Abstract
Purpose
Not many studies have evaluated the adoption and dissemination of evidence-based medicine in rectal cancer radiotherapy (RT). We aimed to analyze the differences by institutional characteristics and geography in adopting evidence-based care for rectal cancer RT and factors affecting the adoption in Korea.
Materials and Methods
Korean National Health Insurance Service claims database was used. All rectal cancer patients treated with radical surgery and adjuvant RT at the same institution in 2005-2016 were included in this study. RT within 3 months before and after surgery was regarded as preoperative and postoperative RT, respectively.
Results
A total of 16,827 patients treated in 83 institutions were included in the analysis. The use of preoperative RT has substantially increased over time, from 40.6% in 2005 to 84.2% in 2016 all over the nation. The proportion of preoperative RT (54.8%) exceeded that of postoperative RT (45.2%) in 2006. However, a wide range of institutional and regional variation was observed. Compared to high-volume institutions, low-volume institutions showed late adoption and variable dissemination patterns of preoperative RT. Busan–Ulsan–Gyeongsangnam-do and Gangwon-do showed slower adoption and less use of preoperative RT than other region.
Conclusion
We demonstrated gradual and steady increase in adoption of preoperative RT in rectal cancer treatment nationally from 2005 to 2016. Institutional variations between high- and low-volume institutions were observed.
Keywords: Rectal neoplasms, Preoperative radiotherapy, Evidence-based medicine, Patterns of care
Introduction
Postoperative radiotherapy (RT) with or without chemotherapy is offered to patients with locally advanced rectal cancer to reduce local recurrences and to improve survival. This is regarded as standard adjuvant treatment for patients with locally advanced rectal cancer [1,2]. Preoperative irradiation provides several potential advantages over postoperative irradiation, including irradiation of nonhypoxic tissue, minimizing radiation-induced injury to the small bowel, and increasing the potential for sphincter-sparing surgery [3]. Based on these rationales, three prospective randomized trials (two in the United States and one in Germany) comparing preoperative with postoperative chemoradiotherapy (CRT) were initiated between 1993 and 1994. While two U.S. trials did not accrue enough patients to draw any conclusion, the German Rectal Cancer Study Group reported that preoperative CRT, as compared with postoperative CRT, was associated with improved local control, increased rates of sphincter preservation, and reduced treatment-related toxicities [4]. Since the German study published these outcomes in 2004, preoperative CRT followed by surgical resection has become the preferred treatment for patients with clinical stage T3-4 or node-positive rectal cancer worldwide; therefore, was considered as practice-changing, high-level evidence.
Practice of evidence-based medicine (EBM), by making treatment decision on the basis of the reliable and up-to-date evidence is the foundation of modern clinical medicine [5]. Although physicians are encouraged to learn new medical knowledge with high level of evidence, there are few reports regarding the adoption or dissemination of this knowledge into real clinical practice in Korea. As for the treatment of locally advanced rectal cancer, a few research groups in the United States were interested in implementation of evidencebased treatment, which was the transition of practice from postoperative to preoperative CRT, and found gradual increase of the use of preoperative RT instead of postoperative RT analyzing Surveillance, Epidemiology, and End Results (SEER) database or National Cancer Database [3,6,7].
We aimed to investigate the adoption and dissemination of EBM in the treatment of locally advanced rectal cancer in Korean using claims data of National Health Insurance Service (NHIS). Furthermore, we examined different patterns of practice change over time according to the characteristics of institutions and the region.
Materials and Methods
1. Data source
National claims data from NHIS of the Republic of Korea (KNHIS) from 2005 to 2016 were analyzed. The NHIS database covers over 98% of the Korean population for over 30 years [8]. Moreover, it has comprehensive information of the diagnoses, treatments, procedures, surgical history, and prescription records for all insured patients. In addition, the insured population, and their dependents are all required to have periodic general health examinations [9].
This study was conducted as part of the Korean Health Map research consortium (KNHIS-ATLAS, 20170603E6E) sponsored by NHIS.
2. Study population and operational definition
Data with the code C20, indicating rectal cancer according to International Classification of Diseases, 10th edition, were primarily screened. We set 2000-2004 as a washout period, and newly diagnosed rectal cancer patients in 2005-2016 were included in study population to minimize confounding effects of previously diagnosed rectal cancer.
As we intended to analyze the adoption of preoperative RT according to institutions over time, 87 institutions with radiation therapy capacity were identified first, and information on each institution was collected without institution’s identifier. With same reason, if a patient had surgery and RT at different institutions, the patient was excluded in this study. And then, the number of patients who had surgical resection, preoperative RT, and postoperative RT was respectively identified according to institutions and each year in 2005-2016. A total of seven geographical groups were defined based on administrative district. And each institution was regrouped in order to evaluate regional variation.
Radical surgical resection for rectal cancer mainly included low anterior resection and abdomino-perineal resection (Miles' Operation). KNHIS reimbursement code QA921-926 (with lymph node dissection) and Q2921-2926 (without lymph node dissection) was used for selection of patients (Table 1). Considering that preoperative RT ends 6-8 weeks before surgery, postoperative RT starts 4-6 weeks after surgery, and claim data is usually generated every month, RT within 3 months before and after surgery was counted for preoperative and postoperative RT, respectively. RT included all types of RT technique. KNHIS reimbursement code HD061 (3-dimensional conformal therapy), HZ271 (intensitymodulated RT, started reimbursed from July 2011), HD110, HD111, HD121, HD211, HD051-059 was used for selection of patients (Table 2).
Table 1.
Korean National Health Insurance Service reimbursement codes for radical surgery of rectal cancer
| Reimbursement code |
Name of radical surgery | |
|---|---|---|
| LND(+) | LND(‒) | |
| QA921 | Q2921 | Rectal and sigmoid resection–anterior resection |
| QA922 | Q2922 | Rectal and sigmoid resection–low anterior resection |
| QA923 | Q2923 | Rectal and sigmoid resection (abdomino-perineal resection) |
| QA924 | Q2924 | Rectal and sigmoid resection–abdominal pull through operation |
| QA925 | Q2925 | Total coloprotectomy–with ileostomy |
| QA926 | Q2926 | Total coloprotectomy (with ileal pouch-anal anastomosis) |
LND, lymph node dissection.
Table 2.
Korean National Health Insurance Service reimbursement codes for radiotherapy of rectal cancer
| Reimbursement code | Name of radiotherapy |
|---|---|
| HD061 | 3-Dimensional conformal therapy |
| HZ271 | Intensity modulated radiation therapy |
| HD110 | Fractionated stereotactic radiotherapy |
| HD111 | Body stereotactic radiosurgery–linear accelerator |
| HD121 | Proton therapy |
| HD211 | Body stereotactic radiosurgery–cyber knife |
| HD051 | Teletherapy–low energy–single port |
| HD052 | Teletherapy–middle energy–single port |
| HD053 | Teletherapy–high energy–single port |
| HD054 | Teletherapy–low energy–paralled opposed ports |
| HD055 | Teletherapy–middle energy–paralled opposed ports |
| HD056 | Teletherapy–high energy–paralled opposed ports |
| HD057 | Rotational irradiation–low energy |
| HD058 | Rotational irradiation–middle energy |
| HD059 | Rotational irradiation–high energy |
We assumed that the patients treated with surgery and adjuvant RT, whether preoperative or postoperative, were locally advanced rectal cancer patients. Because, although claim data we used do not have clinical information, the most widely accepted indication of adjuvant RT for rectal cancer is stage T3-4 or node-positive disease based on German trial’s results.
3. Ethical statement
This study was approved by Institutional Review Board. And, participant consent was not specifically obtained, because this study was based on collected administrative data.
Results
A total of 16,827 rectal cancer patients in 83 institutions treated with radical surgery and pre/postoperative adjuvant RT in a same hospital over a 12-year period, from 2005 to 2016, were identified. Table 3 summarizes the characteristics of all institutions capable of RT in Korea as of year 2016 (four institutions without any adjuvant RT cases for rectal cancer during study period were excluded). Seoul and Incheon–Gyeonggi-do has more than half institutions (46 of 83, 55.4%), and about half capable of RT were tertiary hospitals. As of 2016, six institutions had more than six full-time radiation oncologists, and more than half institutions had only one or two. Six institutions treated more than 1,000 patients of surgery and adjuvant RT over 12 years, of which four are located in Seoul, one in Incheon–Gyeonggi-do, and one in Gwangju–Jeolla-do–Jeju. Among seven institutions having 300-499 cases, four are located in Incheon–Gyeonggi-do, three in Busan–Ulsan–Gyeongsangnam-do, one in Seoul, and one in Daegu–Gyeongsangbuk-do.
Table 3.
Characteristics of 83 institutions
| Characteristic | No. of institutions |
|---|---|
| Region | |
| Seoul | 25 |
| Incheon–Gyeonggi-do | 20 |
| Daejeon–Chungcheong-do | 7 |
| Gwangju–Jeolla-do–Jeju-do | 8 |
| Busan–Ulsan–Gyeongsangnam-do | 11 |
| Daegu–Gyeongsangbuk-do | 8 |
| Gangwon-do | 4 |
| Tertiary hospital | |
| Yes | 42 |
| No | 41 |
| No. of hospital beds | |
| 2,000-2,499 | 1 |
| 1,500-1,999 | 3 |
| 1,100-1,499 | 4 |
| 900-1,099 | 7 |
| 700-899 | 27 |
| 500-699 | 30 |
| ≤ 499 | 11 |
| No. of radiation oncologists | |
| ≥ 6 | 6 |
| 4-5 | 10 |
| 3 | 21 |
| 2 | 25 |
| 1 | 21 |
| No. of RT cases for 12 years | |
| ≥ 1,000 | 6 |
| 500-999 | 0 |
| 300-499 | 7 |
| 200-299 | 10 |
| 100-199 | 22 |
| 50-99 | 12 |
| 10-49 | 21 |
| 1-9 | 5 |
| Total | 83 |
Four institutions without any adjuvant radiotherapy (RT) for rectal cancer during study period were excluded. The results were as of year 2016.
1. Adoption and dissemination of preoperative RT
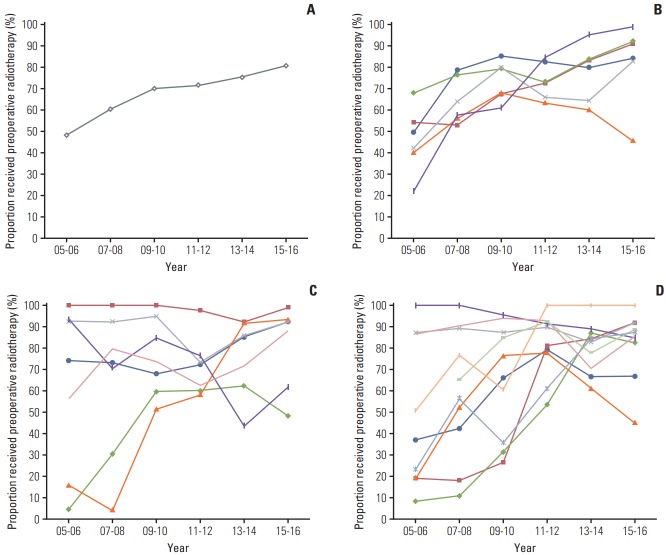
The use of preoperative RT during the study period has substantially increased over time, from 40.6% in 2005 to 84.2% in 2016 in all institutions across the country. Fig. 1A showed a steep adoption of preoperative RT between 2005 and 2010. After 2010, the increasing use of preoperative RT has become steady but less steep. The proportion of patients who received preoperative RT (54.8%) exceeded the proportion who received postoperative RT (45.2%) in 2006 (Fig. 1A).
Fig. 1.
Proportion of rectal cancer patients who received preoperative radiotherapy (RT) by year of diagnosis. (A) National average. (B) Six institutions > 1,000 cases/12 years. (C) Seven institutions with 300-499 cases/12 years. (D) Ten institutions with 200-300 cases/12 years. Vertical axis indicates the number of preoperative RT divided by the number of preoperative and postoperative RT and horizontal axis indicates year of diagnosis.
2. Institutional variation in dissemination of preoperative RT
Table 4 presented proportion of patients who received preoperative RT by institutional characteristics. There were six large-volume institutions, having treated more than 1,000 cases of surgery and adjuvant RT for 12 years. A total number of cases treated in these institutions were 7,298 (43.4% of all cases). Fig. 1B-D presented increasing trends of the use of preoperative RT according to the adjuvant RT cases during study period. All the institutions exhibited overall increasing trend over time, irrespective of the number of cases. In addition, relatively low-volume institutions showed relatively late adoption of preoperative RT in Fig. 1D, the line steeply increased after 2011.
Table 4.
Proportion of patients who received preoperative radiotherapy by hospital characteristics
| Characteristic | 2005-2006 | 2007-2008 | 2009-2010 | 2011-2012 | 2013-2014 | 2015-2016 |
|---|---|---|---|---|---|---|
| Region | ||||||
| Seoul | 373/889 (42.0) | 641/1,087 (59.0) | 924/1,326 (69.7) | 1,112/1,545 (72.0) | 967/1,269 (76.2) | 862/1,095 (78.7) |
| Incheon–Gyeonggi-do | 359/577 (62.2) | 443/653 (67.8) | 444/621 (71.5) | 542/755 (71.8) | 551/694 (79.4) | 645/749 (86.1) |
| Daejeon–Chungcheong-do | 51/153 (33.3) | 91/150 (60.7) | 113/142 (79.6) | 148/169 (87.6) | 104/119 (87.4) | 123/144 (85.4) |
| Gwangju–Jeolla-do–Jeju-do | 43/108 (39.8) | 122/210 (58.1) | 242/319 (75.9) | 232/329 (70.5) | 219/332 (66.0) | 267/324 (82.4) |
| Busan–Ulsan–Gyeongsangnam-do | 119/273 (43.6) | 80/218 (36.7) | 157/259 (60.6) | 204/311 (65.6) | 225/337 (66.8) | 184/265 (69.4) |
| Daegu–Gyeongsangbuk-do | 137/213 (64.3) | 161/221 (72.9) | 158/211 (74.9) | 72/126 (57.1) | 98/127 (77.2) | 87/104 (83.7) |
| Gangwon-do | 6/26 (23.1) | 22/44 (50.0) | 45/87 (51.7) | 58/80 (72.5) | 79/93 (84.9) | 53/73 (72.6) |
| Tertiary hospital | ||||||
| Yes | 821/1,756 (46.8) | 1,166/1,980 (58.9) | 1,700/2,421 (70.2) | 1,928/2,680 (71.9) | 1,716/2,267 (75.7) | 1,648/2,049 (80.4) |
| No | 267/483 (55.3) | 394/603 (65.3) | 383/544 (70.4) | 440/635 (69.3) | 527/704 (74.9) | 573/705 (81.3) |
| No. of hospital bed | ||||||
| ≥ 1,500 | 318/749 (42.5) | 528/855 (61.8) | 747/1,025 (72.9) | 813/1,071 (75.9) | 620/772 (80.3) | 532/644 (82.6) |
| 1,100-1,499 | 95/153 (62.1) | 130/188 (69.1) | 163/231 (70.6) | 206/298 (69.1) | 197/267 (73.8) | 243/300 (81.0) |
| 900-1,099 | 104/131 (79.4) | 103/164 (62.8) | 155/213 (72.8) | 195/285 (68.4) | 147/245 (60.0) | 128/199 (64.3) |
| 700-899 | 335/719 (46.6) | 391/727 (53.8) | 501/761 (65.8) | 592/842 (70.3) | 596/759 (78.5) | 523/654 (80.0) |
| 500-699 | 226/470 (48.1) | 375/595 (63.0) | 463/653 (70.9) | 481/710 (67.7) | 590/803 (73.5) | 721/863 (83.5) |
| ≤ 499 | 10/17 (58.8) | 33/54 (61.1) | 54/82 (65.9 | 81/109 (74.3) | 93/125 (74.4) | 74/94 (78.7) |
| No. of radiation oncologists | ||||||
| ≥ 6 | 475/980 (48.5) | 751/1,149 (65.4) | 943/1,269 (74.3) | 984/1,291 (76.2) | 772/957 (80.7) | 715/844 (84.7) |
| 4-5 | 311/475 (65.5) | 407/595 (68.4) | 516/699 (73.8) | 501/716 (70.0) | 475/681 (69.8) | 561/699 (80.3) |
| 3 | 203/467 (43.5) | 258/497 (51.9) | 389/571 (68.1) | 507/682 (74.3) | 533/671 (79.4) | 454/553 (82.1) |
| 2 | 65/245 (26.5) | 101/256 (39.5) | 176/311 (56.6) | 268/439 (61.0) | 331/454 (72.9) | 327/424 (77.1) |
| 1 | 34/72 (47.2) | 43/86 (50.0) | 59/115 (51.3) | 108/187 (57.8) | 132/208 (63.5) | 164/234 (70.1) |
| No. of RT cases for 12 years | ||||||
| ≥ 1,000 | 496/1,030 (48.2) | 818/1,254 (65.2) | 1,082/1,446 (74.8) | 1,070/1,442 (74.2) | 860/1,105 (77.8) | 859/1,021 (84.1) |
| 300-499 | 303/405 (74.8) | 299/426 (70.2) | 345/457 (75.5) | 333/464 (71.8) | 354/462 (76.6) | 373/448 (83.3) |
| 200-299 | 129/334 (38.6) | 217/387 (56.1) | 292/448 (65.2) | 368/451 (81.6) | 339/435 (77.9) | 291/359 (81.1) |
| 100-199 | 120/343 (35.0) | 161/369 (43.6) | 270/418 (64.6) | 400/612 (65.4) | 464/596 (77.9) | 422/544 (77.6) |
| 1-99 | 40/127 (31.5) | 65/147 (44.2) | 94/196 (48.0) | 197/346 (56.9) | 226/373 (60.6) | 276/382 (72.3) |
Values are presented as the number of patients treated with preoperative radiotherapy/the total number of patients treated with preoperative or postoperative radiotherapy (%).
However, there was an obvious institutional variation. Six high-volume institutions showed gradual and steady increase in general (Fig. 1B). Interestingly, one of them consistently showed low proportion of preoperative RT compared to other five institutions as well as the national average (Fig. 1A), and even decreasing tendency in use of preoperative RT after 2013-2014. On the other hand, low-volume institutions showed drastic changes and wider range of variation in the use of preoperative RT. Among seven institutions with 300-499 cases, three institutions started from top (93%-100%) in 2005-2006, but two started from bottom (4%-16%). The final proportion of receipt of preoperative RT in 2015-2016 also showed wide variation from about 50% to 100% (Fig. 1C). The next 10 institutions also showed drastic changes and wide variation similar to the former seven institutions (Fig. 1D).
3. Regional variation in dissemination of preoperative RT
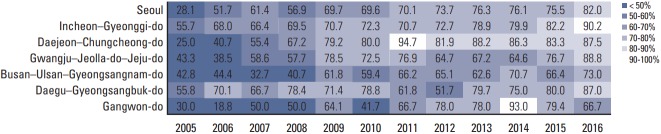
Significant regional variation was also observed when regrouped according to the location of each institution (Table 4). National average showed an intersection between preoperative and postoperative RT in 2006. Daejeon-Chungcheong showed the fastest dissemination and reached over 80% in 2011-2012. On the other hand, Busan–Ulsan–Gyeongsangnam-do and Gangwon-do did not reached 50% of the use of preoperative RT until 2008 (Fig. 2), and still showed about 70% in 2016, which was much lower proportion than the average use of preoperative RT (84.2% at 2016).
Fig. 2.
Dissemination of the use of preoperative radiotherapy by year of diagnosis and location of institutions.
Discussion
EBM is an approach to decision making on the basis of the reliable and up to date best evidence [5]. Early adoption and provision of treatment with high-level of evidence is undoubtedly important. However, dissemination of EBM into clinical practice has been recognized to be problematic [3,10,11].
In 2004, German study group published results of a well-designed randomized controlled trial preoperative RT was associated with improved local control, increased rates of sphincter preservation, and reduced treatment-related toxicities. After this landmark study, preoperative CRT followed by surgical resection has become regarded as a standard treatment for locally advanced rectal cancer. This study presents the adoption and dissemination of EBM in cancer treatment, by evaluating patterns of adjuvant RT for rectal cancer treatment in Korea.
To our knowledge, this is the first study that shows the transition of RT in rectal cancer in Korea, from postoperative to preoperative. It is noteworthy that our data was obtained by complete enumeration from Korean NHIS, which means it was not sampled data. Our results demonstrate that the use of preoperative RT substantially increased over 12 years, from 40.6% in 2005 to 84.2% in 2016. The proportion of patients who received preoperative RT (54.8%) surpassed the proportion who received postoperative RT (45.2%) in 2006. Although the slope of EBM adoption became gradually less steep over time, the change of practice pattern was irreversible. The changing pattern was concordant with results from undermentioned studies [3,6,7].
A few research groups in the United States were interested in implementation of evidence-based treatment, which was the transition of practice from postoperative to preoperative RT, and found gradual increase of the use of preoperative RT instead of postoperative RT analyzing SEER database or National Cancer Database. The first study interested in implementation of evidence-based standards was performed by Fitzgerald et al. [3]. They acquired data from the SEER 17 Registry which represents approximately 26% of the U.S. population, and analyzed patients with stage II/III rectal cancer between 1998 and 2007. In the 65% of patients who did receive adjuvant RT, there was rapid adoption of preoperative approach. Only 17% of patients were treated with preoperative RT in 1998, which increased to 51% by 2007. The preoperative and postoperative RT intersected in 2002, after which preoperative RT became the dominant treatment pattern. Notably, this increasing trend of preoperative RT predated publication of prospective randomized data in 2004. Another U.S. study by Murphy et al. [7] utilized randomly sampled data from SEER program, and included patients diagnosed with stage II/III colorectal cancer in 1990-1991, 1995, 2000, 2005, and 2010. The proportion of patients who received any RT (preoperative or postoperative) increased from 45.5% in 1990-1991 to 66.1% in 2010. Receipt of preoperative RT substantially increased over time, from 2.8% in 1990-1991 to 47.3% in 2010. On the other hand, receipt of postoperative RT increased from 42.7% in in 1990-1991 to 51.3% in 1995 and subsequently decreased to 18.8% in 2010. The most recent study reporting trends of preoperative RT by Reddy et al. [6] acquired data from the National Cancer Database, and all patients diagnosed with rectal cancer from 1998 to 2011 were included. They also presented the use of preoperative RT increased over the study period.
The choice between preoperative and postoperative RT for locally advanced rectal cancer is generally entrusted to an institutional policy or at the discretion of the treating physician’s (typically surgeon’s), rather than the patients’ choice. Therefore, we set institutions as a unit of analysis. Although all institutions exhibited overall onwards and upwards trend in adopting preoperative RT, there was obvious institutional variation when stratified according to the number of treatment cases for 12 years. The institutions having more cases show gradual and steady increasing adoption of preoperative RT. Interestingly, one of largest six institutions shows consistent low proportion of preoperative RT, compared to other institutions or national average (less than 50% in 2015-2016). Presumably this institution has a narrow indication to apply adjuvant RT before surgery. A few studies reported similar findings to ours that volume and facility type of institution affected the adoption of preoperative RT in rectal cancer treatment [6,13]. Reddy et al. [6] reported institutional variation comparing academic and community institutions. In 1998, 51% received preoperative RT in academic centers, but 39% in community institutions. By 2011, 91% received preoperative RT in academic centers, but 84% in community institutions. They concluded that rates of adoption were better in academia, although an increase was seen in both center types. Stewart et al. [13] presented that teaching hospitals were associated with significantly higher utilization of preoperative RT, and improved cancer-specific survival compared with nonteaching hospitals, analyzing private insurance claims data linked with the Pennsylvania Cancer Registry.
Korea Central Cancer Registry (KCCR) provides annual report of cancer statistics to the public. This report presents the incidence of rectal cancer in Korea, which includes rectosigmoid junction cancer (C19) as well as rectal cancer (C20). Table 5 compares study population to the number of cancer incident cases in KCCR in 2005-2014 (KCCR data in 2015-2016 is unavailable). It is reasonable that KCCR has much more cases compared to ours. First, our study exclusively included rectal cancer (C20), because the role of adjuvant RT for recto-sigmoid junction cancer (C19) is still controversial. Second, adjuvant RT is indicated for locally advanced rectal cancers, not for early or metastatic cancers. Third, only patients treated with surgery and RT at the same institution included in our study. Importantly, 12.5%-13.5% of KCCR cases were consistently included in the study population, regardless of year of diagnosis. It could be interpreted that our dataset is stable and reliable enough to perform analyses, and the results from our study could be generalized in Korean rectal cancer population.
Table 5.
Comparison of study population with the number of cancer incident cases in Korea Central Cancer Registry
| Year | No. of patients |
|
|---|---|---|
| KCCR | Our study | |
| 2005-2006 | 17,345 | 2,239 (12.9) |
| 2007-2008 | 19,170 | 2,583 (13.5) |
| 2009-2010 | 22,813 | 2,965 (13.0) |
| 2011-2012 | 25,253 | 3,315 (13.1) |
| 2013-2014 | 23,833 | 2,971 (12.5) |
| Total | 108,414 | 14,073 (13.0) |
KCCR, Korea Central Cancer Registry (source: http://www.ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1) [12].
We acknowledge several limitations of our study. First, our data did not include all the rectal cancer patients treated with surgery and adjuvant RT in 2005-2016, although the data for the analysis was acquired via the whole Korean NHIS database, not a sampled data. The patients who had surgery and RT at different institutions were excluded in this study, because the unit of analysis was institutions. Considering not a few patients receive adjuvant RT at another hospital because of residential area or no RT facility in the institution which surgery was done, the exact number or proportion of preoperative RT could not be presented. Second and perhaps most important limitation was related to the nature of the Korean NHIS data. Because it is basically claim data to get reimbursement, clinical information such as cancer stage was unavailable. The reason we pay attention to variation in EBM dissemination is that a greater use of preoperative RT in an institution may representative higher-quality cancer care. Moreover, this quality of cancer care may affect patients’ treatment outcome. However, there is huge limitation to investigate that assumption without clinical information. Therefore, the next step of our study group is to link Korean NHIS data to KCCR data which has clinical information of registered cancer patients. We hope that relationship between the dissemination of EBM and patients’ outcome will be presented in the near future.
This is the first study to describe changing patterns of adjuvant RT adopting EBM for locally advanced rectal cancer in Korea using a national database. Our findings clearly demonstrated increasing adoption of preoperative RT over 12 years based on high-level evidence. However, institutional variation was observed between high- and low-volume institutions.
Acknowledgments
This study was supported by Korean National Health Insurance Service (project number, 20170603E6E).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Gastrointestinal Tumor Study Group Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–72. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 2.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for highrisk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald TL, Biswas T, O'Brien K, Zervos EE, Wong JH. Neoadjuvant radiotherapy for rectal cancer: adherence to evidence-based guidelines in clinical practice. World J Surg. 2013;37:639–45. doi: 10.1007/s00268-012-1862-z. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Koperny M, Lesniak W, Jankowski M, Bala M. The Cochrane collaboration: the role in the evolution of evidence-based medicine and development of cooperation in Poland. Przegl Epidemiol. 2016;70:508–20. [PubMed] [Google Scholar]
- 6.Reddy SS, Handorf B, Farma JM, Sigurdson ER. Trends with neoadjuvant radiotherapy and clinical staging for those with rectal malignancies. World J Gastrointest Surg. 2017;9:97–102. doi: 10.4240/wjgs.v9.i4.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of colorectal cancer care in the United States: 1990-2010. J Natl Cancer Inst. 2015;107:djv198. doi: 10.1093/jnci/djv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24:63–71. doi: 10.1093/heapol/czn037. [DOI] [PubMed] [Google Scholar]
- 9.Ki YJ, Kim HJ, Kim MS, Park CM, Ko MJ, Seo YS, et al. Association between metformin use and survival in nonmetastatic rectal cancer treated with a curative resection: a nationwide population study. Cancer Res Treat. 2017;49:29–36. doi: 10.4143/crt.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldon TA, Guyatt GH, Haines A. Getting research findings into practice. When to act on the evidence. BMJ. 1998;317:139–42. doi: 10.1136/bmj.317.7151.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiffman RN, Dixon J, Brandt C, Essaihi A, Hsiao A, Michel G, et al. The GuideLine Implementability Appraisal (GLIA): development of an instrument to identify obstacles to guideline implementation. BMC Med Inform Decis Mak. 2005;5:23. doi: 10.1186/1472-6947-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Center . Goyang: National Cancer Center; 2017. Cancer registration statistics data [Internet] [cited 2017 Sep 1]. Available from: http://www.ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1. [Google Scholar]
- 13.Stewart DB, Hollenbeak C, Desharnais S, Camacho F, Gladowski P, Goff VL, et al. Rectal cancer and teaching hospitals: hospital teaching status affects use of neoadjuvant radiation and survival for rectal cancer patients. Ann Surg Oncol. 2013;20:1156–63. doi: 10.1245/s10434-012-2769-5. [DOI] [PubMed] [Google Scholar]