Abstract
The nervous system regulates immunity and inflammation. The molecular detection of pathogen fragments, cytokines, and other immune molecules by sensory neurons generates immunoregulatory responses through efferent autonomic neuron signaling. The functional organization of this neural control is based on principles of reflex regulation. Reflexes involving the vagus nerve and other nerves have been therapeutically explored in models of inflammatory and autoimmune conditions, and recently in clinical settings. The brain integrates neuro-immune communication, and brain function is altered in diseases characterized by peripheral immune dysregulation and inflammation. Here we review the anatomical and molecular basis of the neural interface with immunity, focusing on peripheral neural control of immune functions and the role of the brain in the model of the immunological homunculus. Clinical advances stemming from this knowledge within the framework of bioelectronic medicine are also briefly outlined.
Keywords: nervous system, brain, vagus nerve, immunity, inflammation, cytokines
INTRODUCTION
“Immunological science is in the midst of a revolution”—the opening words of the preface written more than 30 years ago by William Paul, the founding Editor of the Annual Review of Immunology (1). Dr. Paul’s vision of this then new journal was that its timely reviews would have a critical role in helping immunologists keep current with the rapidly changing field, a task that is even more difficult for people in other fields with interest in immunology.
The first 100 years of modern immunology were focused on understanding the role of hematopoietic cells in mediating the response to pathogens, and the acquisition of long-term immunity. More recently, the cytokine theory of disease; the clinical success of monoclonal antibodies in the therapy of rheumatoid arthritis, inflammatory bowel disease (IBD), and other cytokine-mediated diseases in humans; and the continuing successes with the development of immunotherapy to treat a variety of neoplasms have continued to elevate the importance of understanding immunity for the benefit of human health. Immunology has embraced and merged with fields that were previously viewed as “allied” but now occupy a central role in the understanding of immunity, including molecular biology, genetics, and oncology. Preeminent among these is neuroscience. Studying neuro-immune interactions and communication generates conceptually novel insights of interest for therapeutic development. It is increasingly recognized that in addition to the immune system, the nervous system contributes resources to protect the host in injury and infection (2, 3). The intersection of neuroscience and immunology has launched the new field of bioelectronic medicine, focused on mapping molecular mechanisms that can be controlled using bioelectronic devices that modulate specific neural circuits. Pilot clinical trials have reported success using bioelectronic devices targeting a vagus nerve reflex circuit, the inflammatory reflex, in patients with rheumatoid arthritis and IBD (4, 5), and large follow-up trials are under way. Accordingly, now is an ideal time to review the current state of neural regulation in the context of immunology.
Here we present mechanistic insight into the role of peripheral sensory nerves and efferent autonomic nerves in the regulation of immune function and their functional organization in pathways operating in a reflex manner (Figure 1). The integrative role of the brain in this regulation, in the model of the immunological homunculus, and brain functional alterations in inflammatory and autoimmune conditions are summarized. We also point to recent clinical implications of this knowledge. Understandably, an extensive review of the entire neuro-immune dialogue is beyond the scope of this paper. We refer to prior reviews that cover the role of the enteric nervous system in the regulation of immune responses in the gastrointestinal tract (6); the communication between neurons and cells with immune function [microglia and astrocytes in the central nervous system (CNS)] (7–9); and the hypothalamic-pituitary-adrenal (HPA) axis, a major brain-derived immunoregulatory mechanism with neural components (10–12).
Figure 1.
Communication between the nervous system and the immune system. The nervous system and the immune system communicate in response to pathogen invasion, tissue injury, and other homeostatic threats. Macrophages, neutrophils, monocytes, T lymphocytes, and other immune cells detect the presence of pathogen fragments and tissue injury molecules and release cytokines and other signaling molecules. These alterations in peripheral immune homeostasis are detected by sensory neurons residing in the dorsal root ganglia (DRG) and vagus nerve afferent neurons, which signal the spinal cord and the brain. Pathogens also directly activate afferent neurons. These signals are integrated in the central nervous system with descending signaling via sympathetic and efferent vagus nerve fibers with the release of catecholamines and acetylcholine, respectively. These neurotransmitters interact with immune cells and control immune cell function and responses. Other neurotransmitters released from neurons also play a role in immune control. The hypothalamic-pituitary-adrenal (HPA) axis with the release of corticosteroids also provides a conduit of brain–immune regulation.
NEUROCENTRIC PERSPECTIVES
In this section, we provide a brief outline of the nervous system, with a focus on peripheral neurons, whose participation in neuro-immune communication is further reviewed. We also summarize principles of reflex neural regulation and common features of neurons and immune cells that mediate their interactions.
Nervous System Organization
The nervous system comprises the CNS (the brain and the spinal cord) and the peripheral nervous system. The peripheral nervous system has somatic and autonomic components. Somatic nerves originate in the CNS, innervate skeletal muscles, and provide voluntary control of movements. The autonomic nervous system has sympathetic, parasympathetic, and enteric components. Sympathetic neurons localized in the spinal cord project to paravertebral or prevertebral ganglia and synapse with relatively long postganglionic fibers innervating blood vessels, lymphoid tissue and organs, bone marrow, joints, spleen, lungs and airways, gastrointestinal tract, liver, kidneys, and other visceral organs (13, 14). Ganglionic synaptic neurotransmission is cholinergic, while postganglionic neurons release norepinephrine, and to a lesser extent other catecholamines (e.g., epinephrine dopamine), and neuropeptide Y (13, 14). Sympathetic preganglionic fibers also control the secretion of epinephrine (acting as a hormone) from specialized chromaffin cells of the adrenal medulla. Catecholamines, interacting with G protein-coupled β- and α-adrenergic receptors, mediate sympathetic control of heart rate, blood pressure, pulmonary function, hematopoiesis, and other physiological processes (13).
The vagus nerve, with cell bodies residing in the dorsal motor nucleus of the vagus (DMN) and nucleus ambiguus in the brainstem medulla oblongata, is the main nerve of the parasympathetic division of the autonomic nervous system, innervating peripheral visceral sites. Vagus nerve efferent (motor) cholinergic fibers project to visceral organs, including the lungs, heart, liver, gastrointestinal tract, kidneys, and pancreas and form synaptic contacts with postganglionic neurons in proximity to or within these organs. Acetylcholine, the principal neuromediator released from postganglionic fibers, interacts with G protein-coupled muscarinic acetylcholine receptors (mAChRs) that mediate vagus nerve regulation of heart rate, gastrointestinal function, pancreatic exocrine and endocrine secretion, and other physiological functions. Another segment of the parasympathetic part of the autonomic nervous system is represented by cholinergic neurons with cell bodies (somata) localized in the sacral part of the spinal cord (15). The regulatory functions of these fibers making synaptic contacts with postganglionic fibers in the pelvic ganglion are mainly associated with the reproductive organs, large intestine, colon, and bladder (15). The enteric nervous system, with neuronal bodies and projections localized in the gut, controls gastrointestinal functions (16).
Afferent (sensory) neurons transmitting information from peripheral sites to the CNS are also important constituents of the peripheral nervous system. These neurons with cell bodies outside of the CNS are pseudounipolar cells with a single process forming a bidirectional axon. Afferent neurons with cell bodies localized in the dorsal root ganglia are somatosensory and visceral. Somatosensory neurons have their peripheral axons in the skin, joints, and muscles, whereas visceral neurons innervate the gastrointestinal tract, liver, pancreas, lungs, heart, and other organs. Both types of neurons project to the spinal cord via the dorsal horn and synapse with interneurons and relay neurons transmitting the signals to the brain (17, 18). Vagus nerve sensory (visceral) neurons are localized in the nodose and jugular ganglia. These neurons innervate the lungs, heart, gastrointestinal tract, liver, and pancreas and project centrally to the nucleus tractus solitarius (NTS) in the brainstem medulla oblongata (19, 20). Vagus afferent neurons run within the same nerve bundle with vagus efferent neurons and are about 80% of the total neuronal count. Glutamate is the main neurotransmitter released by vagus nerve sensory neurons (20). A well-studied function of vagal afferents is transmitting peripheral signals for alterations in metabolic homeostasis, including cholecystokinin, leptin, and glucose-like peptide 1 to the brain (20, 21).
Neuronal Reflex Regulation
Nervous system regulation of physiological homeostasis is importantly mediated via neuronal reflexes. The origins of neuroscience and reflex regulation date back to the early seventeenth century, when René Descartes proposed that animal behavior might be explained by reflex functions. In his model, a stimulus such as heat would be transmitted to the brain along hydraulic pressure gradients in nerves and this would activate a corresponding signal returning to the body to compensate, in this example, by activating a withdrawal reflex. This reflex theory of neuroscience was the principal dogma until the late nineteenth century, when Santiago Ramón y Cajal identified neurons as individual cells that propagated information in a unidirectional fashion. Thus, by the beginning of the twentieth century reflex circuits had been mapped. This was accomplished by selectively cutting or stimulating sensory or motor neurons from the periphery to the spinal cord and up into the somatosensory cortex of the brain. An understanding emerged that sensory and motor reflex arcs traveling from the brain to the spinal cord and out into the peripheral organs provide an acute control mechanism for physiological homeostasis.
Leading neuroscientists in the early twentieth century spent decades exhaustively mapping reflex circuits controlling physiological homeostasis. Harvey Cushing, the father of modern neurosurgery, discovered the reflex that is named for him by inflating a balloon in the cranium of a dog and observing the corresponding increase in blood pressure and decrease in heart rate and respiratory rate. Interested in understanding the neural circuits that control these physiological responses, he divided the vagus nerves and noted that he could isolate blood pressure and heart rate responses during the increases in intracranial pressure. As a result of these and other similar experiments, by the middle of the twentieth century dozens of reflexes involving the vagus nerve and other nerves had been identified as mechanisms to control the homeostasis of nearly all of the body’s organ systems—but not the immune system.
Reflexes can be relatively simple, e.g., as the axon reflex, or more complex, involving multiple neuronal types and CNS integration. In the axon reflex, sensory neurons both detect environmental changes and generate a response to these changes (22–24). In this structurally simple reflex, action potentials as a result of activation of peripheral axonal endings are propagated in an orthodromic fashion (to the neuronal bodies) and then, at a point of diversion, this neuronal activation is redirected, in an antidromic manner, back to axonal terminals. This results in the release of neuropeptides and other molecules, acting on the endothelium, smooth muscle cells, and other axonal terminals. Axon reflexes mediate vasodilation and other effects with a role in normal physiology and pathophysiological conditions, including asthma (23, 25). In reflexes involving the CNS, an environmental change activates afferent (sensory) neurons that signal to CNS interneurons (integrative centers) and a response via efferent neural output is generated. A variety of CNS-integrated reflexes, including those that are somatic (voluntary), autonomic/visceral (involuntary), or both, have been identified. The vagus nerve plays an essential role in reflexes regulating cardiac function (baroreflex), gastrointestinal function, hepatic metabolic processes, and feeding behavior (26–29). Together NTS (the main brainstem site where vagus nerve afferents terminate), DMN (a major source of vagus nerve efferents), and area postrema (a circumventricular organ in proximity) form the dorsal vagal complex (DVC), an important brainstem integrative and regulatory hub (20). Reflex neural regulation is a key component in maintaining homeostasis, but substantial disruption of nervous system architecture and functional activity, as in spinal cord injury, may result in eliciting detrimental reflexes (30, 31).
Shared Molecular Signaling Between the Nervous and Immune Systems
Niels Kaj Jerne was among the first to point to similarities between the nervous and the immune systems, including recognition components and the capability to learn and form memories (32). In his paper on the network theory of the immune system he noted, “These two systems stand out among all other organs of our body by their ability to respond adequately to an enormous variety of signals” (32, p. 387). We now know that in a reciprocal fashion, the nervous system is a major source of signals that affect immune function and that signals from the immune system have a major impact on the nervous system. These interactions are facilitated by anatomical proximity and sharing molecular vehicles of communication, including receptors and signaling molecules.
Afferent and efferent nerves innervate the skin and visceral organs and are strategically localized to monitor sites of infection and injury. The expression of molecules that in the past were solely assigned to immune regulation, including pattern recognition receptors (such as TLRs) and receptors for TNF, IL-1β, and other cytokines, has been identified on sensory neurons (33–37). In addition, the expression of receptors classically implicated in neural communication in the CNS and in peripheral nerve regulatory function has been identified on immune cells. For instance, muscarinic and nicotinic acetylcholine receptors and α- and β-adrenergic receptors are expressed on monocytes, macrophages, dendritic cells, endothelial cells, and T and B lymphocytes (38–40). In addition, immune cells synthesize and release acetylcholine, catecholamines, and other molecules originally identified as neurotransmitters and neuromodulators (38–41). These newly identified features of neurons and immune cells are of substantial biological importance. The availability of molecular sensors for detecting pathogen fragments and inflammatory molecules on both neurons and immune cells allows their simultaneous involvement in inflammatory responses (42). Immune cells utilize their additional neuron-like “equipment” in close-range paracrine inflammatory regulation and in relay mechanisms in neuroimmunomodulatory circuits (39, 40). Thus, the nervous system and the immune system that evolved seemingly different regulatory mechanisms can join forces in defense against dangers of life-threatening proportions.
FUNCTIONAL NEUROANATOMY FOR COMMUNICATION WITH THE IMMUNE SYSTEM
In this section we review the roles of sensory neurons in communicating alterations in peripheral immune homeostasis to the CNS and efferent neurons in regulating peripheral immune alterations, and their integration in a reflexive manner. Of note, peripheral immune signals can also be communicated to the CNS via nonneuronal humoral mechanisms, through circumventricular organs, or via neutrophil, monocyte, and T cell infiltration of the brain, as previously reviewed (43, 44).
Sensory Neurons and Immune Challenges
Afferent neurons innervate virtually all organs and tissues of the body and provide a vital conduit for communicating peripheral alterations in immune homeostasis to the CNS. Immune molecules and pathogens activate sensory neurons with cell bodies in the dorsal root ganglia and central projections to the spinal cord. In the spinal cord these neurons communicate with spinal interneurons, and relay neurons projecting to the brain (3) (Figure 2). A main group of these neurons, designated nociceptors, specialize in transmitting various forms of pain, which is also a cardinal feature of inflammation (3, 45, 46). The expression of several types of voltage-gated sodium channels, including Nav1.7, Nav1.8, and Nav1.9, and transient receptor potential (TRP) ion channels, including TRPV1, TRPM8, and TRPA1, on sensory neurons mediates depolarization and specific thermal, mechanical, and chemical sensitivities to noxious stimuli (45, 47). Sensory neurons, including nociceptors, also express receptors for cytokines, lipids, and growth factors (3). Cytokines, including TNF, IL-1β, IL-6, IL-17, prostaglandins, and other molecules released from macrophages, neutrophils, mast cells, and other immune cells in the vicinity, interact with sensory neurons through these receptors during infection, allergy, and tissue damage (46, 48–50) (Figure 2). These interactions result in an increased sensitivity of nociceptors to noxious stimuli, known as hyperalgesia, or in direct stimulation of sensory neurons (3, 45, 50). Commensal and pathogenic bacteria also activate sensory neurons through the release of specific metabolic molecules and toxins, acting on Nav1.7, Nav1.8, Nav1.9, TRPV1, and TRPA1 (42, 46).
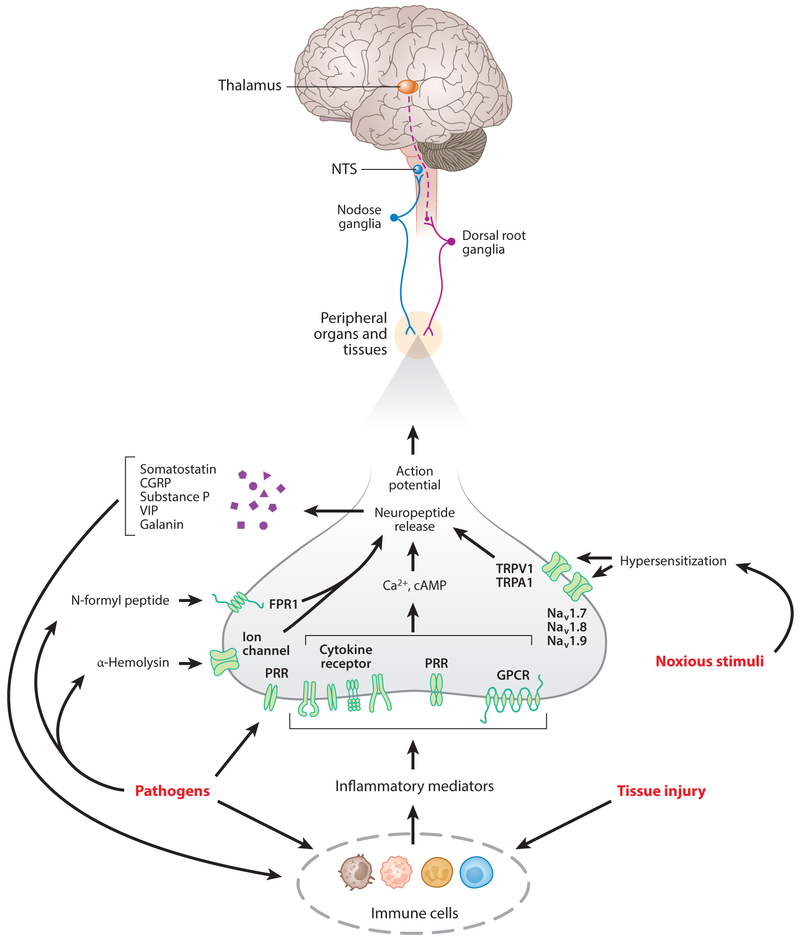
Figure 2.
Functional neuroanatomy and molecular mechanisms of sensing pathogens and immune mediators. Sensory neurons originating from the dorsal root ganglia (DRG) are somatosensory, innervating the skin, joints, and muscles, and visceral, innervating the liver, lungs, gastrointestinal tract, pancreas, heart, and other organs. These neurons enter the spinal cord via the dorsal horn and make synaptic contacts with interneurons and relay neurons (not shown) that signal to the brain, including the thalamus. Sensory vagus nerve fibers originating in the nodose ganglia innervate visceral organs and transmit signals to the nucleus tractus solitarius (NTS), with projections to other brainstem and forebrain areas (not shown). Inflammatory mediators, such as cytokines, are released by immune cells in response to tissue injury or pathogens and activate sensory neurons in the local area. Inflammatory mediators interact with sensory neurons through cognate receptors, expressed on these neurons. Neuronal activation results in a signaling cascade leading to action potential generation and may also decrease the activation threshold for nociceptive receptors [e.g., transient receptor potential cation channel subfamily members (TRPV1, TRPA1)] as well as the voltage-gated sodium channel Nav receptors (Nav1.7, Nav1.8 and Nav1.9) by noxious stimuli. Pathogen fragments can also activate sensory neurons directly by binding to the pattern recognition receptors (PRRs) (such as TLR4) on neurons. In addition, pathogens such as Staphylococcus aureus activate nociceptors by releasing N-formyl peptide and α-hemolysin, which bind to formyl peptide receptor 1 (FPR1) or ion channels. The release of neuropeptides, including calcitonin gene-related peptide (CGRP), galanin, somatostatin, substance P, and vasoactive intestinal peptide (VIP) in an axon-reflex fashion regulates immune responses. Some components of this figure are adapted from Reference 18.
Vagus nerve afferent neurons with cell bodies in the nodose and jugular ganglia (in rats and humans) transmit peripheral immune information to the brain (Figure 2). Most of the vagal afferents are unmyelinated, slow-conducting C-fibers, which can be activated by a variety of mechanical and chemical stimuli (19). Some of these fibers, innervating the lungs, airways, and other organs, express TRPV1, TRPA1, Nav1.7, Nav1.8, and Nav1.9 and function as nociceptors (19, 51, 52). Vagal sensory neurons are activated during bacterial or viral infection, cellular damage, and airway allergenic responses (19, 44, 46, 53, 54). This activation is mediated by TNF, IL-1β, prostaglandins, serotonin, and other molecules released from immune cells, including neutrophils, macrophages, and eosinophils (19, 44). These molecules increase neuronal sensitivity to noxious stimuli by lowering the threshold for evoking action potentials or directly activate sensory neurons (19, 53, 55) (Figure 2). Administration of lipopolysaccharide (LPS), cytokines (including IL-β and TNF), and pathogens such as Campylobacter jejuni to mice and rats stimulates vagus nerve afferent signaling, which can be traced to the NTS and then to other brainstem and forebrain regions (54, 56–59). The expression of pattern recognition receptors (TLR4) on nodose ganglion neurons and cytokines, including IL-1β and TNF receptors, on peripheral axonal endings provides a mediating mechanism for bacterial products, including LPS and cytokines, to activate vagal afferent neurons (33, 34, 37, 55, 57). Recent studies have advanced our understanding of the role of the vagus nerve in transmitting information for peripheral immune alterations to the brain. Intraperitoneal administration of TNF or IL-1β in mice results in increased vagus nerve activity and specific patterns of compound action potentials (37). Electrophysiological recordings of these cytokine-specific alterations in vagus nerve activity, or neurograms, are not obtained in mice with genetically ablated TNF and IL-1β receptors (37). These characteristic electrical patterns are also abrogated in mice with a surgically transected vagus nerve distal to the recording electrode, an observation that indicates that they are associated with afferent vagal nerve fibers (37). These findings indicate that specific peripheral immune signaling may have a characteristic differential impact on afferent vagus nerve activity. Further identification of populations of afferent vagus nerve fibers that are engaged in signaling specific alterations of peripheral immune homeostasis to the brain is of substantial scientific and therapeutic interest.
In addition to sensing immune molecules and pathogens, afferent neurons actively modulate immune responses and inflammation, as demonstrated in experimental IBD (46), arthritis (48), asthma (60), skin inflammation and chronic itch (61, 62), and bacterial infection (3, 42). Sensory neurons release substance P (SP), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), and other molecules interacting with the endothelium, neutrophils, macrophages, and other immune cells in the vicinity of axonal terminals (3, 42, 63) (Figure 2). Recent findings have also implicated the release of the neuropeptide neuromedin U from sensory and enteric neurons in the regulation of group 2 innate lymphoid cell-mediated antibacterial, inflammatory, and tissue protective immune responses (64–66).
Experimental evidence indicates that this dual function of sensory neurons may occur in an axon reflex-like fashion. For instance, in a mouse model of allergic inflammation and bronchial hyperresponsiveness, nociceptors activated by capsaicin release VIP and exacerbate inflammatory responses in the lungs (60). The release of VIP from pulmonary nociceptors can be directly activated by IL-5, produced by activated immune cells. VIP then acts on resident type 2 innate lymphoid cells and CD4+ T cells and stimulates cytokine production and inflammation (60). Selective blockade of these neurons by targeting sodium channels or genetic ablation of Nav1.8+ nociceptors suppresses immune cell infiltration and bronchial hyperresponsiveness in these mice (60). These findings identify lung nociceptors as important contributors to allergic airway inflammation (60). Elements of axon reflex regulation have also been highlighted during Staphylococcus aureus infection (42). The presence of this pathogen triggers local immune cell responses and activation of nociceptors innervating the mouse hind paw. Interestingly, genetic ablation of TLR2 and MyD88 or the absence of neutrophils, monocytes, natural killer (NK) cells, T cells, and B cells mediating innate and adaptive immune responses does not alter nociceptor activation during S. aureus infection. These observations indicate that immune nociceptor activation is not secondary to immune activation caused by the pathogen. This activation occurs directly, via the pathogen’s release of N-formyl peptides and the pore-forming toxin α-hemolysin, which induce calcium flux and action potentials (Figure 2). Nociceptor activation results in pain and the release of CGRP, galanin, and somatostatin, which act on neutrophils, monocytes, and macrophages and suppress S. aureus–triggered innate immune responses (42) (Figure 2). S. aureus–induced pain is abrogated and the local inflammatory responses, including TNF production and lymphadenopathy, are increased in mice with genetically ablated Nav1.8-lineage neurons, including nociceptors (42). These findings indicate the role of sensory nociceptor neurons in the regulation of local inflammatory responses triggered by S. aureus, a bacterial pathogen with an important role in wound- and surgery-related infections. This neuronal immunoregulatory function may be of particular therapeutic interest. Recent findings also point to the role of neural control in antigen trafficking through the lymphatic system, an important process in the generation of lymphocyte antigen-specific responses (67). Direct activation of the neuronal network innervating the lymph nodes results in the retention of antigen within the lymph, whereas blocking the neural activity restores antigen flow in lymph nodes. The antigen restriction is related to nociceptors, because selective ablation of Nav1.8+ sensory neurons significantly reduces antigen restriction in immunized mice (67). Furthermore, genetic deletion of FcγRI/FcεRI also reverses the antigen restriction (67).
Efferent Autonomic Neurons and Neural Reflexes in Immune Regulation
Efferent autonomic sympathetic and vagus nerve fibers have been actively studied in immune regulation. This research has provided evidence for the existence of neural reflex regulation of immunity and inflammation. Many diseases are characterized by peripheral immune dysregulation and excessive and chronic nonresolved inflammation, including sepsis, IBD, arthritis, obesity, and other diseases (21, 68, 69). Therefore, insight into the neural regulation of immunity and inflammation is of interest for developing new treatment options.
Catecholaminergic regulation of immune functions.
Catecholamines released from postganglionic fibers of sympathetic nerves and the adrenal medulla are important regulators of immune cell functions (13, 70–73). Norepinephrine and epinephrine interacting with adrenergic receptors expressed on neutrophils, monocytes, macrophages, T cells, and other immune cells regulate cytokine production and inflammation (Figure 3). This regulation is receptor dependent. Signaling through β-adrenergic receptors, and specifically β2-adrenergic receptors, is associated with anti-inflammatory effects (70). The intracellular cascade downstream of the receptor involves activation of cyclic AMP and protein kinase A and leads to suppression of NF-κB nuclear translocation and inhibition of production of TNF and other proinflammatory cytokines (41, 70) (Figure 3). Catecholamines acting via β2-adrenergic receptors also trigger intracellular signaling, leading to increased production of anti-inflammatory molecules, including IL-10 and TGF-β (13). However, signaling through α-adrenergic receptors on monocytes and macrophages can result in increased production of TNF and other cytokines (70, 74, 75).
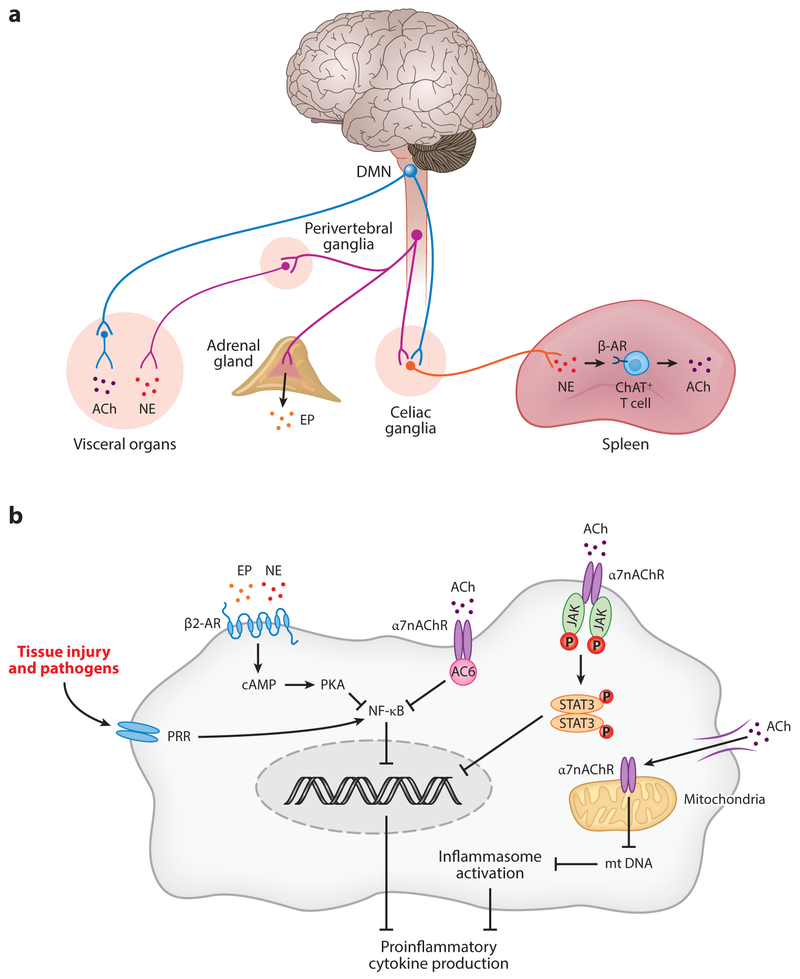
Figure 3.
Functional neuroanatomy and molecular mechanisms of regulating immune responses. (a) Sympathetic preganglionic fibers originating in the spinal cord terminate into paravertebral (not shown) and perivertebral ganglia releasing acetylcholine (ACh; not shown). Postganglionic catecholaminergic fibers innervate visceral organs and release norepinephrine (NE). Efferent sympathetic output to the adrenal medulla induces the secretion of epinephrine (EP) from chromaffin cells. The dorsal motor nucleus of the vagus (DMN) and nucleus ambiguus (not shown) in the brainstem medulla oblongata are major sources of efferent vagus nerve fibers. Cholinergic preganglionic vagus efferent fibers innervate visceral organs, where they interact with postganglionic fibers that release acetylcholine as a principal neurotransmitter. Preganglionic efferent vagus fibers also terminate in the celiac ganglia and the superior mesenteric ganglion, where the splenic nerve originates. The splenic nerve releases norepinephrine, which in turn activates the release of acetylcholine from the choline acetyl transferase (ChAT)-positive CD4+ T cells. (b) Acetylcholine and NE regulate cytokine release by immune cells activated in response to tissue injury or pathogen invasion. Acetylcholine binds to the α7 nicotinic acetylcholine receptor (α7nAChR) expressed on macrophages and other immune cells. This interaction activates intracellular signaling, involving suppression of NF-κB activity and activation of the JAK2/STAT3 pathway, which results in suppression of proinflammatory cytokine production. In addition, acetylcholine binds to the α7nAChR expressed on mitochondria and suppresses mitochondrial DNA release, which in turn inhibits inflammasome activation. Norepinephrine and epinephrine bind to the β2-adrenergic receptors on macrophages and other immune cells and induce intracellular signaling, involving cyclic AMP and protein kinase A (PKA), which results in suppression of NF-κB activity and proinflammatory cytokine release. Some components of this figure are adapted from Reference 18. Other abbreviations: cAMP, cyclic adenosine monophosphate; DMN, dorsal motor nucleus of the vagus; mt, mitochondrial; PRR, pattern recognition receptor.
Recent research has contributed to extending and deepening our knowledge of catecholaminergic regulation of immunity and inflammation. Catecholaminergic signaling via the β2-adrenergic receptor mechanism is implicated in the regulation of hematopoietic stem cell mobilization from bone marrow (76). Catecholaminergic signals originating from postganglionic fibers innervating lymphoid organs play an important role in the regulation of lymphocyte trafficking. Activation of β2-adrenergic receptors expressed on lymphocytes results in their retention in the lymph nodes (77). This regulation is associated with enhanced lymphocyte CXCR4 and CCR7 chemokine receptor responsiveness (77). In mice with T cell-mediated inflammatory conditions, activation of β2-adrenergic receptor-mediated signaling suppresses the egress of antigen-primed T cells from the lymph nodes and their migration to inflamed tissues (77). These specific catecholaminergic regulatory effects may be essential to controlling inflammatory responses (77, 78). Another recent study in mice provided important information about the functional inflammatory programming of macrophages in the gut wall and its neural catecholaminergic control (79). Macrophages localized in the lamina propria, underlying the epithelium, have a proinflammatory programming that is related to their active role in responding to damage in the epithelium (79). In contrast, muscularis macrophages deeper in the gut wall have a predominantly anti-inflammatory profile that is enhanced by the presence of pathogen (Salmonella) in the bowel (79). Catecholaminergic neurons are a major source of regulatory signals for this enhancement, via β2-adrenergic receptors expressed on muscularis macrophages (79). This augmentation of macrophage anti-inflammatory function in the intestinal muscularis balances protective responses against pathogens and other luminal damaging factors (79, 80). The role of neural catecholaminergic signaling in the liver was also evaluated in the context of stroke (81). Following cerebral infarction in mice, the hepatic invariant NK T cells undergo a transition from a proinflammatory T helper 1 (Th1)-type phenotype to an anti-inflammatory Th2-type phenotype, including increased IL-10 production. This is directly implicated in immunosuppression and increased susceptibility to bacterial infection following stroke mediated by catecholaminergic output from hepatic sympathetic innervations (81). Chemical ablation of hepatic catecholaminergic signals alleviates bacterial infection in this stroke mouse model (81). Catecholaminergic signaling also causes significant loss of splenic marginal zone B cells, which results in impaired IgM production and spontaneous bacterial infection in mice with stroke (82). Selective blocking of the β-adrenergic receptor signaling reverses the loss of the marginal zone B cells and suppression of circulating IgM levels, and significantly lowers bacterial burden (82). These findings have major implications for understanding the neural mechanisms of post-stroke immune dysregulation and increased susceptibility to bacterial infection (83–85).
Vagus nerve regulation of cytokine responses and inflammation and the inflammatory reflex.
The discovery of vagus nerve control of immunity occurred during the course of investigating the effects of injecting a cytokine-blocking molecule, CNI 1493, into the brains of rats and mice (86). The expected result was that this molecule would block the production of TNF and other cytokines in the brain; unexpectedly, cytokine production was also blocked in the heart, liver, spleen, and other organs. Cutting the vagus nerve, as Harvey Cushing had done years earlier, enabled isolation of the signals from the brain to the peripheral organs, the result being that cytokine production was not inhibited in the absence of an intact vagus nerve (86). This provided direct evidence that vagus nerve signals inhibit TNF production in the spleen and other organs. If the vagus nerve were transmitting an inhibitory signal to the immune system in the reticuloendothelial system, it would then be possible to utilize specific neural electrode stimulation parameters to drive action potentials in the vagus nerve and inhibit the activity of innate immune responses to endotoxin and other stimuli in the periphery (87).
Vagus nerve stimulation significantly suppresses serum and hepatic TNF levels in murine endotoxemia, and this effect is abrogated in animals with surgical transection of the vagus nerve (vagotomy) (87). Animals with vagotomy also have higher TNF levels (as compared to those who have undergone a sham operation), which is indicative of a tonic anti-inflammatory role of the vagus nerve (87). Stimulation of the nerve below the vagotomy level also results in anti-inflammatory effects (87). Acetylcholine, a major neurotransmitter utilized by vagus nerve efferent fibers, significantly inhibits LPS-stimulated macrophage production of TNF and other proinflammatory cytokines (87). These studies indicated that cholinergic signaling along the efferent vagus nerve has anti-inflammatory functions. Vagus nerve anti-inflammatory and disease-alleviating functions have been shown in endotoxemia (88–90), sepsis (88, 91), arthritis (92), IBD (93–95), hemorrhagic shock (96), postoperative ileus (97), renal ischemia and reperfusion injury (98), and other models of inflammatory and autoimmune conditions (69, 99, 100). The role of vagus nerve cholinergic signaling in endothelial cell activation and leukocyte trafficking has also been demonstrated (101, 102). The α7 nicotinic acetylcholine receptor (α7nAChR), expressed on macrophages and other immune cells, was identified as a main mediator of vagus nerve cholinergic anti-inflammatory output (103, 104). Intracellular mechanisms including suppression of NF-κB nuclear translocation, activation of a JAK2/STAT3 cascade mechanism, and signaling via the inflammasome mediate cholinergic signals through the α7nAChR that suppress production of TNF, IL-1β, and other proinflammatory cytokines (97, 105, 106) (Figure 3).
The resolution of inflammation is an active process of limiting and terminating inflammatory responses in restoring immune homeostasis mediated through the synchronized action of proresolving mediators, including resolvins, protectins, lipoxins, and maresins, and increased neutrophil and macrophage phagocytic activity (107, 108). Experiments utilizing vagotomy in a mouse model of zymosan-induced peritonitis indicated that vagus nerve cholinergic signaling promotes resolution processes in the lungs and abdominal tissues, mediated by netrin-1, an axonal guidance molecule and newly identified proresolving molecule (109). Vagus nerve cholinergic signaling was also implicated in enhancing inflammation resolution in response to bacterial infection (110). This neural control affects peritoneal group 3 innate lymphoid cells and the release of the protective resolvent molecule PCTR1 (110). These findings highlighting a role of the efferent vagus nerve in inflammation resolution in addition to its anti-inflammatory control suggest that the vagus nerve may function as an integrator of inflammatory stages.
Studies on the vagus nerve revealed reflex elements in the neural regulation of immunity. Early observations demonstrated that in addition to stimulating afferent vagus nerve activity, peripheral administration of IL-1β in rats also activates efferent vagus nerve and splenic nerve activity (58). Elucidating the regulatory role of the efferent vagus nerve in inflammation complemented this and other studies on the role of the afferent vagus nerve in communicating peripheral inflammatory signals to the brainstem. The combined sensory arc responding to cytokines and motor arc traveling in the vagus nerve that inhibits cytokines was termed the inflammatory reflex (111). In this reflex, vagal sensory neurons, activated by cytokines, including IL-1β and other inflammatory molecules, send messages to the brainstem NTS that are functionally integrated with efferent vagus nerve anti-inflammatory output originating from the DMN (111). Experimental evidence indicates a functional cooperation between the efferent vagus nerve and the splenic nerve in the inflammatory reflex (40, 88, 112, 113). Vagus nerve fibers from the DMN innervate celiac ganglia and the superior mesenteric ganglion (114, 115), which are a major source of splenic nerve catecholaminergic neurons (116–118). These ganglia provide an anatomical substrate for an interaction between the vagus nerve and the splenic nerve. Activation of the efferent arm of the inflammatory reflex by vagus nerve stimulation results in increased splenic acetylcholine levels (40). Catecholaminergic axonal terminals in the spleen are in proximity to T cells expressing choline acetyltransferase (ChAT), an enzyme responsible for acetylcholine synthesis, and β-adrenergic receptors (40, 119). Under vagus nerve control, splenic nerve catecholaminergic output through β-adrenergic receptor signaling activates the release of acetylcholine from these ChAT-expressing T cells (40). Acetylcholine further interacts with the α7nAChR expressed on macrophages and other immune cells and suppresses release of TNF and other proinflammatory cytokines (40) (Figure 3). ChAT-expressing T cells have a key role in the circuit, because vagus nerve stimulation does not suppress TNF levels in nude mice (lacking T cells) during endotoxemia, and adoptive transfer of ChAT-expressing T cells into nude mice restores the effect (40).
Historically, some have simplified the complex interactions between the parasympathetic and sympathetic divisions of the autonomic nervous system, suggesting that they always work in opposition in the regulation of physiological processes. Advances in neuroimmune communication have challenged this view as imprecise. Studies on the inflammatory reflex highlighted a noncanonical functional cooperation between vagus nerve (parasympathetic) and sympathetic neural signals to achieve regulation of innate immune responses and inflammation (40, 120). This realization is substantiated by studies demonstrating that vagus nerve stimulation suppresses circulating TNF levels and alleviates the severity of renal ischemia and perfusion injury in mice through α7nAChR-mediated signaling in the spleen (98). Interestingly, stimulation of afferent vagus nerve fibers also has protective effects in this model, which suggests other brain-mediated mechanisms (98). Functional integration of the vagus nerve and splenic nerve is also implicated in the regulation of adaptive immune responses and B cell antibody production in the spleen during Streptococcus pneumonia (121) and in controlling T cell activation and egress from the spleen in experimental hypertension (122). Further molecular mapping of these circuits using genetic approaches (120) will advance understanding of their functional organization and indicate new therapeutic approaches.
Broadening the view of reflex regulation in immunity.
Other neuronal reflexes in immune regulation have also been identified, and new interactive relationships and circuits have been evaluated (69, 120, 123). The involvement of somatosensory and somatic neural components in this regulation has been indicated.
Stimulation of somatosensory signaling to the CNS by acupuncture activates a brain muscarinic acetylcholine receptor (mAChR) mechanism that is linked with efferent vagus nerve activity and catecholaminergic regulation that suppresses serum TNF, IL-1β, and IL-6 levels in murine endotoxemia (124). Activation of the sciatic nerve by electroacupuncture via unknown mechanisms triggers stimulation of efferent vagus nerve signaling to the adrenal medulla that results in increased dopamine release (125). This is associated with anti-inflammatory effects mediated through D1 receptors and improved survival in mice with sepsis (125).
A neural gateway reflex has been identified in the regulation of the access of autoreactive T cells to the CNS in an animal model of multiple sclerosis (126, 127). In this circuit, soleus muscle contractions detected by somatosensory neurons result in neural communication with the release of catecholamines in the L5 dorsal blood vessels (126). This signaling stimulates an IL-6-amplifying mechanism of enhanced chemokine CCL20 expression that results in increased T cell entry into the CNS (126). Further elucidation of this reflex may suggest new approaches based on neural modulation to target the entry of autoreactive pathogenic T cells into the CNS for therapeutic benefit (127). Maladaptive, pathogenic spinal reflexes have been elicited in spinal cord injury at the cervical or upper thoracic level in which the control of the brain over spinal autonomic function was lost (30). These reflexes are triggered by activation of afferent neurons due to constriction of splanchnic vasculature (30). These neurons interact with spinal interneurons, resulting in stimulation of sympathetic signaling that causes hypertension and immunosuppression (30, 31, 128). Intervening in these circuitries may provide a new approach for treating post-spinal cord injury visceral complications and immunosuppression (31, 87).
THE ROLE OF THE CENTRAL NERVOUS SYSTEM
Brain regions and neuronal networks integrate and regulate neuro-immune communication. In this section we elaborate on this regulation from the perspective of the immunological homunculus (100). This model proposes identifying brain regions that receive and process afferent information about specific changes in immune homeostasis and orchestrate brain-derived immunoregulatory output (Figure 4). We also summarize evidence for alterations in brain function during disorders characterized by unbalanced peripheral immune responses.
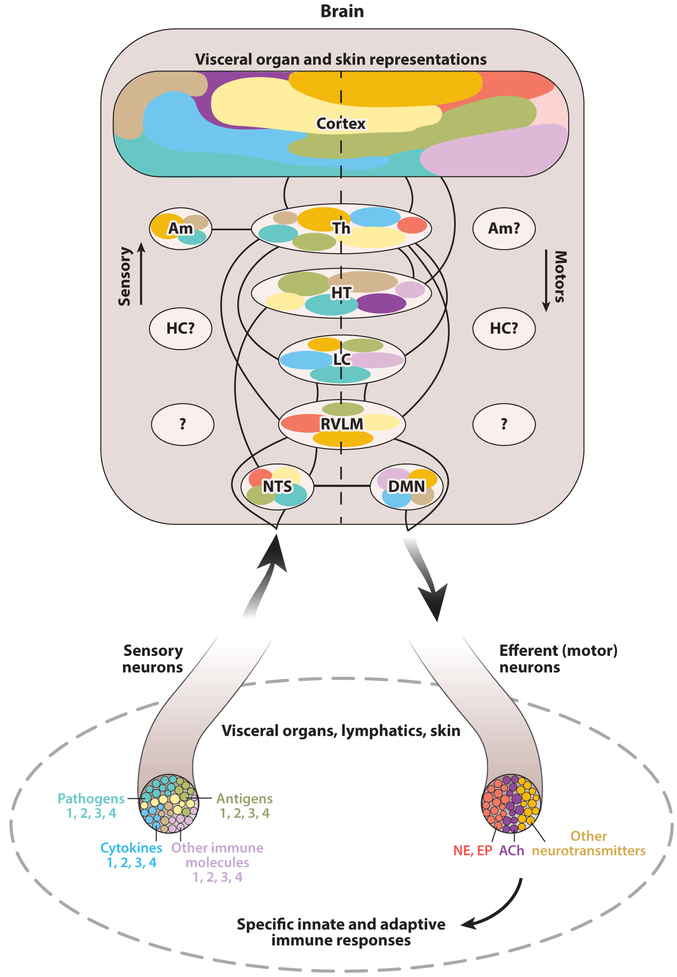
Figure 4.
The model of the immunological homunculus. Alterations in immune homeostasis in visceral organs and the skin are communicated to the spinal cord and the brain via sensory neurons residing in the dorsal root ganglia and vagal afferent neurons. It is important to consider that in this communication, specific neuronal populations (shown in different colors) are engaged in processing signals for the presence of pathogens, antigens, cytokines, and other immune cell–signaling molecules. These are listed as 1, 2, 3, and 4, but theoretically the list could be extended. This sensory information arrives in the nucleus tractus solitaries (NTS), rostroventrolateral medulla (RVLM), locus coeruleus (LC), hypothalamus (HT), thalamus (Th), and different cortex regions. Brain areas including the cortex, Th, HT, LC, RVLM, and dorsal motor nucleus of the vagus (DMN) are interconnected in orchestrating immunoregulatory (motor) output. Most of these brain regions participate in processing both sensory and motor immune-related information. It is possible that specific areas and nuclei in these regions (shown in different colors) are viscerotopically, somatotopically, and functionally organized in relation to peripheral immune information. The amygdala (Am), the hippocampus (HC), and other brain regions (question marks) may also be components of immune-related brain organization. Brain-derived immunoregulatory (motor) output is communicated to the periphery via sympathetic and vagus nerve efferent fibers, releasing norepinephrine (NE) and epinephrine (EP), acetylcholine (ACh), and other neurotransmitters and regulates a myriad of innate and adaptive immune responses in visceral organs, including the lymphatics, and the skin. It is conceivable that peripheral organs with a role in immunity are viscerotopically and somatotopically represented in the cortex by analogy with the classical model of homunculus. This schematic representation aims to present basic principles of the model. Some aspects, including brain neurotransmitter networks with a role in immune regulation, are not presented. The model should be further developed based on molecular mapping of neural circuitries and precise characterization of the roles of these and other unknown brain regions in immune regulation.
The Immunological Homunculus
The classical concept of homunculus proposes a somatotopic brain organization in which specific cortex areas are associated with processing of sensory information from certain parts of the body and other cortex areas are related to motor output and control of peripheral body parts. In this model of “the little man in the brain,” the somatic sensory and motor representation of body parts in the brain cortex is quite disproportional.
The concept of brain sensory and motor representations of immunity, or the immunological homunculus, was proposed in 2007 (100). The brain receives a substantial amount of sensory information for alterations in immune homeostasis via spinal and vagal visceral afferent nerves and spinal somatosensory neurons innervating the skin, as described in a previous section. This sensory information flow arrives in the spinal cord and the brainstem and then, via multisynaptic pathways, reaches forebrain regions and cortex areas (17, 20, 129, 130). Sensory input through spinal afferents is communicated to the thalamus with collateral projections to the rostroventrolateral medulla (RVLM), the locus coeruleus (LC), and other regions (129). These ascending signals are processed and integrated with cognitive functions and behavioral responses via neural projections to the amygdala, the anterior cingulate, and the somatosensory cortex (17, 129, 131). Afferent information via vagal sensory fibers is signaled to the brainstem NTS and then, through direct or multisynaptic projections, is transmitted to the hypothalamus, the cortex, and other forebrain areas (20, 57, 132).
Pathogens and cytokines, such as IL-1β, can directly activate sensory neurons (3, 50). An intriguing and important question is whether sensory neuron activation by pathogens and immune molecules engages specific neuronal populations within a sensory nerve. Another related question is whether these specific peripheral signals have specific brain representations. Identifying selective patterns of afferent vagus nerve activity (neurograms) in response to different cytokines, including IL-1β and TNF, is an important first step toward addressing these questions (37). This line of research may further benefit from molecular tools recently used to perform genetic molecular profiling of vagal afferents with a role in pulmonary and gastrointestinal regulation (133, 134). Brain neuronal activation (c-Fos) following intestinal infection with C. jejuni has been detected in the NTS, the area postrema, the ventrolateral medulla, the LC, the thalamus, different hypothalamic nuclei, the amygdala, and the insular cortex (54). However, it is an open question whether other pathogens generate different brain representations and whether those would be different from stimuli that act through the same nerves but are not directly related to immune homeostasis, e.g., metabolic molecules. The use of neural decoding technology to further decipher pathogen and immune molecule signals via peripheral nerves and in the CNS will be useful in gaining more specific insight (135). Elucidating brain representations of peripheral immune functions and highlighting the specific contribution of sensory immune signaling will enable a broader view of the immunological homunculus. Identifying brain centers with a role in the regulation of the myriad of innate and adaptive immune functions in different peripheral organs will be a challenging task. We should envision cortex and other brain representations of the skin, spleen, thymus, lymphatics, bone marrow, liver, lungs, and other organs and tissues from the perspective of their involvement in immune responses that are communicated to and regulated by the CNS. Thus, a very basic understanding of the immunological homunculus will emerge (Figure 4). This will be a dynamic model that will change, sometimes in a dramatic way, in cases of major homeostatic disruption of a certain immune function or its neural or other forms of communication to the CNS.
CNS control of peripheral immune responses is provided through spinal sympathetic and brainstem vagus nerve efferent signaling. Within the framework of the immunological homunculus, studying the role of brain regions in motor control of peripheral immune function will benefit from current knowledge. Descending projections from the cortex and other forebrain areas including the thalamus through collaterals to the RVLM and LC control spinal sympathetic neural signaling (129). Descending neural projections from the cortex and hypothalamus to the DMC, including the DMN, provide a regulatory control of efferent vagus nerve activity (Figure 4). Of note, ascending and descending pathways are integrated in the brain within reflexes regulating peripheral physiological processes. Many of these reflexes are integrated at the level of the brainstem. The NTS and DMN within the DVC integrate afferent and efferent vagus nerve activity (20, 57). The DVC is anatomically connected with the RVLM and LC, associated with sympathetic regulation and with hypothalamic nuclei (136). One of them, the paraventricular nucleus, plays a major role in the HPA axis (12, 137). These key regions in autonomic regulation receive input from the cortex and other higher forebrain regulatory centers coordinating autonomic visceral reflexes with behavioral responses (27, 29, 138, 139). In the model of the immunological homunculus we need to account for the CNS integration of reflexes controlling immunity and their coordination with cardiometabolic regulation and behavioral responses.
A great deal of experimental evidence has implicated various brain regions, including the brainstem, the limbic system, and the cortex, in the regulation of a myriad of peripheral immune functions (140, 141). We can now begin to organize this abundant but heterogeneous pool of data from the perspective of the immunological homunculus. Understanding can be deepened by providing insight into the role of specific CNS neurotransmitter and neuromodulatory systems in the regulation of immunity. Brain cholinergic mAChR signaling has been implicated in controlling peripheral inflammatory responses through the inflammatory reflex. Central, intracerebroventricular administration of mAChR ligands significantly suppresses serum TNF levels in murine endotoxemia and stimulates efferent vagus nerve activity (89). Centrally acting drugs, including galantamine and other acetylcholinesterase inhibitors, and M1 mAChR agonists suppress peripheral proinflammatory cytokines and improve survival in murine endotoxemia and sepsis and alleviate the severity of IBD in mice (142–144). These effects are mediated through central mAChRs and the vagus nerve. A recent study highlighted the involvement of dopaminergic signaling in the regulation of peripheral immune function (145). Selective chemogenetic stimulation of dopaminergic receptors in the ventral tegmental area results in augmented macrophage and dendritic cell phagocytic activity, macrophage and monocyte bactericidal activation, and suppressed bacterial accumulation in the liver (145). These effects are abrogated in animals with disrupted sympathetic catecholaminergic neurons, pointing to their mediating role in enhancing antibacterial immune activation (145).
These recent studies, demonstrating stimulation of cholinergic and dopaminergic neurons in the brain, began to paint a broader picture of brain immunoregulatory output. These CNS modulations are associated with seemingly different regulatory effects; however, they all center around improving immune homeostasis. Further mapping of brain neural networks that are engaged in these regulatory functions will be an important and challenging task. Knowledge of the anatomical localizations of neurotransmitter and neuromodulatory systems in the brain can be useful in these studies. For instance, basal forebrain cholinergic neurons projecting to cortex areas and the hippocampus were recently shown to play a role in the regulation of peripheral innate immune responses. Optogenetic stimulation targeting these neurons suppresses serum TNF during murine endotoxemia, an effect that is functionally linked to brain regulation of the inflammatory reflex (146). Brainstem C1 neurons were implicated in the regulation of murine kidney ischemia and reperfusion injury, a condition associated with aberrant inflammation (147). Selective optogenetic stimulation of tyrosine hydroxylase–expressing catecholaminergic C1 neurons in the left RVLM confers significant protection against kidney damage (147). This effect is linked to stimulation of both sympathetic and vagus nerve activity to spleen (147).
The brain cholinergic and dopaminergic systems interact with each other and with other brain neurotransmitter systems. Studying these interactions from the perspective of the brain’s control of immunity raises new intriguing questions. We need to consider that brain cholinergic signaling is associated with memory and learning (148) and dopaminergic signaling with reward and control of motor behavior (149), just to mention a few regulatory functions. The biological importance of overlapping regions and networks associated with these functions and with control of peripheral immune responses remains to be uncovered. We also need to account for the possibility of a broader scope of effects while altering these systems for therapeutic benefit.
Further mapping of brain regions and networks with a role in immune regulation is necessary for a more complete understanding of the immunological homunculus. This research will be facilitated by the use of genetic molecular tools, which are currently advancing neuroscience but have yet to be utilized in bridging neuroscience with immunology. These approaches include “barcoding of individual neuronal connections” that provides a selectivity at the level of single-neuron resolution in neuronal circuit mapping (150) and tracing neural connectivity of genetically defined neuronal types by using Cre-dependent viral constructs (151, 152). Specific insight into brain regions and networks in the model of the immunological homunculus will facilitate development of bioelectronics and other selective therapeutic approaches. Current technology, including deep brain stimulation, transcranial magnetic stimulation, and transcranial direct current stimulation (153, 154), may one day be redirected to treating inflammatory and autoimmune conditions. The rapidly growing field of bioelectronic medicine generates breakthrough technological advances in treating paralysis and other diseases by utilizing closed-loop devices (135, 155, 156). It is exciting to envision the future use of closed-loop devices in deciphering and regulating neuro-immune communication. These devices would enable the monitoring of peripheral nerve and brain representations of immunity and their alterations, and generating corrective signaling to treat aberrant fluctuations. These perspectives present exciting and challenging avenues for future research.
Functional Alterations in Brain During Peripheral Inflammation and Autoimmunity
Peripheral inflammation and autoimmune derangements have an impact on brain function. There is evidence that chronic peripheral immune activation and inflammation exacerbate neuroinflammation in neurodegenerative diseases, including Alzheimer disease (157) and schizophrenia (158, 159). Peripheral inflammatory stimuli, including LPS and live bacteria, can also cause proinflammatory signaling in the brain (160, 161). This proinflammatory signaling plays a role in mediating sickness behavior, associated with fever, reduced mobility, social withdrawal, and other symptoms, as demonstrated by peripheral administration of LPS, live bacteria, and cytokines, including TNF and IL-1β (44, 57, 161). Sickness behavior is an important adaptive and protective phenomenon, when it is transient. However, in diseases characterized by excessive or chronic peripheral inflammation and autoimmune derangements, sickness behavior alongside other alterations can be transitioned in brain functional deterioration with debilitating and life-threatening consequences.
Sepsis is such a disease—the number one killer in intensive care units (162, 163). Sepsis develops as a result of a dysregulated host response to infection (162). Innate and adaptive immune dysregulation alongside metabolic and cardiovascular complications are important contributors to sepsis pathology (164, 165). Brain functional impairment in sepsis is known as sepsis-associated encephalopathy (SAE), which is an important constituent of sepsis pathology (166). SAE, defined as brain dysfunction, secondary to infection in the body, and no CNS infection, is relatively frequent and is an independent predictor of mortality (166). An essential component of SAE is delirium (166). This neurobehavioral syndrome is caused by dysregulation of neuronal activity secondary to a broad spectrum of systemic disturbances, including increased release of proinflammatory cytokines and systemic inflammation (166, 167). Brain neurotransmission dysregulation, microglial activation and proinflammatory signaling, endothelial dysfunction, and cerebral blood flow dysregulation have been implicated in SAE (166, 168, 169). Brain cholinergic hypofunction and imbalances in dopaminergic and other neurotransmitter systems have been indicated as a central event in delirium (170, 171). Dysregulation in brain neurotransmission, including cholinergic and orexinergic signaling, has been reported in murine models of sepsis (172, 173). Sepsis has long-term consequences manifested by profound functional disabilities and increased mortality of sepsis survivors after their discharge from the hospital (174, 175). Brain dysfunction and persistent cognitive impairment are also profound manifestations of debilitating long-term sepsis sequelae (174). Despite their substantial clinical importance, events and mechanisms underlying this sepsis chronic illness and cognitive impairment are poorly understood. A key insight with potential therapeutic importance was provided by showing an important role for the peripherally released proinflammatory cytokine HMGB1 in mediating pathogenesis and cognitive deterioration in sepsis survivors in a mouse model (176, 177).
Postoperative conditions and acute and chronic liver diseases are among other disorders in which unbalanced peripheral immune responses and inflammation have been linked to brain functional impairment and cognitive decline (178–180). Postoperative cognitive decline has been related to poor prognoses and higher mortality (181). Systemic inflammation and the peripheral release of IL-1β and TNF have been shown to play a causative role in generating brain neuroinflammation in the hippocampus and cognitive impairment in postorthopedic surgery conditions in mice (178, 182). Acute and chronic liver failure in cirrhosis are associated with hepatic encephalopathy, mediated by increased ammonia levels, astrocyte enlargement and dysfunction in the brain, microglia activation, oxidative stress, and increased proinflammatory cytokine levels (179, 180). Brain cholinergic dysregulation has also been reported in patients with cirrhosis and in models of liver failure (183). Interestingly, alterations in brain cholinergic signaling parallel with lower vagal tone in patients with cirrhosis (184, 185).
Autoimmune diseases, including systemic lupus erythematosus, have an impact on brain function and are associated with neurological complications, including cognitive deterioration and fatigue (186, 187). Peripheral antibodies, released during the disease and targeting the brain glutamatergic system, have been directly implicated in brain derangements (188–191). This insight suggests new therapeutic possibilities. An interesting approach is antibody neutralization by using a decoy antigen to prevent antibody interactions with target tissues (192). A related question is whether cytokine-based or antibody-based therapies can be used in neuroprotective strategies (193) or for improving brain function (187). As recently suggested, antibodies with a role in brain pathology in autoimmune conditions could potentially have beneficial effects in individuals with underlying brain glutamatergic or dopaminergic hypofunction (187).
All of these studies speak to the notion that the brain, severely affected in inflammatory conditions, may lose its regulatory efficiency. The brain’s capabilities to regulate immune function, and other physiological functions, including cardiovascular control and regulation of metabolic homeostasis, may become compromised. Therefore, further insight into the immunological homunculus (Figure 4) and the deviations of the model will be necessary for developing efficient therapeutic approaches, including closed-loop devices.
CLINICAL TRANSLATION OF NEUROSCIENCE IN INFLAMMATORY DISEASE
Ongoing research provides important insight into neuro-immune communication with translational relevance (18, 113). In this section we briefly outline current clinical studies stemming from this research and exploring relevant pharmacological modalities and bioelectronic neuromodulation as novel therapeutics for inflammatory and autoimmune conditions.
Pharmacological Modalities
The discovery of the role of α7nAChR in mediating cholinergic regulation of cytokine production and inflammation (103) generated great interest in α7nAChR agonists in inflammatory settings. Anti-inflammatory and disease-ameliorating effects of GTS-21, choline, and other α7nAChR agonists have been shown in murine endotoxemia, sepsis, postoperative brain inflammation, ischemia and reperfusion injury, and many other models of inflammatory conditions (194–198). Galantamine and other centrally acting acetylcholinesterase inhibitors have also been evaluated as anti-inflammatory agents in endotoxemia, IBD, arthritis, and other disorders (142, 143, 198, 199). Galantamine also exerts anti-inflammatory effects and alleviates insulin resistance and other metabolic derangements in mice with obesity and metabolic syndrome (200).
These preclinical findings and the fact that galantamine is a clinically approved drug for the symptomatic treatment of Alzheimer disease led to studying galantamine in patients with metabolic syndrome. Metabolic syndrome is a constellation of risk factors including obesity, dyslipidemia, and high blood pressure and glucose levels, which together significantly increase the risk of developing type 2 diabetes and cardiovascular disease (201). Metabolic syndrome has become a pandemic, and no efficient treatments for this cluster of risk factors as a whole are available (201). The pathogenesis of this condition is not well understood, but chronic inflammation and interrelated insulin resistance are thought to be essential (201). A recent randomized, double-blind, placebo-controlled trial with 30 patients (15 males and 15 females) per group demonstrated the efficacy of galantamine in alleviating the inflammatory state and insulin resistance in metabolic syndrome (202). A relatively low dosage of galantamine (gradually escalated to 16 mg daily) for 12 weeks suppresses plasma levels of the proinflammatory cytokine TNF and adipokine leptin and increases levels of the anti-inflammatory cytokine IL-10 and adipokine adiponectin (202). Galantamine also significantly suppresses insulin levels and modulates heart rate variability in these patients (202). These findings are of interest for further studying galantamine and other cholinergic modalities in the treatment of metabolic syndrome and other related diseases, including type 2 diabetes.
The New Field of Bioelectronic Medicine
The understanding that electrical vagus nerve stimulation can be therapeutically exploited in preclinical models of arthritis, IBD, and many other inflammatory and autoimmune conditions (69, 92, 93, 100) was pivotal for developing a new field. Bioelectronic medicine seeks to identify molecular mechanisms of disease that can be therapeutically targeted by neural signals using bioelectronic devices. First results from studying the effects of implanted device vagus nerve stimulation in patients with Crohn’s disease and rheumatoid arthritis were recently reported (4, 5).
Crohn’s disease is a form of relapsing IBD that affects children and adults. It has a detrimental impact on quality of life, and treatment with biologics, including anti-TNF antibodies, is expensive and associated with significant adverse effects. A study reported significant clinical remission in five of seven patients with Crohn’s disease subjected to six months of treatment with implanted device vagus nerve stimulation (4). This disease-alleviating effect is accompanied by improved C-reactive protein, fecal calprotectin levels, and vagal tone (determined by analyzing heart rate variability).
Rheumatoid arthritis is a debilitating disease with inflammatory and autoimmune components. The disease has a relatively high prevalence—in 2014 it affected about 1.3 million adults in the United States alone (203)—and is expensive to treat. Currently available treatments, including methotrexate and biologics such as anti-TNF, anti-IL-6 receptor, anti-CD20 antibodies, and T cell costimulation inhibitor, are associated with toxicity and adverse effects. Moreover, some patients do not respond to these treatments. Implanted bioelectronic device vagus nerve stimulation of up to 84 days significantly alleviated the disease in two groups of 17 patients: The first group was in the early stage of disease and was not responding to methotrexate; the second had failed methotrexate treatment or a therapy with two or more biologics (5). This bioelectronic neuromodulation also suppresses TNF production (5). Retraction of the stimulation results in worsening of the disease and reactivation of the stimulation restores the therapeutic benefit (5). Ongoing trials are exploring the effects of bioelectronic vagus nerve stimulation in patients with IBD and rheumatoid arthritis in large-scale multicenter clinical trials.
Preclinical research on neuro-immune communication continues to provide mechanistic insight and to point to new therapeutic targets and approaches that are further explored in clinical settings. This symbiotic relationship has been integral to the development of bioelectronic medicine, in which advances in neurobiology are transformed into novel treatments of inflammatory diseases and a broad spectrum of other diseases with limited treatment options (135, 155, 156).
SUMMARY AND CONCLUSIONS
The nervous system and the immune system provide regulation and defense mechanisms. Here we have reviewed experimental evidence revealing that these two systems communicate with each other in coordinated mechanisms triggered by pathogen invasion, inflammation and autoimmune derangements. Sensory neurons, sending signals to the CNS, are anatomically positioned and equipped with molecular sensors to detect the presence of pathogens and alterations in the state of the immune system, including increased cytokine production. CNS-derived efferent autonomic neurons interact with immune cells through the release of catecholamines and acetylcholine and provide a vital control of cytokine production and many other immune cell functions. Reflexes, including an axon-like reflex within sensory neurons, and CNS-integrated reflex circuits comprising afferent and efferent neurons are integral to the functional organization of neural regulation of immune function. The vagus nerve plays an important role in a physiological mechanism, the inflammatory reflex controlling proinflammatory cytokine production and inflammation. This reflex is an example of a noncanonical cooperation between signals along the vagus nerve, the splenic nerve and a subset of acetylcholine-synthesizing T cells in the spleen. The CNS and the brain integrate and regulate sensory (afferent) and efferent neural signals associated with specific immunoregulatory function. These specific brain representations of immune functions are a foundation principle of the model of the immunological homunculus, which we have also described. Peripheral immune dysregulation and inflammation alter brain function and can be manifested by severe neurological complications in sepsis, systemic lupus erythematosus, and other disorders.
Currently, advances in neuroscience and immunology continue to revolutionize our thinking and understanding of the molecular mechanisms of immune regulation. Gaining mechanistic insight into neuro-immune communication has gone hand in hand with evaluating new therapeutic approaches, including pharmacological cholinergic modalities and electrical vagus nerve stimulation in preclinical settings of sepsis, IBD, arthritis, and many other inflammatory and autoimmune diseases. This research has recently led to successful clinical exploration of a cholinergic drug in metabolic syndrome and bioelectronic neuromodulation in Crohn’s disease and rheumatoid arthritis. The next several years should witness major progress in bioelectronic medicine, a field that links and translates insight about molecular mechanisms of neural regulation and disease pathogenesis into novel treatments of human disease based on neuromodulation of discrete neurocircuitries.
ACKNOWLEDGMENTS
This work was supported by the following grants from the National Institute of General Medical Sciences, National Institutes of Health: R01GM089807 (to K.J.T. and V.A.P.), P01AI102852-01A1 (to S.S.C. and K.J.T.), and R35GM118182 (to KJ.T.).
Footnotes
DISCLOSURE STATEMENT
The authors have the following disclosures: K.J.T. is a consultant to SetPoint Medical, Inc. K.J.T. and V.A.P. are authors on patents broadly related to the topic of this review and have assigned their rights to the Feinstein Institute for Medical Research.
LITERATURE CITED
- 1.Paul WE. 1983. Preface. Annu. Rev. Immunol 1:1. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ. 2010. Understanding immunity requires more than immunology. Nat. Immunol. 11:561–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbot S, Foster SL, Woolf CJ. 2016. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol 34:421–47 [DOI] [PubMed] [Google Scholar]
- 4.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, et al. 2016. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil 28:948–53 [DOI] [PubMed] [Google Scholar]
- 5.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, et al. 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. PNAS 113:8284–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo BB, Mazmanian SK. 2017. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46:910–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Perry VH. 2009. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol 27:119–45 [DOI] [PubMed] [Google Scholar]
- 8.Sofroniew MV. 2015. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci 16:249–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster JI, Tonelli L, Sternberg EM. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol 20:125–63 [DOI] [PubMed] [Google Scholar]
- 11.Chrousos GP. 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med 332:1351–62 [DOI] [PubMed] [Google Scholar]
- 12.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. 2013. Key role of CRF in the skin stress response system. Endocr. Rev 34:827–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. 2000. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev 52:595–638 [PubMed] [Google Scholar]
- 14.Jänig W. 2014. Sympathetic nervous system and inflammation: a conceptual view. Auton. Neurosci 182:4–14 [DOI] [PubMed] [Google Scholar]
- 15.Jänig W, Keast JR, McLachlan EM, Neuhuber WL, Southard-Smith M. 2017. Renaming all spinal autonomic outflows as sympathetic is a mistake. Auton. Neurosci 206:60–62 [DOI] [PubMed] [Google Scholar]
- 16.Goyal RK, Hirano I. 1996. The enteric nervous system. N. Engl. J. Med 334:1106–15 [DOI] [PubMed] [Google Scholar]
- 17.Dubin AE, Patapoutian A. 2010. Nociceptors: the sensors of the pain pathway. J. Clin. Investig 120:3760–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavan SS, Pavlov VA, Tracey KJ. 2017. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46:927–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzone SB, Undem BJ. 2016. Vagal afferent innervation of the airways in health and disease. Physiol. Rev 96:975–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthoud HR, Neuhuber WL. 2000. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci 85:1–17 [DOI] [PubMed] [Google Scholar]
- 21.Pavlov VA, Tracey KJ. 2012. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol 8:743–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaprak M. 2008. The axon reflex. Neuroanatomy 7:17–19 [Google Scholar]
- 23.Barnes PJ. 1986. Asthma as an axon reflex. Lancet 1:242–45 [DOI] [PubMed] [Google Scholar]
- 24.Nieuwenhoff MD, Wu Y, Huygen FJ, Schouten AC, van der Helm FC, Niehof SP. 2016. Reproducibility of axon reflex-related vasodilation assessed by dynamic thermal imaging in healthy subjects. Microvasc. Res 106:1–7 [DOI] [PubMed] [Google Scholar]
- 25.Houghton BL, Meendering JR, Wong BJ, Minson CT. 2006. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J. Physiol 572:811–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abboud FM, Thames MD. 2011 (1983). Interaction of cardiovascular reflexes in circulatory control Comprehensive Physiology, Suppl. 8: Handbook of Physiology; The Cardiovascular System, Peripheral Circulation and Organ Blood Flow, pp. 675–753. New York: Wiley [Google Scholar]
- 27.Dampney RA. 2016. Central neural control of the cardiovascular system: current perspectives. Adv. Physiol. Educ 40:283–96 [DOI] [PubMed] [Google Scholar]
- 28.Mayer EA. 2011. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci 12:453–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travagli RA, Hermann GE, Browning KN, Rogers RC. 2006. Brainstem circuits regulating gastric function. Annu. Rev. Physiol 68:279–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y Guan Z, Reader B Shawler T, Mandrekar-Colucci S, et al. 2013Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J. Neurosci 33:12970–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y. 2016. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci 19:784–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerne NK. 1974. Towards a network theory of the immune system. Ann. Immunol 125C:373–89 [PubMed] [Google Scholar]
- 33.Hosoi T, Okuma Y, Matsuda T, Nomura Y. 2005. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton. Neurosci 120:104–7 [DOI] [PubMed] [Google Scholar]
- 34.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. 2011. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab 301:E187–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu ZZ,Kim YH, Bang S,Zhang Y, Berta T, et al. 2015. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med 21:1326–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CK, Xu ZZ, Berta T, Han Q, Chen G, et al. 2014. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, et al. 2016. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron. Med 3:7–17 [PMC free article] [PubMed] [Google Scholar]
- 38.Kawashima K, Fujii T, Moriwaki Y, Misawa H. 2012. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci 91:1027–32 [DOI] [PubMed] [Google Scholar]
- 39.Kawashima K, Fujii T, Moriwaki Y, Misawa H, Horiguchi K. 2015. Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs. Int. Immunopharmacol 29:127–34 [DOI] [PubMed] [Google Scholar]
- 40.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, et al. 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marino F, Cosentino M. 2013. Adrenergic modulation of immune cells: an update. Amino. Acids 45:55–71 [DOI] [PubMed] [Google Scholar]
- 42.Chiu IM, Heesters BA, Ghasemlou N, von Hehn CA, Zhao F, et al. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. 2003. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med 9:125–34 [PMC free article] [PubMed] [Google Scholar]
- 44.Capuron L, Miller AH. 2011. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther 130:226–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinho-Ribeiro FA, Verri WA Jr., Chiu IM. 2017. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 38:5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai NY, Mills K, Chiu IM. 2017. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Intern. Med 282:5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Julius D. 2013. TRP channels and pain. Annu. Rev. Cell Dev. Biol 29:355–84 [DOI] [PubMed] [Google Scholar]
- 48.Schaible HG. 2014. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther 16:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicol GD, Lopshire JC, Pafford CM. 1997. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J. Neurosci 17:975–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, et al. 2008. Nociceptors are interleukin-1 β sensors. J. Neurosci 28:14062–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, et al. 2008. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol 586:1595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollarik M, Ru F, Brozmanova M. 2010. Vagal afferent nerves with the properties of nociceptors. Auton. Neurosci 153:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Browning KN, Verheijden S, Boeckxstaens GE. 2017. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 152:730–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. 2005. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun 19:334–44 [DOI] [PubMed] [Google Scholar]
- 55.Ek M, Kurosawa M, Lundeberg T, Ericsson A. 1998. Activation of vagal afferents after intravenous injection of interleukin-1 β: role of endogenous prostaglandins. J. Neurosci 18:9471–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. 2004. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav. Immun 18:123–34 [DOI] [PubMed] [Google Scholar]
- 57.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. 2000. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci 85:49–59 [DOI] [PubMed] [Google Scholar]
- 58.Niijima A. 1996. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst 61:287–91 [DOI] [PubMed] [Google Scholar]
- 59.Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. 1998. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res 804:306–10 [DOI] [PubMed] [Google Scholar]
- 60.Talbot S,Abdulnour RE, Burkett PR,Lee S, Cronin SJ, et al. 2015. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87:341–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng J, Yang P, Mack MR, Dryn D, Luo J, et al. 2017. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun 8:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, et al. 2017. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171:217–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu IM, von Hehn CA, Woolf CJ. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci 15:1063–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klose CSN,Mahlakoiv T, Moeller JB, Rankin LC,Flamar AL, et al. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549:282–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, et al. 2017. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549:351–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, et al. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549:277–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanes WM, Olofsson PS, Talbot S, Tsaava T, Ochani M, et al. 2016. Neuronal circuits modulate antigen flow through lymph nodes. Bioelectron. Med 3:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140:871–82 [DOI] [PubMed] [Google Scholar]
- 69.Andersson U, Tracey KJ. 2012. Reflex principles of immunological homeostasis. Annu. Rev. Immunol 30:313–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sternberg EM. 2006. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol 6:318–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Besser MJ, Ganor Y, Levite M. 2005. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFα or both. J. Neuroimmunol 169:161–71 [DOI] [PubMed] [Google Scholar]
- 72.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, et al. 2012. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. 1981. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res. Bull 6:83–94 [DOI] [PubMed] [Google Scholar]
- 74.Miksa M, Das P, Zhou M, Wu R, Dong W, et al. 2009. Pivotal role of the α2A-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLOS ONE 4:e5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. 2011. α1-Adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J. Pharmacol. Exp. Ther 338:648–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, et al. 2006. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124:407–21 [DOI] [PubMed] [Google Scholar]
- 77.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. 2014. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med 211:2583–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tracey KJ. 2014. Lymphocyte called home: β2-adreneric neurotransmission confines T cells to lymph nodes to suppress inflammation. J. Exp. Med 211:2483–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. 2016. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164:378–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335:936–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong CH,Jenne CN, Lee WY, Leger C, Kubes P. 2011. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334:101–5 [DOI] [PubMed] [Google Scholar]
- 82.McCulloch L, Smith CJ, McColl BW. 2017. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat. Commun 8:15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, et al. 2000. Medical complications after stroke: a multicenter study. Stroke 31:1223–29 [DOI] [PubMed] [Google Scholar]
- 84.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. 2005. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci 6:775–86 [DOI] [PubMed] [Google Scholar]
- 85.Prass K, Meisel C, Hoflich C, Braun J, Halle E, et al. 2003. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med 198:725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oke SL, Tracey KJ. 2008. From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex. J. Leukoc. Biol 83:512–17 [DOI] [PubMed] [Google Scholar]
- 87.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, et al. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–62 [DOI] [PubMed] [Google Scholar]
- 88.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, et al. 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203:1623–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, et al. 2006. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. PNAS 103:5219–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olofsson PS, Levine YA, Caravaca A, Chavan SS, Pavlov VA, et al. 2015. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron. Med 2:37–42 [Google Scholar]
- 91.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, et al. 2007. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med 35:2762–68 [DOI] [PubMed] [Google Scholar]
- 92.Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, et al. 2014. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLOS ONE 9:e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meregnani J, Clarencon D, Vivier M, Peinnequin A, Mouret C, et al. 2011. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci 160:82–89 [DOI] [PubMed] [Google Scholar]
- 94.Ghia JE, Blennerhassett P, El Sharkawy RT, Collins SM. 2007. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Gastrointest. Liver Physiol 293:G711–18 [DOI] [PubMed] [Google Scholar]
- 95.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. 2006. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131:1122–30 [DOI] [PubMed] [Google Scholar]
- 96.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, et al. 2004. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc. Res 63:357–65 [DOI] [PubMed] [Google Scholar]
- 97.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, et al. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol 6:844–51 [DOI] [PubMed] [Google Scholar]
- 98.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, et al. 2016. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Investig 126:1939–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pavlov VA. 2008. Cholinergic modulation of inflammation. Int. J. Clin. Exp. Med 1:203–12 [PMC free article] [PubMed] [Google Scholar]
- 100.Tracey KJ. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig 117:289–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, et al. 2005. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med 201:1113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, et al. 2009. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J. Immunol 183:552–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, et al. 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421:384–88 [DOI] [PubMed] [Google Scholar]
- 104.Olofsson PS, Katz DA, Rosas-Ballina M, Levine YA, Ochani M, et al. 2012. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med 18:539–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, et al. 2003. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107:1189–94 [DOI] [PubMed] [Google Scholar]
- 106.Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, et al. 2014. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol. Med 20:350–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Serhan CN, Chiang N, Dalli J. 2015. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol 27:200–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. 2014. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J. Exp. Med 211:1037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalli J, Colas RA, Arnardottir H, Serhan CN. 2017. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity 46:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tracey KJ. 2002. The inflammatory reflex. Nature 420:853–59 [DOI] [PubMed] [Google Scholar]
- 112.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, et al. 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. PNAS 105:11008–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pavlov VA, Tracey KJ. 2015. Neural circuitry and immunity. Immunol. Res 63:38–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berthoud HR, Powley TL. 1993. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst 42:153–69 [DOI] [PubMed] [Google Scholar]
- 115.Berthoud HR, Powley TL. 1996. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech 35:80–86 [DOI] [PubMed] [Google Scholar]
- 116.Bellinger DL, Felten SY, Lorton D, Felten DL. 1989. Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun 3:291–311 [DOI] [PubMed] [Google Scholar]
- 117.Li M, Galligan J, Wang D, Fink G. 2010. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton. Neurosci 154:66–73 [DOI] [PubMed] [Google Scholar]
- 118.Nance DM, Burns J. 1989. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav. Immun 3:281–90 [DOI] [PubMed] [Google Scholar]
- 119.Straub RH. 2004. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci 25:640–46 [DOI] [PubMed] [Google Scholar]
- 120.Pavlov VA, Tracey KJ. 2017. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci 20:156–66 [DOI] [PubMed] [Google Scholar]
- 121.Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al Abed Y, Tracey KJ, Diamond B. 2012. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol. Med 18:618–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, et al. 2016. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun 7:13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chavan SS, Tracey KJ. 2017. Essential neuroscience in immunology. J. Immunol 198:3389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song JG, Li HH, Cao YF, Lv X, Zhang P, et al. 2012. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology 116:406–14 [DOI] [PubMed] [Google Scholar]
- 125.Torres-Rosas R, Yehia G, Pena G, Mishra P, Rocio Thompson-Bonilla M, et al. 2014. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med 20:291–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arima Y, Harada M, Kamimura D, Park JH, Kawano F, et al. 2012. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 148:447–57 [DOI] [PubMed] [Google Scholar]
- 127.Sabharwal L, Kamimura D, Meng J, Bando H, Ogura H, et al. 2014. The Gateway Reflex, which is mediated by the inflammation amplifier, directs pathogenic immune cells into the CNS. J Biochem 156:299–304 [DOI] [PubMed] [Google Scholar]
- 128.Brommer B,Engel O, Kopp MA,Watzlawick R,Muller S,et al. 2016. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 139:692–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ossipov MH, Dussor GO, Porreca F. 2010. Central modulation of pain. J. Clin. Investig 120:3779–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lumpkin EA, Caterina MJ. 2007. Mechanisms of sensory transduction in the skin. Nature 445:858–65 [DOI] [PubMed] [Google Scholar]
- 131.Heinricher MM, Tavares I, Leith JL, Lumb BM. 2009. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev 60:214–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berthoud HR. 2004. Anatomy and function of sensory hepatic nerves. Anat. Rec. A 280:827–35 [DOI] [PubMed] [Google Scholar]
- 133.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. 2015. Vagal sensory neuron subtypes that differentially control breathing. Cell 161:622–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. 2016. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166:209–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bouton C. 2017. Cracking the neural code, treating paralysis and the future of bioelectronic medicine. J. Intern. Med 282:37–45 [DOI] [PubMed] [Google Scholar]
- 136.Monnikes H, Lauer G, Arnold R. 1997. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res 770:277–88 [DOI] [PubMed] [Google Scholar]
- 137.Turnbull AV, Rivier CL. 1999. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev 79:1–71 [DOI] [PubMed] [Google Scholar]
- 138.Shipley MT. 1982. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res. Bull 8:139–48 [DOI] [PubMed] [Google Scholar]
- 139.Benarroch EE. 1993. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc 68:988–1001 [DOI] [PubMed] [Google Scholar]
- 140.Wrona D. 2006. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol 172:38–58 [DOI] [PubMed] [Google Scholar]
- 141.Haas HS, Schauenstein K. 1997. Neuroimmunomodulation via limbic structures—the neuroanatomy of psychoimmunology. Prog. Neurobiol 51:195–222 [DOI] [PubMed] [Google Scholar]
- 142.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, et al. 2009. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun 23:41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. 2014. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 7:335–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosas-Ballina M, Valdes-Ferrer SI, Dancho ME, Ochani M, Katz D,et al. 2015. Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav. Immun 44:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ben Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, et al. 2016. Activation of the reward system boosts innate and adaptive immunity. Nat. Med 22:940–44 [DOI] [PubMed] [Google Scholar]
- 146.Pavlov VA, Lehner KR, Silverman HA, Tsaava T, Chavan SS, Tracey KJ. 2016. Optogenetic stimulation of brain cholinergic networks suppresses inflammation. J. Immunol 196(1 Suppl.):69–24. [Google Scholar]
- 147.Abe C, Inoue T, Inglis MA, Viar KE, Huang L, et al. 2017. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci 20:700–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ballinger EC,Ananth M, Talmage DA, Role LW. 2016. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91:1199–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Girault JA, Greengard P. 2004. The neurobiology of dopamine signaling. Arch. Neurol 61:641–44 [DOI] [PubMed] [Google Scholar]
- 150.Zador AM, Dubnau J, Oyibo HK, Zhan H, Cao G, Peikon ID. 2012. Sequencing the connectome. PLOS Biol 10:e1001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gore BB, Soden ME, Zweifel LS. 2013. Manipulating gene expression in projection-specific neuronal populations using combinatorial viral approaches. Curr. Protoc. Neurosci 65:4–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oh SW, Harris JA, Ng L, Winslow B, Cain N, et al. 2014. A mesoscale connectome of the mouse brain. Nature 508:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cabrera LY, Evans EL, Hamilton RH. 2014. Ethics of the electrified mind: defining issues and perspectives on the principled use of brain stimulation in medical research and clinical care. Brain Topogr 27:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hyam JA, Kringelbach ML, Silburn PA, Aziz TZ, Green AL. 2012. The autonomic effects of deep brain stimulation—a therapeutic opportunity. Nat. Rev. Neurol 8:391–400 [DOI] [PubMed] [Google Scholar]
- 155.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, et al. 2016. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533:247–50 [DOI] [PubMed] [Google Scholar]
- 156.Tracey KJ. 2015. Shock medicine. Sci. Am 312:28–35 [Google Scholar]
- 157.Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, et al. 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kirkpatrick B, Miller BJ. 2013. Inflammation and schizophrenia. Schizophr. Bull 39:1174–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meyer U, Schwarz MJ, Muller N. 2011. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol. Ther 132:96–110 [DOI] [PubMed] [Google Scholar]
- 160.Silverman HA, Dancho M, Regnier-Golanov A, Nasim M, Ochani M, et al. 2014. Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol. Med 20:601–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D.2015. Systemic inflammation and microglial activation: systematic review of animal experiments. J. Neuroinflammation 12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, et al. 2015. Sepsis: a roadmap for future research. Lancet Infect. Dis 15:581–614 [DOI] [PubMed] [Google Scholar]
- 164.Delano MJ, Ward PA. 2016. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J. Clin. Investig 126:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, et al. 2017. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med 45:253–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gofton TE, Young GB. 2012. Sepsis-associated encephalopathy. Nat. Rev. Neurol 8:557–66 [DOI] [PubMed] [Google Scholar]
- 167.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, et al. 2010. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med 38:1513–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Gool WA, van de Beek D, Eikelenboom P. 2010. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375:773–75 [DOI] [PubMed] [Google Scholar]
- 169.Lemstra AW, Groen in’t Woud JC, Hoozemans JJ, van Haastert ES, Rozemuller AJ, et al. 2007. Microglia activation in sepsis: a case-control study. J. Neuroinflammation 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hshieh TT Fong TG, Marcantonio ER Inouye SK. 2008. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J. Gerontol. A 63:764–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trzepacz PT. 2000. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin. Clin. Neuropsychiatry 5:132–48 [DOI] [PubMed] [Google Scholar]
- 172.Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T,et al. 2007. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp. Neurol 204:733–40 [DOI] [PubMed] [Google Scholar]
- 173.Deutschman CS, Raj NR, McGuire EO, Kelz MB. 2013. Orexinergic activity modulates altered vital signs and pituitary hormone secretion in experimental sepsis. Crit. Care Med 41:e368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iwashyna TJ, Ely EW, Smith DM, Langa KM. 2010. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE 2010. Long-term mortality and quality of life in sepsis: a systematic review. Crit. Care Med 38:1276–83 [DOI] [PubMed] [Google Scholar]
- 176.Valdes-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, et al. 2013. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6Chigh inflammatory monocytes in murine sepsis survivors. J. Intern. Med 274:381–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, et al. 2012. HMGB1 mediates cognitive impairment in sepsis survivors. Mol. Med 18:930–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. 2010. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. PNAS 107:20518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Felipo V. 2013. Hepatic encephalopathy: effects of liver failure on brain function. Nat. Rev. Neurosci 14:851–58 [DOI] [PubMed] [Google Scholar]
- 180.Coltart I, Tranah TH, Shawcross DL. 2013. Inflammation and hepatic encephalopathy. Arch. Biochem. Biophys. 536:189–96 [DOI] [PubMed] [Google Scholar]
- 181.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. 2009. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110:548–55 [DOI] [PubMed] [Google Scholar]
- 182.Cibelli M,Fidalgo AR, Terrando N, Ma D, Monaco C, et al. 2010. Role of interleukin-1 β in postoperative cognitive dysfunction. Ann. Neurol 68:360–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garcia-Ayllon MS, Cauli O, Silveyra MX, Rodrigo R, Candela A, et al. 2008. Brain cholinergic impairment in liver failure. Brain 131:2946–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barron HV, Alam I, Lesh MD, Strunk A, Bass NM. 1999. Autonomic nervous system tone measured by baroreflex sensitivity is depressed in patients with end-stage liver disease. Am. J. Gastroenterol. 94:986–89 [DOI] [PubMed] [Google Scholar]
- 185.Mani AR, Montagnese S, Jackson CD, Jenkins CW, Head IM, et al. 2009. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol 296:G330–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mackay M. 2015. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol. Res 63:26–37 [DOI] [PubMed] [Google Scholar]
- 187.Brimberg L, Mader S, Fujieda Y, Arinuma Y, Kowal C, et al. 2015. Antibodies as mediators of brain pathology. Trends Immunol 36:709–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. 2009. Losing your nerves? Maybe it’s the antibodies. Nat. Rev. Immunol 9:449–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Diamond B, Volpe BT. 2012. A model for lupus brain disease. Immunol. Rev 248:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. 2013. Brain-reactive antibodies and disease. Annu. Rev. Immunol 31:345–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, et al. 2015. Selective impairment of spatial cognition caused by autoantibodies to the N-methyl-d-aspartate receptor. EBioMedicine 2:755–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bloom O, Cheng KF, He M, Papatheodorou A, Volpe BT, et al. 2011. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. PNAS 108:10255–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, et al. 2000. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. PNAS 97:10526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, et al. 2007. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med 35:1139–44 [DOI] [PubMed] [Google Scholar]
- 195.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, et al. 2008. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med 14:567–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Norman GJ, Morris JS, Karelina K, Weil ZM, Zhang N, et al. 2011. Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: a role for cholinergic α7 nicotinic receptors. J. Neurosci 31:3446–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Terrando N, Yang T, Ryu JK, Newton PT, Monaco C, et al. 2014. Stimulation of the α7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol. Med 20:667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hoover DB. 2017. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther 179:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gowayed MA, Refaat R, Ahmed WM, El Abhar HS. 2015. Effect of galantamine on adjuvant-induced arthritis in rats. Eur. J. Pharmacol 764:547–53 [DOI] [PubMed] [Google Scholar]
- 200.Satapathy SK, Ochani M, Dancho M, Hudson LK, Rosas-Ballina M, et al. 2011. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol. Med 17:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grundy SM. 2008. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol 28:629–36 [DOI] [PubMed] [Google Scholar]
- 202.Consolim-Colombo F, Sangaleti CT, Costa FO, Morals TL, Lopes HF, et al. 2017. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight 10.1172/jci.insight.93340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hunter TM, Boytsov NN, Zhang X, Schroeder K,Michaud K,Araujo AB. 2017. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol. Int 37:1551–57 [DOI] [PubMed] [Google Scholar]