Abstract
Asymmetric cell division is a common mode of cell differentiation during the invariant lineage of the nematode, C. elegans. Beginning at the four-cell stage, and continuing throughout embryogenesis and larval development, mother cells are polarized by Wnt ligands, causing an asymmetric inheritance of key members of a Wnt/β-catenin signal transduction pathway termed the Wnt/β-catenin asymmetry pathway. The resulting daughter cells are distinct at birth with one daughter cell activating Wnt target gene expression via β-catenin activation of TCF, while the other daughter displays transcriptional repression of these target genes. Here, we seek to review the body of evidence underlying a unified model for Wnt-driven asymmetric cell division in C. elegans, identify global themes that occur during asymmetric cell division as well as highlight tissue-specific variations. We also discuss outstanding questions that remain unanswered regarding this intriguing mode of asymmetric cell division.
Keywords: Wnt, beta-catenin, cell polarity, asymmetry, SYS-1, WRM-1, POP-1
1. INTRODUCTION
Asymmetric cell division (ACD) that results in two daughter cells with different developmental fate potentials is required for proper fate specification and diversification of tissues during development and also regulates self-renewal of these tissues during adulthood. These decisions are coordinated across space and time by cell signaling pathways, such as the well-conserved Wnt signaling pathways. Wnt signal transduction pathways can differ considerably, however they typically share requirements of a Wnt ligand-binding receptors and co-receptors, such as Frizzled and Lrp6, to activate the phosphoprotein Dishevelled. Downstream of Dishevelled the pathways diverge to control several distinct intracellular pathways depending on the protein expression profiles of the recipient cells. These include the β-catenin dependent or “canonical” Wnt signaling pathway, the Wnt planar cell polarity (PCP) pathway, and the Ca2+/Calmodulin pathway (Sokol, 2015, Gao and Chen, 2010). Though Wnt/PCP is often associated with polarizing cells in various contexts including vertebrate convergent extension and the fly eye and wing bristle (Axelrod et al., 1996, Wehrli and Tomlinson, 1998, Klein and Mlodzik, 2005, Gao, 2012) and the Wnt/Ca2+ pathway also polarizes cell movements during gastrulation (Lin et al., 2010), the Wnt/β-catenin canonical pathway regulates mother cell polarity at the time of division and subsequent asymmetry of newly-formed daughter cells in addition to its a well-established role in transcriptional activation, cell proliferation, differentiation and stem cell maintenance (Habib et al., 2013, Sawa and Korswagen, 2013, Murgan and Bertrand, 2015, Munro and Bowerman, 2009, Hardin and King, 2008).
1.1 Canonical Wnt signaling stabilizes β-catenin to activate TCF target genes
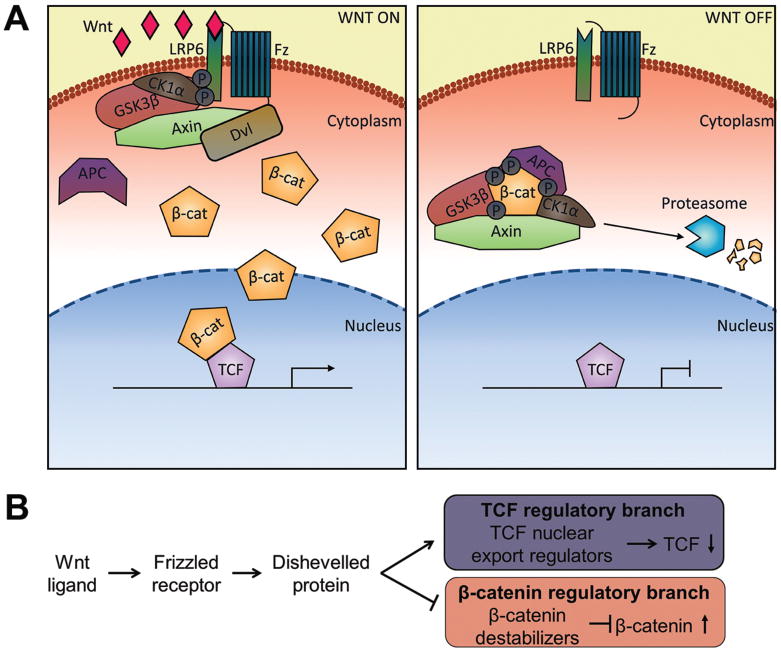
The canonical Wnt pathway affects changes in cell behavior by altering gene expression through regulation of a transcriptional coactivator, β-catenin. In populations of cells that lack Wnt signal transduction, a “destruction complex” forms, consisting of two kinases, Glycogen Synthase Kinase 3β (GSK3β) and Casein Kinase Iα (CKIα) and two scaffold proteins, Axin and Adenomatous Polyposis Coli (APC), binds and phosphorylates cytoplasmic β-catenin, triggering β-catenin ubiquitination and proteasomal degradation (Clevers and Nusse, 2012, Logan and Nusse, 2004, MacDonald and He, 2012). In the absence of β-catenin, the DNA binding protein TCF transcriptionally represses target gene expression, but in the presence of Wnt signal transduction, TCF transcriptional repression is converted to target gene activation. This is accomplished by Wnt activation of Frizzled receptors and LRP5/6 co-receptors, which promotes the activation of Dishevelled (Dvl) and inactivates the destruction complex by physically interacting with its components, thereby inhibiting β-catenin degradation (Clevers and Nusse, 2012, MacDonald and He, 2012). As a result, β-catenin within the cytoplasm is stabilized and translocated into the nucleus, where it binds TCF. This binding converts TCF into a transcriptional activator and promotes the expression of TCF target genes due to β-catenin’s ability to recruit transcriptional activators such as Mediator and chromatin modifiers such as CBP, Brg-1, Bcl-9, and Pygopus (Figure 1A) (Hecht et al., 2000, Takemaru and Moon, 2000, Barker et al., 2001, Kramps et al., 2002, Parker et al., 2002, Thompson et al., 2002, Roose and Clevers, 1999, Yoda et al., 2005).
Figure 1.
The Wnt/β-catenin signing pathway. (A) Canonical Wnt signaling in mammalian systems activates TCF target gene expression by stabilizing β-catenin. (B) Simplified representation of the branched WβA pathway in C. elegans with two outputs: elevated β-catenin levels and decreased nuclear TCF.
1.2 A branched Wnt pathway controls asymmetric divisions in C. elegans
While a similar Wnt/β-catenin signaling pathway is found in nematodes that stabilizes the β-catenin homolog, BAR-1 to generate cell diversity in tissues including the Q neuroblast and vulva precursor cell lineages (Eisenmann et al., 1998, Gleason et al., 2006, Jiang and Sternberg, 1999, Schmid and Hajnal, 2015), a more broadly used Wnt/β-catenin pathway polarizes mother cells and drives serial ACD throughout development, globally generating tissue diversity. In this pathway, termed the Wnt/β-catenin Asymmetry (WβA) pathway, two additional β-catenins, SYS-1 and WRM-1, regulate the transcriptional activity and nuclear levels to the sole C. elegans TCF transcription factor, POP-1. After ACD, the daughter cell whose fate does not depend on Wnt signaling, the “unsignaled daughter”, contains low SYS-1/β-catenin due to destruction complex activity and, similar to the canonical Wnt pathway, the lack of nuclear β-catenin causes POP-1 to repress the transcription of Wnt target genes (Sawa and Korswagen, 2013, Baldwin and Phillips, 2014, Jiang and Sternberg, 1999). The mother cell asymmetrically localizes the destruction complex members (e.g. APC and Axin) such that the unsignaled daughter inherits these negative regulators (Mizumoto and Sawa, 2007b). Conversely, the daughter cell whose fate depends on Wnt signaling (the “signaled daughter”) exhibits lower levels of these negative regulators. In the signaled daughter cell, similar to the canonical pathway, SYS-1/β-catenin accumulates in the cytoplasm, translocates to the nucleus and converts POP-1/TCF into a transcriptional activator. However, the WβA pathway also possesses notable differences compared to the canonical pathway. In addition to the stabilization of SYS-1/β-catenin, a second mechanism downstream of Frizzled and Dvl exports excess nuclear POP-1/TCF. POP-1 export is, somewhat counter-intuitively, necessary for Wnt signal transduction and target gene expression because a decrease in nuclear POP-1 lowers the “free” or repressive POP-1 while retaining sufficient levels to bind SYS-1 and activate gene expression. To complicate matters further, POP-1 nuclear export is carried out with the help of a third β-catenin, called WRM-1, which facilitates TCF phosphorylation (and subsequent nuclear export) by the NEMO-like kinase LIT-1(Yang et al., 2011). In all, the low level of POP-1/TCF in the signaled cell increases the likelihood that most of the POP-1 in the nucleus will be bound by SYS-1/β-catenin, which is increasing in this cell, therefore activating the transcription of genes in the signaled daughter. Conversely, in the Wnt inactive (“unsignaled”) cell, a high level of POP-1 and a low level of SYS-1 leads to more “free” POP-1 and transcriptional repression. (Figure 1B)(Sawa and Korswagen, 2013, Phillips and Kimble, 2009). A detailed discussion of the experimental evidence underlying this model and considerations of future challenges are presented below.
2. WNT POLARIZES THE ENDO-MESODERM LINEAGE
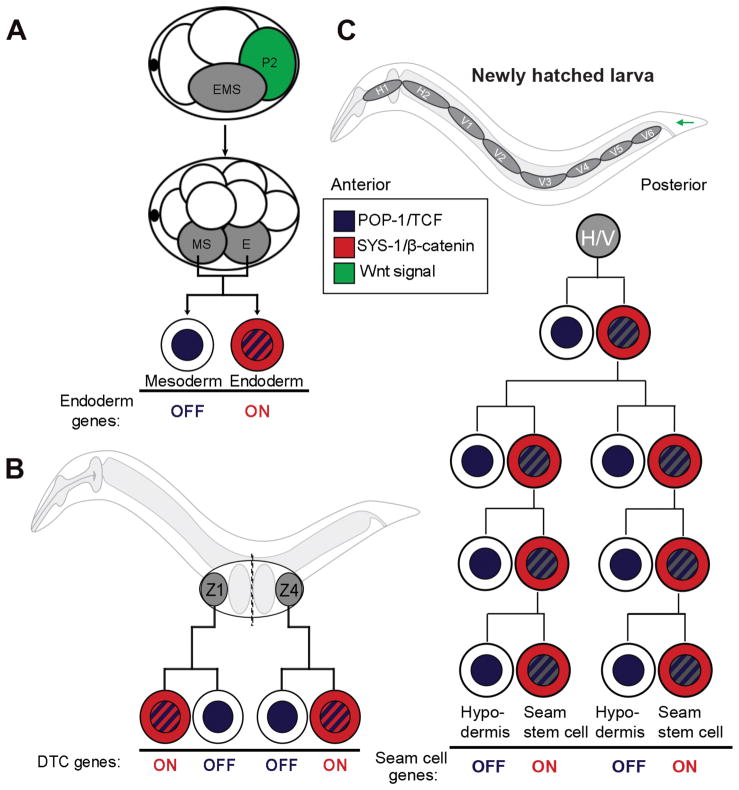
The WβA pathway appears to regulate the many ACDs of C. elegans embryogenesis. Embryonic blastomeres undergo multiple asymmetric divisions, each cell producing daughter cells with different developmental fates. ACDs affect differential cell fate specification as early as the first cell division, where the site of sperm entry determines the first cleavage plane and breaks symmetry by producing a larger somatic cell (AB) and a smaller germ cell (P1). However, the first incidence of Wnt-driven ACD occurs at the four-cell stage, where the posterior daughter of P1, called P2, polarizes its neighbor cell, called EMS, which divides asymmetrically to give rise to the endoderm and mesoderm lineages (Figure 2A) (Munro and Bowerman, 2009, Goldstein and Hird, 1996). EMS polarization by P2-derived Wnt ligand has been well-studied and gives excellent insight into the mechanisms of the WβA pathway.
Figure 2.
Asymmetric cell division in C. elegans. (A) A Wnt signal from P2 polarizes EMS, which divides into endoderm (E) and mesoderm (MS) daughter cells. E displays lowered nuclear POP-1 (blue cross-hatching) and elevated SYS-1 compared to MS. (B) Z1/Z4 somatic gonadal precursor cells divide along the proximal-distal axis (dashed line), each asymmetrically generating a distal tip cell (DTC) lineage daughter and a proximal anchor cell potential lineage. (C) A newly hatched worm contains several seam cells on both sides of the worm. Most seam cells (H1–H2, V1–V6) divide in a stem cell-like manner giving rise to a seam cell daughter and a terminally differentiates hypodermal daughter at each larval molt. In each case (A–C), WβA signaling activates a transcriptional profile specific for the signaled daughter that is repressed in the unsignaled daughter. A symmetric cell division at L2 increases overall seam cell number to 16. Modified from Phillips and Kimble 2007.
2.1 Embryonic blastomere manipulations demonstrate an instructive signal from the germline polarizes EMS
In normal embryo development, polarized EMS divides asymmetrically to produce a posterior E cell and an anterior MS cell, where the decedents of the E cell form endoderm while the decedents of MS cell form mesoderm (Figure 2A) (Rose and Gonczy, 2014, Sulston and Horvitz, 1977). In a technically challenging and revealing series of experiments, Goldstein (1992) studied the interaction between each embryonic cell and its closest neighbor by removing the embryo’s eggshell and physically isolating the blastomeres from the 4-cell stage embryo. These studies found that the decedents of the isolated EMS mother cell do not result in two separate populations of endoderm and mesoderm populations of cells, but rather EMS divides symmetrically into duplicated mesodermal lineages, evident from the absence of gut. Since the decedents of isolated EMS cells fail to differentiate endoderm, it was hypothesized that other cells within the embryo are required for proper ACD of EMS, specifically for specification of the E daughter and/or repression of the MS daughter. To analyze this idea, separated EMS cells were placed next to all remaining embryonic blastomeres, ABa, ABp, or P2 and the decedents of EMS were screened for positive gut differentiation. Only contact with P2 cells induced proper endoderm lineage specification, where the decedents of EMS developed into two populations of cells, one that differentiates into gut and one that fails to do so (Goldstein, 1992). Further experiments from Goldstein found that the orientation of EMS division is highly dependent on contact between P2 and EMS since moving P2 (usually in contact with the posterior end of EMS) to other locations next to EMS results in the EMS daughter that is in contact with P2 to assume the endoderm lineage (i.e.: P2 is moved to the anterior end of EMS will cause the anterior daughter and not the posterior daughter to assume the endoderm fate) (Goldstein, 1993). These studies revealed the likelihood that P2 contact or secretion of a diffusible P2 factor polarized EMS and activated endoderm developmental specification program.
2.2 A Wnt pathway polarizes EMS division
Following the discovery that P2 contact is required for EMS ACD, forward genetic screens in the Priess, Mello, Schnabel, and Bowerman labs identified mutants that are defective for EMS ACD including those that lack endoderm and have the more mesoderm (mom) phenotype. Several of such mutants (including mom-1 through mom-5, pop-1 and lit-1) were mapped and identified as components of a Wnt/β-catenin-related pathway, now known as the WβA pathway (Thorpe et al., 1997, Rocheleau et al., 1997, Mizumoto and Sawa, 2007b, Kaletta et al., 1997, Lin et al., 1995). For instance, MOM-2 is a secreted Wnt ligand that initiates and activates Wnt signaling (Rocheleau et al., 1997, Thorpe et al., 1997). In mom-2 mutants, terminally differentiated embryos lack gut and display an increase in pharyngeal mesodermal cells. Early activity of the mom-2 gene product was shown by laser ablation experiments. In eight cell wild-type embryos, ablating all blastomeres except the E cell results in the remaining E cell descendants producing gut tissues. However, similar experiments in mom-2 mutants found that the remaining E cell descendants produce only pharyngeal muscle and lack gut, an indication that both EMS daughter cells adopt a MS-like fate in the absence of MOM-2/Wnt (Thorpe et al., 1997). Thus, MOM-2 ligand was hypothesized to be the polarizing ligand secreted by P2 to induce asymmetric cell division of the EMS mother cell. To determine if P2-derived MOM-2 non-autonomously regulates EMS, different combinations of isolated wild-type or mom-2 EMS and P2 blastomeres were placed in close contact and the cell fate of the descendent EMS cells were observed (Thorpe et al., 1997). Endoderm failed to develop when an isolated wild type EMS cell was placed next to an isolated mom-2 mutant P2 cell. In contrast, the presence of gut tissue was detected when mom-2 mutant EMS cells contacted wild type P2 cells. These experiments showed that a Wnt ligand, MOM-2, is non-autonomously required for P2 polarization of EMS during mitosis and further facilitates downstream WβA signaling in E and MS daughters (Thorpe et al., 1997).
2.3 WRM-1/β-catenin and LIT-1/Nemokinase control POP-1 nuclear export
The above experiments identified a signal required for EMS polarity, but how does the MOM-2/Wnt ligand polarize the mother cell to control asymmetric fate specification in EMS daughters? MOM-2 polarizing activity results in molecular EMS asymmetry at the time of division producing one signaled daughter cell capable of activating Wnt target genes and one unsignaled daughter cell where Wnt targets are repressed (Thorpe et al., 1997). Initial observations of nuclear POP-1 asymmetry in the two daughters led to the finding that differential WβA pathway activity results in asymmetric regulation of POP-1 nucleo-cytoplasmic distribution in E versus MS resulting in nuclear asymmetry (Maduro et al., 2002, Lin et al., 1998, Lo et al., 2004). Though regulation of TCF levels are apparently not a typical effect of canonical Wnt signal transduction, POP-1 regulation is essential in the worm WβA pathway. POP-1, like other TCF homologs, serves as either transcriptional repressor or activator depending on the availability of corepressors and coactivators (Figure 1) (Sawa and Korswagen, 2013). After EMS ACD, POP-1 is increased in the nucleus of the unsignaled MS daughter compared to the signaled E daughter (Lin et al., 1998). The decrease of POP-1 in E nuclei is due to its interaction with WRM-1/β-catenin, which facilitates POP-1 phosphorylation by LIT-1/NEMO kinase and its subsequent nuclear export (Rocheleau et al., 1999, Meneghini et al., 1999, Ishitani et al., 1999, Shin et al., 1999, Lo et al., 2004, Yang et al., 2011). Counter-intuitively, POP-1 export is required for activation of Wnt target genes, presumably by lowering excess POP-1 that would otherwise repress gene expression. Indeed, the pop-1 mutant EMS phenotype is consistent with the idea that WβA represses POP-1: pop-1 mutants show excess endoderm due to duplication of E fate and loss of MS whereas the Mom phenotype is loss of E and duplication of MS.
2.4 Differential POP-1/TCF regulation by two β-catenins
Asymmetric POP-1 nuclear localization in EMS daughters is dependent on the activity of LIT-1/Nemo kinase where LIT-1 phosphorylates POP-1 to facilitate POP-1 export out of the E nucleus (Lo et al., 2004, Yang et al., 2011). In the embryos of lit-1 mutants, POP-1 display high symmetrical localization in both E and MS nuclei owning to the lack of POP-1 export that is typically seen in the wild type E daughter (Rocheleau et al., 1999, Meneghini et al., 1999, Ishitani et al., 1999, Kaletta et al., 1997). lit-1 mutants, like wrm-1 mutants, also exhibit a lack of endoderm, a phenotype indicative of conversion of the E cell fate to a MS-like cell fate. Furthermore, immunoprecipitation experiments show that LIT-1 binds to WRM-1 for effective POP-1 phosphorylation and this binding is likely in the C-terminal domain of POP-1 rather than the typical “beta-catenin binding domain” located in the N-terminus of TCF proteins (Brunner et al., 1997, Molenaar et al., 1996, Yang et al., 2011). The interaction between WRM-1 and LIT-1 promotes LIT-1 phosphorylation at T220, which is in turn essential for LIT-1 kinase activity required for POP-1 phosphorylation (Yang et al., 2015). The binding of POP-1 with the WRM-1/LIT-1 complex and the binding of POP-1 to its transcriptional coactivator SYS-1 (discussed further in the chapter) use distinct binding domains but are mutually exclusive (Yang et al., 2011). Though recent evidence demonstrates that POP-1 can interact with other DNA binding factors to activate transcription in a WβA-independent manner(Murgan and Bertrand, 2015, Murgan et al., 2015), the above data show that POP-1 contains distinct domains that bind to either its activator SYS-1 or the effector of its export, WRM-1, to control its transcriptional activity and subsequently the expression of WβA target genes.
3. A GENETIC SCREEN OF LARVAL ACDS IDENTIFIES SYS-1/β-CATENIN
Though POP-1 export was shown to be necessary for WβA signal transduction by the aforementioned studies, several lines of evidence suggested that POP-1 nuclear downregulation might be insufficient for target gene activation and that the pathway was missing a TCF coactivator. These include the realization that loss of POP-1 decreases target gene expression levels in E in addition to derepressing them in MS (Shetty et al., 2005). Additionally, transcriptional reporters of POP-1 targets require TCF binding sites for expression, whereas they should be dispensable for or decrease expression if POP-1 was an obligate repressor (Maduro et al., 2005). Finally, a conserved feature of TCF proteins is their ability to switch from repressor mode to transcriptional activation upon β-catenin binding, which displaces corepressors such as Groucho (Roose et al., 1998). These data suggest a transactivator such as β-catenin may be required to activate C. elegans POP-1. In addition to WRM-1, which is a weak transcriptional activator specialized for POP-1 nuclear export (Korswagen et al., 2000, Natarajan et al., 2001), the C. elegans genome contains two β-catenins recognized by sequence similarity to vertebrate and fly β-catenin, HMP-2 and BAR-1, neither of which is required for C. elegans ACD (Sawa and Korswagen, 2013, Phillips et al., 2007, Korswagen et al., 2000). However, SYS-1/β-catenin, first found in a genetic screen to identify factors required for ACDs in the somatic gonad (Miskowski et al., 2001, Siegfried and Kimble, 2002, Siegfried et al., 2004), proved to be the missing TCF activator. Despite being just ~10% identical to human β-catenin, the conclusion that SYS-1 is a β-catenin that acts as the POP-1 coactivator during ACD is supported by the observations that 1) SYS-1 is required for many worm ACDs similar to POP-1 and WRM-1, 2) as with most TCF-β-catenin interactions, SYS-1 physically interacts with POP-1 via the N-terminal β-catenin binding domain of POP-1 (Kidd et al., 2005), and 3) SYS-1 activates TCF target genes, such as ceh-22 and end-1 in worms, but also via the TCF reporter assay TOPLFLASH when coexpressed with POP-1 in human cells (Lam et al., 2006, Kidd et al., 2005, Huang et al., 2007). Finally, 4) SYS-1 expression rescues the defects of loss of a well-established worm β-catenin, BAR-1, which also acts as a POP-1 coactivator (Kidd et al., 2005, Natarajan et al., 2001)(Kidd et al 2005). The sequence divergence of C. elegans SYS-1 therefore concealed its identity as a critical activator of POP-1 transcription until the requirement of SYS-1 for ACD was revealed through the use of forward genetics.
3.1 SYS-1 controls asymmetric division of the somatic gonadal precursors
SYS-1, for symmetric sisters, was identified in a mutagenesis screen for defects in asymmetric divisions of the somatic gonad (Miskowski et al 2001). The two distal tip cells (DTC), which are somatic cells that serve as niches for the germline stem cells and lead gonadal arm elongation, are formed by ACDs tightly controlled by the WβA pathway (Kimble, 1981, Austin and Kimble, 1987, Byrd et al., 2014, Chesney et al., 2009, Siegfried et al., 2004, Kidd et al., 2015). The DTCs are large somatic cells that cap the end of both the gonadal arm of the worm and, coupled with cell-specific GFP markers, serve as an easily screened cell type suitable for high-throughput genetic analyses. The development of the DTCs begin during the L1 larval stage where 2 somatic gonad precursor cells (SGPs), Z1 and Z4, in the gonadal primordium divide asymmetrically to produce a total of 12 cells including the DTCs (Kimble and Hirsh, 1979, Kimble and Ward, 1988). The asymmetric division of the Z1/Z4 somatic gonadal precursor cells requires SYS-1, POP-1 and the WβA pathway; the distal daughter cells of Z1 and Z4 both assume the signaled cell fate and gives rise to the DTCs while the proximal daughters assume the unsignaled fate, including the anchor cells (Figure 2B) (Siegfried and Kimble, 2002, Siegfried et al., 2004, Chang et al., 2005, Miskowski et al., 2001, Lam et al., 2006, Phillips et al., 2007). Further experiments in the somatic gonad show that loss of SYS-1 function via mutation or RNAi causes loss of the signaled fate (DTC) and duplicates the unsignaled proximal fate; SYS-1 overexpression results in an increase in DTCs and loss of proximal fates compared to wild type worms (Kidd et al., 2005). Interestingly, asymmetric SYS-1 expression can be observed in the Z1/Z4 daughters (Phillips et al., 2007). YFP-tagged SYS-1 under the control of the endogenous sys-1 promoter is elevated in the distal daughter (Z1a, Z4p) compared to the proximal daughters (Z1p and Z4a). Thus, SYS-1 is necessary and sufficient for WβA-dependent target gene expression and is elevated in the WβA-dependent daughter cell that requires its activity.
3.2 The SYS-1 crystal structure displays hallmarks of β-catenins
SYS-1’s β-catenin identity was further confirmed by its crystal structure. β-catenins contain twelve tandemly repeated “armadillo” domains that consist of two or three alpha helices connected by flexible linkers (Liu et al., 2008). SYS-1 shares the hallmark twelve armadillo repeats of canonical β-catenin packing together to form a superhelix similar to human β-catenin (Liu et al., 2008, Huber et al., 1997, Xing et al., 2008, Poy et al., 2001). Further, the crystallized SYS-1/POP-1 complex shows similar interactions to the human β-catenin/TCF complex. SYS-1 contains a positively charged groove, spanning armadillo repeats 5–8, that interacts with POP-1, an interaction anchored by a conserved “charged button” lysine-aspartate salt bridge (Liu et al., 2008). This salt bridge is critical for the complex since mutation of either residue abrogates binding and gives a loss of the signaled fate during worm ACD (Siegfried and Kimble, 2002, Liu et al., 2008). Thus, despite significant sequence divergence, SYS-1 retains the functional and structural characteristics of canonical β-catenin. Conversely, the predicted WRM-1 structure shows that, while WRM-1 shares similarity to SYS-1 in the repeat 6–8 region and contains the charged button, in contrast to SYS-1 WRM-1 contains a nearby bulky side chain that prevents WRM-1/POP-1 interactions (Liu et al., 2008).
3.3 Reciprocal POP-1 and SYS-1 asymmetry are widespread in C. elegans
Further analysis of SYS-1 function and expression pattern indicate that SYS-1 activates WβA target gene activity in many of the worm ACDs throughout development, including the aforementioned EMS daughter cells, suggesting that SYS-1 may be the sole TCF coactivator involved in WβA-dependent ACD. SYS-1 asymmetry is observed throughout embryogenesis (Bertrand and Hobert, 2009a, Huang et al., 2007, Zacharias et al., 2015, Phillips et al., 2007), consistent with broad function promoting WβA-dependent cell fate. Further, SYS-1 depletion causes gutlessness and promotes the posterior fate of various embryonic ACDs (Bertrand and Hobert, 2009a, Bertrand and Hobert, 2010, Huang et al., 2007, Phillips et al., 2007). The SYS-1 localization pattern is WβA-dependent as depletion of various Wnt components such as MOM-1/Porcupine, MOM-2/WNT, MOM-5/Frizzled and Dvl homologs induces a loss of nuclear SYS-1 asymmetry in E and MS daughters (Huang et al., 2007). Thus, given the earlier observation that WβA decreases POP-1 levels, SYS-1 and POP-1 show a reciprocal expression in the nucleus of daughters of an asymmetrically dividing cell. For instance, in the EMS division, the signaled E daughter has elevated SYS-1, but decreased POP-1 compared to the unsignaled MS cell (Lin et al., 1995, Huang et al., 2007, Lo et al., 2004) (Figure 2A). Current models posit that WβA activation of target gene expression is controlled by the ratio of SYS-1 and POP-1. In the unsignaled cell nucleus, the SYS-1:POP-1 ratio is low and most of POP-1 is free of SYS-1 activation and therefore in a transcriptionally repressive state. In the signaled cell, the WβA pathway elevates the SYS-1:POP-1 ratio due to increased SYS-1 levels and decreased nuclear POP-1 due to POP-1 nuclear export (Sawa and Korswagen, 2013, Jackson and Eisenmann, 2012, Phillips and Kimble, 2009). Thus, Wnt regulates asymmetric target gene expression via differential regulation of the two branches of the pathway.
4. HYPODERMAL STEM CELL DIVISIONS ELUCIDATE POP-1/TCF AND SYS-1/β-CATENIN REGULATION
While the ratio model explains several features of the WβA pathway and has withstood many tests, the identity of the cytoplasmic fate determinants actually partitioned by mother cell division have been informed through the study of the C. elegans hypodermal tissue known as the seam cells. In addition to EMS and the SGPs, the seam is another tissue that is widely used in the study of the WβA-regulated asymmetric division (Sawa and Korswagen, 2013, Eisenmann, 2005, Mizumoto and Sawa, 2007b, Herman et al., 1995, Eisenmann, 2011, Wildwater et al., 2011, Harandi and Ambros, 2015). A newly hatched worm possesses 10 seams cells (H0, H1–H2, V1–V6, and T) embedded within the hyp7 syncytium on both the left and right sides of the worm (Figure 2C) (Sulston and Horvitz, 1977). With each larval molt, most seam cells divide asymmetrically along the anterior-posterior (A-P) axis to produce a posterior seam cell daughter and an anterior hypodermal cell daughter. In a stem cell-like manner, the anterior hypodermal cell daughter terminally differentiates, and fuses with the hyp7 syncytium while the posterior daughter retains the seam cell fate and the ability to divide at each subsequent larval molt. At the end of the L4 larval molt, the adult worm will have 16 seam cells on each side of the worm due to a symmetric cell division at L2. Seam cell fate acquisition is dependent on active WβA signaling where the signaled posterior seam cell daughter exhibits the characteristic high nuclear localization of SYS-1 and lowered POP-1 and the unsignaled hypodermal cell with a lower nuclear SYS-1 localization but higher POP-1 (Banerjee et al., 2010, Gleason and Eisenmann, 2010, Mizumoto and Sawa, 2007a). The signaled posterior daughter maintains its seam cell fate by upregulating EGL-18 and ELT-6 GATA factors, which are repressed in the unsignaled anterior daughter (Gorrepati et al., 2013, Gorrepati and Eisenmann, 2015, Gorrepati et al., 2015).
4.1 Seam cells show polarized WβA pathway components at mitosis
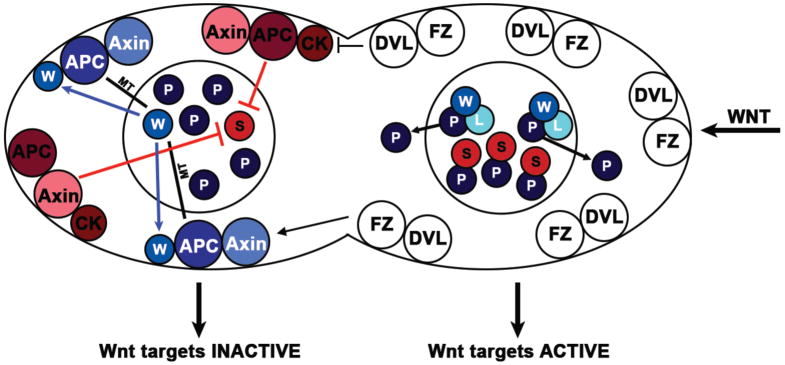
Forward and reverse genetic screens have identified additional WβA components and functional analysis of these players has greatly clarified regulation of the two pathway branches. Loss of negative WβA regulators often render a seam cell division symmetric, inducing a fate change duplicating the seam cell fate at the expense of the hypodermal cell fate. Depletion of negative regulators, KIN-19 (the homolog of CKIα), the APC homolog APR-1 or the Axin homolog PRY-1 results in a significant increase in seam cells (Banerjee et al., 2010, Gleason and Eisenmann, 2010). In the course of WβA-mediated asymmetric seam cell division, multiple cytoplasmic cell fate determinants of the pathway can be observed to asymmetrically localize on the anterior or posterior cortex of the seam mother cells to give rise to two molecularly distinct daughter cells (Mizumoto and Sawa, 2007b). Each daughter cell carries different nuclear, cytoplasmic and cortical localization profiles of the determinants of WβA pathway, as observed by the addition of fluorescent tags (Mizumoto and Sawa, 2007a). The asymmetric localization of negative regulators of the pathway such as APR-1 and PRY-1 can be observed at the anterior cortex while positive regulators such as Frizzled (MOM-5) and Dishevelled proteins (DSH-2 and MIG-5) are distributed to the posterior cortex of the mother seam cell as well as the early embryo (Park et al., 2004, Baldwin et al., 2016, Mizumoto and Sawa, 2007a, Park and Priess, 2003). The asymmetric cortical localization of negative determinants to the anterior cortex is regulated by Wnt ligands as demonstrated by observing APR-1/APC and PRY-1/Axin localization in mutants lacking Wnt ligand EGL-20 (Mizumoto and Sawa, 2007a). In egl-20 mutants, both APR-1 and PRY-1 no longer asymmetrically localized to the anterior cortex but are radialized on the cortex of the entire cell (Figure 3) (Mizumoto and Sawa, 2007a). The finding that a Wnt ligand controls the polarized localization of cell fate-regulating WβA pathway members at mitosis, and likely controls their asymmetric inheritance by daughter cells, suggests these are the critical cytoplasmic cell fate determinants that make the two daughter cells different at birth.
Figure 3.
Model of WβA-controlled asymmetric division. Two separate branches of the pathway, the WRM-1-regulating (blue) and the SYS-1 regulating (red) are balanced by Dishevelled (Dvl) and together generate transcriptional asymmetry in daughter cells following division. The activity of posterior factors including Frizzled and Dishevelled restrict cortical localization or activity of negative factors APC and Axin. In addition, Dishevelled distinguishes between two pools of APC in the unsignaled cell to modulate each downstream branch of the pathway. One pool of APC stabilizes microtubules (MTs) to promote WRM-1/β-catenin (W) export, which results in high nuclear POP-1/TCF (P). The other pool of APC degrades SYS-1/β-catenin in concert with KIN-19/CKIα (CK). Target genes are repressed. In the signaled daughter cell (right), neither pool of APC exists so WRM-1 and SYS-1 are both high. POP-1 is low because of nuclear export, mediated by phosphorylation by LIT-1 (L) through association with WRM-1. Target genes are expressed. Modified version of Baldwin et al 2016.
4.2 Distinct roles for WRM-1/β-catenin at the cortex and nucleus
Interestingly, WRM-1, which shows the aforementioned asymmetric nuclear localization pattern in seam cell daughters, also displays asymmetric cortical localization before division (Takeshita and Sawa, 2005, Mizumoto and Sawa, 2007a). WRM-1 localizes to the anterior cortex of the mother cell in a similar manner as APR-1 and PRY-1 (Figure 3) (Mizumoto and Sawa, 2007a, Takeshita and Sawa, 2005). To test the significance of the WRM-1 cortical localization pattern, WRM-1 was uniformly tethered to the cortex of seam cells through the generation of a WRM-1 transgene containing a C-terminal CAAX cortical localization signal (WRM-1::CAAX) (Mizumoto and Sawa, 2007a). WRM-1::CAAX reduces the posterior nuclear levels of free WRM-1::GFP, resulting in a low symmetrical localization of WRM-1 in both daughter cells. These studies suggest the existence of two pools of WRM-1; one pool asymmetrically localizes to the anterior cortex of the mother cell and, following cell division, prevents the nuclear localization of the second pool of free WRM-1 in the hypodermal cell daughter. The absence of cortical WRM-1 in the posterior seam cell daughter allows nuclear localization of the free WRM-1 (Figure 3) (Mizumoto and Sawa, 2007a).
4.3 WRM-1 asymmetry is regulated by asymmetric nuclear export and differential microtubule stability
The cortical localization of WRM-1 is controlled by APR-1/APC, providing both a link to upstream WβA signaling and the canonical Wnt pathway. APR-1 depletion decreases cortical WRM-1, increases nuclear WRM-1 and results in symmetric seam cell divisions that duplicate seam cell fate. Photobleaching experiments during mother cell telophase show that WRM-1 is rapidly removed from the anterior nucleus, but is stably present in the posterior nucleus. This nuclear export of WRM-1 is dependent on APR-1, which localizes to the anterior cortex (Takeshita and Sawa, 2005, Mizumoto and Sawa, 2007a). WRM-1 in turn is required for APR-1 cortical localization, suggesting the formation of a WRM-1/APR-1 complex at the anterior cortex that recruits additional WRM-1 and APR-1 in a feed-forward manner to amplify WRM-1 export from the unsignaled nucleus. A possible mechanism of APR-1-dependent WRM-1 export was provided by the examination of APR-1 regulation of embryonic microtubule dynamics (Sugioka et al., 2011, Sugioka and Sawa, 2010). Loss of APR-1 preferentially decreased anterior microtubules during EMS division, suggesting that cortical APR-1 promotes microtubule attachment at the anterior cortex and that APR-1 shares this microtubule-regulating role with its vertebrate homolog, APC (Mimori-Kiyosue et al., 2000, Nakamura et al., 2005, Zumbrunn et al., 2001, Clevers and Nusse, 2012, Brocardo et al., 2008, Green and Kaplan, 2003, Barth et al., 2008). The importance of asymmetric microtubule organization for nuclear POP-1 and WRM-1 asymmetry was demonstrated by irradiating the anterior centrosome to disrupt microtubule formation. After centrosome irradiation, the nuclear levels of WRM-1 and POP-1 in both daughters are symmetrical, consistent with the idea of microtubule-dependent WRM-1 nuclear export. Furthermore, depletion of several kinesins decreases the level of POP-1 in the anterior daughter, suggesting nuclear WRM-1 asymmetry is achieved by the active transport of WRM-1 to the cortex in the anterior daughter (Sugioka et al., 2011). Therefore, the emerging model of asymmetric WRM-1 regulation is that APR-1 is restricted to the anterior cortex by upstream WβA signaling, which stabilizes microtubules and promotes WRM-1 transport to the cortex preferentially from the anterior cytoplasm and nucleus. WRM-1 binding to cortical APR-1 stabilizes this localization pattern and increases asymmetric transport. The posterior daughter lacks cortical APR-1 and contains fewer microtubules, thus the posterior nucleus contains more stable WRM-1, facilitating POP-1 nuclear export (Figure 3, blue factors).
4.4 SYS-1 asymmetry is regulated by members of the canonical destruction complex
In canonical Wnt signaling, the destruction complex formed by Axin, APC, CKIα, and GSK3β targets β-catenin for degradation and we have recently begun to address whether the destruction complex regulates the transcriptionally active β-catenin in the WβA pathway, SYS-1. Since APR-1/APC, PRY-1/Axin and KIN-19/CKIα all negatively regulate seam cell fate, we tested the hypothesis that they are required for normal SYS-1 asymmetry. Similar to the embryonic and gonadal divisions described above, SYS-1 is enriched in the signaled (posterior) daughter cell and lost in the unsignaled (anterior) daughter cell (Baldwin and Phillips, 2014, Mizumoto and Sawa, 2007b) (Figure 2C). After depletion of APR-1 or KIN-19, nuclear SYS-1 levels become symmetrically high in both seam cell daughters, suggesting that negative regulation of β-catenin by the destruction complex is conserved in the WβA pathway (Baldwin and Phillips, 2014, Mila et al., 2015). APR-1-dependent negative regulation of SYS-1 is also seen in MS, the unsignaled daughter of EMS, suggesting that destruction complex regulation of SYS-1 may be widespread (Huang et al., 2007). Interestingly, the pry-1/Axin mutant phenotype in the seam cells is distinct from that of apr-1 and kin-19; nuclear SYS-1 and APR-1 asymmetry is still seen in most pry-1 mutant daughter cell pairs, but their polarity is randomized. Since SYS-1 can be lowered in daughter cells in the absence of PRY-1, it appears that Axin is not required for destruction complex activity. Instead, PRY-1 localizes the destruction complex to the anterior cortex and dictates the correct daughter fate in the correct A-P position. Thus, following cell division, APR-1 and SYS-1 exhibit reciprocal asymmetry in their localization pattern. However, this reciprocal asymmetry can be uncoupled by loss of WRM-1, which decreases cortical APR-1 but has no effect on SYS-1 nuclear asymmetry, or loss of KIN-19, which increases APR-1 on the posterior cortex but SYS-1 remains high in the posterior nucleus. An explanation for these observations is that mother cells contain two pools of APR-1: one pool negatively regulates WRM-1 (blue APC in Figure 3) and the other negatively regulates SYS-1 (red APC in Figure 3). Loss of WRM-1, for instance, has no effect on SYS-1 asymmetry because only the WRM-1-regulating pool of APR-1 is affected in wrm-1 mutants. APR-1 entry into the SYS-1-regulating pool appears dependent on KIN-19 because, in the absence of KIN-19, APR-1 can regulate WRM-1 but not SYS-1. By this model, Wnt signaling through PRY-1/Axin not only restricts the localization of the destruction complex, it also controls the balance of the two pathway outputs by differentially regulating the two β-catenins that regulate TCF levels and transcriptional activity (Figure 3).
4.5 Dishevelled controls the balance of the WβA branches
Since WRM-1 and SYS-1 nuclear asymmetry can be used to analyze the relative function of both pools of APR-1, we next sought to test the function of upstream WβA pathway members in controlling the distribution of these pools during asymmetric seam cell division. Dishevelled (Dvl) in particular seemed a likely suspect due to its role as a gatekeeper for the various Wnt signal transduction pathways including Wnt/β-catenin, Wnt/PCP, and Wnt/Calcium (Sokol, 1999, Wallingford and Mitchell, 2011, King et al., 2009, Hingwing et al., 2009). Loss of two C. elegans Dvl paralogs, dsh-2 and mig-5, causes relatively mild changes to overall seam cell numbers but gaps and doublets appear in the seam cell A-P distribution suggested randomized cell fate that resulted in some symmetric divisions (Banerjee et al., 2010, Baldwin et al., 2016). Indeed, APR-1 is mislocalized to the both the anterior and posterior cortex of Dvl mutant seam cells, indicating that Dvl restricts the inheritance of negative regulators to the anterior daughter. To analyze the effects of Dvl mutation on APR-1 function, we examined the levels and localization of SYS-1 (to assay the SYS-1-regulating pool of APR-1) and WRM-1 (to assay the WRM-1-regulating pool of APR-1). Consistent with Dishevelled’s role as a positive regulator of Wnt/β-catenin signaling, Dvl double mutants show symmetrically decreased nuclear SYS-1 in seam cell daughter nuclei while Dvl overexpression shows increased SYS-1 (Baldwin et al., 2016). However, a positive role regulating SYS-1 levels would suggest that Dvl mutants would have a symmetric loss of seam cell fate, rather than the confused or randomized polarity that is observed. The explanation for this discrepancy proved to be a different Dvl role for WRM-1 regulation compared to SYS-1 regulation. In contrast to decreased SYS-1 levels, Dvl mutants show an approximately 2.5-fold increase in nuclear WRM-1, presumably because APR-1 in both daughters is unable to promote WRM-1 nuclear export. These data suggest that, in the absence of Dvl, nuclear TCF is low and unable to activate transcription because Dvl partitions APR-1 into the WRM-1- and SYS-1-regulating pools in addition to its role of restricting the destruction complex to the anterior cortex. Our current model summarized in Figure 3 is that Wnt restricts Frizzled and Dishevelled to the posterior cortex and APC and Axin to the anterior cortex at mitosis. After cytokinesis, the absence of negative regulation in the posterior daughter leads to the accumulation of WRM-1 and SYS-1, export of excess POP-1 and activation of WβA target genes. The anterior daughter exports WRM-1 from the nucleus via stabilized microtubules and degrades SYS-1 through the function of the destruction complex. Thus, the unsignaled daughter exhibits a low SYS-1:POP-1 ratio and repressed target genes compared to target gene expression in the signaled daughter.
4.6 How is PRY-1 asymmetric activity promoted in late seam cell divisions?
In the course of our examination of Dvl function in seam cell ACD, we sought to examine whether Dvl regulates the asymmetric PRY-1/Axin localization pattern seen in earlier L1 seam cell divisions (Mizumoto and Sawa, 2007a). To our surprise, we found that cortical PRY-1::GFP is symmetric throughout the cell cycle and both daughters are born with high levels of cortical PRY-1 (Baldwin et al., 2016). The finding that PRY-1/Axin is symmetrically localized during L4 seam cell divisions begs the question of how PRY-1 drives asymmetric localization of APR-1/. We propose that PRY-1 is qualitatively altered by Wnt signaling in the L4 division to generate two PRY-1 populations, “active” and “inactive” regulators of APR-1/APC. Active PRY-1 resides at the anterior pole and anchors APR-1/APC thus, β-catenin negative regulation occurs in this locale. Inactive PRY-1 in the posterior lacks this ability and as such the posterior daughter lacks negative regulation of both β-catenins. This model is an extension of the pattern seen at L1, where PRY-1 is quantitatively different at either pole of the mother cell (Mizumoto and Sawa, 2007b). However, it is equally possible that “active” PRY-1 is localized at the posterior pole and functions to prevent APR-1 cortical localization. Identifying the qualitative change that regulates PRY-1 activity will address this. Several interesting possibilities are suggested in the vertebrate literature where ubiquitination, SUMOylation, methylation and polymerization have all been observed (Song et al., 2014). Perhaps most intriguingly, mammalian Axin is phosphorylated in the absence of Wnt signaling and phospho-Axin displays greater ability to participate in the destruction complex and bind β-catenin (Song et al., 2014, Jho et al., 1999, Yamamoto et al., 1999, Ikeda et al., 1998, Hart et al., 1998, Rubinfeld et al., 1996). Further, due to Axin’s post-translational modifications and intra-molecular interactions, two Axin-containing complexes have been proposed: the destruction complex that promotes β-catenin degradation and inhibits Wnt signaling and a second Axin population that catalyzes LRP5/6 phosphorylation by binding GSK3β to elevate Wnt signal transduction (Song et al., 2014, Bilic et al., 2007, Strovel et al., 2000, Leung et al., 2002, Yan et al., 2001). Wnt signaling dephosphorylates Axin and is thought to promote a conformational shape changes that promotes association with LRP5/6. Together, these mechanisms suggest that PRY-1 phosphorylation and/or interactions with other Wnt signaling proteins could be responsible for modulating its ability to complex with APR-1/APC.
5. WβA LINKAGE TO THE CELL CYCLE PROVIDES ROBUSTNESS FOR CELL FATE SPECIFICATION
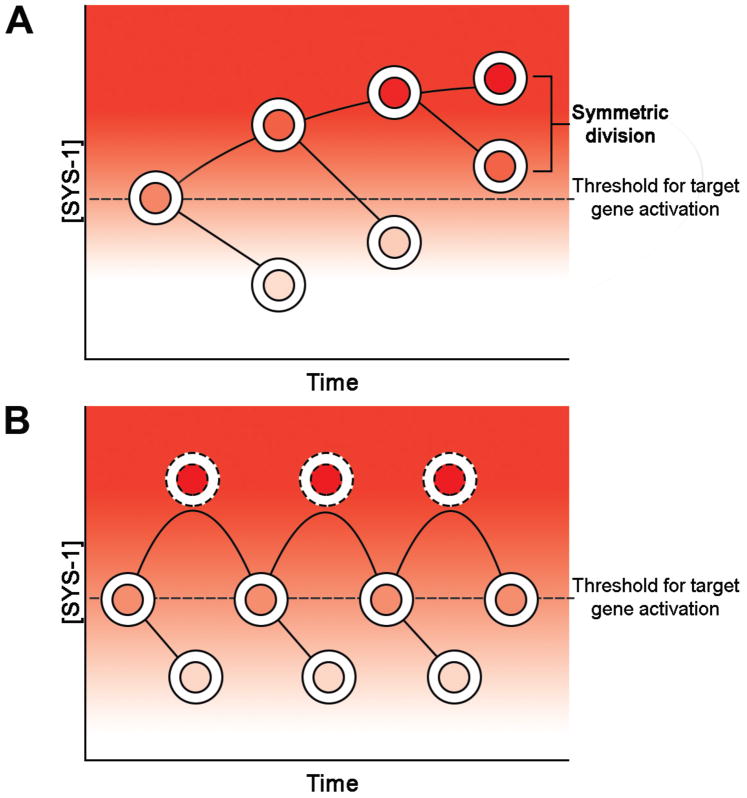
The model depicted in Figure 3 details that polarized localization of destruction complex members in the mother cell just prior to division is likely to be the determining factor that regulates β-catenin nuclear asymmetry for both WRM-1 and SYS-1. However, this model raises a problem that the asymmetrically dividing lineage must solve: mother cells active for Wnt signaling give rise to a signaled daughter that lacks negative SYS-1 regulation, but they also must produce a daughter that can quickly lower its levels of SYS-1. Since SYS-1/β-catenin, at least, is highly expressed at the transcriptional level (Phillips et al., 2007), successive signaled fates would be predicted to have ever-increasing levels of SYS-1 (Figure 4A). In this scenario, the unsignaled daughters inherit a higher and higher level of SYS-1 that would need to be degraded efficiently by the destruction complex, while simultaneously degrading de novo translated SYS-1, to achieve the sub-threshold SYS-1 level needed for target gene repression. Eventually, an unsignaled daughter would be unable to degrade its inherited SYS-1 in a timely manner and the cell would assume the signaled fate, resulting in a symmetric division (Figure 4A). That this problem is always overcome in the invariant C. elegans lineage, and that targets of Wnt signaling are very robust in their asymmetric expression pattern (Figure 4B) (Maduro et al., 2002), suggest that there could be redundant mechanisms limiting the level of SYS-1 in the unsignaled daughter.
Figure 4.
Hypothetical model on SYS-1 robustness. (A) SYS-1 level might be expected to inappropriately increases in the signaled daughter following divisions in the absence of negative regulation. When the level of SYS-1 in the unsignaled daughter of successively signaled daughters reaches the threshold (dashed line) for gene activation, a change of fate will be observed in the cell resulting in a symmetric cell division. (B) Alternatively, following cell division the signaled daughter containing high SYS-1 level (dashed circles) is reduced near its threshold to prepare for subsequent cell division. See text for details of this reduction mechanism.
5.1 Mother cell SYS-1 is cleared by localization to mitotic centrosomes
Though these mechanisms could theoretically include unstable sys-1 transcripts, or an inherently short SYS-1 protein half-life, we identified a mechanism where SYS-1 protein stability is linked to the cell cycle by localization to the peri-centriolar material (PCM) during cell division (Vora and Phillips, 2015). SYS-1 symmetrically localizes to centrosomes specifically during mitosis and is quickly lost after cytokinesis. Fluorescence recovery after photobleach (FRAP) studies show that centrosomal SYS-1 is rapidly turned over and replaced during mitosis. The SYS-1 centrosomal pattern is accomplished by forming a complex with the centrosomal protein RSA-2 that also accumulates with the PCM; in the absence of RSA-2, centrosomes form but do not contain SYS-1. The RSA-2 loss-of-function background can therefore be used to determine the functional significance of SYS-1 centrosomal localization. Mother cells depleted of RSA-2 (and therefore defective in SYS-1 centrosomal localization) divide to produce daughter cells with higher nuclear SYS-1 levels compared to wild type. Interestingly, this elevation in SYS-1 levels is symmetrical, since both daughter cells show ~50% increase in nuclear SYS-1, suggesting that a symmetric negative regulatory process that requires centrosomal localization is normally responsible for decreasing SYS-1 in both daughters. Both the rapid turnover of centrosomal SYS-1 in wild-type cells and the reliance of centrosomal localization for negative regulation implicate a proteolysis mechanism, such as proteasomal degradation, that is linked to the centrosome. Additionally, proteasome components and proteasomal clients localize to centrosomes in C. elegans and other systems (Vora and Phillips, 2015, Vora and Phillips, 2016, Fabunmi et al., 2000, Wigley et al., 1999). To test the idea that the proteasome regulates centrosomal SYS-1, we inhibited proteasomal degradation in C. elegans embryos and found increased SYS-1 centrosomal levels, but decreased SYS-1 turnover in FRAP analyses. These data are consistent with a model where SYS-1 symmetrically localizes to centrosomes during cell division, which leads to SYS-1 clearance from the mother cell before the daughter cells are born. This lowering of inherited SYS-1 allows the unsignaled daughter to properly regulate, via the destruction complex, de novo translated SYS-1 and repress target gene expression (Figures 3, 4B). Wnt signaling inactivates the destruction complex in the signaled daughter cell, leading to elevated de novo SYS-1 levels and activation of target gene expression. Hence, redundant negative regulatory mechanisms for SYS-1/β-catenin during and after division provide robustness for the asymmetric cell fate decision.
6. REITERATIVE SIGNALING PRODUCES MULTIPLE CELL FATES
The WβA pathway is used repeatedly to activate different targets and specify a new cell type in each iteration, yet the pathway has just one apparent outcome: regulation of Wnt target gene expression. How then does signaling specify differential cell fate in so many distinct cell types? One answer to this question is that Wnt signaling regulation of transcription via SYS-1 and POP-1 is layered with WβA-independent lineage-specific transcription factors to give cell-type specific gene expression. These targets include master regulatory genes, such as ceh-22/Nkx 2.1/tinman in the somatic gonad and GATA transcription factor end-1 in the intestine (Lam et al., 2006, Chesney et al., 2009, Maduro et al., 2002, Shetty et al., 2005), that lock in the fate of the cell or lineage expression. A particularly well-established example is the neural lineage that results in specification of a neuron named AIY (Bertrand and Hobert, 2009a, Bertrand and Hobert, 2009b). Terminal AIY fate is driven by the homeodomain transcription factor TTX-3, which directly regulates dozens of target genes to uniquely define AIY fate (Wenick and Hobert, 2004). TTX-3 is initially symmetrically expressed in AIY and its sister cell after mother cell division, but the WβA target gene CEH-10, which is only expressed in AIY, is required for TTX-3 maintenance. Once coexpressed in AIY, CEH-10 and TTX-3 maintain their own expression as well as the AIY-specific pattern of gene expression that drives terminal differentiation. Thus, it can be imagined WβA transcription factors broadly combine with lineage-specific transcriptional regulators to produce a tapestry of cell-specific gene expression.
6.1 Time-lapse imaging identifies a graded response to Wnt signaling
A second mechanism explaining WβA-driven cell type diversity was identified through lineage tracing of cells expressing fluorescently-tagged SYS-1 and POP-1 and transcriptional fusions of their target genes (Zacharias et al., 2015, Zacharias and Murray, 2016). These time-lapse experiments quantifying gene expression demonstrate that, while sister cells always show asymmetry, their absolute expression level is influenced by the prior expression level in the lineage. For instance, cells with higher levels of SYS-1 give rise to daughters and granddaughters that all have higher SYS-1 (termed “cousin enrichment”), though asymmetry between these daughters is maintained. Similar patterns are seen with POP-1 asymmetry indicating variable dosage of the WβA terminal transcription factors in the daughter cells reflect the level present in the mother cell before division. This observation suggests a continuum of Wnt pathway output (for instance, varying from one through ten in terms of WβA pathway activity), rather than a simple binary fate decision (“on-off”) that could drive differential gene expression and result in increased cell type diversity.
The graded WβA response seen by Zacharias et al raises the possibility that this differential effect is due to a Wnt gradient that exists over the A-P length of the embryo. By this model, similar to classic morphogens, cell response would be directly correlated to the distance from ligand source with distinct thresholds that compartmentalize target cell response. In support of this model, the Schnabel lab, in an elegant series of blastomere transplant experiments, showed that the P2 cell and its descendants form a Wnt-secreting polarizing center to direct the cleavage plane of many (and perhaps all) cells of the developing embryo (Bischoff and Schnabel, 2006). They demonstrated that the polarizing activity of the P2 lineage persists over time since cells respond to a shift in the position of the Wnt source even after several divisions. Further, promoter activity of three Wnt ligand loci shows a cumulative posterior enrichment of Wnt expression (Zacharias et al., 2015). Thus, a posterior Wnt signal orients cells at a distance during embryogenesis, suggesting a Wnt gradient across the embryo may be present and could explain the graded response seen by Zacharias et al. However, there is reason to doubt such an attractive model. The Priess lab showed asymmetric POP-1 levels in daughter cells from isolated blastomeres never in contact with the P2 lineage, suggesting these cells have a Wnt-independent ability to activate WβA (Park and Priess, 2003, Park et al., 2004). Additionally, the Murray lab reports many cells with high nuclear SYS-1 in the anterior region of the embryo, despite elevated Wnt expression in the posterior. Indeed, the graded response of cousin enrichment is not a smooth linear distribution from posterior to anterior, but a patchwork pattern seen within lineages (Zacharias et al., 2015). Thus, because even nearby Wnt expression fails to increase WβA activity compared to cells located at a distance, it seems unlikely that a gradient of Wnt ligand is the underlying reason for differential output. Instead, the requirement of Frizzled for cousin enrichment suggests inheritance of signal transduction machinery, perhaps in a post-translationally modified form, underlies the ability of certain lineages to respond differentially to the presence or absence of Wnt polarity, rather than a Wnt gradient (Zacharias and Murray, 2016).
7. DEFINING THE “DEFAULT” STATE
A loss-of-function analysis of the terminal signaling effector in any cell communication pathway is often a useful proxy for the overall function of that pathway, yet POP-1/TCF mutants can show contradictory effects depending on the cell type under analysis. Loss of POP-1/TCF activity gives duplication of either the signaled or unsignaled fates effects depending on the tissue, suggesting the default state of target gene expression varies in a tissue-specific manner. For instance, loss of POP-1 function in the early embryo duplicates the signaled fate since POP-1 targets become deprepressed in MS (Lin et al., 1995, Calvo et al., 2001). Though these targets are expressed at quantitatively lower levels in both E and MS, expression is over the threshold necessary for E fate acquisition (Shetty et al., 2005). In contrast, loss of POP-1 from the somatic gonad duplicates the unsignaled anchor cell fate because the target gene ceh-22 is expressed at sub-threshold levels for fate acquisition (Siegfried and Kimble, 2002, Lam et al., 2006). The molecular mechanism responsible for this is likely a target gene-specific differential requirement for POP-1-mediated repression in the unsignaled cell versus a POP-1-mediated activation in the signaled cell. This differential requirement for activation or repression depends on the level of target gene expression due to the effect of basal transcriptional machinery. In cells where the basal transcriptional machinery causes relatively high level of expression, POP-1 derepression via Wnt-induced nuclear export is largely sufficient for gene expression. Conversely, in cells where basal target gene expression level is low, POP-1 derepression must be coupled with POP-1 activation via SYS-1 (Kidd et al., 2005, Shetty et al., 2005). In addition to basal transcription factors, POP-1 target genes are influenced by a second “Helper” DNA sequence that binds to a distinct POP-1 DNA binding domain termed the C-clamp to specifically facilitate transcriptional activation (Bhambhani et al., 2014, Ravindranath and Cadigan, 2014). The C-clamp and Helper DNA sequences are dispensable for target gene repression, however. It seems likely that the default transcriptional state of POP-1 targets appears to be influenced by atypical enhancer TCF elements including Helper sites and by the chromatin state that differentially recruits basal transcription factors. It should also be mentioned that, since pop-1 is an essential gene, tissue-specific analyses are often performed with different loss of function approaches. These include pop-1 RNAi applied at different developmental times or different methods, and pop-1 alleles with mutant β-catenin binding domains, DNA binding domains or mutant 3′ UTR (Huang et al., 2007, Lin et al., 1995, Siegfried and Kimble, 2002, Kidd et al., 2005), none of which may represent a pop-1 null background. Since POP-1 is a transactivator at intermediate nuclear concentration and a repressor at high levels and these two activities likely result from a distinct use of POP-1 domains, this necessitates a cautious comparison of POP-1 function across tissues. The use of a null pop-1 allele could remove these confounding variables when examining different tissues. By thus removing the activity of the WβA pathway, the role of the underlying basal transcriptional machinery on target gene expression level can be observed.
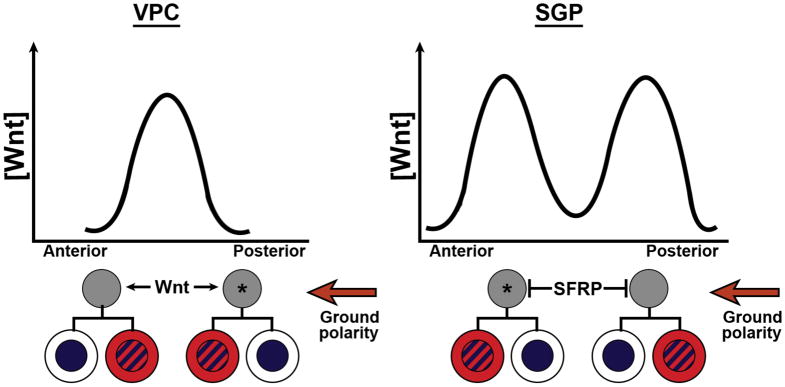
8. THE GLOBAL WNT GRADIENT CAN BE MODIFIED TO PRODUCE MIRROR ASYMMETRY WITHIN A TISSUE
Consistent with the observation that there exists a gradient of Wnt ligand expression at its peak in posterior tissues and at its lowest in anterior (Harterink and Korswagen, 2012, Harterink et al., 2011), most cells in the worm lineage are polarized such that the Wnt-dependent fate is the posterior daughter cell. However, exceptions to this rule include the somatic gonadal precursors (SGPs) and the vuval precursor cells (VPCs) (Figure 2 and 5). Though the divisions are still oriented in the A-P dimension, posterior daughters are not equivalent throughout the tissue. For instance, the signaled fate is not exhibited by both posterior cells, as is typical in the WβA pathway. Instead, ACDs in both tissues occur along an internal proximal-distal axis rather than the more common anterior-posterior axis, meaning that distal daughters have equivalent fates within the tissue independent of their anterior or posterior fates. For the SGPs, WβA pathway activity is required in the distal daughters but not the proximal daughters (Siegfried and Kimble, 2002, Siegfried et al., 2004, Yamamoto et al., 2011, Chang et al., 2005, Jiang and Sternberg, 1999, Lam et al., 2006, Hajduskova et al., 2009, Phillips et al., 2007). This pattern is reversed in the vulva, where WβA signaling is active in the proximal daughters (Green et al., 2008, Schmidt et al., 2005, Phillips et al., 2007). While it could be conceived that this pattern is consistent with a single, central Wnt signal or, in the case of the SGPs a central Wnt antagonist such as secreted-Frizzled related proteins (Harterink et al., 2011), instead evidence from VPC analyses indicate that these tissues as a whole also respond to the overall posterior inducing polarity signal similar to other tissues (termed “ground polarity”). However, posterior vulval cells reorient anteriorly via local Wnt cues based on specific Wnt ligand/receptor combinations (termed “refined polarity”, (Harterink et al., 2011, Green et al., 2008)). Since this Wnt source is posterior to the anterior VPC, it reinforces this cell’s polarity, but the local Wnt signal is sufficient to override the long-range posterior Wnt/EGL-20 signaling effect on the posterior VPC. This is demonstrated by examinations of mutants lacking the refined polarity pathway where the posterior divisions reverse their orientation, resulting in a split vulva phenotype due to posterior orientation of both anterior and posterior vulval ACDs (Figure 5 left)(Green et al., 2008, Deshpande et al., 2005, Inoue et al., 2004). In the absence of both the ground and refined pathways, polarity is random, similar to loss of PRY-1/Axin from the seam cells (Green et al., 2008, Baldwin and Phillips, 2014). Interestingly, the specific effects of the ground and refined polarity pathways are mediated by different Wnt ligands and receptors, including non-Frizzled receptors such as CAM-1/Ror, a receptor tyrosine kinase and LIN-18/Ryk and require Wnt/PCP components in addition to Wnt/β-catenin pathways. This suggests these two polarizing pathways enable differential effects by acting through distinct molecular outputs in the receiving cells that include transcription-independent processes. A similar reorientation could also take place to provide SGP mirror asymmetry, though the geometry would suggest a central depletion of Wnt ligand (Figure 5 right)
Figure 5.
Regulating mirror symmetry in vulval precursor cells (VPCs left) and a hypothetical model regulating the somatic gonadal precursors (SGPs right). A centrally localized Wnt cue reorients the posterior VPC while a hypothesized Wnt antagonist locally depletes the central somatic gonad of Wnt to provide a central proximal-distal axis that promotes mirror asymmetry across a tissue. Asterisks denote ACDs with refined polarity compared to the global ground polarity orienting most ACDs toward the posterior. SFRP, secreted Frizzled-related protein. Color scheme as in Figure 1.
9. INSTRUCTIVE AND PERMISSIVE SIGNALING BY WNT LIGANDS
Though Wnts act instructively to orient polarity in C. elegans EMS and VPC divisions as well as human ES cells (Goldstein et al., 2006, Herman, 2002, Habib et al., 2013, Goldstein, 1993), the situation appears more complex in the seam cells, where a permissive role has been suggested (Yamamoto et al., 2011). In the absence of multiple redundant Wnt signals, early larval seam cell divisions remain polarized but are randomly oriented, resulting in maintenance of the wild-type number of seam cells. Conversely, loss of Wnt receptors Frizzled and Ror lead to symmetric divisions, suggesting the receptors act in a Wnt-independent fashion to generate polarity while the Wnt ligand orients that polarity. Further, misexpression of a Wnt ligand in anterior tissues rescues wild-type polarity at similar rates as posterior misexpression (Yamamoto et al., 2011). Together, these data suggest the presence of a possible second, posterior-orienting, polarizing cue that may be intrinsic to the seam cells. One interesting option is that this cue may be mediated by cell contacts to nearby seam cells. The seam cells are in an epithelium such that each seam cell is in tight contact with its anterior and posterior seam cell neighbors (Altun and Hall, 2009). Seam cell ablations render neighboring seam cell divisions symmetric, suggesting cell contact may also play a polarity role (Austin and Kenyon, 1994). Interestingly, cell adhesion and APC also control the polarity of fly sperm stem cell divisions (Yamashita et al., 2003), suggesting such a cell-contact polarization mechanism may be conserved. Cell contacts are also important for proper Wnt/PCP-induced cell polarity in vertebrates and flies (Mayor and Theveneau, 2014, Bayly and Axelrod, 2011, Ehaideb et al., 2014), so the study of multiple Wnt signaling pathways to direct coordinated molecular outputs promises to be a fruitful area of future investigation.
10. CONCLUDING REMARKS: WβA CONSISTS OF UPSTREAM POLARIZERS AND DOWNSTREAM EFFECTORS
The emerging picture of C. elegans WβA-mediated ACD suggests distinct functional differences of the members of the WβA pathway with regards to their effect on polarity. Upstream components of the pathway serve to localize the downstream components that drive asymmetric gene expression, such as APC, WRM-1, LIT-1, SYS-1, and POP-1 (Sugioka et al., 2011, Shetty et al., 2005, Huang et al., 2007, Baldwin et al., 2016, Baldwin and Phillips, 2014). Loss of upstream members of the pathway (Wnt, Dvl, Pry-1/Axin) generally leads to randomly asymmetric localization of the downstream proteins (SYS-1, WRM-1, POP-1) and random orientation of daughter cell fate, but polarity is often maintained such that two distinct daughters are still generated. Conversely, loss of downstream components of the pathway, either negative or positive acting factors, lead to symmetric divisions presumably because the output of the asymmetric pathway is unable to be completed in either cell such that the two cells show similar transcriptional profiles. Upstream WβA members thus modulate and orient a cell to give a unified polarity orientation across a tissue or even across the whole organism, but the downstream members are required for the actual differential transcriptional output and are therefore essential for asymmetry. Though important aspects of this pathway remain unknown, such as the nature of the Wnt-independent ability to randomly polarize and the cell-type specific response to a global Wnt gradient, the 29 combination of genetics and in vivo cell observations make the study of C. elegans ACD a powerful system to determine how dividing cells sense a polarizing signal and respond to produce two daughter cells with different transcriptional profiles.
Acknowledgments
The authors would like to thank the Phillips lab for helpful comments on the manuscript. This work was supported by the American Cancer Society [grant number RSG-11-140-01-DC]; the Roy J. Carver Charitable Trust [grant number 13-4131]; and the National Science Foundation [grant number IOS-1456941] to B.T.P.
References
- ALTUN ZF, HALL D. Epithelial system, seam cells. WormAtlas 2009 [Google Scholar]
- AUSTIN J, KENYON C. Cell contact regulates neuroblast formation in the Caenorhabditis elegans lateral epidermis. Development. 1994;120:313–23. doi: 10.1242/dev.120.2.313. [DOI] [PubMed] [Google Scholar]
- AUSTIN J, KIMBLE J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- AXELROD JD, MATSUNO K, ARTAVANIS-TSAKONAS S, PERRIMON N. Interaction between wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- BALDWIN AT, CLEMONS AM, PHILLIPS BT. Unique and redundant beta-catenin regulatory roles of two Dishevelled paralogs during C. elegans asymmetric cell division. J Cell Sci. 2016 doi: 10.1242/jcs.175802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN AT, PHILLIPS BT. The tumor suppressor APC differentially regulates multiple beta-catenins through the function of axin and CKIalpha during C. elegans asymmetric stem cell divisions. J Cell Sci. 2014;127:2771–81. doi: 10.1242/jcs.146514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANERJEE D, CHEN X, LIN SY, SLACK FJ. kin-19/casein kinase Ialpha has dual functions in regulating asymmetric division and terminal differentiation in C. elegans epidermal stem cells. Cell Cycle. 2010;9:4748–65. doi: 10.4161/cc.9.23.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER N, HURLSTONE A, MUSISI H, MILES A, BIENZ M, CLEVERS H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20:4935–43. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTH AI, CARO-GONZALEZ HY, NELSON WJ. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin Cell Dev Biol. 2008;19:245–51. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLY R, AXELROD JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–91. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND V, HOBERT O. Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Developmental Cell. 2009a;16:563–75. doi: 10.1016/j.devcel.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND V, HOBERT O. Wnt asymmetry and the terminal division of neuronal progenitors. Cell Cycle. 2009b;8:1973–4. doi: 10.4161/cc.8.13.9024. [DOI] [PubMed] [Google Scholar]
- BERTRAND V, HOBERT O. Lineage programming: navigating through transient regulatory states via binary decisions. Curr Opin Genet Dev. 2010;20:362–8. doi: 10.1016/j.gde.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHAMBHANI C, RAVINDRANATH AJ, MENTINK RA, CHANG MV, BETIST MC, YANG YX, KOUSHIKA SP, KORSWAGEN HC, CADIGAN KM. Distinct DNA binding sites contribute to the TCF transcriptional switch in C. elegans and Drosophila. PLoS Genet. 2014;10:e1004133. doi: 10.1371/journal.pgen.1004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILIC J, HUANG YL, DAVIDSON G, ZIMMERMANN T, CRUCIAT CM, BIENZ M, NIEHRS C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–22. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- BISCHOFF M, SCHNABEL R. A posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLoS Biol. 2006;4:e396. doi: 10.1371/journal.pbio.0040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCARDO M, LEI Y, TIGHE A, TAYLOR SS, MOK MT, HENDERSON BR. Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: regulation of Bcl-2 and implications for cell survival. J Biol Chem. 2008;283:5950–9. doi: 10.1074/jbc.M708775200. [DOI] [PubMed] [Google Scholar]
- BRUNNER E, PETER O, SCHWEIZER L, BASLER K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- BYRD DT, KNOBEL K, AFFELDT K, CRITTENDEN SL, KIMBLE J. A DTC niche plexus surrounds the germline stem cell pool in Caenorhabditis elegans. PLoS One. 2014;9:e88372. doi: 10.1371/journal.pone.0088372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALVO D, VICTOR M, GAY F, SUI G, LUKE MPS, DUFOURCQ P, WEN G, MADURO M, ROTHMAN J, SHI Y. A POP-1 repressor complex restricts inappropriate cell type-specific gene transcription during Caenorhabditis elegans embryogenesis. The EMBO Journal. 2001;20:7197–7208. doi: 10.1093/emboj/20.24.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG W, LLOYD CE, ZARKOWER D. DSH-2 regulates asymmetric cell division in the early C. elegans somatic gonad. Mechanisms of Development. 2005;122:781–789. doi: 10.1016/j.mod.2005.03.005. [DOI] [PubMed] [Google Scholar]
- CHESNEY MA, LAM N, MORGAN DE, PHILLIPS BT, KIMBLE J. C. elegans HLH-2/E/Daughterless controls key regulatory cells during gonadogenesis. Developmental Biology. 2009;331:14–25. doi: 10.1016/j.ydbio.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEVERS H, NUSSE R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- DESHPANDE R, INOUE T, PRIESS JR, HILL RJ. lin-17/Frizzled and lin-18 regulate POP-1/TCF-1 localization and cell type specification during C. elegans vulval development. Dev Biol. 2005;278:118–29. doi: 10.1016/j.ydbio.2004.10.020. [DOI] [PubMed] [Google Scholar]
- EHAIDEB SN, IYENGAR A, UEDA A, IACOBUCCI GJ, CRANSTON C, BASSUK AG, GUBB D, AXELROD JD, GUNAWARDENA S, WU CF, MANAK JR. prickle modulates microtubule polarity and axonal transport to ameliorate seizures in flies. Proc Natl Acad Sci U S A. 2014;111:11187–92. doi: 10.1073/pnas.1403357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMANN D. Wnt signaling. [Accessed June 25, 2005];WormBook. 2005 doi: 10.1895/wormbook.1.7.1. [Online]. Available: http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- EISENMANN DM. C. elegans seam cells as stem cells: Wnt signaling and casein kinase Ialpha regulate asymmetric cell divisions in an epidermal progenitor cell type. Cell Cycle. 2011;10:20–1. [PubMed] [Google Scholar]
- EISENMANN DM, MALOOF JN, SIMSKE JS, KENYON C, KIM SK. The β-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development. 1998;125:3667–3680. doi: 10.1242/dev.125.18.3667. [DOI] [PubMed] [Google Scholar]
- FABUNMI RP, WIGLEY WC, THOMAS PJ, DEMARTINO GN. Activity and regulation of the centrosome-associated proteasome. J Biol Chem. 2000;275:409–13. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- GAO B. Wnt regulation of planar cell polarity (PCP) Curr Top Dev Biol. 2012;101:263–95. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- GAO C, CHEN YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–27. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- GLEASON JE, EISENMANN DM. Wnt signaling controls the stem cell-like asymmetric division of the epithelial seam cells during C. elegans larval development. Dev Biol. 2010;348:58–66. doi: 10.1016/j.ydbio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEASON JE, SZYLEYKO EA, EISENMANN DM. Multiple redundant Wnt signaling components function in two processes during C. elegans vulval development. Dev Biol. 2006;298:442–57. doi: 10.1016/j.ydbio.2006.06.050. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992;357:255–257. doi: 10.1038/357255a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN B. Establishment of gut fate in the E lineage of C. elegans: the roles of lineage-dependent mechanisms and cell interactions. Development. 1993;118:1267–1277. doi: 10.1242/dev.118.4.1267. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN B, HIRD SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN B, TAKESHITA H, MIZUMOTO K, SAWA H. Wnt signals can function as positional cues in establishing cell polarity. Dev Cell. 2006;10:391–6. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORREPATI L, EISENMANN DM. The C. elegans embryonic fate specification factor EGL-18 (GATA) is reutilized downstream of Wnt signaling to maintain a population of larval progenitor cells. Worm. 2015;4:e996419. doi: 10.1080/23723556.2014.996419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORREPATI L, KRAUSE MW, CHEN W, BRODIGAN TM, CORREA-MENDEZ M, EISENMANN DM. Identification of Wnt Pathway Target Genes Regulating the Division and Differentiation of Larval Seam Cells and Vulval Precursor Cells in Caenorhabditis elegans. G3 (Bethesda) 2015;5:1551–66. doi: 10.1534/g3.115.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORREPATI L, THOMPSON KW, EISENMANN DM. C. elegans GATA factors EGL-18 and ELT-6 function downstream of Wnt signaling to maintain the progenitor fate during larval asymmetric divisions of the seam cells. Development. 2013;140:2093–102. doi: 10.1242/dev.091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN JL, INOUE T, STERNBERG PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–56. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN RA, KAPLAN KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol. 2003;163:949–61. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABIB SJ, CHEN BC, TSAI FC, ANASTASSIADIS K, MEYER T, BETZIG E, NUSSE R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–8. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJDUSKOVA M, JINDRA M, HERMAN MA, ASAHINA M. The nuclear receptor NHR-25 cooperates with the Wnt/beta-catenin asymmetry pathway to control differentiation of the T seam cell in C. elegans. J Cell Sci. 2009;122:3051–60. doi: 10.1242/jcs.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARANDI OF, AMBROS VR. Control of stem cell self-renewal and differentiation by the heterochronic genes and the cellular asymmetry machinery in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2015;112:E287–96. doi: 10.1073/pnas.1422852112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDIN J, KING RS. The long and the short of Wnt signaling in C. elegans. Curr Opin Genet Dev. 2008;18:362–7. doi: 10.1016/j.gde.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART MJ, DE LOS SANTOS R, ALBERT IN, RUBINFELD B, POLAKIS P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- HARTERINK M, KIM DH, MIDDELKOOP TC, DOAN TD, VAN OUDENAARDEN A, KORSWAGEN HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development. 2011;138:2915–24. doi: 10.1242/dev.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTERINK M, KORSWAGEN HC. Dissecting the Wnt secretion pathway: key questions on the modification and intracellular trafficking of Wnt proteins. Acta Physiol (Oxf) 2012;204:8–16. doi: 10.1111/j.1748-1716.2011.02287.x. [DOI] [PubMed] [Google Scholar]
- HECHT A, VLEMINCKX K, STEMMLER MP, VAN ROY F, KEMLER R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN MA. Control of cell polarity by noncanonical Wnt signaling in C. elegans. Semin Cell Dev Biol. 2002;13:233–41. doi: 10.1016/s1084-9521(02)00051-4. [DOI] [PubMed] [Google Scholar]
- HERMAN MA, VASSILIEVA LL, HORVITZ HR, SHAW JE, HERMAN RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- HINGWING K, LEE S, NYKILCHUK L, WALSTON T, HARDIN J, HAWKINS N. CWN-1 functions with DSH-2 to regulate C. elegans asymmetric neuroblast division in a beta-catenin independent Wnt pathway. Dev Biol. 2009;328:245–56. doi: 10.1016/j.ydbio.2009.01.025. [DOI] [PubMed] [Google Scholar]
- HUANG S, SHETTY P, ROBERTSON SM, LIN R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator β-catenin SYS-1. Development. 2007;134:2685–95. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- HUBER AH, NELSON WJ, WEIS WI. Three-dimensional structure of the armadillo repeat region of β-catenin. Cell. 1997;90:871–82. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- IKEDA S, KISHIDA S, YAMAMOTO H, MURAI H, KOYAMA S, KIKUCHI A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE T, OZ HS, WILAND D, GHARIB S, DESHPANDE R, HILL RJ, KATZ WS, STERNBERG PW. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- ISHITANI T, NINOMIYA-TSUJI J, NAGAI SI, NISHITA M, MENEGHINI M, BARKER N, WATERMAN M, BOWERMAN B, CLEVERS H, SHIBUYA H, MATSUMOTO K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- JACKSON BM, EISENMANN DM. beta-catenin-dependent Wnt signaling in C. elegans: teaching an old dog a new trick. Cold Spring Harb Perspect Biol. 2012;4:a007948. doi: 10.1101/cshperspect.a007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JHO E, LOMVARDAS S, COSTANTINI F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- JIANG LI, STERNBERG PW. An HMG1-like protein facilitates Wnt signaling in Caenorhabditis elegans. Genes & Development. 1999;13:877–889. doi: 10.1101/gad.13.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALETTA T, SCHNABEL H, SCHNABEL R. Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature. 1997;390:294–298. doi: 10.1038/36869. [DOI] [PubMed] [Google Scholar]
- KIDD AR, 3RD, MUNIZ-MEDINA V, DER CJ, COX AD, REINER DJ. The C. elegans Chp/Wrch Ortholog CHW-1 Contributes to LIN-18/Ryk and LIN-17/Frizzled Signaling in Cell Polarity. PLoS One. 2015;10:e0133226. doi: 10.1371/journal.pone.0133226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD AR, III, MISKOWSKI JA, SIEGFRIED KR, SAWA H, KIMBLE J. A β-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–772. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- KIMBLE J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Developmental Biology. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- KIMBLE J, HIRSH D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- KIMBLE J, WARD S. Germ-line development and fertilization. In: WOOD WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- KING RS, MAIDEN SL, HAWKINS NC, KIDD AR, 3RD, KIMBLE J, HARDIN J, WALSTON TD. The N- or C-terminal domains of DSH-2 can activate the C. elegans Wnt/β-catenin asymmetry pathway. Developmental Biology. 2009;328:234–44. doi: 10.1016/j.ydbio.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN TJ, MLODZIK M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- KORSWAGEN HC, HERMAN MA, CLEVERS HC. Distinct β-catenins mediate adhesion and signalling functions in C. elegans. Nature. 2000;406:527–532. doi: 10.1038/35020099. [DOI] [PubMed] [Google Scholar]
- KRAMPS T, PETER O, BRUNNER E, NELLEN D, FROESCH B, CHATTERJEE S, MURONE M, ZÜLLIG S, BASLER K. WNT/Wingless signaling requires BCL9/Legless-mediated recruitment of Pygopus to the nuclear β-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- LAM N, CHESNEY MA, KIMBLE J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Current Biology. 2006;16:287–95. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG JY, KOLLIGS FT, WU R, ZHAI Y, KUICK R, HANASH S, CHO KR, FEARON ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–65. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- LIN R, HILL RJ, PRIESS JR. POP-1 and anterior-posterior fate decision in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- LIN R, THOMPSON S, PRIESS JR. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell. 1995;83:599–609. doi: 10.1016/0092-8674(95)90100-0. [DOI] [PubMed] [Google Scholar]
- LIN S, BAYE LM, WESTFALL TA, SLUSARSKI DC. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol. 2010;190:263–78. doi: 10.1083/jcb.200912128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, PHILLIPS BT, AMAYA MF, KIMBLE J, XU W. The C. elegans SYS-1 protein is a bona fide β-catenin. Dev Cell. 2008;14:751–61. doi: 10.1016/j.devcel.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LO MC, GAY F, ODOM R, SHI Y, LIN R. Phosphorylation by the β-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell. 2004;117:95–106. doi: 10.1016/s0092-8674(04)00203-x. [DOI] [PubMed] [Google Scholar]
- LOGAN CY, NUSSE R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- MACDONALD BT, HE X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADURO MF, KASMIR JJ, ZHU J, ROTHMAN JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev Biol. 2005;285:510–23. doi: 10.1016/j.ydbio.2005.06.022. [DOI] [PubMed] [Google Scholar]
- MADURO MF, LIN R, ROTHMAN JH. Dynamics of a developmental switch: recursive intracellular and intranuclear redistribution of Caenorhabditis elegans POP-1 parallels Wnt-inhibited transcriptional repression. Developmental Biology. 2002;248:128–142. doi: 10.1006/dbio.2002.0721. [DOI] [PubMed] [Google Scholar]
- MAYOR R, THEVENEAU E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J. 2014;457:19–26. doi: 10.1042/BJ20131182. [DOI] [PubMed] [Google Scholar]
- MENEGHINI MD, ISHITANI T, CARTER JC, HISAMOTO N, NINOMIYA-TSUJI J, THORPE CJ, HAMILL DR, MATSUMOTO K, BOWERMAN B. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature. 1999;399:793–797. doi: 10.1038/21666. [DOI] [PubMed] [Google Scholar]
- MILA D, CALDERON A, BALDWIN AT, MOORE KM, WATSON M, PHILLIPS BT, PUTZKE AP. Asymmetric Wnt Pathway Signaling Facilitates Stem Cell-Like Divisions via the Nonreceptor Tyrosine Kinase FRK-1 in Caenorhabditis elegans. Genetics. 2015;201:1047–60. doi: 10.1534/genetics.115.181412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMORI-KIYOSUE Y, SHIINA N, TSUKITA S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–8. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- MISKOWSKI J, LI Y, KIMBLE J. The sys-1 gene and sexual dimorphism during gonadogenesis in Caenorhabditis elegans. Developmental Biology. 2001;230:61–73. doi: 10.1006/dbio.2000.9998. [DOI] [PubMed] [Google Scholar]
- MIZUMOTO K, SAWA H. Cortical β-catenin and APC regulate asymmetric nuclear β-catenin localization during asymmetric cell division in C. elegans. Dev Cell. 2007a;12:287–99. doi: 10.1016/j.devcel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- MIZUMOTO K, SAWA H. Two βs or not two βs: regulation of asymmetric division by β-catenin. Trends Cell Biol. 2007b;17:465–73. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- MOLENAAR M, VAN DE WETERING M, OOSTERWEGEL M, PETERSON-MADURO J, GODSAVE S, KORINEK V, ROOSE J, DESTRÉE O, CLEVERS H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- MUNRO E, BOWERMAN B. Cellular symmetry breaking during Caenorhabditis elegans development. Cold Spring Harb Perspect Biol. 2009;1:a003400. doi: 10.1101/cshperspect.a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURGAN S, BERTRAND V. How targets select activation or repression in response to Wnt. Worm. 2015;4:e1086869. doi: 10.1080/21624054.2015.1086869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURGAN S, KARI W, ROTHBACHER U, ICHE-TORRES M, MELENEC P, HOBERT O, BERTRAND V. Atypical Transcriptional Activation by TCF via a Zic Transcription Factor in C. elegans Neuronal Precursors. Dev Cell. 2015;33:737–45. doi: 10.1016/j.devcel.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA K, KIM S, ISHIDATE T, BEI Y, PANG K, SHIRAYAMA M, TRZEPACZ C, BROWNELL DR, MELLO CC. Wnt signaling drives WRM-1/β-catenin asymmetries in early C. elegans embryos. Genes & Development. 2005;19:1749–1754. doi: 10.1101/gad.1323705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATARAJAN L, WITWER NE, EISENMANN DM. The divergent Caenorhabditis elegans β-catenin proteins BAR-1, WRM-1 and HMP-2 make distinct protein interactions but retain functional redundancy in vivo. Genetics. 2001;159:159–172. doi: 10.1093/genetics/159.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK FD, PRIESS JR. Establishment of POP-1 asymmetry in early C. elegans embryos. Development. 2003;130:3547–3556. doi: 10.1242/dev.00563. [DOI] [PubMed] [Google Scholar]
- PARK FD, TENLEN JR, PRIESS JR. C. elegans MOM-5/frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr Biol. 2004;14:2252–8. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- PARKER DS, JEMISON J, CADIGAN KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–76. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- PHILLIPS BT, KIDD AR, 3RD, KING R, HARDIN J, KIMBLE J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–6. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS BT, KIMBLE J. A new look at TCF and β-catenin through the lens of a divergent C. elegans Wnt pathway. Developmental Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POY F, LEPOURCELET M, SHIVDASANI RA, ECK MJ. Structure of a human Tcf4-β-catenin complex. Nature Structural Biology. 2001;8:1053–7. doi: 10.1038/nsb720. [DOI] [PubMed] [Google Scholar]
- RAVINDRANATH AJ, CADIGAN KM. Structure-function analysis of the C-clamp of TCF/Pangolin in Wnt/ss-catenin signaling. PLoS One. 2014;9:e86180. doi: 10.1371/journal.pone.0086180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHELEAU CE, DOWNS WD, LIN R, WITTMANN C, BEI Y, CHA YH, ALI M, PRIESS JR, MELLO CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- ROCHELEAU CE, YASUDA J, SHIN TH, LIN R, SAWA H, OKANO H, PRIESS JR, DAVIS RJ, MELLO CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–726. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- ROOSE J, CLEVERS H. TCF transcription factors: molecular switches in carcinogenesis. Biochimica et Biophysica Acta. 1999;97456:M23–M37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- ROOSE J, MOLENAAR M, PETERSON J, HURENKAMP J, BRANTJES H, MOERER P, VAN DE WETERING M, DESTREE O, CLEVERS H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–12. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- ROSE L, GONCZY P. Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook. 2014:1–43. doi: 10.1895/wormbook.1.30.2. [DOI] [PubMed] [Google Scholar]
- RUBINFELD B, ALBERT I, PORFIRI E, FIOL C, MUNEMITSU S, POLAKIS P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- SAWA H, KORSWAGEN HC. Wnt signaling in C. elegans. WormBook. 2013:1–30. doi: 10.1895/wormbook.1.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID T, HAJNAL A. Signal transduction during C. elegans vulval development: a NeverEnding story. Curr Opin Genet Dev. 2015;32:1–9. doi: 10.1016/j.gde.2015.01.006. [DOI] [PubMed] [Google Scholar]
- SCHMIDT DJ, ROSE DJ, SAXTON WM, STROME S. Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations. Mol Biol Cell. 2005;16:1200–12. doi: 10.1091/mbc.E04-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHETTY P, LO MC, ROBERTSON SM, LIN R. C. elegans TCF protein, POP-1, converts from repressor to activator as a result of Wnt-induced lowering of nuclear levels. Developmental Biology. 2005;285:584–92. doi: 10.1016/j.ydbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- SHIN TH, YASUDA J, ROCHELEAU CE, LIN R, SOTO M, BEI Y, DAVIS RJ, MELLO CC. MOM-4, a MAP kinase kinase kinase-related protein, activates WRM-1/LIT-1 kinase to transduce anterior/posterior polarity signals in C. elegans. Molecular Cell. 1999;4:275–280. doi: 10.1016/s1097-2765(00)80375-5. [DOI] [PubMed] [Google Scholar]
- SIEGFRIED K, KIMBLE J. POP-1 controls axis formation during early gonadogenesis in C. elegans. Development. 2002;129:443–453. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- SIEGFRIED KR, KIDD AR, 3RD, CHESNEY MA, KIMBLE J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–86. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOL SY. Wnt signaling and dorso-ventral axis specification in vertebrates. Current Opinion in Genetics and Development. 1999;9:405–410. doi: 10.1016/S0959-437X(99)80061-6. [DOI] [PubMed] [Google Scholar]
- SOKOL SY. Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Semin Cell Dev Biol. 2015;42:78–85. doi: 10.1016/j.semcdb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG X, WANG S, LI L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell. 2014;5:186–93. doi: 10.1007/s13238-014-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROVEL ET, WU D, SUSSMAN DJ. Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem. 2000;275:2399–403. doi: 10.1074/jbc.275.4.2399. [DOI] [PubMed] [Google Scholar]
- SUGIOKA K, MIZUMOTO K, SAWA H. Wnt Regulates Spindle Asymmetry to Generate Asymmetric Nuclear beta-Catenin in C. elegans. Cell. 2011;146:942–54. doi: 10.1016/j.cell.2011.07.043. [DOI] [PubMed] [Google Scholar]
- SUGIOKA K, SAWA H. Regulation of asymmetric positioning of nuclei by Wnt and Src signaling and its roles in POP-1/TCF nuclear asymmetry in Caenorhabditis elegans. Genes Cells. 2010;15:397–407. doi: 10.1111/j.1365-2443.2010.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULSTON JE, HORVITZ HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental Biology. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- TAKEMARU KI, MOON RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKESHITA H, SAWA H. Asymmetric cortical and nuclear localizations of WRM-1/β-catenin during asymmetric cell division in C. elegans. Genes Dev. 2005;19:1743–8. doi: 10.1101/gad.1322805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON B, TOWNSLEY F, ROSIN-ARBESFELD R, MUSISI H, BIENZ M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–73. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- THORPE CJ, SCHLESINGER A, CARTER JC, BOWERMAN B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- VORA S, PHILLIPS BT. Centrosome-Associated Degradation Limits beta-Catenin Inheritance by Daughter Cells after Asymmetric Division. Curr Biol. 2015;25:1005–16. doi: 10.1016/j.cub.2015.02.020. [DOI] [PubMed] [Google Scholar]
- VORA SM, PHILLIPS BT. The benefits of local depletion: The centrosome as a scaffold for ubiquitin-proteasome-mediated degradation. Cell Cycle. 2016:1–11. doi: 10.1080/15384101.2016.1196306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLINGFORD JB, MITCHELL B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEHRLI M, TOMLINSON A. Independent regulation of anterior/posterior and equatorial/polar polarity in the Drosophila eye; evidence for the involvement of Wnt signaling in the equatorial/polar axis. Development. 1998;125:1421–32. doi: 10.1242/dev.125.8.1421. [DOI] [PubMed] [Google Scholar]
- WENICK AS, HOBERT O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell. 2004;6:757–70. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- WIGLEY WC, FABUNMI RP, LEE MG, MARINO CR, MUALLEM S, DEMARTINO GN, THOMAS PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145:481–90. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDWATER M, SANDER N, DE VREEDE G, VAN DEN HEUVEL S. Cell shape and Wnt signaling redundantly control the division axis of C. elegans epithelial stem cells. Development. 2011;138:4375–85. doi: 10.1242/dev.066431. [DOI] [PubMed] [Google Scholar]
- XING Y, TAKEMARU K, LIU J, BERNDT JD, ZHENG JJ, MOON RT, XU W. Crystal structure of a full-length beta-catenin. Structure. 2008;16:478–87. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO H, KISHIDA S, KISHIDA M, IKEDA S, TAKADA S, KIKUCHI A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y, TAKESHITA H, SAWA H. Multiple Wnts redundantly control polarity orientation in Caenorhabditis elegans epithelial stem cells. PLoS Genet. 2011;7:e1002308. doi: 10.1371/journal.pgen.1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMASHITA YM, JONES DL, FULLER MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- YAN D, WIESMANN M, ROHAN M, CHAN V, JEFFERSON AB, GUO L, SAKAMOTO D, CAOTHIEN RH, FULLER JH, REINHARD C, GARCIA PD, RANDAZZO FM, ESCOBEDO J, FANTL WJ, WILLIAMS LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG XD, HUANG S, LO MC, MIZUMOTO K, SAWA H, XU W, ROBERTSON S, LIN R. Distinct and mutually inhibitory binding by two divergent {beta}-catenins coordinates TCF levels and activity in C. elegans. Development. 2011;138:4255–4265. doi: 10.1242/dev.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG XD, KARHADKAR TR, MEDINA J, ROBERTSON SM, LIN R. beta-Catenin-related protein WRM-1 is a multifunctional regulatory subunit of the LIT-1 MAPK complex. Proc Natl Acad Sci U S A. 2015;112:E137–46. doi: 10.1073/pnas.1416339112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YODA A, KOUIKE H, OKANO H, SAWA H. Components of the transcriptional Mediator complex are required for asymmetric cell division in C. elegans. Development. 2005;132:1885–93. doi: 10.1242/dev.01776. [DOI] [PubMed] [Google Scholar]
- ZACHARIAS AL, MURRAY JI. Combinatorial decoding of the invariant C. elegans embryonic lineage in space and time. Genesis. 2016;54:182–97. doi: 10.1002/dvg.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACHARIAS AL, WALTON T, PRESTON E, MURRAY JI. Quantitative Differences in Nuclear beta-catenin and TCF Pattern Embryonic Cells in C. elegans. PLoS Genet. 2015;11:e1005585. doi: 10.1371/journal.pgen.1005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUMBRUNN J, KINOSHITA K, HYMAN AA, NATHKE IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11:44–9. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]