Abstract
Background
One third of travellers to low- and middle-income regions of the tropics and subtropics become colonized by extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE). The risk varies by destination and, for each traveller, may be substantially further increased by travellers’ diarrhoea (TD) and antibiotic use. Despite the risk of TD in Africa, ESBL-PE acquisition rates in all studies are lower there than in Asia. Africa has become increasingly popular as a destination for international travellers, yet minimal data are available from the continent’s subregions and countries.
Methods
We analysed subregion- and country-specific data on carriage and risk factors for ESBL-PE colonization pooled from three prospective studies conducted between 2009 and 2013 among Finnish and Dutch travellers. The data were subjected to multivariable analysis of risk factors. In addition, we compared our data to two recent large investigations reporting data by subregion and country.
Results
Our joint analysis comprised data on 396 travellers. The ESBL-PE colonization rate was highest in Northern Africa, followed by Middle and Eastern Africa, and lowest in Southern and Western Africa. Of individual countries with more than 15 visitors, the highest rates were seen for Egypt (12/17; 70.6%), Ghana (6/23; 26.1%), and Tanzania (14/81; 17.3%); the rates among travellers to Egypt were comparable to those reported in South and Southeast Asia. In a pooled multivariable analysis, travel destination, age, overnight hospitalisation abroad, TD, and use of fluoroquinolones were independently associated with increased ESBL-PE colonization rates.
Conlusions
Even in areas with relatively low risk of colonization, antimicrobials clearly predispose to colonization with ESBL-PE. Travellers to Africa should be cautioned against unnecessary use of antibiotics.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3245-z) contains supplementary material, which is available to authorized users.
Keywords: ESBL, MDR, Extended-spectrum beta-lactamase, Enterobacteriaceae, Africa, Antimicrobials, Travel
Background
Every third traveller from industrialised countries that visits developing regions becomes colonized by extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) [1–11]. While rates as high as 88% have been reported for travellers to South Asia [4–8, 10, 11] and 69% to Southeast Asia [4, 6–8, 10, 11], considerably lower risks have been detected among travellers to the African continent, varying between 12 and 45% [3, 4, 6–8, 10, 11].
Data on colonization among visitors to various African countries or subregions remain scarce, as the vast majority of prospective studies report acquisition rates either for the whole continent [1, 9] or only part of the subregions [3, 4, 6, 8–10, 12, 13]; a few investigations provide data on a number of individual countries [7, 11]. Travel destination, antibiotic use, age, and travellers’ diarrhoea (TD) have been identified as major risk factors. Several other additional factors have been shown in single studies: type of travel, meal location and consumption of certain food products, such as ice cream and pastries [1, 3–7, 9–11, 14]. While these factors all predispose to ESBL-PE colonization in general, studies presenting colonization risk factors by individual geographic areas are few [5, 11], and none have focused exclusively on Africa. As the continent attracts increasing numbers of travellers [15], we decided to review the data published and pool subregion-derived findings of our three earlier investigations [4 6, 10]. Combining these data with subregional carriage rates from two recent studies [7, 11], our paper offers an insight into the subregion-related colonization risk of travellers to Africa.
Methods
Study design, volunteers and samples
To assess the colonization rates of ESBL-PE in Africa, we combined the data of travellers to Africa from three large studies:
Finnish study by Kantele et al. [6]. The volunteers travelled in 2009–2010; 196 of the 430 (45.6%) travelled in Africa. Faecal samples were used for analyses.
Dutch study I by Paltansing et al. [4]. The volunteers travelled in 2011; 103 of 338 (30.5%) travelled in Africa. Rectal swabs were used for analyses.
Dutch study II by Reuland et al. [10]. The volunteers travelled in 2012–2013 (of the travellers reviewed here, all but one travelled in 2012); 97 of 418 (23.2%) travelled in Africa; 63 (64.9%) faecal samples and 34 (35.1%) rectal swabs were analysed. In the original article, the authors report that the colonization rates were similar regardless of sample technique.
For all three prospective studies, the volunteers provided both pre- and post-travel stool samples/rectal swabs. Of the 14 (3.5%) travellers with pre-travel samples positive for ESBL-PE, three had the same strain detected in post-travel samples. In six volunteers, the post-travel sample was negative; these were included in the ESBL-PE(−) group. The five that contracted a different type of ESBL-PE during travel were included in the ESBL-PE(+) group. Travellers who contracted a new ESBL-PE strain during travel constituted the ESBL-PE(+) group, while all others belonged to the ESBL-PE(−) group. The following information was available from all three studies in comparable format: travel itinerary, travel duration, travel dates, age, sex, antimicrobial usage, occurrence of TD, and possible hospitalisation abroad (overnight stay or more).
In all studies, written informed consent was obtained from all participants and the Ethics Committees in the respective organisations approved the study protocols.
Collection of stool samples and identification of ESBL-PE strains
We have described earlier in detail the approaches to collection of samples (stools or swabs) and methods used for identification of ESBL-PE and carbapenemase-producing Enterobacteriaceae (CPE) [4, 6, 10].
Definition of TD and geographical subregions
For the purpose of the present study, TD was defined as three or more loose or liquid stools per day. Geographical subregions in Africa were defined according to the United Nations [16]: Southern Africa, Western Africa, Middle Africa, Eastern Africa, and Northern Africa. Travellers visiting more than one subregion in Africa were categorised on the basis of longest stay.
Statistical analyses
Statistical analyses were carried out with SPSS software version 24 (IBM Corp, Armonk, NY) and Stata version 15.1 (StataCorp. College Station, TX). Binomial regression model was used to obtain profile likelihood confidence intervals for the proportions of travellers with given risk factors and positive for ESBL-PE. The chi-square test, Fisher’s exact test or binary logistic regression analysis were used to compare categorical variables when applicable. Binary logistic regression was used with continuous variables. Variables with a p-value < 0.2 in the univariate analysis for ESBL-PE colonization were subjected to multivariable analysis together with doxycycline as antimalarial, gender and duration of travel in days. The shape of the form for travel duration and age were assessed by cubic splines and appeared log-linear. The interaction between variables of interest and studies was assessed. The final model was built using binary logistic regression analysis with a stepwise backward selection of variables by Akaike Information Criteria (AIC). Factors with 95% confidence intervals ranging only either above or below 1 were considered significant. The three studies pooled in the present paper [4, 6, 10] and the two others [7, 11] used for comparisons were all brought together to produce a forest plot analysis. Heterogeneity between studies in forest plot was measured with I2; values above 75% were considered high, 25–75% moderate, and below 25%. For our pooled data, the interaction between studies and geographical subregions was analysed in the multivariable model.
Results
Demographic data, background characteristics, and occurrence of TD
Demographic data on travellers are presented in Table 1. Of the 396 travellers included in this study, 237 (59.8%) were women. The median age was 36 years (IQR 27–53) and the median duration of travel 19 days (IQR 14–25). One fourth of the travellers (n = 105; 26.5%) had visited more than one country in Africa. The majority of the travellers visited either Western (27.8%) or Eastern (46.7%) Africa. Twenty-three (5.8%) had visited more than one subregion in Africa. In addition to Africa (or Europe en route to Africa), two volunteers (0.5%) had visited Jordania and two (0.5%) United Arab Emirates.
Table 1.
Demographics and risk factors of ESBL-PE acquisition in pooled data on 396 travellers from Finland and the Netherlands
| Univariate analysis | Multivariable analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | total n (% of all) | ESBL-PE(+) n (%) | 95% CI (%)a | P | OR | 95% CI | P | AOR | 95% CI |
| Total | 396 (100.0) | 61 (15.4) | |||||||
| Gender | |||||||||
| Male | 159 (40.2) | 24 (15.1) | 10.1–21.2 | 1.0 | |||||
| Female | 237 (59.8) | 37 (15.6) | 11.4–20.6 | 0.889 | 1.0 | 0.6–1.8 | |||
| Ageb | |||||||||
| Age, median, years | 36 (IQR 27–53) | 38.5 (IQR 25.5–55.5) | 0.071 | 1.0 | 1.0–1-0 | 0.002 | 1.0 | 1.0–1.1 | |
| Study | |||||||||
| Kantele et al. [6] | 196 (49.5) | 25 (12.8) | 8.6–17.9 | 1.0 | 1.0 | ||||
| Paltansing et al. [4] | 103 (26.0) | 29 (28.2) | 20.1–37.3 | 0.001 | 2.7 | 1.5–4.9 | 0.303 | 3.7 | 0.3–50.0 |
| Reuland et al. [10] | 97 (24.5) | 7 (7.2) | 3.2–13.5 | 0.158 | 0.5 | 0.2–1.3 | 0.962 | 1.1 | 0.1–25.1 |
| Sampling method | |||||||||
| Rectal swab | 137 (34.6) | 32 (23.4) | 16.8–30.9 | 0.001 | 2.4 | 1.4–4.2 | 0.648 | 1.5 | 0.3–7.8 |
| Stool sample | 259 (65.4) | 29 (11.2) | 7.7–15.4 | 1.0 | |||||
| Year of travelb | |||||||||
| (year of travel as a continuous variable) | 0.909 | 1.0 | 0.8–1.2 | 0.609 | 0.8 | 0.3–2.1 | |||
| 2009 | 122 (30.8) | 17 (13.9) | 8.6–20.8 | ||||||
| 2010 | 74 (18.7) | 8 (10.8) | 5.1–19.2 | ||||||
| 2011 | 103 (26.0) | 29 (28.2) | 20.1–37.3 | ||||||
| 2012–2013 | 97 (24.5) | 7 (7.2) | 3.2–13.5 | ||||||
| Destination subregion | |||||||||
| Southern Africa | 58 (14.6) | 4 (6.9) | 2.2–15.3 | 1.0 | 1.0 | ||||
| Northern Africa | 28 (7.1) | 12 (42.9) | 25.8–61.2 | < 0.001 | 10.1 | 2.9–35.8 | 0.001 | 12.4 | 3.1–57.3 |
| Middle Africa | 15 (3.8) | 4 (26.7) | 9.2–51.5 | 0.042 | 4.9 | 1.1–22.7 | 0.056 | 5.6 | 0.9–33.6 |
| Eastern Africa | 185 (46.7) | 30 (16.2) | 11.4–22.0 | 0.084 | 2.6 | 0.9–7.8 | 0.058 | 3.1 | 1.1–11.2 |
| Western Africa | 110 (27.8) | 11 (10.0) | 5.3–16.5 | 0.505 | 1.5 | 0.5–4.9 | 0.528 | 1.5 | 0.4–6.2 |
| Antibiotics | |||||||||
| no AB | 352 (88.9) | 44 (12.5) | 9.3–16.2 | 1.0 | |||||
| AB | 44 (11.1) | 17 (38.6) | 25.2–53.4 | < 0.001 | 4.4 | 2.2–8.7 | |||
| AB: beta-lactams | |||||||||
| No | 388 (98.0) | 57 (14.7) | 11.4–18.4 | 1.0 | 1.0 | ||||
| Yes | 8 (2.0) | 4 (50.0) | 19.1–80.9 | 0.022 | 5.8 | 1.4–23.9 | 0.118 | 3.4 | 0.5–21.9 |
| AB: fluoroquinolones | |||||||||
| No | 371 (93.7) | 51 (13.7) | 10.5–17.5 | 1.0 | 1.0 | ||||
| Yes | 25 (6.3) | 10 (40.0) | 22–5-59.5 | 0.002 | 4.2 | 1.8–9.8 | 0.005 | 4.7 | 1.5–13.9 |
| AB others (other than beta-lactams or FQ)/unknown | |||||||||
| No | 382 (96.5) | 56 (14.7) | 11.4–18.4 | 1.0 | 1.0 | ||||
| Yes | 14 (3.5) | 5 (35.7) | 14.6–61.7 | 0.048 | 3.2 | 1.0–10.0 | 0.059 | 3.8 | 0.9–14.6 |
| Doxycycline as antimalarial | |||||||||
| No | 362 (91.4) | 56 (15.5) | 12.0–19.4 | 1.0 | |||||
| Yes | 34 (8.6) | 5 (14.7) | 5.5–29.0 | 0.906 | 0.9 | 0.4–2.5 | |||
| Travellers’ diarrhoea (TD) | |||||||||
| no TD | 243 (61.4) | 30 (12.3) | 8.6–16.9 | 1.0 | |||||
| TD | 153 (38.6) | 31 (20.3) | 14.4–27.1 | 0.034 | 1.8 | 1.0–3.1 | 0.033 | 2.1 | 1.1–4.1 |
| Overnight hospitalisation abroad (information missing n = 1) | |||||||||
| No | 389 (98.5) | 57 (14.7) | 11.4–18.4 | 1.0 | |||||
| Yes | 6 (1.5) | 4 (66.7) | 28.1–100 | 0.006 | 11.6 | 2.1–65.1 | 0.004 | 16.5 | 2.5–140.5 |
| Duration of travel, (information missing n = 1)b | |||||||||
| Median, days | 19 (IQR 14–25) | 18 (IQR 15–23) | 0.828 | 1.0 | 1.0–1.0 | 0.276 | 1.0 | 1.0–1.0 | |
a95% confidence intervals are profile likelihood confidence intervals for proportion of ESBL(+) with given risk factor
bstudied as a continuous variable in statistical analysis
TD rates were lowest in Southern Africa (15/58; 25.9%); in other areas 37.8–46.7% of travellers contracted TD (Table 2.). Of the 44 (11.1% of all travellers) courses of antibiotics, 30 (68.2%) were taken for TD. Eight (2.0% of all travellers) used beta-lactam antibiotics and 25 (6.3%) used fluoroquinolone antibiotics during travel.
Table 2.
ESBL-PE colonization rates, occurrence of TD and antibiotic use in the pooled data on 396 travellers from Finland and the Netherlands in relation to geographical subregion visited
| All | Northern Africa | Middle Africa | Eastern Africa | Western Africa | Southern Africa | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Kantele et al. (Finnish study) [6] | ||||||
| total no. of travellers to subregion (% of all) | 196 | 3 (1.5) | 4 (2.0) | 86 (43.9) | 78 (39.8) | 25 (12.8) |
| ESBL-PE (+) | 25 (12.8) | 2 (66.7) | 1 (25.0) | 14 (16.3) | 5 (6.4) | 3 (12.0) |
| AB | 34 (17.3) | 1 (33.3) | 1 (25.0) | 13 (15.1) | 15 (19.2) | 4 (16.2) |
| TD | 71 (36.2) | 1 (33.3) | 1 (25.0) | 34 (39.5) | 29 (37.2) | 6 (24.0) |
| Paltansing et al. (Dutch study I) [4] | ||||||
| total no. of travellers to subregion (% of all) | 103 | 13 (12.6) | 7 (6.8) | 54 (52.4) | 12 (11.7) | 17 (16.5) |
| ESBL-PE (+) | 29 (28.2) | 7 (53.8) | 3 (42.9) | 14 (25.9) | 4 (33.3) | 1 (5.9) |
| AB | 8 (7.8) | 2 (15.4) | 1 (14.3) | 3 (5.6) | 2 (16.7) | 0 (0.0) |
| TD | 39 (37.9) | 7 (53.8) | 4 (57.1) | 19 (35.2) | 5 (41.7) | 4 (23.5) |
| Reuland et al. (Dutch study II) [10] | ||||||
| total no. of travellers to subregion (% of all) | 97 | 12 (12.4) | 4 (4.1) | 45 (46.4) | 20 (20.6) | 16 (16.5) |
| ESBL-PE (+) | 7 (7.2) | 3 (25.0) | 0 (0.0) | 2 (4.4) | 2 (10.0) | 0 (0.0) |
| AB | 2 (2.1) | 0 (0.0) | 0 (0.0) | 2(4.4) | 1 (16.7) | 0 (0.0) |
| TD | 43 (44.3) | 5 (41.7) | 2 (50.0) | 17 (37.8) | 14 (70.0) | 5 (31.3) |
| Combined total of the three studies | ||||||
| total no. of travellers to subregion (% of all) | 396 | 28 (7.1) | 15 (3.8) | 185 (46.7) | 110 (27.8) | 58 (14.6) |
| ESBL-PE (+) | 61 (15.4) | 12 (42.9) | 4 (26.7) | 30 (16.2) | 11 (10.0) | 4 (6.9) |
| AB | 45 (11.4) | 3 (10.7) | 2 (13.3) | 18 (9.7) | 18 (16.4) | 4 (6.9) |
| TD | 153 (38.6) | 13 (46.4) | 7 (46.7) | 70 (37.8) | 48 (43.6) | 15 (25.9) |
AB antibiotic use, TD travellers’ diarrhoea
ESBL-PE acquisition rates by subregion in the pooled data
In the pooled data (Table 2, Fig. 1), 61 (15.4%) travellers became colonized by ESBL-PE; one Dutch traveller to Egypt became colonized by CPE. The highest ESBL-PE colonization rates were seen among travellers to Northern Africa (12/28; 42.9%), followed by Middle Africa (4/15; 26.7%) and Eastern Africa (30/185; 16.2%). Of travellers to Western and Southern Africa, 10.0% (11/110) and 6.9% (4/58), respectively, acquired ESBL-PE (Tables 1 and 2).
Fig. 1.
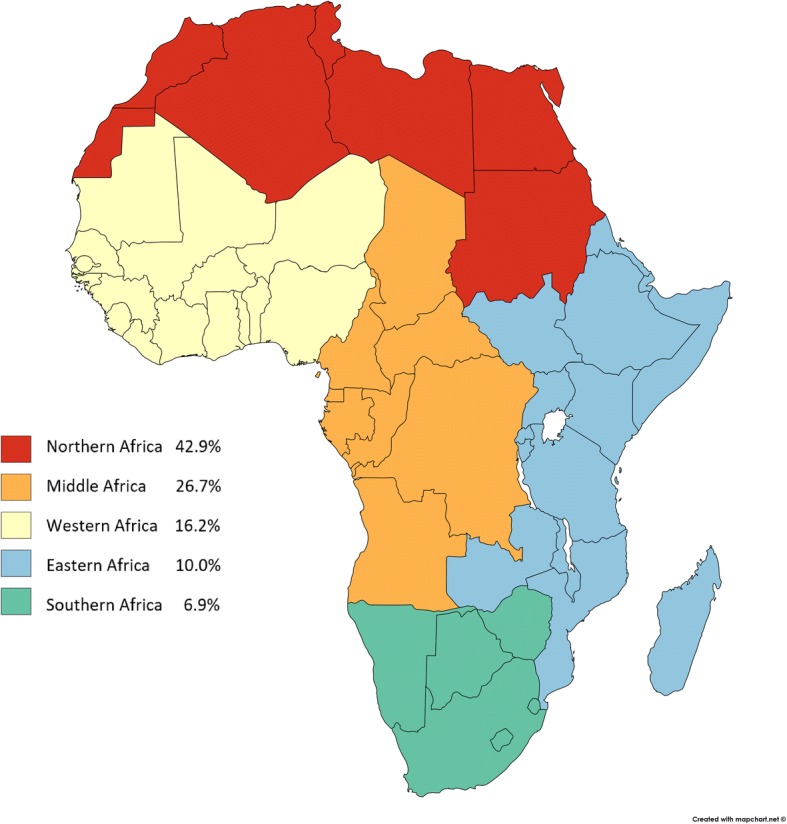
ESBL-PE acquisition rates in five African subregions; joint data on 396 travellers from Finland and the Netherlands. (Created with Mapchart.net)
Of the nine countries with more than 15 visitors (Table 3), the highest ESBL-PE acquisition rates were seen among travellers to Egypt (12/17; 70.6%), Ghana (6/23; 26.1%), Tanzania (14/81; 17.3%), Uganda (4/26; 15.4%) and Kenya (12/82; 14.6%). As for the lowest colonization rates, of the 26 travellers to Senegal, three (11.5%) became colonized by ESBL-PE, in South Africa, 5/49 (10.2%) became colonized and in the Gambia the rate was only 3.4% (2/58); none of the 21 visitors to Namibia acquired ESBL-PE.
Table 3.
ESBL-PE colonization rates from our pooled data of 396 travellers by country visited presented with the respective figures from studies by Ruppé et al. [7] and Arcilla et al. [11]
| Country | Data pooled from three studiesa: Total number of travellers (% of all visitors to Africa) |
Data pooled from three studiesa: ESBL-PE (+) cases/all visitors to country (%) |
Data published by Ruppé et al.b ESBL-PE (+) cases/all visitors to country (%) |
Data published by Arcilla et al.c ESBL-PE (+) cases/all visitors to country (%) |
|---|---|---|---|---|
| Northern Africa | ||||
| Egypt | 17 (4.3) | 12/17 (70.6) | – | 24/30 (80.0) |
| Morocco | 10 (2.5) | 1/10 (10.0) | – | 8/36 (22.2) |
| Tunisia | 3 (0.8) | 0/3 (0) | – | – |
| Middle Africa | ||||
| Cameroon | 7 (1.8) | 1/7 (14.3) | 13/24 (54.2) | – |
| Central African Republic | – | – | 0/1 (0.0) | – |
| Democratic Republic of Congo | 8 (2.0) | 3/8 (37.5) | – | – |
| Republic of Congo | 6 (1.5) | 2/6 (33.3) | 8/13 (61.5) | – |
| Gabon | 1 (0.3) | 0/1 (0) | 2/3 (66.7) | – |
| Sao Tome and Principe | – | – | 0/1 (0.0) | – |
| Eastern Africa | ||||
| Djibouti | 1 (0.3) | 1/1 (100.0) | – | – |
| Ethiopia | 14 (3.5) | 2/14 (14.3) | 2/4 (50.0) | – |
| Kenya | 82 (20.7) | 12/82 (14.6) | 4/6 (66.7) | 10/30 (33.3) |
| Madagascar | 3 (0.8) | 1/3 (33.3) | 4/7 (57.1) | – |
| Malawi | 14 (3.5) | 2/14 (14.3) | – | – |
| Mauritius | 1 (0.3) | 1/1 (100.0) | – | – |
| Mozambique | 8 (2.0) | 1/8 (12.5) | 0/1 (0.0) | – |
| Rwanda | 5 (1.3) | 0/5 (0) | – | – |
| Tanzania | 81 (20.5) | 14/81 (17.3) | 7/11 (63.6) | 14/57 (24.6) |
| Uganda | 26 (6.6) | 4/26 (15.4) | – | 12/27 (44.4) |
| Western Africa | ||||
| Benin | 13 (3.3) | 1/13 (7.7) | 4/11 (36.4) | – |
| Burkina Faso | 2 (0.5) | 0/2 (0) | 4/8 (50.0) | – |
| Cote d Ivoire | 1 (0.3) | 0/1 (0) | 8/17 (47.1) | – |
| Gambia | 58 (14.6) | 2/58 (3.4) | – | 8/49 (16.3) |
| Ghana | 23 (5.8) | 6/23 (26.1) | 1/1 (100.0) | 8/20 (40.0) |
| Guinea Bissau | 1 (0.3) | 0/1 (0) | 0/3 (0.0) | – |
| Liberia | 3 (0.8) | 0/3 (0) | – | – |
| Mali | 4 (1.0) | 1/4 (25.0) | 1/5 (20.0) | – |
| Nigeria | 7 (1.8) | 1/7 (14.3) | 1/1 (100.0) | – |
| Senegal | 26 (6.6) | 3/26 (11.5) | 17/45 (37.8) | – |
| Sierra Leone | 3 (0.8) | 1/3 (33.3) | – | – |
| Togo | 6 (1.5) | 2/6 (33.3) | 9/12 (75.0) | – |
| Southern Africa | ||||
| Angola | 1 (0.3) | 0/1 (0) | 1/3 (33.3) | – |
| Botswana | 10 (2.5) | 1/10 (10.0) | – | – |
| Lesotho | 2 (0.5) | 1/2 (50.0) | – | – |
| Namibia | 21 (5.3) | 0/21 (0) | – | – |
| South Africa | 49 (12.4) | 5/49 (10.2) | 0/1 (0.0) | 3/66 (4.5) |
| Swaziland | 8 (2.0) | 2/8 (25.0) | – | – |
| Zambia | 13 (3.3) | 1/13 (7.7) | – | – |
| Zimbabwe | 7 (1.8) | 1/7 (14.3) | – | – |
There were no travellers to Burundi, Cape Verde, Chad, Equatorial Guinea, Eritrea, Guinea, Libya, Mauritania, Niger, Reunion, Seychelles, Somalia, South Sudan, and Sudan
aThe same traveller may have visited several countries
bOnly travellers that had visited only one country
cThe colonization rates of all individual countries visited were not published
Results of the multivariable analysis for risk factors of ESBL-PE colonization in Africa
The initial univariate analysis (Table 1) detected the following factors with p < 0.2: age, original study, sampling technique, subregion in Africa, use of fluoroquinolones, beta-lactams or other AB/regimen not known, TD, and overnight hospitalisation abroad. When all of these factors, together with gender, duration of travel, and use of doxycycline as antimalarial were subjected to multivariable analysis, the following were found to be independently associated with increased risk: travel to Northern Africa, overnight hospitalisation abroad, age, TD and use of fluoroquinolones (Table 1).
Results of meta-analysis of five studies
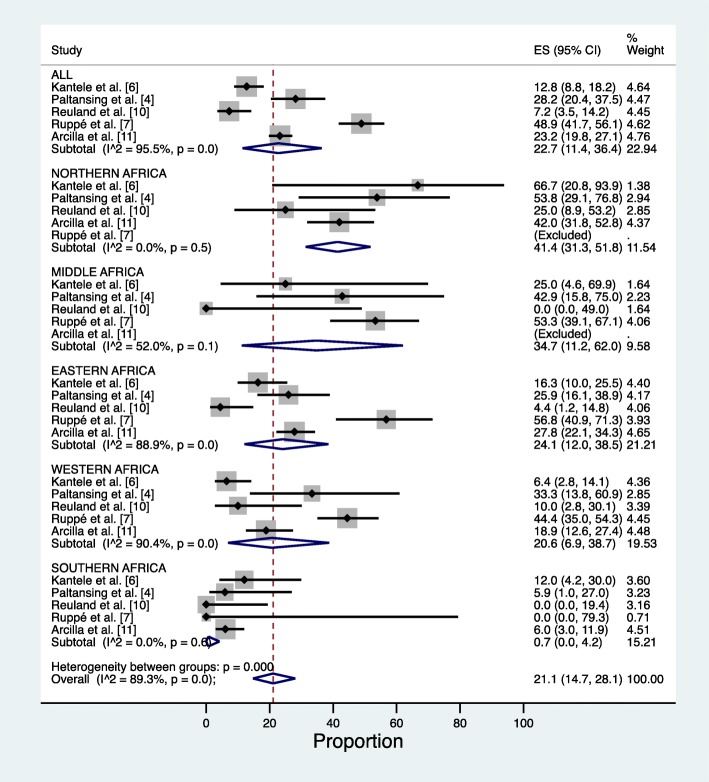
Table 4 and forest plot analysis (Fig. 2) show the ESBL-PE colonization rates from the three studies pooled [4, 6, 10], together with investigations by Ruppé et al. and Arcilla et al. [7, 11] in relation to geographical subregion visited. For Southern and Northern Africa, heterogeneity between the five studies appeared low (I2 = 0.0 and 0.0%, respectively), for Middle Africa moderate (I2 52.0%), and Eastern and Western Africa high (I2 88.9 and 90.4%, respectively). In the multivariable regression model of our pooled data, the interaction between subregions and the three studies was not found significant at 5% significance level.
Table 4.
ESBL-PE colonization rates from the five studies [4, 6, 7, 10, 11] in relation to geographical subregion visited
| All | Northern Africa | Middle Africa | Eastern Africa | Western Africa | Southern Africa | |
|---|---|---|---|---|---|---|
| Kantele et al. [6] (Finnish study) 2009–2010 | ||||||
| ESBL-PE (+) among travellers n (% of all visitors to subregion) | 25/196 (12.8) | 2/3 (66.7) | 1/4 (25.0) | 14/86 (16.3) | 5/78 (6.4) | 3/25 (12.0) |
| Paltansing et al. [4] (Dutch study I) 2011 | ||||||
| ESBL-PE (+) among travellers n (% of all visitors to subregion) | 29/103 (28.2) | 7/13 (53.8) | 3/7 (42.9) | 14/54 (25.9) | 4/12 (33.3) | 1/17 (5.9) |
| Reuland et al. [10] (Dutch study II) 2012–2013 | ||||||
| ESBL-PE (+) among travellers n (% of all visitors to subregion) | 7/97 (7.2) | 3/12 (25.0) | 0/4 (0.0) | 2/45 (4.4) | 2/20 (10.0) | 0/16 (0.0) |
| Ruppé et al. [7]2012–2013 (data on travellers visiting only one country) | ||||||
| ESBL-PE (+) among travellers n (% of all visitors to subregion) | 89/182 (48.9) | N/A | 24/45 (53.3) | 21/37 (56.8) | 44/99 (44.4) | 0/1 (0) |
| Arcilla et al. [11]2012–2013 | ||||||
| ESBL-PE (+) among travellers n (% of all visitors to subregion) | 118/508 (23.2) | 34/81 (42.0) | N/A | 57/205 (27.8) | 20/106 (18.9) | 7/116 (6.0) |
| Combined total: ESBL-PE colonization rates | 268/1086 (24.7) | 46/109 (42.2) | 28/60 (46.7) | 108/427 (25.3) | 75/315 (23.8) | 11/175 (6.3) |
Fig. 2.
Forest plots of ESBL-PE acquisition rates from five studies in relation to geographical subregions. Excluded = no travellers to subregion in study
Discussion
Africa is a continent with increasing numbers of travellers [15]. When pooling subregion-/country-specific data from three traveller studies [4, 6, 10], we found the risk of contracting ESBL-PE to vary significantly between the various parts of Africa. In addition, comparing our joint data with two recent large reports [7, 11] providing subregion- and country-specific data enabled us to investigate the current subregion- and country-specific knowledge about ESBL-PE acquisition by travellers to Africa.
ESBL-PE colonization rates in northern Africa
Our pooled data showed the highest acquisition rates (12/28; 42.9%) among visitors to Northern Africa, which accords with the results from the study by Arcilla et al. (42.0%) [11]; Ruppé et al. [7] did not report visitors to this subregion. Similar (43–44%) rates have been reported among Swedish travellers [3, 12]. Visitors to Egypt appear to be at particularly high risk; 70.6% (12/17) of our subjects, 80.0% (23/40) of those in a study by Arcilla et al., and 50% (19/38) of those in another by Tham et al. became colonized [11, 14]. Moreover, all 12 travellers colonized by ESBL-PE in Northern Africa had visited Egypt. It is noteworthy that these proportions are as high as those among travellers to India/South Asia in various investigations [4–8, 11]. Bassyouni et al. reported carriage rates as low as 21% among healthcare workers in Egypt [17].
ESBL-PE colonization rates in middle Africa
To our knowledge, only one previous study has reported ESBL-PE acquisition rates among visitors to Middle Africa; Ruppé et al. [7] found 53.3% (24/45) of travellers to be colonized. In our pooled data, colonization rates in Middle Africa ranked second (4/15; 26.7%) among the subregions. In nonclinical samples obtained from local populations, carriage rates as high as 59% have been shown among healthy children in the Central African Republic [18], and 44–57% among inpatient carers, hospital workers and their household members in Cameroon [19].
ESBL-PE rates in eastern Africa
Colonization rates among travellers to Eastern Africa (30/185; 16.2%) were lower than those reported by Arcilla et al. (57/205; 27.8%) [11], Lubbert et al. (12/47; 25.5%) [8], and Ruppé et al. (17/29; 56.8%,) [7]. Our moderate colonization rates are supported by findings among local populations: ESBL-PE carriage rates between 11.6 and 16.5% have been reported for healthy community children in Tanzania, [20, 21] and 5.3% for locals in Uganda, [22].
ESBL-PE colonization rates in western Africa
ESBL-PE acquisition rates in Western Africa appear moderately low, but the results differ between studies: our pooled data showed proportions (11/110; 10.0%) close to those presented by Arcilla et al. (20/106; 18.9%) [11], while higher rates have been found by Ruppé et al. (44/99; 44.4%) [7] and Lubbert et al. (5/12; 38.5%) [8] among German travellers to Western and Middle Africa. Moreover, in the research by Frickmann et al., 27.1% (13/48) of European military personnel with diarrhoea in Mali became colonized by ESBL-PE [23]. As for local populations, colonization rates of 22% have been reported for healthy volunteers in Burkina Faso [24] and 33% for healthy community children in Guinea-Bissau [25].
ESBL-PE colonization rates in southern Africa
Our low rates in Southern Africa (4/58; 6.8%) accord with those found by Arcilla et al. (7/116; 6.0%) [11] and Lubbert et al. (2/18; 11%) [8]. In our pooled data, the vast majority had visited South Africa or Namibia. Consistent with the low ESBL-PE acquisition rates, one study exploring local populations in South Africa reported maternal faecal carriage rates of 4.4% in South Africa [26].
Findings from multivariable analysis
Travellers’ diarrhoea
ESBL-PE acquisition rates among those who contracted TD during travel (31/153; 20.3%) were higher than among those without TD (30/243; 12.3%) (AOR 2.1; 95% CI 1.1–4.1). This was expected, since TD was identified as a risk factor in two of the three original studies [6, 10] and numerous others [1, 3, 7, 8, 11, 12].
Antimicrobial medication
Forty-four (11.1%) travellers had taken antimicrobial medications during travel. Of the Finns, 17.3% (34/196) took antibiotics while this proportion was 5.0% (10/200) among the Dutch. In multivariable analyses, fluoroquinolone antibiotics were an independent risk factor for ESBL-PE colonization (ESBL-PE(+) 40.0%; AOR 4.7; 95% CI 1.5–13.9). Other antibiotic groups did not reach statistical significance in the risk factor analysis, yet the numbers of travellers using each individual antibiotic type were small; eight had taken beta-lactams (ESBL-PE(+) 50.0%; AOR 3.4, CI 0.5–21.9) and 14 other antimicrobials (ESBL-PE(+) 35.7%; AOR 3.8 CI 0.9–14.6). Ruppé et al. found beta-lactam usage to predispose to colonization by ESBL-PE (20/25; 80%) [7].
Even though taken by 34 (8.6%) travellers as an antimalarial, doxycycline was not associated with increased ESBL-PE rates (ESBL-PE(+) 5/34; 14.7%; AOR 0.9, 95% CI 0.4–2.5). This finding accords with other studies [7, 11]. However, these data do not allow conclusions on the total impact of doxycycline on antimicrobial resistance, as these investigations only analysed the ESBL or CPE feature of the Enterobacteriaceae; the potential to select doxycycline-resistant strains in general or other types of multidrug-resistant bacteria was not explored. Indeed, we recently showed that fluoroquinolone intake predisposes selectively to colonization by fluoroquinolone-resistant bacteria [27]. Thus, the effect of doxycycline on other bacteria and travellers’ microbiota deserves further research.
Increasing age as risk factor
Increasing age proved an independent risk factor for ESBL-PE colonization in Africa. Only two earlier reports [3, 6] have described similar results, as opposed to several others [7, 8, 11]. Moreover, in one study conducted among returning travellers with diarrhoea, increasing age even appeared protective [28]. The role of age remains unclear. There may be other factors associated with increasing age, such as co-medications/comorbidities or altered immune response not covered in these studies that interfere with the analyses in either direction. As the risk of bacteraemic infections caused by resistant Enterobacteriaceae increases with age [29], the risk factors in the older age groups warrant further studies.
Overnight hospitalisation
In our joint data, overnight hospitalisation predisposed to colonization with ESBL-PE. Although numerous retrospective studies have shown high colonization rates by multiresistant bacteria among travellers hospitalized in high-prevalence countries [30–32], to our knowledge, this is the first study to actually show in a prospective setting hospitalisation abroad as a risk factor for ESBL-PE acquisition. In previous prospective traveller studies, overnight hospitalisations has either not been analysed separately from other health care contacts in the risk factor analyses [7, 11] or the proportion of travellers requiring a stay in hospital for treatment has been small or negligible (0–0.5%) [3, 8]. In our data, six (1.5% of all subjects) needed overnight hospitalisation.
Travel destination
In multivariable analysis, when compared to Southern Africa, travel to Northern Africa was associated with higher colonization rates. The rates presumably vary between subregions and countries according to the background prevalence of the local populations [33]. They may also depend on several other factors, such as local culture-related food production and preparation habits and hygienic conditions and, of course, whether the traveller contracts TD and takes antibiotics (see above).
Other risk factors
Even though multiresistant Enterobacteriaceae have become increasingly prevalent globally [33], colonization rates were not found to increase during the study period (2009–2013). Neither individual studies nor sampling techniques were found statistically significant factors in the multivariable analysis. Travel duration was not seen to be associated with increased risk in univariate or multivariable analysis. This may be explained by a proportion of travellers becoming colonized already on arrival and the carriage resolving while abroad (Professor Kantele, unpublished observation).
Limitations of the study
As the data for the joint risk factor analysis were derived from three separate studies, some data had been collected in differing formats rendering the results incomparable. Moreover, although pooling served to increase the validity and precision of study results, the data remained insufficient in some occasions for analysis in any great detail: In Additional file 1: Table S1, we present the factors available from two out of three studies [4, 6]: purpose of travel, diet (omnivore or vegetarian), type of accommodation, use of medications (antidiarrhoeals, proton-pump inhibitors, and antiemetics) and contact with local health care (other than hospitalisation). The five investigations appeared heterogeneous in the forest plot analysis, however, in the multivariable analysis of the pooled data, the interaction between subregions and studies was not found statistically significant.
Information concerning mild gastrointestinal symptoms in the ‘no TD’ group was only available for the Finnish volunteers (48.8% of all ‘no TD’ cases). To pool the three studies, we had to define TD as three or more stools per 24 h; milder diarrhoea cases were categorised as ‘no TD’, although even mild TD also may predispose to ESBL-PE acquisition.
Conclusions
ESBL-PE colonization rates in African subregions appear moderate, with the exception of Northern Africa, especially Egypt. Also on this continent, however, TD and antibiotic use increase the risk of individual travellers acquiring ESBL-PE.
Additional file
Table S1. Factors available in same format only from the studies of Kantele et al. [6] and Paltansing et al. [4] and not included in the pooled data of this report. (DOCX 16 kb)
Acknowledgements
We thank Jukka Ollgren for expert advice in statistical analyses.
Funding
The three original studies were funded by: Finnish government subsidy for health science research (grant numbers: TYH2012141, TYH 2013218 and TYH 2014216), the SSAC Foundation (grant number SLS-504141) and the Paulo Foundation; Research and Development fund GGD Amsterdam and ZonMw, the Netherlands Organisation for Health Research and Development (grant 125020011); the European Society of Clinical Microbiology and Infectious Diseases (ESCMID Study Group Research Grant) and by the Department of Medical Microbiology of the Leiden University Medical Center, the Netherlands. None of study sponsors were not involved in the study design, collection, analysis or interpretation of data.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to ongoing further analyses on the data but are available from the corresponding author on reasonable request.
Abbreviations
- AB
Antibiotic
- ESBL
Extended-spectrum beta-lactamase
- ESBL-PE
Extended-spectrum beta-lactamase-producing Enterobacteriaceae
- TD
Travellers’ diarrhoea
Authors’ contributions
Study concept and design TL, JV, GS, LV, AK; acquisition of data TL, JV, APvD, HH, GS, LV, AK; analysis and interpretation of results TL, JV, AK; drafting of manuscript TL, AK; statistical analysis TL; Critical comments of the manuscript JV, APvD, HH, GS, LV; final approval of version published TL, JV, APvD, HH, GS, LV, AK. All authors have read and approved the manuscript.
Ethics approval and consent to participate
In all studies, written informed consent was obtained from all participants and the Ethics Committees in the respective organisations approved the study protocols. (METc, NL29769.029.09) of the VU University Medical Centre (NTR Trial ID NTR2453); Ethics Committee of the Department of Medicine in Helsinki University Hospital (406/13/03/01/08); Medical Ethics Committee of the Leiden University Medical Center. (P11.036).
Consent for publication
Not applicable
Competing interests
TL, JV, APvD, HH, GS, LV, declare no competing interests. AK has received honorary for lectures (Pfizer, MSD, Valneva, Immuron) and membership in advisory board (Valneva), and an investigator-initiated grant (Pfizer), none of these relevant to the current manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3245-z) contains supplementary material, which is available to authorized users.
Contributor Information
Tinja Lääveri, Email: tinja.laaveri@hus.fi.
Jessica A. Vlot, Email: J.A.Vlot@lumc.nl
Alje P. van Dam, Email: avdam@ggd.amsterdam.nl
Hanni K. Häkkinen, Email: hanni.hakkinen@helsinki.fi
Gerard J. B. Sonder, Email: gsonder@ggd.amsterdam.nl
Leo G. Visser, Email: L.G.Visser@lumc.nl
Anu Kantele, Phone: +358-50-309 7640, Email: anu.kantele@hus.fi.
References
- 1.Tängden T, Cars O, Melhus A, Lowdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–3568. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham J, Walder M, Melander E, Odenholt I. Duration of colonization with extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand J Infect Dis. 2012;44:573–577. doi: 10.3109/00365548.2011.653582. [DOI] [PubMed] [Google Scholar]
- 3.Östholm-Balkhed A, Tarnberg M, Nilsson M, Nilsson LE, Hanberger H, Hällgren A, Travel Study Group of Southeast Sweden Travel-associated faecal colonization with ESBL-producing Enterobacteriaceae: incidence and risk factors. J Antimicrob Chemother. 2013;68:2144–2153. doi: 10.1093/jac/dkt167. [DOI] [PubMed] [Google Scholar]
- 4.Paltansing S, Vlot JA, Kraakman ME, Mesman R, Bruijning ML, Bernards AT, et al. Extended-Spectrum beta-lactamase-producing Enterobacteriaceae among travelers from the Netherlands. Emerg Infect Dis. 2013;19:1206–1213. doi: 10.3201/eid1908.130257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuenzli E, Jaeger VK, Frei R, Neumayr A, DeCrom S, Haller S, et al. High colonization rates of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli in Swiss travellers to South Asia- a prospective observational multicentre cohort study looking at epidemiology, microbiology and risk factors. BMC Infect Dis. 2014;14:528. doi: 10.1186/1471-2334-14-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantele A, Lääveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, et al. Antimicrobials increase travelers' risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis. 2015;60:837–846. doi: 10.1093/cid/ciu957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruppé E, Armand-Lefevre L, Estellat C, Consigny PH, El Mniai A, Boussadia Y, et al. High rate of acquisition but short duration of carriage of multidrug-resistant Enterobacteriaceae after travel to the tropics. Clin Infect Dis. 2015;61:593–600. doi: 10.1093/cid/civ333. [DOI] [PubMed] [Google Scholar]
- 8.Lubbert C, Straube L, Stein C, Makarewicz O, Schubert S, Mossner J, et al. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol. 2015;305:148–156. doi: 10.1016/j.ijmm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Angelin M, Forsell J, Granlund M, Evengard B, Palmgren H, Johansson A. Risk factors for colonization with extended-spectrum beta-lactamase producing Enterobacteriaceae in healthcare students on clinical assignment abroad: a prospective study. Travel Med Infect Dis. 2015;13:223–229. doi: 10.1016/j.tmaid.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Reuland EA, Sonder GJ, Stolte I, Al Naiemi N, Koek A, Linde GB, et al. Travel to Asia and traveller's diarrhoea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum beta-lactamase-producing Enterobacteriaceae-a prospective cohort study. Clin Microbiol Infect. 2016;22:731. doi: 10.1016/j.cmi.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MC, van Genderen PJ, Goorhuis A, et al. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17:78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 12.Vading M, Kabir MH, Kalin M, Iversen A, Wiklund S, Naucler P, et al. Frequent acquisition of low-virulence strains of ESBL-producing Escherichia coli in travellers. J Antimicrob Chemother. 2016;71:3548–3555. doi: 10.1093/jac/dkw335. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno Y, Miura Y, Yamaguchi T, Matsumoto T. Extended-spectrum beta-lactamase-producing Enterobacteriaceae colonisation in long-term overseas business travellers. Travel Med Infect Dis. 2016;14:561–567. doi: 10.1016/j.tmaid.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Tham J, Odenholt I, Walder M, Brolund A, Ahl J, Melander E. Extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand J Infect Dis. 2010;42:275–280. doi: 10.3109/00365540903493715. [DOI] [PubMed] [Google Scholar]
- 15.United Nations World Tourism Organization (UNWTO), World Tourism Barometer. Available at: http://mkt.unwto.org/barometer.Accessed 15 Oct 2017.
- 16.United Nations, Department of Economic and Social Affairs. Definition of major areas and regions (World Population Prospects, Revision 2015). 2015.Available at: https://esa.un.org/unpd/wpp/General/Files/Definition_of_Regions.pdf. Accessed 15 Oct 2017.
- 17.Bassyouni RH, Gaber SN, Wegdan AA. Fecal carriage of extended-spectrum beta-lactamase- and AmpC- producing Escherichia coli among healthcare workers. J Infect Dev Ctries. 2015;9:304–308. doi: 10.3855/jidc.5633. [DOI] [PubMed] [Google Scholar]
- 18.Farra A, Frank T, Tondeur L, Bata P, Gody JC, Onambele M, et al. High rate of faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in healthy children in Bangui, Central African Republic. Clin Microbiol Infect. 2016;22:891. doi: 10.1016/j.cmi.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Magoue CL, Melin P, Gangoue-Pieboji J, Okomo Assoumou MC, Boreux R, De Mol P. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Ngaoundere, Cameroon. Clin Microbiol Infect. 2013;19:E416–E420. doi: 10.1111/1469-0691.12239. [DOI] [PubMed] [Google Scholar]
- 20.Tellevik MG, Blomberg B, Kommedal O, Maselle SY, Langeland N, Moyo SJ. High prevalence of Faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar Es Salaam. Tanzania PLoS One. 2016;11:e0168024. doi: 10.1371/journal.pone.0168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mshana SE, Falgenhauer L, Mirambo MM, Mushi MF, Moremi N, Julius R, et al. Predictors of blaCTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect Dis. 2016;16:187. doi: 10.1186/s12879-016-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najjuka CF, Kateete DP, Kajumbula HM, Joloba ML, Essack SY. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res Notes. 2016;9:235. doi: 10.1186/s13104-016-2049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frickmann H, Warnke P, Frey C, Schmidt S, Janke C, Erkens K, et al. Surveillance of food- and smear-transmitted pathogens in European soldiers with diarrhea on deployment in the tropics: experience from the European Union training mission (EUTM) Mali. Biomed Res Int. 2015;2015:573904. doi: 10.1155/2015/573904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouedraogo AS, Sanou S, Kissou A, Poda A, Aberkane S, Bouzinbi N, et al. Fecal carriage of Enterobacteriaceae producing extended-Spectrum Beta-lactamases in hospitalized patients and healthy community volunteers in Burkina Faso. Microb Drug Resist. 2017;23:63–70. doi: 10.1089/mdr.2015.0356. [DOI] [PubMed] [Google Scholar]
- 25.Isendahl J, Turlej-Rogacka A, Manjuba C, Rodrigues A, Giske CG, Naucler P. Fecal carriage of ESBL-producing E coli and K pneumoniae in children in Guinea-Bissau: a hospital-based cross-sectional study. PLoS One. 2012;7:e51981. doi: 10.1371/journal.pone.0051981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaba M, Manenzhe RI, Moodley C, Zar HJ, Nicol MP. Epidemiology of extended-spectrum beta-lactamase- and carbapenemase-producing bacteria in stool from apparently healthy children. South Africa Int J Infect Dis. 2016;45:95. [Google Scholar]
- 27.Kantele A, Mero S, Kirveskari J, Lääveri T. Fluoroquinolone antibiotic users select fluoroquinolone-resistant ESBL-producing Enterobacteriaceae (ESBL-PE) - data of prospective traveller study. Travel Med Infect Dis. 2017;16:23–30. doi: 10.1016/j.tmaid.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Barreto Miranda I, Ignatius R, Pfuller R, Friedrich-Janicke B, Steiner F, Paland M, et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med. 2016;23:tav024. doi: 10.1093/jtm/tav024. [DOI] [PubMed] [Google Scholar]
- 29.Bou-Antoun S, Davies J, Guy R, Johnson AP, Sheridan EA, Hope RJ. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Euro Surveill. 2016:21. 10.2807/1560-7917.ES.2016.21.35.30329. [DOI] [PMC free article] [PubMed]
- 30.Nemeth J, Ledergerber B, Preiswerk B, Nobile A, Karrer S, Ruef C, et al. Multidrug-resistant bacteria in travellers hospitalized abroad: prevalence, characteristics, and influence on clinical outcome. J Hosp Infect. 2012;82:254–259. doi: 10.1016/j.jhin.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Kaspar T, Schweiger A, Droz S, Marschall J. Colonization with resistant microorganisms in patients transferred from abroad: who needs to be screened. Antimicrob Resist Infect Control. 2015;4:31. doi: 10.1186/s13756-015-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khawaja T, Kirveskari J, Johansson S, Väisänen J, Djupsjobacka A, Nevalainen A, et al. Patients hospitalized abroad as importers of multiresistant bacteria-a cross-sectional study. Clin Microbiol Infect. 2017;23(9):673. doi: 10.1016/j.cmi.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum Beta-lactamase-producing Enterobacteriaceae and risk factor among healthy individuals: a systematic review and Metaanalysis. Clin Infect Dis. 2016;63:310–318. doi: 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Factors available in same format only from the studies of Kantele et al. [6] and Paltansing et al. [4] and not included in the pooled data of this report. (DOCX 16 kb)
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to ongoing further analyses on the data but are available from the corresponding author on reasonable request.