Abstract
Purpose
We evaluated the impact of postoperative body mass index (BMI) shifts on the quality of life (QoL) following total gastrectomy in patients with gastric cancer.
Materials and Methods
QoL data collected from the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 and QLQ-STO22 questionnaires were obtained from 417 patients preoperatively and 1 year after surgery. Patients were divided into two groups based on changes in BMI: group 1 comprised patientswhose BMIrange category dropped, and group 2 included patients who maintained or rose to a higher category compared to their preoperative BMI category.
Results
There were 276 patients in group 1 and 141 in group 2. QoLs with respect to the global health status and functional scales were not significantly different between the groups 1 year after surgery. However, there were significantly greater decreases in QoL in group 1 due to gastrointestinal symptoms, such as nausea and vomiting (p=0.008), appetite loss (p=0.001), and constipation (p=0.038). Of the QLQ-STO22 parameters, dysphagia (p=0.013), pain (p=0.012), reflux symptoms (p=0.017), eating restrictions (p=0.007), taste (p=0.009), and body image (p=0.009) were associated with significantly worse QoL in group 1 than in group 2 1 year after surgery.
Conclusion
Patients have significantly different QoLs depending on the BMI shift after total gastrectomy. Efforts to reduce the gap in QoL should include intensive nutritional support and restoration of dietary behaviors. Appropriate clinical and institutional approaches, plus active medical interventions, are required for maintaining patients’ BMIs after surgery.
Keywords: Quality of life, Stomach neoplasms, Gastrectomy, Body mass index
Introduction
Surgical resection of the primary tumor, as well as regional lymph node dissection, constitute the only curative treatment for gastric cancer [1]. Although surgical treatment is the only method to gain disease-free status in patients with gastric cancer, most patients who undergo gastrectomy may experience a deteriorated quality of life (QoL), which is affected by a variety of functional and nutritional problems [2]. Patients who undergo total gastrectomy in particular tend to have greater deterioration of QoL, including poor social functioning, nausea/vomiting, dysphagia, dietary restrictions, reflux, and taste compared to patients who undergo distal gastrectomy [3,4]. The QoL after gastrectomy tends to be lowest during the first year after surgery [5-7]. Additionally, weight loss is unavoidable in patients who undergo gastrectomy; it usually lasts for the duration of the first year after surgery, and most patients do not regain their preoperative body weights [8-10]. Various symptomatic nutritional or functional maladies after gastrectomy might lead to weight loss, and postoperative QoL changes in patients with gastric cancer might be closely connected to such weight loss. Thus, it is crucial to investigate any correlations between QoL and weight changes in patients who underwent total gastrectomy.
Among the diverse tools to assess QoL in patients with gastric cancer, the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30, along with the gastric cancer-specific module (QLQ-STO22), have been used most extensively [11,12]. The entire questionnaire has been translated into Korean and validated [13].
There have been several investigations of QoL changes after gastrectomy in patients with gastric cancer using the EORTC QLQ-C30 and QLQ-STO22. However, most such studies compared QoL changes according to the different types of surgical procedures or the progression of QoL changes throughout the postsurgical survival period [2,6,7]. However, little is known about QoL changes according to body mass index (BMI) shifts during the postoperative period. Therefore, we investigated QoL changes during the first year after surgery in relation to BMI shifts, with the goal of providing better personalized medical care by revealing additional factors that could improve the QoL of patients who underwent a total gastrectomy.
Materials and Methods
1. Patients
Patients with gastric cancer who underwent curative total gastrectomy between January 2011 and December 2014 at the Kyungpook National University Hospital (KNUH) and the Kyungpook National University Medical Center (KNUMC) were enrolled. We excluded patients who experienced a recurrence within 1 year after surgery as well as those who died of other causes. Ultimately, 417 patients who completed the entire series of QoL assessments during the first year were analyzed.
The BMI was calculated as body weight/height2 (kg/m2), and BMI values were classified as underweight (< 18.50 kg/m2), normal (18.50-22.99 kg/m2), overweight (23.00-24.99 kg/m2), and obese (≥ 25.00 kg/m2) [14]. Fig. 1 shows the baseline BMIs of patients preoperatively and 1 year after surgery. Patients were divided into two groups based on their BMI shifts: group 1 (n=276) included patients whose BMIs were in lower categories compared to their preoperative classifications; group 2 (n=141) comprised patients who had maintained their BMI categories (n=137) or had shifted to a higher range compared to their preoperative BMIs (n=4). We also performed subgroup analysis. Subgroup analysis was classified as follows. Subgroup 1 (n=69) included patients with BMI change from normal to underweight. Subgroup 2 (n=163) included patients with BMI change from obese to normal BMI. Subgroup 3 (n=137) included patients who had maintained their BMI.
Fig. 1.
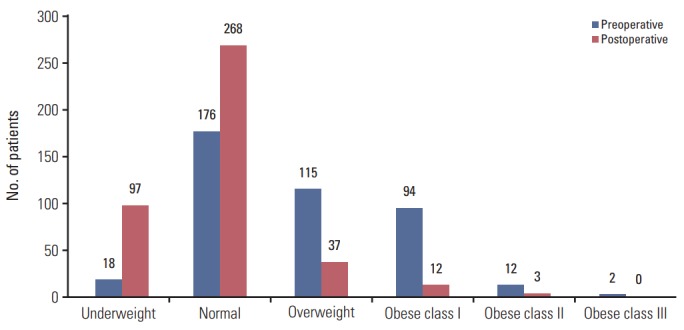
Body mass index shift after total gastrectomy.
2. Surgery
Curative total gastrectomy, D2 lymph node dissection including total omentectomy, and Roux-en-Y esophagojejunostomy were performed in all patients. Reconstruction procedures were performed extracorporeally using circular staplers [15,16]. After surgery, patients were managed according to a clinical protocol that included the intake of drinking water on the third postoperative day, followed by the initiation of a liquid diet on the fourth postoperative day and consumption of a soft diet on the fifth postoperative day. Patients were scheduled to be discharged on the sixth postoperative day.
3. QoL assessments
The Korean versions of the EORTC QLQ-C30 and EORTC QLQ-STO22 were used to assess the QoL. Patients were asked to complete the QLQ-C30 and QLQ-STO22 questionnaires preoperatively and at 1 year after surgery. The QoL assessment was based on the responses to all items in questionnaires as completed by the responders themselves when visiting the outpatient department. If questionnaires were returned with any responses missing, patients were instructed to complete such items. The raw values were linearly transformed into assessment scores ranging from 0 to 100 according to the manual provided by the EORTC. For global health status/QoL and multi-item function scales, a higher score is interpreted as a high QoL and high/healthy level of functioning; however, on symptom scales and single parameters, higher scores reflect additional symptoms/problems. On the EORTC QLQ-STO22, a higher score is interpreted as a low QoL.
4. Statistical analysis
Differences in baseline characteristics between two groups were analyzed with Student’s t test for continuous variables and the chi-square test for categorical variables.
Linear mixed models were used to assess how the surgery affected the changes in QoL between groups and over time. A p-value of < 0.05 was regarded as statistically significant. All statistical analysis was conducted using SPSS software ver. 22 (IBM Corp., Armonk, NY).
5. Ethical statement
The study was approved by the Institutional Review Board of KNUH and KNUMC (approval numbers: 2016-08-025 and 2016-08-019, respectively) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived.
Results
1. Patients characteristics
Table 1 shows the clinicopathological characteristics of the patients. There were no statistical differences in age, sex, comorbidity, previous surgical history, type of surgery, length of hospital stay, number of harvested lymph nodes, and pathological stage between groups 1 and 2.
Table 1.
Characteristics of patients
| Characteristic | Group 1 (n=276) | Group 2 (n=141) | p-value |
|---|---|---|---|
| Age (yr) | 59.2±11.1 | 58.7±11.9 | 0.664 |
| Sex | |||
| Female | 81 (29.3) | 41 (29.1) | 0.954 |
| Male | 195 (70.7) | 100 (70.9) | |
| Comorbidity | |||
| Cardiovascular | 37 (13.4) | 16 (11.3) | 0.108 |
| Cerebrovascular | 4 (1.4) | 1 (0.7) | |
| Diabetes | 14 (5.1) | 3 (2.1) | |
| Hepatic | 4 (1.4) | 2 (1.4) | |
| Renal | 1 (0.4) | 0 | |
| Pulmonary | 0 | 3 (2.1) | |
| Thyroid | 3 (1.1) | 2 (1.4) | |
| Combined disease | 14 (5.1) | 2 (1.4) | |
| Previous operation history | |||
| Gastrectomy | 3 (1.1) | 7 (5.0) | 0.060 |
| Lower anterior resection | 2 (0.7) | 0 | |
| Hysterectomy | 1 (0.4) | 0 | |
| Type of surgery | |||
| OTG | 253 (91.7) | 128 (90.8) | 0.668 |
| LATG | 22 (8.0) | 11 (7.8) | |
| OTG with Whipple’s operation | 1 (0.4) | 2 (1.4) | |
| Hospital stay (day) | 12.4±9.9 | 11.3±4.8 | 0.107 |
| Depth of invasion | |||
| T1a | 72 (26.1) | 22 (15.6) | 0.001 |
| T1b | 92 (33.3) | 40 (28.4) | |
| T2 | 25 (9.1) | 19 (13.5) | |
| T3 | 52 (18.8) | 22 (15.6) | |
| T4a | 35 (12.7) | 38 (27.0) | |
| Lymph node metastasis | |||
| N0 | 205 (74.3) | 90 (63.8) | 0.040 |
| N1 | 19 (6.9) | 19 (13.5) | |
| N2 | 27 (9.8) | 12 (8.5) | |
| N3 | 25 (9.1) | 20 (14.2) | |
| Stage | |||
| IA | 156 (56.5) | 59 (41.8) | 0.085 |
| IB | 26 (9.4) | 16 (11.3) | |
| IIA | 28 (10.1) | 15 (10.6) | |
| IIB | 15 (5.4) | 14 (9.9) | |
| IIIA | 15 (5.4) | 8 (5.7) | |
| IIIB | 21 (7.6) | 14 (9.9) | |
| IIIC | 15 (5.4) | 15 (10.6) | |
| Harvested lymph nodes | 44.6±17.3 | 46.6±20.1 | 0.280 |
| Chemotherapy | |||
| Yes | 61 (22.1) | 51 (36.2) | 0.002 |
| No | 215 (77.9) | 90 (63.8) |
Values are presented as number (%) or mean±standard deviation. Stage grouping by 7th edition of the American Joint Committee on Cancer classification. OTG, open total gastrectomy; LATG, laparoscopic assisted total gastrectomy.
2. Changes of QoL according to BMI shift
Mean QoL scores preoperatively and 1 year after surgery in the two groups are shown in Table 2. The global health status/QoL was improved after surgery in both groups. Functional scales―except for the emotional scale―showed worse outcomes after surgery in both groups; the mean scores of group 1 were lower than those of group 2 at 1 year after surgery. Moreover, all symptom scales/category scores of group 1 patients were higher compared to their preoperative scores. Group 2 patients showed similar outcomes to group1; however, the mean scores for nausea and vomiting, insomnia, appetite loss, and constipation decreased after surgery. With respect to the EORTC-STO22, the mean scores of all scales and items increased after surgery, but the degree of increase was greater in group 1 than in group 2.
Table 2.
Comparison of QoL changes according to BMI shifting
| Variable | Group 1 (n=276) |
Group 2 (n=141) |
||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | p-value | Preoperative | Postoperative | p-value | |
| EORTC QLQ-C30 | ||||||
| Global health status/QoLa) | 61.7 | 64.4 | 0.744 | 61.0 | 66.5 | 0.416 |
| Functional scalesa) | ||||||
| Physical functioning | 86.3 | 79.8 | 0.495 | 87.3 | 81.1 | 0.436 |
| Role functioning | 90.8 | 79.6 | 0.389 | 89.2 | 81.4 | 0.381 |
| Emotional functioning | 79.6 | 80.9 | 0.199 | 82.3 | 82.7 | 0.328 |
| Cognitive functioning | 89.2 | 82.4 | 0.631 | 88.4 | 84.2 | 0.296 |
| Social functioning | 83.8 | 82.6 | 0.415 | 83.6 | 81.6 | 0.645 |
| Symptom scales/itemsb) | ||||||
| Fatigue | 21.6 | 32.2 | 0.802 | 22.1 | 30.5 | 0.393 |
| Nausea and vomiting | 9.0 | 16.2 | 0.071 | 12.4 | 11.8 | 0.035 |
| Pain | 11.8 | 15.5 | 0.967 | 11.7 | 12.2 | 0.058 |
| Dyspnea | 12.2 | 15.0 | 0.602 | 11.1 | 13.0 | 0.372 |
| Insomnia | 15.7 | 17.5 | 0.607 | 17.0 | 15.1 | 0.332 |
| Appetite loss | 14.4 | 20.2 | 0.025 | 20.0 | 13.0 | 0.003 |
| Constipation | 12.4 | 15.5 | 0.112 | 16.1 | 13.2 | 0.307 |
| Diarrhea | 13.4 | 26.9 | 0.588 | 12.3 | 22.5 | 0.080 |
| Financial difficulties | 18.7 | 21.1 | 0.271 | 15.8 | 19.4 | 0.521 |
| EORTC QLQ-STO22b) | ||||||
| Dysphagia scale | 6.6 | 16.9 | 0.140 | 8.5 | 14.6 | 0.118 |
| Pain scale | 15.2 | 22.2 | 0.125 | 18.0 | 18.5 | 0.120 |
| Reflux symptoms scale | 11.1 | 17.9 | 0.078 | 13.9 | 15.4 | 0.190 |
| Eating restrictions scale | 8.7 | 22.9 | 0.008 | 13.0 | 21.1 | 0.362 |
| Anxiety scale | 26.1 | 36.3 | 0.773 | 26.8 | 32.4 | 0.092 |
| Having a dry mouth | 18.4 | 25.8 | 0.623 | 19.6 | 23.2 | 0.320 |
| Taste | 6.4 | 15.1 | 0.165 | 9.0 | 10.9 | 0.051 |
| Body image | 19.3 | 37.4 | 0.225 | 22.9 | 32.9 | 0.113 |
| Hair loss | 18.9 | 29.5 | 0.552 | 21.5 | 27.1 | 0.558 |
Values are presented as mean score. Group 1: Patients whose BMIs were in lower categories compared to their preoperative classifications. Group 2: Patients who had maintained their BMI categories or had shifted to a higher range compared to their preoperative BMIs. QoL, quality of life; BMI, body mass index; EORTC QLQ, European Organization for Research and Treatment Quality of Life Questionnaire.
A higher score represents a better QoL,
A higher score represents a worse QoL.
We also plotted the mean QoL score changes between the groups over time. Fig. 2 shows the changes in global health status and functional scales between the two groups; global health status and emotional functioning increased in both groups with no significant difference. Scales other than emotional functioning were lower in both groups after surgery, without significant differences. Fig. 3 shows the changes of symptom scales and categories between the two groups. The QoL due to gastrointestinal symptoms, such as nausea and vomiting (p=0.008), appetite loss (p=0.001), and constipation (p=0.038), decreased significantly more in group 1 than in group 2. Fig. 4 shows the changes in QLQ-STO22 categories in both groups. Of these categories, dysphagia (p=0.013), pain (p=0.012), reflux symptoms (p=0.017), dietary restrictions (p=0.007), taste (p=0.009), and body image (p=0.009) were associated with significantly worse QoLs in group 1 than in group 2.
Fig. 2.
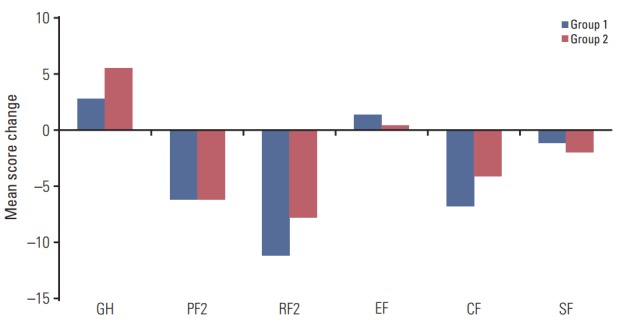
Changes in the quality of life (QoL) according to body mass index shifts 1 year after total gastrectomy, as assessed by the global health status/QoL and functional scales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30. GH, global health status; PF2, physical functioning; RF2, role functioning; EF, emotional functioning; CF, cognitive functioning; SF, social functioning.
Fig. 3.

Changes in the quality of life according to body mass index shifts 1 year after total gastrectomy, as assessed by symptom scales/items of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30. FA, fatigue; NV, nausea and vomiting; PA, pain; DY, dyspnea; SL, insomnia; AP, appetite loss; CO, constipation; DI, diarrhea; FI, financial difficulties. *p < 0.05.
Fig. 4.
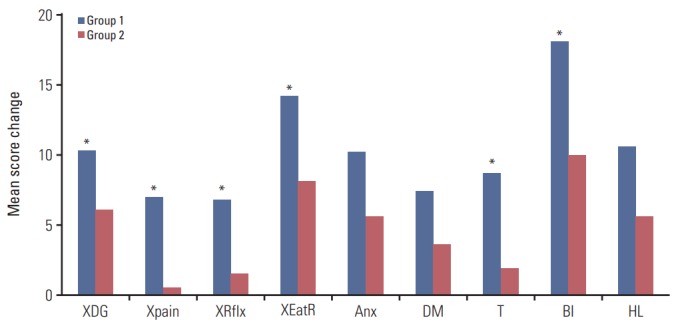
Changes in the quality of life according to body mass index shifts 1 year after total gastrectomy, as assessed by symptom scales/items of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire STO22. XDG, dysphagia; Xpain, pain; XRflx, reflux symptoms; XEatR, eating restrictions; Anx, anxiety; DM, having a dry mouth; T, taste; BI, body image; HL, hair loss. *p < 0.05.
Comparing between subgroup 1 and subgroup 2, age was significantly older in subgroup 1 than subgroup 2 (62.1±10.7 and 58.3±11.5, respectively) and hospital stay was significantly shorter in subgroup 1 than subgroup 2 (10.6±3.3 and 12.8±10.5, respectively). In the aspect of QoL, the global health status/QoL (p=0.011), physical functioning (p=0.011), and role functioning (p=0.026) were significantly decreased in subgroup 1 than in subgroup 2. Also, when comparing between subgroup 2 and subgroup 3, characteristic of patients were significantly different in depth of invasion (p=0.001), stage (p=0.048), and chemotherapy (p=0.004) between two groups. In the aspect of QoL, nausea and vomiting (p=0.010), appetite loss (p=0.001), constipation (p=0.031), pain (p=0.016), eating restrictions (p=0.026), taste (p=0.015), and body image (p=0.034) showed significantly better QoL in subgroup 3 than in subgroup 2.
Discussion
The postoperative QoL in patients who undergo a gastrectomy is dependent on various factors, such as the type of reconstruction, extent of gastric resection, and surgical approach [4,17-20]. In terms of the extent of gastric resection, it is expected that patients who undergo total gastrectomy experience significantly worse QoLs than patients who undergo distal gastrectomy during the postoperative period [3,4]. Furthermore, patients who undergo total gastrectomy more often experience impaired nutritional intake due to the absence of the stomach, which limits the amount of food that can be consumed in a single session and results in significant body weight loss [21]. However, the degree of body weight change after total gastrectomy may vary due to patients’ individual factors such as preoperative individual characteristics, pathological characteristics, and the postoperative course. Additionally, the preoperative BMI status may influence nutritional outcomes.
Consistent with previous studies [4,22], our results showed that the overall global health status improved after surgery, and that the functional scale (except emotional function) decreased after surgery regardless of shifts in BMI. As has previously been shown [23], emotional functioning might be related to depression caused by the initial diagnosis of cancer, and patients experience a feeling of relief from depression attributed to their disease over time. Our study showed improvements in emotional functioning after surgery in both groups. Functional scales might be more affected by the psychic domain than the somatic domain compared to symptom scales or QLQ-STO22. Our results showed no significant differences in functional scales regardless of BMI shifts after total gastrectomy.
With respect to symptom scales/categories and the QLQ-STO22, previous studies showed that patients who underwent total gastrectomy experienced nausea and vomiting, dysphagia, reflux symptoms, eating restrictions, dry mouth, and taste to a greater extent than those who underwent distal gastrectomy [3,4]. A poorer QoL after total gastrectomy might be affected by the surgical procedure itself, but the degree to which the QoL worsens might be different depending on BMI shifts. Deteriorating QoL scales/categories were related to gastrointestinal symptoms and eating problems, such as nausea and vomiting, appetite loss, constipation, dysphagia, reflux symptoms, dietary restrictions, and taste. These symptoms are related to various conditions, such as preoperative patient characteristics, surgical methods, pathological characteristics, and the postoperative course. As our study design was to adjust for various conditions and other potential confounding factors, the differences in QoL between the two groups may be related to symptoms of body weight loss. Hence, patients who shifted to lower BMI categories after total gastrectomy ought to receive medical intervention to improve their gastrointestinal symptoms and eating problems. Moreover, it is important to reassure patients who fear eating because of dysphagia or who restrict their diets, and to assist them in changing their eating habits post-surgery to including smaller amounts of food while increasing the frequency of their meals.
Our data showed that body image was significantly poorer in patients whose BMIs fell after total gastrectomy. The EORTC QLQ-STO22 questionnaire included the query “Have you felt physically less attractive as a result of your disease or treatment?” Patients might regard weight loss as negatively contributing to their body image; this is true even if patients are in the normal BMI range after surgery. We posit that this might be related to concern about weight loss, which is one of the symptoms of malnourishment. Thus, patients who have a worse body image because of weight loss should be accurately assessed in order to clarify their BMI range. Additionally, it is critical that surgeons provide precise body composition data to patients, and identify those patients who have shifted to the underweight BMI category while reporting on their poorer body image score after surgery. Nutritional education and medical support are required for these patients. Moreover, reassuring patients regarding their body image is more important in those who shift to a normal or above normal BMI category after surgery.
Most patients who undergo total gastrectomy experience a BMI shift after surgery. The degrees of such shifts vary among patients with different characteristics. Our data regarding QoL differences according to BMI shifts after surgery might be clinically significant, as most patients who underwent total gastrectomy were in the underweight or normal BMI ranges. Approximately 20% of the patients were in the underweight BMI category after total gastrectomy; these patients ought to receive active medical intervention to overcome nutritional deficits and deteriorated QoL. The majority of patients were in the normal BMI range after total gastrectomy, including those who shifted to this group and those who maintained their BMI categories. However, even patients with normal BMI ranges after surgery may have different QoL statuses. Therefore, patients whose BMIs shifted to a normal range after surgery should receive reassurance concerning their QoL instead of symptom control and nutritional education; the latter is more important in patients who are underweight after surgery.
The subgroup analysis also supports the results of our study. When comparing subgroup 1 between subgroup 2, global health status/QoL and functional scales showed significantly better QoL in subgroup 2 than in subgroup 1. However, there were no significant differences between two groups in symptoms scale and STO 22. These results might be affected by the significant low age in subgroup 2 than in subgroup 1. In subgroup 2 and subgroup 3 analysis, subgroup 3 showed significant better QoL of similar items in our initial results than in subgroup 2. From the 2 subgroup analyses, it could be concluded that maintaining the BMI after surgery showed better QoL than decreased BMI after surgery. Some of the obese patients might experience relief of symptoms related to overweight because of loss of weight. Also, in a healthy population, they can experience a better QoL by reducing their weight. However, in cancer patients, patients with decreased weight after surgery might believe that patients’ preoperative weight is considered to be a healthy weight, and it seems to have worse QoL than whose weight is maintained. Differences in the QoL due to weight changes may require a different approach to healthy population and cancer patients, as well as, maintaining BMI after surgery is crucial to patients who underwent gastrectomy for gastric cancer.
Notably, we did not observe a causal relationship between body weight loss and QoL changes post-surgery. Although our study design was to observe QoL change according to BMI shifting after surgery, poorer QoL might have an inverse correlation with body weight. In the first postsurgical year, significant changes in body weight and QoL might influence each other; therefore, it is difficult to determine a clear correlation or causal relationship between them. Since BMI is an objective index of body weight, we classified the patients by BMI shifts after surgery rather than by body weight loss. Further study is required to clarify the interrelationship between nutritional parameters (including body weight and BMI) and QoL after surgery.
As the distributions of BMI in Asian populations are distinctly left-shifted compared to those in Western populations, the results of our study should be validated in Western countries. However, we focused on the BMI shifts, not actual distributions, to study correlations with QoL changes.
In this study, there was significant difference in the percentage of patients who received adjuvant chemotherapy between the two groups. Adjuvant chemotherapy might affect to QoL. However, as postoperative 1 year is considered to be the time since the end of the adjuvant chemotherapy, the impact of adjuvant chemotherapy on QoL might be not significant. Also, there are few studies on the effect of adjuvant chemotherapy on QoL in patients with gastric cancer. Further study is needed to explore the impact of the adjuvant chemotherapy after gastrectomy on QoL.
In conclusion, patients have significantly different QoLs depending on their BMI shifts after total gastrectomy. Efforts to reduce such declinations in QoL should include intensive nutritional support and the restoration of dietary behaviors. Appropriate clinical and institutional approaches, as well as active medical interventions, are required for maintaining BMI after surgery. Moreover, patients should be well informed that their QoL may change after surgery, and surgeons should make an effort to reassure patients postoperatively.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P'eng FK. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg. 2000;24:465–72. doi: 10.1007/s002689910074. [DOI] [PubMed] [Google Scholar]
- 2.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256:1008–13. doi: 10.1097/SLA.0b013e31827661c9. [DOI] [PubMed] [Google Scholar]
- 3.Lee SS, Chung HY, Kwon OK, Yu W. Long-term quality of life after distal subtotal and total gastrectomy: symptom- and behavior-oriented consequences. Ann Surg. 2016;263:738–44. doi: 10.1097/SLA.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 4.Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014;14:32–8. doi: 10.5230/jgc.2014.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong H, Kwon OK, Yu W. Changes of quality of life after gastric cancer surgery. J Gastric Cancer. 2012;12:194–200. doi: 10.5230/jgc.2012.12.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karanicolas PJ, Graham D, Gonen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039–46. doi: 10.1097/SLA.0b013e31828c4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer. Cancer Res Treat. 2016;48:1030–6. doi: 10.4143/crt.2015.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran FT, Hill GL. Failure of nutritional recovery after total gastrectomy. Br J Surg. 1990;77:1015–7. doi: 10.1002/bjs.1800770920. [DOI] [PubMed] [Google Scholar]
- 9.Braga M, Molinari M, Zuliani W, Foppa L, Gianotti L, Radaelli G, et al. Surgical treatment of gastric adenocarcinoma: impact on survival and quality of life: a prospective ten year study. Hepatogastroenterology. 1996;43:187–93. [PubMed] [Google Scholar]
- 10.Liedman B, Andersson H, Berglund B, Bosaeus I, Hugosson I, Olbe L, et al. Food intake after gastrectomy for gastric carcinoma: the role of a gastric reservoir. Br J Surg. 1996;83:1138–43. doi: 10.1002/bjs.1800830835. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 12.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–71. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 13.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–8. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598–605. doi: 10.1007/s00464-013-2796-8. [DOI] [PubMed] [Google Scholar]
- 16.Wada N, Kurokawa Y, Takiguchi S, Takahashi T, Yamasaki M, Miyata H, et al. Feasibility of laparoscopy-assisted total gastrectomy in patients with clinical stage I gastric cancer. Gastric Cancer. 2014;17:137–40. doi: 10.1007/s10120-013-0235-0. [DOI] [PubMed] [Google Scholar]
- 17.Bae JM, Kim S, Kim YW, Ryu KW, Lee JH, Noh JH, et al. Health-related quality of life among disease-free stomach cancer survivors in Korea. Qual Life Res. 2006;15:1587–96. doi: 10.1007/s11136-006-9000-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 19.Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: short- and long-term consequences. Am J Surg. 2012;204:203–9. doi: 10.1016/j.amjsurg.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Misawa K, Fujiwara M, Ando M, Ito S, Mochizuki Y, Ito Y, et al. Long-term quality of life after laparoscopic distal gastrectomy for early gastric cancer: results of a prospective multiinstitutional comparative trial. Gastric Cancer. 2015;18:417–25. doi: 10.1007/s10120-014-0374-y. [DOI] [PubMed] [Google Scholar]
- 21.Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313–9. doi: 10.1016/j.gassur.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357–64. doi: 10.1007/s00268-010-0860-2. [DOI] [PubMed] [Google Scholar]
- 23.Park JY, Eom BW, Jo MJ, Yoon HM, Ryu KW, Kim YW, et al. Health-related quality of life after robot-assisted distal gastrectomy in early gastric cancer. World J Surg. 2014;38:1112–20. doi: 10.1007/s00268-013-2390-1. [DOI] [PubMed] [Google Scholar]


