Abstract
Objective:
We aimed to examine the general health and intestinal physiology of young and old non-human primates with comparable life histories and dietary environments.
Design:
Vervet monkeys (Chlorcebus aethiops sabaeus) in stable and comparable social and nutritional environments were selected for evaluation. Health phenotype, circulating cytokines and biomarkers of microbial translocation (MT) were measured (n=26–44). Subsets of monkeys additionally had their intestinal motility, intestinal permeability, and fecal microbiomes characterized. These outcomes document age-related intestinal changes present in the absence of nutritional stressors, which are all known to affect gastrointestinal motility, microbiome, and MT.
Results:
We found that old monkeys have greater systemic inflammation and poor intestinal barrier function as compared to young monkeys. Old monkeys have dramatically reduced intestinal motility, and all changes in motility and MT are present without large differences in fecal microbiomes.
Conclusion:
We conclude that deteriorating intestinal function is a feature of normal aging and could represent the source of inflammatory burden yet to be explained by disease or diet in normal aging human primate populations. Intestinal changes were seen independent of dietary influences and aging within a consistent environment appears to avoid major microbiome shifts. Our data suggests interventions to promote intestinal motility and mucosal barrier function have the potential to support better health with aging.
Keywords: Intestinal microbial translocation, microbiome, inflammation, aging, monkey, motility
Introduction
Health variability increases dramatically as age increases for reasons that are still undefined. Successful aging is consistently associated with low inflammatory cytokine levels and preservation of insulin sensitivity. Chronic low-grade sterile inflammation is associated with age-related diseases, such as diabetes and cardiovascular disease, and has been postulated to have a central role in accelerating multi-organ disease (1–3). In elderly persons without chronic disease, measures of pro-inflammatory cytokine levels were higher in both vascular cells and serum, supporting the dogma that inflammation occurs with ‘normal’ aging and be involved in the pathogenesis of age-related diseases (4). However the underlying source of this inflammation has been yet to be fully elucidated. Changed body composition, including greater visceral adiposity, has been suggested to be a common source of systemic inflammation in older patients (5). Interleukin 6 (IL-6) is the inflammatory cytokine that has been most frequently associated with loss of health (1, 2, 6, 7). However, other studies have identified tumor necrosis factor-α [TNF-α] (8) or other inflammatory biomarkers (9) as being more closely associated with health than IL-6. The tissue sources and stimuli for these cytokine profiles are multiple and still being characterized.
Intestinal barrier dysfunction leads to microbial translocation (MT) of live bacteria or bacterial products present in the gastrointestinal tract to extra-intestinal sites, thus inciting inflammatory responses through toll-like receptor signaling. MT biomarkers measured in the circulation include lipopolysaccharide binding protein 1 (LBP1) and the co-receptor soluble CD14, both involved in the presentation of antigens to toll like receptors present on most cell membranes. MT has been observed in healthy older people who were overweight and associates with higher inflammatory biomarkers (10–12). Recent data demonstrates that intestinal barrier dysfunction correlates with lifespan across a range of Drosophila genotypes and environmental conditions (13). Critically, intestinal barrier dysfunction was a better predictor of age-onset mortality than chronological age. The intact intestinal epithelium forms a selective barrier that permits the absorption and limits host contact with harmful entities including microorganisms, dietary antigens, and environmental toxins.
Intestinal epithelial cells are in constant interaction with intestinal luminal content and contribute to host defense through several physical barrier and immune-based mechanisms (14). The mechanisms underlying the loss of normal intestinal barrier function with healthy aging are unknown. Age is known to affect the intestinal microbiome, and is speculated to be partly responsible for the increased inflammation and health decline observed in elderly populations (15, 16). Immune senescence, dietary changes, and reduced intestinal motility are other factors that may contribute to the loss of barrier function and increased MT seen with aging (16–18). Other “environmental factors” such as dietary fat and fructose, and obesity are known to increase MT and endotoxemia and thus confound human studies of aging and intestinal barrier function (19–21). Using a monkey model of human aging, we aim to describe age-related changes in intestinal function and microbiome in a relevant animal model, not confounded by environmental influences such as disease, diet, or social environment. We hypothesize that aged monkeys will have reduced intestinal integrity and therefore have evidence of greater systemic inflammation. We examine intestinal microbiomes and motility as factors that may underpin changes in intestinal barrier integrity with aging.
Methods
Animal procedures
All study procedures were approved by and performed in accordance with the Wake Forest University Institutional Animal Care and Use Committee. The study populations were sourced from a multigenerational pedigreed colony of vervet monkeys (Chlorcebus aethiops sabaeus). A total of 44 premenopausal female monkeys were included in the primary study. Subsets of these animals were included in studies of motility, intestinal permeability, and microbiome differences. The age range extended from 6.1 years to 21.9 years (maximum lifespan ≈ 27 years; Table 1). “Young” is equivalent to approximately 25 to 30 year old, and the “old” to that of 60 to 65 year old humans. Menopause is seen rarely, and only at extreme ages (22). Animals were housed in indoor/outdoor corrals varying from 380 to 1482 square feet with elevated perches, platforms, and climbing structures. During blood sampling, motility assessments, and fecal collection, monkeys were housed singly for approximately four days. All monkeys were fed commercial low fat, high carbohydrate primate laboratory chow (Diet 5038, Lab Diet, Purina, St. Louis, MO) supplemented with five time weekly fresh produce for the duration of the primary study. The microbiome was determined from a subset of young and old monkeys under this dietary condition and a more westernized diet described below. All animals had ad libitum access to food and opportunities for exercise resulting in equivalent and stable life-long environmental, dietary, and social conditions.
Table 1.
Demographic, health, and microbial translocation indices of the monkeys. Mean ± SEM shown, with p-value for ANOVA
| N (young/old) | Young | Old | p-value | |
|---|---|---|---|---|
| Age (yrs) | 20/24 | 9.2 (0.43) | 20.3 (0.53) | <0.001 |
| BW (kg) | 20/24 | 5.18 (0.21) | 5.38 (0.20) | 0.52 |
| Waist (cm) | 20/24 | 33.3 (1.19) | 37.9 (1.20) | 0.01 |
| Glucose (mg/dL) | 19/24 | 75.2 (15.3) | 89.8 (14.8) | 0.52 |
| Insulin (uIU/mL) | 8/12 | 23.4 (3.40) | 18.9 (2.40) | 0.28 |
| HOMA | 8/12 | 3.42 (0.80) | 3.89 (0.96) | 0.73 |
| Interleukin-6 (pg/mL) | 8/10 | 14.9 (4.49) | 60.3 (51.7) | 0.45 |
| C-reactive protein (ng/L) | 20/24 | 1.20 (0.31) | 3.91 (0.51) | <0.001 |
| Lipopolysaccheride binding protein-1 (ng/L) | 12/14 | 2.36 (1.13) | 14.5 (4.54) | 0.02 |
| Soluble CD14 (mg/mL) | 16/19 | 95.8 (45.0) | 133 (70.3) | 0.34 |
| Citrulline (ug/mL) | 15/17 | 1.82 (0.19) | 1.71 (0.25) | 0.74 |
For blood collection, animals were fasted overnight prior to being sedated with ketamine and dexmedetomidine (5 mg/kg and 25 ug/kg intramuscularly, respectively) to enable femoral venipuncture. Plasma was processed and stored at −80°C. All analyses were measured by ELISA in plasma and endpoints included: LBP1 (Hycult Biotech, Netherlands), citrulline (MyBioSource Inc., San Diego CA); C reactive protein (CRP; ALPCO Inc., Salem, NH), IL-6 and soluble CD14 (sCD14; R&D Systems, Minneapolis, MN).
All monkeys had waist circumference measured by placing a measuring tape around the abdomen at the level of the umbilicus and the circumference measured in centimeters. Fasting glucose concentrations were measured in all monkeys and insulin concentrations were available for 20 monkeys, enabling homeostasis model assessment index calculations. All endpoints were measured as previously described (21).
Intestinal motility
Twenty two monkeys were administered Barium Impregnated Polyethylene Spheres (BIPs) via orogastric intubation under ketamine and dexmedetomidine sedation. The BIPs dose was confirmed radioagraphically. Whole body serial radiographs were taken at 24, 48, and 72 hours post-dose and the number of markers counted and expressed as the percent of total BIPs dosed.
Intestinal permeability
Seven monkeys (4 old and 3 young) were orally administered fluorescein-isothiocyanate conjugated dextran 40kD (FITC-Dextran) to determine intestinal integrity. Orogastric intubation facilitated dosing of 500 mg/kg (200 mg/mL) and was coupled with blood collection at baseline and at 24, 48, and 72 hours post-administration. Fluorescence presence was measured in duplicate at each time point and was defined as being positive when the mean count was more than 4 standard deviations above the blank.
Microbiome
Ten vervet monkeys (6 old and 4 young) from the same colony of vervet monkeys were moved to pair-housing for evaluation before and after a short-term dietary fructose challenge (21). They had approximately 400 grams of fecal material per monkey opportunistically collected at weekly intervals over a 5 week period. Samples were flash frozen in liquid nitrogen and stored at −80°C until DNA extraction. DNA extraction was performed via QIAGEN ® QIAmp® Fast DNA Stool Mini Kit and DNA quality assessed by agarose gel. Eluted DNA was quantitated using UV spectrometry with a Nano-Drop ND-1000. For gene sequencing, the amplicon primers for the 16S variable V6 regions were synthesized for the gene-specific region preparation of the feces. Illumina Nextera XT indices (Illumina Inc. San Diego, CA) were used for the preparation of the library from multiple pooled samples. 16S rRNA sequences from two of the timepoints from this study have been previously published (21). The details of methodology used for taxonomic assignment and data analysis are presented in the Supplementary Data file.
Data analysis
Data are presented as mean +/− standard error of mean (SEM) for each group. Group sizes for each measure are indicated in figure and tables. Data was analyzed for normality and logarithmically transformed where necessary (LBP1, glucose) before analysis of variance was performed to assess for group differences. CRP and sCD14 data was analyzed using z-scores as we utilized kits from different lots run on different days. Actual values for CRP and sCD14 are supplied in the results. FITC-Dextran permeability detection frequency was examined by Fishers exact test (1-tailed). Statistical analysis was performed using Statistica v10 (StatSoft, Inc. Tulsa, OK). Significance was set at α≤0.05 for group differences, and ≤0.1 for trends.
Four elderly animals were classified as diabetic or with impaired fasting glucose (based on current American Diabetes Association Guidelines). Analysis of data was performed with and without these individuals, and outcomes were not different. Data shown includes raw values with all monkeys included. Co-varying results for body weight and waist circumference was also conducted and did not change any outcomes reported.
Results
Old monkeys exposed to comparable diets have similar body weights and metabolic health as young monkeys (Table 1). Glucose, insulin and insulin sensitivity were equivalent and monkeys were observed to be in overall good health, mobile, have normal appetite and fecal consistency. Redistribution of body fat is present as evidenced by old monkeys having significantly but moderately enlarged waist circumferences (14% greater than young monkeys). Obesity in this species has been defined as waist circumference > 40.5cm and the study population mean was 38cm, thus this cohort of young and old monkeys are of normal adiposity (23). Despite their general good health, old monkeys demonstrate more than a 3-fold greater inflammatory burden as measured by CRP (Figure 1A). IL-6 levels were highly variable in both age groups, and thus not significantly different despite a 4-fold greater group mean in old monkeys (Table 1). Intestinal barrier dysfunction was seen in the old monkeys with significantly elevated LBP1, which is a surrogate biomarker for exposure to greater amounts of intestinally-sourced endotoxin (Figure 1B). sCD14 is the co-receptor for TRL4 and interacts with LBP1 to generally promote innate cellular responses to endotoxin. sCD14 was non-significantly higher in old monkeys but there was a very strong positive association between LBP1 and sCD14 (r = 0.72, p < 0.001, n=26) that supports this biology. This positive association was present and statistically significant when the young and old animals are separately evaluated. Citrulline was evaluated as a marker of mucosal health and mass (24) and was not statistically different between groups. The elevation of LBP1 as a circulating biomarker of increased MT was supported by observation that intestinal permeability to large molecules in old monkeys was higher (p=0.039; see Supplementary Table 2). FITC-dextran was specific for age-related barrier dysfunction, with all of the young monkeys able to efficiently exclude this marker from translocating from the intestinal lumen into the general circulation.
Figure 1.
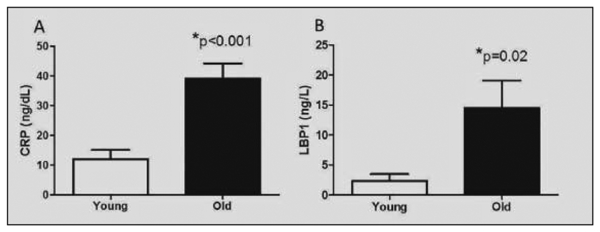
A) C-reactive protein (CRP) concentrations in plasma of young (n=20) and old (n=24) monkeys. B) Lipopolysaccheride binding protein (LBP)l concentrations in plasma of young (n=12) and old (n=14) monkeys
Evaluation of intestinal motility revealed dramatic age-related differences (Figure 2A). Evaluations show that at all timepoints post-dosing, marker clearance was delayed in old monkeys. A representative radiograph at 72 hours is shown (Figure 2B). Observed differences in motility likely represent predominantly large bowl motility reductions with age, as percentage differences become more exaggerated as time passed and markers were retained in the colon.
Figure 2.
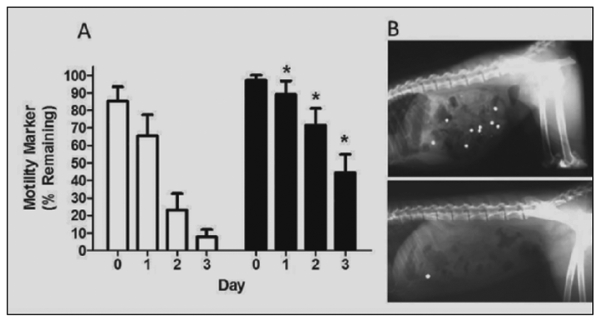
A) Gastro-colic motility rates in young (open bars) and old (black bars) monkeys (n=l1/age group). Values reflect the percentage of total administered markers (depicted in B) observed at each day’s radiograph. Loss of markers at Time 0 presumed to result of unaccounted emesis/regurgitation, retention of marker in cheek pouch or esophagus. *p<0.05 versus young monkey group at the same time point. B) Representative radiographic comparison of a young and old monkey at 72 hours post-dose
In order to determine whether age was associated with changes to the microbial community, we sampled 10 monkeys longitudinally, sampling each individual 5 times over a time-period of 5weeks. As described previously (21), the monkeys had shared common environmental, genetic, and dietary life experiences prior to this experimental comparison of microbiomes while consuming either a control or experimental high-fructose western diet. Terms for effects of age were not significant in either models built individually for animals on the control diet (see Supplementary Table 2), the high-fructose diet (see Supplementary Table 3) or in a model in which the data from the animals on the two diets is combined (Table 2). We saw that either on the control diet (Figure 3A) or the high-fructose diet (Figure 3B), the microbial signature of each individual monkey was highly constant, with highly significant p-values associated with stability of the microbial community in our statistical models (Table 2, see Supplementary Tables 2 and 3). We also found significant p-values indicating that the microbial community shifted with time across the entire population of monkeys during the course of the experiments (Table 2, see Supplementary Tables 2 and 3). This presumably indicates the presence of a modest, but statistically significant, degree of taxonomic drift within the small population of physically isolated animals from which we are sampling. Our measurements of the fecal microbiome do not preclude the possibility that mucosal microbiome interactions do differ between young and the old. However, the gross equivalence in the fecal microbiome across age groups from these monkeys points to the importance that other age-related factors, such as intestinal motility, play in permitting microbial translocation and inflammation in the aged population.
Table 2.
Regression results for microbiome analysis of monkeys with diet and age included as fixed term and animal nested within cage as a random tenn. P-values shown are for significance of the coefficients of the linear model for the first five MDS axes at the family level
| MDS Axis | Percent variation explained | Age p-value | Days p-value | Diet p-value | Cage/Animal Effect |
|---|---|---|---|---|---|
| 1 | 34.50% | 0.168 | 5.72 × 10−5 | 0.178 | 0.00 |
| 2 | 15.65% | 0.589 | 0.294 | 0.077 | 4.33 × 10−15 |
| 3 | 9.92% | 0.148 | 0.006 | 0.336 | 7.59 × 10−7 |
| 4 | 7.71% | 0.319 | 5.80 × 10−5 | 0.649 | 1.71 × 10−5 |
| 5 | 5.24% | 0.169 | 0.042 | 0/593 | 0.192 |
Figure 3.
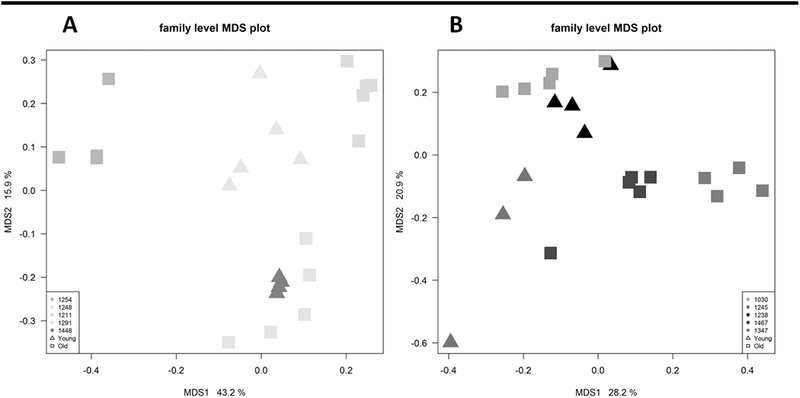
A) Multidimensional scaling (MDS) fecal microbiomic profiles at the family level from old and young monkeys while consuming a chow diet. The plot is colored by monkey, and their age indicated by marker shape. Old animals are denoted by squares (■) and young by triangles (■). No large differences between age groups were observed. B) Multidimensional scaling (MDS) fecal microbiomic profiles at the family level from old and young monkeys while consuming a high fructose diet. The plot is colored by monkey, and their age indicated by marker shape. Old animals are denoted by squares (■) and young by triangles (■). No large differences between age groups were observed
Discussion
Our study points to intestinal dysfunction being an important and yet to be fully recognized age-related phenotype. This is the first study to measure microbial translocation marker coincidently with motility and direct measures of permeability. We see evidence of microbial translocation, and permeation of macromolecules across the mucosal barrier without gross signs of intestinal disease. These results are important to disseminate as Old World non-human primates have comparable gastrointestinal and cardiometabolic physiologies to humans, and age-associated changes are present despite consumption of an optimized diet (low fat, few calories as simple sugars, and adequate dietary fiber). Consequentially these animals are non-obese (23), however this is not the nutritional context of westernized nations. High fat, sugar diets and obesity increase microbial translocation and these factors combined with increasing gut leakiness with age may underpin why the prevalence of metabolic syndrome and related disorders in people increases with age (25).
C-reactive protein is an acute phase protein primarily produced in the liver, which is the first tissue exposed to the portal circulation with its microbial contents, and is circulating at much higher levels in old monkeys. We suggest that this inflammatory background will augment negative Western diet-related effects on vascular and insulin-responsive tissues. Aged persons have exaggerated vascular and insulin resistant responses to diet challenges (26, 27) consistent with this concept. Histological assessments of aged baboon colon supports loss of intestinal integrity and barrier function in the absence of clinical of histological evidence of disease (28). Histological quantitation of colonic smooth muscle in people and rats do not show reductions with age (29, 30). We cannot determine whether the loss of normal propulsive activity precedes loss of barrier function, or if barrier dysfunction leads to motility changes. Either scenario is plausible as reduced motility leads to alterations in the intestinal contents and the mucosal interface (31, 32). Motility reduction with aging isn’t fully understood but is a clinically significant problem, with constipation rates in older people estimated at more than double the prevalence in the general population, with females being disproportionately affected (33). Reduced intestinal motility may be the result of compounding effects of neuromuscular changes seen with age. In the colons of human patients, age-related neuronal loss is associated with a greater proportion of abnormal myenteric ganglia, appearing with cavities (31). The enteric nervous system coordinates sensing, secretory, barrier and motility functions of the gut. The loss of neuronal abundance with aging is not universally observed, but degenerative changes in some neural populations is likely with loss of glial cells shown to affect barrier function (34).
Multiple deficits in contractile signaling pathways also exist on top of reduced neurotransmitter signaling (35). Diabetes, seen commonly with aging, is known to result in poor intestinal motility. We did not observe further reductions in intestinal motility in the few diabetic monkeys included in our study, possibly due to the supportive dietary conditions (18). Aged myocytes from skeletal tissue show reduced contractile ability (36) an effect that may be reflected also in smooth muscle.
Changed intestinal motility will alter both the environment and kinetics of the microbiome at the mucosal surface. The citrulline data reported herein grossly suggests that mucosal health is comparable between age groups (37, 38) but does not indicate the intactness of the mucosal barrier, or its immune competence. A leaky mucosa exposes the sub-mucosal tissues to antigenic materials. The local inflammatory response can sensitize afferent and efferent neurons of enteric nervous system and alter neuromuscular signaling, pain sensation and eventually intestinal motility (32). This vicious cycle of altered motility, mucosal inflammatory responses and degraded barrier competency has been described for inflammatory bowel diseases but has yet to be applied to normal aging of the intestines. Infiltration of bacteria or their byproducts initiates dysfunctional intestinal immune responses which has been reported to cause gastrointestinal disorders such as colitis (39). Even independently of aging, mucosal barrier injury directly triggers a systemic inflammatory response that precedes the onset of bacteremia.
Immunity additionally wanes with age, contributing to inflammatory burdens (40). Human and nonhuman primates share age-related shifts in immune cell profiles and greater systemic inflammation, most commonly associated with increased production of IL-6 and TNF-α (17). Without a competent mechanism to manage locally and systemically detected microbial antigens, negative effects of circulating antigens on all organ systems can be expected. The multi-systemic functional decline seen with aging has been traditionally captured in animal studies as mortality rates, but a promising surrogate marker in people and animal models is physical function indices (1, 13, 16). LBP1, the marker of microbial translocation is elevated in elderly monkeys and people (10), and has been related to physical functioning in healthy aged people. Microbial translocation also correlated positively with inflammatory marker in this study of healthy aging.
Chronic low-grade sterile inflammation is associated with age-related diseases, such as diabetes, cancer, and cardiovascular disease, and postulated to have a central role in accelerating these disease processes. Interestingly, proinflammatory cytokines are elevated in the vascular cells and serum of elderly persons with no overt signs of disease, suggesting inflammation accompanying the natural aging process may contribute to the onset of age-related diseases (4). A growing body of evidence, which we subscribe to, implicates decline in gastrointestinal barrier function as a cause of increased systemic inflammation. High impact invertebrate studies that demonstrate intestinal barrier function lead to functional decline and mortality, also demonstrate that preservation of barrier function through improved proteostasis can lead to lifespan and healthspan extension (13, 41). Translation of these exciting findings to an appropriate mammalian animal model, such as the aged non-human primate that we describe, is required.
The microbiomic profile was not appreciably different in our young and old monkeys. This is not surprising due to the consistency of the shared genetics, diet and dietary fiber amounts, social community, lack of medication effects, and housing exposures (42–44). The fecal microbiome represents a “catch-all” approach for estimation of colonic contents and may not represent location-specific microbiomes. The microbiome in close association with the mucosal surface may differ between young and old, and is a sub-population of the general microbiome that cannot be captured with gross fecal analysis (15). Microbes able to colonize the outer intestinal mucus layer, and the presence of this protective mucus, are both features that prevent metabolic disease development in animal experiments (14, 45). Intestinal epithelial cell-surface modulation (glycosylation) occurs by bacteria, and pathogenic gram negative bacteria are able to degrade mucin and tight junction proteins that modulate paracellular translocation (46). Microbiome manipulations can alter mucosal permeability in mice (47) and one potential mechanism is stimulation of cannabinoid receptors which facilitate increased permeability of the intestinal barrier (48).
The microbiome in aging people displays greater inter-individual variation than that of younger adults and clinical studies have repeatedly demonstrated that geography, diet, and medication affects the microbiome making generalizations about age difficult (16, 25). Slower intestinal motility such as we observed can alter the microbiome by virtue of reducing the amount of nutritive substance that reaches the colon, resulting in selective expansion of unfavorable anaerobes (15). Probiotics and fiber supplementation have been widely recommended to combat motility reductions and restore colonic health but conclusive results are not available. We have evidence from this and prior studies that short-term dietary changes do not greatly modulate the microbiome, while the components of a western diet (high fat, high simple sugars, caloric excess) all have been shown to reduce intestinal barrier function and increase microbial translocation/endotoxemia (19, 21, 49, 50). This could mean that minor microbiomic population changes or altered metabolism of the microbiome can affect the mucosa greatly. Our monkey study indicates a dramatic difference in LBP1 between young and old individuals and to contextualize the significance of this, the magnitude of the observed difference far exceeds that seen between healthy and diabetic patients (50). Endotoxemia has gained a high level of interest as it pertains to metabolic disease risk, but its contribution to age-associated multi-organ disease is possibly of greater public health importance. Invertebrate models link gut integrity with muscle function and insulin sensitivity (13, 41), and rodents with altered microbiomes have reduced integrity in both intestinal epithelium and the vascular endothelium of the brain (47). Barrier function and microbial translocation is a multi-systemic process. The older citizens of westernized countries typically consume western diets that drive greater microbial translocation, and such diets have been related to microbiomic changes and frailty in the elderly (16). Poor diets will potentially exacerbate age-related reductions in barrier function of the gut and extra-intestinal tissues such as the blood brain barrier.
Our monkey study has some limitations to acknowledge. It is a small study, but the homogeneity of the monkey’s life experiences and genetics enabled meaningful comparisons regarding physiological changes with age. We have only evaluated monkeys cross-sectionally, generating a phenotype that can be intervened on or followed longitudinally. The aging monkey model presented also substantiates its use for evaluating intestinal immune profiles, and interventions aimed to improve intestinal barrier function. We did not evaluate the mucosal microbiome which is more specific to microbial translocation than the fecal microbiome. Although food intake was not measured, reduced activity and thus caloric expenditure was seen in old monkeys despite comparable bodyweights, reduced food intake may be inferred. Our study has strengths in that it evaluates age-related changes in a relevant primate model unconfounded by diet or environment, and points to intestinal aging as an important and conserved biogerontological phenomena. In doing so, we translate findings from invertebrate studies and substantiate the gut as under-appreciated organ to support in the aging process. We conclude that impairment of gastrointestinal function (motility and barrier integrity) as a result of aging may be a source of low grade endotoxemia that initiates inflammation in healthy, normal aging populations. Maintaining gastrointestinal motility may be a primary preventive factor in age-related diseases. Interventions to normalize motility and mucosal integrity may improve health in older people.
Supplementary Material
Acknowledgments
Funding: Funding for this study came from K01AG033641, R21AG049160, P40OD010965, the Claude D. Pepper Older Americans Independence Center of Wake Forest School of Medicine (P30AG21332), T35HL092618 (K.B.), T35OD010946 (M.D.) and Wake Forest School of Medicine.
Footnotes
Conflict of interest: No conflicts of interest exist for any of the authors.
Ethical Standards: All experimental procedures were reviewed and in compliance with the ethical standards of Wake Forest School of Medicine, and in line with the recommendations of the United States Department of Agriculture and Office of Laboratory Animal Welfare.
References
- 1.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, and Kirkwood TB Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev 2012; 133, 456–466 [DOI] [PubMed] [Google Scholar]
- 2.Adriaensen W, Mathei C, Vaes B, van Pottelbergh G, Wallemacq P, and Degryse JM Interleukin-6 predicts short-term global functional decline in the oldest old: results from the BELFRAIL study. Age 2014;36, 9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, and Barzilai N Clinical phenotype of families with longevity. J Am Geriatr Soc 2004;52, 274–277 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu I, Yoshida Y, Suda M, and Minamino T (2014) DNA damage response and metabolic disease. Cell metabolism 2014;20, 967–977 [DOI] [PubMed] [Google Scholar]
- 5.Levine ME, and Crimmins EM The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring) 2012;20, 2101–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PW, Abad LW, and Harris T Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc 2003;51, 1237–1243 [DOI] [PubMed] [Google Scholar]
- 7.Souza RB, and Powers CM Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther 2009;39, 12–19 [DOI] [PubMed] [Google Scholar]
- 8.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, and Visser M Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64, 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa K, Kim HK, Shimizu T, Abe S, Shiga Y, and Calderwood SK Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 2012; 17, 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Lertwattanarak R, Garduno Jde J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, and Musi N Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 2015;70, 232–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stehle JR Jr., Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, and High KP Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci 2012;67, 1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Miguelez P, Fernandez-Gonzalo R, Collado PS, Almar M, Martinez-Florez S, de Paz JA, Gonzalez-Gallego J, and Cuevas MJ Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech Ageing Dev 2015; 150, 12–19 [DOI] [PubMed] [Google Scholar]
- 13.Rera M, Clark RI, and Walker DW Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A 2012; 109, 21528–21533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, and Cani PD Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110, 9066–9071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiihonen K, Ouwehand AC, and Rautonen N Human intestinal microbiota and healthy ageing. Ageing research reviews 2010; 9, 107–116 [DOI] [PubMed] [Google Scholar]
- 16.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, and O’Toole PW Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488, 178–184 [DOI] [PubMed] [Google Scholar]
- 17.Haberthur K, Engelman F, Barron A, and Messaoudi I Immune senescence in aged nonhuman primates. Exp Gerontol 2010;45, 655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley JE Constipation and irritable bowel syndrome in the elderly. Clinics in geriatric medicine 2007;23, 823–832, vi–vii [DOI] [PubMed] [Google Scholar]
- 19.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, and Ferrieres J Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 20.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, and Burcelin R Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, and Cullen JM Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. American Journal of Clinical Nutrition 2013;98, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins HM, Willson CJ, Silverstein M, Jorgensen M, Floyd E, Kaplan JR, and Appt SE Characterization of ovarian aging and reproductive senescence in vervet monkeys (Chlorocebus aethiops sabaeus). Comparative medicine 2014;64, 55–62 [PMC free article] [PubMed] [Google Scholar]
- 23.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, and Wagner JD Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007; 15, 1666–1674 [DOI] [PubMed] [Google Scholar]
- 24.Beaufrere AM, Neveux N, Patureau Mirand P, Buffiere C, Marceau G, Sapin V, Cynober L, and Meydinal-Denis D Long-term intermittent glutamine supplementation repairs intestinal damage (structure and functional mass) with advanced age: assessment with plasma citrulline in a rodent model. The journal of nutrition, health & aging 2014; 18, 814–819 [DOI] [PubMed] [Google Scholar]
- 25.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, and Heymsfield SB The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2003; 163, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einstein FH, Huffman DM, Fishman S, Jerschow E, Heo HJ, Atzmon G, Schechter C, Barzilai N, and Muzumdar RH Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci 2010;65, 800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, and Volpi E Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010;59, 2764–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran L, and Greenwood-Van Meerveld B Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 2013;68, 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDougal JN, Miller MS, Burks TF, and Kreulen DL Age-related changes in colonic function in rats. The American journal of physiology 1984;247, G542–546 [DOI] [PubMed] [Google Scholar]
- 30.Southwell BR, Koh TL, Wong SQ, King SK, Ong SY, Lee M, Farmer PJ, Peck CJ, Sutcliffe JR, Stanton MP, Keck J, Cook DJ, Chow CW, and Hutson JM Decrease in nerve fibre density in human sigmoid colon circular muscle occurs with growth but not aging. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2010;22, 439–445, el06 [DOI] [PubMed] [Google Scholar]
- 31.Bitar K, Greenwood-Van Meerveld B, Saad R, and Wiley JW Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2011;23, 490–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez C, Gonzalez-Castro A, Vicario M, and Santos J Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut and liver 2012;6, 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costilla VC, and Foxx-Orenstein AE Constipation: understanding mechanisms and management. Clinics in geriatric medicine 2014;30, 107–115 [DOI] [PubMed] [Google Scholar]
- 34.Saffrey MJ Aging of the mammalian gastrointestinal tract: a complex organ system. Age 2014;36, 9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bitar KN, and Patil SB Aging and gastrointestinal smooth muscle. Mech Ageing Dev 125, 907–910 [DOI] [PubMed] [Google Scholar]
- 36.Choi SJ, Shively CA, Register TC, Feng X, Stehle J, High K, Ip E, Kritchevsky SB, Nicklas B, and Delbono O Force-generation capacity of single vastus lateralis muscle fibers and physical function decline with age in African green vervet monkeys. J Gerontol A Biol Sci Med Sci 2012;68, 258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blijlevens NM, Donnelly JP, and DePauw BE Inflammatory response to mucosal barrier injury after myeloablative therapy in allogeneic stem cell transplant recipients. Bone marrow transplantation 2005;36, 703–707 [DOI] [PubMed] [Google Scholar]
- 38.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, and Messing B Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000; 119, 1496–1505 [DOI] [PubMed] [Google Scholar]
- 39.Gunther C, Buchen B, Neurath MF, and Becker C Regulation and pathophysiological role of epithelial turnover in the gut. Seminars in cell & developmental biolog 2014;35, 40–50 [DOI] [PubMed] [Google Scholar]
- 40.DelaRosa O, Pawelec G, Peralbo E, Wikby A, Mariani E, Mocchegiani E, Tarazona R, and Solana R Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology 2006;7, 471–481 [DOI] [PubMed] [Google Scholar]
- 41.Ulgherait M, Rana A, Rera M, Graniel J, and Walker DW AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell reports 2014;8, 1767–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, and Ley RE Human genetics shape the gut microbiome. Cell 2014; 159, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, and Lewis JD Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334, 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakshminarayanan B, Stanton C, O’Toole PW, and Ross RP Compositional dynamics of the human intestinal microbiota with aging: implications for health. The journal of nutrition, health & aging 2014; 18, 773–786 [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Yang Z, Rey FE, Ridaura VK, Davidson NO, Gordon JI, and Semenkovich CF Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell host & microbe 2012; 11, 140–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma R, Young C, and Neu J Molecular modulation of intestinal epithelial barrier: contribution of microbiota. Journal of biomedicine & biotechnology 2010, 305879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E. t., Taylor CM, Welsh DA, and Berthoud HR Obese-type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biological psychiatry 2015;77, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez M, Panahi S, and Tremblay A Childhood obesity: a role for gut microbiota? International journal of environmental research and public health 2015; 12, 162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, and Burcelin R Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56, 1761–1772 [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, and Fernandez-Real JM Circulating lipopolysaccharide-binding protein (EBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2011;36, 1442–1449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


