Trichomoniasis, a prevalent sexually transmitted infection, is commonly symptomatic in women. The causative agent is Trichomonas vaginalis, an extracellular protozoan parasite.
KEYWORDS: aggregation-promoting factor, Lactobacillus, Trichomonas vaginalis, host cell adhesion, host-parasite, protozoa
ABSTRACT
Trichomoniasis, a prevalent sexually transmitted infection, is commonly symptomatic in women. The causative agent is Trichomonas vaginalis, an extracellular protozoan parasite. The host-protective mechanisms and molecules of vaginal lactobacilli that counteract this pathogen are largely unknown. This study examines the inhibition promoted by Lactobacillus gasseri against the adhesion of T. vaginalis to host cells, a critical virulence aspect of this pathogen. We observed that the vaginal strain L. gasseri ATCC 9857 is highly inhibitory by various contact-dependent mechanisms and that surface proteins are largely responsible for this inhibitory phenotype. We found that the aggregation-promoting factor APF-2 from these bacteria significantly contributes to inhibition of the adhesion of T. vaginalis to human vaginal ectocervical cells. Understanding the molecules and mechanisms used by lactobacilli to protect the host against T. vaginalis might help in the development of novel and specific therapeutic strategies that take advantage of the natural microbiota.
INTRODUCTION
Trichomoniasis is a prevalent sexually transmitted infection (STI) worldwide. It is responsible for about 143 million cases each year, affecting mostly women of childbearing age (1). Besides nonspecific vaginitis, this infection can lead to the premature rupture of the placental membrane, preterm birth, and the birth of low-weight infants (2–4). In addition, trichomoniasis increases the transmissibility of the human immunodeficiency virus (HIV), and it is linked to the development of cervical and prostate cancers (5–7). Metronidazole or tinidazole is used to treat trichomoniasis. However, drug resistance is proving to be a cause of concern among medical practitioners (8, 9).
Trichomonas vaginalis, the causative agent of this STI, is an extracellular parasitic protozoan that adheres to the surface of host vaginal cells. Host cell adhesion is critical to initiate infection, and during this process, this flagellated parasite undergoes drastic morphological changes, switching from a free-swimming ovoid shape to a host-adherent amoeboid shape (10–12). Several surface proteins, as predicted from the T. vaginalis genome and proteome, may provide functions that allow it to adhere to host cells and substrates; these include the large family of BspA-like proteins (13–15). Other proteins claimed to be involved in cytoadherence include cysteine proteinases and a number of moonlighting proteins (16–20). The role of the latter in host cell adhesion, however, is highly disputed (21, 22). More recently, a rhomboid protease was found to mediate parasite adhesion (23). T. vaginalis lipoglycan (TvLG), a polysaccharide that coats the outer surface of the parasite (24), has also been shown to promote attachment of the protozoa to human vaginal ectocervical cells (hVECs) via host galectin-1 (25, 26).
On the other hand, the vagina of asymptomatic women of childbearing age is naturally colonized by commensal bacteria at a concentration of 107 to 108 CFU/g of vaginal fluid (27). These microbiota have been classified into 5 community state types (CSTs). Of these communities, four (CST-I, -II, -III, and -V) are dominated by one species of Lactobacillus (Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, or Lactobacillus jensenii, respectively) and present in ∼75% of women. The fifth community (CST-IV), present in the remaining ∼25% of women, does not show a specific dominant species. It is instead represented by a more diversified community which is composed mostly of anaerobic bacteria, such as Prevotella, Atopobium, and Gardnerella (28). Among the four Lactobacillus-dominant CSTs, L. gasseri is claimed to be the most stable community over time, rarely transiting to other community types (27, 29). Hence, deciphering the host-protective characteristics of L. gasseri is important for understanding how lactobacilli help maintain the healthy state of the vagina.
T. vaginalis infections were found to be associated with CST-IV, the more diversified community of vaginal bacteria (30, 31), suggesting that lactobacilli and T. vaginalis could counteract each other as natural microbial competitors in the vagina (32). Recently, T. vaginalis was shown to adversely affect populations of vaginal lactobacilli (33), while lactobacilli were shown to inhibit T. vaginalis adhesion to hVECs in a profound and a contact-dependent manner (34). These initial observations provided the first piece of evidence that this microbial interaction might be influential to the outcome of T. vaginalis infection.
Lactobacilli have been repeatedly implicated in mitigating infections. These bacteria can inhibit the growth of various sexually transmitted pathogens such as Gardnerella vaginalis, Neisseria gonorrhoeae, and Candida albicans (35–37). Besides secreting general molecules that have a broad spectrum of inhibitory activity (e.g., lactic acid, bacteriocins, and biosurfactants) (38, 39), these bacteria also utilize surface molecules that target pathogens in a more specific manner. These include lipoteichoic acids (40), mucin-binding proteins (41), aggregation-promoting factors (42), and S-layer proteins (43), which were shown to prevent attachment of specific pathogens to host cells and substrates. In addition, lectins from different lactobacilli have been found to prevent biofilm formation by uropathogenic Escherichia coli and adhesion to endocervical cells (44, 45). The highly mannose-specific lectin Msl from Lactobacillus plantarum CMPG5300, in particular, is capable of binding to HIV-1 glycoprotein gp120 and C. albicans (44).
S-layer proteins and aggregation-promoting factors (APFs) of lactobacilli are implicated in bacterial shape and integrity as well as adhesive phenotypes (42, 43, 46–48). S-layer proteins from lactobacilli share similarities at the genetic, physical, and biochemical levels to APFs of L. gasseri (43). For example, because of their noncovalent association to the cell wall, they are both extractable by high molar concentrations of LiCl (43, 48, 49). The S-layer proteins of Lactobacillus acidophilus mediate attachment to mucin, fibronectin (50), and human colon HT-29 cells (51), competing with bacterial pathogens for binding sites (51–54). The APFs of L. gasseri 4B2 are critically necessary for the maintenance of its shape and integrity (48). APF-1 of L. gasseri LG2055, in particular, mediates competitive exclusion of the pathogen Campylobacter jejuni from poultry, likely due to its properties of adhesion to host cells and substrates (42).
Here, we investigated further the remarkable inhibitory phenotype of L. gasseri against the host cytoadherence of T. vaginalis, as demonstrated by our group previously (34). In this new study, our goals were (i) to describe the underlying mechanisms of this inhibitory phenotype and (ii) to reveal molecules that confer to ATCC 9857, a L. gasseri strain of human vaginal origin, the ability to inhibit the adhesion of T. vaginalis to human vaginal ectocervical cells. Our findings point to novel mechanisms and molecules employed by L. gasseri to protect the vagina against T. vaginalis infection.
RESULTS
The L. gasseri strain of vaginal origin ATCC 9857 is highly inhibitory toward T. vaginalis adhesion to hVECs.
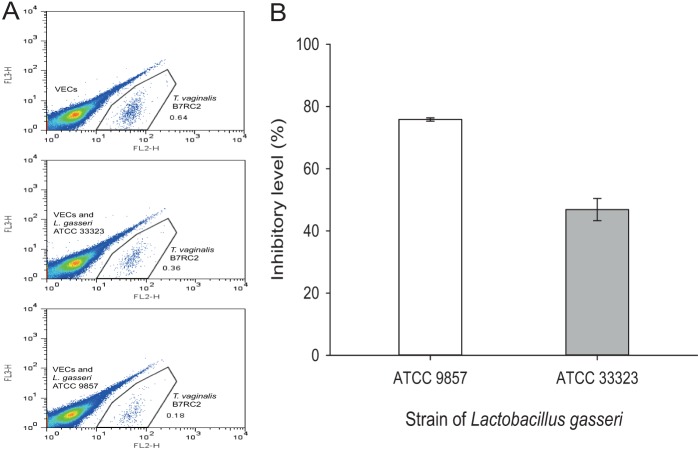
Our previous study had shown that host cytoadherence of the very adhesive T. vaginalis strain B7RC2 can be significantly inhibited by lactobacilli in a species/strain-dependent manner (34). Because L. gasseri is a dominant species of the human vaginal microbiota (28), we further examined here the inhibitory properties of L. gasseri strains ATCC 9857 and ATCC 33323, originally isolated from human vagina and intestine, respectively (55–57), against host cytoadherence of T. vaginalis B7RC2 (Fig. 1). This evaluation was done by flow cytometry (34, 58), where the absolute number of fluorescence-labeled parasites attached to hVECs was counted (Fig. 1A). We compared the levels of parasite cytoadherence in the presence of lactobacilli (preincubated with hVECs prior to addition of parasites) and in the absence of bacteria. The strain of vaginal origin, ATCC 9857, had the highest level of adhesion inhibition (Fig. 1B). Almost 80% of the B7RC2 parasites were inhibited from binding to hVECs by ATCC 9857, which indicates that ATCC 9857 is about two times more inhibitory than the strain of intestinal origin, ATCC 33323 (P < 0.001).
FIG 1.
L. gasseri ATCC 9857 is highly inhibitory toward T. vaginalis B7RC2 adhesion to hVECs. In this assay, lactobacilli were preincubated with hVECs, followed by addition of CMTMR-stained T. vaginalis. As a control, hVECs and stained T. vaginalis cells were incubated without bacteria. At the end of the incubation, unbound parasites were removed and bound parasites only were collected after trypsinization and counted by flow cytometry (see Materials and Methods). The number of hVEC-bound T. vaginalis cells in the presence of L. gasseri strain ATCC 9857 or ATCC 33323 (originally isolated from the human vagina and intestine, respectively) was compared to that for the control in order to determine the percent inhibition of T. vaginalis adhesion to hVECs. (A) Flow cytometry data depicting the separation of CMTMR-stained, hVEC-bound T. vaginalis (FL-2 channel) from a mixed population of unstained lactobacilli and/or hVECs. (B) Percent inhibition of T. vaginalis adhesion to hVECs by the two Lactobacillus strains. The differences between both samples and the control were statistically significant (P < 0.01). Lactobacillus strain ATCC 9857 was more inhibitory to T. vaginalis cytoadhesion than strain ATCC 33323 (P < 0.001).
L. gasseri inhibits T. vaginalis adhesion to hVECs by various modes.
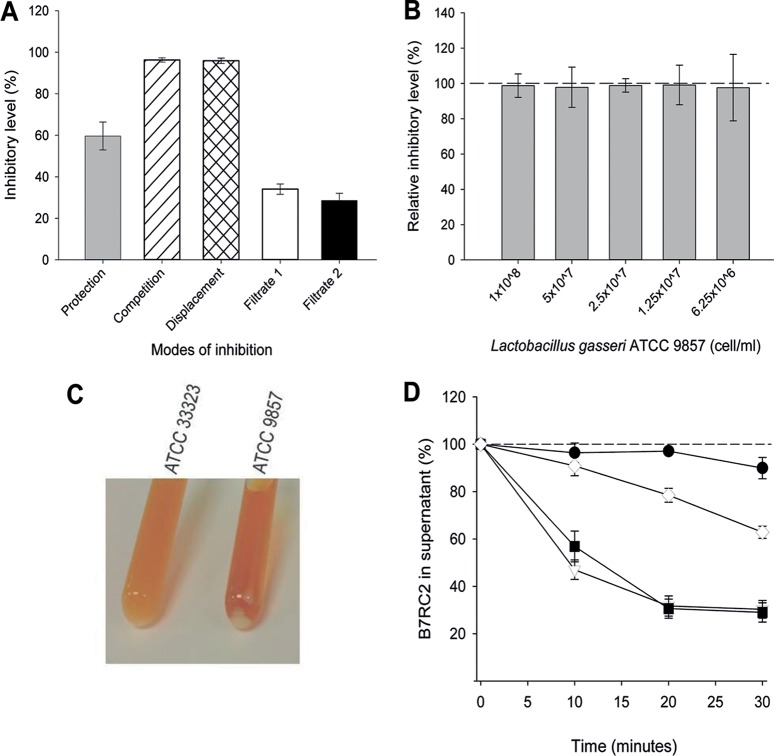
Inhibition of T. vaginalis adhesion to host cells by lactobacilli was shown to be dependent on cell contact (34). Until this point, these experiments were performed by preincubating hVECs with lactobacilli, thus protecting host cells from T. vaginalis attachment. However, lactobacilli can inhibit pathogens in a contact-dependent manner by protection, competition, and displacement (35, 59). Therefore, the mode of inhibition was assessed by changing the order in which lactobacilli and the pathogen (T. vaginalis) were added to host cells in the adhesion assays. To investigate if there was a preferential mode of inhibition of T. vaginalis adhesion, L. gasseri ATCC 9857 was added to hVECs either before T. vaginalis B7RC2 was added, with T. vaginalis B7RC2, or after T. vaginalis B7RC2 was added (Fig. 2A). In addition, we examined if the supernatant from lactobacilli only (filtrate 1) or lactobacilli preincubated with hVECs without parasites (filtrate 2) would be capable of inhibition. Surprisingly, L. gasseri ATCC 9857 was capable of inhibiting T. vaginalis adhesion significantly, regardless of the mode of incubation. In fact, L. gasseri could compete with and even displace T. vaginalis more efficiently than it could protect or prevent the adhesion of parasites to hVECs. The culture supernatants (filtrates 1 and 2) also displayed a modest but significant level of inhibition without a significant difference between the filtrates.
FIG 2.
L. gasseri inhibits T. vaginalis B7RC2 adhesion to hVECs in different ways. (A) Inhibition is achieved by L. gasseri ATCC 9857 cells or culture supernatant by different modes. When using bacterial cells, lactobacilli were added either before T. vaginalis was added (Protection), with T. vaginalis (Competition), or after T. vaginalis was added (Displacement). When using culture supernatant, cell-free medium recovered from lactobacilli only (Filtrate 1) or lactobacilli incubated with hVECs (Filtrate 2) was added to the adhesion assay mixtures prior to parasite addition. The results for all samples were compared to those for the controls, which consisted of hVECs incubated with T. vaginalis only (no bacteria or culture supernatant). Data are expressed as the percent inhibition of parasite adhesion to cells. The differences between all samples and their respective controls were statistically significant (P < 0.01). The levels of inhibition achieved in the competition and displacement modes were significantly higher (P < 0.01) than the level of inhibition achieved in the protection mode. (B) hVEC-bound lactobacilli are sufficient for the maximum level of inhibition via the protection mode. The relative level of inhibition of T. vaginalis cytoadherence by L. gasseri ATCC 9857 was compared between hVEC-bound lactobacilli and the total lactobacilli (i.e., lactobacilli obtained by removing or not removing the supernatant prior to addition of T. vaginalis, respectively). For comparison, inhibition by total lactobacilli was considered 100%, as indicated by the dashed line. Twofold serial dilutions of bacteria were tested, starting from 1 × 108 lactobacilli per ml. Removing the unbound lactobacilli did not change the level of inhibition at any bacterial dilution. (C) L. gasseri strain ATCC 9857 is more self-aggregative than strain ATCC 33323. Overnight cultures of ATCC 9857 displayed a more evident cell pellet than ATCC 33323, whereby the culture of the latter was distinctively more turbid than that of the former. (D) L. gasseri and T. vaginalis coaggregate. Coaggregation was measured as a drop in the number of swimming parasites in the medium, when recovered from the top of the culture tube, over the course of the experiment, and the percent coaggregation was calculated in reference to that at time zero. Controls were done with T. vaginalis alone (i.e., no bacteria) at different temperatures. At 37°C, the number of motile parasites remained constant (black circles). In contrast, when motility was slowed by ice-temperature incubation, a linear drop in the number of swimming parasites was seen across the time course (white diamonds). In contrast to the results obtained with parasites alone, coincubation of parasites and lactobacilli ATCC 9857 and ATCC 33323 (black squares and white triangles, respectively) in warm medium caused a significant drop in the numbers of swimming parasites, indicating coaggregation (P < 0.01). The differences between the L. gasseri strains were not significant.
In the standard protection mode, where hVECs were precolonized with lactobacilli prior to infection, T. vaginalis was added subsequently without the removal of any unbound bacteria. We tested if removing the supernatant and, hence, the unbound L. gasseri prior to adding T. vaginalis would result in a reduction of adhesion inhibition (Fig. 2B). To test if any reduction of inhibition is proportional to the initial bacterial inoculum, this experiment was done with 2-fold serial dilutions of L. gasseri ATCC 9857 (from 1 × 108 to 6.1 × 106 bacteria/ml). We have shown previously that this is the concentration range (number of bacteria per milliliter) where inhibition of T. vaginalis cytoadhesion behaves in a dose-dependent manner (34). We observed that removing the unbound lactobacilli did not significantly reduce the level of inhibition of T. vaginalis adhesion to hVECs at any bacterial concentration compared to that achieved in the standard assay (without removing the supernatant) (Fig. 2B). Therefore, the hVEC-bound lactobacilli are sufficient to achieve the maximum level of inhibition of T. vaginalis adhesion to host cells.
In addition to the physical contact between microbes and host cells, microbial cells (bacteria and protozoa) are likely to contact each other in the vaginal secretions as well as in the adhesion assay. Noticeably, on the basis of the extent of cell pelleting formed spontaneously from the overnight cultures, ATCC 9857 displayed a stronger aggregation phenotype than ATCC 33323 (Fig. 2C). Besides self-aggregation, which has been reported for lactobacilli (42, 47, 60), these bacteria might also coaggregate with pathogens (36, 61). Next, we tested if L. gasseri and T. vaginalis were able to coaggregate (Fig. 2D). We reasoned that the slowing of motility causes the flagellated parasites to pellet at the bottom of the conical tube. This was demonstrated by slowing parasite motility in ice-temperature medium, which reduced the number of parasites swimming in the supernatant (Fig. 2D, white diamonds), in contrast to the warm medium (i.e., 37°C), which maintained a stable number of swimming parasites across the time course of the experiment (Fig. 2D, black circles). Therefore, if lactobacilli and parasites coaggregate, aggregates of bacteria-parasites would result in a drop in the numbers of swimming parasites in the supernatant from the coincubation cultures in a time-dependent manner. This experiment indeed showed a noticeable drop in parasite counts during the course of the experiment (Fig. 2D). At as early as 10 min of coincubation, parasites were evidently drowned to the bottom of the tube only in the presence of lactobacilli (Fig. 2D, black squares and white triangles). Notably, there was no significant difference in the level of T. vaginalis coaggregation with either L. gasseri ATCC 9857 or ATCC 33323, despite the former being evidently more self-aggregative (Fig. 2C).
Surface proteins of L. gasseri contribute to inhibition of T. vaginalis adhesion to hVECs.
Because the inhibitory activity of L. gasseri toward the adhesion of T. vaginalis to hVECs was largely dependent on cell contact, we investigated the contribution of bacterial surface molecules to this inhibition (Fig. 3). L. gasseri protoplasts (i.e., bacterial cells devoid of cell walls) were very inefficient at inhibiting adhesion, about 5 times less efficient than integral bacterial cells (Fig. 3, top). When lactobacilli were treated with LiCl or proteinase K, a dose-dependent reduction in inhibition was observed, with the reduction in inhibition being more pronounced for the proteinase K-digested bacteria (Fig. 3, top). As expected, the combination of proteinase K digestion with LiCl treatment of the lactobacilli had a cumulative effect, with the inhibitory effect being similar to that achieved with the protoplast. In other words, lactobacilli became less inhibitory against T. vaginalis with the increase of either or both treatments. These results indicate that surface proteins, including those noncovalently associated with the surface of lactobacilli (i.e., the proteins removed by the LiCl treatment), contribute significantly to the inhibitory properties of these bacteria against adhesion of T. vaginalis to hVECs.
FIG 3.
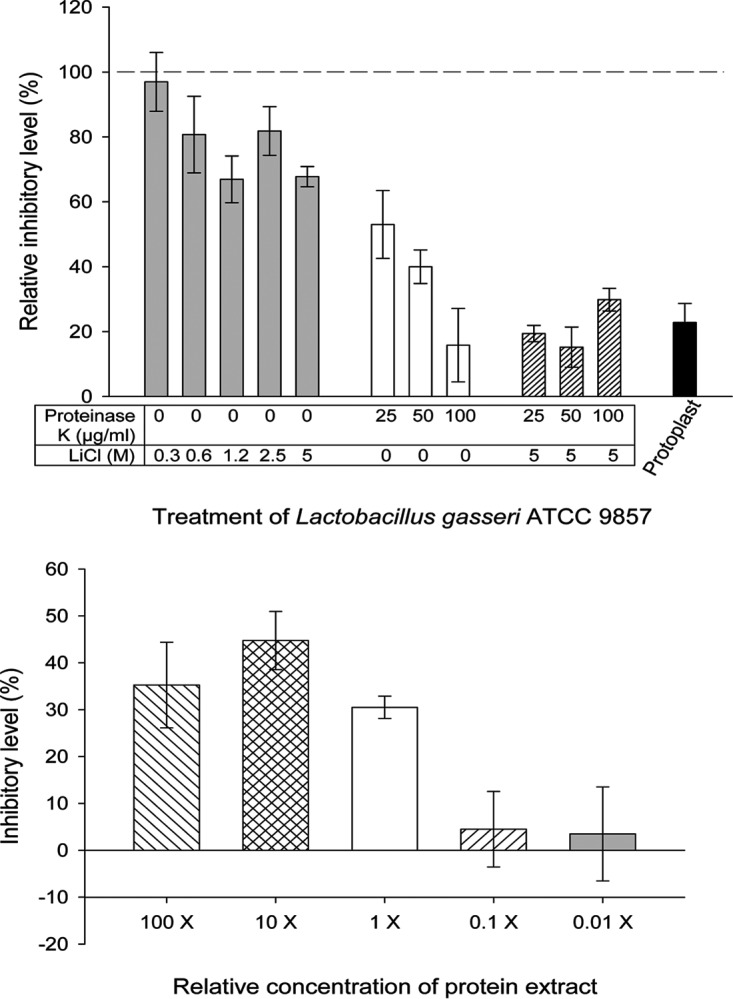
Surface molecules of L. gasseri ATCC 9857 contribute to inhibition of T. vaginalis B7RC2 adhesion to hVECs. These adhesion assays were done under the protection mode, where bacteria or protein extracts were added to hVECs prior to addition of the parasites. (Top) Surface proteins of L. gasseri contribute to inhibition of parasite cytoadherence. Prior to the adhesion assays, lactobacilli were subjected to chemical and/or enzymatic treatments either to prepare protoplasts or to remove surface-associated molecules (see Materials and Methods). As indicated in the figure, samples were either protoplasts or lactobacilli treated with increasing concentrations of LiCl, proteinase K, or a combination of both. Mock-treated lactobacilli were used as controls (see Materials and Methods). The inhibition of T. vaginalis adhesion to hVECs in the presence of mock-treated lactobacilli was considered 100% (dashed line). On the other hand, the inhibition of T. vaginalis adhesion to hVECs in the presence of treated lactobacilli was compared to that of their controls, and the relative inhibitory level was expressed as a percent change. All treatments caused a decrease in inhibition in a dose-dependent manner. For each type of treatment, the decrease in the relative inhibitory level was statistically significant compared to the result for the respective control (P < 0.05). (Bottom) Effects of noncovalently associated surface proteins of L. gasseri ATCC 9857 on the adhesion of T. vaginalis B7RC2 to hVECs. This protein extract was obtained by treatment of L. gasseri with 5 M LiCl. The concentration of the protein extract was relative to the number of lactobacilli (1×, equivalent to 2.5 × 107 lactobacilli/ml). Compared to the inhibition achieved at the 1× concentration, inhibition was lost at concentrations of 0.1× and below, and it did not change significantly at concentrations of 10× and above.
Aggregation-promoting factors (APFs) are noncovalently associated with the surface of L. gasseri via electrostatic interactions, and treating these bacterial cells with 5 M LiCl releases these surface proteins (50, 62, 63). The reduction in inhibition of T. vaginalis adhesion by simply treating lactobacilli with LiCl, particularly at concentrations of 1.2 M and above (Fig. 3, top), suggests that these proteins could be involved in the inhibition. To confirm this observation, protein extracts obtained from LiCl treatment of L. gasseri ATCC 9857 were prepared and tested in the T. vaginalis adhesion assays (Fig. 3, bottom). To ascertain the significance of such inhibition, 10-fold serial dilutions of the protein extract, including concentrations that were above and below the equivalent number of lactobacilli used in our standard adhesion assays (2.5 × 107 lactobacilli/ml), were tested in parallel. This number of lactobacilli reduces T. vaginalis adhesion to hVECs by ∼80% (Fig. 1), whereas the protein extract alone at a concentration equivalent to this cell number (1×) inhibited T. vaginalis adhesion by ∼30% (Fig. 3, bottom). This inhibition was apparently saturated, since it did not increase significantly with the concentration of the extract and it virtually disappeared if the extract was diluted 10-fold or greater (Fig. 3, bottom).
Aggregation-promoting factor 2 of L. gasseri ATCC 9857 participates in the inhibition of T. vaginalis adhesion to hVECs.
Summarizing from above, the previous observations that noncovalently associated proteins on the L. gasseri cell surface interfere with T. vaginalis cytoadherence (Fig. 3) suggest the involvement of L. gasseri APFs in this inhibitory phenotype. Genetic depletion of these genes in L. gasseri has been proven to be either deleterious or highly detrimental to the physiology of these bacteria (48). In addition, APFs and S-layer proteins are naturally self-aggregative (42, 43) and therefore notoriously insoluble for recombinant expression in Escherichia coli. To circumvent these issues, we employed the lactic acid bacterium (LAB) Lactococcus lactis as a heterologous system for the expression of L. gasseri APFs. L. lactis is a nonautochthonous species of the vagina and a LAB model for which genetic tools, including heterologous expression of surface proteins, are readily available (64).
In the genome of reference strain ATCC 33323, APFs are found as two apf-like genes, LGAS_1547 (apf-1) and LGAS_1546 (apf-2), which were recently relabeled as hypothetical proteins LGAS_RS07600 and LGAS_RS076005, respectively. Their two homologues in vaginal strain ATCC 9857 (GenBank accession numbers KY778774 and KY778775, respectively), which were identified here, share 96.6% and 86.7% similarity at the amino acid sequence level, respectively (data not shown). Repetitive attempts to clone these homologues from ATCC 9857 for surface expression on L. lactis recovered only the apf-2 recombinant L. lactis (L. lactis apf-2). Unlike nontransformed L. lactis, L. lactis apf-2 expresses a ∼32-kDa protein, detectable in cell wall extracts by use of the His tag antibody (Fig. 4, inset). The nisin expression system proved to be leaky in L. lactis apf-2, as previously reported for other genes (65, 66), since APF-2 expression could be detected even in the absence of the inducer nisin (Fig. 4, inset). Therefore, as a negative control for this experiment, protoplasts were prepared. With the removal of the cell wall, protoplasts should lack the exogenously expressed cell wall-anchored APF-2; hence, the effect of this protein on adhesion inhibition could be evaluated. We examined the inhibition of T. vaginalis adhesion by either nontransformed L. lactis or apf-2-transformed L. lactis (Fig. 4). By comparing the inhibition achieved with whole bacterial cells to that achieved with protoplasts, a significant fold increase in inhibition was evident only for recombinant L. lactis apf-2 and not for nonrecombinant L. lactis. As expected, when the expression of recombinant APF-2 was induced by nisin, the enhancement of inhibition of T. vaginalis adhesion became more pronounced (∼2.5-fold). Under this condition, ∼75% of T. vaginalis B7RC2 cells were inhibited from attaching to the human cells by the recombinant APF-2-expressing L. lactis strain. These results indicate that expression of L. gasseri APF-2 on the cell wall of L. lactis confers inhibition of T. vaginalis adhesion to hVECs.
FIG 4.
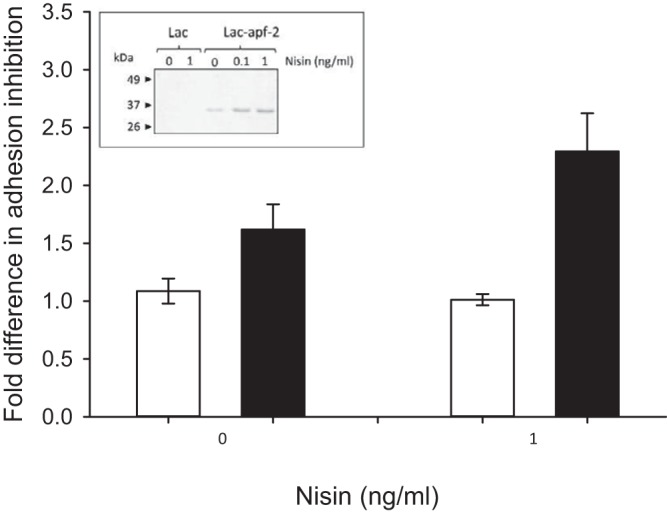
Expression of L. gasseri APF-2 on the surface of L. lactis enhances inhibition of T. vaginalis B7RC2 adhesion to hVECs. (Inset) Heterologous expression of APF-2 of L. gasseri ATCC 9857 on the cell wall of L. lactis, as detected by Western blotting. A discrete band of the expected size (∼32 kDa) was seen in the cell wall extracts derived from nisin-induced apf-2-transformed L. lactis (Lac/apf-2) but not from nontransformed L. lactis (Lac). Expression of APF-2 from L. lactis apf-2 was also detected in the absence of nisin (leaky expression) and increased from 0 to 1 ng/ml of nisin. Adhesion of T. vaginalis to hVECs was done in the presence of nontransformed L. lactis (white bars) and apf2-transformed L. lactis (black bars) either induced or not induced with nisin. The fold changes in the inhibition of T. vaginalis adhesion were measured between cells and protoplasts for nontransformed L. lactis and for apf2-transformed L. lactis. Significant fold changes between cells and protoplasts were seen only for apf2-transformed L. lactis. Expression of APF-2 on the surface of L. lactis promotes inhibition of T. vaginalis adhesion to hVECs, which was more pronounced upon nisin induction at 1 ng/ml (P < 0.01).
DISCUSSION
T. vaginalis is a protozoan parasite of the urogenital tract of humans and the causative agent of trichomoniasis, a major STI which is symptomatic more often in women than in men. In agreement with clinical observations, metagenomics studies suggest that lactobacilli are reduced in the vagina of women infected with T. vaginalis (30), implying that these microbes are competitors in the microecology of the human vagina. T. vaginalis lives extracellularly; hence, it must attach to the vaginal mucosa in order to establish infection. In this study, we examined the role of L. gasseri surface molecules on the inhibition of T. vaginalis adhesion to human vaginal ectocervical cells (hVECs).
In our previous study, L. gasseri ATCC 9857 ranked as one of the lactobacilli most inhibitory against T. vaginalis cytoadherence among a panel of 34 strains and isolates of lactobacilli tested (34). Here, we compared the L. gasseri strains ATCC 9857 and ATCC 33323 (of vaginal and intestinal origins, respectively) and showed that the former is indeed very inhibitory and is about two times more inhibitory than the latter, suggesting a possible niche-specific adaptation. Therefore, we focused on ATCC 9857 to further examine the nature of this inhibitory phenotype. Our findings indicate that L. gasseri ATCC 9857 inhibits T. vaginalis adhesion to hVECs profoundly through various contact-dependent mechanisms. These bacteria can prevent, compete with, and displace T. vaginalis from hVECs. This observation demonstrates that T. vaginalis attachment to host cells is dynamic, can be modulated, and can be reversed by a major species of the human vaginal microbiota. The cell-free filtrates also contribute to inhibition, suggesting the production of some soluble inhibitory factor. Overall, the inhibitory properties of L. gasseri are largely dependent on cell contact.
Inhibition of pathogen colonization by vaginal lactobacilli has been described elsewhere (39) and reviewed more recently (61). However, very little is known about the interactions between lactobacilli and T. vaginalis in particular (33, 34). Additionally, the inhibitory mechanisms and the molecules from lactobacilli that confer such a protective role remain largely unknown. Here, we further investigated the mechanisms and the involvement of specific molecules from L. gasseri in the inhibition of T. vaginalis adhesion to host cells.
Despite being predominantly dependent on cell contact, the inhibitory activity of L. gasseri against T. vaginalis adhesion to hVECs has many facets. Our findings indicate various modes of inhibition, including microbial coaggregation. Coaggregation has been described to be a potential mechanism for inhibiting the virulence of pathogens, preventing them from binding to or invading host cells and tissues (36, 47, 54, 61, 67). It is possible that microbial adhesion to host cells as well as bacterium-protozoan contact might trigger substantial changes on their surface or secretory profile. This means that host cells, bacteria, and protozoa are likely to sense each other in the environment and adapt through behavioral responses. Removing proteins from the surface of L. gasseri, for instance, could disrupt such interactions.
In fact, we observed that removing surface proteins from L. gasseri significantly alleviated inhibition against T. vaginalis. However, even with protoplasts, inhibition was not completely abolished. Also, depletion of surface proteins with proteinase K or LiCl reduced the level inhibition but to different levels. While proteinase K trims off proteins regardless of their nature of association with the cell surface, LiCl removes only proteins that are associated with the surface of lactobacilli by electrostatic interactions (i.e., noncovalently bound proteins). Together, these findings indicate that inhibition of T. vaginalis adhesion to host cells by lactobacilli involves multiple factors. In other words, various surface proteins from lactobacilli contribute differently to the inhibition of T. vaginalis adhesion to hVECs. In addition, our findings cannot exclude the possibility of the involvement of nonproteinaceous molecules in this inhibition.
Lactobacilli are known for using both broad-spectrum molecules (e.g., lactic acid) and pathogen-specific molecules that are inhibitory to pathogens (39, 61). Additionally, lactobacilli can affect a pathogen directly by interfering with host cell attachment or invasion but also indirectly by regulating pathways on the host mucosa (such as mucin production, barrier function, and cytokine expression) which help with immune function (39, 61). Therefore, the observation here that various molecules and mechanisms likely contribute to this protective phenotype against T. vaginalis is not surprising.
Notably, our experiments revealed that about one-third of the inhibition of pathogen adhesion was derived from proteins that are noncovalently associated with the surface of L. gasseri. The level of inhibition of T. vaginalis adhesion to hVECs compared to that achieved by untreated lactobacilli decreased when lactobacilli were treated with increasing concentrations of LiCl in a molar concentration-dependent manner, reaching a saturation. This decrease in the level of inhibition achieved by treating lactobacilli with LiCl was identical to the level of inhibition achieved by the LiCl-extractable surface proteins alone. The level of inhibition by these surface proteins alone (∼30%) also reached saturation, which was equivalent to the cell number used in our experiments. This is consistent with the findings from our previous study, which showed that the concentration of lactobacilli used here, similar to the one found in the vagina, provides the maximum level of inhibition of T. vaginalis adhesion to hVECs (34).
Among possible noncovalently bound surface proteins of L. gasseri (68), the aggregation-promoting factors (APFs) became our immediate candidate. APFs, similarly to S-layer proteins, have been implicated in adhesive phenotypes (42, 69). Recently, APFs of L. gasseri LG2055 were shown to confer aggregation phenotypes that contribute to the inhibition of C. jejuni colonization in poultry (42). Attempts to express the APFs of L. gasseri ATCC 9857 in a soluble recombinant form in E. coli failed due to a lack of expression or insolubility (data not shown). Attempts to express these APFs in L. lactis could recover only the apf-2 transfectant. Expression was achieved using a nisin-inducible system (64) and a vector that provides the elements necessary for anchoring the protein of interest to the cell wall of L. lactis (70). This system proved to be inducible but leaky, as previously observed (65, 66). By comparing cells to protoplasts (which are devoid of cell walls and, hence, have no APF-2), it became evident that expression of APF-2 on the surface of L. lactis promotes inhibition of T. vaginalis adhesion to hVECs, particularly upon nisin induction. In conclusion, APF-2 is at least one of the noncovalently bound surface proteins of L. gasseri that has a role in the inhibition of adhesion of the pathogen T. vaginalis to human vaginal cells.
This study is the first to reveal the protective nature of L. gasseri against T. vaginalis with the description of the mechanisms and molecules involved in this microbial interaction. In addition to the microbiota, T. vaginalis is often found hosting Trichomonasvirus and mycoplasmas as endosymbionts. T. vaginalis endosymbionts modify host responses and may even have an impact on the urogenital microbiome (71–73). Hence, the microbiota, trichomonas, and its endosymbionts make up a singular microbial entity which is influential to disease outcomes. The identification of health-promoting factors derived from host-protective lactobacilli, the dominant species of the vaginal microbiota, should have practical implications for the prevention and treatment of sexually transmitted infections such as trichomoniasis.
MATERIALS AND METHODS
Culture of microorganisms and human cells.
Human vaginal ectocervical cells (hVECs; ATCC CRL-2614), Lactobacillus gasseri ATCC 9857, and Lactobacillus gasseri ATCC 33323 were obtained from the American Type Culture Collection. Highly adherent Trichomonas vaginalis strain B7RC2 was given to us by Peter and Jacqui Upcroft from the Queensland Institute of Medical Research, Brisbane, Australia. T. vaginalis was cultured in Diamond's or TYM medium (74) supplemented with 10% horse serum, 10 U/ml penicillin, and 10 μg/ml streptomycin (Invitrogen) with no agitation at 37°C. The hVECs were cultivated in keratinocyte serum-free medium (K-SFM) complemented with growth factor and the same penicillin-streptomycin mix described above under a 5% CO2 atmosphere at 37°C (75). These cells were grown to 80 to 90% confluence before using them in the adhesion assays. Lactococcus lactis strain NZ9000 (pepN::nisR nisK), kindly donated by Philippe Langella (INRA, France), was grown in SGM17 (3.75% [wt/vol] M17, 10% glucose, 0.5 M sucrose) medium at 30°C overnight without agitation. E. coli strain DH5α (Invitrogen) was grown in Luria-Bertani medium with agitation at 37°C.
Flow cytometry.
Fluorescently labeled T. vaginalis cells were enumerated by flow cytometry as previously described (58), with some modifications here. T. vaginalis parasites (2.5 × 104 cells/ml, unless otherwise stated) were stained with Invitrogen CellTracker Orange CMTMR (5-[and 6]{[(4-chloromethyl)benzoyl]amino} tetramethylrhodamine) dye (1 μM) in serum-free Diamond's medium and finally counted and analyzed in a BD Accuri C6 flow cytometer. No liquid counting beads were used in the assays, as the Accuri C6 flow cytometer accurately measures the volume of sample being analyzed, in addition to the absolute number of cells being counted. Overnight cultures of Lactobacillus or Lactococcus cells were stained using a BD cell viability kit containing thiazole orange (TO) and propidium iodide (PI) per the manufacturer's instruction and counted by the BD Accuri C6 flow cytometer before being used in the adhesion assays.
Adhesion assays.
The adhesion assays were performed as previously described (34). Overnight cultures of L. gasseri were counted by flow cytometry, as described above, and diluted down to 2.5 × 107 cells/ml (unless otherwise stated) in antibiotic-free K-SFM. T. vaginalis cells, prestained with Orange CMTMR dye (Invitrogen), were counted under a hemocytometer and diluted to appropriate dilutions in antibiotic-free K-SFM. Following the aspiration of the medium from the confluent monolayer of hVECs grown in a 48-well plate, lactobacilli were added and the plate was incubated for 30 min at 37°C under a 5% CO2 atmosphere. The stained T. vaginalis cells were added, and incubation proceeded for another 30 min under the same conditions. Finally, the supernatant was discarded, and trichomonas cells that remained bound to hVECs were removed by trypsinization and counted by flow cytometry (58). In this standard mode of the assay (protection), the monolayers were precolonized with L. gasseri prior to addition of T. vaginalis and without removal of the unbound bacteria (34). Where indicated, this assay varied by changing the order in which the microbes were added to the hVECs, either with the microbes being added simultaneously (competition) or with L. gasseri being added after T. vaginalis was added (displacement). Incubation of hVECs with each microbe or both microbes was done for 30 min at 37°C under a 5% CO2 atmosphere. The standard mode of incubation was also varied where indicated by removing from hVECs the supernatant containing unbound L. gasseri prior to adding T. vaginalis. Adhesion of T. vaginalis in the presence of L. lactis also followed the standard mode of the assay. In this case, as described below, L. lactis was induced for expression, and entire cells and protoplasts were tested in these experiments. Bacterial and T. vaginalis concentrations were 2.5 × 107 cells/ml and 2.5 × 104 cells/ml, respectively, unless stated otherwise.
Adhesion of T. vaginalis was measured in the presence of a culture supernatant and protein extracts, and this assay also used the standard mode of incubation. In the first case, the culture supernatant was prepared by incubating L. gasseri either alone or with hVECs in K-SFM under the same conditions used for the adhesion assays. The supernatant was collected, spun down, and filtered through a 0.22-μm-pore-size membrane to remove the cells. In the second case, extracts of noncovalently bound surface proteins were obtained after LiCl treatment of L. gasseri cells, as described below. The supernatant containing these proteins was extensively dialyzed (10-kDa exclusion) against deionized water with 5 water changes (>500 volumes of water in relation to the sample volume) for 24 h at 4°C. After dialysis, the protein extracts were lyophilized and resuspended in water for use. Inhibition of T. vaginalis adhesion to hVECs was always compared to that for a control without the presence of the inhibitory bacterium, supernatant, or protein extract. Adhesion assays were done at least three independent times (i.e., biological replicas). For each time, a replica of three samples was carried along (i.e., experimental replicas).
Chemical and enzymatic treatment of bacterial cells.
Overnight cultures of L. gasseri ATCC 9857 were washed and resuspended in 1× phosphate-buffered saline (PBS; pH 7.4). Three types of treatments were performed. First, to remove noncovalently associated surface proteins, these cells were treated with a high molar concentration of LiCl as previously described (76, 77). The concentration of LiCl was 5 M when preparing the protein extracts used in the adhesion assays. Otherwise, concentrations of 0.3, 0.6, 1.2, 2.5, and 5 M were tested to selectively strip these proteins off the surface of the L. gasseri cells. LiCl-treated cells were washed, resuspended in K-SFM, and immediately used in the adhesion assays. Second, proteinase K (in 50 mM Tris-HCl, 0.1% SDS, pH 8.0) was employed to digest the surface proteins on these bacteria. Lactobacilli were digested with 25 μg/ml, 50 μg/ml, and 100 μg/ml of proteinase K for 2 h at 37°C. Third, both treatments were combined in the order mentioned above. After the treatments, the cells were washed three times in 1× PBS by centrifugation, resuspended in K-SFM, and immediately used in the adhesion assays. Controls consisted of lactobacilli treated with PBS only or the proteinase K buffer only without the enzyme (mock treatment).
Cell wall extracts and protoplasts from bacterial cells were obtained as described previously (78), with minor modifications here. Briefly, 1.5 ml of overnight cultures was centrifuged at 2,000 × g for 10 min, resuspended in an equal volume of prewarmed culture medium, and incubated for 2 h. Cells were washed once with Tris-buffered saline (TBS) buffer (25% [wt/vol] sucrose, 1 mM EDTA, pH 7.4) by centrifugation and resuspended in 1 ml of TBS buffer containing 20 mg/ml lysozyme (Roche, New Zealand) and 20 μg/ml mutanolysin (Sigma-Aldrich, New Zealand). After incubation for 1 h, 0.5 ml of 0.25 M EDTA was added and the mixture was incubated on ice for 5 min. This was applied to both L. lactis and L. gasseri at their optimal incubation temperatures (30 and 37°C, respectively). For L. lactis, after centrifugation, the supernatant containing the cell wall extracts was collected for Western blot analysis. The protoplast pellets were washed, resuspended in K-SFM, and immediately used in the adhesion assays. Controls consisted of bacteria treated with the buffer but in the absence of the enzymes (mock treatment).
Coaggregation of Lactobacillus gasseri and Trichomonas vaginalis.
Overnight cultures of L. gasseri (ATCC 9857 and ATCC 33323) and T. vaginalis B7RC2 were washed in K-SFM by centrifugation. T. vaginalis cells were labeled with CMTMR (Life Technologies) as previously described (58). The CMTMR-labeled T. vaginalis and lactobacilli were resuspended in prewarmed K-SFM and mixed together to final concentrations of 1 × 106 and 1 × 109 cells/ml, respectively, in a final volume of 15 ml. These mixed cultures were incubated at 37°C for up to 30 min in a 15-ml conical tube. At each time point (0, 10, 20, and 30 min), a volume of 0.5 ml was carefully pipetted out from the top of the mixed cultures. The number of CMTMR-labeled T. vaginalis cells in these samples was quantified by flow cytometry (58). The experiments were done in triplicate.
PCR and DNA cloning.
Alignment of the nucleotide sequences of the apf genes from L. gasseri and Lactobacillus johnsonii (including sequences upstream and downstream of the coding region), available from the NCBI database, allowed us to perform PCR and sequence the full open reading frames of apf-1 and apf-2 of L. gasseri ATCC 9857. Primers specific for the coding regions of these apf genes were designed to remove the secretion peptide, as this was provided in plasmid pVE5547 (kindly donated by Philippe Langella at INRA, France). This plasmid also carries a C-terminal cell wall anchor domain in frame with the gene of interest, allowing the protein product to be translocated and anchored on the cell wall of L. lactis (70). These primers were apf-1 forward (GATAGTCGACCACCACCACCACCACCACGCAGAAGTTACTGTTGGTC), apf-1 reverse (GATAGATATCGCGTACCAGCCATTTGCTTGCC), apf-2 forward (GAGAGTCGACCACCACCACCACCACCACGCTACTATTGTTCAAAATGATAC), and apf-2 reverse (GATAGATATCGCGTACCAACCGTTAGTTTGCC). The underlined sequences correspond to the restriction enzyme sites used for cloning in pVE5547 (70). The italicized sequences in the forward primers indicate the addition of a His tag for the purpose of immune detection. PCR amplification of the apf genes from ATCC 9857 genomic DNA was performed using high-fidelity Phusion DNA polymerase (Thermo Fisher). The amplicons from pVE5547 were cloned into E. coli DH5α and fully sequenced. The final plasmids carrying the apf genes were electroporated into L. lactis NZ9000. Transformed L. lactis cells were selected by erythromycin and induced for expression with nisin, as described previously (70).
Western blotting.
Extracts of cell wall proteins were obtained from the same number of transformed and nontransformed L. lactis cells, as described above. These extracts were resolved by electrophoresis on a 12% SDS-polyacrylamide gel and blotted onto a 0.45-μm-pore-size polyvinylidene difluoride (PVDF) membrane (Bio-Rad) using a Trans-Blot apparatus (Owl VEP-2), as recommended by the manufacturer (Thermo Fisher). The blot was blocked and probed in TBS-Tween 20 (TBST; 20 mM Tris, 150 mM NaCl, 0.1% [vol/vol] Tween 20, pH 7.6) containing 5% (wt/vol) skim milk. Blocking was done for 5 to 6 h at room temperature. Probing was done with a 1:10,000 dilution of the mouse anti-His antibody (Invitrogen) at 4°C overnight, followed by a 1:50,000 dilution of the goat horseradish peroxidase-conjugated anti-mouse immunoglobulin antibody (Invitrogen) at room temperature for 1 h. Between the time of addition of antibodies and before development, the blot was washed five times with TBST. At the end, the blot was developed using a SuperSignal West Pico chemiluminescent substrate kit (Thermo Fisher), and the signal was detected with an Amersham Imager 600 instrument.
Statistical analysis.
One-way analysis of variance and Dunnett's pairwise multiple-comparison tests were used to statistically analyze the data via the software R (https://www.r-project.org/). Means were considered significant when P values were less than 0.05 (P < 0.05) and highly significant when they were less than 0.01 (P < 0.01).
Accession number(s).
The homologues of hypothetical proteins LGAS_RS07600 and LGAS_RS07605 from Lactobacillus gasseri ATCC 33323 in vaginal strain Lactobacillus gasseri ATCC 9857 have been deposited in the GenBank database under accession numbers KY778774 and KY778775, respectively.
ACKNOWLEDGMENTS
We all contributed to and reviewed the manuscript. None of us has a competing financial interest or conflicting interests in any aspect of the research reported here.
We thank Philippe Langella (INRA, France) for donating L. lactis strain NZ9000 and plasmid pVE5547. We are also grateful to Kathy Ruggiero (Statistical Consulting Centre, University of Auckland) for her help with statistical analysis using R. We acknowledge the helpful assistance from the technical team of the DNA Sequencing Core Facility at the University of Auckland.
We are also grateful for the research funders, more specifically, the Health Research Council of New Zealand (HRC11/314 to A.S.-B.), the Maurice & Phyllis Paykel Trust (to A.S.-B.), and the Faculty of Science Research Development Fund, the University of New Zealand (to A.S.-B.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.World Health Organization. 2015. Sexually transmitted infections (STIs). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Cotch MF, Pastorek JG II, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey CJ, Regan JA, Krohn MA, Klebanoff MA, Rao VA, Rhoads GG. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hardy P, Nell EE, Spence M, Hardy J, Graham D, Rosenbaum R. 1984. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet 324:333–337. doi: 10.1016/S0140-6736(84)92698-9. [DOI] [PubMed] [Google Scholar]
- 4.Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, Clark L, Pringle G, McCormack WM. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 150:965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 5.Gram IT, Macaluso M, Churchill J, Stalsberg H. 1992. Trichomonas vaginalis (TV) and human papillomavirus (HPV) infection and the incidence of cervical intraepithelial neoplasia (CIN) grade III. Cancer Causes Control 3:231–236. doi: 10.1007/BF00124256. [DOI] [PubMed] [Google Scholar]
- 6.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M, Heyward WL, Ryder RW, Piot P. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, Platz EA, Sutcliffe S, Fall K, Kurth T, Ma J, Stampfer MJ, Mucci LA. 2009. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J Natl Cancer Inst 101:1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ, Pathela P, Schwebke JR, Evan Secor W, Workowski KA, Davis D, Braxton J, Weinstock HS. 2012. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009-2010. Emerg Infect Dis 18:939–943. doi: 10.3201/eid1806.111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meri T, Jokiranta TS, Suhonen L, Meri S. 2000. Resistance of Trichomonas vaginalis to metronidazole: report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J Clin Microbiol 38:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rendón-Maldonado JG, Espinosa-Cantellano M, González-Robles A, Martínez-Palomo A. 1998. Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol 89:241–250. doi: 10.1006/expr.1998.4297. [DOI] [PubMed] [Google Scholar]
- 11.Elmendorf HG, Hayes RD, Srivastava S, Johnson P. 2010. New insights into the composition and function of the cytoskeleton in Giardia lamblia and Trichomonas vaginalis, p 119–156. In Clark CG, Johnson P, Adam RD (ed), Anaerobic parasitic protozoa. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 12.Gonzalez-Robles A, Lazaro-Haller A, Espinosa-Cantellano M, Anaya-Velazquez F, Martinez-Palomo A. 1995. Trichomonas vaginalis: ultrastructure bases of the cytopathic effect. J Eukaryot Microbiol 42:641–651. doi: 10.1111/j.1550-7408.1995.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 13.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UCM, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Müller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu C-L, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik S-B, Logsdon JM, Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu C-H, Lee Y-S, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ. 2007. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirt RP, de Miguel N, Nakjang S, Dessi D, Liu YC, Diaz N, Rappelli P, Acosta-Serrano A, Fiori PL, Mottram JC. 2011. Trichomonas vaginalis pathobiology: new insights from the genome sequence. Advances in parasitology, 1st ed Elsevier Ltd., Oxford, United Kingdom. [DOI] [PubMed] [Google Scholar]
- 15.De Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ. 2010. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics 9:1554–1566. doi: 10.1074/mcp.M000022-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia AF, Alderete J. 2007. Characterization of the Trichomonas vaginalis surface-associated AP65 and binding domain interacting with trichomonads and host cells. BMC Microbiol 7:116. doi: 10.1186/1471-2180-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engbring JA, Alderete JF. 1998. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiology 144:3011–3018. doi: 10.1099/00221287-144-11-3011. [DOI] [PubMed] [Google Scholar]
- 18.Hernández HM, Marcet R, Sarracent J. 2014. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 21:54. doi: 10.1051/parasite/2014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan CM, De Miguel N, Johnson PJ. 2011. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem 51:161–175. doi: 10.1042/bse0510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H. 2016. Influence of 120 kDa pyruvate: ferredoxin oxidoreductase on pathogenicity of Trichomonas vaginalis. Korean J Parasitol 54:71–74. doi: 10.3347/kjp.2016.54.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addis MF, Rappelli P, Fiori PL. 2000. Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect Immun 68:4358–4360. doi: 10.1128/IAI.68.7.4358-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL. 2007. Trichomonas vaginalis surface proteins: a view from the genome. Trends Parasitol 23:540–547. doi: 10.1016/j.pt.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Riestra AM, Gandhi S, Sweredoski MJ, Moradian A, Hess S, Urban S, Johnson PJ. 2015. A Trichomonas vaginalis rhomboid protease and its substrate modulate parasite attachment and cytolysis of host cells. PLoS Pathog 11:1–25. doi: 10.1371/journal.ppat.1005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan CM, Mehlert A, Richardson JM, Ferguson MAJ, Johnson PJ. 2011. Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (β1-4/3) N-acetylglucosamine repeats in host cell interaction. J Biol Chem 286:40494–40508. doi: 10.1074/jbc.M111.280578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. 2005. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell 4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumura CYM, Baum LG, Johnson PJ. 2008. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol 10:2078–2090. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boris S, Barbés C. 2000. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2:543–546. doi: 10.1016/S1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 28.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajer P, Brotman R, Bai G, Sakamoto J, Schutte U, Koenig S, Zhanshan M, Zhou X, Abdo Z, Forney L, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra152. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brotman RM, Bradford L, Conrad M, Gajer P, Ault K, Peralta L, Forney LJ, Carlton JM, Abdo Z, Ravel J. 2012. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 39:807–812. doi: 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shipitsyna E, Roos A, Datcu R, Hallén A, Fredlund H, Jensen JS, Engstrand L, Unemo M. 2013. Composition of the vaginal microbiota in women of reproductive age—sensitive and specific molecular diagnosis of bacterial vaginosis is possible? PLoS One 8:e60670. doi: 10.1371/journal.pone.0060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bär A-K, Phukan N, Pinheiro J, Simoes-Barbosa A. 2015. The interplay of host microbiota and parasitic protozoans at mucosal interfaces: implications for the outcomes of infections and diseases. PLoS Negl Trop Dis 9:e0004176. doi: 10.1371/journal.pntd.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB. 2013. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 89:460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phukan N, Parsamand T, Brooks AES, Nguyen TNM, Simoes-Barbosa A. 2013. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex Transm Infect 89:455–459. doi: 10.1136/sextrans-2013-051039. [DOI] [PubMed] [Google Scholar]
- 35.Coudeyras S, Jugie G, Vermerie M, Forestier C. 2008. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect Dis Obstet Gynecol 2008:549640. doi: 10.1155/2008/549640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Wang Q, Xiao B, Zhang R, Wang B, Liao Q, Zhuang H, Li T. 2015. Characterization of vaginal Lactobacillus strains and their potential antagonistic effects on Candida albicans. Br Microbiol Res J 6:185–195. doi: 10.9734/BMRJ/2015/15116. [DOI] [Google Scholar]
- 37.Graver MA, Wade JJ. 2011. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 10:8. doi: 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borges S, Silva J, Teixeira P. 2014. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 289:479–489. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 39.Spurbeck RR, Arvidson CG. 2011. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol 6:567–582. doi: 10.2217/fmb.11.36. [DOI] [PubMed] [Google Scholar]
- 40.Granato D, Perotti F, Masserey I, Rouvet M, Golliard M, Servin A, Brassart D. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl Environ Microbiol 65:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, Roos S, Walter J, Juge N. 2010. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156:3368–3378. doi: 10.1099/mic.0.043265-0. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama K, Nakazato A, Ueno S, Seto Y, Kakuda T, Takai S, Yamamoto Y, Mukai T. 2015. Cell surface-associated aggregation-promoting factor from Lactobacillus gasseri SBT2055 facilitates host colonization and competitive exclusion of Campylobacter jejuni. Mol Microbiol 98:712–726. doi: 10.1111/mmi.13153. [DOI] [PubMed] [Google Scholar]
- 43.Åvall-Jääskeläinen S, Palva A. 2005. Lactobacillus surface layers and their applications. FEMS Microbiol Rev 29:511–529. [DOI] [PubMed] [Google Scholar]
- 44.Malik S, Petrova MI, Imholz NCE, Verhoeven TLA, Noppen S, Van Damme EJM, Liekens S, Balzarini J, Schols D, Vanderleyden J, Lebeer S. 2016. High mannose-specific lectin Msl mediates key interactions of the vaginal Lactobacillus plantarum isolate CMPG5300. Sci Rep 6:37339. doi: 10.1038/srep37339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrova MI, Lievens E, Verhoeven TLA, MacKlaim JM, Gloor G, Schols D, Vanderleyden J, Reid G, Lebeer S. 2016. The lectin-like protein 1 in Lactobacillus rhamnosus GR-1 mediates tissue-specific adherence to vaginal epithelium and inhibits urogenital pathogens. Sci Rep 6:37437. doi: 10.1038/srep37437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carasi P, Ambrosis NM, De Antoni GL, Bressollier P, Urdaci MC, Serradell MDLA. 2014. Adhesion properties of potentially probiotic Lactobacillus kefiri to gastrointestinal mucus. J Dairy Res 81:16–23. doi: 10.1017/S0022029913000526. [DOI] [PubMed] [Google Scholar]
- 47.Kmet V, Lucchini F. 1997. Aggregation-promoting factor in human vaginal Lactobacillus strains. FEMS Immunol Med Microbiol 19:111–114. doi: 10.1111/j.1574-695X.1997.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 48.Jankovic I, Ventura M, Meylan V, Rouvet M, Elli M, Zink R. 2003. Contribution of aggregation-promoting factor to maintenance of cell shape in Lactobacillus gasseri 4B2. J Bacteriol 185:3288–3296. doi: 10.1128/JB.185.11.3288-3296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura M, Jankovic I, Walker DC, David R, Pridmore RD, Zink R. 2002. Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl Environ Microbiol 68:6172–6181. doi: 10.1128/AEM.68.12.6172-6181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hymes JP, Johnson BR, Barrangou R, Klaenhammer TR. 2016. Functional analysis of an S-layer associated fibronectin-binding protein in Lactobacillus acidophilus NCFM. Appl Environ Microbiol 82:2676–2685. doi: 10.1128/AEM.00024-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng J, Zhu X, Gao SM, Zhang QX, Sun Z, Lu RR. 2014. Characterization of surface layer proteins and its [sic] role in probiotic properties of three Lactobacillus strains. Int J Biol Macromol 65:110–114. doi: 10.1016/j.ijbiomac.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Wang H, Liu J, Zhao Y, Gao K, Zhang J. 2013. Adhesive ability means inhibition activities for lactobacillus against pathogens and S-layer protein plays an important role in adhesion. Anaerobe 22:97–103. doi: 10.1016/j.anaerobe.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Kos B, Šušković J, Vuković S, Sǐmpraga M, Frece J, Matošić S. 2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Xu J, Shuai J, Chen J, Zhang Z, Fang W. 2007. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is [sic] responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int J Food Microbiol 115:307–312. doi: 10.1016/j.ijfoodmicro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Charteris WP, Kelly PM, Morelli L, Collins JK. 2001. Antibacterial activity associated with Lactobacillus gasseri ATCC 9857 from the human female genitourinary tract. World J Microbiol Biotechnol 17:615–625. doi: 10.1023/A:1012405821202. [DOI] [Google Scholar]
- 56.Azcarate-Peril MA, Altermann E, Yong JG, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O'Flaherty S, Buck BL, Dobson A, Duong T, Miller MJ, Barrangou R, Klaenhammer TR. 2008. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl Environ Microbiol 74:4610–4625. doi: 10.1128/AEM.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chagnaud P, Jenkinson HF, Tannock GW. 1992. Cell surface-associated proteins of gastrointestinal strains of lactobacilli. Microb Ecol Health Dis 5:121–131. doi: 10.3109/08910609209141306. [DOI] [Google Scholar]
- 58.Brooks AES, Parsamand T, Kelly RW, Simoes-Barbosa A. 2013. An improved quantitative method to assess adhesive properties of Trichomonas vaginalis to host vaginal ectocervical cells using flow cytometry. J Microbiol Methods 92:73–78. doi: 10.1016/j.mimet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Vielfort K, Sjölinder H, Roos S, Jonsson H, Aro H. 2008. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect 10:1325–1334. doi: 10.1016/j.micinf.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira CL, Grzeœkowiak L, Collado MC, Salminen S. 2011. In vitro evaluation of Lactobacillus gasseri strains of infant origin on adhesion and aggregation of specific pathogens. J Food Prot 74:1482–1487. doi: 10.4315/0362-028X.JFP-11-074. [DOI] [PubMed] [Google Scholar]
- 61.Younes JA, Lievens E, Hummelen R, van der Westen R, Reid G, Petrova MI. 2018. Women and their microbes: the unexpected friendship. Trends Microbiol 26:16–32. doi: 10.1016/j.tim.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Sára M, Sleytr UB. 2000. S-layer proteins. J Bacteriol 182:859–868. doi: 10.1128/JB.182.4.859-868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hynönen U, Palva A. 2013. Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol 97:5225–5243. doi: 10.1007/s00253-013-4962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 65.Brøndsted L, Pedersen M, Hammer K. 2001. An activator of transcription regulates phage TP901-1 late gene expression. Appl Environ Microbiol 67:5626–5633. doi: 10.1128/AEM.67.12.5626-5633.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Renye JA, Somkuti GA. 2010. Nisin-induced expression of pediocin in dairy lactic acid bacteria. J Appl Microbiol 108:2142–2151. doi: 10.1111/j.1365-2672.2009.04615.x. [DOI] [PubMed] [Google Scholar]
- 67.Ocana VS, Nader-Macias ME. 2002. Vaginal lactobacilli: self- and co-aggregating ability. Br J Biomed Sci 59:183–190. doi: 10.1080/09674845.2002.11783657. [DOI] [PubMed] [Google Scholar]
- 68.Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, Bron PA. 2010. The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34:199–230. doi: 10.1111/j.1574-6976.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- 69.Goh YJ, Klaenhammer TR. 2010. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl Environ Microbiol 76:5005–5012. doi: 10.1128/AEM.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribeiro LA, Azevedo V, Le Loir Y, Oliveira SC, Dieye Y, Piard JC, Gruss A, Langella P. 2002. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl Environ Microbiol 68:910–916. doi: 10.1128/AEM.68.2.910-916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirt RP, Sherrard J. 2015. Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr Opin Infect Dis 28:72–79. doi: 10.1097/QCO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 72.Fichorova R, Fraga J, Rappelli P, Fiori PL. 2017. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res Microbiol 168:882–891. doi: 10.1016/j.resmic.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dessì D, Rappelli P, Diaz N, Fiori P. 2006. Mycoplasma hominis and Trichomonas vaginalis: a unique case of symbiotic relationship between two obligate human parasites. Front Biosci 11:2028. doi: 10.2741/1944. [DOI] [PubMed] [Google Scholar]
- 74.Clark CG, Diamond LS. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fichorova R, Rheinwald J, Anderson D. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 76.Johnson B, Selle K, O'Flaherty S, Goh YJ, Klaenhammer T. 2013. Identification of extracellular surface-layer associated proteins in Lactobacillus acidophilus NCFM. Microbiology 159:2269–2282. doi: 10.1099/mic.0.070755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lortal S, Van Heijenoort J, Gruber K, Sleytr UB. 1992. S-layer of Lactobacillus helveticus ATCC 12046: isolation, chemical characterization and re-formation after extraction with lithium chloride. J Gen Microbiol 138:611–618. doi: 10.1099/00221287-138-3-611. [DOI] [Google Scholar]
- 78.Calandra GB, Cole RM. 1980. Lysis and protoplast formation of group B streptococci by mutanolysin. Infect Immun 28:1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]