Infect Immun 67:3580–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. 2009. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol 128:53–59. doi: 10.1016/j.vetimm.2008.10.295. [DOI] [PubMed] [Google Scholar]
- 19.Langridge GC, Fookes M, Connor TR, Feltwell T, Feasey N, Parsons BN, Seth-Smith HM, Barquist L, Stedman A, Humphrey T, Wigley P, Peters SE, Maskell DJ, Corander J, Chabalgoity JA, Barrow P, Parkhill J, Dougan G, Thomson NR. 2015. Patterns of genome evolution that have accompanied host adaptation in Salmonella. Proc Natl Acad Sci U S A 112:863–868. doi: 10.1073/pnas.1416707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146(Part 12):3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 21.McArthur MA, Sztein MB. 2012. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One 7:e38408. doi: 10.1371/journal.pone.0038408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhuiyan S, Sayeed A, Khanam F, Leung DT, Rahman Bhuiyan T, Sheikh A, Salma U, LaRocque RC, Harris JB, Pacek M, Calderwood SB, LaBaer J, Ryan ET, Qadri F, Charles RC. 2014. Cellular and cytokine responses to Salmonella enterica serotype Typhi proteins in patients with typhoid fever in Bangladesh. Am J Trop Med Hyg 90:1024–1030. doi: 10.4269/ajtmh.13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson GS, Miles AA, Topley WCC. 1964. Topley and Wilson's principles of bacteriology and immunity, 5th ed Edward Arnold, London, United Kingdom. [Google Scholar]
- 24.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med 192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrow PA, Lovell MA. 1991. Experimental infection of egg-laying hens with Salmonella enteritidis phage type 4. Avian Pathol 20:335–348. doi: 10.1080/03079459108418769. [DOI] [PubMed] [Google Scholar]
- 26.Berndt A, Pieper J, Methner U. 2006. Circulating γδ T cells in response to Salmonella enterica serovar Enteritidis exposure in chickens. Infect Immun 74:3967–3978. doi: 10.1128/IAI.01128-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setta A, Barrow PA, Kaiser P, Jones MA. 2012. Immune dynamics following infection of avian macrophages and epithelial cells with typhoidal and non-typhoidal Salmonella enterica serovars; bacterial invasion and persistence, nitric oxide and oxygen production, differential host gene expression, NF-kappaB signalling and cell cytotoxicity. Vet Immunol Immunopathol 146:212–224. doi: 10.1016/j.vetimm.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. 1999. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol 163:3771–3777. [PubMed] [Google Scholar]
- 30.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 31.Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. 2013. Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe 14:171–182. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rath M, Muller I, Kropf P, Closs EI, Munder M. 2014. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front Biosci 13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 35.Mastroeni P. 2002. Immunity to systemic Salmonella infections. Curr Mol Med 2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 36.Mastroeni P, Menager N. 2003. Development of acquired immunity to Salmonella. J Med Microbiol 52:453–459. doi: 10.1099/jmm.0.05173-0. [DOI] [PubMed] [Google Scholar]
- 37.Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA, Tani H, Sasai K, Baba E, Lillehoj EP. 2005. Differential responses of macrophages to Salmonella enterica serovars Enteritidis and Typhimurium. Vet Immunol Immunopathol 107:327–335. doi: 10.1016/j.vetimm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Johanns TM, Ertelt JM, Rowe JH, Way SS. 2010. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog 6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. 1997. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6:411–417. doi: 10.1016/S1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 40.Selvaraj RK. 2013. Avian CD4+ CD25+ regulatory T cells: properties and therapeutic applications. Dev Comp Immunol 41:397–402. doi: 10.1016/j.dci.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Shanmugasundaram R, Selvaraj RK. 2011. Regulatory T cell properties of chicken CD4+ CD25+ cells. J Immunol 186:1997–2002. doi: 10.4049/jimmunol.1002040. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P. 2004. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- 43.Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I. 2011. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect Immun 79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matulova M, Varmuzova K, Sisak F, Havlickova H, Babak V, Stejskal K, Zdrahal Z, Rychlik I. 2013. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet Res 44:37. doi: 10.1186/1297-9716-44-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz SM, Köhler G, Holscher C, Iwakura Y, Alber G. 2008. IL-17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol 20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 46.Raffatellu M, Santos RL, Chessa D, Wilson RP, Winter SE, Rossetti CA, Lawhon SD, Chu H, Lau T, Bevins CL, Adams LG, Baumler AJ. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect Immun 75:4342–4350. doi: 10.1128/IAI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wigley P, Hulme SD, Bumstead N, Barrow PA. 2002. In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes Infect 4:1111–1120. doi: 10.1016/S1286-4579(02)01635-0. [DOI] [PubMed] [Google Scholar]
- 48.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Velden AW, Copass MK, Starnbach MN. 2005. Salmonella inhibit T cell proliferation by a direct, contact-dependent immunosuppressive effect. Proc Natl Acad Sci U S A 102:17769–17774. doi: 10.1073/pnas.0504382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pullinger GD, Paulin SM, Charleston B, Watson PR, Bowen AJ, Dziva F, Morgan E, Villarreal-Ramos B, Wallis TS, Stevens MP. 2007. Systemic translocation of Salmonella enterica serovar Dublin in cattle occurs predominantly via efferent lymphatics in a cell-free niche and requires type III secretion system 1 (T3SS-1) but not T3SS-2. Infect Immun 75:5191–5199. doi: 10.1128/IAI.00784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones MA, Wigley P, Page KL, Hulme SD, Barrow PA. 2001. Salmonella enterica serovar Gallinarum requires the Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect Immun 69:5471–5476. doi: 10.1128/IAI.69.9.5471-5476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones MA, Hulme SD, Barrow PA, Wigley P. 2007. The Salmonella pathogenicity island 1 and Salmonella pathogenicity island 2 type III secretion systems play a major role in pathogenesis of systemic disease and gastrointestinal tract colonization of Salmonella enterica serovar Typhimurium in the chicken. Avian Pathol 36:199–203. doi: 10.1080/03079450701264118. [DOI] [PubMed] [Google Scholar]
- 54.Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun 76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, Bernard S, Casadesus J, Platt DJ, Olsen JE. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol Infect 125:229–255. doi: 10.1017/S0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Callaghan D, Maskell D, Liew FY, Easmon CS, Dougan G. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun 56:419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chausse AM, Grepinet O, Bottreau E, Robert V, Hennequet-Antier C, Lalmanach AC, Lecardonnel J, Beaumont C, Velge P. 2014. Susceptibility to Salmonella carrier-state: a possible Th2 response in susceptible chicks. Vet Immunol Immunopathol 159:16–28. doi: 10.1016/j.vetimm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Vogelsang TM, Boe J. 1948. Temporary and chronic carriers of Salmonella typhi and Salmonella paratyphi B. J Hyg (Lond) 46:252–261. doi: 10.1017/S0022172400036378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young D, Hussell T, Dougan G. 2002. Chronic bacterial infections: living with unwanted guests. Nat Immunol 3:1026–1032. doi: 10.1038/ni1102-1026. [DOI] [PubMed] [Google Scholar]
- 60.Nath G, Singh YK, Maurya P, Gulati AK, Srivastava RC, Tripathi SK. 2010. Does Salmonella Typhi primarily reside in the liver of chronic typhoid carriers? J Infect Dev Ctries 4:259–261. doi: 10.3855/jidc.820. [DOI] [PubMed] [Google Scholar]
- 61.Hinton M, Williams BM. 1977. Salmonella dublin infection in adult cattle: a slaughter house and knackery survey in South West Wales. J Hyg (Lond) 78:121–127. doi: 10.1017/S002217240005600X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wigley P, Jones MA, Barrow PA. 2002. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathol 31:501–506. doi: 10.1080/0307945021000005879. [DOI] [PubMed] [Google Scholar]
- 63.Barrow PA. 1991. Experimental infection of chickens with Salmonella enteritidis. Avian Pathol 20:145–153. doi: 10.1080/03079459108418749. [DOI] [PubMed] [Google Scholar]
- 64.Barrow PA, Lovell MA, Berchieri A. 1991. The use of two live attenuated vaccines to immunize egg-laying hens against Salmonella enteritidis phage type 4. Avian Pathol 20:681–692. doi: 10.1080/03079459108418807. [DOI] [PubMed] [Google Scholar]
- 65.Hughes S, Poh TY, Bumstead N, Kaiser P. 2007. Re-evaluation of the chicken MIP family of chemokines and their receptors suggests that CCL5 is the prototypic MIP family chemokine, and that different species have developed different repertoires of both the CC chemokines and their receptors. Dev Comp Immunol 31:72–86. doi: 10.1016/j.dci.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.