Abstract
The role of vitamin D beyond its classical function in calcium homeostasis has been of significant interest in recent years. There has been expanding research on the pleiotropic role of vitamin D in pregnancy and the implications of its deficiency on maternal-fetal outcomes. Several studies have associated low maternal vitamin D status to adverse outcomes in pregnancy, including preeclampsia, gestational diabetes, preterm births, low birth weight, and others. Several randomized controlled clinical trials of Vitamin D supplementation during pregnancy have also been conducted. Though some of the studies found improvement in pregnancy outcomes with vitamin D supplementation, others have not shown any association. In this article, we have critically reviewed the observational and interventional studies, published primarily within the past two years (January 2014 to February 2016) on the influence of vitamin D deficiency on pregnancy and the impact of its supplementation. The potential underlying mechanisms of vitamin D in regulating each of the outcomes have also been discussed.
Keywords: Pregnancy, Vitamin D, Maternal outcomes, Neonatal outcomes, Vitamin D supplementation
Introduction
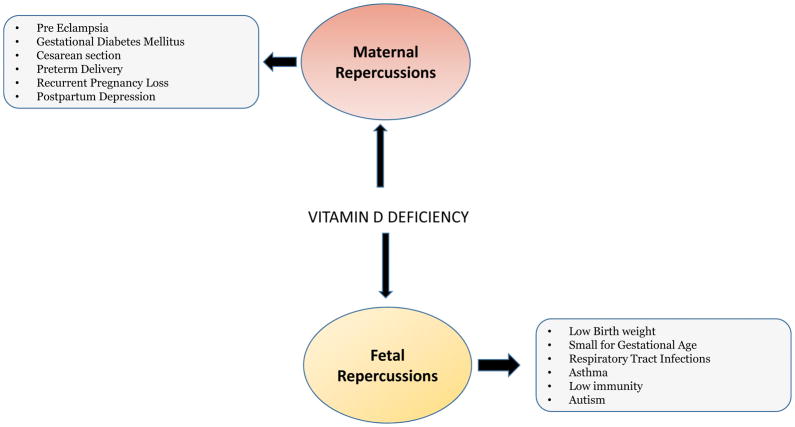
Vitamin D is a secosteroid, which is also considered an important prohormone (Weinert et al, 2015). Since vitamin D receptors (VDRs) are present in many cells and tissues throughout the body, many studies support the role of vitamin D in several physiological functions beyond bone and muscle health (Joergensen et al, 2014). During pregnancy, vitamin D plays a vital role in embryogenesis, especially fetal skeletal development and calcium homeostasis (Hollis et al, 2011). Vitamin D deficiency is a growing health concern worldwide in both adults and children (Weinert et al, 2015). Indeed, the findings from several studies suggest the increasing prevalence of vitamin D deficiency in pregnancy and the associated adverse maternal and fetal outcomes, such as gestational diabetes mellitus (GDM), preeclampsia, small for gestational age (SGA), preterm births among others (Palaniswamy et al, 2016) (Figure 1).
Figure 1.
The schematic diagram depicts the adverse maternal and fetal outcomes associated with vitamin D deficiency.
To further investigate the role of vitamin D in pregnancy and the improvement of outcomes on its supplementation, many interventional and observational studies have been undertaken but the results have not been consistent. Heterogeneity in the findings could be attributed, but not limited, to the differences in ethnicity, geographical locations, amount of vitamin D supplementation, and duration of supplementation. The methodology of the studies is not consistent and cannot be compared since the studies were conducted at different stages of gestation. It is still not well defined as to how vitamin D supplementation modifies the risk of the outcomes and whether vitamin D contributes to the etiopathogenesis of these diseases or it is just a marker (Yin et al, 2014). Given the low cost of vitamin D supplementation, if proven effective in pregnancy, it could bring about some major improvements in public health worldwide (Christensen et al, 2012).
The impact of vitamin D deficiency during pregnancy has been discussed in a few recent reviews and metaanalyses (Perez-Lopez et al, 2015; Harvey et al, 2014; Wei et al, 2015); however, more randomized clinical trials (RCTs) and observational studies have been conducted after the publication of the last review. Hence, this review article summarizes the recent studies on the influence of vitamin D deficiency during pregnancy. The impact of vitamin D supplementation on various maternal and fetal clinical endpoints has also been reviewed with critical discussion on the clinical and translational application of these findings and identified gaps in our knowledge to conduct future prospective studies.
Methods
Literature search was performed using PubMed Database of the National Library of Medicine, with date limits from January 2014 to February 2016, as the major emphasis of our review was to critically review the recent findings on Vitamin D in pregnancy. We used the keywords: Vitamin D, pregnancy, vitamin D supplementation, hypovitaminosis D, preeclampsia, gestational diabetes, preterm birth, and other related terms. The studies of interest included original papers and review articles on the influence of vitamin D deficiency in pregnancy and the impact of vitamin D supplementation on the maternal and fetal outcomes. References in these articles were searched for any other related articles. Duplicate articles were excluded. Articles were also excluded if they were not published in English language; were unavailable in full text, or if the studies did not involve human subjects. Sixty studies fit the criteria and hence, were critically evaluated in this review.
Physiology of vitamin D and vitamin D deficiency
Vitamin D is a fat-soluble vitamin with two major physiological forms: Ergocalciferol, vitamin D2 and Cholecalciferol vitamin D3 (DeLuca, 2014). These two forms basically differ in their side chain structure. While D2 is mainly obtained from plants and vegetable sources, D3 is majorly synthesized in the skin on exposure to sunlight (UVB radiation) from 7 dehydrocholesterol (De-Regil et al, 2016). Both forms of vitamin D are also available as dietary supplements. All the forms of vitamin D are activated only on enzyme-mediated hydroxylation. The first hydroxylation reaction is mediated by 25α-hydroxylase, to produce 25 hydroxyvitamin D (25OHD) or calcidiol and takes place in the liver (Harvey et al, 2014). This is followed by the next hydroxylation step in the kidney, mediated by 1α-hydroxylase, to produce 1,25-dihydroxycholecalciferol (1,25-DHCC) or calcitriol (Wagner et al, 2012). While 25OHD is the most abundant circulating form of vitamin D, 1,25-DHCC is the most active form (Sahota, 2014). The hydroxylation reactions and the availability of active vitamin D are regulated by serum calcium and phosphorus and parathyroid hormone (PTH) (Lips P, 2006).
Apart from its critical role in the regulation of calcium and phosphorus homeostasis for skeletal development, vitamin D also mediates other functions, including anti-inflammation, regulation of innate and acquired immunity, and prevention of cardiovascular diseases (Christakos et al, 2016).
Based on the recommendations by the Institute of Medicine (IOM), the recommended daily intake of vitamin D during pregnancy and lactation is 600 IU, taking into account the fetal demands as well (Grant et al, 2014). The serum 25OHD is regarded as the best stable and circulating biomarker for vitamin D status (Brannon et al, 2014). The cut-off value for vitamin D deficiency is set at 20 ng/ml while for vitamin D insufficiency it ranges from 20–30 ng/ml (Ross CA et al IOM, 2011).
Vitamin D deficiency is a global epidemic affecting people of all age groups with the principal cause being the lack of exposure to sunlight (Holick et al, 2008). Others include darker skin (high melanin content), aging, use of sunscreen and winters, all associated with decreased dermal synthesis of vitamin D (Holick et al, 2008). Some pathological conditions associated with low vitamin D include kidney failure, intestinal malabsorption, chronic inflammation and liver failure and use of concurrent medications (Dusilova-Sulkova, 2009). As a consequence, there is decreased bone density and greater susceptibility to fractures. Children suffer from rickets, while adults develop osteopenia or osteoporosis. Additionally, vitamin D deficiency is also associated with other co-morbid conditions like type 1 diabetes mellitus, neoplasms, cardiovascular diseases and others (Dusilova-Sulkova, 2009).
Vitamin D in pregnancy
During pregnancy, mobilization of maternal calcium increases to meet the demands of adequate fetal bone mineralization (Olmos-Ortiz et al, 2015). As a consequence, a number of physiological adaptations take place, including increased maternal serum calcitriol, vitamin D binding protein (DBP), placental VDR and renal and placental CYP27B1 activity to maintain normal serum levels of 25OHD and calcium (Olmos-Ortiz et al, 2015). Maternal 25OHD crosses the placenta and is the main form of vitamin D for the fetus (Marshall et al, 2016). Calcitriol rises during pregnancy, almost doubled by the end of third trimester and then returns to normal levels after delivery (Marshall et al, 2016). During pregnancy, even placenta and fetal kidney express 1α-hydroxylase stimulated by prolactin and placental lactogen, though the major function is still performed by the maternal renal hydroxylase(Olmos-Ortiz et al, 2015). Maternal serum calcium levels fall during pregnancy, which can be attributed to a decrease in the serum albumin, but the ionized calcium levels remain unchanged (Olausson et al, 2012). In fact, fetal serum calcium levels are higher than maternal serum calcium levels, thereby requiring specific trans-placental carriers to transfer calcium against the concentration gradient (Olmos-Ortiz et al, 2015). This is mediated by the expression of calcium binding proteins in placenta, including calbindin D-9k and D-28k (Halhali et al, 2010).
The most important contribution of vitamin D during pregnancy is to escalate calcium absorption and placental calcium transport (Olmos-Ortiz et al, 2015). Additionally, vitamin D also regulates immune system and inhibits inflammation by restraining inflammatory cytokines. including TNF-α, IFN-γ, IL-6, while promoting the release of antimicrobial peptide cathelicidin hCTD in the placenta (Olmos-Ortiz et al, 2014). Calcitriol also plays an important role in placental physiology. It stimulates endometrial decidualization, synthesis of estradiol and progesterone and regulation of the expression of human chorionic gonadotrophin (hCG) and human placental lactogen(hPL) in the placenta(Olmos-Ortiz et al, 2014).
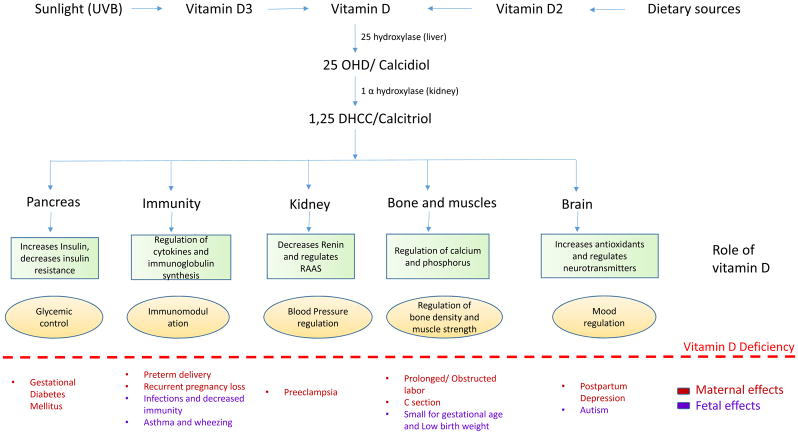
All these effects indicate the vital importance of vitamin D during gestation and the potential role of its deficiency on adverse maternal-fetal outcomes (Figure 2). Each maternal and fetal outcome investigated in the recent papers has been discussed in detail below with a tabular representation of the recent observational studies in Table 1 and Table 2. The impact of vitamin D supplementation has also been reviewed with a depiction of the recent Randomized Controlled Trials shown in Table 3. Table 4 summarizes the level of evidence of the impact of vitamin D on various maternal-fetal outcomes based on the GRADE system.
Figure 2.
The sxhematic diagram depicts the activation of vitamin D by two hyroxylation steps, one each in liver and kidney, followed by the normal physiological role of vitamin D in each organ. The specific maternal and fetal repercussion of vitamin D deficiency are mentioned below the dotted line.
Table 1.
Observational studies of vitamin D status in pregnancy and maternal outcomes
| Study design | Place of study | Sample size | Gestational age at Vit D assay | Vit D Assay method | Outcome analyzed | Statistics (95% CI) | Study authors | Quality of evidence (GRADE system) |
|---|---|---|---|---|---|---|---|---|
| Case control | India | 74 cases and 100 controls | On enrollment | ELISA | Preeclampsia | p= 0.0001 | Singla et al, 2015 | Moderate |
|
| ||||||||
| Case control | Iran | 59 cases and 59 controls | Before the onset of labor | ELISA, HPLC | Preeclampsia | OR 24.04 (2.10–274.8) | Abedi et al, 2014 | Low |
|
| ||||||||
| Case control | Iran | 41 cases and 50 controls | Onset of labor | ELISA | Preeclampsia | P=0.0001 | Mohagegh et al, 2015 | Moderate |
|
| ||||||||
| Case control | USA | 717 cases and 2986 controls | <26 weeks | LC-MS | Preeclampsia | Adjusted RR 0.65 (0.43–0.98) | Bodnar et al, 2014 | Moderate |
|
| ||||||||
| Case control | Iran | 40 cases and 40 controls | Anytime | CLIA | Preeclampsia | P=0.002 | Sadin et al, 2015 | Moderate |
|
| ||||||||
| Case control | USA | 1126 cases and 2327 controls | <20 weeks | LC-MS | PTL | P<0.01 | Bodnar et al, 2015 | Moderate |
|
| ||||||||
| Case control | USA | 79 cases and 113 controls | At delivery | CLIA, RIA | PTL | Thota et al, 2014 | Moderate | |
| (Caucasian) | P<0.01 | |||||||
| (African American) | P<0.04 | |||||||
|
| ||||||||
| Case control | USA | 839 cases and 2629 controls | <26 weeks | LC-MS | PTL | - | Bodnar et al, 2015 | Moderate |
|
| ||||||||
| Case control | China | 821 | On admission | ELISA | PTL | P<0.001 | Zhu et al, 2015 | High |
|
| ||||||||
| Case control | India | 46 cases and 46 controls | At delivery | ECLIA | C-section | OR 2.31 (0.77–6.92) | Sebastian et al, 2015 | Moderate |
|
| ||||||||
| Nested case control | Sweden | 39 cases and 120 controls | 12 weeks | ELISA | Preeclampsia | p=0.3 | Gidlof et al, 2015 | High |
|
| ||||||||
| Nested case control | Canada | 169 cases and 1975 controls | <20 weeks | ELISA | Preeclampsia | OR 2.23 (1.29–3.83) | Achkar et al, 2015 | Moderate |
|
| ||||||||
| Nested case control | USA | 400 | 15–21 weeks | LC-MS | Preeclampsia | OR 1.4 (0.7–3.0) | Wetta et al, 2014 | Moderate |
| Spontaneous preterm birth | OR 1.3 (0.6–3.0) | |||||||
|
| ||||||||
| Nested case control | Australia | 5109 | 10–14 weeks | CLIA | Adverse pregnancy outcomes (SGA, preterm births, preeclampsia, GDM, miscarriage and stillbirth) | OR 1.20 (0.69–2.07) | Schnueur et al, 2014 | High |
|
| ||||||||
| Nested case control | USA | 135 cases and 517 controls | On enrollment (Avg 16 weeks) | LC-MS/MS | GDM | P<0.05 | Arnold et al, 2015 | Low |
|
| ||||||||
| Cross sectional study | Poland | 280 | 11–13 weeks | ECLIA | Preeclampsia | Not significant | Bomba Opon et al, 2014 | Low |
|
| ||||||||
| Cross sectional study | USA | 1591 | 16.4–36.9 weeks (mean 27.9 weeks) | CLIA/RIA | Preeclampsia | Adjusted OR 1.14 (0.77–1.67) | Burris et al, 2014 | Moderate |
| Gestational hypertension | Adjusted OR 1.32 (1.01–1.72) | |||||||
|
| ||||||||
| Cross sectional study | Egypt | 160 | On enrollment | RIA | Glycemic control in GDM | P<0.05 | Lithy et al, 2014 | Low |
|
| ||||||||
| Cross sectional study | UK | 1467 | 26 weeks | LC-MS | GDM | NS | Whitelaw et al, 2014 | Moderate |
|
| ||||||||
| Cross sectional study | Turkey | 687 | 24–28 weeks | ELISA | Postpartum depression at | Gur et al, 2015 | Moderate | |
| 1 week | P=0.003 | |||||||
| 6 weeks | P=0.004 | |||||||
| 6 months | P<0.001 | |||||||
|
| ||||||||
| Retrospective cross sectional study | US | 133 | - | LC-MS/MS | Recurrent pregnancy loss | - | Ota et al, 2014 | Low |
|
| ||||||||
| Prospective study | Canada | 655 | 6–13 weeks | LC-MS/MS | GDM | OR 1.48, p=0.04 | Lacroix et al, 2014 | Moderate |
|
| ||||||||
| Prospective study | Korea | 523 | 12–14, 20–22, 32–34 weeks | ECLIA | GDM and pregnancy outcomes (Apgar, miscarriage, birth weight) | Adjusted OR 0.647. | Park et al, 2014 | Moderate |
|
| ||||||||
| Prospective cohort study | Netherlands | 2074 | 12 weeks | ELISA | Pregnancy related hypertensive disorders | OR 1.88 (0.79–4.48) | Weert et al, 2016 | Low |
|
| ||||||||
| Prospective cohort study | Spain | 2382 | Avg 13.5 weeks | HPLC | GDM | RR=0.92 (0.55,1.55) | Rodriguez et al, 2015 | High |
| PTL | p>0.4 | |||||||
| C-section | RR=0.60 (0.37,0.97) | |||||||
| FGR, SGA, HC, B wt, length | p>0.4 | |||||||
|
| ||||||||
| Prospective cohort study | Australia | 796 | 18 weeks | ELISA, LC-MS | Postpartum depression | OR 2.19 (1.26–3.78) | Robinson et al, 2014 | Moderate |
|
| ||||||||
| Cohort study | Australia | 1040 | At delivery | LC-MS/MS | Postpartum depression at | Gould et al, 2015 | Moderate | |
| 6 weeks | Adjusted RR 0.92, p=0.11 (0.84–1.02) | |||||||
| 6 months | Adjusted RR=0.96, p=0.41 (0.88–1.05) | |||||||
|
| ||||||||
| Cohort study | Singapore | 940 | 26–28 weeks | ID-LC-MS | GDM | (0.01–0.14) | Loy et al, 2015 | Moderate |
| C-section | OR 1.39 (0.95–2.05) | |||||||
|
| ||||||||
| Cohort study | China | 213 | 24–48 hrs after delivery | LC-MS/MS | Postpartum depression | P<0.0001, OR 7.17 (3.81–12.94) | Fu et al, 2015 | Moderate |
|
| ||||||||
| Retrospective cohort study | USA | 235 | 5–12 weeks | ELISA | Pre- eclampsia, PTL, GDM, spontaneous abortion | Adjusted OR 1.01 (0.961–1.057) | Flood-Nichols et al, 2015 | Low |
|
| ||||||||
| Cohort study | US | 2798 | <26 weeks | LC-MS/MS | C-section, prolonged labor and instrumental delivery | Gernand et al, 2015 | Moderate | |
|
| ||||||||
| Retrospective cohort study | UK | 995 | 11–13 weeks | LC-MS/MS | Mode of delivery | P=0.53 | Savvidou et al, 2012 | Moderate |
Abbreviations used: Vit D (vitamin D), ELISA (Enzyme Linked Immune sorbent Assay), CLIA (Chemiluminescent Immunoassay), LC-MS/MS (Liquid Chromatography tandem Mass Spectrometry), HPLC (High performance liquid chromatography), RIA (Radio Immune sorbent Assay), GDM (gestational diabetes mellitus), PTL (Preterm labor), SGA (small for gestational age), B Wt (birth weight), HC (head circumference), C-section (Cesarean section), FGR (Fetal growth rate)
Table 2.
Observational studies of vitamin D status in pregnancy and fetal outcomes
| Study design | Place of study | Sample size | GA at vit D assay | Vit D assay method | Fetal Outcome | Statistics (CI) | Study authors | Quality of evidence (GRADE system) |
|---|---|---|---|---|---|---|---|---|
| Case control | Iran | 40 cases | At delivery | ELISA | SGA | P=0.003 | Mirzaei et al, 2015 | Moderate |
|
| ||||||||
| Case control | Turkey | 30 cases and 30 controls | On admission | LC-MS/MS | LRTI in infants | P=0.001 | Dinlen et al, 2016 | Moderate |
|
| ||||||||
| Case control | India | 60 cases and 60 controls | At delivery | ELISA | Birth weight | P=0.004 | Nandal et al, 2015 | Moderate |
|
| ||||||||
| Nested case control | Canada | 607 cases and 1027 controls | At delivery | CLIA | Neonatal outcomes (LBW, SGA, preterm births) | OR 0.47 (0.23–0.97) | Morgan et al, 2016 | Moderate |
|
| ||||||||
| Cohort study | China | 1491 | At delivery | RIA | Birth weight and risk of SGA | P<0.001 | Zhu et al, 2015 | Moderate |
|
| ||||||||
| Cohort study | China | 3658 | Anytime during pregnancy | RIA | Birth weight | r=0.477, p<0.001 | Chen et al, 2015 | Moderate |
|
| ||||||||
| Cohort study | Spain | 2358 | 13–15 weeks | HPLC | Fetal growth and risk of overweight. | Morales et al, 2015 | High | |
| AC >= 90th percentile | OR 1.50, P=0.041 (1.01–2.21) | |||||||
| EFW >= 90th percentile | OR 1.47, P=0.046 (1.00–2.16) | |||||||
|
| ||||||||
| Cohort study | Taiwan | 164 | Before delivery | LC-MS/MS | Allergic disorders | Chiu et al, 2015 | Moderate | |
| Eczema (at age 4) | OR 0.12, p=0.012 (0.02–0.63) | |||||||
| Asthma (at age 4) | OR 0.22, p=0.038 (0.06–0.92) | |||||||
|
| ||||||||
| Cohort study | Denmark | 32456 | Mid pregnancy | - | Allergic diseases and asthma | P=0.21 | Maslova et al, 2014 | Low |
|
| ||||||||
| Prospective cohort | Germany | 777 | At delivery | EIA | Risk of LRTIs | Adjusted RR 1.32 (1.00, 1.73) | Luczynska et al, 2014 | Moderate |
|
| ||||||||
| Cross sectional | Turkey | 152 | Anytime during pregnancy | ELISA | Perinatal outcomes | Aydogmus et al, 2015 | Low | |
| C-section | p=0.176 | |||||||
| Perinatal complications | OR 4.30 (1.85–9.99) | |||||||
| SGA | OR 4.7 (1.41–15.78) | |||||||
|
| ||||||||
| Cross sectional | Ireland | 94 | At delivery | Vit D total automated competitive binding protein assay | Respiratory outcomes in preterm infants | Onwuneme et al, 2015 | Low | |
| Oxygen requirement | P=0.008 | |||||||
| Need for assisted ventilation | P=0.13 | |||||||
| Duration of IPPV | P=0.32 | |||||||
|
| ||||||||
| Cross sectional | Iran | 102 | At delivery | EIA | LBW | P=0.001 | Khalessi et al, 2015 | Moderate |
|
| ||||||||
| Cross sectional | Australia | 225 | At delivery | LC-MS/MS | Immunity and Allergies | Jones et al, 2015 | Moderate | |
| Response to HDM by | ||||||||
| IL-13 | P=0.002 | |||||||
| IL-5 | P=0.001 | |||||||
| IL-5 response to OVA | P=0.009 | |||||||
|
| ||||||||
| Cohort study | US | 792 | 12–26 weeks | LC-MS/MS | SGA | P=0.028 | Gernand et al, 2014 | Moderate |
Abbreviations used: GA (Gestational age), Vit D (vitamin D), ELISA (Enzyme Linked Immune sorbent Assay), CLIA (Chemiluminescent Immunoassay), LC-MS/MS (Liquid Chromatography tandem Mass Spectrometry), HPLC (High performance liquid chromatography), RIA (Radio Immune sorbent assay), EIA (Enzyme Immune assay), GDM (gestational diabetes mellitus), GHTN (Gestational Hypertension), SGA (small for gestational age), LBW (low birth weight), HC (head circumference), CI (Confidence Interval), OR (Odd’s Ratio), RR (Risk Ratio), IPPV (Intermittent positive pressure ventilation), AC (Abdominal circumference), ADHD (attention deficit hyperactivity disorder), EFW (estimated fetal weight), LRTI (Lower respiratory tract infections), C-section (Cesarean section), HDM (House dust mites), OVA (ovalbumin).
Table 3.
Randomized Controlled Trials of vitamin D supplementation in pregnancy
| Place of study | No. of participants | Intervention | Vit D assay method | Duration of intervention | Outcomes | Study authors | Quality of evidence (GRADE system) |
|---|---|---|---|---|---|---|---|
| Iran | 50 | Placebo Calcium 1000 mg/day + Vit D3 50,000 U on day 1 and 21 |
ELISA | 6 weeks | Metabolic profile of women with GDM | Asemi et al, 2013 | High |
| Australia | 209 | LD vit D3 400 U/day HD vit D3 5,000 U/day |
CLIA | till delivery | Primary: GDM Secondary: maternal and neonatal outcomes |
Yap et al, 2014 | High |
| Iran | 500 | LD vit D 400 U/day HD vit D 50,000 U/day |
- | till delivery | GDM, GHTN, pre- eclampsia, preterm, LBW. | Mojibian et al, 2015 | High |
| Pakistan | 193 | FeS04 200mg + Calcium 600mg/day + Vit D3 4,000 U/day | CLIA | 20 weeks to delivery | Pregnancy outcomes (GDM, GHTN, preterm labor, SGA, B wt, length, HC, Apgar at 1 and 5 min) | Hossain et al, 2014 | High |
| India | 180 | No intervention Vit D3 120,000 IU at 20, 24, 28 and 32 weeks |
ELISA | Preterm, pre- eclampsia, GDM, LBW and Apgar scores at 1 and 5 minutes | Sablok et al, 2015 | High | |
| Iran | 60 | Placebo Calcium 1000mg/day + vit D3 50,000 IU every 2 weeks |
ELISA | 12 weeks | Metabolic profiles in women at risk of pre- eclampsia | Samimi et al, 2015 | High |
| Iran | 46 | Placebo MM 800mg Ca+ 200mg Mg+ 8mg Zn + Vit D3 400 IU |
ELISA | 9 weeks | Outcomes in Pre- eclampsia patients | Asemi et al, 2015 | High |
| Iran | 60 | Placebo Vit D3 50,000 IU every 2 wks |
ELISA | 12 weeks | Metabolic profile and outcomes in pre-eclampsia patients | Karamali et al, 2015 | High |
| US | 440 | Placebo+ MV with Vit D 400 IU/day Vit D +MV with Vit D 4400 IU/day |
CLIA, LC-MS | till delivery | Asthma and recurrent wheezing in offspring | Litonjua et al, 2016 | High |
| Japan | 164 | Placebo Vit D3 800 IU/day |
6 weeks | Allergies like infantile eczema, atopic dermatitis, food allergy and wheeze | Norizoe et al, 2014 | High | |
| Iran | 130 | MVI + Ca 400 IU/day MVI+ Ca 400 IU/day + Vit D3 50,000 IU/ week |
ELISA | 8 weeks | Fetal growth (length, HC, weight) | Hashemipour et al, 2013 | Moderate |
Abbreviations used: Vit D (vitamin D), MVI (Multivitamin Injection), ELISA (Enzyme Linked Immune sorbent Assay), CLIA (Chemiluminescent Immunoassay), LC-MS/MS (Liquid Chromatography tandem Mass Spectrometry), GDM (gestational diabetes mellitus), GHTN (Gestational Hypertension), SGA (small for gestational age), LBW (low birth weight), HC (Head circumference),
Table 4.
Summary of the various maternal-fetal outcomes studies with the level of evidence of association with vitamin D.
| Outcomes | Total no. of studies (observational) | Total no. of studies (interventional) | Quality of evidence (GRADE system) |
|---|---|---|---|
| Preeclampsia | 11 | 3 | High |
| Gestational Diabetes Mellitus | 8 | 6 | High |
| Cesarean section | 5 | 0 | Moderate |
| Preterm Delivery | 5 | 4 | Moderate |
| Recurrent pregnancy loss | 1 | 0 | Low |
| Postpartum Depression | 4 | 0 | Moderate |
| Low Birth weight and Small for gestational age | 9 | 2 | High |
| Respiratory infections | 5 | 0 | Moderate |
| Immunity and allergies | 4 | 2 | Moderate |
| Anthropometry | 1 | 4 | High |
| Autism | 0 | 1 | Moderate |
Adverse Maternal Outcomes associated with vitamin D deficiency
Preeclampsia
Preeclampsia is defined as newly diagnosed hypertension (systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg) after 20 weeks of gestation along with proteinuria (>300mg/day), and other organ dysfunction, including liver involvement, hematological disturbance, neurological or renal complications (Roberts et al, 2016). Pathogenesis is related to the release of angiogenic factors into maternal circulation, which causes inadequate remodeling and trophoblastic invasion of spiral arteries, and consequently shallow implantation and hypoxia, and even release of inflammatory mediators (Sircar et al, 2015; Singla et al, 2015). The protective role of vitamin D in preeclampsia can be explained by multiple mechanisms. One of them is the immunomodulatory role of calcitriol in regulating immune response. Defective control of effector T cells by regulatory T cells can lead to poor placental invasion, thereby leading to the release of placenta-derived vasoconstrictive factors, and consequently maternal hypertension and proteinuria (Hyppönen et al, 2013). Vitamin D helps in maintaining the immune homeostasis and thus prevents placental vasoconstriction and ultimately, preeclampsia (Hyppönen et al, 2013). Also, vitamin D regulates the endothelial and vascular smooth muscle cell proliferation and therefore, plays a role in regulating blood pressure through RAAS (Renin-Angiotensin-Aldosterone system) (Hyppönen et al, 2013). Vitamin D has also been found to regulate angiogenesis via its receptor elements in VEGF promoter and stimulating the expression of VEGF in vascular smooth muscle cells. Calcitriol also helps to prevent cholesterol uptake by the macrophages and vascular smooth muscle cells of the arterial walls, which is the observed pathology in utero placental vessels of patients with preeclampsia (Hyppönen et al, 2013). Despite these findings, in other studies no causal relationship was found between vitamin D supplementation and improvement/prevention of preeclampsia. These studies are discussed below.
Several observational studies were recently performed to investigate an association between vitamin D deficiency and preeclampsia but the findings have been inconsistent. Eleven observational studies have examined the association between preeclampsia and vitamin D; Achkar et al (2015) carried out a nested case control study in Canada and concluded that vitamin D deficiency (<30 ng/ml) was associated with increased risk of preeclampsia (adjusted Odd’s Ratio (OR), 2.23; 95% Confidence Interval (CI) 1.29–3.83). Similar results were also reported by other recent studies (Singla et al, 2015; Mohaghegh et al, 2015; Abedi et al, 2014; Sadin et al, 2015). On the contrary, Bomba-Opon et al (2014) measured the 25OHD levels in 280 pregnant women in Poland and found no association between vitamin D levels and markers of preeclampsia, including Pregnancy-associated plasma protein A (PAPP-A), Placenta Growth Factor (PlGF), uterine artery pulsatility index and mean arterial pressure. Similarly, Schneuer et al(2014) reported no increased risk of preeclampsia in vitamin D deficient pregnant women in a nested case control study conducted in 5109 pregnant women. Some other studies also described comparable outcomes (Gidlof et al, 2015; van Weert et al, 2016; Wetta et al, 2014; Burris et al, 2014).
Many randomized controlled trials (RCT) have been undertaken to determine the impact of vitamin D supplementation in preeclampsia patients. In the past two years, three of the investigations took place in Iran (Mojibian et al, 2015; Samimi et al, 2015; Asemi et al, 2015)and one each in India (Sablok et al, 2015) and Pakistan (Hossain et al, 2014). Positive impact was found in some (Samimi et al, 2015; Asemi et al. 2015; Sablok et al, 2015)while no significant impact was reported in others (Hossain et al, 2014). Similarly, Karamali et al (2015) conducted a double blind placebo-controlled RCT in pregnant women at risk of preeclampsia determined by abnormal uterine artery waveform, and reported no significant change in blood pressure with vitamin D supplementation. When combined supplementation of vitamin D and calcium was given in an interventional study to at-risk preeclampsia patients, significant improvement in the outcomes with reduced systolic and diastolic blood pressure was observed in the vitamin D and calcium supplemented group compared to the placebo group (Samimi et al, 2015). Similarly, Asemi et al (2015) also reported positive outcomes on supplementation with vitamin D (400 IU) and multi-minerals (800 mg calcium, 200 mg magnesium, 8 mg zinc) over 9 weeks compared to placebo in at-risk preeclampsia patients. Recently, the published Cochrane review on vitamin D supplementation studies reported that women who receive vitamin D supplementation had lower risk of preeclampsia but with only borderline significance (RR 0.52, CI 0.25–1.05), whereas combined vitamin D and calcium supplementation significantly reduces the risk of preeclampsia (Risk Ratio (RR): 0.51, CI 0.32–0.80) (De-Regil et al, 2016). Our analysis also supports the conclusion of Cochrane review that vitamin D supplementation reduces the risk of preeclampsia.
Gestational Diabetes Mellitus (GDM)
Gestational diabetes mellitus is defined as hyperglycemia from glucose intolerance that develops or is first diagnosed during pregnancy (Baz et al, 2016). Vitamin D plays a role in glucose homeostasis by multiple mechanisms (Lithy et al, 2014). Firstly, it regulates the calcium levels, which in turn regulates insulin production and secretion by the endocrine pancreas (Al-Shoumer et al, 2015). It also improves the sensitivity of the target cells like adipose tissue, liver and skeletal muscles to insulin (Lithy et al, 2014). Through its role in regulation of immune cells, it protects β cells from detrimental immune attacks and even enhances their function (Al-Shoumer et al, 2015). Vitamin D deficiency or the dysfunction of vitamin D receptors relates to the pathogenesis of type 1 and type 2 diabetes, but its role in GDM remains inconclusive.
Eight observational studies have been recently conducted to study the association of vitamin D with GDM. Arnold et al(2015) conducted a nested case control study in the United States among pregnant women and reported an inverse relationship between vitamin D status in early pregnancy and risk of gestational diabetes. A 5 ng/ml increase in vitamin D3 levels was associated with reduction in GDM risk by 14%. Similar effects were reported by Lacroix and colleagues (2014) in a prospective observational study in Canada and Lithy et al(2014) in a cross sectional observational study in Arab women. On the contrary, multiple studies failed to establish a role of vitamin D in the prevention of GDM (Schneuer et al, 2014; Sunmin et al, 2014; Whitelaw et al, 2014; Loy et al, 2015; Flood-nichols et al, 2015). The lack of consistent findings calls for large-scale prospective studies to evaluate the role of vitamin D in GDM.
To test the effectiveness of vitamin D supplementation in the prevention or reduction of risk of GDM, many randomized controlled clinical trials have been conducted. Six RCTs have been conducted in this regard from January 2014 onwards (Mojibian et al, 2015; Sablok et al, 2015; Hossain et al, 2014; Asemi et al, 2014; Asemi et al, 2013; Yap et al, 2014). Asemi et al(2014) performed a randomized placebo controlled trial in Iranian pregnant women and found positive impact of vitamin D and calcium supplementation on the metabolic profiles of GDM patient with decrease in free plasma glucose, serum insulin, HOMA–IR, LDL cholesterol and total cholesterol. Few other studies, on the contrary, reported no beneficial effects of vitamin D supplementation on GDM outcome (Sablok et al, 2015; Hossain et al, 2014). To compare and contrast the efficacy of different dosing patterns of vitamin D on GDM, recently two clinical trials were undertaken; Yap et al (2014) conducted the study in women with 25OHD levels less than 32 ng/ml before 20 weeks and randomized them to receive oral vitamin D3 at 5,000 IU daily or 400 IU daily from 14 weeks until delivery, and found no difference in the outcomes. Mojibain et al (2015) randomized pregnant women with 25OHD less than 30 ng/ml to receive 400 IU daily or 50,000 IU every 2 weeks until delivery, and found improved outcomes with high dose vitamin D supplementation. The difference in the outcomes could be attributed to different dosages of vitamin D used for intervention, as well as the varying dosing schedules in the two studies. Two recently published meta-analyses of RCTs (De-Regil et al, 2016; Pérez-López et al, 2015), however, showed same incidence of GDM with and without vitamin D supplementation. While studies have shown varying results, the overall level of evidence is high for vitamin D supplementation playing no role in the prevention of GDM.
Cesarean section (C-section)
Vitamin D receptors are present on smooth muscle cells, including uterine muscles, and skeletal muscle cells. Vitamin D regulates the contractile proteins of uterine myometrial cells (Loy et al, 2015). Vitamin D deficiency, therefore, may decrease the strength of the contractile muscles, causing prolonged labor or obstructed labor, indicating the need for C-section (Sebastian et al, 2015). Vitamin D deficiency has also been postulated to cause malformation of pelvis, which is yet another indication for C-section (Gernand et al, 2015).
Few studies have analyzed the association between vitamin D levels and cesarean sections. Recently, five observational studies were conducted with mixed outcomes (Loy et al, 2015; Sebastian et al, 2015; Gernand et al, 2015; Rodriguez et al, 2015). In a prospective cohort study conducted in Spain in 2,382 mother child pairs, Rodriguez et al (2015) reported that adequate vitamin D (25 OHD ≥ 30ng/ml) decreased the risk of C-section by obstructed labor (RR=0.60, 95% CI 0.37, 0.97). In a multiethnic Asian cohort study conducted by GUSTO study group (Loy et al, 2015), it was found that low vitamin D levels were associated with increased risk of emergency C-sections in Chinese and Indian women, and not in Malay women and overall there was no significant association. No association between vitamin D deficiency and risk of C-section was reported in other recent studies (Sebastian et al, 2015; Gernand et al, 2015). The inconsistency in the findings might be due to the difference in defining C-section in terms of indication, primary or secondary, emergency or elective (Gernand et al, 2015).
There have not been any interventional studies undertaken to determine the association between the two. Although analysis of the recent observational studies suggests that vitamin D deficiency can increase the risk of C section, there is a need for investigators to conduct RCTs to study the impact of vitamin D supplementation on C-section rates.
Preterm Delivery
One of the most common causes for preterm delivery is infection (Sangkomkamhang et al, 2015). Vitamin D, through its role in anti-inflammatory pathways via nuclear factor-kB inhibition, could play a role in decreasing the incidence of infections thereby, preterm births (Thota et al, 2014). But the exact role of vitamin D in the pathogenesis of preterm birth has not yet been clearly defined. Five observational and four interventional studies have investigated their association with lack of consistency in the results. Bodnar et al (2015) conducted a case control study with 1,126 cases and 2,327 controls in Pittsburgh, PA, USA. Serum 25OHD was measured using liquid chromatography- tandem mass spectroscopy and a protective association of vitamin D sufficiency and preterm birth was found after adjusting confounding factors. Thota et al (2014) reported similar results. However, other studies did not support the above findings (Schneuer et al, 2014; Wetta et al, 2014; Rodriguez et al, 2015).
Few recent interventional studies on vitamin D supplementation examined its role in the prevention of preterm births. Hossain et al(2014) carried out a RCT of routine care (200 mg ferrous sulfate and 600 mg calcium) vs vitamin D3 supplementation (4,000 IU daily) from 20 weeks of gestation till delivery in 207 pregnant women and reported comparable outcomes in both the groups (p >0.05). Mojibian et al(2015) also reported similar findings. On the contrary, Sablok et al (2015) found decreased incidence of preterm delivery in the group with vitamin D supplementation (8.3%) compared to no intervention (21.1%) though it was not statistically significant. Wagner et al(2015) carried out a post hoc analysis to measure the strength of association between serum 25OHD levels at 3 times, one in each trimester and preterm birth. They found that maternal vitamin D status closest to the delivery was most significantly associated with preterm birth, thereby proposing that later intervention could be used as a rescue treatment to decrease the risk of preterm deliveries (Wagner et al, 2015). Meta-analysis carried out by Pérez López et al (2015) showed no significant impact of vitamin D supplementation on the prevention of preterm deliveries. Though the level of evidence is moderate, our analysis shows no significant association between vitamin D and preterm deliveries.
Recurrent pregnancy loss
One of the non-classical functions of vitamin D is the regulation of immune system. To maintain maternal fetal interaction for a successful pregnancy, a dominant Th2 cell response is required (Garrido-gimenez et al, 2015). Therefore, dysregulated immune function and autoimmunity could alter the immune response leading to pregnancy loss. Vitamin D reportedly inhibits Th1 cells-mediated immune response and also the production of their cytokines, including IFN-γ, IL-2 and TNF-α, while promoting the proliferation of Th2 cells and their cytokines, including IL-4, IL-5, IL-6, IL-9, IL-13 (Wei et al, 2015). Such an effect of vitamin D supports its role in the immunoregulation during implantation. This hypothesis was examined by Ota et al, (2014) who conducted a retrospective study of 133 pregnant women with a history of three or more pregnancy loss before 20 weeks of gestation. Serum vitamin D levels and the markers of immunity were measured. The 47.4% of these women had low vitamin D levels (<30 ng/ml). The levels of autoantibodies were significantly higher in vitamin D-deficient group. These include anti-phospholipid antibody (APA) (p<0.005, adjusted OR 2.22, CI 1.0–4.7), antinuclear antigen antibody, anti-ssDNA and thyroperoxidase antibody. This was also followed by in vitro experiments and significantly decreased ratio of TNF-α/IL-10 expressing CD3+/CD4+ cells with 100 nM of vitamin D3 (31.3 ± 9.4 ng/ml, p<0.05) was found in cases compared to controls (40.4± 11.3 ng/ml). Although no conclusion can be drawn based on the findings of a single study, but this calls for more observational and interventional studies to establish the association between the two.
Postpartum depression
Postpartum depression is the most common psychiatric condition occurring in the postpartum period (Cherry et al, 2014). Vitamin D is believed to be a neurosteroid and has been linked to the occurrence of depression (Anglin et al, 2013). There are various plausible mechanisms relating postpartum depression with vitamin D deficiency. Firstly, VDRs are located throughout the body including brain, so, in case of vitamin D-deficiency, the VDRs in the brain may affect the hormones that are involved in the occurrence of mood disorders (Fu et al, 2015). Vitamin D also plays a role in brain processes like neurotransmission, neuro-immunomodulation and neuroprotection (Christesen et al, 2012). Thirdly, vitamin D is involved in the synthesis of norepinephrine and dopamine, which gets imbalanced in mood disorders (Eyles et al, 2013). Additionally, vitamin D maintains the antioxidant glutathione in brain, thereby protecting brain from oxidative damage. Lastly, VDRs are present in the areas of brain involved in planning, processing and formation of new memories (Eyles et al, 2013).
Many studies have been designed to investigate an association between postpartum depression and vitamin D. Recently, four observational studies were performed in this regard. Fu et al(2015) conducted a prospective cohort study in 248 pregnant women in China to examine an association between serum vitamin D levels 24 hours after delivery and postpartum depression. The investigators reported that serum 25OHD levels were significantly higher in women with no postpartum depression (p<0.001). After adjusting for confounders and using multivariate analysis, they found increased risk of postpartum depression with 25OHD levels ≤ 10.2 ng/ml (OR 7.17, CI 3.81–12.94, p< 0.001). Gur et al(2015) reported a significant association between mid-pregnancy vitamin D levels and postpartum depression. They conducted this study in 678 Turkish pregnant women, measured serum 25OHD level between 24–28 weeks of gestation and then assessed these women for postpartum depression using Edinburgh Postnatal Depression Scale (EPDS) at 1 week, 6 weeks and 6 months postpartum. The investigators also found significant negative correlation between vitamin D levels and EDPS at all the three follow-up periods (r= −0.2, −0.2, −0.3, respectively). Robinson et al (2014) also demonstrated a positive association between vitamin D deficiency and postpartum depression. On the contrary, Gould et al(2015) performed a similar study on a large cohort of 1,040 women in Australia. They measured cord blood 25OHD and then evaluated these women for postpartum depression symptoms using EPDS at six weeks and six months postpartum. They reported no association between cord blood vitamin D levels and postpartum depression. Although the results from various studies are conflicting, postpartum depression might be associated with vitamin D deficiency but the studies have been inconsistent in their results and warrant more extensive studies in this regard.
Adverse fetal outcomes associated with vitamin D deficiency
Low birth weight (LBW) and Small for Gestational Age (SGA)
Vitamin D plays a role in fetal growth by regulating calcium homeostasis and parathyroid hormone levels (Hollis et al, 2011). Maternal vitamin D adequacy improves the fetal outcomes in the form of birth weight sufficiency that has been proven by many but not all studies. Most of them are observational studies. In our search, we found 9 studies that reported this correlation; 3 were cross sectional studies, 3 were case control studies and 3 were prospective cohorts. Chen et al(2015) performed a population-based cohort, recruiting 3,658 mother-fetus pairs. Maternal serum 25OHD level was measured at anytime during pregnancy. A positive correlation was found between maternal 25OHD levels and neonatal birth weight (r = 0.477, p <0.001). Similar results were reported by other investigators (Khalessi et al, 2015; Morgan C et al, 2016; Gernand et al, 2014; Mirzaei et al, 2015; Aydogmus et al, 2016). Another study undertaken in China by Zhu et al(2015) measured the cord blood 25OHD levels and its association with low birth weight and SGA in 1,491 neonates. They reported that per 10 nmol/L increase in the cord blood 25OHD level, birth weight increased by 61.0 gm at concentration less than 40 nmol/L of 25OHD, and then decreased by 68.5 gm at concentrations from 40–70 nmol/L. This is the first study to report inverted U-shaped relationship between neonatal vitamin D status and fetal growth. However, other studies failed to establish any association between vitamin D levels and fetal birth weight (Schneuer et al, 2014; Rodriguez et al, 2015). Conflicting results were found in the interventional studies for vitamin D supplementation and its impact on offspring birth weight. One of these studies conducted by Mojibian et al (2015) in Iranian pregnant women found no difference on vitamin D supplementation on the fetal outcomes. Another RCT carried out by Hossain et al(2014) found improved outcomes with vitamin D supplementation, but the results did not reach statistical significance. Thus, most of the findings suggest that vitamin D deficiency increases the risk of low birth weight infants. However, large scale well-designed interventional studies are required to further establish the role of vitamin D supplementation to improve fetal outcomes.
Respiratory infections
Vitamin D receptors (VDRs) are present on almost every immune cells, and activation of VDRs play a critical role in adaptive and innate immunity, along with the regulation of inflammatory response (Esposito et al, 2015). There are multiple mechanisms by which vitamin D could regulate inflammatory response: (i) vitamin D controls the activity of macrophages and dendritic cells and various events in response to the activation of Toll-like receptors in neutrophils (Greiller et al, 2015), (ii)vitamin D inhibits the function of dendritic cells by restricting their maturation, antigen presentation and the production of cytokines, like IL-12 and IL-23 (Esposito et al, 2015), (iii) vitamin D induces the expression of two antimicrobial peptides, cathelicidin and β-defensin, which are critical in innate immunity through chemotactic action and neutralization of toxins (Sá et al, 2015), and (iv) vitamin D shifts the cytokine expression from type 1 to type 2 cells response, thus contributing to the maintenance of self-tolerance (Wei et al, 2015). Thus, based on the potent immunomodulatory effect of vitamin D, several studies have linked vitamin D deficiency to the increased risk of respiratory tract infections in children. It has been hypothesized that maternal vitamin D deficiency increases the risk of respiratory tract infections in their offspring. Few investigators recently studied this association. Five observational studies have been conducted. Onwuneme et al(2015) performed a study in 94 preterm infants and found that low serum 25OHD levels (<30 nmol/L) in pre-terms were associated with increased oxygen requirements (p=0.008), increased duration of intermittent positive pressure ventilation (p=0.032), and more requirement of assisted ventilation (p=0.013). Luczynska et al (2014) performed a prospective cohort study on 777 mother-child pairs in Germany and found a statistically significant association between 25OHD levels in cord blood and susceptibility to lower respiratory tract infection (LRTI). The adjusted risk-ratio for vitamin D deficient infants (<25 nmol/L) was 1.32 in comparison to the reference group (>50 nmol/L). In Turkey, Dinlen et al(2016) reported the same conclusions. They performed a study in 60 infants, 30 with acute LRTI term infants and 30 controls matched for gestational age, weight and gender and reported lower serum 25OHD levels in mothers of the study group (p=0.0001). However, Jongh et al(2014) reported no association between the two based on a cohort study performed on 2,025 mother child pairs in Southampton, UK with mother’s serum 25OHD measured at 34 weeks’ gestation and infants followed up at 6,12 and 24 months for respiratory symptoms. In summary, maternal vitamin D deficiency can increase the risk of respiratory infections in infants. This conclusion is yet to be confirmed by interventional studies.
Immunity and Allergies
As mentioned earlier, Vitamin D plays an important role in the immunomodulation through its effect on the function of T and B lymphocytes. While in T cells, it promotes Th2 cell-mediated anti-inflammatory response, in B cells vitamin D promotes the production of immunoglobulins and inhibition of memory B cells and generation of plasma cells (Szymczak et al, 2016).
To validate the association between maternal vitamin D status and development of allergies, including eczema and asthma in their offspring, recently four observational studies and two interventional studies have been carried out. In Taiwan, a study was conducted in 164 mother-child pairs cohort to study this association (Chiu et al, 2015) and reported protective effect of vitamin D in protecting children from allergies, eczema and asthma after following up children for up to 4 years of age. Maslova et al(2014) conducted a prospective cohort study in Denmark in 965 pregnant women and their offspring were followed until adulthood for airway allergic diseases and lung function. In this study, higher levels of maternal vitamin D ≥ 125 nmol/L was found to be associated with higher incidence of allergies in children (Hazard Ratio, HR=1.81, 95% CI = 0.78–4.16). In UK, Miller et al(2015) studied the association between maternal vitamin D and neonatal inflammatory and allergic response by studying the response of neonatal nasal airway epithelial cells (AECs). They reported that increased maternal vitamin D was associated with increased release of IL-10 by AECs after stimulation with TNF-α/IL-1β (p=0.024) or house dust mite (p=0.049). It was concluded that there exists an association between maternal micronutrient intake and the function of neonatal airway cells and hence may impact the development of asthma or allergies later in life. Jones et al (2015) selected a cohort of mothers with history of atopy and investigated the association of vitamin D in them to the immune response in their children and found that improving serum 25OHD level during pregnancy or early infancy can lower the risk of allergies by inhibiting the inflammatory cytokines associated with allergies.
This association has been of interest for carrying out interventional studies as well. Vitamin D Antenatal Asthma Reduction Trial (VDAART) (Litonjua et al, 2016) was a randomized, double blind, placebo controlled trial conducted across three centers in the United States. A total of 881 pregnant women at 10–18 weeks’ gestation at high risk of having children with asthma were included in the study. They reported that the risk of asthma and wheeze-related disorders were lower in vitamin D supplemented women by 6.1% compared to the placebo group, although it did not reach statistical significance. Another interventional study took place in Japan (Norizoe et al, 2014) which studied the impact of maternal vitamin D supplementation during lactation to improve the outcomes in infantile allergic disorders like eczema. They concluded that although vitamin D supplementation did not decrease the incidence of eczema, but it enhanced the risk of food allergies up to 2 years of age (RR 3.42, 95% CI: 1.02–11.77; p=0.030). In conclusion, there is still no definite evidence of whether vitamin D supplementation increases or decreases the risk of allergies and therefore, requires further exploration.
Anthropometry
Vitamin D plays a primary role in bone remodeling, calcium homeostasis and muscle functioning. Its deficiency in pregnancy is associated with impaired bone development, fetal growth and other unfavorable effects on musculoskeletal system of the offspring (Kovacs et al, 2014).
Some studies have shown adverse effects of maternal vitamin D deficiency on the anthropometric measures of the offspring. This includes one cohort study (Morales et al, 2015) and four RCTs (Hashemipour et al, 2013; Hossain et al, 2014; Nandal et al 2015; Roth et al, 2015). Morales and colleagues(2015) conducted a study in a cohort of 2,358 pregnant women in Spain and measured serum 25OHD levels at any time during gestation. Child’s femur length, bi-parietal diameter and abdominal circumference were noted through ultrasound findings at 12, 20 and34 weeks of gestation. They found no association of maternal serum 25OHD level with femur length and a weak inverse association with bi-parietal diameter at 34 weeks.
A single center randomized clinical trial in Pakistan reported no favorable outcomes on fetal growth by vitamin D supplementation (Hossain et al, 2014). Nandal et al(2015) conducted a case control study of 120 pregnant women in India and found that the women’s offspring supplemented with vitamin D had higher birth weight and crown heel length compared to the non-supplemented group (3.1 ± 0.485 kg vs 2.8 ± 0.705 kg and 49.35 ± 1.36 cm vs 48.67 ± 2.12 cm, respectively). Similarly, Hashemipour et al (2013) conducted a randomized controlled trial in 130 Iranian pregnant women and reported that mean length (p=0.01), head circumference (p=0.001) and weight (p=0.01) were higher in the intervention group compared to the control group. There is an ongoing trial in Dhaka, Bangladesh to estimate the effects of prenatal and postnatal supplementation of different doses of vitamin D vs placebo on infant length at 1 year of age (Roth et al, 2015). In this study, healthy pregnant women in second trimester have been randomized to receive either vitamin D 4,200 IU/wk, 16,800 IU/wk or 28,000 IU/wk and placebo in the postpartum period or 28,000 IU/wk in the prenatal and postpartum period, and the infants will be followed up at regular intervals for growth monitoring upto 1 year of age. A recently published meta-analysis of RCTs by Pérez-López et al (2015) also concludes positive outcomes of vitamin D supplementation in pregnancy on offspring birth weight and length. Thus, our analysis of the published findings also supports that vitamin D supplementation in pregnancy has a positive impact on fetal growth.
Vitamin D and autism
A recent meta-analysis by Wang et al (2015) investigated the possible mechanisms by which vitamin D could potentially influence the occurrence of autism spectrum disorders in children. Firstly, it influences the early brain development in children. It plays a role in neuronal differentiation, neurotransmission and synaptic functions (Wang et al, 2015). Secondly, as vitamin D is involved in the immune system homeostasis and autism is considered an autoimmune disorder, so it is considered that vitamin D deficiency may alter T cell activation profile and affect the adaptive immunity and cause preponderance to autism (Currenti, 2010). Also, oxidative stress may increase the susceptibility to autism by their lethal interaction with genetically susceptible genes. Glutathione is an antioxidant that protects the brain from the oxidative stress and vitamin D increases the level of glutathione in the brain, thereby protecting it from preventable conditions like autism (Cannell, 2008). Serotonin plays an essential role in controlling emotions. Vitamin D activates the serotonin-synthesizing gene in the brain and inversely affects the serotonin production in peripheral tissues (Patrick et al, 2014). It is seen that autistic individuals have lower concentration of serotonin in brain and higher in their blood, indicating that vitamin D could be potentially involved in the underlying pathogenesis. Lastly, vitamin D deficiency could increase the risk of genetic mutations by inhibiting DNA repair of early mutations, and thus could contribute to the appearance of autism (Wang et al, 2015).
An interventional study recently reviewed the benefits of vitamin D supplementation in pregnant women to decrease the incidence of autism in the offspring. This was conducted by Stubbs et al(2016) whereby the investigators supplemented pregnant women with previous autistic child, with 5,000 IU/day of vitamin D followed by supplementation of newborn with 1,000 IU/day vitamin D for first three years of life. These children were followed up at 18 and 36 months of age. The results were promising, with only 1 out of 19 children developing autism (5%), compared to the general recurrence risk of 20%. This single recent study suggests that vitamin D supplementation in pregnancy could reduce the risk of autism in children, but more studies are warranted to confirm the conclusion.
CONCLUSION
Vitamin D deficiency is increasing worldwide, both in general population and in pregnant women. In pregnancy, it has been found to be associated with increased incidence of adverse maternal and fetal outcomes, primarily preeclampsia, GDM, low birth weight and preterm births. Other outcomes are still under study and no definite conclusions have been drawn yet. The critical evaluation of the published findings on the maternal and fetal outcomes in a large number of observational studies and critical trials in a large cohort provide high level of evidence for an association between vitamin D and preeclampsia, GDM in maternal outcomes and, small for gestational age and anthropometry in fetal outcomes (Table 4). Other maternal-fetal outcomes have moderate level of evidence of association with vitamin D, as reported in other reports and meta analyses. In our review of the findings, we also found association between vitamin D and recurrent pregnancy loss and association between vitamin D and autism, although the level of evidence remains low, thereby requiring additional studies to confirm the association. However, the question still remains whether there is any causal relationship between vitamin D deficiency and the maternal and fetal outcomes or it is just one of the manifestations of these conditions. The etiology of the various maternal and fetal outcomes is complex and multifactorial with many confounding factors. To determine the benefits of vitamin D supplementation in pregnancy would require further evaluation through large, multicenter double-blind randomized controlled clinical trials, with a focus on specific adverse pregnancy outcomes. Additionally, dose-response randomized trials would be helpful in identifying the optimal dosage for supplementation and potential long-term side effects of vitamin D therapy.
Acknowledgments
This work was supported by research grants R01HL116042, R01HL112597, and R01HL120659 to DK Agrawal from the Office of the Dietary Supplement, NIH Director’s Office and the National Heart, Lung and Blood Institute, NIH, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- Abedi P, Mohaghegh Z, Afshary P, Latifi M. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern Child Nutr. 2014;10(2):206–12. doi: 10.1111/mcn.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achkar M, Dodds L, Giguère Y, Forest J, Armson BA, Woolcott C, et al. Am J Obstet Gynecol [Internet] 4. Vol. 212. Elsevier Inc; 2015. Apr, Vitamin D status in early pregnancy and risk of preeclampsia; pp. 511.e1–7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-shoumer KAS, Al-essa TM. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J Diabetes. 2015;6(8):1057–64. doi: 10.4239/wjd.v6.i8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin RES, Samaan Z, Walter SD, Mcdonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Pschiatry. 2013;202:100–7. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Enquobahrie DA, Qiu C, Huang J. Early Pregnancy Maternal Vitamin D Concentrations and Risk of Gestational Diabetes Mellitus. Paediatr Perinat Epidemiol. 2015;29(3):200–10. doi: 10.1111/ppe.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi Z, Esmaillzadeh A. The Effect of Multi mineral – Vitamin D Supplementation on Pregnancy Outcomes in Pregnant Women at Risk for Pre – eclampsia. Int J Prev Med. 2015;6:62. doi: 10.4103/2008-7802.160975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial 1 – 3. Am J Clin Nutr. 2013;98(6):1425–32. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium – vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. 2014;57(9):1798–806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- Aydogmus S, Kelekci S, Aydogmus H, Eriş S, Yilmaz B, Sağlam G, et al. High prevalence of vitamin D deficiency among pregnant women in a Turkish population and impact on perinatal outcomes High prevalence of vitamin D deficiency among pregnant women in a Turkish population and impact on perinatal outcomes. J Matern Fetal Neonatal Med. 2015;28(15):1828–32. doi: 10.3109/14767058.2014.969235. [DOI] [PubMed] [Google Scholar]
- Baz B, Riveline J. Gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur J Endocrinol. 2015;174(2):R43–51. doi: 10.1530/EJE-15-0378. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Platt RW, Simhan HN. Early-Pregnancy Vitamin D Deficiency and Risk of Preterm Birth Subtypes. Obstet Gynecol. 2015;125(2):439–47. doi: 10.1097/AOG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomba-Opon DA, Brawura-Biskupski-Samaha R, Kozlowski S, Kosinski P, Bartoszewicz Z, Bednarczuk T, et al. First trimester maternal serum vitamin D and markers of preeclampsia. J Matern Fetal Neonatal Med [Internet] 2014 Jul;27(10):1078–9. doi: 10.3109/14767058.2013.846318. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24050181. [DOI] [PubMed] [Google Scholar]
- Brannon PM, Picciano MF. Vitamin D in Pregnancy and Lactation in Humans. Annu Rev Nutr. 2011;31:89–115. doi: 10.1146/annurev.nutr.012809.104807. [DOI] [PubMed] [Google Scholar]
- Burris HH, Rifas-Shiman SL, Huh SY, Kleinman K, Litonjua AA, Oken E, et al. Ann Epidemiol [Internet] 5. Vol. 24. Elsevier; 2014. May, Vitamin D status and hypertensive disorders in pregnancy; pp. 399–403.e1. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70(4):750–9. doi: 10.1016/j.mehy.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu L, Hao J, Yu Z, Zhu P, Wang H. Elevates the Risks of Small for Gestational Age and Low Birth Weight Infants in Chinese Population. J Clin Endocrinol Metab. 2015;100(5):1912–9. doi: 10.1210/jc.2014-4407. [DOI] [PubMed] [Google Scholar]
- Cherry AS, Mccaffree MA, Gillaspy SR. Postpartum depression on the neonatal intensive care unit: current perspectives. Int J Womens Health. 2014;6:975–87. doi: 10.2147/IJWH.S54666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Huang S, Peng Y, Tsai M, Hua M, Yao T, et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol. 2015;26(4):337–43. doi: 10.1111/pai.12384. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. VITAMIN D: METABOLISM, MOLECULAR MECHANISM OF ACTION, AND PLEIOTROPIC EFFECTS VITAMIN D ANALOGS. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christesen HT, Falkenberg T, Lamont RF, Jørgensen JANS, Christesen HT, Christian H. The impact of vitamin D on pregnancy: a systematic review. Acta Obstet Gynecol Scand. 2012;91(12):1357–67. doi: 10.1111/aogs.12000. [DOI] [PubMed] [Google Scholar]
- Currenti SA. Understanding and Determining the Etiology of Autism. Cell Mol Neurobiol. 2010;30(2):161–71. doi: 10.1007/s10571-009-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca HF. History of the discovery of vitamin D and its active metabolites. BoneKey Reports. 2014;3(479):1–8. doi: 10.1038/bonekey.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane database Syst Rev [Internet] 2016;1(1):CD008873. doi: 10.1002/14651858.CD008873.pub3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26765344. [DOI] [PubMed] [Google Scholar]
- Dinlen N, Zenciroglu A, Beken S, Dursun A, Dilli D. Association of vitamin D deficiency with acute lower respiratory tract infections in newborns Association of vitamin D deficiency with acute lower respiratory tract infections in newborns. J Matern Fetal Neonatal Med. 2016;29(6):928–32. doi: 10.3109/14767058.2015.1023710. [DOI] [PubMed] [Google Scholar]
- Dusilová-Sulková S. Vitamin D metabolism and vitamin D traditional and nontraditional, target organs: Implications for kidney patients. J Ren Care. 2009;35(SUPPL 1):39–44. doi: 10.1111/j.1755-6686.2009.00066.x. [DOI] [PubMed] [Google Scholar]
- Esposito S, Lelii M. Vitamin D and respiratory tract infections in childhood. BMC Infect Dis [Internet]. BMC Infectious Diseases. 2015:1–10. doi: 10.1186/s12879-015-1196-1. Available from: [DOI] [PMC free article] [PubMed]
- Eyles DW, Burne THJ, Mcgrath JJ. Frontiers in Neuroendocrinology Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol [Internet] 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001. Available from: [DOI] [PubMed] [Google Scholar]
- Flood-nichols SK, Tinnemore D, Huang RR. Vitamin D Deficiency in Early Pregnancy. 2015;1:1–15. doi: 10.1371/journal.pone.0123763. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Liu J, Tu W, Yang J, Cao Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG. 2015;122(12):1688–94. doi: 10.1111/1471-0528.13111. [DOI] [PubMed] [Google Scholar]
- Garrido-gimenez C, Alijotas-reig J. Recurrent miscarriage: causes, evaluation and management. Potgrad Med J. 2015;91(1073):151–62. doi: 10.1136/postgradmedj-2014-132672. [DOI] [PubMed] [Google Scholar]
- Gernand AD, Klebanoff MA, Simhan HN, Bodnar LM. Maternal vitamin D status, prolonged labor, cesarean delivery and instrumental delivery in an era with a low cesarean rate. J Perinatol. 2015;35(1):23–8. doi: 10.1038/jp.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Simhan HN, Bodnar LM, Caritis S. Maternal Vitamin D Status and Small-for-Gestational-Age Offspring in Women at High Risk for Preeclampsia. Obstet Gynecol. 2014;123(1):40–8. doi: 10.1097/AOG.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlöf S, Silva AT, Gustafsson S, Lindqvist PG. Vitamin D and the risk of preeclampsia--a nested case-control study. Acta Obstet Gynecol Scand [Internet] 2015 Aug;94(8):904–8. doi: 10.1111/aogs.12658. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25884254. [DOI] [PubMed] [Google Scholar]
- Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Gibson RA, et al. Association of cord blood vitamin D at delivery with postpartum depression in Australian women. Aust N Z J Obstet Gynaecol. 2015;55(5):446–52. doi: 10.1111/ajo.12344. [DOI] [PubMed] [Google Scholar]
- Grant ACC, Alistair W. Vitamin D During Pregnancy and Infancy and Infant Serum 25-Hydroxyvitamin D Concentration. Pediatrics. 2014;133(1):e143–53. doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- Greiller CL, Martineau AR. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients. 2015;7(6):4240–70. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur EB, Genc M, Eskicioglu F. The effect of vitamin D level in pregnancy on postpartum depression. Arch Womens Ment Health. 2015;18(2):263–4. doi: 10.1007/s00737-015-0509-0. [DOI] [PubMed] [Google Scholar]
- Halhali A, Figueras AG, Díaz L, Avila E, Barrera D, Hernández G, et al. J Steroid Biochem Mol Biol [Internet] 1–2. Vol. 121. Elsevier Ltd; 2010. Journal of Steroid Biochemistry and Molecular Biology Effects of calcitriol on calbindins gene expression and lipid peroxidation in human placenta; pp. 448–51. Available from: [DOI] [PubMed] [Google Scholar]
- Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18(45):1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemipour S, Lalooha F, Mirdamadi SZ, Ziaee A, Ghaleh TD. Effect of vitamin D administration in vitamin D-deficient pregnant women on maternal and neonatal serum calcium and vitamin D concentrations: a randomised clinical trial. Br J Nutr. 2013;110(9):1611–6. doi: 10.1017/S0007114513001244. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health. Am J Clin Nutr. 2008;87(4):1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. J BMR Vitamin D Supplementation During Pregnancy: and Effectiveness. J Bone Miner Res. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Vitamin D and Pregnancy: Skeletal Effects, Nonskeletal Effects, and Birth Outcomes. Calcif Tissue Int. 2013;92(2):128–39. doi: 10.1007/s00223-012-9607-4. [DOI] [PubMed] [Google Scholar]
- Hossain N, Kanani FH, Ramzan S, Kausar R. Obstetric and Neonatal Outcomes of Maternal Vitamin D Supplementation: Results of an Open- Label, Randomized Controlled Trial of Antenatal Vitamin D Supplementation in Pakistani Women. J Clin Endocrinol Metab. 2014;99(7):2448–55. doi: 10.1210/jc.2013-3491. [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, et al. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab [Internet] 2013;63(4):331–40. doi: 10.1159/000358338. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24603503. [DOI] [PubMed] [Google Scholar]
- Joergensen JS, Lamont RF, Torloni MR. Vitamin D and gestational diabetes: an update. Curr Opin Clin Nutr Metab Care. 2014;17(4):360–7. doi: 10.1097/MCO.0000000000000064. [DOI] [PubMed] [Google Scholar]
- Jones AP, Vaz ND, Meldrum S, Palmer DJ, Zhang G, Prescott SL. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin Exp Allergy. 2015;45(1):220–31. doi: 10.1111/cea.12449. [DOI] [PubMed] [Google Scholar]
- Jongh RT, Crozier SR, D’Angelo S, Pike KC, Roberts G, et al. Maternal 25 hydroxyvitamin D levels in relation to offspring respiratory symptoms and infections. Eur Respir J. 2014;43:1181–1183. doi: 10.1183/09031936.00116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of High-Dose Vitamin D Supplementation on Metabolic Status and Pregnancy Outcomes in Pregnant Women at Risk for Pre-Eclampsia. Horm Metab Res = Horm und Stoffwechselforsch = Horm métabolisme [Internet] 2015 Nov;47(12):867–72. doi: 10.1055/s-0035-1548835. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25938886. [DOI] [PubMed] [Google Scholar]
- Khalessi N, Kalani M, Araghi M, Farahani Z. The Relationship between Maternal Vitamin D Deficiency and Low Birth Weight Neonates. J Family Reprod Health. 2015;9(3):113–7. [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS. BONE DEVELOPMENT AND MINERAL HOMEOSTASIS IN THE FETUS AND NEONATE: ROLES OF THE CALCIOTROPIC AND PHOSPHOTROPIC HORMONES. Physiol Rev. 2014;94(4):1143–218. doi: 10.1152/physrev.00014.2014. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Myriam MB. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014;51(4):609–16. doi: 10.1007/s00592-014-0564-4. [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Lithy A, El Abdella RM, El-faissal YM, Sayed AM, Samie RMA. The relationship between low maternal serum vitamin D levels and glycemic control in gestational diabetes assessed by HbA1c levels: an observational cross-sectional study. BMC Pregnancy Childbirth. 2014;14:362. doi: 10.1186/1471-2393-14-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, Mcelrath TF, Connor GTO, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years The VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy SL, Lek N, Yap F, Soh SE, Padmapriya N, Tan H, et al. Association of Maternal Vitamin D Status with Glucose Tolerance and Caesarean Section in a Multi-Ethnic Asian Cohort: The Growing Up in Singapore Towards Healthy Outcomes Study. PLoS One. 2015;10(11):e0142239. doi: 10.1371/journal.pone.0142239. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynska A, Łuczyn A, Brenner H, Rothenbacher D. Cord blood 25 ( OH ) D levels and the subsequent risk of lower respiratory tract infections in early childhood: the Ulm birth cohort. Eur J Epidemiol. 2014;29(8):585–94. doi: 10.1007/s10654-014-9918-z. [DOI] [PubMed] [Google Scholar]
- Marshall I, Mehta R, Petrova A, Marshall I, Mehta R, Petrova A. Vitamin D in the maternal – fetal – neonatal interface: clinical implications and requirements for supplementation and requirements for supplementation. J Matern Fetal Neonatal Med. 2013;26(7):633–8. doi: 10.3109/14767058.2012.746306. [DOI] [PubMed] [Google Scholar]
- Maslova E, Hansen S, Thorne-lyman AL, Jensen CB, Cohen A, Nielsen NO, et al. Predicted vitamin D status in mid-pregnancy and child allergic disease. Pediatr Allergy Immunol. 2014;25(7):706–13. doi: 10.1111/pai.12295. [DOI] [PubMed] [Google Scholar]
- Miller DR, Turner SW, Scaife AR, Danielian PJ, Devereux GS, Walsh GM, et al. Maternal vitamin D and E intakes during early pregnancy are associated with airway epithelial cell responses in neonates. Clin Exp Allergy. 2015;45(5):920–7. doi: 10.1111/cea.12490. [DOI] [PubMed] [Google Scholar]
- Mirzaei F, Moghadam TA, Arasteh P. Comparison of serum 25-hydroxy vitamin D levels between mothers with small for gestational age and appropriate for gestational age newborns in Kerman. Iran J Reprod Med. 2015;13(4):203–8. [PMC free article] [PubMed] [Google Scholar]
- Mohaghegh Z, Abedi P, Dilgouni T, Namvar F, Ruzafza S. The relation of preeclampsia and serum level of 25-hydroxyvitamin D in mothers and their neonates: a case control study in Iran. Horm Metab Res. 2015;47(4):284–8. doi: 10.1055/s-0034-1395607. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25611206. [DOI] [PubMed] [Google Scholar]
- Mojibian M, Soheilykhah S, Moghadam J. The effects of vitamin D supplementation on maternal and neonatal outcome: A randomized clinical trial. Iran J Reprod Med. 2015;13(11):687–96. [PMC free article] [PubMed] [Google Scholar]
- Morales E, Rodriguez A, Valvi D, Iñiguez C, Esplugues A, Vioque J, et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int J Obes. 2015;39(1):61–8. doi: 10.1038/ijo.2014.165. [DOI] [PubMed] [Google Scholar]
- Morgan C, Dodds L, Langille DB, Weiler HA. Cord blood vitamin D status and neonatal outcomes in a birth cohort in Quebec, Canada. Arch Gynecol Obstet. 2016;293(4):731–8. doi: 10.1007/s00404-015-3899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandal R, Chhabra R, Sharma D, Lallar M, Maheshwari P. Comparison of cord blood vitamin D levels in newborns of vitamin D supplemented and unsupplemented pregnant women: a prospective, comparative study Comparison of cord blood vitamin D levels in newborns of vitamin D supplemented and unsupplemented pregnancy. J Matern Fetal Neonatal Med. 2015;25:1–5. doi: 10.3109/14767058.2015.1064106. Available from: [DOI] [PubMed] [Google Scholar]
- Norizoe C, Akiyama N, Segawa T, Tachimoto H, Mezawa H, Ida H, et al. Increased food allergy and vitamin D: Randomized, double-blind, placebo-controlled trial. Pediatr Int. 2014;56(1):6–12. doi: 10.1111/ped.12207. [DOI] [PubMed] [Google Scholar]
- Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LMA, Prentice A. Calcium economy in human pregnancy and lactation Nutrition Research Reviews. Nutr Res Rev. 2012;25(1):40–67. doi: 10.1017/S0954422411000187. [DOI] [PubMed] [Google Scholar]
- Olmos-ortiz A, Avila E, Durand-carbajal M, Díaz L. Regulation of Calcitriol Biosynthesis and Activity Focus on Gestational Vitamin D Deficiency and Adverse Pregnancy Outcomes. Nutrients. 2015;7(1):443–80. doi: 10.3390/nu7010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwuneme C, Martin F, Mccarthy R, Carroll A, Segurado R, Murphy J, et al. J Pediatr [Internet] 5. Vol. 166. Elsevier Inc; 2015. The Association of Vitamin D Status with Acute Respiratory Morbidity in preterm Infants; pp. 1175–80e1. Available from: [DOI] [PubMed] [Google Scholar]
- Palaniswamy S, Williams D, Sebert S. Vitamin D and the Promotion of Long-Term Metabolic Health from a Programming Perspective. Nutr Metab Insights. 2016;8(Suppl 1):11–21. doi: 10.4137/NMI.S29526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- Pérez-López FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril [Internet] 2015 May;103(5):1278–88.e4. doi: 10.1016/j.fertnstert.2015.02.019. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25813278. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Thangaratinam S, Magee LA, De Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387(10022):999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- Robinson M, Whitehouse AJO, Newnham JP, Gorman S, Jacoby P, Holt BJ, et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Womens Ment Health. 2014;17(3):213–9. doi: 10.1007/s00737-014-0422-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, García-Esteban R, Basterretxea M, Lertxundi A, Rodríguez-Bernal C, Iñiguez C, et al. Associations of maternal circulating 25-hydroxyvitamin D3 concentration with pregnancy and birth outcomes. BJOG [Internet] 2015 Nov;122(12):1695–704. doi: 10.1111/1471-0528.13074. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25208685. [DOI] [PubMed] [Google Scholar]
- Ross CA, Talylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for clacium and vitamin D. Washington DC: 2011. IOM (Institute of Medicine) 2011. [Google Scholar]
- Roth DE, Gernand AD, Morris SK, Pezzack B, Islam MM, Dimitris MC, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh ( MDIG trial ): study protocol for a randomized controlled trial. Trials [Internet] Trials. 2015;16:300. doi: 10.1186/s13063-015-0825-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá F, De Fiol D, Barberato-filho S, Lopes LC, Bergamaschi CDC. Review Vitamin D and respiratory infections. J Infect Dev Ctries. 2015;9(4):355–61. doi: 10.3855/jidc.5711. [DOI] [PubMed] [Google Scholar]
- Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endrocinol (Oxf) 2015;83(4):536–41. doi: 10.1111/cen.12751. [DOI] [PubMed] [Google Scholar]
- Sadin B, Gargari BP, Pourteymour F, Tabrizi F. Vitamin D Status in Preeclamptic and Non-preeclamptic Pregnant Women: A Case-Control Study in the North West of Iran. Tabriz Univ Med Sci [Internet] 2015;5(3):183–90. doi: 10.15171/hpp.2015.022. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi M, Kashi M, Foroozanfard F, Karamali M, Bahmani F, Asemi Z, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J Hum Nutr Diet. 2015 doi: 10.1111/jhn.12339. [DOI] [PubMed] [Google Scholar]
- Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery ( Review ) Cochrane Database Syst Rev. 2015;2:CD006178. doi: 10.1002/14651858.CD006178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvidou MD, Makgoba M, Castro PT, Akolekar R, Nicolaides KH. Short Communication First-trimester maternal serum vitamin D and mode of delivery British Journal of Nutrition. Br J Nutr. 2012;108(11):1972–5. doi: 10.1017/S0007114512000207. [DOI] [PubMed] [Google Scholar]
- Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. 2014;99(2):287–95. doi: 10.3945/ajcn.113.065672. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Vijayaselvi R, Nandeibam Y, Natarajan M. A Case Control Study to Evaluate the Association between Primary Cesarean Section for Dystocia and Vitamin D Deficiency. J Clin Diagn Res. 2015;9(9):QC05–8. doi: 10.7860/JCDR/2015/14029.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla R, Gurung P, Aggarwal N. Arch Gynecol Obstet [Internet] 6. Vol. 291. Springer; Berlin Heidelberg: 2015. Relationship between preeclampsia and vitamin D deficiency: a case control study; pp. 1247–51. Available from: [DOI] [PubMed] [Google Scholar]
- Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24(2):131–8. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- Stubbs G, Henley K, Green J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses. 2016;88:74–8. doi: 10.1016/j.mehy.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Sunmin P, Hyun-koo Y, Hyun-mee R, HYJ, LSW, PBK, et al. Maternal Vitamin D Deficiency in Early Pregnancy Is Not Associated with Gestational Diabetes Mellitus Development or Pregnancy Outcomes in Korean Pregnant Women in a Prospective Study. J Nutr Sci Vitaminol. 2014;60(4):269–75. doi: 10.3177/jnsv.60.269. [DOI] [PubMed] [Google Scholar]
- Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol [Internet] 2016;83(2):83–91. doi: 10.1111/sji.12403. Available from: http://doi.wiley.com/10.1111/sji.12403\nhttp://www.ncbi.nlm.nih.gov/pubmed/26678915. [DOI] [PubMed] [Google Scholar]
- Thota C, Menon R, Fortunato SJ, Brou L, Lee J, Al-hendy A. 1, 25-Dihydroxyvitamin D Deficiency Is Associated With Preterm Birth in African American and Caucasian Women. Reprod Sci. 2014;21(2):244–50. doi: 10.1177/1933719113493513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert B, van den Berg D, Hrudey EJ, Oostvogels AJJM, de Miranda E, Vrijkotte TGM. Midwifery [Internet] Vol. 34. Elsevier; 2016. Is first trimester vitamin D status in nulliparous women associated with pregnancy related hypertensive disorders? pp. 117–22. Available from: [DOI] [PubMed] [Google Scholar]
- Wagner CL, Baggerly C, Mcdonnell SL, Baggerly L, Hamilton SA, Winkler J, et al. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. J Steroid Biochem Mol Biol. 2015;148:256–60. doi: 10.1016/j.jsbmb.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Shan L, Du L, Feng J, Xu Z, Staal WG. Serum concentration of 25 – hydroxyvitamin D in autism spectrum disorder: a systematic review and meta – analysis. Eur Child Adolesc Psychiatry [Internet] 2015 doi: 10.1007/s00787-015-0786-1. Available from: “. [DOI] [PubMed]
- Wei R, Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015;7(10):8251–60. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert S, Silveiro P. Maternal – Fetal Impact of Vitamin D Deficiency: A Critical Review. Matern Child Health J. 2015;19(1):94–101. doi: 10.1007/s10995-014-1499-7. [DOI] [PubMed] [Google Scholar]
- Wetta LA, Biggio JR, Cliver S, Barnes S, Tita ATN, Abramovici A. Is Midtrimester Vitamin D Status Associated with Spontaneous Preterm Birth and Preeclampsia? Am J Perinatol. 2014;31(6):541–6. doi: 10.1055/s-0033-1356483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw DC, Scally AJ, Tuffnell DJ, Davies TJ, Fraser WD, Bhopal RS, et al. Associations of Circulating Calcium and 25-Hydroxyvitamin D With Glucose Metabolism in Pregnancy: A Cross-Sectional Study in European and South Asian Women. J Clin Endocrinol Metab. 2014;99(3):938–46. doi: 10.1210/jc.2013-2896. [DOI] [PubMed] [Google Scholar]
- Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A. Vitamin D Supplementation and the Effects on Glucose Metabolism During Pregnancy: A Randomized Controlled Trial. Diabetes Care. 2014;37(7):1837–44. doi: 10.2337/dc14-0155. [DOI] [PubMed] [Google Scholar]
- Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Tong S, Hu W, Hao J, Tao R, Huang K. Cord Blood 25-hydroxyvitamin D and Fetal Growth in the China- Anhui Birth Cohort Study. Sci Rep. 2015;5:14930. doi: 10.1038/srep14930. [DOI] [PMC free article] [PubMed] [Google Scholar]