Abstract
Eye movement desensitization and reprocessing (EMDR) is a popular treatment for posttraumatic stress disorder. However, little is known about the memory effects of EMDR. Using a misinformation paradigm, we examined whether lateral eye movements, as used in EMDR, enhance susceptibility to false memories. Undergraduates (N = 82) saw a video depicting a car crash. Subsequently, participants either performed eye movements or held their eyes stationary. Afterward, all participants received misinformation in the form of an eyewitness narrative. The results indicate that eye movement participants were less accurate and were more susceptible to the misinformation effect than controls. Our finding suggests EMDR may have risky drawbacks in an eyewitness context and therefore urgently needs follow-up research.
Keywords: eye movements, EMDR, false memory, misinformation paradigm
Eye movement desensitization and reprocessing (EMDR; Shapiro, 1989) is currently one of the most frequently used interventions to treat individuals with mental disorders, such as posttraumatic stress disorder (PTSD; van den Hout & Engelhard, 2012). The crux of EMDR is that a client is instructed to perform eye movements while retrieving a traumatic event. The purpose of these eye movements is to reduce the vividness and emotionality linked to the traumatic memory. Despite its popularity and the numerous studies examining symptom reduction after EMDR, little is known about the potential drawbacks of eye movements (Lilienfeld, 2007). We examined one potential drawback. Specifically, we tested whether eye movements might enhance the susceptibility to misinformation.
Experimental work has confirmed that performing eye movements significantly reduces the vividness and emotionality of traumatic memories (Lee & Cuijpers, 2013). One explanation for this is that performing eye movements while focusing on the most salient aspects of a traumatic memory requires working memory capacity and performing this dual task interferes with the capacity for memory recall (van den Hout & Engelhard, 2012). As a result, both vividness and emotionality of the memory decline. The rationale for this decline is the following. When imagining an event, a phenomenon called imagination inflation emerges, and the subjective confidence that the imagined event had occurred (e.g., by vivid images) increases (Goff & Roediger, 1998; Otgaar, Scoboria, Howe, Moldoveanu, & Smeets, 2016). The reverse is said to occur during eye movements. Eye movements make the memory less vivid, thereby making them less distinctive (i.e., imagination deflation; van den Hout & Engelhard, 2012).
Before and after an EMDR session, therapists frequently engage in a comprehensive conversation (e.g., asking follow-up questions) to clarify certain aspects that surfaced during the session (de Jongh & ten Broeke, 2016). Oftentimes, therapists have poor knowledge about issues related to memory functioning compared with what we know from the science of memory (Patihis, Ho, Tingen, Lilienfeld, & Loftus, 2014). Given this state of affairs, follow-up questions during a therapeutic interview might unintentionally have a misinformation potential. Hence, it is surprising that little research has focused on whether eye movements might enhance susceptibility to misinformation (see Devilly & Brown, 2011). This is the focus of the current study.
The misinformation paradigm (Loftus, Miller, & Burns, 1978) is a well-established procedure in which participants first witness an event (e.g., mock crime) and then receive suggestive misinformation about the event (e.g., the robber used a knife, while no knife was present). Next, participants are interviewed about the details of the event. Research has consistently shown that exposure to misinformation can make participants include that misinformation as part of their own memory reports (i.e., reporting misinformation and thus creating false memories), an effect that has been termed the misinformation effect (Loftus, 2005). In the study of Parker, Buckley, and Dagnall (2009), participants had to listen to a story and viewed pictures simultaneously that illustrated the narrative. Participants received misinformation by means of a postevent misinformation questionnaire. Hereafter, they were randomly assigned to a horizontal eye movement, vertical eye movement, or a control condition. Participants in the eye movement conditions were more resistant to the misinformation effect than the controls. However, this study cannot be applied to situations in which clients are first engaged in eye movements and receive misinformation afterward.
Theoretically, participants might be most susceptible to misinformation when their memory of an event has become vague. In such a situation, it is difficult to determine the source of misinformation and so the misinformation might be experienced as part of the original record. The source monitoring framework (Johnson, Hashtroudi, & Lindsay, 1993) explains the misinformation effect as a failure to accurately identify the source of information. Divided attention can compromise this source attribution. Of relevance to the current experiment is research showing that divided attention can increase the probability of false memories (Otgaar, Peters, & Howe, 2012). Performing eye movements and simultaneously retrieving a memory can be seen as a dual task in which both tasks tax attentional resources. Our predictions are in line with the tenets of several false memory theories, such as fuzzy trace theory (FTT; Brainerd, Reyna, & Ceci, 2008). FTT postulates that memories are stored along two independent memory traces: verbatim (i.e., item-specific characteristics) and gist (i.e., meaning of the information) traces. False memories arise as a result of reliance on gist traces, when verbatim traces are unable to be retrieved. With respect to the current experiment, this could indicate the following. As previous research has shown that eye movements decrease the vividness (and emotionality) of a memory (Lee & Cuijpers, 2013), this could imply that the memory becomes less detailed. If so, after eye movements, individuals will rely more on gist traces, thereby making them more prone to accept misinformation. Also, associative activation theory (Howe, Wimmer, Gagnon, & Plumpton, 2009) posits that false memories are the result of spreading activation. Recent research has shown that when misinformation is associatively related to an experienced event, misinformation is easily accepted as being part of this event (Otgaar, Howe, Brackmann, & Smeets, 2016). When eye movements make an experienced event less distinctive, misinformation might be more easily accepted as it is less likely to be seen as discrepant and unrelated to the event.
We examined whether eye movements increase the susceptibility to the misinformation effect. After watching a car crash video and performing eye movements or not, participants received misinformation. In line with previous research (Lee & Cuijpers, 2013), we hypothesized that performing eye movements reduces the vividness and emotionality of the memory. In addition, we expected that participants who had performed eye movements would show heightened levels of misinformation acceptance compared with control participants who did not engage in the eye movement task.
Method
Participants
On the basis of previous research (Parker & Dagnall, 2007; study on eye movements and false memories with an effect size of d = 0.6), an a priori analysis using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007), with a medium to large effect size (d = 0.6) and a power of 0.80, indicated a sample size of 72 participants. Ten extra participants were tested in case any dropouts took place. Thus, 82 second-year undergraduate psychology students from Maastricht University (age M = 21.42 years, SD = 2.08, range = 19–31, 54 women) were tested. Participants received course credits for their participation. The experiment was approved by the standing ethical committee for the Faculty of Psychology and Neuroscience, Maastricht University.
Materials
All material is available at the Open Science Framework at https://osf.io/j479p/.
Beck Depression Inventory
The Beck Depression Inventory (BDI; Beck & Steer, 1987) is a self-reported questionnaire that contains 21 items, including several statements of which participants have to select the statement that describes them best (e.g., Item 1: “I do not feel sad—I feel sad—I am sad all the time and I cannot snap out of it—I am so sad and unhappy that I cannot stand it”). The BDI was included purely for exploratory analyses. As EMDR is used for individuals who experienced a traumatic event, or are depressed as a result of this trauma (Shapiro & Forrest, 2004), we want to explore whether participants with high BDI scores also show higher ratings on vividness and emotionality.
Video
Participants saw a video that has previously been used in the trauma film paradigm (Holmes & Bourne, 2008; Strange & Takarangi, 2012). It depicts a road accident where several cars crash into one another. Specifically, three women are in a car arguing about a text message that the driver is texting. As the driver does not pay attention to the road, they crash into another vehicle. As the car stops, another car crashes into the two vehicles. At least five people, including a baby, are injured and unconscious. Emergency services arrive and the video ends with a close-up of the driver’s face.
Eye movement task
To simulate the eye movement component of EMDR, we employed a computerized eye movement task that has been described in van den Hout, Bartelski, and Engelhard (2013). A gray dot was presented on a black background via E-Prime and moved from left to right with 1 s per cycle, during four intervals of 24 s with a 10-s interval. The stationary dot was presented via PowerPoint, also during four intervals of 24 s with a 10-s interval. This duration is in line with previous research (van den Hout & Engelhard, 2012) and largely converges to what is usually done in EMDR therapy. All participants sat at a 30 cm distance from the computer screen. All participants were instructed to think about the video and the emotions that they felt during the video presentation. The researcher checked whether the participant complied with the instructions by monitoring the participant.
Misinformation
Misinformation was provided in the form of an eyewitness narrative and was presented on paper. The narrative contained 10 true statements (e.g., “the girls were driving a small car”) and 5 false statements about the video (e.g., “the driver was texting to John” instead of James; “I saw the injured mother” instead of father).
Recognition task
The recognition task contained 15 questions, including 10 questions with true and foil answering options (e.g., “What vehicle were the girls driving?”; true answer: a small car, foil answer: a van) and five critical questions pertaining to misinformation that was presented earlier (e.g., “To whom were the girls writing a text message?”; true answer: James, misinformation answer: John). In line with previous research (Parker et al., 2009), the recognition test was presented orally to the participants.
Design and procedure
The current experiment used a between-subjects design and included one independent variable with two levels: horizontal eye movements versus control (i.e., central fixation). The dependent variables were (a) correct answers and (b) endorsement of misinformation. All participants were tested individually in a quiet lab room. After obtaining written informed consent, participants completed the BDI. Then, they were told they would see a violent car crash. They were instructed to view the video carefully, as if they were an eyewitness. After the video had finished, they were asked to complete a visual analogue scale (VAS; Lee & Cuijpers, 2013) for vividness and emotionality. The VAS ranged from 0 (not vivid at all) to 10 (extremely vivid) for vividness and ranged from 0 (extremely negative) to 10 (extremely positive) for emotionality. From this point, participants were randomly assigned to either the eye movement (EM) condition or the control condition. EM participants were instructed to follow the moving dot on the computer screen with their eyes without moving their head, for four episodes of 24 s. They were instructed to think about the video and the emotions that they felt during the video presentation. Control participants were instructed to watch the stationary dot for four episodes of 24 s while thinking about the video and the emotions that they felt during the video presentation. Immediately after the four episodes of 24 s, participants were asked to rate the vividness and emotionality on a VAS again. Hereafter, participants had to complete a filler task (e.g., playing Bejeweled) for 5 min. Then, participants read a narrative from another eyewitness containing the misinformation. After completing another filler task for 5 min, and to end the session, participants received an oral recognition task. Participants were debriefed and received their course credit.
Results
Vividness and emotionality
Table 1 shows mean scores for vividness and emotionality. A repeated measures ANOVA for vividness revealed no significant interaction effect, F(1, 80) = 1.32, p = .26. No effect of condition, F(1, 80) = 1.025, p = .31, was found. A significant effect of time emerged, F(1, 80) = 21.05, p < .001. Specifically, vividness scores significantly decreased within both conditions (t = 4.84, 95% CI = [0.50, 1.21], p < .001, Cohen’s d = 0.66 for the EM condition, and t = 2.14, 95% CI = [0.03, 1.00], p = .039, Cohen’s d = 0.35 for the control condition).
Table 1.
Mean Scores (and SDs) and Confidence Intervals (CIs) on Vividness and Emotionality, Before and After Performing Eye Movements or Control
| Vividness | Emotionality | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Pre | CI | Post | CI | Pre | CI | Post | CI |
| EM (n = 41) | 7.73 (1.16) | 7.36, 8.10 | 6.88 (1.40) | 6.44, 7.32 | 2.20 (1.63) | 1.68, 2.71 | 2.68 (1.69) | 2.15, 3.22 |
| Control (n = 41) | 7.83 (1.34) | 7.40, 8.26 | 7.31 (1.56) | 6.83, 7.81 | 2.76 (1.95) | 2.14, 3.37 | 2.85 (1.77) | 2.30, 3.41 |
EM = eye movement condition.
Emotionality scores increased from pre- to posttest in both conditions. A repeated measures ANOVA for emotionality revealed a nonsignificant interaction effect, F(1, 80) = 3.79, p = .055. The effect of condition was nonsignificant, F(1, 80) = 0.94, p = .33. A significant effect of time, F(1, 80) = 8.53, p = .005, was found, showing that the video at posttest was experienced as more positive than at pretest. Again, the effect size for the EM condition was larger than for the control condition (EM condition: t = −3.48, 95% CI = [−0.77, 0.20], p = .001, Cohen’s d = −0.30; control: t = −0.68, 95% CI = [0.39, 0.19], p = .50, Cohen’s d = −0.05).
As predicted, both vividness and emotionality ratings decreased. However, the results did not reveal a difference between both conditions. This decrease in vividness and negativity could be attributed to the time interval or nonspecific factors. To examine whether there is a relation between vividness and emotionality, Pearson’s r was calculated. A correlation was found between post vividness and emotionality ratings for the EM condition (r = −.38, n = 41, p < .05), but not for the control condition (r = −.08, n = 41, p > .05).
Misinformation
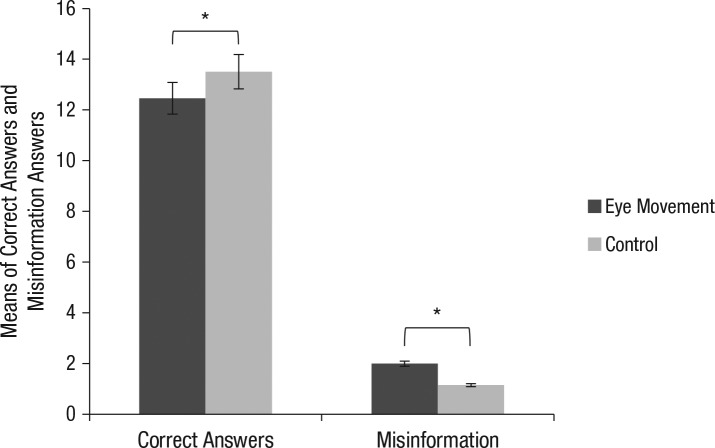
Means of correct answers and endorsed misinformation answers are depicted in Figure 1. An independent samples t test revealed a statistically significant difference in the number of correct answers and the endorsement of misinformation. That is, confirming our hypothesis, control participants answered questions correctly more often than EM participants, t(80) = 4.00, 95% CI = [0.53, 1.75], p < .001, Cohen’s d = −0.88. EM participants significantly more often accepted misinformation than control participants, t(80) = −3.47, 95% CI = [−1.34, −0.36], p = .001, Cohen’s d = 0.77. A correlation was also found between the number of correct answers and number of endorsed misinformation items for the EM condition (r = −.90, n = 41, p < .001) and for the control condition (r = −.86, n = 41, p < .001).We also calculated a Bayes factor (i.e., a comparison of how well two hypotheses predict the data) using a default prior of 0.71. The larger this factor deviates from zero, the more support for the alternative hypothesis (Goodman, 1999). We found a BF10 = 35.26 indicating that our eye movement-misinformation effect is more in line with the alternative hypothesis than with the null hypothesis. Next to that, we examined the post hoc power of our experiment. A post hoc observed power analysis indicated a power of 0.93 for the condition effect.
Fig. 1.
Means of correct answers and misinformation items for eye movement condition and control condition. Error bars show 95% confidence intervals.
Exploratory analysis
We explored whether BDI scores served as a covariate. An independent samples t test revealed no significant difference in BDI between the two conditions (EM: M = 3.93, SD = 4.09; control: M = 4.59, SD = 3.71; t = 0.77, 95% CI = [–1.06, 2.37], p = .45, Cohen’s d = −0.17). An ANCOVA with BDI scores as covariate revealed no significant interaction effect on the number of correct answers, F(1, 78) = 2.42, p = .12, η2partial = .03, and no significant main effect of BDI scores, F(1, 78) = 0.002, p = .97, η2partial = .000. A main effect of condition emerged, F(1, 78) = 14.60, p < .001, η2partial = .158. For misinformation acceptance, a similar pattern was found. An ANCOVA with BDI scores as covariate revealed no significant interaction effect on misinformation acceptance, F(1, 78) = .82, p = .37, η2partial = .01, and no significant main effect of BDI scores, F(1, 78) = 0.001, p = .98, η2partial = .000. A main effect of condition emerged, F(1, 78) = 8.79, p = .004, η2partial = .101. BDI scores were not statistically correlated with VAS ratings (pre, post, and mean change), number of correct answers, or number of endorsed misinformation items (all ps > .05), which suggests that BDI scores did not affect memory scores.
Discussion
Individuals who are treated with EMDR may be unintentionally exposed to misinformation incorporated in follow-up questions of a therapist. The aim of the current study was to examine susceptibility to misinformation after performing eye movements. We found an unwanted drawback of such eye movements. That is, participants who had engaged in eye movements more often endorsed misinformation than control participants did.
Our finding seems to contradict the results of Parker and colleagues (2009). As previously stated, participants in the eye movement conditions were more resistant to the misinformation effect than the controls. Our design differs from Parker et al., as we intended to use a sequence that mimics what might happen during an EMDR session. Specifically, in such sessions, after (and not before) performing eye movements, clients might be confronted with (suggestive) misinformation during the follow-up interview. Thus, the important question is whether eye movements may make people more susceptible to misinformation. We are the first showing that this is, indeed, the case. Eye movements might have an undesirable drawback.
Furthermore, the results could also be explained in terms of the discrepancy detection principle (Tousignant, Hall, & Loftus, 1986). According to this principle, the larger the discrepancy between an actual memory and misleading information, the less susceptible individuals will be to create false memories. It is likely that this discrepancy can decrease as the memory becomes less vivid, and hence it will be more difficult to detect the discrepancy. The consequence is that participants will become more susceptible to incorporate misinformation in their memory reports. It is reasonable to assume that eye movements increase reporting of misinformation because they interfere with the vividness of true memories (Lee & Cuijpers, 2013).
In our study, participants also rated vividness and emotionality of the video before and after performing eye movements. The video was perceived as less vivid and more positive at posttest for both the eye movement and control conditions. Often, such effects are more pronounced in participants performing eye movements (van den Hout & Engelhard, 2012). In line with this, effect sizes for decreases in vividness and negative emotions in our study were substantially larger for the eye movement than for the control condition.
Our study was based on previous work on EMDR, and hence we used effect sizes found in that research. A limitation of this could be that although our study had adequate power and although the sample size was based on previous effect sizes, recent research has suggested that reported effect sizes in studies are likely to be an overestimation of actual effect sizes (Matzke et al., 2015). Hence, future studies might attempt to replicate this study using a larger sample size.
To conclude, research has suggested that EMDR and thus eye movements are effective as a treatment for PTSD, but the current work is the first showing that eye movements can have adverse effects. This finding combined with recent work showing that individuals with PTSD are at increased risk to create false memories (Otgaar, Muris, Howe, & Merckelbach, 2017) and work showing that therapists have poor knowledge on issues concerning memory (Patihis et al., 2014) stresses the importance of conducting follow-up research on the drawbacks of EMDR.
Supplemental Material
Supplemental material, Houben_Open_Practices_Disclosure for Lateral Eye Movements Increase False Memory Rates by Sanne T. L. Houben, Henry Otgaar, Jeffrey Roelofs and Harald Merckelbach in Clinical Psychological Science
Footnotes
Author Contributions: S. T. L. Houben and H. Otgaar developed the study concept. S. T. L. Houben performed the data analysis under the supervision of H. Otgaar and J. Roelofs. H. Otgaar, J. Roelofs, and H. Merckelbach provided critical revisions. All the authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Open Practices:


All data and materials have been made publicly available via the Open Science Framework and can be accessed at https://osf.io/j479p/. The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/2167702618757658. This article has received badges for Open Data and Open Materials. More information about the Open Practices badges can be found at https://www.psychologicalscience.org/publications/badges.
References
- Beck A. T., Steer R. A. (1987). Beck Depression Inventory: Manual. San Antonio, TX: Psychiatric Corporation. [Google Scholar]
- Brainerd C. J., Reyna V. F., Ceci S. J. (2008). Developmental reversals in false memory: A review of data and theory. Psychological Bulletin, 134, 343–382. doi: 10.1037/0033-2909.134.3.343 [DOI] [PubMed] [Google Scholar]
- de Jongh A., ten Broeke E. (2016). Handboek EMDR: Een geprotocolleerde behandelmethode voor de gevolgen van psychotrauma [EMDR handbook: A treatment protocol for the consequences of psychotrauma]. Amsterdam, the Netherlands: Pearson. [Google Scholar]
- Devilly G. J., Brown L. (2011). The role of imagery rehearsal with and without eye movements in the creation of false memories. Psychology, Crime, & Law, 17, 529–543. doi: 10.1080/10683160903397524 [DOI] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioural, and biomedical sciences. Behavior Research Methods, 39, 175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Goff L. M., Roediger H. L. (1998). Imagination inflation for action events: Repeated imaginings lead to illusory recollections. Memory & Cognition, 26, 20–33. doi: 10.3758/BF03211367 [DOI] [PubMed] [Google Scholar]
- Goodman S. N. (1999). Toward evidence-based medical statistics 2: The Bayes factor. Annals of Internal Medicine, 130, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Bourne C. (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica, 127, 553–566. doi: 10.1016/j.actpsy.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Howe M. L., Wimmer M. C., Gagnon N., Plumpton S. (2009). An associative-activation theory of children’s and adults’ memory illusions. Journal of Memory and Language, 60, 229–251. doi: 10.1016/j.jml.2008.10.002 [DOI] [Google Scholar]
- Johnson M. K., Hashtroudi S., Lindsay D. S. (1993). Source monitoring. Psychological Bulletin, 114, 3–28. doi: 10.1037/0033-2909.114.1.3 [DOI] [PubMed] [Google Scholar]
- Lee C. W., Cuijpers P. (2013). A meta-analysis of the contribution of eye movements in processing emotional memories. Journal of Behavior Therapy and Experimental Psychiatry, 44, 231–239. doi: 10.1016/j.jbtep.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Lilienfeld S. O. (2007). Psychological treatments that cause harm. Perspectives on Psychological Science, 2, 53–70. doi: 10.1111/j.1745-6916.2007.00029.x [DOI] [PubMed] [Google Scholar]
- Loftus E. F. (2005). Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learning & Memory, 12, 361–366. doi: 10.1101/lm.94705 [DOI] [PubMed] [Google Scholar]
- Loftus E. F., Miller D. G., Burns H. J. (1978). Semantic integration of verbal information into a visual memory. Journal of Experimental Psychology: Human Learning and Memory, 4, 19–31. doi: 10.1037//0278-7393.4.1.19 [DOI] [PubMed] [Google Scholar]
- Matzke D., Nieuwenhuis S., van Rijn H., Slagter H. A., van der Molen M. W., Wagenmakers E. J. (2015). The effect of horizontal eye movements on free recall: A preregistered adversarial collaboration. Journal of Experimental Psychology: General, 144, e1–e15. doi: 10.1037/xge000038 [DOI] [PubMed] [Google Scholar]
- Otgaar H., Howe M. L., Brackmann N., Smeets T. (2016). The malleability of developmental trends in neutral and negative memory illusions. Journal of Experimental Psychology: General, 145, 31–55. doi: 10.1037/xge0000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otgaar H., Muris P., Howe M. L., Merckelbach H. (2017). What drives false memories in psychopathology? A case for associative activation. Clinical Psychological Science, 5, 1048–1069. doi: 10.1177/2167702617724424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otgaar H., Peters M., Howe M. L. (2012). Dividing attention lowers children’s but increases adult’s false memories. Journal of Experimental Psychology, 38, 204–210. doi: 10.1037/a0025160 [DOI] [PubMed] [Google Scholar]
- Otgaar H., Scoboria A., Howe M. L., Moldoveanu G., Smeets T. (2016). Challenging memories in children and adults using an imagination inflation procedure. Psychology of Consciousness: Theory, Research, and Practice, 3, 270–283. doi: 10.1037/cns0000087 [DOI] [Google Scholar]
- Parker A., Buckley S., Dagnall N. (2009). Reduced misinformation effect following saccadic bilateral eye movements. Brain and Cognition, 69, 89–97. doi: 10.1016/j.bands.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Parker A., Dagnall N. (2007). Effects of bilateral eye movements on gist based false recognition in the DRM paradigm. Brain and Cognition, 63, 221–225. doi: 10.1016/j.bandc.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Patihis L., Ho L. Y., Tingen I. W., Lilienfeld S. O., Loftus E. F. (2014). Are the “memory wars” over? A scientist-practitioner gap in beliefs about repressed memory. Psychological Science, 25, 519–530. doi: 10.1177/0956797613510718 [DOI] [PubMed] [Google Scholar]
- Shapiro F. (1989). Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. Journal of Traumatic Stress, 2, 199–223. doi: 10.1002/jts.2490020207 [DOI] [Google Scholar]
- Shapiro F., Forrest M. S. (2004). EMDR: The breakthrough therapy for overcoming anxiety, stress, and trauma. New York, NY: Basic Books. [Google Scholar]
- Strange D., Takarangi M. K. (2012). False memories for missing aspects of traumatic events. Acta Psychologica, 141, 322–326. doi: 10.1016/j.actpsy.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Tousignant J. P., Hall D., Loftus E. F. (1986). Discrepancy detection and vulnerability to misleading postevent information. Memory & Cognition, 14, 329–338. [DOI] [PubMed] [Google Scholar]
- van den Hout M., Bartelski N., Engelhard I. M. (2013). On EMDR: Eye movements during retrieval reduce subjective vividness and objective memory accessibility during future recall. Cognition & Emotion, 27, 177–183. doi: 10.1080/02699931.2012.691087 [DOI] [PubMed] [Google Scholar]
- van den Hout M., Engelhard I. M. (2012). How does EMDR work? Journal of Experimental Psychopathology, 3, 724–738. doi: 10.5127/jep.028212 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Houben_Open_Practices_Disclosure for Lateral Eye Movements Increase False Memory Rates by Sanne T. L. Houben, Henry Otgaar, Jeffrey Roelofs and Harald Merckelbach in Clinical Psychological Science